Abstract
Gait control becomes more demanding in healthy older adults, yet what cognitive or motor process leads to this age‐related change is unknown. The present study aimed to investigate whether it might depend on specific decay in the quality of gait motor representation and/or a more general reduction in the efficiency of lower limb motor control. Younger and older healthy participants performed in fMRI a virtual walking paradigm that combines motor imagery (MI) of walking and standing on the spot with the presence (Dynamic Motor Imagery condition, DMI) or absence (pure MI condition) of overtly executed ankle dorsiflexion. Gait imagery was aided by the concomitant observation of moving videos simulating a stroll in the park from a first‐person perspective. Behaviorally, older participants showed no sign of evident depletion in the quality of gait motor representations, and absence of between‐group differences in the neural correlates of MI. However, while younger participants showed increased frontoparietal activity during DMI, older participants displayed stronger activation of premotor areas when controlling the pure execution of ankle dorsiflexion, regardless of the imagery task. These data suggest that reduced automaticity of lower limb motor control in healthy older subjects leads to the recruitment of additional premotor resources even in the absence of basic gait functional disabilities.
Keywords: fMRI, foot movements, gait motor control, healthy aging, motor imagery, premotor cortex
1. INTRODUCTION
Age‐related changes in gait control are well known, yet their central neural correlates are largely unexplored. On the one hand, gait disturbances have a substantial impact in healthy aging because falls during locomotion constitute a major source of injury and limited mobility, thus deeply affecting the quality of life (Alexander, 1996; Gillespie et al., 2012). On the other hand, previous studies have shown that cortical control over supraspinal centers consolidates during childhood and resumes being more prominent with senescence, possibly because of impoverished automaticity of motor control in the later decades of life (Boisgontier et al., 2013; Ruffieux, Keller, Lauber, & Taube, 2015). Cortical control is responsible for the adaptation of automatic gait patterns to environmental cues (Liston, Mickelborough, Bene, & Tallis, 2003; Nutt, 2013), allowing obstacle negotiation or daily‐life activities like walking while talking. It mainly involves the recruitment of an extensive frontoparietal network, which is especially vulnerable to age‐related physiological decay (Seidler et al., 2010): this may justify why the above‐mentioned everyday‐life functions become more demanding even in healthy older adults (Saimpont, Malouin, Tousignant, & Jackson, 2013). However, this issue has been scarcely investigated at the neurophysiological level and by using functional magnetic resonance (fMRI) techniques.
Exploration of the neural correlates of walking per se, let alone the effects of aging, using functional imaging poses some obvious practical challenges due to the constraints of the fMRI setting.1 Motor Imagery (MI) represents a useful tool for addressing this issue while overcoming some pragmatic limitations of the imaging setting. MI is defined as a mental state in which real movements and the corresponding neural activity are internally evoked without overt muscular contraction (Jeannerod & Frak, 1999; Munzert & Zentgraf, 2009). According to the simulation theory (Jeannerod, 2001), common motor representations guide MI and movement planning, as shown by evidence that MI and overt movement execution share common temporal features (“isochrony,” Decety & Michel, 1989) and similar anatomo‐functional correlates (see Hétu et al., 2013 for a review). Thus, MI has become a widely‐used proxy to study and train motor representations in healthy subjects and clinical populations (see Munzert, Lorey, & Zentgraf, 2009 for a review), because it allows for the simulation of complex motor acts within constrained experimental settings as those required by the application of neuroimaging techniques (see, for instance, Lotze, Scheler, Tan, Braun, & Birbaumer, 2003; Ruby & Decety, 2001; Sacco et al., 2006). Although being an indirect measure of overt motor behaviors, MI proved to be highly reliable. With regard to gait behavior, for instance, previous studies have consistently shown that gait MI tasks show neural correlates that are highly overlapping with the cortical and subcortical structures involved in gait motor control and navigation (Bakker et al., 2008; Bakker, Verstappen, Bloem, & Toni, 2007; Jahn et al., 2004, 2008).
MI has also been widely applied to track changes in motor representations across the life‐span (see Saimpont et al., 2013 for a review). Behaviorally, a good correlation between imagined and executed movement timings in older individuals has been widely reported (Personnier, Paizis, Ballay, & Papaxanthis, 2008; Skoura, Papaxanthis, Vinter, & Pozzo, 2005), suggesting that isochrony might be preserved in aging, at least for simple movements. Yet, imagery might be less vivid in healthy older individuals when imagining more complex actions (Mulder, Hochstenbach, van Heuvelen, & den Otter, 2007; Zapparoli, Gandola, Banfi, & Paulesu, 2019), with lower reliance on kinesthetic features in favor of more visual strategies (Zapparoli et al., 2013; Zapparoli et al., 2016). With regard to gait, isochrony seems to be preserved in “young” older adults (up to 70 years of age, Skoura et al., 2005; Schott & Munzert, 2007), although gait control may require additional computational resources, especially when gait is performed in parallel with a cognitive task, as suggested by the poor performance that healthy older adults (Boisgontier et al., 2013; Ruffieux et al., 2015) and neurological patients affected by movement disorders (Nieuwhof et al., 2017) typically show at “dual‐task” paradigms. The reasons for this performance decay are still poorly understood, and a description of which supplemental neurocognitive resources might overcome it is lacking.
Neurofunctional evidence accumulated so far is insufficient to shed light on this issue. Previous fMRI studies comparing gait MI in younger and older participants show that, in spite of similar behavioral outcomes, additional neural resources might be required by older adults to maintain performance at a juvenile‐like level (Allali et al., 2014; Wai et al., 2012; Zwergal et al., 2012) in line with compensatory hypotheses of graceful aging (see for instance Reuter‐Lorenz & Cappell, 2008; Berlingeri et al., 2010). Importantly, to count as properly “compensatory,” these additional neural resources should show a linear relation with the individuals' level of performance (i.e., the higher an area is activated in older participants, the higher the performance should be, Cabeza et al., 2018). Different distributions of possibly compensatory patterns of brain activity have been reported: while some studies have shown hyper‐activations in older as compared to younger participants in prefrontal regions (Allali et al., 2014; Blumen, Holtzer, Brown, Gazes, & Verghese, 2014), others have highlighted the involvement of more posterior activations in somatosensory, vestibular, and visual brain regions (Wai et al., 2012; Zwergal et al., 2012), although none of these studies reported correlations between brain activations and the participants' behavioral performance in gait imagery or gait execution.
The practical implications of this contradictory evidence remain limited, especially concerning the potential role that gait MI might have for the prevention of falls and for motor training in patients who tend to be older (like neurological and orthopedic patients). Previous studies do not bring evidence on what eventually makes the gait imagery task more difficult for older adults. For instance, on the one hand, one might hypothesize that it is the more “cognitive” side of gait motor control to be more affected in aging, that is, those motor cognitive processes related to navigation and obstacle negotiation that are mainly controlled by frontoparietal areas (Liston et al., 2003; Nutt, 2013). On the other hand, it might well be that aging affects to a greater extent lower levels of gait motor control, for example, the efficiency of the brain network controlling the execution of rhythmic lower limb movements. These two possibilities, which are not mutually exclusive, can be seen as a “higher” versus “lower” level of age‐related changes in gait motor control. Along the same line, one might wonder whether the addition of internal (motor) and external (visual) cues might ease the task for older individuals and thus reduce the need for additional resources permitting to achieve a better outcome at mental training based on gait MI.
1.1. Aims of the study and working hypotheses
To overcome the aforementioned ambiguities and give a novel look at healthy aging and gait representations, in this study we applied a recently devised paradigm (Sacheli et al., 2018) that combines MI of walking and standing on the spot with the presence (dynamic motor imagery [DMI] condition) or absence (pure MI condition) of overtly executed ankle dorsiflexion. DMI has been defined as a MI task that includes overt movements or body configurations mimicking those mentally rehearsed to provide proprioceptive feedbacks that resemble those of the imagined movements (Guillot, Moschberger, & Collet, 2013). As a proxy of actual walking patterns, we selected ankle dorsiflexion because it is applicable to the fMRI environment, and its neural underpinnings proved to correlate with actual walking performance (Dobkin, Firestine, West, Saremi, & Woods, 2004).
This design allowed us to test two main hypotheses. First, the direct comparison between younger and older participants' neural activations in a purely imaginative but visually‐guided gait imagery task (MI condition) allowed us to test whether it requires additional neural resources in older participants and particularly the recruitment of prefrontal cortices (see Allali et al., 2014; Blumen et al., 2014), which might indicate that the internal simulation of “higher level” processes involved in gait motor control (like those required for simulating navigation) become more cognitively demanding in older participants. Second, by comparing the neural correlates of purely imaginative (MI) and DMI tasks between younger and older participants, we aimed to test whether the additional proprioceptive feedbacks and increasing motor demands characterizing DMI facilitate the recruitment of brain motor resources during gait imagery in all participants and particularly in healthy older participants. The availability of a baseline condition in which ankle dorsiflexion is performed without the request of imagining to walk allowed us to finally test whether “lower level” processes involved in gait control like the mere performance of simple rhythmic foot movements become more cognitively demanding for the older adults. If so, one would expect stronger recruitment of cortical regions in older than younger participants for the mere execution of the motor task. Before fMRI examination, the participants also performed a mental chronometry task to measure the quality of gait motor representations and guide the interpretation of our fMRI findings.
We had the following expectations compatible with two alternative scenarios. A former “representational impairment” hypothesis, whereby voluntary recall of gait kinesthetic sensations through imagery is deficient in healthy older participants, would be satisfied if they showed performance decay at the behavioral task, and if the neural correlates of the MI task would show in the older group hypoactivation of motor and premotor regions and hyperactivation of alternative neural resources, possibly in prefrontal cortices (see Allali et al., 2014; Blumen et al., 2014). In this former scenario, proprioceptive signals provided by DMI might then facilitate the recruitment of gait‐related motor areas during the imagery task. On the contrary, a “reduced efficiency in lower limb motor control” hypothesis would be satisfied if the mere execution of rhythmic foot movements, independently of imagery of walking, was sufficient to saturate the motor/premotor network, suggesting that older participants need more cognitive resources to control the execution of simple foot movements and that this might be independent from more “higher level” motor cognitive processes involved in walking tasks.
We had no a priori expectation in favor of one or the other scenario, but what counts here is that our experimental design allowed us to compare the two possibilities analytically.
2. MATERIALS AND METHODS
2.1. Participants
Twenty‐one healthy older participants (12 males, age 66.33 ± 6.83; education 13.14 ± 4.19 years) and twenty‐one younger adults (10 males, age 25.48 ± 3.22; education 15.52 ± 2.69 years) were included in the study. The older participants were the same who were included in the healthy sample of a previous paper from our group (Sacheli et al., 2018). None of the participants reported any current or previous motor hindrance, or any history of neurologic or psychiatric disease. All subjects were right‐handed according to the Edinburgh Handedness Inventory (Oldfield, 1971).
All volunteers were preliminarily screened with two tests of global cognitive functioning, the mini mental state examination (Folstein, Folstein, & McHugh, 1975) and the Raven's colored progressive matrices (Raven, Bulheller, & Häcker, 1984), to exclude the presence of between‐group differences. Older participants were also screened at long‐term and short‐term verbal memory tests (Novelli et al., 1986) to exclude deficits due to pathological aging. One participant in the older group did not perform the Raven's colored progressive matrices and one in the younger group did not perform both neuropsychological tests; all tested participants scored in the normal range. Importantly, the two groups did not differ for education (p uncorr = .09), nor in MMSE (p uncorr = .60) and Raven's colored progressive matrices scores (p uncorr = .27) once correction for age and education was performed.
We also administered the vividness of movement imagery questionnaire (VMIQ, Isaac, Marks, & Russell, 1986), in order to assess whether self‐reported MI general abilities were different between the two groups; the VMIQ scores of younger and older subjects were not significantly different (p uncorr = .21).
The experimental protocol was approved by the Local Ethics Committee (Comitato Etico Ospedale San Raffaele) and carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki and later amendments. All participants provided written informed consent to take part in the study and had no contraindication to MRI.
2.2. Procedure
Both groups underwent the same experimental procedure, that included motor execution and MI tasks performed outside the scanner and a MI “virtual walking” paradigm executed during fMRI, which was either associated or not with overt foot movements (Sacheli et al., 2018).
2.2.1. Behavioral task and data analysis
The behavioral tests aimed to assess the quality of gait MI in both groups. This was accomplished by measuring the duration of motor execution and MI of a short walk during a standardized test, the Timed‐Up‐and‐Go (TUG; Podsiadlo & Richardson, 1991), in the version developed by Beauchet et al. (2010). Subjects were seated, allowed to use the armrests to stand up and instructed to walk 3 m, turn around, walk back to the chair and sit down saying “stop.” Times for each condition were recorded with a stopwatch to the nearest 0.01 s. The stopwatch was started on the command “ready–set–go” and stopped as the subject sat down and said “stop.” For the imagined condition, subjects sat in the chair and were instructed to imagine performing the TUG (imagined TUG, iTUG) with their eyes closed and to say “stop” when they were finished. Participants performed both the TUG and the iTUG twice and we averaged the times of the two trials. To assess mental chronometry abilities (CA) we calculated the time discrepancy between the TUG and the iTUG with the following formula (Allali et al., 2014): CA = (TUG − iTUG)/[(TUG + iTUG)/2]. CA was separately calculated per trial and the results were averaged to obtain one outcome measure. The lower the CA score, the smaller the difference between times recorded during the TUG and the iTUG, which would index better isochrony and higher MI abilities.
To test group differences in TUG and iTUG time durations, a 2 × 2 factorial analysis of variance (ANOVA) was performed, with group (older vs. younger adults) as a between‐subjects factor and task (MI vs. ME) as a within‐subjects factor. Group differences in CA were measured by means of a t test for independent samples. All data were normally distributed according to Kolmogorov–Smirnov and Shapiro–Wilks test (all ps > .1) after log‐transformation of raw values was applied. The Levene's test was also not significant (all ps > .1), indicating no violation of between‐group equality of variance.
2.2.2. fMRI task
The gait imagery task performed in the MRI scanner was the same as described in the paper by Sacheli et al. (2018).
The task required participants to perform MI of walking in two different conditions, in which overt foot movements were either associated (“DMI” condition) or not (pure MI condition, MI) with the imagery tasks. Gait imagery was aided by in‐motion visual stimuli of a path in a park shown in first‐person perspective. As a baseline condition, stationary movies were shown, and participants were required to imagine “standing on the spot”. In a full‐factorial design, we examined age‐related differences in brain activations during gait imagery (as compared to imagery of standing still) and modulation thereof introduced by DMI. Our full factorial design also allowed us to measure, in a control analysis, between‐group differences in the neural correlates of foot movement execution.
Stimuli and procedure
During the fMRI session, the participants watched 15 s naturalistic videos of a path leading through a park in two conditions: (a) in‐motion, “virtual walking” condition (Walk), or stationary, standing condition (Stand) that served as baseline. Throughout the experiment, participants were asked to imagine standing (Stand) or walking along the path (Walk) as if the camera were “their own eyes” (see also Iseki, Hanakawa, Shinozaki, Nankaku, & Fukuyama, 2008). In the Walk condition, the scene moved forward at a speed compatible with slow human walking rhythm (≈ 1.1 m/s). Naturalistic scenarios depicted two different paths that were shown as either ascending or descending (see Sacheli et al., 2018). At the beginning of each video, a written prompt with either the instructions “no foot movement” or “move your feet” was displayed for 3 s. In the “move your feet” condition (50% of the trials), the participants executed alternate ankle dorsiflexion (Dobkin et al., 2004) in‐step with the rhythm of their imagined gait pattern (in the Walk condition). The participants were instructed to maintain this rhythm throughout the experiment whenever cued to make foot movements (i.e., also in the Stand condition): when the Stand condition was combined with foot movements, participants were told that a daily life analogue of the task would have been stepping on the spot. The experimenter monitored the participants' foot movements during the entire session to ensure they followed the instructions. Scans in which a participant failed to respond to the prompt, that is, did not move the feet when cued to “move your feet,” or vice‐versa, were discarded from the analysis (10 scans in one participant and 20 scans in a second one in the group of older participants). Finally, eight times per run (two per experimental condition), the participants were asked whether the path that they had just seen ascended or descended. The purpose of these attention‐getter questions was to keep participants focused on the videos.
The fMRI run lasted 11.5 min and 230 scans were acquired. The first 10 scans, corresponding to visualization of task instructions, were discarded from the analysis. The run included 32, 15 s videos, that is, eight per experimental condition (i.e., Walk‐MI, Stand‐MI, Walk‐DMI, and Stand‐DMI), for a total of 40 scans acquired per experimental condition.
Before starting the fMRI session, the participants practiced the task outside the scanner tube (while seated on a chair) for about 10 min, so that they could familiarize with the videos and learn to correctly execute ankle dorsiflexion when prompted. Afterwards, they shortly familiarized with the rhythmic ankle dorsiflexion movements while lying down before entering into the scanner tube.
Foam padding was applied around the head to minimize head movements; a semicircular cushion supporting the legs was provided, so that the participants could freely move their ankles without bending their knees.
Stimuli presentation was controlled by Cogent 2000 MATLAB Toolbox (MathWorks). Visual stimuli were delivered using VisuaStim fiber‐optic goggles (800 × 600 pixel resolution). Responses were recorded through a response box placed under the participant's right hand (Resonance Technology Inc., Northridge, CA). Subjects' ankle dorsiflexion movements were video‐recorded, in order to calculate the movement frequency and to compare it across different conditions (Walk‐DMI/Stand‐DMI) and groups (Young/Old).
fMRI data acquisition
MRI scans were acquired using a Siemens Magnetom Avanto 1.5 T scanner (Siemens AG, Erlangen, Germany), equipped with gradient‐echo echoplanar imaging. Two hundred thirty functional volumes were acquired for each subject (flip angle 90°, TE 60 ms, TR 3000 ms, FoV 250 mm, matrix 64 × 64, 31 slices, slice thickness 4 mm, interleaved acquisition). The first two brain images (TR periods) from the functional run were necessary to allow for steady‐state tissue magnetization and were thus not collected. A MPRAGE high‐resolution T1‐weighted structural image was also acquired for each subject (flip angle 35°, TE 5 ms, TR 21 ms, FOV 256 × 192 mm, matrix 256 × 256, TI 768, for a total of 160 axial slices with 1 × 1 × 1 mm voxels).
Preprocessing
Raw functional data were reconstructed and converted from the DICOM to the NIfTI format using the MRIcron software (http://www.mccauslandcenter.sc.edu/crnl/mricron/). The subsequent image manipulations and statistical analyses were performed in the MATLAB platform (2016b, Math Works, Natick, MA) with the Statistical Parametric Mapping software package (SPM12 ‐ Wellcome Department of Imaging Neuroscience, London, UK). Functional images were first realigned to the first acquired volume and unwarped to minimize the effect of the subjects' movement during the session. The high‐resolution T1‐weighted structural image of each participant was segmented and normalized to the Montreal Neurological Institute (MNI) stereotactic space to allow between‐subject comparison (Ashburner & Friston, 2005), and it was then co‐registered to the realigned and unwarped functional volumes. The functional images were then normalized by applying the Deformation Fields estimated during the segmentation of the structural data, and the data matrix was interpolated to produce voxels 2 × 2 × 2 mm in dimension. The normalized scans were finally smoothed using a Gaussian filter with 10 × 10 × 10 mm as full width at half‐maximum (FWHM) value, to improve the signal‐to‐noise ratio in the data.
An additional step was included in order to reduce the impact of movement artifacts by using the Artifact detection Tools (ART, Withfield‐Gabrieli https://www.nitrc.org/projects/artifact_detect/). This toolbox allows identifying and discarding from the analyses the scans that could lead to artefactual statistical effects due to excessive movement. Thresholds were set at 1 mm scan‐to‐scan head movement and 3 SD of scan‐to‐scan global signal intensity change. Experimental subjects that exhibited more than 20% outlier scans in the whole experimental run or more than 20% outlier scans in at least one of the relevant experimental conditions would have been excluded from the subsequent statistical analyses. No participant included in the final sample (21 younger and 21 older adults) exceeded these thresholds. Overall, we excluded 5.3 ± 3.3% of scans in the Younger group and 6.15 ± 3.3% of scans in older group, and the number of excluded scans did not differ between the groups (p = .44).
Statistical analysis
Preprocessed functional volumes were entered in a two‐level statistical analysis procedure based on the General Linear Model. This allowed testing for statistical differences in the blood‐oxygen‐level dependent (BOLD) signal within the different experimental conditions in the two groups. The signal was analyzed by a convolution with a canonical hemodynamic response function (Worsley & Friston, 1995). No global normalization was performed. The time series was high‐pass filtered at 128 s to remove artifactual contributions to the fMRI signal such as noise from cardiac and respiratory cycles, and it was pre‐whitened by means of an autoregressive model AR(1). Each of the videos during which the participants performed the gait imagination task represented a single trial in a block design, identified by its specific onset and a fixed duration of 15 s.
At the first level, a within‐subjects fixed‐effect analysis allowed the condition‐specific effects to be estimated. In particular, each of the following conditions corresponded to a specific regressor: Walk‐MI, Stand‐MI, Walk‐DMI, Stand‐DMI. Moreover, specific regressors of no interest were defined for the written prompts (lasting 3 s each) and the attention‐getter questions regarding the slope of the scenarios (lasting 8 s each). Realignment parameters from the preprocessing steps of the analysis were also included in the GLM as regressors of no interest, as well as specific regressors generated by the ART toolbox to exclude the outlier scans that exceeded the movement thresholds.
At the second level of analysis (group analysis), two orthogonal 2 × 2 ANOVAs were employed. First, we evaluated groups differences and similarities in the activation patterns associated to MI and DMI (Virtual Walking analysis); second, in a control analysis we measured group differences and similarities in the cortical activations associated with actual foot movements (Foot Movement analysis).
Virtual walking analysis
We investigated age‐related differences in the neural correlates of MI of gait by comparing the brain responses during the in‐motion videos (Walk videos) with those collected while the participants imagined standing on the spot (Stand videos that served as baseline), and by separately analyzing the trials where explicit ankle dorsiflexion was present (MI condition) or absent (DMI condition). The group‐analysis was thus based on the following linear contrasts (calculated at the single‐subject level): (a) “Walk(MI) > Stand(MI)”, and (b) “Walk(DMI) > Stand(DMI)”.
Both the MI and DMI contrasts carry the implicit motor component of the imaginative task. Importantly, in the “Walk(DMI) > Stand(DMI)” contrasts the explicit motor component (linked to overt movement) is canceled out by the subtraction between two conditions (Walk [DMI] and Stand [DMI]) both associated with overt foot movements, while the implicit motor component of the imaginative task is preserved. Thus, after removal of the pure motor component, the DMI contrasts convey, relative to the MI ones, the additional neural resources necessary for combining the gait imagery task with overt foot movement execution.
The (a) “Walk(MI) > Stand(MI)”, and (b) “Walk(DMI) > Stand(DMI)” contrast images for both the young and the old groups were entered into a 2 × 2 factorial ANOVA, with Group (older vs. younger adults) as between‐subject factor and the Imagery‐type (MI vs. DMI) as a within‐subject factor. This allows evaluating (a) general group differences in gait imagery (main effect of Group), (b) general difference between MI and DMI (main effect of Imagery‐type), and (c) group differences in the way MI and DMI are dealt with by the two groups (interaction effect).
We also mapped the overall main effect of virtual walking (linear contrast: 1 1 1 1) to identify the brain regions associated gait imagery, and the between‐group differences and similarities separately per each Imagery‐type (DMI and MI) by calculating simple between‐group effect contrasts and the conjunction effects.
Foot movement analysis
As a control analysis, we mapped age‐related differences in the motor network responsible for foot movement execution. At the first‐level of statistical analysis (single‐subject level), we calculated the following linear contrasts: (a) “Stand(DMI) > Stand(MI)”, and (b) “Walk(DMI) > Walk(MI)”. These contrasts convey information on the cortical activations recorded during explicit foot movement execution during the stand versus walk condition, where the implicit motor component of the imagery task is subtracted out by the baselines. These contrast images were entered into a 2 × 2 factorial ANOVA, with Group (old vs. young) as a between‐group factor and the imaginative Task (walk vs. stand) as a within‐subject factor. In this analysis, we were specifically interested in testing simple between‐group effects, and we thus evaluated age‐related differences in the neural correlates of foot movement execution, both as a main effect and separately for the Stand and Walk condition. We also mapped the overall main effect of Foot Movements (linear contrast: 1 1 1 1) to identify the brain regions associated with the execution of lower limb movements compatible with gait behaviors (i.e., ankle dorsiflexion, see Dobkin et al., 2004).
All reported results survive a cluster‐wise family‐wise error rate (FWER) correction for multiple comparisons (p FWER < .05). The cluster‐wise correction was applied to data having applied a 10 × 10 × 10 Gaussian smoothing and at p uncorr < .001 at the voxel‐level, as recommended by Flandin and Friston (2019). All tables also report which activation peaks also survived the FWER correction for multiple comparisons at the voxel‐level.
3. RESULTS
3.1. Behavioral results: Gait motor performance and mental CA
The ANOVA on TUG and iTUG times showed a main effect of Task (F[1,40] = 47.51, p < .001), indicating that iTUG times were shorter than real TUG times in both groups (mean TUG times 7.96 ± 1.44 s; mean iTUG times 6.64 ± 2.18 s, Figure 1). No significant effect of Group (p = .67) or Group by Task interaction (p = .62) was found. The t‐test indicated the absence of significant between‐group differences in CA (t[40] = .42, p = .68, Figure 1). To sum up, the group of older subjects did not show neither dysfunctions of basic gait functional mobility, nor impairment in MI abilities.
Figure 1.
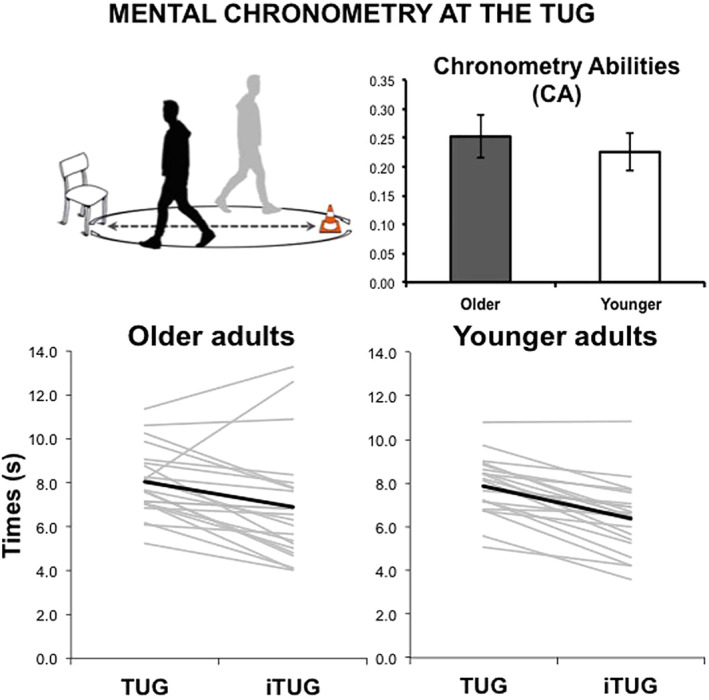
Behavioral results at the timed up and go (TUG) task. Upper panel: a representation of the TUG and group comparison of chronometry abilities (CA) (indexed by the mean of within‐subject differences between duration of gait imagery and execution, see main text). Error bars indicate SEM Lower panel: the timings required by participants to execute (TUG) and imagine (iTUG) the task. Gray lines indicate the singlesubject data and black thick lines indicate the group means
3.2. Behavioral results: Ankle dorsiflexion movements
Non‐parametric independent sample tests showed a significant difference between the two groups in terms of ankle‐flexion frequencies: movement frequency was lower in the older subjects compared to the younger ones; however, this was true for both the Walk(DMI) (Mann–Whitney test's U = 32, p uncorr = .049, Cohen's d [effect size] = −0.96; mean frequency young 1.70 +/− 0.83 movements per second [mov/s]; mean frequency old 1.08 +/− 0.27 mov/s) and Stand(DMI) conditions (Mann–Whitney test's U = 32, p uncorr = 0.042, Cohen's d [effect size] = −1.01; mean frequency young 1.71 ± 0.83 mov/s; mean frequency old 1.05 ± 0.28 mov/s).
3.3. fMRI results. Virtual walking analysis
3.3.1. Main effect of virtual walking (contrast 1 1 1 1)
Overall, the main effect of task activated an extensive network in the occipital, parietal and frontal lobe, including, on the one hand, the frontoparietal regions of the median wall, basal ganglia and mesencephalic regions responsible for foot movement motor control, and, on the other hand, dorsal premotor and posterior parietal regions associated with the adaptation of gait patterns to environmental cues (Table S1; Figure 2).
Figure 2.
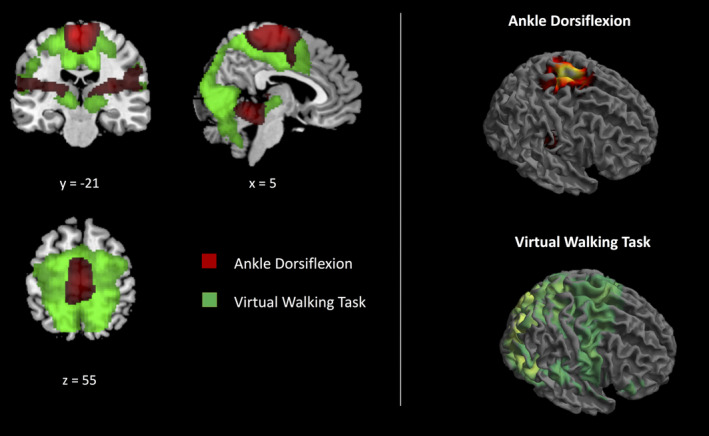
The main effects of the tasks. The main effect of virtual walking (green) and the main effect of foot movements (red) have been superimposed (left) and illustrated separately (right). Only clusters surviving FWER‐correction for multiple comparisons have been reported
3.3.2. Main effect of group
The ANOVA showed no significant main effect of Group.
3.3.3. Main effect of imagery‐type
Five clusters (having their peaks in the supplementary motor area, cerebellum, right inferior frontal gyrus, and bilaterally in the parietal opercula) showed a main effect of Imagery‐type, being more active in the MI than the DMI task (Table S2). No area showed a significant effect in the opposite direction as a main effect (DMI > MI).
3.3.4. Interaction effect
The main effect of Imagery‐type was further specified by a significant Group × Imagery‐type interaction, showing that group modulated the difference between brain activations in MI as compared to DMI in frontoparietal regions and in the cerebellum (Table 1). In particular, the left and right premotor cortices were more active during MI as compared to DMI in the older participants, and in DMI as compared to MI in the younger participants (Figure 3b,c). This suggests that younger and older participants applied different neurocognitive strategies to deal with the DMI task. No area showed a significant interaction effect in the opposite direction, that is, no area was more strongly recruited by older adults in the DMI as compared to the MI task, or by younger adults in the MI as compared to the DMI task.
Table 1.
Interaction effect in the virtual walking analysis (contrast 1 ‐1 ‐1 1)
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| Sup. frontal gyrus (6) | −22 | −10 | 48 | 5.0* | 24 | −8 | 64 | 3.8 |
| SMA | −8 | −18 | 58 | 3.8 | ||||
| SMA (6) | −8 | 4 | 52 | 4.3 | ||||
| −4 | 6 | 66 | 4.0 | |||||
| Precentral gyrus | 32 | −20 | 42 | 4.6 | ||||
| Precentral gyrus (6) | −32 | −10 | 52 | 4.6 | 26 | −10 | 56 | 4.8* |
| 32 | −24 | 70 | 4.6 | |||||
| 42 | −12 | 56 | 4.4 | |||||
| 18 | −18 | 60 | 3.8 | |||||
| 28 | −18 | 56 | 3.6 | |||||
| 20 | −22 | 60 | 3.6 | |||||
| Central operculum | 56 | 0 | 6 | 4.3 | ||||
| Parietal lobe | ||||||||
| Postcentral gyrus | −24 | −42 | 50 | 4.2 | 30 | −28 | 70 | 4.7* |
| 30 | −24 | 40 | 4.5 | |||||
| 50 | −14 | 48 | 4.0 | |||||
| Postcentral gyrus (3) | −56 | −22 | 46 | 4.1 | 46 | −20 | 58 | 4.3 |
| −52 | −16 | 42 | 3.8 | 38 | −30 | 54 | 3.9 | |
| Paracentral lobule (4) | −6 | −22 | 58 | 3.8 | ||||
| Sup. parietal lobule (2) | −36 | −42 | 66 | 4.7* | ||||
| Sup. parietal lobule (40) | −34 | −36 | 44 | 4.2 | 40 | −46 | 62 | 4.8* |
| Temporal lobe | ||||||||
| Sup. temporal gyrus (22) | 54 | 0 | −8 | 3.3 | ||||
| Cerebellum | ||||||||
| Cerebellum‐III lobule | 14 | −30 | −22 | 4.9* | ||||
Note: x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space. Voxel‐level statistical threshold p < .001uncorr. All reported voxels are included in clusters surviving the family‐wise error rate (FWER) correction at the cluster‐level. (*) Z‐scores statistically significant also after FWER correction at the voxel‐level.
Figure 3.
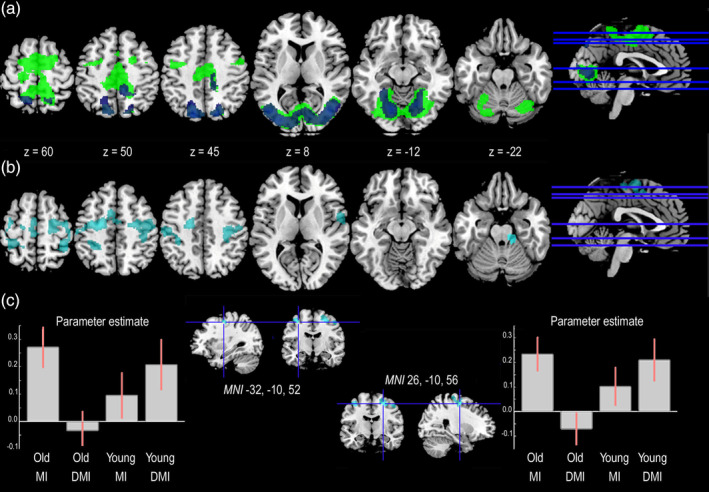
The conjunction and interaction effects in the Virtual Walking analysis. (a) The areas commonly activated by younger and older participants during MI (green) and DMI (blue) as shown by the results of the Virtual Walking analysis. (b) The areas showing an interaction effect in the Virtual Walking analysis. (c) The plots report the parameter estimate (beta values) for the two clusters included in the interaction effect that also showed a significant effect of group in DMI (in this figure, we used the simple group effect of DMI [DMI_Young > DMI_Old contrast] as an explicit mask). Only activations surviving FWER‐correction for multiple comparisons at either the cluster or voxel level have been reported [Color figure can be viewed at http://wileyonlinelibrary.com]
Direct group comparisons separately tested in the two imagery conditions supported these results by showing hypo‐activations in DMI in older as compared to younger participants bilaterally in frontoparietal regions and in the superior temporal gyrus (Table 2 and Figure 4). Older participants showed no hypo‐ or hyper‐activation as compared to younger participants in MI, where no group difference was found.
Table 2.
Between‐group differences in the Virtual Walking analysis, Younger > Older in DMI
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Insula | ||||||||
| Insula | 44 | 4 | −4 | 3.5 | ||||
| 36 | −18 | 2 | 3.1 | |||||
| Frontal lobe | ||||||||
| Precentral gyrus | 38 | −12 | 34 | 3.8 | ||||
| Precentral gyrus (6) | −26 | −18 | 50 | 4.2 | 30 | −24 | 70 | 4.5 |
| −34 | −12 | 54 | 3.9 | 24 | −14 | 62 | 4.3 | |
| 26 | −10 | 56 | 4.2 | |||||
| 48 | −12 | 50 | 4.0 | |||||
| 40 | −8 | 34 | 3.7 | |||||
| 24 | −12 | 50 | 3.6 | |||||
| Parietal lobe | ||||||||
| Precentral (4)/postcentral gyrus | 36 | −26 | 52 | 4.4 | ||||
| 34 | −22 | 48 | 4.2 | |||||
| 44 | −20 | 46 | 3.7 | |||||
| Postcentral gyrus (3) | −32 | −30 | 56 | 3.8 | 36 | −20 | 44 | 4.2 |
| 44 | −20 | 56 | 4.1 | |||||
| 34 | −26 | 46 | 4.1 | |||||
| 56 | −8 | 34 | 3.6 | |||||
| Supramarginal gyrus | 50 | −30 | −30 | 3.6 | ||||
| Occipital lobe | ||||||||
| Precuneus (5) | 14 | −46 | 58 | 4.0 | ||||
| Temporal lobe | ||||||||
| Sup. temporal gyrus | 56 | −2 | 4 | 4.2 | ||||
| 58 | −8 | 4 | 4.1 | |||||
| 50 | 2 | −6 | 3.4 | |||||
| 42 | −16 | 4 | 3.4 | |||||
| 46 | −18 | 2 | 3.2 | |||||
| 46 | −14 | 4 | 3.2 | |||||
| Sup. temporal gyrus (21) | 54 | 2 | −10 | 3.5 | ||||
Note: x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space. Voxel‐level statistical threshold p < .001uncorr. All reported voxels are included in clusters surviving the family‐wise error rate (FWER) correction at the cluster‐level.
Figure 4.
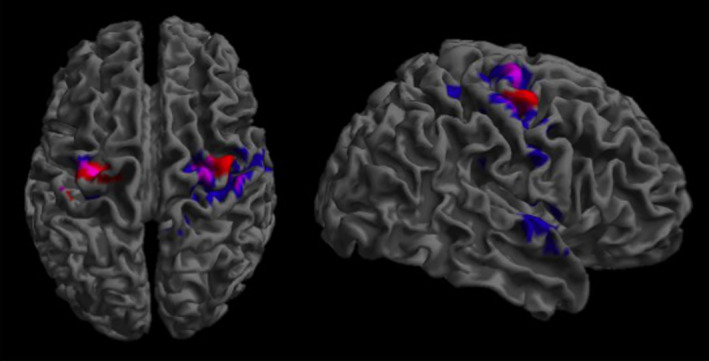
Dorsal premotor regions involved in lower limb motor control. The premotor regions that were significantly more active during DMI in younger than older participants (Virtual Walking analysis, blue), and during ankle dorsiflexion in older as compared to younger participants (Foot Movements analysis, red) have been superimposed. The violet areas represent the overlaps. Only clusters surviving FWER‐correction for multiple comparisons have been reported
Direct within‐group comparisons between Imagery‐types were also coherent with the pattern that emerged from the interaction effect. Older adults recruited the cortical regions responsible for gait motor control more strongly during MI than DMI: these activations included, bilaterally, the superior frontal gyrus and the insula, the left inferior and middle frontal gyri, the precentral gyrus, and the supplementary motor area; they also included parietal, occipital and temporal regions and the cerebellum (Table S3). On the contrary, younger adults showed hyper‐activation in DMI as compared to MI bilaterally in two clusters extending from the precentral to the postcentral and inferior parietal gyri (Table S4).
Importantly, these effects were independent of the specific features of the visual stimuli observed by participants, because stimuli were identical in the MI and DMI conditions.
3.3.5. Conjunction analyses
Finally, conjunction analyses complemented the findings reported above by showing that the areas in common between older and younger participants differed depending on the Imagery‐type. While both visual and motor areas (including medial and lateral premotor cortices) were commonly recruited by younger and older participants to perform the MI task, mainly visual and visuo‐motor areas in the occipital and parietal lobes were commonly recruited during the DMI task (Table S5 and Figure 3a).
3.4. fMRI results. Foot movements analysis
3.4.1. Main effect of foot movement (contrast 1 1 1 1)
The execution of foot movements recruited, bilaterally, the insula, the middle cingulum, the paracentral lobule, the supramarginal gyrus, the parietal operculum, and the thalamus, the basal ganglia and the cerebellum (Table S6 and Figure 2).
3.4.2. Between‐group difference in foot movement execution
The analysis revealed no significant main effect of Group in either direction. However, the simple effect of Group during imagery of Standing on the spot revealed bilateral hyper‐activation of premotor areas (including the left superior frontal gyrus and supplementary motor area and, bilaterally, the precentral gyrus) in older as compared to younger participants (Table 3 and Figure 4). No between‐group difference in the neural correlates of foot movement execution was present during imagery of Walking.
Table 3.
Between‐group differences in the Foot Movements analysis, Older > Younger during imagery of Standing on the spot
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| Sup. frontal gyrus | −20 | −8 | 44 | 3.6 | ||||
| SMA | −16 | −4 | 46 | 3.6 | ||||
| −10 | −12 | 46 | 3.4 | |||||
| −12 | −8 | 46 | 3.4 | |||||
| Precentral gyrus (6) | −34 | −14 | 54 | 4.7* | 42 | −10 | 58 | 4.5 |
| −26 | −14 | 52 | 4.7* | 38 | −10 | 56 | 4.5 | |
| −24 | −14 | 56 | 4.3 | 32 | −16 | 70 | 4.3 | |
| 26 | −16 | 52 | 3.6 | |||||
| 22 | −16 | 54 | 3.4 | |||||
| 24 | −12 | 50 | 3.4 | |||||
| Parietal lobe | ||||||||
| Postcentral gyrus | −44 | −22 | 60 | 3.5 | ||||
Note: x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space. Voxel‐level statistical threshold p < .001uncorr. All reported voxels are included in clusters surviving the family‐wise error rate (FWER) correction at the cluster‐level. (*) Z‐scores statistically significant also after FWER correction at the voxel‐level.
4. DISCUSSION
Motor representations map the kinematic and proprioceptive features of bodily movements: they develop when actions are actually executed (Jeannerod, 2001; Mylopoulos & Pacherie, 2017) and are somatotopically anchored to the cerebral cortex, in primary motor, premotor and parietal areas. The brain regions involved are prone to the anatomical and physiological challenges associated with aging (Seidler et al., 2010). Since a decline in the quality of motor representations or an impoverished motor control might enhance the risk of traumatic injuries in older subjects (Sleet, Moffett, & Stevens, 2008), a systematic description of age‐related changes in motor representations and their neurophysiological underpinnings acquires primary importance, especially with regard to motor acts strictly linked to the quality of daily living like walking.
We applied a visually‐cued MI task (Sacheli et al., 2017; Sacheli et al., 2018) to examine age‐related differences in the neural correlates of gait motor representations by testing a group of younger and older healthy volunteers. In our full‐factorial design, we tested whether the presence or absence of overt foot movements compatible with gait motor patterns during a DMI condition might facilitate the recruitment of neural motor resources during the gait imagery task.
It is crucial to remember that both the MI and DMI conditions had a matched baseline that involved imagining to stand on a spot, which was associated (DMI) or not (MI) with ankle dorsiflexion. Thus, the activations shown in the DMI condition report the areas that were significantly more active during gait MI associated with ankle dorsiflexion as compared to ankle dorsiflexion associated with imagery of standing on the spot.
Altogether, our design allowed us to better reveal the nature of age‐related differences in gait motor control by testing whether these are mainly due to impairment in “higher level” cognitive processes linked to walking behavior, including the ability to mentally evoke kinesthetic sensations linked to gait and to simulate navigation during the virtual walking task (“representational impairment” hypothesis), and/or to reduced efficiency in “lower level” motor control involved in the execution of rhythmic limb movements (“reduced efficiency in lower limb motor control” hypothesis).
Our results revealed two main findings. First, older participants showed, outside the MRI scanner, mental CA similar to those measured in younger ones, indicating no sign of evident depletion in the quality of gait motor representations; this is in line with previous studies on young‐old individuals (i.e., older adults in their 60s, Skoura et al., 2005; Saimpont et al., 2013). These behavioral results were paralleled by the absence of between‐group differences in the neural correlates of MI at our Virtual Walking task performed during fMRI. Second, the request to coordinate gait MI with compatible overt foot movements during the DMI task determined what appeared as hypoactivation in older as compared to younger participants in premotor regions. However, the control analysis performed on foot movement execution revealed that this apparent hypo‐activation emerged at least partly because older participants showed stronger recruitment of premotor cortical regions in the baseline condition when controlling the mere execution of rhythmic ankle dorsiflexion.
Taken together, our results indicate that older adults might find it difficult to deal with the simultaneous control of gait imagery and ankle dorsiflexion because of lower efficiency of lower limb motor control and because the mere execution of ankle dorsiflexion is a cognitively demanding task for them. Below, we elaborate on these suggestions and discuss implications for gait rehabilitation.
4.1. Virtual walking (MI) in healthy aging
Our results suggest that gait motor representations per se might be preserved in “young” healthy older individuals with no walking disturbances, and that gait MI might thus require no additional neural resources in this population. These results are in contrast with the (limited) previous literature available so far, indicating compensatory hyper‐activation in older as compared to younger participants either in prefrontal (Allali et al., 2014; Blumen et al., 2014) or vestibular, somatosensory and visual cortices (Wai et al., 2012; Zwergal et al., 2012). Crucially, however, previous studies applied MI tasks that differed from the present one. For instance, while Zwergal et al. (2012) asked older participants to perform the imagery task with closed eyes, Allali et al. (2014) employed a visually‐aided task that yet implied static images.
On the contrary, the present study implemented a virtual walking task (see also Iseki et al., 2008) that uses first‐person ecological moving stimuli to facilitate the identification with the situation of performing a quiet stroll in the park. This discrepancy might justify the lack of consistency with previous findings: obviously, the results of the present study might have changed in case no visual cues were provided. This might be assessed by future studies that directly compare gait imagery in the presence versus absence of dynamic visual cues and that also include an explicit assessment of participants' compliance during the task. However, we believe that our results suggest that virtual walking stimuli might constitute a (low‐cost) valuable tool to facilitate the imagery task in healthy aging. Indeed, both younger and older participants recruited the whole network involved in gait motor control during the MI virtual walking condition: it largely overlapped with the frontoparietal regions of the median wall responsible for foot movement motor control but also included premotor and parietal areas involved in navigation (Figure 2). Overall, our results indicate that performing visually‐guided gait MI might not be particularly challenging in healthy older participants, and that healthy older subjects with no walking disturbances might be characterized by the preserved ability to internally simulate both the kinesthetic sensations evoked by gait behaviors and navigation (i.e., they might be characterized by the absence of higher level “ representational impairment”).
4.2. DMI of gait and dorsal premotor activations in young subjects
The scenario impressively changed in the DMI condition. Here, younger participants showed bilateral hyperactivations (as compared to older adults) of dorsal premotor (dPM) areas. Although the dPM cortex is not typically included in the network responsible for gait execution (La Fougère et al., 2010), its role in locomotor control has long been suggested (Freund & Hummelsheim, 1985; see also Nakajima, Fortier‐Lebel, & Drew, 2019). dPM activations similar to those described here have been previously reported during gait MI (Malouin et al., 2003), and it has been shown that dPM recruitment mediates gait recovery after stroke (Miyai et al., 2003) and might be crucial for visually‐guided gait control. Interestingly, it also plays a role in mediating visually‐guided improvements of gait initiation in patients with Parkinson's disease (Hanakawa, Fukuyama, Katsumi, Honda, & Shibasaki, 1999), and lesions to this area result in an impaired ability to adapt gait to turns or navigate through narrow passages, with inadequate responses to external cues (Liston et al., 2003; Nutt, 2013). Finally, the portion of dPM described here has also been reported by Buccino et al. (2001) for the observation of foot movements, and by Sahyoun, Floyer‐Lea, Johansen‐Berg, and Matthews (2004) during the execution of active as compared to passive foot movements, supporting the hypothesis that the dPM is endowed with a motor representation of lower limb actions.
DMI has been recently proposed as a suitable tool for gait rehabilitation (Fusco et al., 2014; Fusco et al., 2016), but, to our knowledge, its neurofunctional underpinnings were yet unexplored. This study provides the first evidence that, in younger participants, DMI is indeed effective in recruiting similar neural resources as compared to MI, at least as far as MI of gait is concerned. However, it also requires additional resources, most likely to face the requirement for the integration of multiple motor representations. Within this line, DMI might be interpreted as a “motorically more demanding” task. For instance, Kanthack et al. (2016) reported that in young basketball players the advantage of DMI over a pure MI mental rehearsal vanished in a condition of physical fatigue, that is, in a situation characterized by a reduction of the available resources.
4.3. DMI and foot movement control in older adults
So far, no study specifically applied DMI in healthy older adults nor the neural correlates were measured. We anticipated two alternative scenarios, with divergent implications for gait rehabilitation. According to a “representational impairment” hypothesis, older participants should have shown performance decay at the behavioral task coupled with hypoactivation of premotor regions and hyperactivation of alternative neural resources, possibly in prefrontal cortices (see Allali et al., 2014; Blumen et al., 2014), during the MI task. This would have suggested that older adults have a specific difficulty in voluntarily recalling the kinesthetic sensations linked to gait motor patterns and in simulating navigation, and that a motor trigger might have a beneficial effect for rehabilitation purposes. Our data do not support this scenario at the neurofunctional level, at least when subjects are tested without previous training.
On the contrary, our results indicate that the absence of age‐related differences in MI was coupled with a relative hypo‐activation for DMI in older as compared to younger participants in two bilateral dPM clusters extending from the superior frontal to the precentral gyri. These regions correspond to those that are recruited during active as compared to passive or electrically stimulated ankle dorsiflexion (Francis et al., 2009) and they are largely overlapping with those hyper‐activated in older as compared to younger participants during repetitive ankle dorsiflexion (Figure 4). This indicates that the apparent hypoactivation shown by older participants during the DMI condition was due to the fact that premotor activations were actually subtracted out in this condition by the strong recruitment of premotor cortical regions during the “imagine to stand (or, better, to step on the spot)” condition that served as baseline in the DMI contrast (see Methods). This is in line with the “reduced efficiency in lower limb motor control” hypothesis. Indeed, this pattern suggests that a reduced efficiency in the motor control of the lower limbs led older adults to recruit additional premotor resources to execute the mere ankle dorsiflexion. In the DMI condition, the further request of combining ankle dorsiflexion with gait imagery resulted in a comparative drop of neural activations because additional motor cognitive resources were not available to deal with the more demanding task, due to a competition within the same neural circuitry (Nijboer, Borst, van Rijn, & Taatgen, 2014). This pattern is in line with claims that aging is characterized by a reduction of available resources (Cabeza, 2002), at least in the motor domain.
Since the pattern of results was associated with preserved isochrony in the gait mental chronometry task in older participants, the motor difficulty was not associated, we argue, with decay in the quality of gait motor representations. On the contrary, results speak in favor of a “reduced efficiency in lower limb motor control” hypothesis. The higher cognitive load required by gait in healthy aging, which leads to decay in performance during concurrent cognitively demanding tasks like walking while talking (Blumen et al., 2014), might not be due to a specific deficit in the gait motor control network; rather it might reflect a higher cognitive control generally required by older adults to execute rhythmic lower limb movements. Gait rehabilitation in healthy aging might thus take advantage of the spared motor representations to train and improve automaticity in motor control. This acquires primary importance in the face of evidence that lower limb motor control might show a lower efficiency even in older adults with no disturbances of central or peripheral origin.
5. CONCLUSIONS
DMI has shown positive effects in younger adults in terms of higher imagery vividness, temporal coupling with motor execution, and better performance (Fusco et al., 2014; Guillot et al., 2013; Kanthack et al., 2016). It is a potential tool to enhance the quality of MI in MI‐based training protocols. In a previous study (Sacheli et al., 2018), we have shown that DMI might also be effective in engaging in a mental practice task orthopedic patients who had lost the ability to recall the kinesthetic and proprioceptive sensations linked to gait execution due to a long‐lasting functional limitation. In this case, the motor trigger proved to facilitate the engagement of motor‐related processes during imagery. Moreover, a recent large multicenter randomized controlled trial has demonstrated that, in older adults with a high‐risk of falls, intensive training with a virtual walking task similar to the one used in our DMI condition (i.e., actual gait execution in a nonimmersive virtual reality—VR‐environment), had a significant effect on reducing the rate of falls with respect to the control treatment based only on a treadmill stroll without the virtual reality component (Mirelman et al., 2016). This evidence is encouraging on the possibility of applying virtual reality enhanced training strategies for primary or even secondary prevention of falls. However, a word of caution is needed here for the DMI, which is not fully equivalent to the VR enhanced treadmill exercise: our results show that in older subjects, who still have no functional limitations and good MI abilities, a DMI task, without prior training, may be too demanding. It remains an open issue whether specific training at the DMI task and similar ones can be associated with better performance with clinical implications for rehabilitation and prevention of falls.
More generally, our findings complement previous evidence on young subjects (Kanthack et al., 2016) and suggest that—in a life‐span perspective—a wise use of DMI as a proxy to study and/or rehabilitate motor functions shall involve an assessment of the individual and contextual conditions (e.g., aging, cognitive status, physical fatigue) possibly affecting the resources available for its performance.
CONFLICT OF INTEREST
The authors declare no conflict of interest that could have direct or potential influence on the work.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The study was funded by the Italian Ministry of Health ([Grant N. RF‐2011‐02350666—Can mental training based on MI speed up the rehabilitation of walking? Efficacy of a controlled procedure and neurofunctional bases of recovery in patients with total knee arthroplasty] and [Ricerca Corrente: protocol L3008]) and by the Milan Center for Neuroscience (NeuroMi).
Sacheli LM, Zapparoli L, Bonandrini R, et al. How aging affects the premotor control of lower limb movements in simulated gait. Hum Brain Mapp. 2020;41:1889–1903. 10.1002/hbm.24919
Funding information Italian Ministry of Health and Fondi di Ricerca Corrente, Grant/Award Number: RF‐2011‐02350666
Endnote
One possible exception is the clever use of FDG‐PET activation paradigms that permit the acquisition of the data after walking and the accumulation of the tracer in the brain. However, this method is limited in terms of number of observations that can be reasonably made in each individual, typically not more than two (see for instance La Fougère et al., 2010).
Contributor Information
Lucia Maria Sacheli, Email: lucia.sacheli@unimib.it.
Eraldo Paulesu, Email: eraldo.paulesu@unimib.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon request.
REFERENCES
- Alexander, N. B. (1996). Gait disorders in older adults. Journal of the American Geriatrics Society, 44(4), 434–451. [DOI] [PubMed] [Google Scholar]
- Allali, G. , Van Der Meulen, M. , Beauchet, O. , Rieger, S. W. , Vuilleumier, P. , & Assal, F. (2014). The neural basis of age‐related changes in motor imagery of gait: An fMRI study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Bakker, M. , De Lange, F. P. , Helmich, R. C. , Scheeringa, R. , Bloem, B. R. , & Toni, I. (2008). Cerebral correlates of motor imagery of normal and precision gait. NeuroImage, 41(3), 998–1010. [DOI] [PubMed] [Google Scholar]
- Bakker, M. , Verstappen, C. C. P. , Bloem, B. R. , & Toni, I. (2007). Recent advances in functional neuroimaging of gait. Journal of Neural Transmission, 114(10), 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet, O. , Annweiler, C. , Assal, F. , Bridenbaugh, S. , Herrmann, F. R. , Kressig, R. W. , & Allali, G. (2010). Imagined timed up & go test: A new tool to assess higher‐level gait and balance disorders in older adults? Journal of the Neurological Sciences, 294, 102–106. [DOI] [PubMed] [Google Scholar]
- Berlingeri, M. , Bottini, G. , Danelli, L. , Ferri, F. , Traficante, D. , Sacheli, L. , … Paulesu, E. (2010). With time on our side? Task‐dependent compensatory processes in graceful aging. Experimental Brain Research, 205(3), 307–324. [DOI] [PubMed] [Google Scholar]
- Blumen, H. M. , Holtzer, R. , Brown, L. L. , Gazes, Y. , & Verghese, J. (2014). Behavioral and neural correlates of imagined walking and walking‐while‐talking in the elderly. Human Brain Mapping, 35(8), 4090–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier, M. P. , Beets, I. A. , Duysens, J. , Nieuwboer, A. , Krampe, R. T. , & Swinnen, S. P. (2013). Age‐related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neuroscience & Biobehavioral Reviews, 37(8), 1824–1837. [DOI] [PubMed] [Google Scholar]
- Buccino, G. , Binkofski, F. , Fink, G. R. , Fadiga, L. , Fogassi, L. , Gallese, V. , … Freund, H. J. (2001). Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience, 13(2), 400–404. [PubMed] [Google Scholar]
- Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The Harold model. Psychology and Aging, 17(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , Albert, M. , Belleville, S. , Craik, F. I. M. , Duarte, A. , Grady, C. L. , … Rajah, M. N. (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat Rev Neurosci, 19(11), 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J. , & Michel, F. (1989). Comparative analysis of actual and mental movement times in two graphic tasks. Brain and Cognition, 11(1), 87–97. [DOI] [PubMed] [Google Scholar]
- Dobkin, B. H. , Firestine, A. , West, M. , Saremi, K. , & Woods, R. (2004). Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage, 23(1), 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin, G. , & Friston, K. J. (2019). Analysis of family‐wise error rates in statistical parametric mapping using random field theory. Human Brain Mapping, 40(7), 2052–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini‐mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Francis, S. , Lin, X. , Aboushoushah, S. , White, T. P. , Phillips, M. , Bowtell, R. , & Constantinescu, C. S. (2009). fMRI analysis of active, passive and electrically stimulated ankle dorsiflexion. NeuroImage, 44(2), 469–479. [DOI] [PubMed] [Google Scholar]
- Freund, H. J. , & Hummelsheim, H. (1985). Lesions of premotor cortex in man. Brain, 108(3), 697–733. [DOI] [PubMed] [Google Scholar]
- Fusco, A. , Gallotta, M. C. , Iosa, M. , Morone, G. , Iasevoli, L. , Trifoglio, D. , … Guidetti, L. (2016). The dynamic motor imagery of locomotion is task‐dependent in patients with stroke. Restorative Neurology and Neuroscience, 34(2), 247–256. [DOI] [PubMed] [Google Scholar]
- Fusco, A. , Iosa, M. , Gallotta, M. C. , Paolucci, S. , Baldari, C. , & Guidetti, L. (2014). Different performances in static and dynamic imagery and real locomotion. An exploratory trial. Frontiers in Human Neuroscience, 8, 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, L. D. , Robertson, M. C. , Gillespie, W. J. , Sherrington, C. , Gates, S. , Clemson, L. M. , & Lamb, S. E. (2012). Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews, 9, CD007146 10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot, A. , Moschberger, K. , & Collet, C. (2013). Coupling movement with imagery as a new perspective for motor imagery practice. Behavioral and Brain Functions, 9(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa, T. , Fukuyama, H. , Katsumi, Y. , Honda, M. , & Shibasaki, H. (1999). Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Annals of Neurology, 45, 329–336. [DOI] [PubMed] [Google Scholar]
- Hétu, S. , Grégoire, M. , Saimpont, A. , Coll, M. P. , Eugène, F. , Michon, P. E. , & Jackson, P. L. (2013). The neural network of motor imagery: An ALE meta‐analysis. Neuroscience & Biobehavioral Reviews, 37(5), 930–949. [DOI] [PubMed] [Google Scholar]
- Isaac, A. , Marks, D. F. , & Russell, D. G. (1986). An instrument for assessing imagery of movement: The vividness of movement imagery questionnaire (VMIQ). Journal of Mental Imagery, 10, 23–30. [Google Scholar]
- Iseki, K. , Hanakawa, T. , Shinozaki, J. , Nankaku, M. , & Fukuyama, H. (2008). Neural mechanisms involved in mental imagery and observation of gait. NeuroImage, 41, 1021–1031. [DOI] [PubMed] [Google Scholar]
- Jahn, K. , Deutschländer, A. , Stephan, T. , Kalla, R. , Wiesmann, M. , Strupp, M. , & Brandt, T. (2008). Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage, 39(2), 786–792. [DOI] [PubMed] [Google Scholar]
- Jahn, K. , Deutschländer, A. , Stephan, T. , Strupp, M. , Wiesmann, M. , & Brandt, T. (2004). Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage, 22(4), 1722–1731. [DOI] [PubMed] [Google Scholar]
- Jeannerod, M. (2001). Neural simulation of action: A unifying mechanism for motor cognition. NeuroImage, 14(1), S103–S109. [DOI] [PubMed] [Google Scholar]
- Jeannerod, M. , & Frak, V. (1999). Mental imaging of motor activity in humans. Current Opinion in Neurobiology, 9(6), 735–739. [DOI] [PubMed] [Google Scholar]
- Kanthack, T. F. D. , Guillot, A. , Altimari, L. R. , Nagy, S. N. , Collet, C. , & Di Rienzo, F. (2016). Selective efficacy of static and dynamic imagery in different states of physical fatigue. PLoS One, 11(3), e0149654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fougère, C. , Zwergal, A. , Rominger, A. , Forster, S. , Fesl, G. , Dieterich, M. , … Jahn, K. (2010). Real versus imagined locomotion: A [18F]‐FDG PET‐fMRI comparison. NeuroImage, 50, 1589–1598. [DOI] [PubMed] [Google Scholar]
- Liston, R. , Mickelborough, J. , Bene, J. , & Tallis, R. (2003). A new classification of higher level gait disorders in patients with cerebral multi‐infarct states. Age and Ageing, 32(3), 252–258. [DOI] [PubMed] [Google Scholar]
- Lotze, M. , Scheler, G. , Tan, H. R. , Braun, C. , & Birbaumer, N. (2003). The musician's brain: Functional imaging of amateurs and professionals during performance and imagery. NeuroImage, 20(3), 1817–1829. [DOI] [PubMed] [Google Scholar]
- Malouin, F. , Richards, C. L. , Jackson, P. L. , Dumas, F. , & Doyon, J. (2003). Brain activations during motor imagery of locomotor‐related tasks: A PET study. Human Brain Mapping, 19, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman, A. , Rochester, L. , Maidan, I. , Del Din, S. , Alcock, L. , Nieuwhof, F. , … Hausdorff, J. M. (2016). Addition of a non‐immersive virtual reality component to treadmill training to reduce fall risk in older adults (V‐TIME): A randomised controlled trial. Lancet, 388(10050), 1170–1182. [DOI] [PubMed] [Google Scholar]
- Miyai, I. , Yagura, H. , Hatakenaka, M. , Oda, I. , Konishi, I. , & Kubota, K. (2003). Longitudinal optical imaging study for locomotor recovery after stroke. Stroke, 34(12), 2866–2870. [DOI] [PubMed] [Google Scholar]
- Mulder, T. , Hochstenbach, J. B. , van Heuvelen, M. J. , & den Otter, A. R. (2007). Motor imagery: The relation between age and imagery capacity. Human Movement Science, 26, 203–211. [DOI] [PubMed] [Google Scholar]
- Munzert, J. , Lorey, B. , & Zentgraf, K. (2009). Cognitive motor processes: The role of motor imagery in the study of motor representations. Brain Research Reviews, 60(2), 306–326. [DOI] [PubMed] [Google Scholar]
- Munzert, J. , & Zentgraf, K. (2009). Motor imagery and its implications for understanding the motor system. Progress in Brain Research, 174, 219–229. [DOI] [PubMed] [Google Scholar]
- Mylopoulos, M. , & Pacherie, E. (2017). Intentions and motor representations: The interface challenge. Review of Philosophy and Psychology, 8(2), 317–336. [Google Scholar]
- Nakajima, T. , Fortier‐Lebel, N. , & Drew, T. (2019). Premotor cortex provides a substrate for the temporal transformation of information during the planning of gait modifications. Cerebral Cortex, 29, 4982–5008. 10.1093/cercor/bhz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwhof, F. , Bloem, B. R. , Reelick, M. F. , Aarts, E. , Maidan, I. , Mirelman, A. , … Helmich, R. C. (2017). Impaired dual tasking in Parkinson's disease is associated with reduced focusing of cortico‐striatal activity. Brain, 140(5), 1384–1398. [DOI] [PubMed] [Google Scholar]
- Nijboer, M. , Borst, J. , van Rijn, H. , & Taatgen, N. (2014). Single‐task fMRI overlap predicts concurrent multitasking interference. NeuroImage, 100, 60–74. [DOI] [PubMed] [Google Scholar]
- Novelli, G. , Papagno, C. , Capitani, E. , Laiacona, M. , Vallar, G. , & Cappa, S. F. (1986). Three clinical tests for the assessment of verbal long‐term memory function: Norms from 320 normal subjects. Archivio di Psicologia, Neurologia e Psichiatria, 47(2), 278–296. [Google Scholar]
- Nutt, J. G. (2013). Higher‐level gait disorders: An open frontier. Movement Disorders, 28(11), 1560–1565. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Personnier, P. , Paizis, C. , Ballay, Y. , & Papaxanthis, C. (2008). Men‐ tally represented motor actions in normal aging. II. The influence of the gravito‐inertial context on the duration of overt and covert arm movements. Behavioral Brain Research, 186, 273–283. [DOI] [PubMed] [Google Scholar]
- Podsiadlo, D. , & Richardson, S. (1991). The timed “up & go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39(2), 142–148. [DOI] [PubMed] [Google Scholar]
- Raven, J. , Bulheller, S. , & Häcker, H. (1984). CPM. Coloured Progressive Matrices. OS, Firenze.
- Reuter‐Lorenz, P. A. , & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. [Google Scholar]
- Ruby, P. , & Decety, J. (2001). Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience, 4(5), 546–550. [DOI] [PubMed] [Google Scholar]
- Ruffieux, J. , Keller, M. , Lauber, B. , & Taube, W. (2015). Changes in standing and walking performance under dual‐task conditions across the lifespan. Sports Medicine, 45(12), 1739–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco, K. , Cauda, F. , Cerliani, L. , Mate, D. , Duca, S. , & Geminiani, G. C. (2006). Motor imagery of walking following training in locomotor attention. The effect of ‘the tango lesson’. NeuroImage, 32(3), 1441–1449. [DOI] [PubMed] [Google Scholar]
- Sacheli, L. M. , Zapparoli, L. , De Santis, C. , Preti, M. , Pelosi, C. , Ursino, N. , … Paulesu, E. (2017). Mental steps: Differential activation of internal pacemakers in motor imagery and in mental imitation of gait. Human Brain Mapping, 38(10), 5195–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli, L. M. , Zapparoli, L. , Preti, M. , De Santis, C. , Pelosi, C. , Ursino, N. , … Paulesu, E. (2018). A functional limitation to the lower limbs affects the neural bases of motor imagery of gait. NeuroImage Clinical, 20, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun, C. , Floyer‐Lea, A. , Johansen‐Berg, H. , & Matthews, P. M. (2004). Towards an understanding of gait control: Brain activation during the anticipation, preparation and execution of foot movements. NeuroImage, 21(2), 568–575. [DOI] [PubMed] [Google Scholar]
- Saimpont, A. , Malouin, F. , Tousignant, B. , & Jackson, P. L. (2013). Motor imagery and aging. Journal of Motor Behavior, 45(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Schott, N. , & Munzert, J. (2007). Temporal accuracy of motor im‐ agery in older women. International Journal of Sport Psychology, 38, 304–320. [Google Scholar]
- Seidler, R. D. , Bernard, J. A. , Burutolu, T. B. , Fling, B. W. , Gordon, M. T. , Gwin, J. T. , … Lipps, D. B. (2010). Motor control and aging: Links to age‐related brain structural, functional, and biochemical effects. Neuroscience & Biobehavioral Reviews, 34(5), 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura, X. , Papaxanthis, C. , Vinter, A. , & Pozzo, T. (2005). Mentally represented motor actions in normal aging. I. Age effects on the temporal features of overt and covert execution of actions. Behavioral Brain Research, 165, 229–239. [DOI] [PubMed] [Google Scholar]
- Sleet, D. A. , Moffett, D. B. , & Stevens, J. (2008). CDC's research portfolio in older adult fall prevention: A review of progress, 1985‐2005, and future research directions. Journal of Safety Research, 39(3), 259–267. [DOI] [PubMed] [Google Scholar]
- Wai, Y. Y. , Wang, J. J. , Weng, Y. H. , Lin, W. Y. , Ma, H. K. , Ng, S. H. , … Wang, C. H. (2012). Cortical involvement in a gait‐related imagery task: Comparison between Parkinson's disease and normal aging. Parkinsonism & Related Disorders, 18(5), 537–542. [DOI] [PubMed] [Google Scholar]
- Worsley, K. , & Friston, K. (1995). Analysis of fMRI time‐series revisited—Again. NeuroImage, 2, 173–181. [DOI] [PubMed] [Google Scholar]
- Zapparoli, L. , Gandola, M. , Banfi, G. , & Paulesu, E. (2019). A breakdown of imagined Visuomotor transformations and its neural correlates in young elderly subjects. Cerebral Cortex, 29(4), 1682–1696. [DOI] [PubMed] [Google Scholar]
- Zapparoli, L. , Invernizzi, P. , Gandola, M. , Verardi, M. , Berlingeri, M. , Sberna, M. , … Paulesu, E. (2013). Mental images across the adult lifespan: A behavioural and fMRI investigation of motor execution and motor imagery. Experimental Brain Research, 224(4), 519–540. [DOI] [PubMed] [Google Scholar]
- Zapparoli, L. , Saetta, G. , De Santis, C. , Gandola, M. , Zerbi, A. , Banfi, G. , & Paulesu, E. (2016). When I am (almost) 64: The effect of normal ageing on implicit motor imagery in young elderlies. Behavioural Brain Research, 303, 137–151. [DOI] [PubMed] [Google Scholar]
- Zwergal, A. , Linn, J. , Xiong, G. , Brandt, T. , Strupp, M. , & Jahn, K. (2012). Aging of human supraspinal locomotor and postural control in fMRI. Neurobiology of Aging, 33(6), 1073–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


