Abstract
About 50% of attention deficit hyperactivity disorder (ADHD) patients suffer from comorbidity with oppositional defiant disorder/conduct disorder (ODD/CD). Most previous studies on structural morphology did not differentiate between pure (ADHD‐only) and comorbid ADHD (ADHD+ODD/CD). Therefore, we aimed to investigate the structural profile of ADHD‐only versus ADHD+ODD/CD spanning the indices subcortical and cortical volume, cortical thickness, and surface area. We predicted a reduced total gray matter, striatal, and cerebellar volume in both patient groups and a reduced amygdalar and hippocampal volume for ADHD+ODD/CD. We also explored alterations in prefrontal volume, thickness, and surface area. We acquired structural images from an adolescent sample ranging from 11 to 17 years, matched with regard to age, pubertal status, and IQ—including 36 boys with ADHD‐only, 26 boys with ADHD+ODD/CD, and 30 typically developing (TD) boys. We analyzed structural data with FreeSurfer. We found reductions in total gray matter and total surface area for both patient groups. Boys with ADHD+ODD/CD had a thicker cortex than the other groups in a right rostral middle frontal cluster, which was related to stronger ODD/CD symptoms, even when controlling for ADHD symptoms. No group differences in local cortical volume or surface area emerged. We demonstrate the necessity to carefully differentiate between ADHD and ADHD+ODD/CD. The increased rostral middle frontal thickness might hint at a delayed adolescent cortical thinning in ADHD+ODD/CD. Patients with the double burden ADHD and ODD or CD seem to be even more affected than patients with pure ADHD.
Keywords: ADHD, adolescence, conduct disorder, FreeSurfer, oppositional defiant disorder, structural morphology, structural MRI
1. INTRODUCTION
One of the most commonly diagnosed childhood psychiatric disorders affecting 3–9% of children and adolescents is attention deficit hyperactivity disorder (ADHD; Barkley, 2014). ADHD is defined by developmentally inappropriate levels of inattention, and/or hyperactivity/impulsivity (American Psychiatric Association, 1994). ADHD is related to academic and work‐related difficulties and represents a major public health burden (Pardini & Fite, 2010).
Around 48–67% of ADHD patients suffer from comorbid oppositional defiant disorder (ODD) or conduct disorder (CD, Connor, Steeber, & McBurnett, 2010). ODD is defined by developmentally inappropriate, negativistic, defiant, and disobedient behavior (American Psychiatric Association, 1994) and is often a precursor for the more severe CD (Maughan, Rowe, Messer, Goodman, & Meltzer, 2004), which is characterized by aggression, deceitfulness, property destruction and rules violation. Compared with patients with pure ADHD, patients with ADHD and comorbid ODD/CD show an earlier age of onset for ADHD symptoms, and have a worse prognosis (Connor & Doerfler, 2008; Loeber, Burke, Lahey, Winters, & Zebra, 2000).
ADHD patients show neuropsychological deficits in attention and “cool” executive function such as inhibition and switching (Halperin & Schulz, 2006), while patients with ODD/CD seem to have difficulties in “hot” executive functions including reward and emotion processing (Byrd, Loeber, & Pardini, 2014). In line with these neuropsychological findings, morphological neuroimaging studies have shown abnormalities for ADHD in frontostriatal and frontocerebellar circuits and for ODD/CD in frontolimbic circuits (for a review see Rubia, 2011). Importantly, recent accounts stress the multifactorial causation of ADHD, which leads to heterogeneous neurocognitive and structural abnormalities (Faraone et al., 2015).
In detail, volume reductions for ADHD have been most consistently reported for the striatum, the cerebellum, and total gray matter (GM; Greven et al., 2015; for meta‐analyses and reviews see Hoogman et al., 2017; Nakao, Radua, Rubia, & Mataix‐Cols, 2011; Valera, Faraone, Murray, & Seidman, 2007; Faraone et al., 2015). Regarding frontal alterations, studies reported a decreased (Ambrosino, de Zeeuw, Wierenga, van Dijk, & Durston, 2017) or increased (Garrett et al., 2008; Semrud‐Clikeman, Pliszka, Bledsoe, & Lancaster, 2014) volume of the prefrontal cortex.
Some studies also found a reduction in prefrontal cortical thickness (CT; Almeida et al., 2010; Hoekzema et al., 2012; Shaw et al., 2006; Yang, Carrey, Bernier, & MacMaster, 2015) or a delayed cortical thinning (Shaw et al., 2007). In contrast, further research shows an increased CT in other areas (occipital or temporal cortex, presupplementary motor area; Almeida Montes et al., 2013; Duerden, Tannock, & Dockstader, 2012) or no CT alterations (Ambrosino et al., 2017; Wolosin, Richardson, Hennessey, Denckla, & Mostofsky, 2009).
The few recent studies to investigate an additional dimension of cortical morphology, namely surface area (SA) have reported reduced total, frontal, temporal or parietal SA (Dirlikov et al., 2015; Noordermeer et al., 2017; Wolosin et al., 2009). Taken together, while overall frontostriatal and frontocerebellar circuits seem to be affected in ADHD, previous studies partly remain inconsistent, mainly for the frontal alterations (Stevens & Haney‐Caron, 2012).
For ODD/CD, volume reductions were found in the striatum, amygdala, hippocampus, or prefrontal cortex (Noordermeer, Luman, & Oosterlaan, 2016; Rogers & Brito, 2016). Reports on prefrontal volume also include increases (De Brito et al., 2009). Very few ODD/CD studies investigated CT and SA. These reported CT reductions for temporal and parietal (Hyatt, Haney‐Caron, & Stevens, 2012; Wallace et al., 2014) or prefrontal regions (Fahim et al., 2011). Prefrontal SA reductions (Fairchild et al., 2015; Sarkar et al., 2015) or no SA differences (Wallace et al., 2014) have also been shown. Summarizing these findings, while ODD/CD alterations are mainly evident in frontolimbic circuits, several inconsistencies are evident regarding the specific frontal alterations.
Divergent findings for both ADHD and ODD/CD studies might stem from previous samples that partly included ADHD patients comorbid with ODD/CD (Wolosin et al., 2009) or vice versa (see Stevens & Haney‐Caron, 2012 for a summary). Thus, the contribution of the respective other disorder could only—if at all—be statistically controlled for. Studies on ADHD+ODD/CD that try to disentangle each disorder's specific contribution are rare. One study contrasted adolescent ADHD versus CD volume abnormalities and found total GM volume reductions reflecting frontal, temporal, parietal, and subcortical volume reductions in CD but not ADHD patients (Stevens & Haney‐Caron, 2012). The authors therefore questioned previously reported frontal abnormalities in ADHD and speculated they might stem from ODD/CD comorbidity in previous ADHD samples. To date, only one recent study compared structural morphology (volume and thickness) in adolescents with pure ADHD to those with ADHD+ODD and found both similar and differential cortical volume (CV) alterations (Noordermeer et al., 2017). Both patient groups showed alterations from TD in the prefrontal cortex. Patients with ADHD+ODD had a stronger reduction in the orbito‐, middle‐, and superior frontal cortex, which is in line with stronger impairments in executive functions (Connor & Doerfler, 2008; Loeber et al., 2000). Differential alterations were found for ADHD+ODD differing from pure ADHD and TD in some prefrontal regions of interest (ROIs) and the precuneus, and for ADHD+ODD differing from TD in the left middle temporal gyrus. No thickness or subcortical volume alterations emerged, possibly due to the rather old sample (mean age 16–17 years) and wide age range (7–29 years). Overall, inconsistencies in previous ADHD studies might stem from large age differences ranging from child (Dirlikov et al., 2015; Wolosin et al., 2009; Yang et al., 2015) to adolescent (Almeida Montes et al., 2013; Ambrosino et al., 2017; Duerden et al., 2012) and adult samples (Fairchild et al., 2015) or samples spanning all three age groups (Greven et al., 2015).
An exhaustive review on ADHD has stressed that age largely influences findings on structural alterations (Faraone et al., 2019). Brain development, specifically of the frontal cortex, continues well into adolescence (Gogtay et al., 2004). Gray matter volume and thickness of the fontal cortex peaks around puberty onset followed by a thinning across adolescence (Giedd et al., 1999; Tamnes et al., 2010). This shows that specifically for the frontal cortex age may confound disorder‐related effects. Investigations on adolescent ADHD are rare (for a review see Lin & Roth, 2017). Therefore, fostering knowledge on the structural alterations of ADHD during this age phase by assessing adolescent samples with a more narrow age range would shed light on the neurodevelopment of this disorder. Overall, more investigations on the structural abnormalities associated with ADHD+ODD/CD compared to pure ADHD are warranted to reveal similarities and differences in the morphological alterations of the disorders. This might have important implications for disorder etiology and treatment. Therefore, our major aim was to provide knowledge to disentangle morphological characteristics of adolescents with pure ADHD (ADHD‐only) and ADHD+ODD/CD. Few previous studies analyzed several cortical and subcortical morphological markers simultaneously to reach an overall picture on alterations. We therefore aimed at investigating the structural profile of both ADHD‐only and ADHD+ODD/CD spanning (sub)cortical volume, CT, and SA. We assessed a well‐matched sample spanning the adolescent age phase (11–17 years). We included only boys due to the larger prevalence for males in ADHD (Willcutt, 2012) and the gender‐specific morphology in ADHD (Seymour et al., 2017)
First, we predicted a reduced total GM volume in patient groups similar to Noordermeer et al. (2017). Second, we predicted a reduced striatum and cerebellum (see mega‐analysis by Hoogman et al., 2017) with a stronger reduction due to stronger symptoms and neuropsychological deficits observed in ADHD+ODD/CD. In line with previous studies on ODD/CD (Rogers & Brito, 2016) we predicted for ADHD+ODD/CD a reduced volume of the amygdala and hippocampus.
We additionally explored, on a voxel‐based whole‐brain basis, cortical alterations in volume, thickness, and SA. Given previous inconsistent findings and large age heterogeneities we did not have a clear hypothesis about the form of the alterations, that is, if we would find a de‐ or increase. We expected ADHD+ODD/CD to result in an addition of structural alterations of ADHD‐only and ODD‐only, respectively CD‐only (Schachar & Tannock, 1995) and thus yield more severe cortical alterations than each disorder alone (McAlonan et al., 2007). We expected such alterations mainly in the prefrontal cortex (see also Noordermeer et al., 2017; Stevens & Haney‐Caron, 2012).
2. METHODS
2.1. Participants and study design
This study was part of larger neuroimaging study on ADHD and comorbid ODD/CD using another subsample than our previous publications (Backhausen et al., 2016; Vetter et al., 2018). It was carried out according to the latest version of the Declaration of Helsinki and approved by the ethics committee of the TU Dresden. Participants and parents or legal guardians gave their written informed consent and participants received around 40 €.
We recruited three groups of boys within the age range of 11–17 years: First, ADHD‐only with 51 boys diagnosed only with ADHD; second, ADHD+ODD/CD with 29 boys diagnosed with ADHD and comorbid ODD/CD, and third, TD with 40 boys without any psychiatric diagnosis. Exclusion criteria consisted of IQ < 80 or any other additional axis‐I disorder. We recruited patients within the local in‐ and outpatient clinics and TD boys in schools, doctors' offices and a parish. Diagnoses were made according to the ICD‐10 (World Health Organization, 1992) by board certified child and adolescent psychiatrists and verified by the Mini International Neuropsychiatric Interview for Children and Adolescents (Sheehan et al., 1998).
We had to exclude nine boys due to motion (three ADHD‐only, one ADHD+ODD/CD, two TD), ferromagnetic artifacts (one ADHD‐only), or gross neuroanatomical abnormalities (one ADHD‐only, one TD). The remaining sample (n = 46 ADHD‐only, n = 28 ADHD+ODD/CD, and n = 37 TD) was not matched regarding age, IQ, socioeconomic or pubertal status. To achieve a matched group at least for the most important variables (age, pubertal status, and IQ), we had to exclude those ADHD+ODD/CD patients younger than 13.5 years (n = 2), those ADHD‐only patients with an IQ lower than 94 (n = 7) and older than 17 years (n = 3), as well as those TD with an IQ higher than 123 (n = 3) and older than 16.2 years (n = 4). This approach was necessary due to a low age (11.37–15.39) and high IQ (82–133) variance of the ADHD+ODD/CD group, which was the smallest group due to recruitment problems. This resulted in a sample of n = 36 ADHD‐only, n = 26 ADHD+ODD/CD, and n = 30 TD (Table 1).
Table 1.
Demographics, clinical characteristics, and group comparisons (n = 92)
| Parameter | ADHD‐only (n = 36) | ADHD+ODD/CD (n = 26) | TD (n = 30) | Group comparison | Post hoc | |||
|---|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | F | df | p | |||
| Age in years | 13.02 (1.57) | 12.99 (1.17) | 13.58 (1.58) | 1.46 | 2, 89 | n.s. | ||
| Socioeconomic statusa | 12.41 (3.83) | 9.79 (4.07) | 15.65 (3.82) | 13.67 | 2, 73 | <.001 | ADHD‐only<TD**, ADHD+ODD/CD<TD** | |
| IQb | 107 (8) | 106 (13) | 110 (8) | 1.46 | 2, 89 | n.s. | ||
| Pubertal statusc | 2.45 (.94) | 2.13 (.82) | 2.68 (1) | 1.4 | 2, 81 | n.s. | ||
| CBCLd | Attention problems | 66.13 (7.016) | 70.61(8.68) | 54.41(5.71) | 35.26 | 2, 77 | <.001 | ADHD‐only>TD**, ADHD+ODD/CD>TD** |
| Rule‐breaking behavior | 56.10 (6.71) | 66.52 (7.43) | 52.93 (4.34) | 31.64 | 2, 77 | <.001 | ADHD+ODD/CD>ADHD‐only**, ADHD+ODD/CD>TD** | |
| Aggressive behavior | 60.93 (9.68) | 73.83 (9.43) | 54.48(6.39) | 31.97 | 2, 77 | <.001 | ADHD‐only>TD*, ADHD+ODD/CD>TD**, ADHD‐only<ADHD+ODD/CD** | |
| Total | 61.37 (7.25) | 72.04 (6.5) | 51.44 (9.46) | 42.49 | 2, 77 | <.001 | ADHD‐only>TD**, ADHD+ODD/CD>TD**, ADHD‐only<ADHD+ODD/CD** | |
Note: F values and p level refer to the main effect of group in ANOVA. **p < .01. *p < .05. Post hoc tests are t tests. ADHD‐only, attention‐deficit hyperactivity disorder group; ADHD+ODD/CD, comorbid attention‐deficit hyperactivity disorder and conduct disorder/oppositional defiant disorder group, TD, typically developing group. Please note that due to assessment difficulties some values are missing for a few participants (n range from 92 of 92 to 80 of 92).
Calculation of socioeconomic status included parents' school education. Professional education. Recent professional status and family income following the procedure suggested by Winkler and Stolzenberg (2009). Scores for mothers and fathers were averaged into a family‐based measure of socioeconomic background. The score ranges from 3 to 21 with higher values indicating higher socioeconomic status.
To estimate general cognitive ability the subtests Vocabulary, Letter‐Number Sequencing, Matrix Reasoning and Symbol Search from the Wechsler Intelligence Scale For Children [WISC‐IV. German adaptation; (Petermann & Petermann, 2010)] were used.
Pubertal status ranges from 1 for prepubertal to 5 for postpubertal status, measured with the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988).
CBCL—Child Behavior Checklist (Achenbach, 1991). Reported are CBCL t scores.
Eighteen boys with ADHD were regularly taking methylphenidate (MPH; for a mean duration of about 33 months). Five boys with ADHD had taken MPH previously (for a mean duration of about 28 months) but stopped a few years before the study. Thirteen boys with ADHD had never been pharmacologically treated for ADHD. Three boys with ADHD had a history of other medication.
Twenty‐one boys with ADHD and comorbid ODD/CD were regularly taking medication for a mean duration of about 31 months (15 thereof MPH and six MPH and/or atypical antipsychotics). Five boys with ADHD and comorbid ODD/CD had never been pharmacologically treated for ADHD or ODD/CD.
All participants taking MPH and/or atypical antipsychotics when participating in the study were taken off medication for at least 48 hr prior to scanning.
2.2. Structural imaging
2.2.1. Image acquisition and processing
We acquired magnetic resonance imaging scans on a 3T whole‐body MR tomograph (Magnetom TRIO, Siemens, Erlangen, Germany) equipped with a 12‐channel head coil and processed T1‐weighted images using the cross‐sectional pipeline of the segmentation and image analysis software FreeSurfer (Version 5.1, https://surfer.nmr.mgh.harvard.edu/). We applied detailed visual quality control for raw and processed images (Backhausen et al., 2016). See Supplement S1 for further details.
2.2.2. Statistical analysis
Statistical analyses on demographic and clinical measures were performed with SPSS (IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY) using analyses of variance (ANOVAS). Using FreeSurfer's volume‐based stream we analyzed global measures (including intracranial volume, [ICV], total, cortical, and subcortical GM volume, mean CT, and total SA) and volume‐based ROIs (including the cerebellum, thalamus, putamen, pallidum, hippocampus, amygdala, nucleus accumbens, and caudate). We ran ANOVAs followed by post hoc t tests to compare groups.
As cortical measures, we analyzed CV, SA, and CT using FreeSurfer's surface‐based stream applying a voxel‐based whole brain approach with general linear modeling. First, to enable comparability with previous publications, we reported clusters surviving a cluster‐forming threshold (CFT) of p < .05 with a precomputed Monte Carlo Z simulation with 10,000 iterations and a full‐width at half‐maximum Gaussian smoothing kernel of 15 mm. Then we performed robustness analyses. First, we applied new stringent thresholds (Greve & Fischl, 2018): CFT p < .005 for CT and CV, and p < .001 for SA with a cluster‐wise threshold (CWP) of p < .05 for CV, CT, and SA. Second, for CV and SA we added ICV as a covariate (Greve & Fischl, 2018).
2.3. Results
2.4. Global volume, thickness, and surface area
We found 4–6% decreases for both patient groups compared to TD in the ICV (5% ADHD‐only vs. TD: p = .011, 5% ADHD+ODD/CD vs. TD: p = .0013), total GM volume (4% ADHD‐only vs. TD: p = .013, 5% ADHD+ODD/CD vs. TD: p = .009), right cortical GM volume (4% ADHD‐only vs. TD: p = .034, 5% ADHD+ODD/CD vs. TD: p = .0035), and total left and right SA (left: 5% ADHD‐only vs. TD: p = .014, 6% ADHD+ODD/CD vs. TD: p = .007; right: 4% ADHD‐only vs. TD: p = .024, 6% ADHD+ODD/CD vs. TD: p = .006). Patient groups did not differ from each other. There were no group differences for subcortical GM volume or mean CT (see Table S1, available online).
2.5. Volume‐based ROIs
Analyses including ICV as a covariate showed cerebellar volume decreases of 5–6% (right: ADHD‐only vs. TD: p = .041, ADHD+ODD/CD vs. TD: p = .005; left: ADHD+ODD/CD vs. TD, p = .033; Table S1; Figure 1). After applying false discovery rate (FDR) correction, no group difference remained significant.
Figure 1.
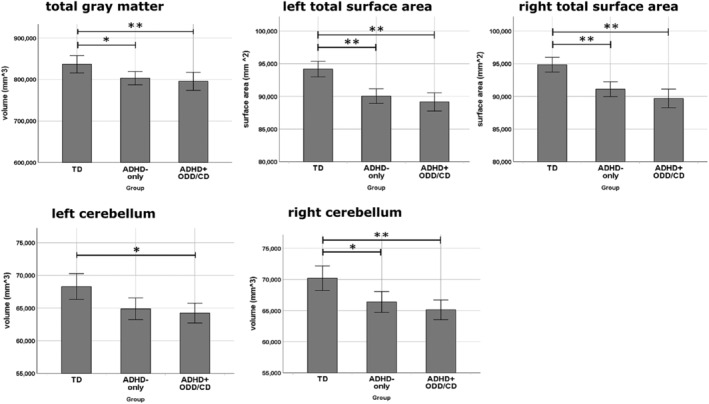
Global and subcortical volume differences. Note: **p < .01. *p < .05. Error bars denote standard errors. ADHD‐only, group of boys diagnosed with ADHD only. ADHD+ODD/CD, group of boys diagnosed with ADHD and comorbid ODD/CD. TD, group of typically developing boys
2.6. Cortical thickness
We report anatomical regions according to the Desikan‐Kiliany atlas (Desikan et al., 2006). For a liberal threshold (CFT p < .05), a main effect of group in a cluster of the right rostral middle frontal cortex emerged (see Table S2 for post hoc tests, available online). When applying new stringent thresholds with a CFT p < .005 and CWP of p < .05 no cluster emerged for the main effect of group (Table 2). Since we had a priori hypotheses about differences between ADHD‐only and TD, ADHD+ODD/CD and TD as well as between ADHD‐only and ADHD+ODD/CD, we performed post hoc analyses. These revealed a 6.5% increased CT for ADHD+ODD/CD versus TD and 7% increase for ADHD+ODD/CD versus ADHD‐only in a rostral middle frontal cluster (Table 2, Figure 2).
Table 2.
Differences in cortical thickness
| Cortical region | L/R | MNI coordinates | Cluster size (mm2) | Number of vertices | Clusterwise p‐value (CWP) | Post hoc group comparisons | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| ADHD‐only versus ADHD+ODD/CD versus TD | ||||||||
| Rostral middle frontal | R | 36 | 39 | 10 | 283 | 534 | .41 | |
| R | 44 | 23 | 29 | 205 | 353 | .67 | ||
| R | 44 | 35 | 26 | 63 | 103 | 1 | ||
| Caudal middle frontal | R | 41 | 0 | 34 | 137 | 240 | .9 | |
| Parahippocampal | R | 46 | 17 | −39 | 46 | 153 | 1 | |
| Supramarginal | L | −43 | −42 | 39 | 49 | 136 | 1 | |
| ADHD‐only versus TD | ||||||||
| Parahippocampal | R | 17 | −39 | −3 | 80 | 250 | .98 | ADHD‐only>TD |
| Supramarginal | L | −43 | −42 | 39 | 117 | 315 | .93 | ADHD‐only>TD |
| ADHD+ODD/CD versus TD | ||||||||
| Rostral middle frontal* | R | 44 | 23 | 30 | 871 | 1,525 | <.001 | ADHD+ODD/CD≥TD |
| R | 36 | 42 | 24 | 101 | 151 | .96 | ADHD+ODD/CD>TD | |
| Precentral | R | 42 | 0 | 34 | 158 | 271 | .84 | ADHD+ODD/CD>TD |
| Precuneus | R | 10 | −59 | 43 | 107 | 270 | .95 | ADHD+ODD/CD>TD |
| Superior parietal | R | 31 | −71 | 19 | 56 | 141 | 1 | ADHD+ODD/CD>TD |
| Superior frontal | R | 10 | −3 | 67 | 38 | 77 | 1 | ADHD+ODD/CD>TD |
| L | −12 | −5 | 65 | 81 | 190 | .98 | ADHD+ODD/CD>TD | |
| L | −19 | 10 | 61 | 28 | 44 | 1 | ADHD+ODD/CD>TD | |
| Inferior parietal | L | −34 | −61 | 39 | 51 | 112 | 1 | ADHD+ODD/CD>TD |
| ADHD‐only versus ADHD+ODD/CD | ||||||||
| Rostral middle frontal* | R | 37 | 40 | 10 | 669 | 1,180 | .01812 | ADHD‐only<ADHD+ODD/CD |
| Caudal middle frontal | R | 40 | 0 | 34 | 150 | 267 | .86 | ADHD‐only<ADHD+ODD/CD |
| Insula | R | 37 | −18 | −7 | 82 | 235 | .98 | ADHD‐only<ADHD+ODD/CD |
| Frontal pole | R | 11 | 58 | −14 | 107 | 133 | .95 | ADHD‐only<ADHD+ODD/CD |
| Medial orbito frontal | R | 10 | 33 | −11 | 39 | 93 | 1 | ADHD‐only<ADHD+ODD/CD |
| Inferior parietal | R | 34 | 41 | −66 | 34 | 60 | 1 | ADHD‐only<ADHD+ODD/CD |
| Medial orbito frontal | L | −12 | 41 | −9 | 85 | 169 | .98 | ADHD‐only<ADHD+ODD/CD |
| Isthmus cingulate | L | −7 | −46 | 10 | 38 | 110 | 1 | ADHD‐only<ADHD+ODD/CD |
Note: Smoothing kernel = 15 mm FWHM, cluster‐forming threshold p < .005, Monte Carlo Z simulation with 10,000 iterations; *and underlined = CWP < .05, CWP = clusterwise p‐value. ADHD‐only, attention‐deficit hyperactivity disorder group; ADHD+ODD/CD, comorbid attention‐deficit hyperactivity disorder and conduct disorder/oppositional defiant disorder group; TD, typically developing group.
Figure 2.
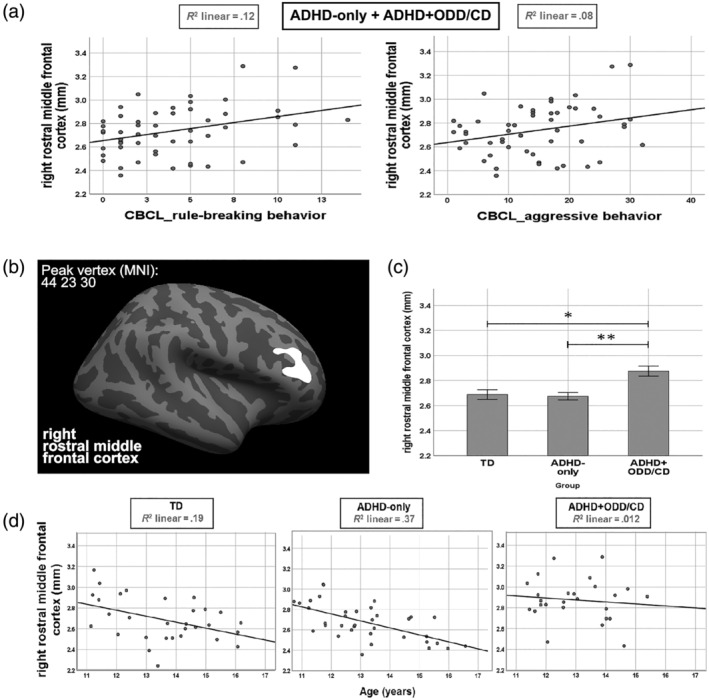
Cluster with group differences in cortical thickness. Note: (a) For contrasting ADHD‐only versus ADHD+ODD/CD the only significant cluster is depicted (cluster forming threshold p < .005 and clusterwise p < .05) on the lateral surface of an inflated brain. (b) Mean cortical thickness for TD, ADHD‐only and ADHD+ODD/CD extracted from the cluster shown in (a). Error bars denote standard errors. (c) Associations of mean cortical thickness in the cluster shown in (a) with raw scores of the rule‐breaking and aggressive behavior scales as assessed with the CBCL. (d) Correlations with age of mean cortical thickness in the cluster shown in (a). **p < .01. *p < .05. ADHD‐only, group of boys diagnosed with ADHD only. ADHD+ODD/CD, group of boys diagnosed with ADHD and comorbid ODD/CD. TD, group of typically developing boys
2.7. Cortical volume and surface area
See Table S2 (available online) for results with a liberal threshold (CFT p < .05) and Table S3 (available online) for a stringent threshold (for CV: CFT p < .005; for SA: CFT p < .001). These results did not survive a CWP of p < .05 or robustness analyses when including ICV as a covariate.
2.7.1. Correlations of cortical thickness in the right rostral middle frontal cortex with symptom severity and age
To disentangle the contribution of disorder‐specific symptoms to altered CT, we correlated in both patient groups mean CT from the right rostral middle frontal cluster resulting for ADHD+ODD/CD versus ADHD‐only with ADHD symptoms (scale “attention problems”) controlling for ODD/CD symptoms (scale “externalizing behavior” consisting of “aggressive behavior” and “rule‐breaking behavior”) and with ODD/CD symptoms controlling for ADHD symptoms as assessed with the CBCL.
CT correlated with ODD/CD symptoms, (aggressive behavior: r(50) = .28, p = .046 and rule‐breaking behavior: r(50) = .34, p = .013) controlling for ADHD symptoms (Figure 2). CT and ADHD symptoms did not correlate when controlling for ODD/CD symptoms, r(50) = −.087, p = .54.
Regarding previous reports on a developmental delay of cortical thinning for ADHD (Shaw et al., 2007), we explored correlations of age and CT of the right rostral middle frontal cluster resulting for ADHD+ODD/CD versus ADHD‐only (Figure 2) separately for the groups. Results showed cortical thinning in the ADHD‐only, r(34) = −.61, p < .001, and TD, r(28) = −.44, p = .016, but not the ADHD+ODD/CD group, r(24) = −.11, p = .59. Fisher r‐to‐z transformation revealed a significant difference only between the correlation coefficients of the ADHD+ODD/CD and ADHD‐only groups (z = −2.2, p = .028) but not of the ADHD+ODD/CD and TD (z = −1.25, p = .21) or ADHD‐only and TD (z = −.93, p = .3) groups.
3. DISCUSSION
In accordance with two recent reports (Noordermeer et al., 2017; Stevens & Haney‐Caron, 2012) this study is one of the first to provide knowledge to disentangle the related conditions ADHD‐only and ADHD+ODD/CD. Going beyond most previous studies, we analyzed several morphological markers, that is, subcortical and cortical volume as well as CT and SA by contrasting a matched sample of ADHD‐only with ADHD+ODD/CD. We found similar and differential alterations for the two patient groups. Similarly, both groups had reductions in total GM volume, right CV, and total SA. Differentially, ADHD+ODD/CD diverged from both TD and ADHD‐only in a rostral middle frontal cluster. The increased thickness was related to ODD/CD symptoms under control of ADHD symptomatology. Further, we observed thinning across adolescence in this cluster in the ADHD‐only and TD groups but not in the comorbid ADHD+ODD/CD group. No other alterations for CV or SA survived stringent thresholding and ICV correction (Greve & Fischl, 2018).
In line with our first hypothesis, both diagnostic groups had reductions in total GM volume with 4% for ADHD‐only and 5% for ADHD+ODD/CD. Both groups also had reductions in right cortical GM volume (4% for ADHD‐only and 5% for ADHD+ODD/CD), and total SA (4–5% for ADHD and 6% for ADHD+ODD/CD). This study adds evidence that for ADHD+ODD/CD total and cortical GM volume reductions are in a similar range as for ADHD‐only (see Ambrosino et al., 2017 and Greven et al., 2015 for samples including comorbidity; see Noordermeer et al., 2017 for ODD). For SA, we extend previous findings that did not exclude ODD comorbidity (Wolosin et al., 2009) in showing that in both pure and comorbid ADHD similar reductions are evident in total SA.
Contrary to our second hypothesis, we did not find reductions in the cerebellum after applying FDR correction. Interestingly, some recent studies did not assess (Greven et al., 2015; Hoogman et al., 2017) or did not find cerebellum alterations (Noordermeer et al., 2017; for a meta‐analysis see also Frodl & Skokauskas, 2012), while others showed increased gray matter concentration of the cerebellum in CD (De Brito et al., 2009). Together with our findings, this suggests that alterations in the cerebellum might not be robustly found in (comorbid) ADHD. Overall, results are largely dependent in the a priori selected ROIs and thus the chance to survive robustness analyses. This should be kept in mind and made transparent in future reports. Specifically for comorbid ADHD, the lack of a stronger reduction in the cerebellum for comorbid versus pure ADHD might be due to the absence of stronger ADHD symptoms in comorbid compared to pure ADHD.
We found no alterations for neither patient group for subcortical volumes similarly to two other adolescent studies (Ambrosino et al., 2017; Noordermeer et al., 2017). Sample age seems to be important as effect sizes for subcortical reductions were shown to be larger in child samples (see mega‐analysis by Hoogman et al., 2017) and two meta‐analyses showed that basal ganglia reductions tended to decrease with increasing age (Frodl & Skokauskas, 2012; Nakao et al., 2011). Again, specifically for comorbid ADHD, the lack of a stronger reduction in the striatum for comorbid versus pure ADHD might be due to the absence of stronger ADHD symptoms in comorbid compared to pure ADHD.
Our data of underlying structural regions cannot directly speak to the differentiation of neuropsychological deficits in “cool” executive functions in ADHD versus deficits in “hot” executive functions in ODD/CD. We did not find alterations for “cool” striatal and cerebellar regions for neither patient group. We also did not find alterations for “hot” limbic regions such as the amygdala for neither patient group. Therefore, this distinction although very indirectly is not supported by the current data. It has to be stressed that a pure ODD and CD group is missing and most importantly “hot” and “cool” executive function tests have to be employed in future studies. Also, as has been stated in Rubia (2011) combined structure–function studies are a major aim of shedding more light on a possible distinction of the two disorder types.
Furthermore, we explored cortical alterations. Regarding CT, the ANOVA comparing all three groups was not significant on a stringent, but on a liberal threshold. According to our a priori hypotheses, we performed direct comparisons between groups. For these comparisons, we applied a stringent threshold and found that ADHD+ODD/CD had a 6–7% thicker cortex than both ADHD‐only and TD in a rostral middle frontal cluster. The region is in line with neuropsychological impairments in inhibition, a cognitive function that is dependent on the (inferior) frontal cortex and has been shown for both ADHD and with worse neuropsychological problems in ODD/CD (Loeber et al., 2000). The increased thickness in this cluster seems to be related to the ODD/CD and not ADHD component, since it correlated with ODD/CD symptoms when controlling for ADHD symptoms. Our results are in contrast to a study that found no CT alterations in ADHD with comorbid ODD (Noordermeer et al., 2017). A possible reason is that our sample was younger (mean age 16 vs. 13, age range 7–29 vs. 10–17). Two ADHD+ODD/CD studies with a similar age range found an increase in about the same location in the right prefrontal cortex in volume (that closely relates to thickness, Garrett et al., 2008; Semrud‐Clikeman et al., 2014). The increased thickness in our cluster might also be related to a slower cortical thinning in the ADHD+ODD/CD group since thickness did not decrease across adolescence in ADHD+ODD/CD. In contrast, for the TD and ADHD‐only group a thinning was observed—as would be expected for typical adolescent development. Interestingly, a delay of attaining peak CT before adolescence in a similar middle frontal region was reported for ADHD at age 10.5 versus age 7.5 (Shaw et al., 2007). Almost half of the sample of the Shaw et al. (2007) study (42%) suffered from ODD/CD comorbidity. Probably, specifically the added burden with ODD/CD might relate to a delayed prefrontal cortical maturation in thickness. Data from our study cannot answer the question whether the increased frontal CT might be due to the comorbidity, that is, sum of ADHD and ODD/CD, or due to the disorder of ODD respectively CD alone. Previous findings of a study of pure CD and pure ADHD demonstrated frontal volume alterations in CD but not ADHD (Stevens & Haney‐Caron, 2012). Similarly, current findings of altered frontal brain development might be related to the pure form of ODD or to the pure form of CD, respectively, instead of ADHD (Stevens & Haney‐Caron, 2012). Future research could longitudinally follow cortical maturation of (1) pure ODD, (2) pure CD, (3) pure ADHD as well as (4) ADHD with comorbid ODD, and (5) ADHD with comorbid CD. Hence, the supposed later thinning could be verified to be connected specifically to the respective pure disorder, that is, CD, ODD, ADHD, or to the comorbid occurring disorders, that is, ADHD with comorbid ODD or ADHD with comorbid CD.
We found no evidence of local alterations in CV and SA that survived robustness analyses. About only three previous studies report local SA alterations from which two assessed child samples and all three included participants with ODD comorbidity (Dirlikov et al., 2015; Silk et al., 2016; Wolosin et al., 2009). In line with these reports, for children but not adults with ADHD SA reductions have been shown (Hoekzema et al., 2012). Differences to previous studies demonstrating frontal volume alterations might also be related to sample age and inclusion of comorbidity in the ADHD sample (e.g., Ambrosino et al., 2017).
Overall, comparisons to previous reports remain difficult because—as was reported by a recent review (Noordermeer et al., 2016)—several previous studies using a voxel‐based whole brain approach did not control for multiple comparisons, nor applied cluster thresholding.
Limiting generalization of present findings, we assessed boys only; replication is warranted in mixed samples. Due to the small size of the ADHD+ODD/CD‐group, we could not differentiate between ODD and CD alterations. Future studies are needed, that disentangle the specific contribution of ODD versus CD (Noordermeer et al., 2016; Noordermeer et al., 2017) and systematically compare ADHD‐only, ADHD with comorbid ODD or with comorbid CD and the pure forms of ODD and CD. Due to the cross‐sectional nature of our study, conclusions about a possible delayed cortical thinning in ADHD+ODD/CD remain to be verified by longitudinal studies.
4. CONCLUSION
We demonstrate the necessity to carefully differentiate between ADHD and ADHD+ODD/CD since we found similarities (reduced volume of the total GM and SA) but also differences in brain morphology between the disorders (increase in rostral middle frontal thickness for ADHD+ODD/CD). The increased rostral middle frontal thickness hints at a specific developmental brain alteration in ADHD+ODD/CD, probably related to a delayed cortical thinning. Implications for treatment could be to specifically focus on the (adolescent) boys with a “double burden” of ADHD and ODD or CD that seem to be even more affected than boys with ADHD‐only.
Supporting information
Table S1 Global volume and volume‐based ROIs: results of group comparisons
Table S2 Group comparisons applying liberal CFT thresholds
Table S3 Group comparisons applying stringent CFT thresholds
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This research was supported by the Bundesministerium für Bildung und Forschung under the Grant Numbers 01EV0711 and Aerial 01EE1406B; by the Deutsche Forschungsgemeinschaft under the Grant Numbers SFB 940/1, SFB 940/2, and VE 892/2‐1; and by the Faculty of Medicine at the Technische Universität Dresden under the MeDDrive Grant. The authors would like to thank Stefan Ehrlich and Fabio Bernardoni (both Division of Psychological and Social Medicine and Developmental Neurosciences, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany), Franziska Böhme (Department of Psychiatry and Neuroimaging Center, Technische Universität Dresden, Germany) and Sandy Schramm (FernUniversität in Hagen, Germany) for their helpful methodical discussion and advice. Resources of The Center for Information Services and High Performance Computing (ZIH) at TU Dresden were used for fast data processing.
Vetter NC, Backhausen LL, Buse J, Roessner V, Smolka MN. Altered brain morphology in boys with attention deficit hyperactivity disorder with and without comorbid conduct disorder/oppositional defiant disorder. Hum Brain Mapp. 2020;41:973–983. 10.1002/hbm.24853
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Numbers: 01EV0711, Aerial 01EE1406B; Deutsche Forschungsgemeinschaft, Grant/Award Numbers: SFB 940/1, SFB 940/2, VE 892/2‐1; Faculty of Medicine at the Technische Universität Dresden, Grant/Award Number: MeDDrive Grant
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Achenbach, T. M. (1991). Manual for the child behavior checklist/4‐18 and 1991 profile. Burlington, VT: University of Vermont, Dept. of Psychiatry. [Google Scholar]
- Almeida, L. G. , Ricardo‐Garcell, J. , Prado, H. , Barajas, L. , Fernández‐Bouzas, A. , Ávila, D. , & Martínez, R. B. (2010). Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross‐sectional study. Journal of Psychiatric Research, 44(16), 1214–1223. 10.1016/j.jpsychires.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Almeida Montes, L. G. , Prado Alcántara, H. , Martínez García, R. B. , De La Torre, L. B. , Ávila Acosta, D. , & Duarte, M. G. (2013). Brain cortical thickness in ADHD: Age, sex, and clinical correlations. Journal of Attention Disorders, 17(8), 641–654. 10.1177/1087054711434351 [DOI] [PubMed] [Google Scholar]
- Ambrosino, S. , de Zeeuw, P. , Wierenga, L. M. , van Dijk, S. , & Durston, S. (2017). What can cortical development in attention‐deficit/hyperactivity disorder teach us about the early developmental mechanisms involved? Cerebral Cortex, 27(9), 4624–4634. 10.1093/cercor/bhx182 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (1994). Diagnostic and statistical manual of mental disorders. DSM‐IV. Washington, DC: American Psychiatric Press. [Google Scholar]
- Backhausen, L. L. , Herting, M. M. , Buse, J. , Roessner, V. , Smolka, M. N. , & Vetter, N. C. (2016). Quality control of structural MRI images applied using FreeSurfer—A hands‐on workflow to rate motion artifacts. Frontiers in Neuroscience, 10, 558. 10.3389/fnins.2016.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley, R. A. (2014). Attention‐deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: Guilford Publications. [Google Scholar]
- Byrd, A. L. , Loeber, R. , & Pardini, D. A. (2014). Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clinical Child and Family Psychology Review, 17(2), 125–156. 10.1007/s10567-013-0159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, D. F. , & Doerfler, L. A. (2008). ADHD with comorbid oppositional defiant disorder or conduct disorder: Discrete or nondistinct disruptive behavior disorders? Journal of Attention Disorders, 12(2), 126–134. [DOI] [PubMed] [Google Scholar]
- Connor, D. F. , Steeber, J. , & McBurnett, K. (2010). A review of attention‐deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. Journal of Developmental & Behavioral Pediatrics, 31(5), 427–440. 10.1097/DBP.0b013e3181e121bd [DOI] [PubMed] [Google Scholar]
- De Brito, S. A. , Mechelli, A. , Wilke, M. , Laurens, K. R. , Jones, A. P. , Barker, G. J. , … Viding, E. (2009). Size matters: Increased grey matter in boys with conduct problems and callous–unemotional traits. Brain, 132(4), 843–852. 10.1093/brain/awp011 [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Ségonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dirlikov, B. , Shiels Rosch, K. , Crocetti, D. , Denckla, M. B. , Mahone, E. M. , & Mostofsky, S. H. (2015). Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage: Clinical, 7, 222–229. 10.1016/j.nicl.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden, E. G. , Tannock, R. , & Dockstader, C. (2012). Altered cortical morphology in sensorimotor processing regions in adolescents and adults with attention‐deficit/hyperactivity disorder. Brain Research, 1445, 82–91. 10.1016/j.brainres.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Fahim, C. , He, Y. , Yoon, U. , Chen, J. , Evans, A. , & Pérusse, D. (2011). Neuroanatomy of childhood disruptive behavior disorders. Aggressive Behavior, 37(4), 326–337. 10.1002/ab.20396 [DOI] [PubMed] [Google Scholar]
- Fairchild, G. , Toschi, N. , Hagan, C. C. , Goodyer, I. M. , Calder, A. J. , & Passamonti, L. (2015). Cortical thickness, surface area, and folding alterations in male youths with conduct disorder and varying levels of callous–unemotional traits. NeuroImage: Clinical, 8, 253–260. 10.1016/j.nicl.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone, S. V. , Asherson, P. , Banaschewski, T. , Biederman, J. , Buitelaar, J. K. , Ramos‐Quiroga, J. A. , … Franke, B. (2015). Attention‐deficit/hyperactivity disorder. Nature Reviews Disease Primers, 1, 15020 10.1038/nrdp.2015.20 [DOI] [PubMed] [Google Scholar]
- Faraone, S. V. , Rostain, A. L. , Blader, J. , Busch, B. , Childress, A. C. , Connor, D. F. , & Newcorn, J. H. (2019). Practitioner review: Emotional dysregulation in attention‐deficit/hyperactivity disorder—Implications for clinical recognition and intervention. Journal of Child Psychology and Psychiatry, 60(2), 133–150. 10.1111/jcpp.12899 [DOI] [PubMed] [Google Scholar]
- Frodl, T. , & Skokauskas, N. (2012). Meta‐analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica, 125(2), 114–126. 10.1111/j.1600-0447.2011.01786.x [DOI] [PubMed] [Google Scholar]
- Garrett, A. , Penniman, L. , Epstein, J. N. , Casey, B. , Hinshaw, S. P. , Glover, G. , … Reiss, A. L. (2008). Neuroanatomical abnormalities in adolescents with attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 47(11), 1321–1328. 10.1097/CHI.0b013e318185d285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd, J. N. , Blumenthal, J. , Jeffries, N. O. , Castellanos, F. X. , Liu, H. , Zijdenbos, A. , … Rapoport, J. L. (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, A. C. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2018). False positive rates in surface‐based anatomical analysis. NeuroImage, 171, 6–14. 10.1016/j.neuroimage.2017.12.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven, C. U. , Bralten, J. , Mennes, M. , O'Dwyer, L. , van Hulzen, K. J. E. , Rommelse, N. , … Buitelaar, J. K. (2015). Developmentally stable whole‐brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention‐deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry, 72(5), 490–499. 10.1001/jamapsychiatry.2014.3162 [DOI] [PubMed] [Google Scholar]
- Halperin, J. M. , & Schulz, K. P. (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention‐deficit/hyperactivity disorder. Psychological Bulletin, 132(4), 560–581. 10.1037/0033-2909.132.4.560 [DOI] [PubMed] [Google Scholar]
- Hoekzema, E. , Carmona, S. , Ramos‐Quiroga, J. A. , Fernández, V. R. , Picado, M. , Bosch, R. , … Vilarroya, O. (2012). Laminar thickness alterations in the Fronto‐parietal cortical mantle of patients with attention‐deficit/hyperactivity disorder. PLoS One, 7(12), e48286 10.1371/journal.pone.0048286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , … Franke, B. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. The Lancet Psychiatry, 4(4), 310–319. 10.1016/S2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt, C. J. , Haney‐Caron, E. , & Stevens, M. C. (2012). Cortical thickness and folding deficits in conduct‐disordered adolescents. Biological Psychiatry, 72(3), 207–214. 10.1016/j.biopsych.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. , & Roth, R. M. (2017). The status of structural and functional MRI in adolescents with attention‐deficit/hyperactivity disorder. Psychological Injury and Law, 10(3), 209–222. 10.1007/s12207-017-9296-4 [DOI] [Google Scholar]
- Loeber, R. , Burke, J. D. , Lahey, B. B. , Winters, A. , & Zebra, M. (2000). Oppositional defiant and conduct disorder: A review of the past 10 years, part I. Journal of the American Academy of Child & Adolescent Psychiatry, 39(12), 1468–1484. 10.1097/00004583-200012000-00007 [DOI] [PubMed] [Google Scholar]
- Maughan, B. , Rowe, R. , Messer, J. , Goodman, R. , & Meltzer, H. (2004). Conduct disorder and oppositional defiant disorder in a national sample: Developmental epidemiology. Journal of Child Psychology and Psychiatry, 45(3), 609–621. 10.1111/j.1469-7610.2004.00250.x [DOI] [PubMed] [Google Scholar]
- McAlonan, G. M. , Cheung, V. , Cheung, C. , Chua, S. E. , Murphy, D. G. M. , Suckling, J. , … Ho, T. P. (2007). Mapping brain structure in attention deficit‐hyperactivity disorder: A voxel‐based MRI study of regional grey and white matter volume. Psychiatry Research: Neuroimaging, 154(2), 171–180. 10.1016/j.pscychresns.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Nakao, T. , Radua, J. , Rubia, K. , & Mataix‐Cols, D. (2011). Gray matter volume abnormalities in ADHD: Voxel‐based meta‐analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry, 168(11), 1154–1163. 10.1176/appi.ajp.2011.11020281 [DOI] [PubMed] [Google Scholar]
- Noordermeer, S. D. , Luman, M. , Greven, C. U. , Veroude, K. , Faraone, S. V. , Hartman, C. A. , … Oosterlaan, J. (2017). Structural brain abnormalities of attention‐deficit/hyperactivity disorder with oppositional defiant disorder. Biological Psychiatry, 82(9), 642–650. 10.1016/j.biopsych.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Noordermeer, S. D. , Luman, M. , & Oosterlaan, J. (2016). A systematic review and meta‐analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention‐deficit hyperactivity disorder (ADHD) into account. Neuropsychology Review, 26(1), 44–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini, D. A. , & Fite, P. J. (2010). Symptoms of conduct disorder, oppositional defiant disorder, attention‐deficit/hyperactivity disorder, and callous‐unemotional traits as unique predictors of psychosocial maladjustment in boys: Advancing an evidence base for DSM‐V. Journal of the American Academy of Child and Adolescent Psychiatry, 49(11), 1134–1144. 10.1016/j.jaac.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann, F. , & Petermann, U. (2010). HAWIK‐iv (3rd extended ed.). Bern: Huber. [Google Scholar]
- Petersen, A. C. , Crockett, L. , Richards, M. , & Boxer, A. (1988). A self‐report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Rogers, J. C. , & Brito, S. A. D. (2016). Cortical and subcortical gray matter volume in youths with conduct problems: A meta‐analysis. JAMA Psychiatry, 73(1), 64–72. 10.1001/jamapsychiatry.2015.2423 [DOI] [PubMed] [Google Scholar]
- Rubia, K. (2011). “Cool” inferior frontostriatal dysfunction in attention‐deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal‐limbic dysfunction in conduct disorder: A review. Biological Psychiatry, 69(12), e69–e87. 10.1016/j.biopsych.2010.09.023 [DOI] [PubMed] [Google Scholar]
- Sarkar, S. , Daly, E. , Feng, Y. , Ecker, C. , Craig, M. C. , Harding, D. , … Murphy, D. G. M. (2015). Reduced cortical surface area in adolescents with conduct disorder. European Child & Adolescent Psychiatry, 24(8), 909–917. 10.1007/s00787-014-0639-3 [DOI] [PubMed] [Google Scholar]
- Schachar, R. , & Tannock, R. (1995). Test of four hypotheses for the comorbidity of attention‐deficit hyperactivity disorder and conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 34(5), 639–648. 10.1097/00004583-199505000-00016 [DOI] [PubMed] [Google Scholar]
- Semrud‐Clikeman, M. , Pliszka, S. R. , Bledsoe, J. , & Lancaster, J. (2014). Volumetric MRI differences in treatment Naïve and chronically treated adolescents with ADHD‐combined type. Journal of Attention Disorders, 18(6), 511–520. 10.1177/1087054712443158 [DOI] [PubMed] [Google Scholar]
- Seymour, K. E. , Tang, X. , Crocetti, D. , Mostofsky, S. H. , Miller, M. I. , & Rosch, K. S. (2017). Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Research: Neuroimaging, 261, 20–28. 10.1016/j.pscychresns.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Eckstrand, K. , Sharp, W. , Blumenthal, J. , Lerch, J. P. , Greenstein, D. , … Rapoport, J. L. (2007). Attention‐deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104(49), 19649–19654. 10.1073/pnas.0707741104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Lerch, J. , Greenstein, D. , Sharp, W. , Clasen, L. , Evans, A. , … Rapoport, J. (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 63(5), 540–549. 10.1001/archpsyc.63.5.540 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Silk, T. J. , Beare, R. , Malpas, C. , Adamson, C. , Vilgis, V. , Vance, A. , & Bellgrove, M. A. (2016). Cortical morphometry in attention deficit/hyperactivity disorder: Contribution of thickness and surface area to volume. Cortex, 82, 1–10. 10.1016/j.cortex.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Stevens, M. C. , & Haney‐Caron, E. (2012). Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. Journal of Psychiatry & Neuroscience, 37(6), 389–398. 10.1503/jpn.110148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes, C. K. , Østby, Y. , Fjell, A. M. , Westlye, L. T. , Due‐Tønnessen, P. , & Walhovd, K. B. (2010). Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and White matter volume and microstructure. Cerebral Cortex, 20(3), 534–548. 10.1093/cercor/bhp118 [DOI] [PubMed] [Google Scholar]
- Valera, E. M. , Faraone, S. V. , Murray, K. E. , & Seidman, L. J. (2007). Meta‐analysis of structural imaging findings in attention‐deficit/hyperactivity disorder. Biological Psychiatry, 61(12), 1361–1369. 10.1016/j.biopsych.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Vetter, N. C. , Buse, J. , Backhausen, L. L. , Rubia, K. , Smolka, M. N. , & Roessner, V. (2018). Anterior insula hyperactivation in ADHD when faced with distracting negative stimuli. Human Brain Mapping, 39(7), 2972–2986. 10.1002/hbm.24053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, G. L. , White, S. F. , Robustelli, B. , Sinclair, S. , Hwang, S. , Martin, A. , & Blair, R. J. R. (2014). Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous‐unemotional traits. Journal of the American Academy of Child & Adolescent Psychiatry, 53(4), 456–465. 10.1016/j.jaac.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt, E. G. (2012). The prevalence of DSM‐IV attention‐deficit/hyperactivity disorder: A meta‐analytic review. Neurotherapeutics, 9(3), 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, J. , & Stolzenberg, H. (2009). Adjustierung des Sozialen‐Schicht‐Index für die Anwendung im Kinder‐ und Jugendgesundheitssurvey (KiGGS) (Wismar Discussion Paper No. 07/2009). Retrieved from Hochschule Wismar, Wismar Business School website: http://econpapers.repec.org/paper/zbwhswwdp/072009.htm.
- Wolosin, S. M. , Richardson, M. E. , Hennessey, J. G. , Denckla, M. B. , & Mostofsky, S. H. (2009). Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping, 30(1), 175–184. 10.1002/hbm.20496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (1992). International statistical classification of diseases and related health problems (ICD‐10) (10th revision ed.). Geneva: WHO. [Google Scholar]
- Yang, X.‐R. , Carrey, N. , Bernier, D. , & MacMaster, F. P. (2015). Cortical thickness in young treatment‐naive children with ADHD. Journal of Attention Disorders, 19(11), 925–930. 10.1177/1087054712455501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Global volume and volume‐based ROIs: results of group comparisons
Table S2 Group comparisons applying liberal CFT thresholds
Table S3 Group comparisons applying stringent CFT thresholds
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


