Abstract
Background
Culture-independent next generation sequencing has identified diverse microbial communities within the cystic fibrosis (CF) airway. The study objective was to test for differences in the upper airway microbiome of children with CF and healthy controls and age-related differences in children with CF.
Methods
Oropharyngeal swabs and clinical data were obtained from 25 children with CF and 50 healthy controls aged ≤6 years. Bacterial DNA was amplified and sequenced for the V4 region of 16S rRNA marker-gene. Alpha diversity was measured using operational taxonomic units (OTUs), Shannon diversity, and the inverse Simpson's index. Beta diversity was measured using Morisita-Horn and Bray-Curtis and Jaccard distances. General linear models were used for comparison of alpha diversity measures between groups to account for differences in demographics and exposures. Mixed effects general linear models were used for longitudinal comparisons 1) between children with CF of different ages and 2) between children with CF receiving CF transmembrane conductance regulator (CFTR) modulators, children with CF not receiving CFTR modulators, and healthy controls to adjust for repeated measures per subject.
Results
Children with CF were more likely to have received antibiotics in the prior year than healthy controls (92% vs 24%, p < 0.001). Controlling age, race, ethnicity, length of breastfeeding, and having siblings, children with CF had a lower richness than healthy controls: OTUs 62.1 vs 83, p = 0.022; and trended toward lower diversity: Shannon 2.09 vs 2.35, p = 0.057; inverse Simpson 5.7 vs 6.92, p = 0.118. Staphylococcus, three Rothia OTUs, and two Streptococcus OTUs were more abundant in CF children versus healthy controls (all p < 0.05). Bray-Curtis and Jaccard distances, which reflect overall microbial community composition, were also significantly different (both p = 0.001). In longitudinally collected samples from children with CF, Morisita-Horn trended toward more similarity in those aged 0–2 years compared to those aged 3–6 years (p = 0.070). In children >2 years of age, there was a significant trend in increasing alpha diversity measures between children with CF not receiving CFTR modulators, children with CF receiving CFTR modulators, and healthy controls: OTUs 63.7 vs 74.7 vs 97.6, p < 0.001; Shannon 2.11 vs 2.34 vs 2.56, p < 0.001; inverse Simpson 5.78 vs 7.23 vs 7.96, p < 0.001.
Conclusions
Children with CF have lower bacterial diversity and different composition of organisms compared with healthy controls. This appears to start in early childhood, is possibly related to the use of antibiotics, and may be partially corrected with the use of CFTR modulators.
Keywords: Health sciences, Respiratory system, Infectious disease, Microbiology, Epidemiology, Pediatrics, Cystic fibrosis, Human microbiome, Pulmonary medicine
Health sciences; Respiratory system; Infectious disease; Microbiology; Epidemiology; Pediatrics; Cystic fibrosis; Human microbiome; Pulmonary medicine.
1. Introduction
Cystic fibrosis (CF), a chronic and progressive suppurative lung disease, affects more than 30,000 people in the Unites States (MacKenzie et al., 2014; Ramsey, 1996). A hallmark of CF disease is declining lung function over time, which has been attributed to recurrent lung infection and lung inflammation (Sagel et al., 2002; Sagel et al., 2012). Bronchiectasis, airway inflammation, and decreased lung function is evident even in infants and young children with CF (Stick et al., 2009, 2014; VanDevanter and Pasta, 2013).While Staphylococcus aureus and Pseudomonas aeruginosa are important drivers of airway inflammation in older children and adults, infants and young children may have negative culture results for these bacterial species (Frayman et al., 2017; Sagel et al., 2009; Zemanick et al., 2010). Recent studies to better understand the role of bacteria in CF disease in infants and young children have used culture-independent next generation sequencing (NGS).
Studies of bronchoalveolar lavage (BAL) fluid from infants and young children with CF show a diverse community of aerobes and anaerobes that normally comprise the flora of the upper airway (Laguna et al., 2016; Muhlebach et al., 2018). Colonization or infection with a recognized CF pathogen is associated with a decrease in bacterial diversity (Frayman et al., 2017; Muhlebach et al., 2018). Reduced bacterial diversity in the lower airway is also associated with the use of prophylactic antibiotics (Pittman et al., 2017).
Comparisons between BAL fluid obtained from infants and young children with CF and non-CF controls have consistently shown that children with CF have decreased microbial diversity in the lower airways (Frayman et al., 2019; Renwick et al., 2014; Zemanick et al., 2017). Differences in microbial community composition appear to be driven by both the prevalence of certain bacteria and their relative abundance (Frayman et al., 2019; Renwick et al., 2014; Zemanick et al., 2017). Differences in community composition are also more extreme when CF children are experiencing a pulmonary exacerbation (Frayman et al., 2019). Studies of the upper airway microbiome in infants and young children with CF and healthy controls using nasopharyngeal swabs have also found differences in community composition, driven in part by antibiotic administration (Mika et al., 2016; Prevaes et al., 2016).
While the above studies primarily explored differences in the upper airways from nasopharyngeal samples or the lower airways from samples obtained by bronchoscopy, national guidelines in the United States recommend quarterly oropharyngeal (OP) swabs as a surrogate of lower airway microbiota (Borowitz et al., 2009; Lahiri et al., 2016). The primary objective of this study is to identify differences in the oropharyngeal microbiome of infants and young children with CF and healthy controls. The secondary objective is to examine longitudinal changes in the oropharyngeal microbiome of infants and young children with CF, and the potential impact of CF transmembrane conductance regulator (CFTR) modulators on microbial diversity.
2. Materials & methods
2.1. Study design and participants
2.1.1. Children with cystic fibrosis
The creation of a bio- and data repository was approved 08 Dec 2015 by the Institutional Review Board (Pro6781) at Children's National Hospital (CNH). Parental permission (written consent) for study participation was obtained prior to respiratory sample collection and extraction of data from electronic medical records.
2.1.1.1. Inclusion criteria
All children and adults with CF, diagnosed by sweat chloride or genetic testing, who receive care at CNH are eligible to participate in the biorepository. Participants are recruited during routinely scheduled clinical encounters in the CNH CF Center. For this study, children had to be between 0-6 years of age with at least four consecutive respiratory samples available in the biorepository.
2.1.1.2. Subject encounters, data collection, and respiratory sample collection
Demographic information for each study participant was collected after study enrollment, including age, gender, race/ethnicity, and CFTR genotype. After each regularly scheduled clinic visit or hospital admission, the participant's medical record is accessed to record visit specific information, including antibiotics received and clinical culture results. Each encounter was noted to be either a well visit or a sick visit, based on documentation of at least 4 of 12 characteristics consistent with Fuchs criteria for pulmonary exacerbation (Fuchs et al., 1994). Respiratory samples (Copan Eswabs in 1 mL liquid Amies transport media collected from the oropharynx) were collected from the microbiology laboratory at CNH after they were no longer needed for clinical purposes and were then processed and stored in the biorepository. The clinical microbiology laboratory uses MicroScan (BeckmanCoulter) for identification and antibiotic susceptibility testing (via broth dilution) of CF respiratory samples. The respiratory samples used in this study were obtained from the biorepository.
2.1.2. Children who served as healthy controls
The recruitment of healthy controls was approved 24 Aug 2016 by the CNH Institutional Review Board (Pro7992). Parental permission (written consent) was obtained for all subjects.
2.1.2.1. Inclusion and exclusion criteria
Children between 0-6 years of age, with no history of chronic upper airway or lung medical conditions, who have not been on antibiotics for the prior 30 days, and who match the age and gender of the comparison subjects with cystic fibrosis were recruited for the study.
2.1.2.2. Potential subject identification
Patients coming to be seen at the Goldberg Center for Community Pediatric Health at CNH for well child checks were identified as potential candidates for study inclusion. Their medical charts were reviewed to determine if 1) they had any chronic medical conditions and 2) they met the demographic profile to match with a CF participant.
2.1.2.3. Subject encounters and respiratory sample collection
The potential subjects were approached by the principal investigator (PI) or other study staff to discuss participation. Screening questions were asked to determine if any exclusion criteria were met, including verification of any chronic upper airway or lung medical conditions and recent use of antibiotics. If they still met enrollment criteria, the study was explained in detail to the family. The PI or study staff completed the informed consent process. The family was asked to complete a study questionnaire and study staff obtained a single oropharyngeal (OP) swab. The electronic medical record was then accessed and the parental responses to the questionnaire were verified against previously documented information, including past medical history and medications.
2.2. Experimental procedures
2.2.1. Data management
All study data were maintained and managed in a unified REDCap database (Harris et al., 2009).
2.2.2. Respiratory sample processing
Copan eSwabs with 1 mL liquid Amies media were used for respiratory sample collection. After collection, the OP swab samples were stored in a 4 °C refrigerator prior to processing. The swab was removed and the Amies media transferred to a sterile 1.5 mL microcentrifuge tube. The media was then pelleted through centrifugation (12,000 g × 10 min). Supernatants were removed and bacterial pellets were frozen at -80 °C until they underwent DNA extraction.
2.2.3. Bacterial DNA extraction
Pelleted bacterial cells were rapidly thawed and mixed with 1 mL of sterile phosphate buffered saline (PBS). Bacterial DNA was extracted using a QIAamp DNA Microbiome kit (Qiagen, Valencia CA), following the protocol as outlined by the company.
2.2.4. Next generation sequencing and bioinformatics
Sequencing was performed by the University of Michigan Host Microbiome Initiative (Ann Arbor MI) (Kozich et al., 2013; Seekatz et al., 2015). Briefly, extracted DNA was amplified for the V4 region of the 16S rRNA marker-gene using PCR primers (forward primer GTGCCAGCMGCCGCGGTAA; reverse primer TAATCTWTGGGVHCATCAGG) (Kozich et al., 2013). The PCR cycle for the amplification of the V4 region was as follows: 95 °C × 2 min (1x); 95 °C × 20 s, 55 °C 15 s, 72 °C 5 min (30x); 72 °C 10 min (1X); 4 °C (until sequencing). Libraries were normalized using SequalPrep Normalization Plate Kit (Life Technologies, Carlsbad CA), and DNA concentration measured using Kapa Biosystems Library Quantification Kit (Kapa Biosystems, Wilmington MA). A MiSeq Reagent Kit V2 (500 cycle) was used to perform the dual-index sequencing. Specifically, 2 × 250 base pair (bp) paired-end reads were produced, allowing for full overlap of the forward and reverse reads of the V4 region (Kozich et al., 2013). ZymoBIOMICS Microbial Community Standards (Zymo Research, Irvine CA), were included as positive sequencing controls and PCR-free water were included as negative sequencing controls in each MiSeq run.
Raw FASTQ files were processed in mothur v.1.39.5 (https://www.mothur.org/wiki/MiSeq_SOP, accessed 7 Jun and 25 Sept 2018) (Kozich et al., 2013; Schloss et al., 2009). As per the standard operating procedure, we first combined paired reads. We next looked across the alignment to see if there were any base pairs that were mismatched, to correct any low quality base pair reads. We next removed any sequences where the base pair length was 275 bp. We then combined all duplicate sequences and aligned the sequences to the SILVA_v123 bacterial reference alignment (obtained from http://www.mothur.org/.) We then removed any sequences that were outside the expected alignment region of V4, and pre-clustered any sequences that were differing by 2 bp or less. We then removed chimeras and any non-bacterial sequences, including those that align to Archaea, Chloroplasts, Eukaryotes, or Archaea. Lastly, we then clustered the sequences into operational taxonomic units (OTUs) at the 0.03 threshold (97% sequence similarity). These final OTU assignments for the sequences in each sample were then taken into further downstream alpha and beta diversity analyses and evaluation of differential abundance of OTUs between comparison groups.
2.3. Statistical analysis
Categorical variables were compared using Pearson's chi-square. As age was skewed, a general linear model was used to first apply a log transformation to normalize the age distribution. Microbiome analyses were performed using all sequences passing quality control. Multiple measures of diversity were used to see the level of agreement across metrics that evaluate a similar quality but using different approaches. Alpha diversity was measured by the number of observed OTUs, Shannon Index, and the inverse Simpson's Index. Observed OTUs provide information about richness (q = 0), while the Shannon index (q = 1) and inverse Simpson index (q = 2) increasingly add information about relative abundance (Wagner et al., 2018). Observed OTUs and the inverse Simpson's index were determined using mothur v.1.39.5 (Schloss et al., 2009). Shannon index was determined using the shannon function of vegan v.2.5–5 in RStudio (Oksanen et al., 2017). Beta diversity was measured using Morisita-Horn (using Explicet v.2.10.5) and Bray-Curtis and Jaccard distances (using phyloseq v.1.28.0 in RStudio) (McMurdie and Holmes, 2013; Robertson et al., 2013). Bray-Curtis distances utilize relative abundance (similar to Shannon and inverse Simpson indices), while Jaccard distances only use presence/absence of community members (similar to richness) (Tuomisto, 2010). Morisita-Horn was used for pairwise comparison (Wagner et al., 2018). Importantly, alpha diversity was calculated without prior subsampling to maximize use of data obtained (McMurdie and Holmes, 2014); however, comparisons were made to subsampled data to ensure validity of our findings. General linear models (using STATA/IC v15.1) were used for comparison of alpha diversity measures between groups to account for differences in demographics and bacterial exposures, including method of delivery, duration of breastfeeding, attendance at daycare, and siblings in the home. Mixed effects general linear models were used for longitudinal comparisons 1) between children with CF of different ages and 2) between children with CF receiving CF transmembrane conductance regulator (CFTR) modulators, children with CF not receiving CFTR modulators, and healthy controls to adjust for repeated measures per subject. OTU and taxonomy tables were imported into RStudio for subsequent analyses using phyloseq v.1.28.0 and DESeq2 v.1.24.0 to determine differential abundance and create principle coordinates analysis (PCoA) plots (Love et al., 2014; McMurdie and Holmes, 2013). Lastly, the adonis function of vegan v.2.5–5 was used to determine significant differences in the centroids and dispersion of the groups by permutational multivariate analysis of variance (PERMANOVA) using Bray-Curtis (“bray”) and Jaccard (“jaccard”) distances (Oksanen et al., 2017). The strata argument was used to constrain permutations when assessing repeated patient samples.
3. Results
3.1. Participant demographics and exposure history
Twenty-five children with CF aged 6 years or younger had clinical data and respiratory samples collected between February 2016 through April 2017, encompassing between 4 and 6 visits. Fifty healthy controls were enrolled and had clinical data and respiratory samples collected between October 2016 and May 2018 from a single visit. No differences were noted between the two groups with regard to demographics, excepting that children with CF were more likely to be white and healthy controls were more likely to be black (p = 0.002, Table 1). There were also no differences in common methods of bacterial exposures, specifically method of delivery, duration of breastfeeding, attendance at daycare or school, or siblings in the home. Children with CF were more likely to have received antibiotics within the last year (p < 0.001, Table 1).
Table 1.
Participant demographics, bacterial exposure, and antibiotic history‡.
| Cystic Fibrosis (n = 25) | Healthy Control (n = 50) | P value | |
|---|---|---|---|
| Age (years) (mean, standard error)∗ | 4.04 (0.29) | 3.72 (0.38) | 0.508 |
| Gender (n, %)† | 0.870 | ||
| Female | 13 (52) | 27 (54) | |
| Male | 12 (48) | 23 (46) | |
| Race (n, %)† | 0.002 | ||
| White | 13 (52) | 19 (38) | |
| Black | 4 (16) | 25 (50) | |
| Other | 8 (32) | 6 (12) | |
| Ethnicity (n, %)† | 0.221 | ||
| Hispanic | 7 (28) | 8 (16) | |
| Not Hispanic | 18 (72) | 42 (84) | |
| Delivery Method (n, %)† | 0.814 | ||
| Vaginal | 15 (65) | 34 (68) | |
| Cesarean | 8 (35) | 16 (32) | |
| Length of Breastfeeding (n,%)† | 0.111 | ||
| None | 9 (36) | 10 (20) | |
| <3 months | 2 (8) | 8 (16) | |
| 3–6 months | 6 (24) | 14 (28) | |
| 6–12 months | 6 (24) | 5 (10) | |
| >12 months | 2 (8) | 13 (26) | |
| Daycare or School (n, %)† | 0.994 | ||
| No | 8 (38) | 19 (38) | |
| Yes | 13 (62) | 31 (62) | |
| Siblings (n, %)† | 0.697 | ||
| No | 5 (20) | 12 (24) | |
| Yes | 20 (80) | 38 (76) | |
| Antibiotics Courses in the 12 months Preceding Sample Collection (n, %)† | <0.001 | ||
| None | 2 (8) | 38 (76) | |
| 1–2 courses | 10 (40) | 12 (24) | |
| 3–4 courses | 9 (36) | 0 (0) | |
| 5 or more courses | 4 (16) | 0 (0) | |
For children with CF, the sample used in this analysis was the first sample collected in the longitudinal series.
Generalized linear model adjusting for age skew.
Chi-squared.
3.2. Results of 16S sequencing controls
A positive and negative sequencing control was included in each MiSeq run (n = 4). A mean of 9 sequences (range 6–12) were detected in the PCR-free water controls. A mean of 21,720 sequences (range 18,289–24,912) were detected in the ZymoBIOMICS Microbial Community Standards, with a mean sequencing error rate of 1.57 percent (range 1.56–1.59).
3.3. Comparisons in bacterial community composition between children with CF versus age similar healthy controls
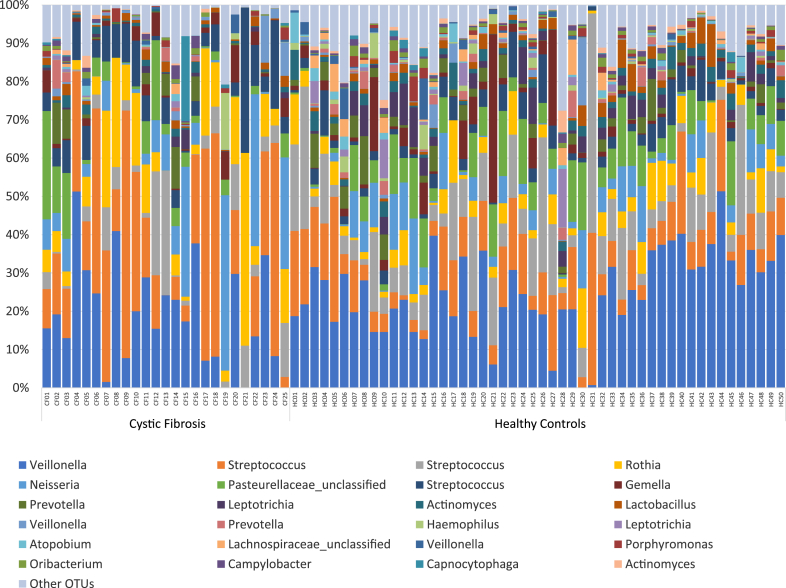
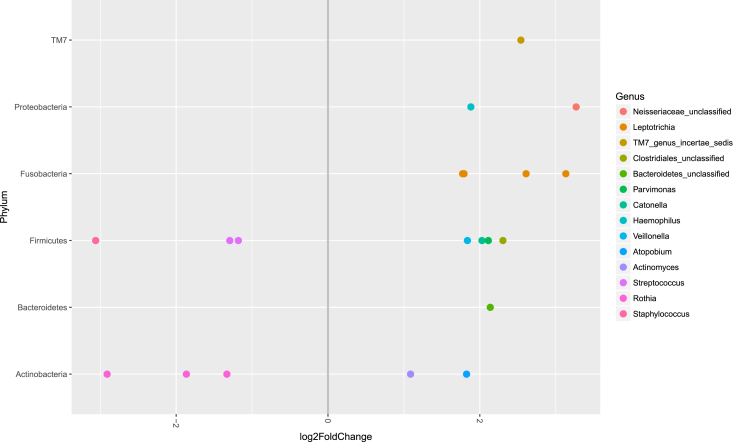
A mean of 21,385 (range 2043–35,696) sequences were available for the CF samples and a mean of 21,218 (range 10,777–34,654) for healthy control samples. The relative abundance of bacteria identified in the upper airway microbiome differed between the baseline samples of children with CF and healthy controls (Figure 1). Using adjusted p values (with false discovery rate correction), six bacterial OTUs were more abundant in children with CF: three Rothia OTUs (OTU66 p < 0.001, OTU3 p = 0.003, and OTU58 p = 0.047), one Staphylococcus OTU (OTU27 p = 0.003), and two Streptococcus OTUs (OTU7 p = 0.004 and OTU2 p = 0.006) (Figure 2). Conversely, fourteen bacterial OTUs were more abundant in healthy controls: four Leptotrichia OTUs (OTU25 p = 0.003, OTU11 p = 0.026, OTU53 p = 0.037, and OTU82 p = 0.049), Neisseriaceae_unclassified (OTU90 p = 0.006), Clostridiales_unclassified (OTU63 p = 0.007), Atopobium (OTU21 p = 0.009), Haemophilus (OTU20 p = 0.010), Catonella (OTU72 p = 0.015), Veillonella (OTU18 p = 0.019), Actinomyces (OTU12 p = 0.023), TM7_genus_incertae_sedis (OTU106 p = 0.038), Bacteroidetes_unclassified (OTU74 p = 0.049), and Parvimonas (OTU65 p = 0.049).
Figure 1.
Relative taxonomic abundance of the upper airway microbiome of children with cystic fibrosis and healthy controls. Only the top 25 OTUs are included.
Figure 2.
Differential abundance of bacterial genera in the upper airway microbiome between children with cystic fibrosis and healthy controls. Bacterial genera on the left side of the graph with a negative fold change have a higher relative abundance in the healthy control samples compared to the cystic fibrosis samples. Bacterial genera on the right side of the graph with a positive fold change have a higher relative in the cystic fibrosis samples compared to the healthy control samples. All genera shown had adjusted p values <0.05.
3.4. Comparisons in microbial diversity between children with CF and age similar controls
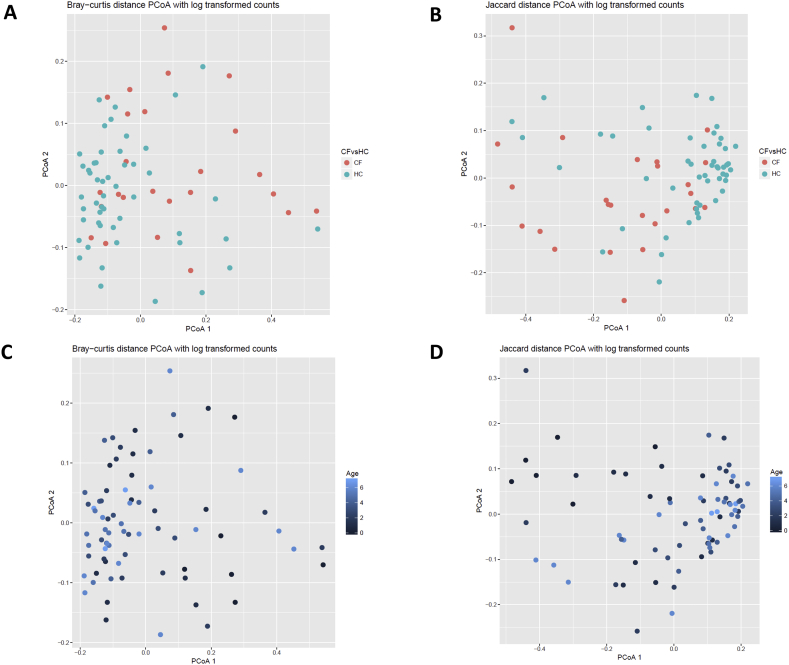
Children with CF had on average lower upper airway diversity in their baseline sample than age similar controls. Controlling age, race, ethnicity, length of breastfeeding, and having siblings, the number of OTUs was lower in children with CF compared to healthy controls (62.1 vs 83, p = 0.022; Table 2). There were similar trends for the Shannon diversity index (p = 0.057) and the inverse Simpson index (p = 0.118). Furthermore, alpha diversity measures of subsampled data (2043 sequences) showed congruent results (Table 2). Therefore, all available sequence data was used for subsequent diversity analyses. Beta diversity as measured by both Bray-Curtis and Jaccard distances were also different between children with CF and healthy controls (PERMANOVA R2 = 0.071, p = 0.001 and R2 = 0.054, p = 0.001 respectively, Figure 3).
Table 2.
Alpha diversity Measures between children with CF and healthy controls.
| Cystic Fibrosis (n = 25)† | Healthy Control (n = 50)† | P value† | Cystic Fibrosis (n = 25)‡ | Healthy Control (n = 50)‡ | P value‡ | |
|---|---|---|---|---|---|---|
| Number of OTUs∗ (mean, standard error) | 62.1 (7.4) | 83 (4.4) | 0.022 | 36.0 (3.2) | 43.0 (1.9) | 0.080 |
| Shannon Diversity Index∗ (mean, standard error) | 2.09 (0.11) | 2.35 (0.07) | 0.057 | 2.07 (0.11) | 2.34 (0.07) | 0.054 |
| Inverse Simpson Index∗ (mean, standard error) | 5.70 (0.63) | 6.92 (0.38) | 0.118 | 5.70 (0.63) | 6.92 (0.38) | 0.118 |
OTUs, operational taxonomic units.
Generalized linear model, adjusting for age, race, ethnicity, length of breastfeeding, and siblings.
Using all available sequencing data.
Using subsampled sequencing data (2043 sequences).
Figure 3.
Differences in community composition between children with cystic fibrosis versus healthy controls. Dissimilarities in bacterial genera using log transformed counts are shown based on cystic fibrosis versus control and age of the participant. (A) Bray-Curtis distance PCoA for cystic fibrosis versus healthy control samples. (B) Jaccard distance PCoA for cystic fibrosis versus healthy control samples. (C) Bray-Curtis distance PCoA based on age. (D). Jaccard distance PCoA based on age. PERMANOVA showed a significant difference for both cystic fibrosis versus healthy control samples (R2 = 0.071, p = 0.001 Bray, R2 = 0.054, p = 0.001 Jaccard) and based on age (R2 = 0.042, p = 0.001 Bray, R2 = 0.033, p = 0.002 Jaccard).
3.5. Comparisons in demographics and clinical characteristics of children with CF based on age
There were no significant differences in demographics, use of cycled inhaled antibiotics, or antibiotic courses received in the 12 months preceding the study period between those children with CF 0 to <3 years of age at study enrollment (n = 12) and those who were ≥3–6 years of age at study enrollment (n = 13) (Table 3). Those in the older age group were more likely to be on a CFTR modulator than those in the younger group (p = 0.039), likely due to the FDA guidance for approved use of this product during the study period. When exploring visit specific information, those in the younger age group (n = 50 samples, n = 12 subjects) were more likely to have normal flora detected in clinical respiratory culture than children in the older age group (n = 59 samples, n = 13 subjects, OR 0.29, p = 0.023; Table 3). Pseudomonas aeruginosa (OR 2.72, p = 0.217) and other bacteria (OR 3.59, p = 0.110) were identified more often in clinical respiratory cultures in those in the older age group than those in the younger age group, although these results were not significant. Those in the older age group also had more sick visits during the 12 month study period (OR 2.09, p = 0.191), but again this did not achieve significance.
Table 3.
Demographics, medications, treatments, and diversity measures based on age group.
| Age 0 to <3 years (n = 12 study participants) | Age ≥3–6 years (n = 13 study participants) | Odds Ratio | P value | |
|---|---|---|---|---|
| Gender (n, % female)∗ | 7 (58) | 6 (46) | NA | 0.695 |
| Race (n, %)∗ | NA | 0.751 | ||
| White | 7 (58) | 6 (46) | ||
| Black | 1 (8) | 3 (23) | ||
| Other | 4 (33) | 4 (31) | ||
| Ethnicity (n, %)∗ | NA | >0.999 | ||
| Hispanic or Latino | 4 (33) | 4 (31) | ||
| Not Hispanic or Latino | 8 (67) | 9 (69) | ||
| CFTR Genotype (n, %) | NA | 0.683 | ||
| F508del homozygous | 5 (42) | 6 (46) | ||
| F508del heterozygous | 5 (42) | 3 (23) | ||
| Other | 2 (17) | 4 (31) | ||
| Inhaled antibiotics (n, % yes)∗ | 3 (25) | 2 (15) | NA | 0.645 |
| Antibiotics Courses in the 12 months Preceding Sample Collection (n, %)∗ | NA | 0.537 | ||
| None | 2 (17) | 0 (0) | ||
| 1–2 courses | 5 (42) | 5 (38.5) | ||
| 3–4 courses | 4 (33) | 5 (38.5) | ||
| 5 or more courses | 1 (8) | 3 (23) | ||
| CFTR modulator† (n, % yes) | 0 (0) | 5 (38) | NA | 0.039 |
| Age 0 to <3 years (n = 50 study samples) | Age ≥3–6 years (n = 59 study samples) | Odds Ratio¶ | P value | |
| Respiratory Culture (n, % yes)‡ | ||||
| Normal respiratory flora | 44 (88) | 40 (68) | 0.29 | 0.023 |
| Staphylococcus aureus | 2 (4) | 3 (5) | 1.29 | 0.802 |
| Pseudomonas aeruginosa | 2 (4) | 6 (10) | 2.72 | 0.217 |
| Other bacteria§ | 3 (6) | 11 (19) | 3.59 | 0.110 |
| Number of Sick Visits (n, % yes) | 7 (14) | 15 (25) | 2.09 | 0.191 |
| Oral Antibiotics (n, % yes) | 7 (14) | 13 (22) | 1.74 | 0.305 |
| IV Antibiotics (n, % yes) | 0 (0) | 1 (2) | NA | NA |
| Steroids (n, % yes) | 0 (0) | 4 (7) | NA | NA |
| Number of OTUs∗∗ (mean, standard error) | 63.9 (5.9) | 70.1 (5.6) | NA | 0.455 |
| Shannon Diversity Index∗∗(mean, standard error) | 2.15 (0.08) | 2.25 (0.08) | NA | 0.371 |
| Inverse Simpson Index∗∗ (mean, standard error) | 6.13 (0.41) | 6.52 (0.39) | NA | 0.482 |
| Morisita-Horn between subsequent encounters∗∗ (mean, standard error) | 0.78 (0.04) | 0.69 (0.04) | NA | 0.070 |
CFTR, cystic fibrosis transmembrane conductance regulator.
Fisher's exact test.
Ivacaftor n = 3, lumacaftor/ivacaftor n = 2.
Logistic regression, adjusting for repeated patient visits.
OR less than 1 more likely in Age 0 to <3 years, greater than 1 more likely in Age ≥3–6 years.
Other bacteria include Stenotrophomonas maltophilia (x5), Streptococcus pyogenes (x4), Streptococcus agalactiae (x3), Chryseobacterium species (x2), and Elizabethkingia meningoseptica.
Mixed effects generalized linear model, adjusting for repeated patient visits (p-value reported in the table) and whether the sample was collected at a sick or well visit (starting with OTUs, p-value was 0.223, 0.194, 0.334, and 0.028).
3.6. Comparisons in microbial diversity and community composition of children with CF based on age
Alpha diversity measures were similar between the younger (age 0 to <3 years) and older (age ≥3–6 years) children with CF, incorporating all visits and controlling for repeated patient measures (Table 3). Community composition tended to remain more similar between visits in younger children with CF compared to older children (Morisita-Horn 0.78 versus 0.69, p = 0.070). When we controlled for nature of the visit (sick versus well) at the time of sample collection instead of for repeated patient samples, no significant differences were noted in alpha diversity measures. However, the difference in Morisita-Horn reached significance (p = 0.028).
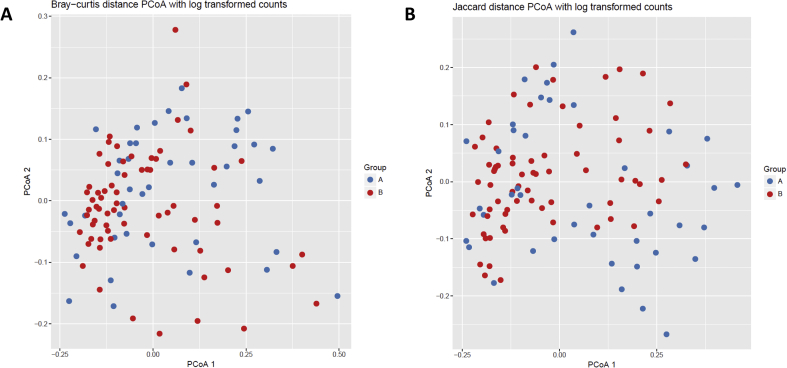
No significant differences were noted in beta diversity between the younger and older children with CF as measured by both Bray-Curtis and Jaccard distances (PERMANOVA R2 = 0.016, p > 0.999 and R2 = 0.015, p > 0.999 respectively, Figure 4). No differences in the relative abundance of specific taxa were identified between the younger and older children with CF (p > 0.05).
Figure 4.
Differences in community composition in children with cystic fibrosis based on age. Dissimilarities in bacterial genera using log transformed counts are shown based on age 0 to <3 years (Group A) versus age ≥3–6 years (Group B). (A) Bray-Curtis distance PCoA (PERMANOVA R2 = 0.016, p > 0.999). (B) Jaccard distance PCoA (PERMANOVA R2 = 0.015, p > 0.999).
3.7. Effects of CFTR modulation in microbial diversity and community composition
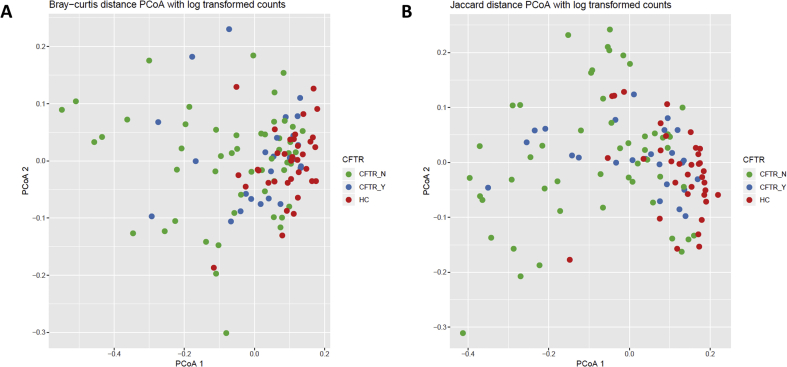
To assess any impacts of CFTR modulation on the differences in microbial diversity, we performed a sub-analysis of children ≥2 years of age (the youngest FDA approved age to start CFTR modulators during the study period). Those children on CFTR modulation (n = 5) had significantly more study visits during the study period compared to children not receiving CFTR modulators (n = 12) (p = 0.029), but had no increase in the number of pulmonary exacerbations or antibiotic treatment courses during the study period (Table 4). Likewise, there was no significant difference in inhaled antibiotic use or antibiotic treatment courses in the 12 months preceding the study period between the two groups. Respiratory samples from 35 healthy controls were compared to 25 samples from 5 children with CF receiving CFTR modulators and 51 samples from 12 children with CF not receiving CFTR modulators. While respiratory samples from healthy controls retained the highest levels of alpha diversity, samples from children with CF receiving CFTR modulators had higher alpha diversity compared to children with CF not receiving CFTR modulators (Table 4), and was significantly different between groups for all measures (all p < 0.001). However, community composition as assessed by Bray-Curtis and Jaccard distances showed no differences between healthy children, children with CF on modulator therapy, or children with CF not receiving CFTR modulators (PERMANOVA Bray-Curtis R2 = 0.071, p > 0.999 and Jaccard R2 = 0.058, p > 0.999, Figure 5).
Table 4.
Demographics and Effect of CFTR modulators on alpha diversity.
| CFTR no (n = 12 study participants) | CFTR yes (n = 5 study participants) | HC (n = 35 study participants) | P value | |
|---|---|---|---|---|
| Inhaled antibiotics (n, % yes) | 2 (17) | 1 (20) | NA | >0.999 |
| Antibiotics Courses in the 12 months Preceding Sample Collection (n, %)† | <0.001 (all) >0.999 (CFTR no vs yes) |
|||
| None | 0 (0) | 0 (0) | 27 (77) | |
| 1–2 courses | 5 (42) | 2 (40) | 8 (23) | |
| 3–4 courses | 4 (33) | 2 (40) | 0 (0) | |
| 5 or more courses | 3 (25) | 1 (20) | 0 (0) | |
| Total Visits during the Study Period (mean, SD)‡ | 4.3 (0.45) | 5 (0.71) | NA | 0.029 |
| Sick Visits during the Study Period (mean, SD)‡ | 1.1 (1.24) | 0.8 (0.84) | NA | 0.781 |
| Antibiotic Courses in the Study Period (mean, SD)‡ | 1.1 (1.24) | 0.8 (0.84) | NA | 0.781 |
| CFTR no (n = 51 study samples) | CFTR yes (n = 25 study samples) | HC (n = 35 study samples) | P value | |
| Number of OTUs∗ (mean, standard error) | 63.7 (4.9) | 74.7 (7.4) | 97.6 (4.4) | <0.001 |
| Shannon Diversity Index∗ (mean, standard error) | 2.11 (0.07) | 2.34 (0.10) | 2.56 (0.07) | <0.001 |
| Inverse Simpson Index∗ (mean, standard error) | 5.78 (0.41) | 7.23 (0.59) | 7.96 (0.43) | <0.001 |
CFTR, cystic fibrosis transmembrane conductance regulator; HC, healthy control.
Fisher's exact test.
Wilcoxon rank-sum test.
Mixed effects generalized linear model, adjusting for repeated patient visits.
Figure 5.
Effect of CFTR modulation on community composition. Dissimilarities in bacterial genera using log transformed counts are shown for children with CF receiving CFTR modulators (CFTR_Y), children with CF not receiving CFTR modulators (CFTR_N), and age similar healthy controls (HC). (A) Bray-Curtis distance PCoA (PERMANOVA R2 = 0.071, p > 0.999). (B) Jaccard distance PCoA (PERMANOVA R2 = 0.058, p > 0.999).
4. Discussion
In this study, we found that infants and young children with CF have decreased microbial diversity compared to age similar healthy controls. This is consistent with prior studies of the lower airways. One of the early studies focused on differences in microbial richness between infants and young children with CF (n = 13, mean age 3.95 years) and non-CF controls (n = 9, mean age 3.78 years) found an average of 130 species in the BAL fluid of children with CF and 167 species in non-CF controls (Renwick et al., 2014). A more recent study found that microbial richness was reduced in lower airway samples of infants with CF (n = 21, median age 1.8 months) compared to non-CF controls (n = 10, median age 5 months), with a 95% confidence interval of difference of 1.13–11.83 OTUs (Frayman et al., 2019). Larger comparison studies analyzing the upper airway via nasopharyngeal (NP) swab have not found significant differences in alpha diversity; however, these studies were limited to the first year of life (Mika et al., 2016; Prevaes et al., 2016).
We also found differences in microbial composition between our cohort of infants and young children with CF and age similar healthy controls. Our significant differences in beta diversity as measured by Bray-Curtis and Jaccard distances were similar to those described in prior studies of both the lower airway via BAL fluid and the upper airway via NP swabs (Frayman et al., 2019; Mika et al., 2016; Prevaes et al., 2016; Renwick et al., 2014). In particular, Staphylococcus species were more abundant in infants and children with CF than their age-matched counterparts, which has been described by other groups and is consistent with our knowledge that S. aureus is an early pathogen in CF disease (Frayman et al., 2017, 2019; Mika et al., 2016; Prevaes et al., 2016; Sagel et al., 2009). In our cohort, we also found that Rothia and two Streptococcus OTUs were more abundant in infants and young children with CF than age similar healthy controls. Streptococcus is a common oral flora, and has frequently been found to be dominant taxa in the lower respiratory microbiome of young children with CF (Frayman et al., 2017; Laguna et al., 2016; Pittman et al., 2017; Zemanick et al., 2017). Rothia has previously been described as part of the core microbiota of upper airway samples in adults with CF, but has not consistently been identified in younger children (Coburn et al., 2015; Zemanick et al., 2017).
The number of antibiotic courses in the last 12 months was significantly higher in infants and children with CF than the age similar healthy controls in our cohort. This may have contributed in part to the differences we saw in both relative bacterial abundance and alpha diversity between our two groups. Cumulative antibiotic use is associated with decreased diversity in older children and adults with CF (Zhao et al., 2012). A study of the upper airway in infants with CF found that initiation of prophylactic antibiotics significantly modified the microbial community, and the change persisted longitudinally (Mika et al., 2016). Another study of infants with CF found prophylactic antibiotics were related to an increase in gram-negative bacteria (Prevaes et al., 2016). Further studies are required to test the impact of intermittent courses of antibiotics on the microbiome in infants and young children with CF.
When testing for age related differences in longitudinally collected OP swabs from infants and children with CF, we found that younger children (age 0 to <3 years) were more likely to culture normal respiratory flora than older children age (≥3–6 years). Additionally, in sequencing analyses younger children had a trend toward more stability in microbial composition between visits than older children. No differences were noted in alpha diversity between the two groups. This was in contrast to a large multicenter cross-sectional study that found that younger children (<2 years) had higher diversity than preschool and older children (2–5 or 5–10 years), although no significance was noted between those age groups (Zemanick et al., 2017). A single center longitudinal study of 48 children with CF 0–6 years of age found that overall microbial diversity was inversely correlated to age (Frayman et al., 2017).
When examining beta diversity and community composition, we did not find any differences between younger and older children with CF, and no specific taxa were identified as being differentially abundant between the two groups. This is in contrast to a cross-sectional study of BAL fluid from 46 infants and young children with CF, which found that the lower airway microbiome of children 1–2 years of age was dominated by oral flora (Streptococcus, Prevotella, and Veillonella), while the lower airway microbiome of children 3–5 years of age were dominated by CF pathogens (Haemophilus, Staphylococcus, Moraxella, and Pseudomonas) (Muhlebach et al., 2018). Similar differences in community composition based on age were found in a multicenter study of BAL fluid collected for microbiome analyses, with children <2 years of age having approximately 50% of reads attributed to nontraditional taxa (Streptococcus, Neisseria, Porphyromonas, Prevotella, and Veillonella) compared to 30% CF pathogens (Pseudomonas, Staphylococcus, Stenotrophomonas, Haemophilus, and Burkholderia) (Zemanick et al., 2017). Conversely, children 2–5 years of age had BAL fluid microbiomes composed of roughly 50% traditional CF pathogens and 30% nontraditional taxa (Zemanick et al., 2017). From these studies together, it seems likely that diversity remains high when the airway is composed of normal airway flora, but decreases as typical CF pathogens become residents of the microbiome. This pattern would also be consistent with what has previously been established in adults with CF (Coburn et al., 2015; Flight et al., 2015; Zemanick et al., 2017).
CFTR modulators are newly developed and available drugs which aim to improve the function of the CFTR protein (Clancy et al., 2019; Habib et al., 2019). When assessing for an impact of CFTR modulation on airway microbial diversity, we found significant differences between children with CF not receiving CFTR modulators, children with CF receiving CFTR modulators, and healthy controls. While diversity was highest for healthy controls, those receiving CFTR modulators had the next highest levels of diversity. These findings corroborate previously published studies in older children and adults with CF. The first study of changes in the airway microbiome related to ivacaftor use assessed paired sputum from 14 sub-study participants (Rowe et al., 2014). In this older cohort (mean age 27 years), the authors found that while sputum bacterial diversity did not change with treatment, the combined relative abundance of traditional CF bacterial pathogens trended downward (mean change -13.9, p = 0.11) (Rowe et al., 2014). Another study of 3 patients with longitudinally collected sputum samples pre- and post-ivacaftor initiation found that microbial communities became more dissimilar after ivacaftor initiation, suggesting a change in community composition and relative abundance (Bernarde et al., 2015). A more recent study of 12 adult subjects (mean age 29.5 years), 8 of whom were chronically infected with Pseudomonas aeruginosa, found that all experienced a significant decline in P. aeruginosa following ivacaftor initiation that lasted for at least 1 year (Hisert et al., 2017). Additionally, the decline in the relative abundance of P. aeruginosa was accompanied by reciprocal changes in the relative abundance of nonconventional organisms, leading to increased richness and Shannon diversity (Hisert et al., 2017). While this sub-analysis was only performed on small number of patients, it adds to this body of CFTR literature by demonstrating that CFTR modulation is significantly associated with increased microbial richness and diversity, bringing levels closer to those seen in age similar healthy children.
Limitations in our study include the use of 16S rRNA sequencing, which can lead to primer bias and only describes the microbial community at the level of bacterial genera, missing species level differentiation of bacteria (and unknown bacteria) as well as viral and fungal components of the microbiome. While we did not find statistical significance in common causes of bacterial exposures between the children with CF and the healthy controls, this does not signify the absence of confounding in a modest sized study, especially in light of the difference in race identified between the two groups. This study also did not address concurrent viral infection, which also contributes to respiratory health and can impact the microbiome. Future studies would need to control for these confounders. Additionally, the total numbers of children with CF who participated in the longitudinal study was small and likely led to an inability to detect differences that may have been present between the younger and older age groups. In the CFTR modulator sub-analysis, those receiving modulators had significantly more study visits and samples collected during the study period, which may have introduced biases to our analysis. Lastly, this study relied on information obtained from OP swabs and did not include a BAL comparison group, and prior studies have shown discordance between cultures and microbiome analyses of OP swabs versus sputum or BAL (Armstrong et al., 1996; Borowitz et al., 2009; Goddard et al., 2012; Lahiri et al., 2016; Prevaes et al., 2017; Ramsey et al., 1991; Rosenfeld et al., 1999; Zemanick et al., 2015).
5. Conclusions
In summary, we found that children with CF have reduced microbial diversity of the upper airway microbiome compared to age similar healthy controls. The decrease in bacterial diversity appears to start in early childhood and, in addition to the presence of CF disease, it may be associated with antibiotic exposure. Longitudinal samples collected over time of children with CF showed a trend toward less stability in the microbial composition of older children, possibly related to increased exacerbations and infection with traditional CF pathogens. Lastly, the addition of CFTR modulators increased airway microbial diversity closer to the levels seen in healthy controls. We did not find antibiotic use to be a factor affecting differences in microbial diversity among children with CF, but our study was not designed to answer that specific question. Longitudinal studies in a larger cohort are needed to better define the acquisition of the diverse microbial community in young children with CF. More specifically, we need to improve our understanding of how early initiation of CFTR modulators and administration antibiotics early in life affect microbial diversity and clinical outcomes.
Declarations
Author contribution statement
A. Hahn: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Burrell, E. Ansusinha, D. Peng, H. Chaney, I. Sami, G Perez and A. Koumbourlis: Performed the experiments.
R. McCarter, R. Freishtat, K. Crandall and E. Zemanick: Analyzed and interpreted the data.
Funding statement
A. Hahn was supported by a K12 Career Development Award from National Heart, Lung, and Blood Institute of the National Institutes of Health (award number K12HL119994), the Margaret Q. Landenberger Foundation (BBH20170207), and the Cystic Fibrosis Foundation Harry Shwachman Clinical Investigator Award (HAHN18A0-Q). K. Crandall was supported by NIH/NCATS (R21TR002513, UL1TR001876). The creation of the CF biorepository was funded by the Clark Charitable Trust. The recruitment of healthy controls and the sequencing experiments were funded by the Board of Visitors at Children's National Grant Program.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at NCBI SRA under the accession number BioProject PRJNA529405.
Acknowledgements
The authors would like to acknowledge Dr. Linda Fu MD, MS for her assistance in recruiting healthy controls from the Goldberg Center for Community Pediatric Health at Children's National Hospital.
References
- Armstrong D.S., Grimwood K., Carlin J.B., Carzino R., Olinsky A., Phelan P.D. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr. Pulmonol. 1996;21(5):267. doi: 10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K. AID-PPUL1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bernarde C., Keravec M., Mounier J., Gouriou S., Rault G., Férec C.…Héry-Arnaud G. Impact of the CFTR-Potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PloS One. 2015 doi: 10.1371/journal.pone.0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz D., Robinson K.A., Rosenfeld M., Davis S.D., Sabadosa K.A., Spear S.L.…Accurso F.J. Cystic fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J. Pediatr. 2009 doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy J.P., Cotton C.U., Donaldson S.H., Solomon G.M., VanDevanter D.R., Boyle M.P.…Tuggle K.L. CFTR modulator theratyping: current status, gaps and future directions. J. Cyst. Fibros. 2019 doi: 10.1016/j.jcf.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn B., Wang P.W., Diaz Caballero J., Clark S.T., Brahma V., Donaldson S.…Guttman D.S. Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 2015;5(1):10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flight W.G., Smith A., Paisey C., Marchesi J.R., Bull M.J., Norville P.J.…Mahenthiralingam E. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J. Clin. Microbiol. 2015;53(7):2022–2029. doi: 10.1128/JCM.00432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayman K.B., Armstrong D.S., Carzino R., Ferkol T.W., Grimwood K., Storch G.A.…Ranganathan S.C. The lower airway microbiota in early cystic fibrosis lung disease: a longitudinal analysis. Thorax. 2017 doi: 10.1136/thoraxjnl-2016-209279. [DOI] [PubMed] [Google Scholar]
- Frayman K.B., Wylie K.M., Armstrong D.S., Carzino R., Davis S.D., Ferkol T.W.…Ranganathan S.C. Differences in the lower airway microbiota of infants with and without cystic fibrosis. J. Cyst. Fibros. 2019 doi: 10.1016/j.jcf.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W.…Wohl M.E. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N. Engl. J. Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- Goddard A.F., Staudinger B.J., Dowd S.E., Joshi-Datar A., Wolcott R.D., Aitken M.L.…Singh P.K. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl. Acad. Sci. Unit. States Am. 2012 doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.R.R., Kajbafzadeh M., Desai S., Yang C.L., Skolnik K., Quon B.S. Scientific Reports; 2019. A Systematic Review of the Clinical Efficacy and Safety of CFTR Modulators in Cystic Fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.a., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap) - a metadata driven methodology and workflow process for providing translational research informatict support. J. Biomed. Inf. 2009 doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisert K.B., Heltshe S.L., Pope C., Jorth P., Wu X., Edwards R.M.…Singh P.K. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna T.A., Wagner B.D., Williams C.B., Stevens M.J., Robertson C.E., Welchlin C.W.…Harris J.K. Airway microbiota in bronchoalveolar lavage fluid from clinically well infants with cystic fibrosis. PloS One. 2016 doi: 10.1371/journal.pone.0167649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri T., Hempstead S.E., Brady C., Cannon C.L., Clark K., Condren M.E.…Davis S.D. Clinical practice guidelines from the cystic fibrosis foundation for preschoolers with cystic fibrosis. Pediatrics. 2016 doi: 10.1542/peds.2015-1784. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12) doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie T., Gifford A.H., Sabadosa K.A., Quinton H.B., Knapp E.A., Goss C.H., Marshall B.C. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the cystic fibrosis foundation patient registry. Ann. Intern. Med. 2014;161(4):233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 2014 doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika M., Korten I., Qi W., Regamey N., Frey U., Casaulta C.…Hilty M. The nasal microbiota in infants with cystic fibrosis in the first year of life: a prospective cohort study. The Lancet Respiratory Medicine. 2016 doi: 10.1016/S2213-2600(16)30081-9. [DOI] [PubMed] [Google Scholar]
- Muhlebach M.S., Zorn B.T., Esther C.R., Hatch J.E., Murray C.P., Turkovic L.…Wolfgang M.C. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018 doi: 10.1371/journal.ppat.1006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., Mcglinn D.…Wagner H. Vegan: community ecology package. R package version 2.4-4. 2017. https://CRAN.R-project.org/package=veganhttps://github.com/vegandevs/vegan/issues%0Ahttps://github.com/vegandevs/vegan/issues%0Ahttps://github.com/vegandevs/vegan Retrieved from.
- Pittman J.E., Wylie K.M., Akers K., Storch G.A., Hatch J., Quante J.…Ferkol T.W. fibrosiAssociation of Antibiotics, Airway Microbiome, and Inflammation in Infants with Cystics. Annals of the American Thoracic Society; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevaes S.M.P.J., De Steenhuijsen Piters W.A.A., De Winter-De Groot K.M., Janssens H.M., Tramper-Stranders G.A., Chu M.L.J.N.…Bogaert D. Concordance between upper and lower airway microbiota in infants with cystic fibrosis. Eur. Respir. J. 2017 doi: 10.1183/13993003.02235-2016. [DOI] [PubMed] [Google Scholar]
- Prevaes S.M.P.J., De Winter-De Groot K.M., Janssens H.M., De Steenhuijsen Piters W.A.A., Tramper-Stranders G.A., Wyllie A.L.…Bogaert D. Development of the nasopharyngeal microbiota in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2016 doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- Ramsey B.W. Management of pulmonary disease in patients with cystic fibrosis. N. Engl. J. Med. 1996;335(3):179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- Ramsey B.W., Wentz K.R., Smith A.L., Richardson M., Williams-Warren J., Hedges D.L.…Harris K. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am. Rev. Respir. Dis. 1991 doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- Renwick J., McNally P., John B., DeSantis T., Linnane B., Murphy P. The microbial community of the cystic fibrosis airway is disrupted in early life. PloS One. 2014 doi: 10.1371/journal.pone.0109798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.E., Harris J.K., Wagner B.D., Granger D., Browne K., Tatem B.…Frank D.N. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013 doi: 10.1093/bioinformatics/btt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M., Emerson J., Accurso F., Armstrong D., Castile R., Grimwood K.…Wagener J. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr. Pulmonol. 1999;28(5):321. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. AID-PPUL3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rowe S.M., Heltshe S.L., Gonska T., Donaldson S.H., Borowitz D., Gelfond D.…Ramsey B.W. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014 doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagel S.D., Gibson R.L., Emerson J., McNamara S., Burns J.L., Wagener J.S.…Rosenfeld M. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 2009 doi: 10.1016/j.jpeds.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagel S.D., Sontag M.K., Wagener J.S., Kapsner R.K., Osberg I., Accurso F.J. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J. Pediatr. 2002 doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- Sagel S.D., Wagner B.D., Anthony M.M., Emmett P., Zemanick E.T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012 doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B.…Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seekatz A.M., Theriot C.M., Molloy C.T., Wozniak K.L., Bergin I.L., Young V.B. Fecal microbiota transplantation eliminates Clostridium difficile in a murine model of relapsing disease. Infect. Immun. 2015 doi: 10.1128/IAI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick S.M., Brennan S., Murray C., Douglas T., von Ungern-Sternberg B.S., Garratt L.W.…Sly P.D. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J. Pediatr. 2009 doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Stick S.M., Ware R.S., Chen L., Murray C.P., Gangell C.L., Ranganathan S.…Mott L.S. Risk factors for bronchiectasis in children with cystic fibrosis. Surv. Anesthesiol. 2014 doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 2. Quantifying beta diversity and related phenomena. Ecography. 2010 [Google Scholar]
- VanDevanter D.R., Pasta D.J. Evidence of diminished FEV1 and FVC in 6-year-olds followed in the European cystic fibrosis patient registry, 2007-2009. J. Cyst. Fibros. 2013 doi: 10.1016/j.jcf.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Wagner B.D., Grunwald G.K., Zerbe G.O., Mikulich-Gilbertson S.K., Robertson C.E., Zemanick E.T., Harris J.K. On the use of diversity measures in longitudinal sequencing studies of microbial communities. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick E.T., Wagner B.D., Harris J.K., Wagener J.S., Accurso F.J., Sagel S.D. Pulmonary exacerbations in cystic fibrosis with negative bacterial cultures. Pediatr. Pulmonol. 2010 doi: 10.1002/ppul.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick E.T., Wagner B.D., Robertson C.E., Ahrens R.C., Chmiel J.F., Clancy J.P.…Harris J.K. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur. Respir. J. 2017;50(5) doi: 10.1183/13993003.00832-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick E.T., Wagner B.D., Robertson C.E., Stevens M.J., Szefler S.J., Accurso F.J.…Harris J.K. Annals of the American Thoracic Society; 2015. Assessment of Airway Microbiota and Inflammation in Cystic Fibrosis Using Multiple Sampling Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Schloss P.D., Kalikin L.M., Carmody L.A., Foster B.K., Petrosino J.F.…LiPuma J.J. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(15):5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]