Summary
While metabolic syndrome and alcohol consumption are the two main causes of chronic liver disease, one of the two conditions is often predominant, with the other acting as a cofactor of morbimortality. It has been shown that obesity and alcohol act synergistically to increase the risk of fibrosis progression, hepatic carcinogenesis and mortality, while genetic polymorphisms can strongly influence disease progression. Based on common pathogenic pathways, there are several potential targets that could be used to treat both diseases; based on the prevalence and incidence of these diseases, new therapies and clinical trials are needed urgently.
Keywords: Alcohol, ALD, ASH, NASH, NAFLD, metabolic syndrome, obesity, diabetes
Abbreviations: ACC, acetyl-CoA carboxylase; ALD, alcohol-related liver disease; ASH, alcohol-related steatohepatitis; ASK-1, apoptosis signal-regulating kinase 1; BMI, body mass index; CLD, chronic liver disease; CPT, carnitine palmitoyltransferase; DNL, de novo lipogenesis; EASL, European Association for the Study of the Liver; ER, endoplasmic reticulum; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; HSD17B13, hydroxysteroid 17-beta dehydrogenase 13; IL, interleukin; LPS, lipopolysaccharide; MBOAT7, membrane bound O-acyl transferase 7; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OR, odds ratio; PAMP, pathogen-associated molecular pattern; PI3K, phosphatidylinositol-3-kinase; PIP3, phosphatidylinositol 3,4,5-triphosphate; PNPLA3, palatin-like phospholipase domain-containing 3; PRKCE, protein kinase C Epsilon; ROS, reactive oxygen species; SREBP-1c, sterol regulatory element binding protein-1c; TLR, Toll-like receptor; TM6SF2, transmembrane 6 superfamily member 2; TNF-α, tumour necrosis factor-α; WHO, World Health Organization
Key points.
-
•
ALD (alcohol-related liver disease) and NAFLD (non-alcoholic fatty liver disease) are the leading causes of chronic liver disease.
-
•
Obesity and alcohol synergistically increase the progression of fibrosis, mortality and enhance hepatic carcinogenesis.
-
•
Genetic polymorphisms strongly influence disease progression.
-
•
Based on pathogenesis, there are several potential targets that can be used to develop new treatments in these two diseases.
Introduction and disease burden
Around 844 million people suffer from chronic liver disease (CLD) resulting in approximately 2 million deaths per year.1 At present, alcohol and obesity are the leading causes of CLD in Western countries.2 The mean overweight and obesity rates have nearly tripled worldwide since 1975.3 In 2016, the World Health Organization (WHO) reported that more than 1.9 billion adults (39% of the world population) were overweight and that approximately 650 million of them were obese (13% of the world's population).3 In Europe, despite disparities among countries, more than 50% of the population is overweight and the obesity rate varies from 7% (in Italy) to 21% (in the United Kingdom).4,5 As a result, around 422 million patients have diabetes worldwide with a mortality rate of 1.6 million deaths per year.6 The prevalence of the hepatic consequences of metabolic syndrome, termed non-alcoholic fatty liver disease (NAFLD), has also increased and has reached nearly 24% in Western countries.[7], [8], [9]
Despite a global decrease in alcohol consumption in the past few decades, alcohol consumption remains high,10 with 10 litres of pure alcohol consumed per adult each year in Europe. Beer consumption is predominant in Northern and central Europe while in Southern Europe, people mainly drink wine. There has been a significant decrease in per capita consumption in the countries of Southern and Western Europe in the past few years (France, Germany, Greece, Italy etc.) while there has been a significant increase in Eastern and Northern Europe and the UK.10 There have been changes in the patterns of alcohol consumption, especially relating to binge drinking and episodes of heavy consumption. A recent report published in September 2019 by the WHO reveals that 30.4% of people report having consumed more than 60 g of pure alcohol on a single occasion in the last 30 days.11 Meanwhile, the issue of a “safe” quantity of daily alcohol consumption is still controversial. The Global Burden of Diseases group emphasized in its large study on the consequences of alcohol consumption that the risk of death or disability-adjusted life-years (DALYs) became significant from a daily alcohol consumption as low as 10 g/day.12 Thus, no level of alcohol improves health, as underlined by Burton & Sheron in their editorial.13
When the development of cirrhosis is considered to be an endpoint, the risk becomes significant above 12–25 g/day.14,15 However, individuals with excessive alcohol consumption will not all develop CLD, which emphasizes the role of cofactors such as obesity and insulin resistance. Due to the high prevalence of overweight/obesity and alcohol consumption worldwide, the presence of these conditions in the same individual is frequent and the presence of a combination of inflammatory lesions (alcoholic and non-alcoholic steatohepatitis) is plausible. Beside the consequences to the liver, the combination of alcohol and metabolic factors is associated with an increased risk of cardiovascular disease (20.8 million DALYs)12 and cancers.16 In obese patients, high alcohol consumption is associated with a higher risk (>2-fold) of colorectal cancer than in obese patients with low alcohol consumption.17 The identification of patients with excess alcohol consumption and metabolic syndrome is important for the liver because fibrosis progresses faster in this group and they are at a higher risk of liver-related deaths and hepatocellular carcinoma (HCC).[18], [19], [20] The European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases recommend not consuming more than 30 g/day for men and 20 g/day for women.21,22 In fact, these cut-offs are used to differentiate alcohol-related liver disease (ALD) from NAFLD, even though the evidence supporting their use for this purpose is not strong. Patients are often divided into two groups (with ALD or with NAFLD) although this classification is somewhat arbitrary in certain situations.
The present review summarizes the pathophysiology of the metabolic syndrome and alcohol, and their consequences on the liver, focusing on the impact of inflammatory lesions.
Alcoholic steatohepatitis and non-alcoholic steatohepatitis: Definitions, clinical signs and histological features
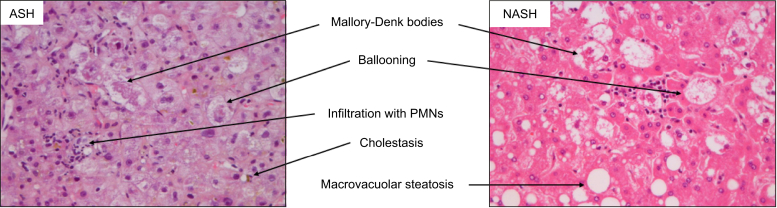
The spectrum of liver injury in ALD and NAFLD is quite similar. It ranges from steatosis, steatohepatitis to fibrosis, cirrhosis and HCC.23,24 Histologically, non-alcoholic steatohepatitis (NASH) and alcohol-related steatohepatitis (ASH) are difficult to distinguish. These two entities include a certain degree of steatosis, lobular inflammation and ballooning. However, some lesions described in ASH are very rare in pure NASH, for example, portal acute inflammation, the presence of large numbers of neutrophils, sclerosing hyaline necrosis and cholestasis.25 Some other lesions such as fibro-obliterative and inflammatory lesions of the outflow veins, alcoholic foamy degeneration, and acute cholestasis are seen during ALD but have not yet been described in NAFLD26 (Fig. 1).
Fig. 1.
Liver biopsy during alcoholic hepatitis and non-alcoholic steatohepatitis.
Staining was performed using haematoxylin, eosin and saffron. Magnification is 400×. Please note the presence of cholestasis during ASH and of steatosis in NASH. Steatosis can disappear during ASH after alcohol withdrawal. Intensity of neutrophil infiltrate and of Mallory-Denk bodies are more prevalent in ASH than in NASH. ASH, alcohol-related steatohepatitis; NASH: non-alcoholic steatohepatitis.
In the early phases, most patients with NASH and ASH are asymptomatic but later jaundice can develop in ASH (especially in its severe form), while this clinical symptom is almost never observed in patients with NASH except if another cause of hyperbilirubinemia is present (e.g. infection, HCC, etc.). These entities can also be difficult to distinguish biologically. Having predominantly elevated aspartate aminotransferase (AST) rather than alanine aminotransferase (ALT) suggests a mainly alcoholic origin. However, the AST/ALT ratio is no longer specific in patients with cirrhosis.27 Elevated gamma glutamyltransferase (GGT), which is often used to assess alcohol consumption, can be difficult to interpret as it also increases along with body mass index (BMI).28 In addition, the sensitivity of GGT for detecting alcohol consumption is only 60%.29
The assessment of fibrosis plays a key role in the management of patients with CLD. Transient elastography is a widely validated tool to assess fibrosis in both ALD and NAFLD,21,29 although its diagnostic accuracy seems better in the former. It should be noted that the cut-offs for detecting advanced fibrosis (≥F3) are different for the two conditions: 12.9 kPa in patients with ALD30 and 9.6 kPa in patients with NAFLD.31 Thus, care should be taken when interpreting liver stiffness measurements in patients with excessive alcohol consumption and obesity or the metabolic syndrome (Table 1).
Table 1.
Cut-off values of liver stiffness in kPa to detect hepatic fibrosis during ALD and NAFLD, according to the METAVIR classification.
ALD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease.
Obesity/metabolic syndrome and heavy alcohol consumption can be observed simultaneously in the same individual and similar histological lesions can be difficult to attribute to ASH or NASH. Thus, in the literature, the term “BASH” for “both alcoholic and non-alcoholic steatohepatitis” has been proposed to define these patients.32,33 This terminology is questionable and probably inappropriate since one of the two conditions is frequently predominant in an individual, while the other is more of a cofactor. In addition, this new terminology accentuates the role of inflammation while ASH, especially severe forms, has a different clinical presentation and a specific course.
Pathogenesis of NASH and ASH
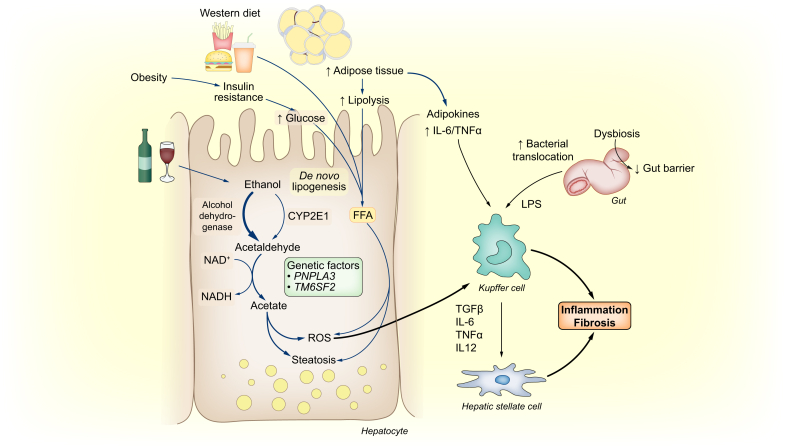
NAFLD and ALD share common pathogenic mechanisms34,35 (Fig. 2). For example, oxidative stress, inflammation and adipose tissue play a role in the development of both alcoholic and non-alcoholic steatohepatitis.
Fig. 2.
Common pathways in the pathogenesis of ALD and NAFLD, from alcohol, high-fat diet or obesity to inflammation and fibrosis.
ALD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease.
Alcohol metabolism
Under normal conditions, ethanol is mainly metabolized into acetaldehyde in an oxidative process driven by alcohol dehydrogenase and a microsomal system based on cytochromes P450, in particular CYP2E1.36,37 This microsomal pathway accounts for approximately 10% of ethanol biotransformation. Acetaldehyde is responsible for the generation of reactive oxygen species (ROS) which cause oxidative stress, endoplasmic reticulum (ER) stress and steatosis. In addition, after oxidation most acetaldehyde is converted into acetate by aldehyde dehydrogenase. This reaction is catalysed by NAD+/NADH and increases the amount of NADH in the liver.38 Alcohol dehydrogenase, CYP2E1 and aldehyde dehydrogenase are mainly expressed in hepatocytes, which explains why ethanol toxicity mainly affects these cells.
Glutathione plays an important role in the mitochondrial defence against constant ROS generation, especially hydrogen peroxide. However, chronic alcohol exposure causes glutathione depletion. Thus, the ethanol detoxification capacities are overtaken, leading to the accumulation of toxic metabolites in hepatocytes39,40 and resulting in lipid peroxidation, organelle damage and enhancement of steatosis.
Diet, insulin resistance and NAFLD
High daily caloric intake (especially intake of sugars, fats and carbohydrates) in modern society results in weight gain, insulin resistance and the development of fatty liver. Not all calories are alike. For example, many studies have confirmed the negative effect of fructose, a common component in sweeteners (sucrose, high-fructose corn syrup, etc.).41 Excess fructose intake leads to steatosis by increasing plasma triglyceride levels and de novo hepatic lipogenesis.42 Insulin resistance is an important step in the development of NASH and the composition of the diet can promote insulin resistance, whatever the BMI. Indeed, free fatty acids originating from dietary sources43 play a major role in the pathogenesis of insulin resistance. Excessive transport of dietary fat to hepatocytes and increased lipogenesis increase hepatic diacylglycerol content and the translocation of protein kinase C Epsilon (PRKCE) to the plasma membrane. PRKCE then impairs insulin signalling and its ability to activate glycogen synthesis and inhibit neoglucogenesis, leading to insulin resistance.44
Under normal circumstances, insulin binds to its receptor on the surface of hepatocytes leading to the autophosphorylation of the tyrosine kinase part of the receptor and its subsequent activation. The kinase part of the insulin receptor then phosphorylates phosphatidylinositol-3-kinase (PI3K)45 which catalyses the conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 then catalyses the autophosphorylation and activation of protein kinase B (AKT). These changes have multiple effects,46 including: stimulation of glycogen production by inhibiting glycogen synthase kinase and thereby increasing glycogen synthase activity,47 suppression of gluconeogenesis and increased glucose transport from the periphery into hepatocytes through glucose transporters. Ethanol can also induce hepatic insulin resistance through the inhibition of the PI3K/AKT pathway by decreasing insulin receptor density, inhibiting the binding affinity between insulin and its receptor, and decreasing receptor phosphorylation.48
Lipid accumulation in the liver in ALD and NAFLD
Whatever the underlying mechanism, common pathways lead to steatosis in both entities through an imbalance in fatty acid synthesis and β-oxidation. In NAFLD, steatosis is the consequence of lipid accumulation whereas in ALD, it is the consequence of direct ethanol toxicity in hepatocytes. Macrovacuolar steatosis is more frequent than microvesicular steatosis in both entities, although the latter is more frequent in ALD and is associated with more severe disease.25,49
Chronic alcohol exposure induces activation of sterol regulatory element binding protein-1c (SREBP-1c) in ALD, which promotes fatty acid synthesis. It also induces downregulation of peroxisome proliferator-activated receptor-α, causing reduced lipid catabolism and leading to fat accumulation in hepatocytes.50 Similar impairments in lipid metabolism are involved in NAFLD. Hyperinsulinaemia—associated with insulin resistance—causes upregulation of the transcription factor SREBP-1c, which is involved in de novo lipogenesis (DNL), while β-oxidation is reduced, promoting lipid accumulation.51
Acetyl-CoA carboxylase (ACC) is implicated in DNL through the production of malonyl-CoA. Carnitine palmitoyltransferase (CPT) is involved in mitochondrial β-oxidation. Both increased ACC and decreased CPT activity result in fat accumulation and steatosis. Chronic alcohol exposure inhibits AMPK (5′ adenosine monophosphate-activated protein kinase) leading to increased ACC and decreased CPT, and consequently to fat accumulation and steatosis.52,53
These mechanisms are potential targets for the treatment of lipid accumulation in both ALD and NAFLD.
Role of adipose tissue
Adipose tissue also plays an important role in the pathogenesis of ALD and NAFLD. At a basal state, adipose tissue is a source of proinflammatory cytokines such as interleukin (IL)-6 and tumour necrosis factor-α (TNF-α) and produces adipokines (leptin, adiponectin). Leptin has proinflammatory actions that normally prevent lipid accumulation in adipose sites and the liver by lowering SREBP-1c expression.54 Adiponectin has anti-inflammatory effects by inhibiting the release of TNF-α and IL-6, secreting anti-inflammatory cytokines and blocking NF-kB activation.55 Adiponectin also improves both hepatic and peripheral insulin resistance.
There is an imbalance in adipokines due to insulin resistance and adipose tissue hypertrophy in most obese patients, with reduced adiponectin and increased leptin levels resulting in steatosis, inflammation and fibrogenesis.56 When the leptin level increases, its profibrogenic role is prevalent, as it activates hepatic stellate cells through the sonic hedgehog and mTOR pathways.57,58
Chronic ethanol exposure induces CYP2E1 in adipose tissue,59 resulting in inflammation. Activation of CYP2E1 causes oxidative stress and ER stress leading to adipokine dysregulation and the progression of ALD.
Dysbiosis, immunity, and inflammation
The role of dysbiosis in the pathogenesis of both ASH and NASH has been shown in many studies. Germ-free mice are resistant to high-fat diet-induced obesity and hepatic steatosis.60 Animals transplanted with faeces from an obese donor accumulate more fat and develop exacerbated NASH compared to those transplanted from lean donors.61,62 In a recent study, Bacteroidetes abundance was shown to be increased in patients with NASH. The proportion of Ruminococcus was lower in these patients and was associated with significant fibrosis.63 In another study in patients with biopsy-proven NAFLD, a decrease in Firmicutes and an increase in Proteobacteria (including E. coli) were observed in patients with advanced NASH-related fibrosis. Ruminococcus obeum was significantly lower in advanced fibrosis than in mild/moderate NAFLD,64 suggesting a specific microbiota signature related to liver damage during NAFLD.65
Although microbial alterations are also found in patients with ALD, they are different to those in patients with NAFLD66 (Table 2). Certain patients with ALD have colonic dysbiosis, including a lower percentage of Bacteroidetes and a higher percentage of Proteobacteria than in non-drinkers.67 Along with the bacterial dysbiosis, a recent study also found changes in the abundance and composition of the faecal mycobiome (commensal fungi) in mice after chronic alcohol administration68 and in alcohol-dependent patients. Daily alcohol consumption for 10 weeks alters colonic mucosa-associated bacterial microbiota composition in rats.69 In another study, mice harbouring the intestinal microbiota from a patient with severe alcoholic hepatitis developed more severe liver inflammation, more liver necrosis, greater intestinal permeability and higher translocation of bacteria after alcohol challenge than mice harbouring the intestinal microbiota from an alcoholic patient without alcoholic hepatitis.70
Table 2.
Dysbiosis during NAFLD and ALD.
| Disease | Dysbiosis |
|
|---|---|---|
| Increased bacteria | Decreased bacteria | |
| NAFLD | Bacteroidetes | Ruminococcus |
| ALD | Proteobacteria | Bacteroidetes |
ALD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease.
Dysbiosis in both ALD and NAFLD leads to gut barrier dysfunction and increased intestinal permeability causing higher levels of endotoxins and enhancing pathogen-associated molecular pattern (PAMP)-induced liver inflammation. In fact, in ALD, chronic alcohol consumption results in increased intestinal TNF-α production causing disruption of intestinal tight junctions and intestinal barrier dysfunction.71 Studies in NAFLD have shown that alterations in the microbiome lead to endogenous production of alcohol72,73 resulting in the same intestinal barrier dysfunction.
The liver is exposed to gut-derived toxins by receiving more than 50% of the blood from the splanchnic district and represents the first line of defence against bacterial-derived products such as lipopolysaccharides (LPS). Chronic alcohol exposure increases circulating LPS concentrations.74,75 LPS and other PAMP molecules are recognised by pathogen-recognition receptors, including Toll-like receptors (TLRs, mainly TLR4 for LPS). After binding, LPS activates Kupffer cells resulting in the activation of mitogen-activated protein kinases (including JNK and p38), NF- κB76 and AP-177 and the release of ROS and proinflammatory cytokines (TNF-α, IL-1 and IL-6).78 Leukocyte recruitment amplifies the inflammatory response to LPS. Besides LPS-TLR4, the activation of TLR2 and TLR6 (which are involved in the recognition of bacterial lipopeptides), and TLR9 (which recognises bacterial DNA-containing unmethylated CpG motifs) leads to an increase in the proinflammatory cascade.79
The altered microbiome in patients with NAFLD, resulting in overgrowth of bacteria producing LPS and endogenous alcohol, induces increased intestinal permeability80 and bacterial translocation. Consequently, circulating levels of PAMPs increase, causing an inflammatory response through activation of liver cells.81 This disruption of the microbiome acts together with changes in adipose tissue to promote liver inflammation in obesity and NASH.
Role of genetics
Common genes have been identified in the pathogenesis of ALD and NAFLD.82 Genome-wide association studies have identified polymorphisms in the palatin-like phospholipase domain-containing 3 (PNPLA3) gene, especially the rs738409[G] variant, in the development and progression of NAFLD and ALD.[83], [84], [85] The PNPLA3 gene normally encodes adiponutrin, a protein involved in lipid metabolism. Patients with the rs738409[G] variant have higher DNL and expression of SREBP-1c and decreased secretion of triglyceride-rich lipoproteins from the liver.86 This leads to steatosis both in NAFLD87,88 and ALD.89 Whatever the mechanism leading to the hepatic insult, patients with the rs738409[G] variant are at an increased risk of cirrhosis. In patients with excess daily alcohol intake, the odds ratio (OR) for cirrhosis is increased by 2-fold in rs738409[G] carriers compared to those not carrying this allele.84,90 The meta-analysis published by Sookoian showed that there was a 3-fold greater risk of developing fibrosis in GG homozygous compared to CC homozygous individuals.85 In patients with NAFLD, GG homozygotes exhibit a 5-fold increased risk of HCC compared to CC homozygotes.91 An increased risk of HCC is also observed in rs738409[G] carriers with alcohol-related cirrhosis.92,93
The transmembrane 6 superfamily member 2 (TM6SF2) gene is a regulator of liver fat metabolism, influencing triglyceride secretion and hepatic lipid droplet content. The TM6SF2 rs58542926 variant has been identified as a disease modifier in both ALD and NAFLD. In patients with ALD and this variant, the risk of cirrhosis is increased by 1.4-fold.94,95 In patients with NAFLD, this variant was also associated with advanced hepatic fibrosis.96 The TM6SF2 rs58542926 variant is associated with a 1.5-fold increased risk of HCC in patients with alcohol-related cirrhosis.92,93 In patients with NAFLD, the link between this variant and HCC is unclear. Liu et al. found an association between the TM6SF2 E167K variant and the risk of NAFLD-related HCC (OR = 1.9). However, this allele was not independently associated with HCC after adjustment for confounding factors.96 The same result was found in a recent study.93
Membrane bound O-acyl transferase 7 (MBOAT7) catalyses the transfer of an acyl-CoA to lysophosphatidylinositol, which regulates the proinflammatory effects caused by free arachidonic acid and eicosanoid. The rs641738 genotype at the MBOAT7-TMC4 locus, encoding for MBOAT7 was associated with more severe liver damage and an increased risk of fibrosis in patients with NAFLD and ALD.94,97
In a study by Abul-Husn et al., which explored the genetic factors underlying CLD from various causes, a variant (rs72613567:TA) in HSD17B13, encoding the hepatic lipid droplet protein hydroxysteroid 17-beta dehydrogenase 13, was found to be associated with a reduced risk of ALD and NAFLD. The risk of alcoholic cirrhosis was reduced by 42% among heterozygotes and by 73% among homozygotes. In addition, the risk of non-alcoholic cirrhosis was reduced by 26% among heterozygotes and by 49% among homozygotes.98 It seems that HSD17B13 modulates liver inflammation and fibrosis but does not play a significant role in lipid accumulation in the liver.
Overall, most evidence linking genetics and ALD or NAFLD underline the critical role of factors associated with lipid metabolism in the liver, from the early stages to HCC.
Combined effects of obesity, diabetes, metabolic syndrome and alcohol in patients with alcohol-induced liver disease
The study by Bellentani et al. in a large cohort of individuals in Northern Italy was one of the first to show the combined effect of alcohol consumption and obesity on the development of hepatic steatosis, evaluated by ultrasonography. The prevalence of hepatic steatosis was 46% in non-obese heavy drinkers (>60 g of alcohol per day) and 76% in obese patients who did not drink. Steatosis was observed in 94% of obese heavy drinkers, leading to a 5.8-fold relative risk of steatosis compared to non-obese controls.7 Several other studies have confirmed this association.
Impact of obesity and diabetes on the progression of ALD
Alcohol and obesity synergistically increase the development of hepatic fibrosis and cirrhosis. The results of a French study published in 1997 showed that being overweight (defined as a BMI ≥25 kg/m2 in women and ≥27 in men) for at least 10 years was independently associated with the risk of steatosis, alcoholic hepatitis and cirrhosis.99 The same team confirmed these results in another study in 268 heavy drinkers.100 After adjusting for daily alcohol intake and total duration of alcohol abuse, the fibrosis score (>F2) was correlated with the BMI. In another study performed in the UK for 6.2 years in 1,230,662 middle-aged women without hepatic disease at baseline, alcohol consumption (>150 g/week) increased the relative risk of cirrhosis by approximately 3-fold. The deleterious association of alcohol consumption and obesity was confirmed by results showing that obese women who drink >150 g of alcohol/week had a more than 5-fold increase in the relative risk of cirrhosis.101 However, obese women who drink moderate amounts of alcohol (<70 g alcohol/week) were not at a higher risk of cirrhosis during the study period.
Obesity and diabetes increase mortality in patients with ALD
Hart et al. analysed 9,559 men in a prospective study. After a median follow-up period of 29 years, the adjusted relative rates of liver disease-related mortality in individuals who drank ≥15 units per week were 3 for underweight/normal weight men, 7 for overweight men, and nearly 19 for obese men. The relative rate in obese men who consumed 1–14 units per week was 5.3.102 In another Japanese study in heavy drinkers, the presence of diabetes was a significant risk factor for mortality in both cirrhosis (OR 8.10) and non-cirrhosis groups (OR 4.38).103
Obesity and diabetes increase the risk of HCC
In a prospective population-based study in 23,712 Taiwanese residents who were followed for 11.6 years, there was an association between alcohol use (defined as those who consume alcohol at least 4 days per week for at least a year) and obesity. The risk of HCC in obese patients increased by 7-fold (unadjusted analysis) and 4-fold (multivariable-adjusted analysis).104 Another Finnish study in 6,732 individuals without baseline liver disease observed that diabetes was the only significant predictor (hazard ratio of 6.79) of a severe liver event in individuals at risk due to alcohol use (≥210 g/week for men, ≥140 g/week for women).105 Another study performed in patients with ALD confirmed the higher risk of cirrhosis and HCC in diabetics compared to non-diabetics.106
Impact of alcohol in patients with NAFLD: The unsolved controversy
While there is no doubt that heavy drinking is harmful for the liver whatever the BMI, the consequence of moderate alcohol consumption in patients with NAFLD is somewhat controversial. Certain studies suggest that it may play a protective role while others report a harmful effect. The controversy is due to the absence of randomized studies, which are impossible for ethical reasons. The controversy also persists depending on the type of alcohol consumed.
In a study based on the Third National Health and Nutrition Examination Survey, 7,211 non-drinkers and 945 modest wine drinkers (alcohol consumption up to 10 g per day) were included. A diagnosis of NAFLD (based on unexplained serum ALT elevation) was observed in 3.2% of non-drinkers and 0.4% of modest wine drinkers. The adjusted OR of NAFLD was 0.15 in modest wine drinkers (95% CI 0.05–0.49) after adjusting for cofounding factors (age, gender, race, neighbourhood, income, education, caffeine intake, and physical activity), suggesting a protective role.107 In a cross-sectional analysis of participants to the NIH NASH Clinical Research Network, 251 lifetime non-drinkers and 331 modest drinkers (under 20 g per day) were included. Compared to lifetime non-drinkers with proven NAFLD, modest drinkers had a significantly lower risk of being diagnosed with NASH, with an OR of 0.56. The OR of NASH decreased as the frequency of alcohol consumption increased within the range of modest consumption. They also had significantly lower ORs for fibrosis (OR 0.56) and ballooning (OR 0.66).108
In a meta-analysis published by Sookoian et al. including 43,175 individuals (30,791 non-drinkers and 12,384 modest drinkers), moderate alcohol consumption was associated with a significantly lower risk of NAFLD (OR 0.68, p <10−8). The effect was greater in women than in men.109 The risk of developing NASH in the 822 patients with NAFLD was lower in those with moderate alcohol consumption with an OR of 0.5. The extent of fibrosis was also less severe in patients with moderate alcohol consumption. The type of alcohol consumed seems to be critical in determining the potential positive effects of moderate alcohol consumption in NAFLD. In an animal model of NAFLD, resveratrol (the wine polyphenol) decreases steatosis in mice fed with a high-calorie diet.110 Dunn et al. found that modest wine consumption was associated with a decreased risk of advanced fibrosis in NAFLD, while there was no benefit in beer drinkers.107 In addition to its potential role on the liver, wine consumption seems to decrease the risk of death from all causes, including cardiovascular and cerebrovascular events. In a large European study, the decrease in risk in wine drinkers was approximately 50% compared to that in non-wine drinkers.111
In another study that was not included in the meta-analysis by Sookoian et al., 71 patients with biopsy-proven NASH or NAFLD with a mean follow-up of 13.2 years showed that limited alcohol intake was independently associated with significant progression of fibrosis. In the same study, two other independent factors for the progression of fibrosis were identified including heavy episodic drinking and insulin resistance.112 In addition, Ascha et al. found an association between alcohol consumption and HCC in patients with NASH-related cirrhosis, with a relative risk of approximately 4.19
In summary, evidence suggesting that moderate daily alcohol consumption is beneficial in patients with NAFLD is limited. This impact, if it exists, seems only to be observed in wine drinkers. However, based on the conclusion of a Danish cross-sectional study, the putative positive effect of moderate wine consumption could be explained by the fact that wine buyers compared to beer buyers make more purchases of healthy food items.113 In addition, it must be remembered that moderate daily alcohol consumption is associated with a higher occurrence of non-hepatic diseases such as breast cancer in women and tuberculosis both in men and women.12 Thus, it is neither safe nor based on strong evidence to recommend moderate alcohol consumption in patients with NAFLD.
Treatments
Lifestyle interventions
The detection of early stages of ALD and NAFLD is essential because certain lesions (i.e. steatosis) may be reversed. Besides pharmacological treatment, certain lifestyle changes can be helpful for both ALD and NAFLD. EASL guidelines state that prolonged abstinence is needed to prevent the progression of fibrosis and the risk of HCC in patients with ALD.22 In addition, the identification and management of cofactors including insulin resistance and obesity are recommended. Consumption of alcohol and of sweet foods share the same reward pathways in the brain.114 To overcome cravings after alcohol withdrawal, patients frequently increase their calorie intake (especially sweet food consumption).115,116 This leads to weight gain117 and eventually obesity. Nutritional assessment along with regular physical exercise is then recommended and should be incorporated in the global strategy. A similar intervention is required for patients with NAFLD, in particular dietary restrictions and regular physical exercise, with EASL advising patients to target weight loss of 7 to 10% of bodyweight.21 This is based on a large body of evidence, such as the study by Vilar-Gomez et al. showing that weight loss through lifestyle changes is associated with the resolution of steatosis and NASH, improvement in insulin resistance and regression of fibrosis. However, only 10% of patients successfully achieve this level of weight loss.118
Coffee drinking seems to have beneficial effects on the risk of cirrhosis. In a recent meta-analysis, drinking up to two cups of coffee per day decreased the risk of alcoholic cirrhosis by nearly half after adjustment for confounding factors including alcohol consumption.119 Other studies confirm the protective effect of caffeine on the risk of NAFLD, NASH and the progression of fibrosis[120], [121], [122], [123] and HCC.124
Medical treatments
Lifestyle changes (i.e. control of body weight and absence of regular drinking) are crucial in the management of patients with NAFLD and ALD. However, as mentioned above, only a minority of patients with NAFLD achieve weight loss and some regular drinkers still consume alcohol despite medical advice. Thus, pharmacological interventions may be needed.
Unfortunately, there are no recommended medical treatments for NAFLD.21 Corticosteroids are the only validated treatment for ALD in the subgroup of patients with severe alcoholic hepatitis.125 No other therapeutic options are recommended in this group except those targeting alcohol addiction. Based on the common pathogenesis of ASH and NASH, new therapies are being evaluated to treat both conditions.
FXR (farnesoid X receptor) agonists have multiple actions on glucose and lipid metabolism. Activation of FXR increases glycogen synthesis and fatty acid oxidation in the liver and has anti-inflammatory and anti-fibrotic effects.126 Obeticholic acid, a synthetic FXR agonist, was the first agent to enter phase III development for NASH after promising results in the phase II FLINT study.127 The interim analysis of this phase III study, REGENERATE, including 931 patients, was completed in February 2019. Although the study reached the primary endpoint of an improvement in fibrosis of ≥1 stage with no worsening of NASH in the intention-to-treat population, there was no significant difference in the proportion of patients achieving NASH resolution without worsening of fibrosis.128 A phase II placebo-controlled randomized clinical trial (NCT02039219) using 10 mg of obeticholic acid daily for 6 weeks has been completed in patients with alcoholic hepatitis and a model for end-stage liver disease (MELD) score of between 12 and 19; the results are awaited.
Apoptosis signal-regulating kinase 1 (ASK-1) activation leads to phosphorylation of p38 and JNK, causing activation of stress response pathways that worsen hepatic inflammation, apoptosis, and fibrosis. Inhibition of ASK1 has been proposed as a target for the treatment of NASH and alcoholic hepatitis. Despite encouraging results from a small phase II study,129 selonsertib failed to improve NASH or fibrosis in the phase III STELLAR study.130 In a phase II clinical trial in 102 patients with severe alcoholic hepatitis, selonsertib was evaluated in combination with prednisolone. There was no survival benefit at 28 days in treated patients compared to those receiving placebo, and no difference in the Lille response or infection rates. Although there was no therapeutic response with selonsertib in NASH or ASH, strategies targeting ASK1 are still interesting.
Many drugs with various targets are currently being evaluated in the field of NAFLD: thyroid hormone receptor-beta agonists, aramchol (a steroyl-CoA desaturase-1 inhibitor), etc.131 The therapeutic landscape is expected to evolve in the coming years.
Other strategies: targeting gut-liver axis and bariatric surgery
Some studies have attempted to modify microbiota during NASH and ASH. While animal studies are encouraging, human data are less clear-cut. A pilot study132 has suggested that giving probiotics to heavy drinkers was associated with improved liver tests, but this has not been confirmed to date. Another pilot study tested the impact of faecal microbiota transplantation in 8 patients with severe alcoholic hepatitis, with encouraging results.133 However, these results must be validated in ongoing trials. In NAFLD, the administration of rifaximin for 6 weeks in patients with biopsy-proven NASH did not modify aminotransferases or hepatic lipid content.134 Thus, strategies targeting dysbiosis during ASH and NASH cannot be regarded as effective at present.
In patients unresponsive to lifestyle changes, bariatric surgery can be considered. Indeed, bariatric surgery is associated with the disappearance of NASH in 85% of morbidly obese patients.135 Many studies have confirmed the increased risk of alcohol use disorder or dependence mainly during the 5 years following bariatric surgery.[136], [137], [138], [139] This underlines the importance of screening alcohol use disorders in candidates for bariatric surgery. In addition, bariatric surgery influences alcohol metabolism due to the increased absorption of ethanol in the stomach and the intestine.140,141
Conclusion
In conclusion, while metabolic syndrome and alcohol consumption are the two main causes of CLD, one of the two conditions is often predominant, with the other acting as a cofactor of morbimortality. While abstinence has clearly been shown to prevent disease progression and complications in patients with ALD, there is still controversy surrounding moderate alcohol consumption in patients with NAFLD. Based on the common pathogenic pathways of ASH and NASH and the lack of effective treatments, new therapies and clinical trials are urgently needed.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Drafting of the manuscript and critical review: LCNW, VG, PM, AL.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100101.
Supplementary data
References
- 1.Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. doi: 10.1186/s12916-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimpin L., Cortez-Pinto H., Negro F., Corbould E., Lazarus J.V., Webber L. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 3.WHO Obesity and overweight. http://wwwwhoint/news-room/fact-sheets/detail/obesity-and-overweight Available at. Accessed 12 April, 2019.
- 4.Gallus S., Lugo A., Murisic B., Bosetti C., Boffetta P., La Vecchia C. Overweight and obesity in 16 European countries. Eur J Nutr. 2015;54(5):679–689. doi: 10.1007/s00394-014-0746-4. [DOI] [PubMed] [Google Scholar]
- 5.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di Angelantonio E. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellentani S., Saccoccio G., Masutti F., Crocè L.S., Brandi G., Sasso F. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 8.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2018. Global Status Report on Alcohol and Health 2018. [Google Scholar]
- 11.Pruckner N., Hinterbuchinger B., Fellinger M., Konig D., Waldhoer T., Lesch O.M. Alcohol-related mortality in the WHO European region: sex-specific trends and predictions. Alcohol Alcohol. 2019;54(6):593–598. doi: 10.1093/alcalc/agz063. [DOI] [PubMed] [Google Scholar]
- 12.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton R., Sheron N. No level of alcohol consumption improves health. Lancet. 2018;392(10152):987–988. doi: 10.1016/S0140-6736(18)31571-X. [DOI] [PubMed] [Google Scholar]
- 14.Corrao G., Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27(4):914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 15.Rehm J., Taylor B., Mohapatra S., Irving H., Baliunas D., Patra J. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 16.Massetti G.M., Dietz W.H., Richardson L.C. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA. 2017;318(20):1975–1976. doi: 10.1001/jama.2017.15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J., Zhu Y., Wang P.P., West R., Buehler S., Sun Z. Interaction between alcohol drinking and obesity in relation to colorectal cancer risk: a case-control study in Newfoundland and Labrador, Canada. BMC Public Health. 2012;12:94. doi: 10.1186/1471-2458-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A., Feldstein A.F., Zein N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto E., Yatsuji S., Tobari M., Taniai M., Torii N., Tokushige K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacSween R.N., Burt A.D. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6(3):221–232. doi: 10.1055/s-2008-1040605. [DOI] [PubMed] [Google Scholar]
- 24.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 25.Tiniakos D.G. Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroenterol Clin Biol. 2009;33(10–11):930–939. doi: 10.1016/j.gcb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Lackner C., Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Nyblom H., Berggren U., Balldin J., Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39(4):336–339. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- 28.Alatalo P.I., Koivisto H.M., Hietala J.P., Puukka K.S., Bloigu R., Niemela O.J. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr. 2008;88(4):1097–1103. doi: 10.1093/ajcn/88.4.1097. [DOI] [PubMed] [Google Scholar]
- 29.European Association for the Study of the Liver. European Association for the Study of the Liver EASL clinical Practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Khac E., Thiele M., Voican C., Nahon P., Moreno C., Boursier J. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(9):614–625. doi: 10.1016/S2468-1253(18)30124-9. [DOI] [PubMed] [Google Scholar]
- 31.Cassinotto C., Boursier J., de Ledinghen V., Lebigot J., Lapuyade B., Cales P. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 32.Becker U., Deis A., Sorensen T.I., Gronbaek M., Borch-Johnsen K., Muller C.F. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 33.Rinella M.E., Loomba R., Caldwell S.H., Kowdley K., Charlton M., Tetri B. Controversies in the diagnosis and management of NAFLD and NASH. Gastroenterol Hepatol (N Y) 2014;10(4):219–227. [PMC free article] [PubMed] [Google Scholar]
- 34.Louvet A., Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 35.Wree A., Broderick L., Canbay A., Hoffman H.M., Feldstein A.E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10(11):627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 36.Cederbaum A.I. Alcohol metabolism. Clin Liver Dis. 2012;16(4):667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieber C.S. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21(Suppl 3):S3–S6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirano T., Kaplowitz N., Tsukamoto H., Kamimura S., Fernandez-Checa J.C. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology. 1992;16(6):1423–1427. doi: 10.1002/hep.1840160619. [DOI] [PubMed] [Google Scholar]
- 41.Tappy L., Le K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 42.Chong M.F., Fielding B.A., Frayn K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85(6):1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 43.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumashiro N., Erion D.M., Zhang D., Kahn M., Beddow S.A., Chu X. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108(39):16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J., Liu F. Tissue-specific insulin signaling in the regulation of metabolism and aging. IUBMB Life. 2014;66(7):485–495. doi: 10.1002/iub.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carr R.M., Correnti J. Insulin resistance in clinical and experimental alcoholic liver disease. Ann N Y Acad Sci. 2015;1353:1–20. doi: 10.1111/nyas.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang M., de la Monte S.M., Longato L., Tong M., He J., Chaudhry R. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 2009;50(6):1192–1201. doi: 10.1016/j.jhep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip W.W., Burt A.D. Alcoholic liver disease. Semin Diagn Pathol. 2006;23(3-4):149–160. doi: 10.1053/j.semdp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Ji C., Chan C., Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45(5):717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Kohjima M., Higuchi N., Kato M., Kotoh K., Yoshimoto T., Fujino T. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21(4):507–511. [PubMed] [Google Scholar]
- 52.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68(6):2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You M., Matsumoto M., Pacold C.M., Cho W.K., Crabb D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 54.Kakuma T., Lee Y., Higa M., Wang Z., Pan W., Shimomura I. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci U S A. 2000;97(15):8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 56.Adolph T.E., Grander C., Grabherr F., Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18(8) doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aleffi S., Navari N., Delogu W., Galastri S., Novo E., Rombouts K. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G210–G219. doi: 10.1152/ajpgi.00047.2010. [DOI] [PubMed] [Google Scholar]
- 58.Choi S.S., Syn W.K., Karaca G.F., Omenetti A., Moylan C.A., Witek R.P. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285(47):36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang H., Sebastian B.M., Axhemi A., Chen X., Hillian A.D., Jacobsen D.W. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res. 2012;36(2):214–222. doi: 10.1111/j.1530-0277.2011.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 63.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loomba R., Seguritan V., Li W., Long T., Klitgord N., Bhatt A. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25(5):1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knudsen C., Neyrinck A.M., Lanthier N., Delzenne N.M. Microbiota and nonalcoholic fatty liver disease: promising prospects for clinical interventions? Curr Opin Clin Nutr Metab Care. 2019;22(5):393–400. doi: 10.1097/MCO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 66.Starkel P., Leclercq S., de Timary P., Schnabl B. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond) 2018;132(2):199–212. doi: 10.1042/CS20171055. [DOI] [PubMed] [Google Scholar]
- 67.Mutlu E.A., Gillevet P.M., Rangwala H., Sikaroodi M., Naqvi A., Engen P.A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang A.M., Inamine T., Hochrath K., Chen P., Wang L., Llorente C. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mutlu E., Keshavarzian A., Engen P., Forsyth C.B., Sikaroodi M., Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llopis M., Cassard A.M., Wrzosek L., Boschat L., Bruneau A., Ferrere G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 71.Chen P., Starkel P., Turner J.R., Ho S.B., Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61(3):883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boursier J., Diehl A.M. Nonalcoholic fatty liver disease and the gut microbiome. Clin Liver Dis. 2016;20(2):263–275. doi: 10.1016/j.cld.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Yuan J., Chen C., Cui J., Lu J., Yan C., Wei X. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–688.e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Mathurin P., Deng Q.G., Keshavarzian A., Choudhary S., Holmes E.W., Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32(5):1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 75.Parlesak A., Schafer C., Schutz T., Bode J.C., Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32(5):742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 76.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 77.Wheeler M.D., Thurman R.G. Up-regulation of CD14 in liver caused by acute ethanol involves oxidant-dependent AP-1 pathway. J Biol Chem. 2003;278(10):8435–8441. doi: 10.1074/jbc.M212076200. [DOI] [PubMed] [Google Scholar]
- 78.Zima T., Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29(11 Suppl):110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]
- 79.Gustot T., Lemmers A., Moreno C., Nagy N., Quertinmont E., Nicaise C. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43(5):989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 80.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 81.Rivera C.A., Adegboyega P., van Rooijen N., Tagalicud A., Allman M., Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anstee Q.M., Seth D., Day C.P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 2016;150(8):1728–1744.e7. doi: 10.1053/j.gastro.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 83.Kotronen A., Johansson L.E., Johansson L.M., Roos C., Westerbacka J., Hamsten A. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52(6):1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- 84.Salameh H., Raff E., Erwin A., Seth D., Nischalke H.D., Falleti E. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am J Gastroenterol. 2015;110(6):846–856. doi: 10.1038/ajg.2015.137. [DOI] [PubMed] [Google Scholar]
- 85.Sookoian S., Pirola C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 86.Pirazzi C., Adiels M., Burza M.A., Mancina R.M., Levin M., Stahlman M. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57(6):1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 87.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu R., Tao A., Zhang S., Deng Y., Chen G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: a HuGE review and meta-analysis. Sci Rep. 2015;5:9284. doi: 10.1038/srep09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian C., Stokowski R.P., Kershenobich D., Ballinger D.G., Hinds D.A. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42(1):21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 90.Trepo E., Gustot T., Degre D., Lemmers A., Verset L., Demetter P. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55(4):906–912. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y.L., Patman G.L., Leathart J.B., Piguet A.C., Burt A.D., Dufour J.F. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Stickel F., Moreno C., Hampe J., Morgan M.Y. The genetics of alcohol dependence and alcohol-related liver disease. J Hepatol. 2017;66(1):195–211. doi: 10.1016/j.jhep.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Yang J., Trepo E., Nahon P., Cao Q., Moreno C., Letouze E. PNPLA3 and TM6SF2 variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases. Int J Cancer. 2019;144(3):533–544. doi: 10.1002/ijc.31910. [DOI] [PubMed] [Google Scholar]
- 94.Buch S., Stickel F., Trepo E., Way M., Herrmann A., Nischalke H.D. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47(12):1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 95.Stickel F., Buch S., Nischalke H.D., Weiss K.H., Gotthardt D., Fischer J. Genetic variants in PNPLA3 and TM6SF2 predispose to the development of hepatocellular carcinoma in individuals with alcohol-related cirrhosis. Am J Gastroenterol. 2018;113(10):1475–1483. doi: 10.1038/s41395-018-0041-8. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y.L., Reeves H.L., Burt A.D., Tiniakos D., McPherson S., Leathart J.B. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P. A protein-Truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378(12):1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naveau S., Giraud V., Borotto E., Aubert A., Capron F., Chaput J.C. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 100.Raynard B., Balian A., Fallik D., Capron F., Bedossa P., Chaput J.C. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35(3):635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 101.Liu B., Balkwill A., Reeves G., Beral V., Million Women Study C. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hart C.L., Morrison D.S., Batty G.D., Mitchell R.J., Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yokoyama A., Matsushita S., Ishii H., Takagi T., Maruyama K., Tsuchiya M. The impact of diabetes mellitus on the prognosis of alcoholics. Alcohol Alcohol. 1994;29(2):181–186. [PubMed] [Google Scholar]
- 104.Loomba R., Yang H.I., Su J., Brenner D., Barrett-Connor E., Iloeje U. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177(4):333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aberg F., Helenius-Hietala J., Puukka P., Farkkila M., Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67(6):2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 106.Raff E.J., Kakati D., Bloomer J.R., Shoreibah M., Rasheed K., Singal A.K. Diabetes mellitus Predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J Clin Transl Hepatol. 2015;3(1):9–16. doi: 10.14218/JCTH.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dunn W., Xu R., Schwimmer J.B. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. 2008;47(6):1947–1954. doi: 10.1002/hep.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dunn W., Sanyal A.J., Brunt E.M., Unalp-Arida A., Donohue M., McCullough A.J. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57(2):384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sookoian S., Castano G.O., Pirola C.J. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63(3):530–532. doi: 10.1136/gutjnl-2013-305718. [DOI] [PubMed] [Google Scholar]
- 110.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gronbaek M., Deis A., Sorensen T.I., Becker U., Schnohr P., Jensen G. Mortality associated with moderate intakes of wine, beer, or spirits. BMJ. 1995;310(6988):1165–1169. doi: 10.1136/bmj.310.6988.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ekstedt M., Franzen L.E., Holmqvist M., Bendtsen P., Mathiesen U.L., Bodemar G. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44(3):366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 113.Johansen D., Friis K., Skovenborg E., Gronbaek M. Food buying habits of people who buy wine or beer: cross sectional study. BMJ. 2006;332(7540):519–522. doi: 10.1136/bmj.38694.568981.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leggio L., Addolorato G., Cippitelli A., Jerlhag E., Kampov-Polevoy A.B., Swift R.M. Role of feeding-related pathways in alcohol dependence: a focus on sweet preference, NPY, and ghrelin. Alcohol Clin Exp Res. 2011;35(2):194–202. doi: 10.1111/j.1530-0277.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 115.Junghanns K., Backhaus J., Tietz U., Lange W., Rink L., Wetterling T. The consumption of cigarettes, coffee and sweets in detoxified alcoholics and its association with relapse and a family history of alcoholism. Eur Psychiatry. 2005;20(5–6):451–455. doi: 10.1016/j.eurpsy.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 116.Kampov-Polevoy A.B., Eick C., Boland G., Khalitov E., Crews F.T. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28(9):1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- 117.Krahn D., Grossman J., Henk H., Mussey M., Crosby R., Gosnell B. Sweet intake, sweet-liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav. 2006;31(4):622–631. doi: 10.1016/j.addbeh.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 118.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 119.Kennedy O.J., Roderick P., Buchanan R., Fallowfield J.A., Hayes P.C., Parkes J. Systematic review with meta-analysis: coffee consumption and the risk of cirrhosis. Aliment Pharmacol Ther. 2016;43(5):562–574. doi: 10.1111/apt.13523. [DOI] [PubMed] [Google Scholar]
- 120.Anty R., Marjoux S., Iannelli A., Patouraux S., Schneck A.S., Bonnafous S. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012;57(5):1090–1096. doi: 10.1016/j.jhep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 121.Birerdinc A., Stepanova M., Pawloski L., Younossi Z.M. Caffeine is protective in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35(1):76–82. doi: 10.1111/j.1365-2036.2011.04916.x. [DOI] [PubMed] [Google Scholar]
- 122.Klatsky A.L., Morton C., Udaltsova N., Friedman G.D. Coffee, cirrhosis, and transaminase enzymes. Arch Intern Med. 2006;166(11):1190–1195. doi: 10.1001/archinte.166.11.1190. [DOI] [PubMed] [Google Scholar]
- 123.Molloy J.W., Calcagno C.J., Williams C.D., Jones F.J., Torres D.M., Harrison S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55(2):429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 124.Inoue M., Yoshimi I., Sobue T., Tsugane S., Group J.S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 2005;97(4):293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 125.Louvet A., Thursz M.R., Kim D.J., Labreuche J., Atkinson S.R., Sidhu S.S. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155(2):458–468.e8. doi: 10.1053/j.gastro.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 126.Ma K., Saha P.K., Chan L., Moore D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 129.Loomba R., Lawitz E., Mantry P.S., Jayakumar S., Caldwell S.H., Arnold H. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67(2):549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.https://www.gilead.com/news-and-press/press-room/press-releases/2019/4/gilead-announces-topline-data-from-phase-3-stellar3-study-of-selonsertib-in-bridging-fibrosis-f3-due-to-nonalcoholic-steatohepatitis-nash Available at. Accessed 25 April, 2019.
- 131.Wong V.W., Singal A.K. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol. 2019;4:53. doi: 10.21037/tgh.2019.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kirpich I.A., Solovieva N.V., Leikhter S.N., Shidakova N.A., Lebedeva O.V., Sidorov P.I. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42(8):675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Philips C.A., Pande A., Shasthry S.M., Jamwal K.D., Khillan V., Chandel S.S. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017;15(4):600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 134.Cobbold J.F.L., Atkinson S., Marchesi J.R., Smith A., Wai S.N., Stove J. Rifaximin in non-alcoholic steatohepatitis: an open-label pilot study. Hepatol Res. 2018;48(1):69–77. doi: 10.1111/hepr.12904. [DOI] [PubMed] [Google Scholar]
- 135.Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388. doi: 10.1053/j.gastro.2015.04.014. quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 136.Ivezaj V., Benoit S.C., Davis J., Engel S., Lloret-Linares C., Mitchell J.E. Changes in alcohol use after metabolic and bariatric surgery: predictors and mechanisms. Curr Psychiatry Rep. 2019;21(9):85. doi: 10.1007/s11920-019-1070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.King W.C., Chen J.Y., Courcoulas A.P., Dakin G.F., Engel S.G., Flum D.R. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1392–1402. doi: 10.1016/j.soard.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.King W.C., Chen J.Y., Mitchell J.E., Kalarchian M.A., Steffen K.J., Engel S.G. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–2525. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Svensson P.A., Anveden A., Romeo S., Peltonen M., Ahlin S., Burza M.A. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity (Silver Spring) 2013;21(12):2444–2451. doi: 10.1002/oby.20397. [DOI] [PubMed] [Google Scholar]
- 140.Klockhoff H., Naslund I., Jones A.W. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54(6):587–591. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steffen K.J., Engel S.G., Pollert G.A., Li C., Mitchell J.E. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(3):470–473. doi: 10.1016/j.soard.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.