Abstract
White matter hyperintensities (WMH) have been extensively associated with cognitive impairment and reductions in gray matter volume (GMv) independently. This study explored whether WMH lesion volume mediates the relationship between cerebral patterns of GMv and cognition in 521 (mean age 57.7 years) cognitively unimpaired middle‐aged individuals. Episodic memory (EM) was measured with the Memory Binding Test and executive functions (EF) using five WAIS‐IV subtests. WMH were automatically determined from T2 and FLAIR sequences and characterized using diffusion‐weighted imaging (DWI) parameters. WMH volume was entered as a mediator in a voxel‐wise mediation analysis relating GMv and cognitive performance (with both EM and EF composites and the individual tests independently). The mediation model was corrected by age, sex, education, number of Apolipoprotein E (APOE)‐ε4 alleles and total intracranial volume. We found that even at very low levels of WMH burden in the cohort (median volume of 3.2 mL), higher WMH lesion volume was significantly associated with a widespread pattern of lower GMv in temporal, frontal, and cerebellar areas. WMH mediated the relationship between GMv and EF, mainly driven by processing speed, but not EM. DWI parameters in these lesions were compatible with incipient demyelination and axonal loss. These findings lead to the reflection on the relevance of the control of cardiovascular risk factors in middle‐aged individuals as a valuable preventive strategy to reduce or delay cognitive decline.
Keywords: aging, cognition, hypertension, prevention, vascular risk factors, white matter lesions
1. INTRODUCTION
White matter hyperintensities (WMH) are commonly detected in the brain of elderly individuals through magnetic resonance imaging (MRI) (Longstreth et al., 1996) and are thought to have a vascular etiology, although their histopathological substrate might be heterogeneous as shown by pathological studies (Mortamais, Artero, & Ritchie, 2014; Prins & Scheltens, 2015) as well as by microstructural characterization of WMH with diffusion‐weighted MRI imaging (Bastin et al., 2009; Wardlaw, Valdés Hernández, & Muñoz‐Maniega, 2015; Zhong & Lou, 2016). Even they are relatively frequent in asymptomatic individuals (Arvanitakis et al., 2016; Birdsill et al., 2014; Brugulat‐Serrat et al., 2017; Kloppenborg, Nederkoorn, Geerlings, & van den Berg, 2014), WMH burden has been shown to exert a negative impact on cognition (Arvanitakis et al., 2016; Bolandzadeh, Davis, Tam, Handy, & Liu‐Ambrose, 2012; Jiang et al., 2018; Lampe et al., 2017) mainly in executive function (EF) (Desmond, 2002; de Groot et al., 2000; Jiang et al., 2018; Kloppenborg et al., 2014; Ramirez, McNeely, Scott, Stuss, & Black, 2014). WMH also increase the risk of cognitive decline and Alzheimer's disease (AD), contributing to its progression and severity (Habes et al., 2016; Smith et al., 2008). Risk factors of WMH overlap with those of AD such as aging, hypertension, hypercholesterolemia, and diabetes (Christiane et al., 2010; Habes et al., 2016; Jeerakathil et al., 2004; Kivipelto et al., 2006; Murray et al., 2005; Salvadó et al., 2019). Regarding hereditary risk factors for AD, Apolipoprotein E (APOE)‐ε4 allele is the major known genetic risk factor for AD (Corder et al., 1993; Farrer et al., 1997; Izaks et al., 2011; Jack et al., 2015; Lim et al., 2015) and has also been associated with increased WMH load (Brickman et al., 2014; Rojas et al., 2017; Schilling et al., 2013).
Normal aging is also characterized by gray matter volume (GMv) loss (Ramanoël et al., 2018) with the frontal and temporal lobes being the regions with the highest degree of GM loss (Driscoll et al., 2009), which is also considered to be marker of neurodegeneration (Dong et al., 2015). Cross‐sectional studies have also revealed a regional relationship between GMv and cognition in cognitively unimpaired individuals (Duarte et al., 2006; Tuladhar et al., 2015). In this context, the brain structural networks supporting EM and EF appeared to be spatially segregated and did not show overlap cerebral networks (Cacciaglia, Molinuevo, Sánchez‐Benavides, et al., 2018). As efforts to reduce the global burden of dementia progressively shift toward prevention, a better understanding of the mechanisms linking WMH with neurodegeneration is of utmost importance (Prins & Scheltens, 2015; Winblad et al., 2016).
Prior studies have shown that high WMH burden is associated with GM atrophy (Wen, Sachdev, Chen, & Anstey, 2006) in the temporal lobe (Habes et al., 2016; Rizvi et al., 2018; Swardfager et al., 2018) and the frontal cortex (Raji et al., 2012; Rizvi et al., 2018). However, the regional patterns of brain atrophy associated with higher WMH burden are still not widely understood in cognitively unimpaired young individuals.
Nonetheless, few studies have explored the effects on cognition of both WMH and GMv take into account simultaneously. A recent cross‐sectional study found that global and regional cortical thickness mediated the relationship between WMH and global cognition in a mixed sample of cognitively healthy individuals, mild cognitive impairment (MCI), and AD patients (Rizvi et al., 2018). Authors also looked for the effect in AD‐related regions, such as the entorhinal cortex and the hippocampus and showed an indirect effect in the association of frontal, parietal and occipital WMH with memory performance. Previous studies showed that GMv mediated the association between WMH burden with EF and EM, again in nondemented individuals (Knopman et al., 2015), and in mixed populations of individuals with cardiovascular risk factors and AD patients (Swardfager et al., 2018) (details of these previous studies are shown in Table 1). However, the extent to which these associations are present in cognitively unimpaired individuals has not yet been fully assessed.
Table 1.
Previous cross‐sectional studies that apply mediation analysis between WMH, cortical thickness, and cognition
| Reference (year) | Study sample | Population | N | Age, years (SD) | Women (%) | Education, years (SD) | WMH measurement | WMH volume (cm3) | Cortical thickness measurement | Cortical thickness (mm) | Cognition | Mediation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rizvi et al. (2018) | Washington Heights‐Inwood Columbia aging project (WHICAP) |
CU MCI AD |
519 | 73.98 (5.6) | 56.2 | 12.8 (4.5) | Volumetric | 5.5 (7.1) | Global ROI‐based | 2.434 (0.115) |
Global Memory |
X: WMH Y: Cognition M: Cortical thinninga |
| Swardfager et al. (2018) | Sunnybrook dementia study |
CU MCI AD |
702 | 70.7 (9.4) | 46.2 | 14.1 (3.7) | Volumetric | 7.7 (12.4) | Temporal ROI‐based | Inferred as BPFb | Memory |
X: WMH Y: Verbal recall M1: Left temporal atrophy M2: Verbal learningc |
| Knopman et al. (2015) | Atherosclerosis risk in communities (ARIC) | CU | 1906 | 75.55 (5.21) | 58 | 37% <11 years | Volumetric | 17.29 (16.86) | ROI‐based |
Hippocampal = 6.89 (0.93) Posterior ROI = 59.07 (6.90) Frontal ROI = 150.21 (16.00) |
Memory Executive function Language |
X: WMH Y: Cognition M: Cortical thinningd |
Abbreviations: AD, Alzheimer's disease; BPF, brain parenchymal fraction; CU, cognitively normal; MCI, mild cognitive impairment; M, mediation; ROI, region of interest; TIV, total intracranial volume; WMH, white matter hyperintensities.
Model adjusted by age, education, and TIV.
Model adjusted by age, sex, race, education, history of diabetes mellitus, history of hypertension, history of alcohol use, history of smoking, APOE‐ε4 genotype, and TIV.
Left temporal atrophy = 0.78 (0.06).
Model adjusted by age, education, sex, and TIV.
Studies using mediation pathway analysis have typically selected WMH as predictor, whose association with cognition was mediated by GMv under the assumption that there is a causal relationship between the presences of WMH with brain atrophy and between both of them with cognition. However, it is worth noting that causality cannot be inferred from correlations observed in cross‐sectional observational studies. Therefore, the difference between selecting one variable or another as mediator is the main pairwise association that is intended to be explained by the mediation (MacKinnon, Fairchild, & Fritz, 2007). Unlike previous literature, here we focus on explaining the association between patterns of GMv and cognition as this association is stronger than the one between WMH and cognition, as shown in previous studies with the same cohort (Brugulat‐Serrat et al., 2019; Cacciaglia, Molinuevo, Sánchez‐Benavides, et al., 2018).
Therefore, in this work, we examine in cognitively unimpaired middle‐aged participants the mediating role of WMH lesion volume in the relationship between cognition and topographical patterns of GMv. To this end, we used the Multilevel Mediation and Moderation (M3) toolbox (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008; Wager et al., 2009) for voxel‐based morphometry (VBM) imaging data. This method enables the evaluation of mediation effects in a topographically unbiased way. To better understand the pathological substrate of WMH in our healthy sample, we further characterized WMH lesions by means of diffusion‐weighted magnetic resonance (DWI) parameters.
2. METHODS
2.1. Participants
The ALFA (for ALzheimer and FAmilies) cohort, established by the Barcelonaβeta Brain Research Center (BBRC), is composed of 2,743 cognitively normal participants aged between 45 and 75 years (Molinuevo et al., 2016). A subset of 608 participants was selected to participate in the present study (http://clinicaltrials.gov Identifier: NCT02198586) that was approved by the Ethics Committee of the “Parc de Salut Mar” (Barcelona, Spain; MRI/FBB2014v1.0). A detailed description of the inclusion criteria can be found in (Cacciaglia, Molinuevo, Falcón, et al, 2018) but in brief, those subjects who were carriers of APOE‐ε4 and APOE‐ε2, and those with family history of AD were prioritized. All participants accepted the study procedures by signing the informed consent. 576 participants provided valid MRI scans, out of which 32 had to be discarded due to MRI incidental findings (n = 10 meningioma, n = 3 hydrocephaly, n = 6 Chiari malformation type I, n = 4 mega cisterna magna, n = 4 posterior fossa cyst, n = 3 anterior fossa cyst and n = 1 not specified) and 11 due to poor image quality. From 533 the remaining participants, 12 were excluded due to WMH segmentation failure, rendering a final sample of 521 participants. The mean age of the final sample was 57.7 years and 60.5% were women. Sixty‐four participants (12.3%) were APOE‐ε4 homozygotes, 201 (38.6%) were APOE‐ε4 heterozygotes, and 256 (49.2%) were ε4 allele noncarriers. The median WMH load was 3.2 mL. The main characteristics of the participants are displayed in Table 2.
Table 2.
Characteristics of the study population (N = 521)
| Age, years, mean (SD) (range) | 57.7 (7.4) (44–75 years) |
| Sex, female, no. (%) | 315 (60.5) |
| Education, years, mean (SD) | 13.7 (3.6) |
| Number of APOE‐ε4 alleles, no. (%) | |
| None | 256 (49.2) |
| One APOE‐ ε4 alleles | 201 (38.6) |
| Two APOE‐ ε4 alleles | 64 (12.3) |
| TIV, mL (Q1–Q3) | 1416 (1328–1,490) |
| WMH volume, mL (Q1–Q3) | 3.2 (1.09–3.69) |
| Periventricular WMH volume, mL (Q1–Q3) | 1.23 (0.53–1.55) |
| Deep WMH volume, mL (Q1–Q3) | 1.10 (0.25–1.14) |
| Juxtacortical WMH, mL (Q1–Q3) | 0.68 (0.16–0.75) |
| Cognitive evaluation, mean (SD) | |
| Episodic memory | 0.01 (0.9) |
| Executive function | 0.01 (0.6) |
| Memory Binding Test | |
| Total paired recall (0–32) | 24.1 (4.6) |
| Total free recall (0–32) | 16.5 (5.2) |
| Paired recall pairs (0–16) | 9.2 (3.4) |
| Total delayed free recall (0–32) | 16.9 (5.3) |
| Total delayed paired recall (0–32) | 23.9 (4.7) |
| Pairs in delayed free recall (0–16) | 6.4 (3.1) |
| Semantic proactive interference (%) | 75.3 (18.8) |
| WAIS‐IV subtests | |
| Visual puzzles (0–26) | 13.3 (4.3) |
| Digit span forward (0–16) | 8.5 (2.1) |
| Digit span backward (0–16) | 8.0 (2.1) |
| Digit span sequencing (0–16) | 8.4 (2.1) |
| Matrix reasoning (0–26) | 16.4 (4.3) |
| Similarities (0–36) | 22.6 (4.7) |
| Coding (0–135) | 65.6 (15.1) |
2.2. Cognitive measures
The Memory Binding Test (MBT) (Buschke, 2014) was used to evaluate verbal EM. This test assesses immediate and delayed retention of verbal information (after a lapse of 25–35 min) through a controlled learning process of two lists of 16 words belonging to 16 different semantic categories presented in the same order. Further detail on the administration procedure of the MBT and an exhaustive description of each of the variables can be found in (Gramunt et al., 2015). We analyzed seven MBT main outcomes corresponding to two main areas: learning and immediate recall, and delayed recall. EF was assessed by means of five WAIS‐IV subtests: the digit span (immediate and working memory): forward, backward and sequencing; coding subtest (processing speed); matrix reasoning and visual puzzles (fluid intelligence); and similarities (abstract verbal reasoning).
2.3. MRI acquisition
MRI scans for all participants were acquired on the same 3.0T scanner (GE Discovery MR750 W 3T) using the same protocol that included a T1‐, three T2‐weighted sequences (fluid‐attenuated inversion recovery [FLAIR], fast spin echo [FSE], and gradient echo [GRE]) and diffusion‐weighted imaging (DWI) sequence. The T1‐weighted sequence had an isotropic voxel size of 1 mm3 with a matrix size of 256 × 256 × 160 (TR/TE/TI = 8.0/3.7/450 ms, NSA = 1, flip angle = 8°). T2‐ and T2*‐weighted sequences, with a voxel size of 1 × 1 × 3 mm, were as follows: fluid attenuation inversion recovery (FLAIR: TR/TE/TI = 11,000/90/2600 ms, flip angle = 160°), fast spin echo (TR/TE = 5000/85 ms, flip angle = 110°), and gradient echo (GRE: TR/TE = 1300/23 ms, flip angle = 15°). Finally, DW volumes were acquired with 64 distinct diffusion‐encoding directions (b = 1,000 s mm− 2). The field of view was 256 × 256 mm, and the imaging matrix was 128 × 128 with 56 slices and slice thickness 2 mm, giving 2‐mm isotropic voxels.
2.4. T1‐weighted images processing
The 3D‐T1w images were segmented into GM and WM tissue using the new segment function implemented in Statistical Parametric Mapping software (SPM 12, Wellcome Department of Imaging Neuroscience, London, UK), and located into a common space for subsequent normalization using a 9‐affine parameter transformation. Segmented images were then used to generate a reference template object of the sample, which was warped into a standard Montreal Neurological Institute (MNI) space using the high dimensional DARTEL toolbox (Ashburner, 2007). The generated flow fields and normalization parameters were then implemented to normalize the native GM and WM images to the MNI space. In order to preserve the native local amount of GM as well as WM volume, we applied a modulation step, where each voxel signal's intensity was multiplied by the Jacobian determinants derived from the normalization procedure (Good et al., 2001). Quality control of normalization was assured by checking the sample homogeneity with the computational anatomy toolbox (CAT12) (http://dbm.neuro.uni-jena.de/cat/) using nonsmoothed data, which did not return errors in the registration procedure in any subject. Finally, images were spatially smoothed with an 8 mm full‐width at half maximum (FWHM) Gaussian kernel. Total intracranial volume (TIV) was computed by summing the segmented GM, WM, and CSF for each individual.
2.5. WMH segmentation and quantification
WMH were automatically segmented using a Bayesian algorithm (Sudre et al., 2015) and quality control of this segmentation was performed visually for each participant by a trained rater. In short, T1‐weighted, T2‐weighted and T2‐FLAIR images are rigidly coregistered using the NiftyReg package (Modat et al., 2014). The data is then modeled as a multivariate Gaussian mixture model that simultaneously accounts for healthy tissue and unexpected observations and is constrained by participant‐specific statistical tissue priors derived from the Geodesic Information Flows algorithm (Cardoso et al., 2015). The number of required Gaussian components is dynamically determined on a patient level to ensure a balance between model fit and complexity using the Bayesian Inference Criterion. Once the model has converged, a postprocessing step is applied to extract probability maps of candidate lesion voxels that are then further corrected for spurious false positive detection using the output of the parcellation algorithm to avoid regions prone to artifacts. Volumetric measurements are derived as the sum of this probability map over a region of interest (ROI). WMH volumes were also calculated in periventricular WMH (PVWMH), deep WMH (DWMH), and juxtacortical WMH (JCWMH) as described previously (Sudre et al., 2017).
2.6. DWI processing
The WMH probability maps first registered to T1‐space were then registered to DWI space using ANTs' nonlinear algorithm (Avants, Tustison, & Song, 2009). DWI images were used to generate fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), and radial diffusivity (RD) maps using the FSL Diffusion Toolbox (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). DWI images were normalized to the MNI standard space by coregistering all participants' FA data to a common space using FMRIB's Software Library (http://www.fmrib.ox.ac.uk/fsl) Nonlinear Image Registration Tool. These same parameters were applied to DWI‐space WMH probability maps to warp them to the same MNI‐space group template. Then, DWI parameters were recorded in voxels categorized as WMH as well as in normally appearing white matter (NAWM) in equivalent white matter locations across the group. This last step is explained further in Section 2.7.3.
2.7. Statistical analysis
2.7.1. Cognitive outcomes
First, we computed two global z‐scores for the cognitive measures: EM from MBT and EF from WAIS‐IV subtests. These global measures were calculated by averaging normalized raw scores of all subtests in each domain. Figure S1 shows the cross‐correlation and statistical significance between pairs of cognitive scores.
2.7.2. Mediation analysis
First, we tested the normality distribution of WMH load (Figure S2). Given the great skewness of the distribution of WMH load, which log‐transformation did not solve, all the following analyses were performed with nonparametric techniques.
In the mediation analysis, we defined GMv as the predictor (X), WMH (global and divided by distance to the ventricle; PVWMH, DWMH, and JCWMH, or by lobes) as the mediator (M) and cognitive performance as outcome (Y). See Figure 1 for a schematic representation of the mediation analysis and accompanying definitions. The VBM pairwise and mediation analyses were performed using the M3 Toolbox of SPM (Wager et al., 2008; Wager et al., 2009) with bootstrapping techniques to address the non‐normal distribution of WMH volumes. This method partitions the variance shared by X and Y (total effect, denoted as c) into two components: one mediated by M (indirect effect; ab) and another one independent from M (direct effect; c′). Cognitive performance was analyzed by both global z‐scores (EM and EF) and all the outcomes of MBT and WAIS‐IV individually. Age, sex, education, TIV, and APOE (number of ε4 alleles) were introduced as confounders in the VBM analysis adjusting all the paths associations between mediators and outcomes.
Figure 1.
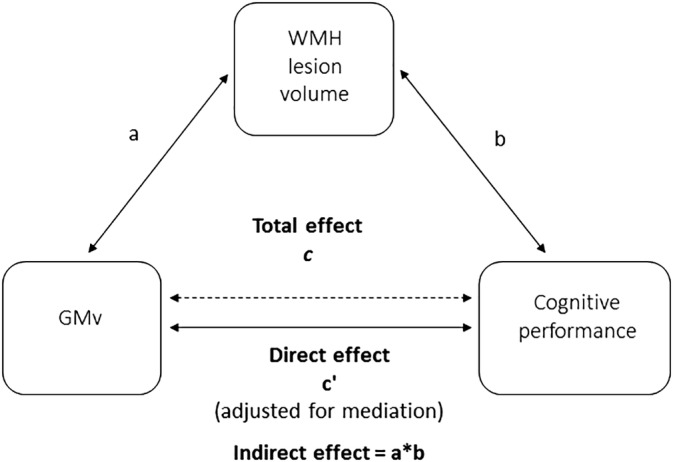
Schematic illustration of the mediation statistical model running in the study. All paths were adjusted for age, sex, education, TIV, and number of APOE‐ε4 alleles. GMv, gray matter volume; TIV, total intracranial volume; WMH, white matter hyperintensities
We studied all the relationships or paths as they all give some relevant information. First, we looked at the total association between GMv and cognition (total effect; c) by the VBM analysis. Secondly, we evaluated pairwise associations between GMv‐WMH burden (path a) and WMH burden‐cognition (path b) independently. The correlation between WMH burden and cognition (path b) was the only path not to include GMv, for this reason we tested it independently with Spearman's rank test (divided by TIV and adjusting for the same covariates as in the rest of the analysis). Next, we tested the mediation effect of the WMH load on the association between GMv and cognition (path ab). And, finally, we assessed the association between GMv and cognition after removing the mediation effect (direct effect; c′). Statistical significance threshold was set to p < .005. This threshold is the one most commonly used with the bootstrapping implementation in the M3 toolbox and provides a good balance between control of false positives and sensitivity (Wager et al., 2008; Wager et al., 2009). For total, direct and indirect effects we computed the mean effect size dividing the β coefficients by standard deviation within the areas that showed a significant mediation effect. Afterward, the proportion mediated was calculated; (being ∧ the absolute value) (Fleming & DeMets, 1996).
For all paths that included GMv, we only reported as significant areas those that were bigger than 5% of the ROI from Neuromorphometrics atlas, and that included more than 100 voxels. The maximum Z effect was computed for each significant brain regions.
2.7.3. DWI parameters
To better characterize WMH in our sample, we compared DWI parameters (FA, MD, AxD, and RD) between WMH and NAWM in the same brain locations. In order to restrict the subsequent analysis only to voxels with the highest class probability (of being WMH or NAWM) and to ensure that interclass comparisons would be performed on sufficient participants across the group, we filtered voxels based on the two following rules. First, voxels with a probability of being WMH higher than 0.9 (lower than 0.1) were classified as WMH (NAWM, respectively). Voxels with probabilities between 0.1 and 0.9 were left out of this analysis. Then, for every voxel in white matter, we counted the numbers of participants in which the voxel was classified either as a WMH or as NAWM. Voxels with a minimum of 10 participants classified as WMH and a minimum of 10 classified as NAWM were included in the final mask. In these voxels only, values of the DWI parameters were compared between WMH and NAWM using t test. Differences in DWI parameters in WMH tissue were calculated as the percentage of change with respect NAWM: (DWIWMH‐DWINAWM)/ DWINAWM; and a two‐sample t test and a threshold for significance of p < .05 were carried out.
2.7.4. Additional analysis
As complementary analyses, we replicated the mediation analysis defining WMH as predictor (X) and GM volume as mediator (M), after adjusting by the previously described confounders. Finally, for total, direct and indirect effects we computed the effect size of significant regions in which the mediation effect (ab) was significative (p < .005) in the main model for comparability.
3. RESULTS
3.1. Pairwise associations
3.1.1. GM versus WMH load (path a)
We found that a greater WMH lesion volume was associated with lower GMv (Figure 2) in specific brain regions, mainly in the temporal and in frontal areas (Table S2). The regions with greater ROI percentage of this direct effect were the bilateral nucleus accumbens (R = 59.0%, L = 55.98%), right amygdala (55.02%), and right caudate nucleus (48.41%).
Figure 2.
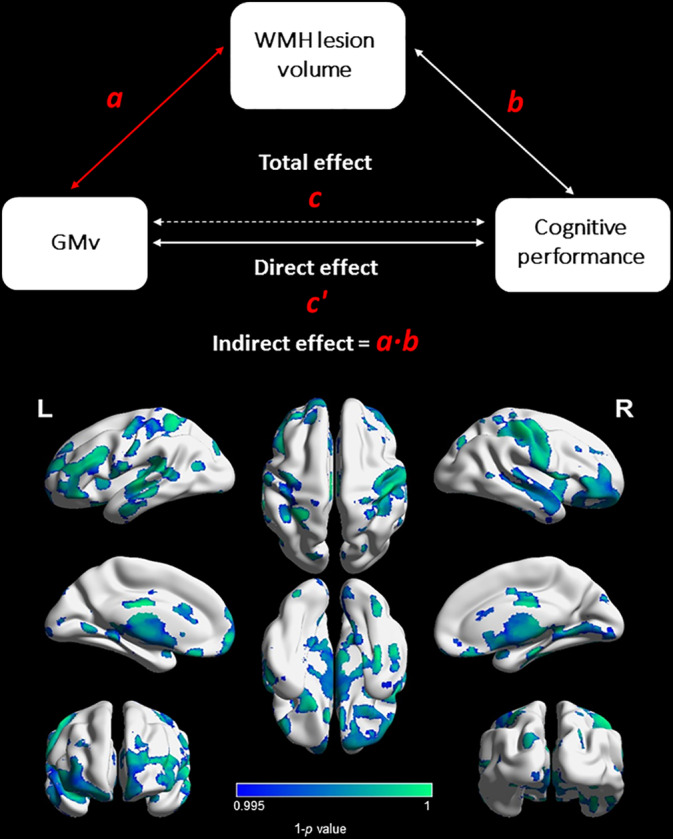
Association between GM volume and WMH burden (path a). A greater WMH lesion volume was associated with lower GMv in specific brain regions, mainly in parietal, temporal, and frontal areas. Cold color bar = negative relationship. GMv, gray matter volume; WMH, white matter hyperintensities
3.1.2. WMH load versus cognition (path b)
Global WMH was significantly associated with lower performance on EM (Rho = −0.07, p = 0.04) and EF (Rho = −0.07, p = 0.04) (Table 3). Some subtests showed also a significant correlation with WMH: digit span backwards (DSBs) (Rho = −0.09, p = 0.01), total delayed free recall (Rho = −0.07, p = 0.04), total delayed paired recall (Rho = −0.07, p = 0.04), pairs in delayed free recall (Rho = −0.08, p = 0.03) and semantic proactive interference (Rho = −0.08, p = 0.03). Spearman's rho values for correlations between regional WMH load divided distance to the ventricles and cognition are shown in Table S1.
Table 3.
Correlation between global WMH load and cognitiona
| Rho [95% CI] | p | |
|---|---|---|
| Cognitive z‐score composites | ||
| Episodic memory | −0.07 [−0.156 to 0.001] | 0.04* |
| Executive function | −0.07 [−0.152 to 0.012] | 0.04* |
| Memory Binding Test | ||
| Total free recall | −0.05 [−0.131 to 0.029] | 0.12 |
| Total delayed free recall | −0.07 [−0.156 to 0.001] | 0.04* |
| Total paired recall | −0.05 [−0.130 to 0.037] | 0.14 |
| Total delayed paired recall | −0.07 [−0.155 to −0.001] | 0.04* |
| Paired recall pairs | −0.06 [−0.146 to 0.019] | 0.07 |
| Pairs in delayed free recall | −0.08 [−0.166 to −0.001] | 0.03* |
| Semantic proactive interference | −0.08 [−0.161 to 5.5e−05] | 0.03* |
| Subtests of WAIS‐IV | ||
| Digit span forward | −0.01[−0.090 to 0.079] | 0.42 |
| Digit span backward | −0.09 [−0.174 to −0.001] | 0.01* |
| Digit span sequencing | 0.03 [−0.056 to 0.110] | 0.28 |
| Coding | −0.06 [−0.135 to 0.023] | 0.08 |
| Visual puzzles | −0.01 [−0.095 to 0.070] | 0.39 |
| Matrix reasoning | −0.05 [−0.129 to 0.031] | 0.12 |
| Similarities | −0.06 [−0.143 to 0.024] | 0.08 |
Cognition adjusted by age, sex, education and number of APOE‐ε4 alleles. WMH also adjusted by TIV.
*p < .05.
3.1.3. GM vs cognition (Total effect; path c)
A significant association between GMv and cognition was found with nonoverlapping patterns linked to EF and EM (Figure 3). We found a significant total effect between GMv and EF (effect size = 0.821), but not in EM (Table 4).
Figure 3.
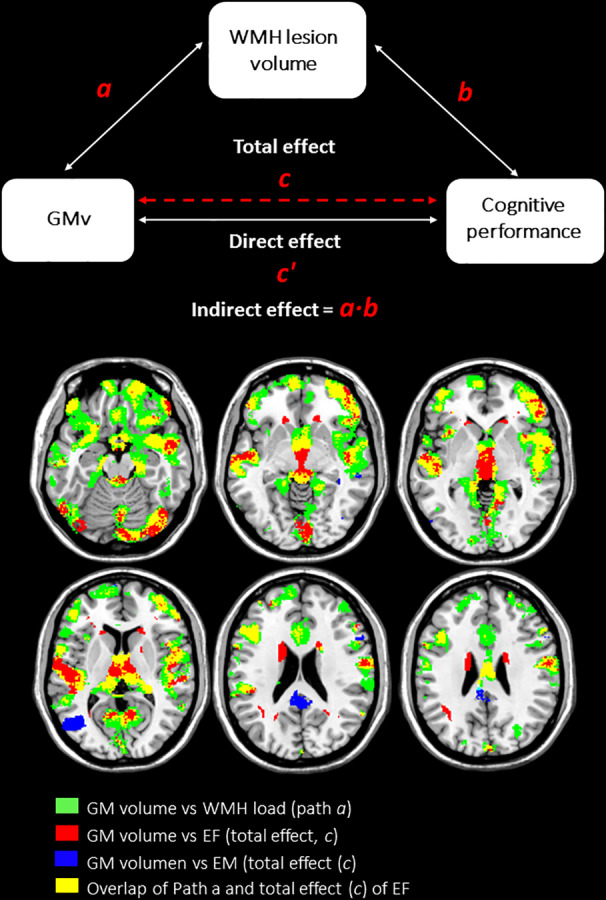
Regions with significant WMH mediation effect on EF performance. WMH load mediate the relationship between GM volume and brain regions involved in EF performance. Mainly in frontal and temporal regions, the indirect effect (path ab) is overlapped with the regions with significant GM volume‐WMH load association. The statistical significance was set at p < .005. The paths were adjusted for mediator‐outcomes confounders: age, sex, education, TIV, and number of APOE‐ε4 alleles. EF, executive function; EM, episodic memory; GMv, gray matter volume; WMH, white matter hyperintensities
Table 4.
Effect sizes of total (c), direct (c′), and mediated (ab) paths within regions with significant mediation effect (p < .005)
| Total effect (c) | Direct effect (c′) | Indirect effect (ab) | PMa | |
|---|---|---|---|---|
| Effect size | Effect size | Effect size | % | |
| Cognition composites | ||||
| Episodic memory | NS | NS | NS | – |
| Executive function | 0.821 | 0.441 | 2.020 | 82.0 |
| Executive function outcomes | ||||
| Coding | 0.378 | 0.019 | 2.010 | 99.1 |
| Digit span backward | −0.300 | −0.670 | 1.958 | IMMb |
| Matrix reasoning | −0.951 | −1.375 | 1.901 | IMMb |
| Similarities | −1.149 | −1.580 | 2.052 | IMMb |
| Mediation effect of WMH on EF by distance to ventricles | ||||
| PVWMH | 0.188 | −0.194 | 2.126 | IMMb |
| DWMH | 1.102 | 0.728 | 1.976 | 68.1 |
| JCWMH | −1.208 | −1.399 | 1.367 | IMMb |
Abbreviations: PVWM, periventricular white matter hyperintensities; DWMH, deep white matter hyperintensities; JCWMH, juxtacortical white matter hyperintensities; PM, percentage of mediation; NS, nonsignificant; IMM, inconsistent mediation model.
PM (proportion of mediation) = (Fleming & DeMets, 1996).
IMM, inconsistent mediation model (MacKinnon, Krull, & Lockwood, 2000).
3.2. Mediation effects across GMv and cognitive performance through WMH load
3.2.1. Indirect effect (ab)
We found a significant partial mediation effect (effect size = 2.020) of WMH volume in the relationship between GMv and EF performances (Figure 4). This significant mediation effect was mainly driven by coding, which displayed an effect size (effect size = 2.010) that was one order of magnitude higher than the rest of outcomes; about 99.1% of the total effect of GMv on coding was mediated by WMH load (Table 4, Figure 4 and Figure S6). Specifically, in temporal and frontal regions was mainly driven by the mediation effects (ab) of DWMH (Table S4 and Figure S3). The significant brain regions with greater ROI percentage of mediation effect were right frontal regions (orbital gyrus: anterior, 87.6%; medial, 57.1%; lateral, 87.6%; and posterior, 61.8%) and left frontal pole (76.9%), but also right accumbens area (68.0%) and right planum polare (48.6) (Table S3). On the other hand, we did not find a significant mediation effect of WMH in GMv of regions involved in EM performance (Figure S4).
Figure 4.
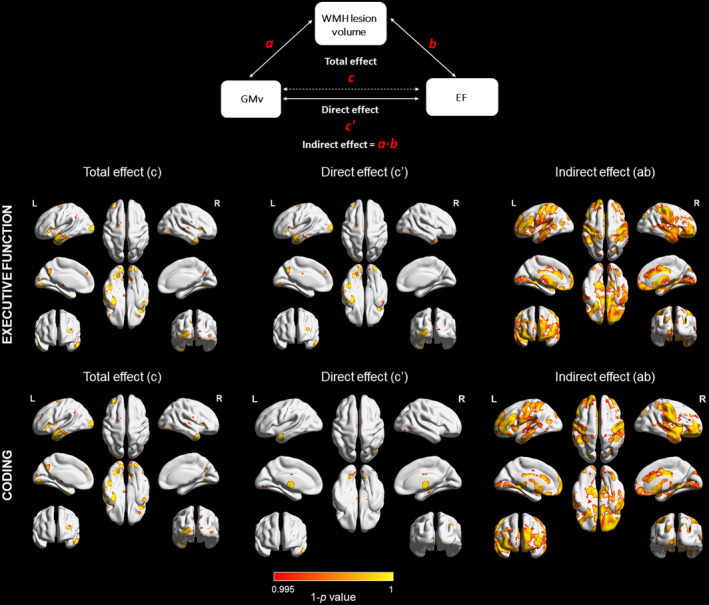
Mediation results from EF and coding performance. Total effect (path c): GMv‐coding relationship. Direct effect (path c′): effect of GMv in cognitive domain performance after removing the mediation effect. Indirect effect (path ab): mediation effect across GMv and EF and coding performance through WMH load. After accounting for the mediation effect, a direct significant association (path c′) remained between GMv in the temporal pole, inferior temporal and the insular in coding. Statistical significance was set at p < .005. Paths were adjusted for mediator‐outcomes confounders: age, sex, education, TIV, and number of APOE‐ε4 alleles. Hot color bar = positive relationship. GMv, gray matter volume; WMH, white matter hyperintensities
When we repeated the mediation analysis for EF including as mediator WMH as distance to the ventricle, we found that the indirect effect of WMH on EF through GMv showed more significant regions for DWMH (effect size = 1.976) than for PVWMH (effect size = 2.126) and JCWMH (effect size = 1.367) (Figure S3). Specifically, about 68.1% of the total effect of GMv on EF was mediated by DWMH load mainly in temporal and frontal regions (Table 4).
Regarding other specific cognitive tests, we found a significant mediation effect of WMH in DSB (effect size = 1.958) (Figure S5), matrix reasoning (effect size = 1.901), and similarities (effect size = 2.052) (Table 4). The results of PVWMH and JCWMH mediation effect on DSB, matrix, and similarities performance showed inconsistent mediation models (MacKinnon et al., 2000).
3.2.2. Direct effect (c′)
After discounting the mediation effect, a direct significant association remained between GMv and EF performance in the temporal pole, inferior temporal and the insulae (effect size = 0.441) (Figure 4). The total effect found between GMv and EM remained significant in the same locations as direct effect (Figure S4).
3.3. WMH characterization by DWI parameters
The mean percentage of change of DWI metrics compared for all voxels in equivalent brain locations between WMH and NAWM is represented in Figure 5. WMH showed lower FA (−8.46%; 95%CI: [−7.84%, −9.08%]; p < .001) and increased diffusivity, particularly in the radial direction (MD: +4.40%; 95% CI: [3.68%, 5.12%]; p < .001; AxD: +1.40%; 95% CI: [0.82%, 1.97%]; p < .001; RD: +7.08%; 95% CI: [6.23%, 7.93%]; p < .001).
Figure 5.
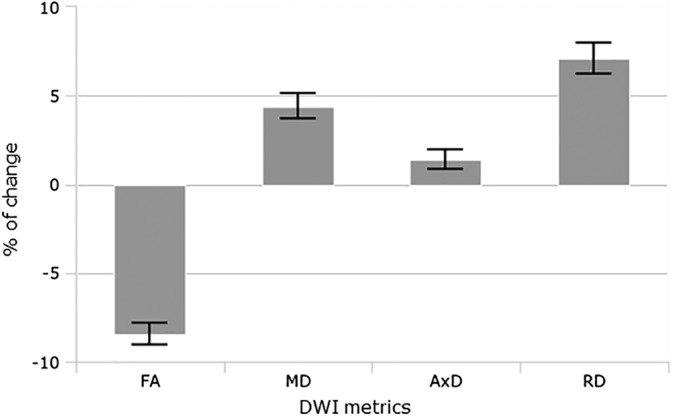
Mean percentage of change of DWI metrics compared in equivalent brain locations between WMH and NAWM. WMH showed significant lower FA and increased diffusivity. RD shows significantly larger changes than in AxD in WMH. All the differences were statistically significant at p < .001. Error bars show 95% of CI. AxD, axial diffusivity; DWI, diffusion‐weighted imaging; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity
3.4. Additional analysis
In the mediation analysis defining WMH as predictor (X) and GMv as mediator (M) (Figure 6), we did not find a GMv mediation effect across the relationship between WMH load and EF performance (effect size = −0.459) ( Table 5).
Figure 6.
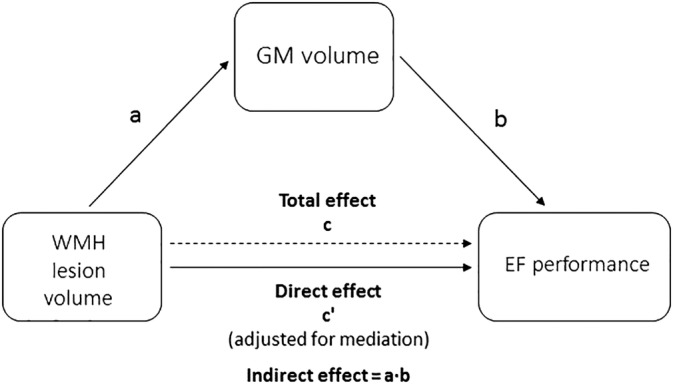
Schematic illustration of the alternative mediation model All paths were adjusted for age, sex, education, TIV, and number of APOE‐ε4 alleles. GMv, gray matter volume; TIV, total intracranial volume; WMH, white matter hyperintensities
Table 5.
Effect sizes of total (c), direct (c′), and mediated (ab) paths within regions with significant mediation effect (p < .005) of the alternative mediation model (X = WMH volume; M = GMv; Y = EF)
| Total effect (c) | Direct effect (c′) | Indirect effect (ab) | PM | |
|---|---|---|---|---|
| Effect size* | Effect size* | Effect size* | % | |
| Executive function | −2.854 | −2.731 | −0.459 | 14.4 |
Abbreviations: PM, proportion of mediation = (Fleming & DeMets, 1996).
*Effect size in significant brain regions in which the mediation effect (ab) was significant (p < .005) in the main model.
4. DISCUSSION
In this work, we have studied whether WMH volume mediates the relationship between GMv and cognition in cognitively unimpaired middle‐aged participants to extend previous findings in older populations with additional comorbidities (Knopman et al., 2015; Rizvi et al., 2018; Swardfager et al., 2018; Tuladhar et al., 2015), to a younger and healthier cohort both from the cognitive and cerebrovascular standpoint. Our results show that, even in such a low‐risk population, higher WMH lesion volume is significantly associated with a widespread pattern of lower GMv in frontal, occipital, and temporal regions, including the hippocampus, as well as in the thalamus and cerebellum. In turn, WMH volume mediates the relationship between GMv and EF in frontal and temporal regions, including the hippocampus, as well as in the thalamus. Specifically, the most significant WMH mediating effect was found for processing speed as measured by the Coding subtest. Concerning EM, even though we found significant relationship between GMv and WMH in regions involved in this cognitive domain, such as hippocampus, we did not find a significant mediation effect. This result is in line with previous work (Cacciaglia, Molinuevo, Sánchez‐Benavides, et al., 2018) suggesting that this area shows a relatively low involvement in MBT performance.
We found that WMH showed significantly low FA values and an increased diffusivity compared with NAWM in equivalent brain locations. In addition, the WMH changes in RD were significantly larger than in AxD compared with NAWM. These results may reflect a reduction of WM microstructural organization in these brain locations being the results compatible with axonal loss and/or demyelination (Alexander, Lee, Lazar, & Field, 2007; Scholz, Tomassini, & Johansen‐Berg, 2009; Song et al., 2002; Zhang, Aggarwal, & Mori, 2012). Taken together, there may be several mechanistic explanations for our findings. On the one hand, ischemic injury to the axons might impair the connectivity of distal cortical and subcortical regions and eventually lead to reduced GMv and/or neuronal loss. On the other hand, there is also ample evidence that WMH may be the result of degenerative axonal loss secondary to neuronal damage due to Wallerian degeneration. Alternatively, ischemia could be leading to both vascular brain injury and neurodegeneration. Even though implicit in the mediation pathway model, analysis of cross‐sectional data cannot establish a causal link between the studied variables. In addition, the interpretation of the microstructural alterations leading to altered DWI parameters should be taken with caution as they can be related to multiple causes (Scholz et al., 2009). Previous studies in disease and in model systems suggested that changes in DWI metrics are unspecific and sensitive to a variety of biological phenomena (Alexander et al., 2007; Melhem et al., 2002; Song et al., 2002; Zhang et al., 2012).
For mediation to be considered as significant, paths a, b, and ab are required to show significant associations (MacKinnon et al., 2007). These requirements are met in our own findings and are also supported by previous literature. We found that greater WMH volume related to lower GMv in extensive brain regions comprising temporal, frontal and occipital areas, thalamus and cerebellum. Previous studies showed a relationship between WMH and global GM atrophy also in the temporal (Habes et al., 2016; Swardfager et al., 2018), and frontal cortex (Raji et al., 2012), and in the bilateral hippocampus (Habes et al., 2016). However, to the best of our knowledge, no previous research has looked at for the joint impact of both WMH and GMv on cognition using voxel‐wise approach (Rizvi et al., 2018; Wen et al., 2006). Regarding path c, we had previously established in this cohort that EM and EF rely on nonoverlapping cerebral structural networks (Cacciaglia, Molinuevo, Sánchez‐Benavides, et al., 2018).
In the present study, the mediation analysis (path ab) showed a significant indirect effect of GMv on EF performance through WMH volume regionally, such as in frontal and temporal areas. Particularly, we found that the behavior of the EF composite was mainly driven by the indirect effects (ab) of DWMH, particularly for processing speed. These results are consistent with an earlier study that found that processing speed was partially mediated by posterior cortical regions atrophy, such as hippocampus, parahippocampus gyrus, and entorhinal cortex (Knopman et al., 2015). In line with this, we found significant mediation effects in regions belonging to the executive control network, such as the dorsolateral prefrontal cortex, the anterior cingulate gyrus (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), and the thalamus (Marzinzik et al., 2008). Regarding the regional pattern of the WMH mediating effect, it was more significant for deep than in periventricular WMH. Previous literature has determined that WMH progression extends from the PVWMH to DWMH in subcortical white matter (Prins & Scheltens, 2015). DWMH is functionally more relevant (Wen et al., 2006) and related to reduced cognitive function than PVWMH, which is in line with our findings.
Previous cross‐sectional studies in older nondemented samples (Knopman et al., 2015) or including MCI and AD patients (Rizvi et al., 2018; Swardfager et al., 2018) that used mediation pathway analysis selected WMH as predictor, whose association with cognition was mediated by GMv. For comparability with these previous reposts, we built an alternative mediation model with GMv as the mediator of the association between WMH load and cognitive performance (Figure 6). In this alternative model, we could not find a significant mediating effect of GMv (in the same regions where the mediating effect of WMH was significant in the main model) in the relationship between WMH load and EF performance (Table 5). While in our main model the indirect effect (path ab) could account for most of the total observed effect (path c), this was not the case for the alternative model. Given that path a is identical in both models, this indicates that path b in our main model (i.e., WMH volume after accounting for the effect of GMv) showed a higher effect size (effect size of path ab of the main model: 0.2020) that could explain a significantly higher percentage of the total effect (percentage of mediation path ab of the main model: 82.0%) than path b in the alternative model (i.e., GMv after accounting for WMH) with an effect size of (−0.459) that only explained 14.4% of the total effect (Table 5). The comparison of the results of both models suggests that WMH has an independent effect on EF which cannot be accounted by GMv in middle‐aged cognitively intact individuals with low WMH burden. On the other hand, the previous results in older and/or cognitively impaired individuals indicate that in these individuals with a higher WMH burden, GMv exerts an additional effect of cognitive function that is independent of WMH burden. This may also explain why, in contrast to previous findings (Rizvi et al., 2018; Swardfager et al., 2018), we did not find a significant mediation effect with EM performance in our main model. An additional factor that could explain such a discrepancy might be the test that we used to measure EM (MBT), whose characteristics may differ from those of the other memory tests used in similar reports. We previously showed that, in cognitively unimpaired middle‐aged participants, better EM performance was significantly associated with lower GMv in several brain regions modulated by aging (Cacciaglia, Molinuevo, Sánchez‐Benavides, et al., 2018). This result is consistent with earlier research in which WMH are mainly related to frontal‐type dysfunction including impairments in attention, and processing speed (Desmond, 2002; Kloppenborg et al., 2014).
One of the main strengths of this study is that, even though our participants are younger and cognitively unimpaired with a lower WMH burden than in previous studies, we found a strong impact of WMH on GMv, which, in turn, also affected executive functioning. This result provides additional evidence to support the early control of modifiable risk factors in middle‐aged individuals as a useful preventive strategy to reduce or delay the onset of dementia. Another methodological strength of our work is the use of a voxel‐wise mediation analysis which allowed us to detect topographical patterns of GMv in an unbiased fashion compared with previous studies that used ROI‐based approaches.
Our work is not free from limitations. The highly non‐normal distribution of WMH load, due to the high percentage of WMH‐free participants, prevented us from using parametric statistics. Even though the bootstrap method implemented in the M3 toolbox is robust against deviations from normality, the use of a nonparametric test is expected to have lowered the statistical power in this study. Another limitation is the lack of core AD biomarkers. Prior research suggested that WMH load might interact with the hallmarks of AD pathology, such as abnormal deposition of β‐amyloid (Aβ) (Gold et al., 2017) and tau‐protein (McAleese et al., 2017) in cognitively unimpaired participants. Therefore, the study of the impact of core AD pathology on the mediation effects here described represents an important topic for future research.
5. CONCLUSION
In middle‐aged cognitively unimpaired participants we found that higher WMH lesion volume was significantly associated with a widespread pattern of lower GMv in temporal, frontal and occipital areas as well as in the thalamus and cerebellum. WMH volume significantly mediated the association between processing speed, and GMv in extensive brain regions encompassing frontal and temporal regions, including the thalamus. Concerning EM, we did not find a significant mediation effect. This study provides novel evidence of the impact of WMH on GM and cognition even in a healthy middle‐aged population. In light of these results, the control of modifiable risk factors in individuals at higher risk of developing WMH might represent a valuable preventive strategy to reduce or delay the onset of cognitive decline.
DISCLOSURES
Research conducted by José Luis Molinuevo receives support from: the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD grant agreement #115952; the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement #115736 and the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AETIONOMY grant #115568. He is/has been a scientific consultant and/or attended scientific advisory boards of Alergan, Roche diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, Raman Health.
Research conducted by Frederik Barkhof receives support from AMYPAD (IMI); EuroPOND (H2020); UK MS Society; Dutch MS Society; PICTURE (IMDI‐NWO); NIHR UCLH; Biomedical Research Centre (BRC) and ECTRIMS‐MAGNIMS. He has received consultancy fees from Bayer Pharma, Biogen‐IDEC, TEVA, Merck, Novartis, Roche, Jansen Research, Genzyme‐Sanofi, IXICO Ltd, GeNeuro and Apitope Ltd.
The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGMENTS
This publication is part of the ALFA study (Alzheimer and Families). The authors would like to express their most sincere gratitude to the ALFA project participants, without whom this research would have not been possible. With recognition and heartfelt gratitude to Mrs. Blanca Brillas for her outstanding and continued support to the Pasqual Maragall Foundation to make possible a Future without Alzheimer's. Collaborators of the ALFA study are: Alba Cañas, Marta Crous‐Bou, Carme Deulofeu, Ruth Dominguez, Marta Félez‐Sánchez, José M. González de Echevarri, Xavi Gotsens,Laura Hernandez, Gema Huesa, Jordi Huguet, María León, Paula Marne, Eider M. Arenaza‐Urquijo, Tania Menchón, Marta Milà‐Alomà, Maria Pascual, Albina Polo, Sandra Pradas, Aleix Sala‐Vila, Mahnaz Shekari, Anna Soteras, Laia Tenas, Marc Vilanova and NataliaVilor‐Tejedor.
Brugulat‐Serrat A, Salvadó G, Operto G, et al. White matter hyperintensities mediate gray matter volume and processing speed relationship in cognitively unimpaired participants. Hum Brain Mapp. 2020;41:1309–1322. 10.1002/hbm.24877
Anna Brugulat‐Serrat, Gemma Salvadó contributed equally to the manuscript
The complete list of collaborators of the ALFA Study can be found in the acknowledgments section
Funding information Alzheimer's Society, Grant/Award Number: AS‐JF‐17_011; European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska‐Curie action, Grant/Award Number: 752310; Instituto de Salud Carlos III, Grant/Award Number: PI12/00326; La Caixa Foundation, Grant/Award Number: LCF/PR/GN17/10300004; NIHR UCLH biomedical research centre, Grant/Award Number: NA; Secretaría de Estado de Investigación, Desarrollo e Innovación, Grant/Award Numbers: IEDI‐2016‐00690, RYC‐2013‐13054; EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD, Grant/Award Numbers: 115568, #115736, #115952
Contributor Information
José L. Molinuevo, Email: jlmolinuevo@barcelonabeta.org.
Juan D. Gispert, Email: jdgispert@barcelonabeta.org.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4, 316–329. http://www.ncbi.nlm.nih.gov/pubmed/17599699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis, Z. , Fleischman, D. A. , Arfanakis, K. , Leurgans, S. E. , Barnes, L. L. , & Bennett, D. A. (2016). Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Structure & Function, 221, 2135–2146. http://surfer.nmr.mgh.harvard.edu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. http://www.ncbi.nlm.nih.gov/pubmed/17761438 [DOI] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. , & Song, G. (2009). Advanced normalization tools (ANTS). Insight J, 2, 1–35. http://www.picsl.upenn.edu/ANTS [Google Scholar]
- Bastin, M. E. , Clayden, J. D. , Pattie, A. , Gerrish, I. F. , Wardlaw, J. M. , & Deary, I. J. (2009). Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiology of Aging, 30, 125–136. https://www.sciencedirect.com/science/article/pii/S0197458007002126 [DOI] [PubMed] [Google Scholar]
- Birdsill, A. C. , Koscik, R. L. , Jonaitis, E. M. , Johnson, S. C. , Okonkwo, O. C. , Hermann, B. P. , … Bendlin, B. B. (2014). Regional white matter hyperintensities: Aging, Alzheimer's disease risk, and cognitive function. Neurobiology of Aging, 35, 769–776. http://www.ncbi.nlm.nih.gov/pubmed/24199958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolandzadeh, N. , Davis, J. C. , Tam, R. , Handy, T. C. , & Liu‐Ambrose, T. (2012). The association between cognitive function and white matter lesion location in older adults: A systematic review. BMC Neurology, 12, 1 http://www.biomedcentral.com/1471-2377/12/126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman, A. M. , Schupf, N. , Manly, J. J. , Stern, Y. , Luchsinger, J. A. , Provenzano, F. A. , … Portet, F. (2014). APOE ε4 and risk for Alzheimer's disease: Do regionally distributed white matter hyperintensities play a role? Alzheimer's Dement, 10, 619–629. http://linkinghub.elsevier.com/retrieve/pii/S1552526014027678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugulat‐Serrat, A. , Rojas, S. , Bargalló, N. , Conesa, G. , Minguillón, C. , Fauria, K. , … Gispert, J. D. (2017). Incidental findings on brain MRI of cognitively normal first‐degree descendants of patients with Alzheimer's disease: A cross‐sectional analysis from the ALFA (Alzheimer and families) project. BMJ Open, 7, e013215 http://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2016-013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugulat‐Serrat, A. , Salvadó, G. , Sudre, C. H. , Grau‐Rivera, O. , Suárez‐Calvet, M. , Falcon, C. , … Gispert, J. D. (2019). Patterns of white matter hyperintensities associated with cognition in middle‐aged cognitively healthy individuals. Brain Imaging and Behavior. http://link.springer.com/10.1007/s11682-019-00151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke, H. (2014). The rationale of the Memory Binding Test. In Dementia and Memory, Nilsson LG, Ohta N, eds. Psychology Press, New York, pp. 55–71. [Google Scholar]
- Cacciaglia, R. , Molinuevo, J. L. , Falcón, C. , Brugulat‐Serrat, A. , Sánchez‐Benavides, G. , Gramunt, N. , … Gispert, J. D. (2018). Effects of APOE‐ε4 allele load on brain morphology in a cohort of middle‐aged healthy individuals with enriched genetic risk for Alzheimer's disease. Alzheimer's Dement, 14, 902–912 http://linkinghub.elsevier.com/retrieve/pii/S155252601830044X [DOI] [PubMed] [Google Scholar]
- Cacciaglia, R. , Molinuevo, J. L. , Sánchez‐Benavides, G. , Falcón, C. , Gramunt, N. , Brugulat‐Serrat, A. , … Gispert, J. D. (2018). Episodic memory and executive functions in cognitively healthy individuals display distinct neuroanatomical correlates which are differentially modulated by aging. Human Brain Mapping, 39, 4565–4579. http://doi.wiley.com/10.1002/hbm.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, M. J. , Modat, M. , Wolz, R. , Melbourne, A. , Cash, D. , Rueckert, D. , & Ourselin, S. (2015). Geodesic information flows: Spatially‐variant graphs and their application to segmentation and fusion. IEEE Transactions on Medical Imaging, 34, 1976–1988. [DOI] [PubMed] [Google Scholar]
- Christiane, R. , Ming‐Xin, T. , Schupf, N. , Manly, J. J. , Richard, M. , & Luchsinger, J. A. (2010). A summary risk score for the prediction of Alzheimer disease in elderly persons. Archives of Neurology, 67, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder, E. H. , Saunders, A. M. , Strittmatter, W. J. , Schmechel, D. E. , Gaskell, P. C. , Small, G. W. , … Pericak‐Vance, M. A. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science, 261, 921–923 http://www.ncbi.nlm.nih.gov/pubmed/8346443 [DOI] [PubMed] [Google Scholar]
- de Groot, J. C. , de Leeuw, F. E. , Oudkerk, M. , van Gijn, J. , Hofman, A. , Jolles, J. , & Breteler, M. M. (2000). Cerebral white matter lesions and cognitive function: The Rotterdam scan study. Annals of Neurology, 47, 145–151 http://www.ncbi.nlm.nih.gov/pubmed/10665484 [DOI] [PubMed] [Google Scholar]
- Desmond, D. W. (2002). Cognition and white matter lesions. Cerebrovascular Diseases, 13(Suppl. 2), 53–57 https://www.karger.com/Article/FullText/49151 [DOI] [PubMed] [Google Scholar]
- Dong, C. , Nabizadeh, N. , Caunca, M. , Cheung, Y. K. , Rundek, T. , Elkind, M. S. V. , … Wright, C. B. (2015). Cognitive correlates of white matter lesion load and brain atrophy: The northern Manhattan study. Neurology, 85, 441–449 http://www.ncbi.nlm.nih.gov/pubmed/26156514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll, I. , Davatzikos, C. , An, Y. , Wu, X. , Shen, D. , Kraut, M. , & Resnick, S. M. (2009). Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology, 72, 1906–1913 http://www.ncbi.nlm.nih.gov/pubmed/19487648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, A. , Hayasaka, S. , Du, A. , Schuff, N. , Jahng, G. H. , Kramer, J. , … Weiner, M. (2006). Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer's disease. Neuroscience Letters, 406, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer, L. A. , Cupples, L. A. , Haines, J. L. , Hyman, B. , Kukull, W. A. , Mayeux, R. , … van Duijn, C. M. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer disease meta analysis consortium. JAMA, 278, 1349–1356 http://www.ncbi.nlm.nih.gov/pubmed/9343467 [PubMed] [Google Scholar]
- Fleming, T. R. , & DeMets, D. L. (1996). Surrogate end points in clinical trials: Are we being misled? Annals of Internal Medicine, 125, 605–613 http://www.ncbi.nlm.nih.gov/pubmed/8815760 [DOI] [PubMed] [Google Scholar]
- Gold, B. T. , Brown, C. A. , Hakun, J. G. , Shaw, L. M. , Trojanowski, J. Q. , & Smith, C. D. (2017). Clinically silent Alzheimer's and vascular pathologies influence brain networks supporting executive function in healthy older adults. Neurobiology of Aging, 58, 102–111 http://www.ncbi.nlm.nih.gov/pubmed/28719854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. A. , Friston, K. J. , & Frackowiak, R. S. J. (2001). A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Gramunt, N. , Buschke, H. , Sánchez‐Benavides, G. , Lipton, R. B. , Peña‐Casanova, J. , Diéguez‐Vide, F. , … Garre‐Olmo, J. (2015). Reference data of the Spanish memory binding test in a midlife population from the ALFA STUDY (Alzheimer's and family). Journal of Alzheimer's Disease, 48, 613–625. [DOI] [PubMed] [Google Scholar]
- Habes, M. , Erus, G. , Toledo, J. B. , Zhang, T. , Bryan, N. , Launer, L. J. , … Davatzikos, C. (2016). White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain, 139, 1164–1179 http://www.ncbi.nlm.nih.gov/pubmed/26912649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaks, G. J. , Gansevoort, R. T. , van der Knaap, A. M. , Navis, G. , Dullaart, R. P. F. , & Slaets, J. P. J. (2011). The association of APOE genotype with cognitive function in persons aged 35 years or older. Ed. Rochelle E. Tractenberg. PLoS One, 6, e27415 http://dx.plos.org/10.1371/journal.pone.0027415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R. , Wiste, H. J. , Weigand, S. D. , Knopman, D. S. , Vemuri, P. , Mielke, M. M. , … Petersen, R. C. (2015). Age, sex, and APOE ε4 effects on memory, brain structure, and β‐amyloid across the adult life span. JAMA Neurology, 72, 511 http://archneur.jamanetwork.com/article.aspx?doi=10.1001/jamaneurol.2014.4821–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil, T. , Wolf, P. A. , Beiser, A. , Massaro, J. , Seshadri, S. , D'Agostino, R. B. , & DeCarli, C. (2004). Stroke risk profile predicts white matter hyperintensity volume: The Framingham study. Stroke, 35, 1857–1861 http://www.ncbi.nlm.nih.gov/pubmed/15218158 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62, 782–790 http://www.ncbi.nlm.nih.gov/pubmed/21979382 [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Paradise, M. , Liu, T. , Armstrong, N. J. , Zhu, W. , Kochan, N. A. , … Wen, W. (2018). The association of regional white matter lesions with cognition in a community‐based cohort of older individuals. NeuroImage Clinical, 19, 14–21 https://linkinghub.elsevier.com/retrieve/pii/S2213158218301050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto, M. , Ngandu, T. , Laatikainen, T. , Winblad, B. , Soininen, H. , & Tuomilehto, J. (2006). Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population‐based study. Lancet Neurology, 5, 735–741 http://www.ncbi.nlm.nih.gov/pubmed/16914401 [DOI] [PubMed] [Google Scholar]
- Kloppenborg, R. P. , Nederkoorn, P. J. , Geerlings, M. I. , & van den Berg, E. (2014). Presence and progression of white matter hyperintensities and cognition: A meta‐analysis. Neurology, 82, 2127–2138 http://www.ncbi.nlm.nih.gov/pubmed/24814849 [DOI] [PubMed] [Google Scholar]
- Knopman, D. S. , Griswold, M. E. , Lirette, S. T. , Gottesman, R. F. , Kantarci, K. , Sharrett, A. R. , … ARIC Neurocognitive Investigators . (2015). Vascular imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities‐neurocognitive study. Stroke, 46, 433–440 https://www.ahajournals.org/doi/10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe, L. , Kharabian‐Masouleh, S. , Kynast, J. , Arelin, K. , Steele, C. J. , Löffler, M. , … Bazin, P.‐L. (2017). Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. Journal of Cerebral Blood Flow & Metabolism, 39(1), 36–43. http://www.ncbi.nlm.nih.gov/pubmed/29106319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y. Y. , Villemagne, V. L. , Pietrzak, R. H. , Ames, D. , Ellis, K. A. , Harrington, K. , … Australian Imaging B and L (AIBL) RG . (2015). APOE ε4 moderates amyloid‐related memory decline in preclinical Alzheimer's disease. Neurobiology of Aging, 36, 1239–1244 http://www.ncbi.nlm.nih.gov/pubmed/25559335 [DOI] [PubMed] [Google Scholar]
- Longstreth, W. T. , Manolio, T. A. , Arnold, A. , Burke, G. L. , Bryan, N. , Jungreis, C. A. , … Fried, L. (1996). Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke, 27, 1274–1282 http://www.ncbi.nlm.nih.gov/pubmed/8711786 [DOI] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Fairchild, A. J. , & Fritz, M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614 http://www.ncbi.nlm.nih.gov/pubmed/16968208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Krull, J. L. , & Lockwood, C. M. (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science, 1, 173–181 http://www.ncbi.nlm.nih.gov/pubmed/11523746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzinzik, F. , Wahl, M. , Schneider, G.‐H. , Kupsch, A. , Curio, G. , & Klostermann, F. (2008). The human thalamus is crucially involved in executive control operations. Journal of Cognitive Neuroscience, 20, 1903–1914 http://www.ncbi.nlm.nih.gov/pubmed/18370599 [DOI] [PubMed] [Google Scholar]
- McAleese, K. E. , Walker, L. , Graham, S. , Moya, E. L. J. , Johnson, M. , Erskine, D. , … Attems, J. (2017). Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathologica, 134, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem, E. R. , Mori, S. , Mukundan, G. , Kraut, M. A. , Pomper, M. G. , & van Zijl, P. C. M. (2002). Diffusion tensor MR imaging of the brain and white matter Tractography. American Journal of Roentgenology, 178, 3–16 http://www.ncbi.nlm.nih.gov/pubmed/11756078 [DOI] [PubMed] [Google Scholar]
- Modat, M. , Cash, D. M. , Daga, P. , Winston, G. P. , Duncan, J. S. , & Ourselin, S. (2014). Global image registration using a symmetric block‐matching approach. Journal of Medical Imaging, 1, 024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo, J. L. , Gramunt, N. , Gispert, J. D. , Fauria, K. , Esteller, M. , Minguillon, C. , … Camí, J. (2016). The ALFA project: A research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimer's Dementia: Translational Research and Clinical Interventions, 2, 82–92 http://linkinghub.elsevier.com/retrieve/pii/S2352873716000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais, M. , Artero, S. , & Ritchie, K. (2014). White matter Hyperintensities as early and independent predictors of Alzheimer's disease risk. Journal of Alzheimer's Disease, 42, 393–400 https://content-iospress-com.sire.ub.edu/download/journal-of-alzheimers-disease/jad141473?id=journal-of-alzheimers-disease%2Fjad141473 [DOI] [PubMed] [Google Scholar]
- Murray, A. D. , Staff RT , Shenkin, S. D. , Deary, I. J. , Starr, J. M. , & Whalley, L. J. (2005). Brain white matter hyperintensities: Relative importance of vascular risk factors in nondemented elderly people. Radiology, 237, 251–257. [DOI] [PubMed] [Google Scholar]
- Prins, N. D. , & Scheltens, P. (2015). White matter hyperintensities, cognitive impairment and dementia: An update. Nature Reviews. Neurology, 11, 157–165 http://www.ncbi.nlm.nih.gov/pubmed/25686760 [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT, Becker JT (2012): White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging, 33, 834.e7–834.e16. http://www.ncbi.nlm.nih.gov/pubmed/21943959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanoël, S. , Hoyau, E. , Kauffmann, L. , Renard, F. , Pichat, C. , Boudiaf, N. , … Baciu, M. (2018). Gray matter volume and cognitive performance during Normal aging. A voxel‐based Morphometry study. Frontiers in Aging Neuroscience, 10, 1–10 https://www.frontiersin.org/article/10.3389/fnagi.2018.00235/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, J. , McNeely, A. A. , Scott, C. J. , Stuss, D. T. , & Black, S. E. (2014). Subcortical hyperintensity volumetrics in Alzheimer's disease and normal elderly in the Sunnybrook dementia study: Correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimer's Research & Therapy, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi, B. , Narkhede, A. , Last, B. S. , Budge, M. , Tosto, G. , Manly, J. J. , … Brickman, A. M. (2018). The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiology of Aging, 64, 25–32 10.1016/j.neurobiolaging.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, S. , Brugulat‐Serrat, A. , Bargalló, N. , Minguillón, C. , Tucholka, A. , Falcon, C. , … Gispert, J. D. (2017). Higher prevalence of cerebral white matter hyperintensities in homozygous APOE‐ɛ4 allele carriers aged 45–75: Results from the ALFA study. Journal of Cerebral Blood Flow and Metabolism, 38, 250–261 http://journals.sagepub.com/doi/10.1177/0271678X17707397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadó, G. , Brugulat‐Serrat, A. , Sudre, C. H. , Grau‐Rivera, O. , Suárez‐Calvet, M. , Falcon, C. , … Gispert, J. D. (2019). Spatial patterns of white matter hyperintensities associated with Alzheimer's disease risk factors in a cognitively healthy middle‐aged cohort. Alzheimer's Research & Therapy, 11, 12 https://alzres.biomedcentral.com/articles/10.1186/s13195-018-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling S, DeStefano AL, Sachdev PS, Choi SH, Mather KA, DeCarli CD, Wen W, H??gh P, Raz N, Au R, Beiser A, Wolf PA, Romero JR, Zhu YC, Lunetta KL, Farrer L, Dufouil C, Kuller LH, Mazoyer B, Seshadri S, Tzourio C, Debette S (2013): APOE genotype and MRI markers of cerebrovascular disease. Neurology 81:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, J. , Tomassini, V. , & Johansen‐Berg, H. (2009). Individual differences in white matter microstructure in the healthy brain. In: Diffusion MRI Second Edi. Elsevier. pp 237–249. 10.1016/B978-0-12-396460-1.00014-7. [DOI]
- Smith, E. E. , Egorova, S. , Blacker, D. , Killiany, R. J. , Muzikansky, A. , Dickerson, B. C. , … Guttmann, C. R. G. (2008). Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Archives of Neurology, 65, 94–100 http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- Song, S.‐K. , Sun, S.‐W. , Ramsbottom, M. J. , Chang, C. , Russell, J. , & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17, 1429–1436 http://www.ncbi.nlm.nih.gov/pubmed/12414282 [DOI] [PubMed] [Google Scholar]
- Sudre, C. H. , Cardoso, M. J. , Bouvy, W. H. , Biessels, G. J. , Barnes, J. , & Ourselin, S. (2015). Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Transactions on Medical Imaging, 34, 2079–2102. [DOI] [PubMed] [Google Scholar]
- Sudre, C. H. , Gomez Anson, B. , Davagnanam, I. , Schmitt, A. , Mendelson, A. F. , Prados, F. , … Ourselin, S. (2017). Bullseye's representation of cerebral white matter hyperintensities. Journal of Neuroradiology, 45, 114–122 http://linkinghub.elsevier.com/retrieve/pii/S0150986117302249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager, W. , Cogo‐Moreira, H. , Masellis, M. , Ramirez, J. , Herrmann, N. , Edwards, J. D. , … Black, S. E. (2018). The effect of white matter hyperintensities on verbal memory. Neurology, 90, e673–e682 http://www.neurology.org/lookup/doi/10.1212/WNL.0000000000004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar, A. M. , Reid, A. T. , Shumskaya, E. , De Laat, K. F. , Van Norden, A. G. W. , Van Dijk, E. J. , … De Leeuw, F. E. (2015). Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke, 46, 425–432. [DOI] [PubMed] [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a Frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100, 3328–3342 http://jn.physiology.org/cgi/doi/10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Davidson, M. L. , Hughes, B. L. , Lindquist, M. A. , & Ochsner, K. N. (2008). Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050 https://www.sciencedirect.com/science/article/pii/S0896627308007538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Waugh, C. E. , Lindquist, M. , Noll, D. C. , Fredrickson, B. L. , & Taylor, S. F. (2009). Brain mediators of cardiovascular responses to social threat. Part I: Reciprocal dorsal and ventral sub‐regions of the medial prefrontal cortex and heart‐rate reactivity. Neuroimage, 47, 821–835 http://www.ncbi.nlm.nih.gov/pubmed/19465137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Valdés Hernández, M. C. , & Muñoz‐Maniega, S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. Journal of the American Heart Association, 4, 001140 http://www.ncbi.nlm.nih.gov/pubmed/26104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, W. , Sachdev, P. S. , Chen, X. , & Anstey, K. (2006). Gray matter reduction is correlated with white matter hyperintensity volume: A voxel‐based morphometric study in a large epidemiological sample. NeuroImage, 29, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Winblad, B. , Amouyel, P. , Andrieu, S. , Ballard, C. , Brayne, C. , Brodaty, H. , … Zetterberg, H. (2016). Defeating Alzheimer's disease and other dementias: A priority for European science and society. The Lancet Neurology, 15, 455–532. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Aggarwal, M. , & Mori, S. (2012). Structural insights into the rodent CNS via diffusion tensor imaging. Trends in Neurosciences, 35, 412–421 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3591520/pdf/nihms381371.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, G. , & Lou, M. (2016). Multimodal imaging findings in normal‐appearing white matter of leucoaraiosis: A review. BMJ, 1, 59–63 http://www.ncbi.nlm.nih.gov/pubmed/28959465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


