Abstract
Emerging evidence has associated autism spectrum disorder (ASD) with static functional connectivity abnormalities between multiple brain regions. However, the temporal dynamics of intra‐ and interhemispheric functional connectivity patterns remain unknown in ASD. Resting‐state functional magnetic resonance imaging data were analyzed for 105 ASD and 102 demographically matched typically developing control (TC) children (age range: 7–12 years) available from the Autism Brain Imaging Data Exchange database. Whole‐brain functional connectivity was decomposed into ipsilateral and contralateral functional connectivity, and sliding‐window analysis was utilized to capture the intra‐ and interhemispheric dynamic functional connectivity density (dFCD) patterns. The temporal variability of the functional connectivity dynamics was further quantified using the standard deviation (SD) of intra‐ and interhemispheric dFCD across time. Finally, a support vector regression model was constructed to assess the relationship between abnormal dFCD variance and autism symptom severity. Both intra‐ and interhemispheric comparisons showed increased dFCD variability in the anterior cingulate cortex/medial prefrontal cortex and decreased variability in the fusiform gyrus/inferior temporal gyrus in autistic children compared with TC children. Autistic children additionally showed lower intrahemispheric dFCD variability in sensorimotor regions including the precentral/postcentral gyrus. Moreover, aberrant temporal variability of the contralateral dFCD predicted the severity of social communication impairments in autistic children. These findings demonstrate altered temporal dynamics of the intra‐ and interhemispheric functional connectivity in brain regions incorporating social brain network of ASD, and highlight the potential role of abnormal interhemispheric communication dynamics in neural substrates underlying impaired social processing in ASD.
Keywords: autism spectrum disorder, dynamic functional connectivity, interhemisphere, intrahemisphere, resting‐state functional magnetic resonance imaging
1. INTRODUCTION
Autism spectrum disorder (ASD), featuring impairments in social and communication abilities, and repetitive behaviors, is an early‐onset neurodevelopmental condition (American Psychiatric Association, 2013). It occurs roughly 1 in 59 children and persists through adulthood (Baio et al., 2018). Despite divergent findings in functional magnetic resonance imaging (fMRI) studies of ASD, damaged intrinsic functional organizations involving multiple brain regions and networks have been increasingly recognized in the development of ASD (Belmonte et al., 2004; Cerliani et al., 2015; Duan et al., 2017).
Arising from preliminary neuroimaging evidence on connectivity abnormalities in ASD, a prevailing notion of “under‐connectivity theory” was proposed for ASD (Belmonte et al., 2004; Just, Cherkassky, Keller, Kana, & Minshew, 2007; Just, Keller, Malave, Kana, & Varma, 2012). Specifically, this hypothesis posited decreased long‐distance combined with increased local connectivity patterns in ASD (Belmonte et al., 2004). The under‐connectivity account in ASD was further supported by a substantial body of fMRI research on disrupted functional connectivity (Just et al., 2012; Just, Cherkassky, Keller, & Minshew, 2004; Kana, Libero, & Moore, 2011). For example, particularly implicated in long‐range disconnection in ASD is between the frontal and posterior cortical regions (Damarla et al., 2010; Just et al., 2012), anterior and posterior default mode network (DMN) (Kennedy & Courchesne, 2008), interhemispheric connectivity (Anderson et al., 2011) and social brain networks (Hagen, Stoyanova, Baron‐Cohen, & Calder, 2012). Although the neurobiological mechanisms underlying impaired functional connectivity in ASD are not yet fully understood, emerging evidence revealed that early brain overgrowth, especially in the frontal and temporal lobes, disrupted the formation and fine‐tuning of neural connections in ASD (Courchesne et al., 2007). Such deficits in neural circuits may lead to the skewed ratio of short‐ over long‐distance functional connectivity between brain regions in autistic brain (Courchesne et al., 2007; Yenkoyan, Grigoryan, Fereshetyan, & Yepremyan, 2017). Furthermore, structural connectivity studies in ASD demonstrated that the overgrowth primarily impaired the intrahemispheric connectivity (Minshew & Williams, 2007). However, our understanding of the intrahemispheric and interhemispheric functional processing in ASD remains relatively limited (Anderson et al., 2011; Di Martino et al., 2014; Dinstein et al., 2011; Hahamy, Behrmann, & Malach, 2015; Kozhemiako et al., 2019; Lee, Kyeong, Kim, & Cheon, 2016). Notably, most of these studies have focused on the interhemispheric functional connectivity by evaluating the neural synchronization between homologous voxels and showed consistently reduced interhemispheric connections in ASD (Anderson et al., 2011; Di Martino et al., 2014; Dinstein et al., 2011; Kozhemiako et al., 2019). To the best of our knowledge, only two fMRI studies have investigated both intra‐ and interhemispheric functional connectivity at rest in ASD (Hahamy et al., 2015; Lee et al., 2016). Individuals with ASD were found to have functional connectivity abnormalities at both intra‐ and interhemispheric levels (Hahamy et al., 2015; Lee et al., 2016). Collectively, these findings point to the impairments in intra‐ and interhemispheric functional coordination in the autistic brain.
Nevertheless, all these intra‐ and/or interhemispheric functional connectivity abnormalities in ASD have been identified with an assumption that functional connections are static during the scan. Recent advances in functional connectivity demonstrated that brain networks exhibits meaningful time‐varying interplay over time (Allen et al., 2014; Hutchison et al., 2013). Capturing the functional connectivity dynamics has been validated to provide new insights into the brain networks of neuropsychiatric disorders, including epilepsy (Li et al., 2018), depression (Liao et al., 2018) and schizophrenia (Damaraju et al., 2013). Investigations of dynamic functional connectivity in ASD have revealed aberrant dynamics of functional connectivity (Chen, Nomi, Uddin, Duan, & Chen, 2017; Guo et al., 2019; He et al., 2018). For example, enhanced temporal variability of intrinsic functional connectivity was observed in individuals with ASD, implying increased intra‐individual variance of brain networks across time (Falahpour et al., 2016). Moreover, such hypervariant dynamics of the functional connectivity were associated with ASD symptom severity (Chen et al., 2017). To date, temporal dynamics of the intra‐ and interhemisphere functional connectivity in the resting‐state remains unknown in ASD.
Recent developments in complex network analysis have demonstrated that graph theoretical analyses offer new ways to quantifying the brain network organization (Bullmore & Sporns, 2009). Functional connectivity density (FCD) mapping is a voxel‐wise data‐driven graph theory approach, which allows for identification of the distribution of highly connected hubs in brain networks (Tomasi & Volkow, 2010, 2011). Network hubs facilitate efficient functional integration of information processing both within and between particular neural systems (Avena‐Koenigsberger, Misic, & Sporns, 2018). Subsequent FCD studies confirmed its sensitivity in detecting abnormalities of the functional connectivity hubs in psychiatric and neurological diseases (Lee et al., 2016; Li et al., 2019; Tomasi & Volkow, 2012).
In the present study, we incorporated sliding‐window analysis into FCD mapping to investigated the temporal variability of the intra‐ and interhemispheric dynamic FCD (dFCD) in ASD and typically developing control (TC) children obtained from the open‐access Autism Brain Imaging Data Exchange (ABIDE, http://fcon_1000.projects.nitrc.org/indi/abide/) database. Specifically, the whole‐brain functional connectivity within each window was decomposed into ipsilateral and contralateral parts, denoting the intra‐ and interhemispheric functional connectivity, respectively. Then we compared the temporal changes of the intra‐ and interhemisphere dFCD between ASD and TC children to assess whether there is ASD‐related abnormality in dynamic interactions between brain networks. Based on previous findings on intra‐ and interhemispheric functional connectivity abnormalities in ASD (Hahamy et al., 2015; Lee et al., 2016), we hypothesized that autistic children would display altered temporal dynamics of the intra‐ and interhemispheric FCD compared with TC group.
2. MATERIALS AND METHODS
2.1. Participants
Original resting‐state fMRI data were drawn from the open‐access ABIDE I and ABIDE II database (Di Martino et al., 2014; Di Martino et al., 2017). Specifically, analyses were limited to (a) children aged between 7 and 12 years; (b) male subjects, because they represented more than 90% of the aggregate datasets; (c) individuals with available full IQ (FIQ), handedness and eye status values (eyes open/closed); (d) subjects without excessive head motion in resting‐state scan (i.e., motion within 2 mm translation and 2 degree rotation and less than 50% frames with large frame‐wise displacement [FD], as indicated in Preprocessing); (e) subjects with complete cortical coverage in resting‐state scan; (f) subjects with full brain anatomical images and without apparent motion artifact; (g) a well‐matched dataset of ASD and TC groups within each site created by using a data‐driven algorithm that maximized p values of group difference on age, handedness, FIQ, eye status and mean FD; (h) sites with more than 10 subjects per group after the above‐mentioned selection procedure. In view of the potential effects of eyes conditions on resting‐state functional connectivity (Nair et al., 2018; Patriat et al., 2013; Zou et al., 2009), eye status (eyes open or eyes closed) were regarded as a covariate in dataset selection and data processing. These criteria left us with a well‐matched dataset of 207 children (ASD/TC: 105/102) from six sites. Demographic data were summarized in Table 1.
Table 1.
Demographics and clinical characteristics of the participants
| ASD (n = 105) | TC (n = 102) | p value | |
|---|---|---|---|
| Age (years) | 10.15 ± 1.26 | 10.02 ± 1.38 | .48a |
| Handedness (right/left/mixed) | 83/9/13 | 82/5/15 | .54b |
| FIQ | 110.53 ± 17.42 | 113.78 ± 11.98 | .12a |
| Mean FD (mm) | 0.17 ± 0.08 | 0.16 ± 0.08 | .16a |
| Eye state (open/closed) | 91/14 | 88/14 | .93b |
| ADOS | |||
| Communication | 3.13 ± 1.59 | ‐ | ‐ |
| Social | 8.01 ± 2.51 | ‐ | ‐ |
| RRB | 2.23 ± 1.63 | ‐ | ‐ |
| Total | 11.33 ± 3.83 | ‐ | ‐ |
Abbreviations: ADOS, the Autism Diagnostic Observation Schedule (available for 82 ASD subjects); ASD, autism spectrum disorder; FD, frame‐wise displacement; FIQ, the full‐scale intelligence quotient; RRB, restricted and repetitive behaviors; TC, typical‐developing controls.
Indicates p values for two sample t test.
Indicates p values for χ 2 test.
All autistic children had a clinical diagnosis of Autistic Disorder, Asperger's Disorder, or Pervasive Developmental Disorder Not‐Otherwise‐Specified. Typically developing children showed no history of psychiatric or neurological disorders. All experimental protocols were approved by the local Institutional Review Boards. Written informed consent was obtained from a parent/guardian and assent was obtained from each child. Detailed information on scanning information, diagnostic protocols and ethical statements can be found at http://fcon_1000.projects.nitrc.org/indi/abide/.
2.2. Data preprocessing
Resting‐state fMRI data preprocessing was performed using the advanced edition of Data Processing Assistant for Resting‐State fMRI (DPARSF A v4.1, http://rfmri.org/DPARSF) toolbox in MATLAB (Yan & Zang, 2010). Image preprocessing was performed as follows. The first 10 volumes from each subject were discarded, and the remaining consecutive volumes were then slice‐timing corrected, spatial realigned (participants with translational or rotational motion higher than 2 mm or 2° were excluded), normalized to standard Montreal Neurological Institute stereotaxic space, and resampled to 3 × 3 × 3 mm3. Next, linear trends were removed. The resulting images were despiked using the 3dDespike algorithm in Analysis of Functional NeuroImaging (https://afni.nimh.nih.gov/afni/) to address potential motion artifacts. Signal outliers higher than median absolute deviation were replaced with the best estimate by using a third‐order spline fit to clean portions of the time series. This method retains temporal information of signals and improves root‐mean‐square of temporal derivative of time courses (Allen et al., 2014; Marusak et al., 2017; Nomi et al., 2016). Finally, Friston 24 head motion parameters (Friston, Williams, Howard, Frackowiak, & Turner, 1996; Satterthwaite et al., 2012; Yan et al., 2013) as well as white matter and cerebrospinal fluid signals were regressed out as nuisance covariates, and band‐pass filtering was carried out at 0.01–0.1 Hz. Additionally, we examined high‐motion frame percentage of each subject to reduce unwanted effects from head motion artifacts on functional connectivity analyses (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Time points with FD > 0.5 mm with preceding one and subsequent two time points obtained in realignment were labeled as high‐motion frames (Chen et al., 2019). Subjects with less than 50% high‐motion frames were included in the following analyses (Chen et al., 2017; Guo et al., 2019).
2.3. dFCD estimation and temporal variability
Global, contralateral and ipsilateral dFCD values were calculated for each voxel using a sliding window approach. This analysis was limited to a cerebral gray matter mask which is obtained from the automated anatomical labeling template excluding the cerebellum (Tzourio‐Mazoyer et al., 2002). According to the rule of thumb, the minimum window length should exceed 1/f min, where f min denoted the minimum frequency of time courses (Leonardi & Van De Ville, 2015; Liao et al., 2018). Here, the minimum repetition time (TR) value of the selected dataset is 2 s. Therefore, the entire time course for each subject was divided into windows of 50 TRs shifting with a step size of 2 TRs. Previous dynamic functional connectivity studies demonstrated that too short window length can increase the risk of introducing artifacts in dynamic functional connectivity analysis and reducing reliability of functional connectivity, and too long window lengths can obscure the temporal variations of dynamic functional connectivity (Preti, Bolton, & Ville, 2016). Thus 50 TRs was selected as the window length in the current study to balance the specificity and sensitivity for dynamic functional connectivity calculation (He et al., 2018; Li, Liao, et al., 2018). To verify the robustness of the sliding‐window analysis, we replicated our findings with different window lengths (30 TRs and 80 TRs) and shifting step (1TR) (Figures S1 and S2, Supporting Information). In each window, we first calculated the global FCD at each voxel as the number of functional connectivity between this voxel and all the other voxels in the brain mask (Tomasi & Volkow, 2010). Then we decomposed the global FCD into contralateral and ipsilateral FCD based on the relative positions of seed and target voxels. This division method was identical to a previous static FCD study in ASD (Lee et al., 2016). Contralateral (or interhemispheric) FCD was computed as the number of functional edges of a given voxel connected with all voxels in the opposite hemisphere. Analogously, ipsilateral (or intrahemispheric) FCD at each voxel referred to the number of functional connectivity with all voxels in the same hemisphere. Pearson correlation was used to compute the functional connectivity of a given voxel and other voxels, and the correlation threshold was determined by p < .05, uncorrected. Therefore, a voxel i is thought to be functionally connected with a voxel j only if their correlation coefficient is larger than correlation threshold. Validation analyses of different correlation thresholds (p < .01, p < .001, R > 0.6 and sparsity thresholds of 0.2, 0.3 and 0.4) were established to demonstrate the robustness of our findings (Figures S3 and S4, Supporting Information). Temporal variability in global, contralateral and ipsilateral dFCD was quantified by the standard deviation (SD) of the time‐varying FCD patterns over time. For the purpose of standardization, original dFCD variance maps were transformed into z score by minus the global mean value and then divided by the standard variation to minimize the individual variability. Finally, all the transformed variance maps were spatially smoothed with an isotropic Gaussian kernel of 6 mm full width at half maximum.
2.4. Statistical analyses
Random effect voxel‐wise two‐sample t test was performed with statistical parametric mapping (SPM 12, http://www.fil.ion.ucl.ac.uk/spm/) to ascertain the group differences in global, contralateral and ipsilateral dFCD variance between autism and TC groups, after adjustment for age, handedness, FIQ, mean FD, eye status and sites (using a dummy coding scheme). Multiple comparisons correction was performed using Monte Carlo simulations (1,000 iterations). Statistical significance was set at cluster‐level threshold of p < .05 and voxel‐level threshold of p < .005. Average variance values of all voxels within each cluster from corrected statistical maps were extracted for post‐hoc analyses.
2.5. Prediction analysis of autism symptom severity
We then explored whether the abnormal dFCD variance patterns were related to autism symptom severity as assessed by the Autism Diagnostic Observation Schedule (ADOS) subscale scores (i.e., communication, social and restricted and repetitive behaviors) (Lord et al., 2000). To achieve this goal, voxel‐wise contralateral and ipsilateral dFCD variance within aberrant brain regions were extracted, separately. Age, handedness, FIQ, mean FD, eye status and sites (using a dummy coding scheme) were first regressed out from the dFCD variance. Only autistic subjects with available ADOS subscores were included in the following brain‐behavior analyses (n = 82). Multivariate support vector regression approach was then applied to model the relationship between dependent variables (ADOS subscores) and multiple independent variables (voxel‐wise contralateral or ipsilateral dFCD variance values) (Drucker, Burges, Kaufman, Smola, & Vapnik, 1996; Guo et al., 2017). Both dependent and independent variables were normalized into the interval [0, 1] for standardization. Compared with conventional univariate correlation analysis, multivariate regression analysis integrates dFCD variance information in a voxel‐wise manner rather than a simple average strategy. Thus, it is more likely to uncover the relationship between brain imaging biomarkers and behavioral manifestations. Library for support vector machines (LIBSVM) (http://www.csie.ntu.edu.tw/~cjlin/libsvm/), an integrated toolbox for support vector classification/regression analyses, was utilized in analysis with default c parameter (Chang & Lin, 2011). Leave‐one‐out cross validation (LOOCV) was subsequently performed to assess the performance of regression algorithm in predicting symptom severity (Scholkopf & Smola, 2002). For instance, we assumed the presence of n samples in total. In each LOOCV trial, we first selected one subject as testing set, and remaining samples were used as training sets. Regression model was built for each training dataset and then used to predict its corresponding testing dataset. The above procedure was repeated n times, and the correlation coefficient value R was computed between predicted and observed values. Statistical significance was finally evaluated by a nonparametric permutation test (Golland & Fischl, 2003; Liu et al., 2013). In permutation test, observed values were permuted randomly, and the whole regression procedure was implemented to obtain an R perm value based on the shuffled dataset; the procedure was repeated 1,000 times in the current study. Statistical significance (p value) was determined by the proportion of times that R perm was higher than the original R value with respect to total permutation times (here is 1,000).
3. RESULTS
3.1. dFCD variance
Group‐level global, contralateral and ipsilateral dFCD variance maps for autism and TC groups were presented in Figure 1. DFCD variance patterns in TC group were maximal in the bilateral occipital regions including the calcarine and cuneus, and middle temporal gyrus, dorsolateral prefrontal cortex and supplementary motor area, whereas minimal in the temporal pole (TPO), hippocampus, fusiform gyrus (FG), inferior temporal gyrus (ITG), caudate and posterior cingulate cortex/precuneus.
Figure 1.
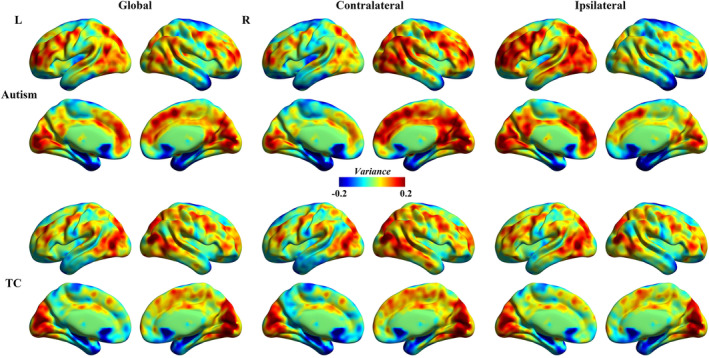
Average dynamic functional connectivity density (dFCD) variance for autism and typically developing controls (TC) children [Color figure can be viewed at http://wileyonlinelibrary.com]
Compared with TC group, autistic children showed decreased global dFCD variance in the temporal cortex including the FG, ITG and middle TPO, as well as precentral gyrus and postcentral gyrus, while increased global dFCD variance in the anterior DMN regions including the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) (Figure 2, Table 2). Moreover, in the comparisons of the contralateral dFCD variance maps, autistic children showed decreased temporal variability in the FG/ITG, and increased variability in the ACC/mPFC. Group comparisons of the ipsilateral dFCD variance maps revealed similar patterns to global dFCD variance (Figure 2, Table 2). Compared with TC group, autistic children showed decreased ipsilateral dFCD variance in the FG/ITG/middle TPO and precentral gyrus and postcentral gyrus, and increased variance in the ACC/mPFC (Figure 2, Table 2).
Figure 2.
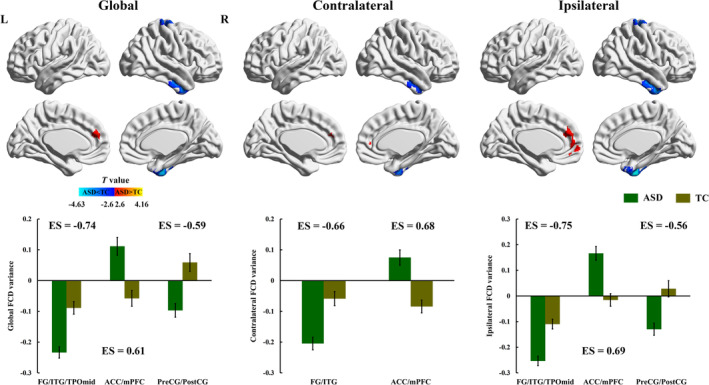
Between‐group differences for global, contralateral and ipsilateral dFCD variance. ACC, anterior cingulate cortex; ASD, autism spectrum disorder; dFCD, dynamic functional connectivity density; ES, Cohen's d effect size; FG, fusiform gyrus; ITG, inferior temporal gyrus; mPFC, medial prefrontal cortex; PostCG, postcentral gyrus; PreCG, precentral gyrus; TC, typically developing controls; TPOmid, middle temporal pole [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Altered dynamic FCD variability in ASD group
| Brain areas | Hemi | Voxels | BA | MNI coordinates | T value | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Global FCD variance | ASD < TC | |||||||
| Cluster 1 | Fusiform gyrus | R | 141 | 20 | 33 | −3 | −39 | −4.16 |
| Inferior temporal gyrus | R | |||||||
| Temporal pole: middle | R | |||||||
| Cluster 2 | Precentral gyrus | R | 73 | 3/4/6 | 24 | −15 | 81 | −3.62 |
| Postcentral gyrus | R | |||||||
| ASD > TC | ||||||||
| Cluster 1 | Medial prefrontal cortex | L/R | 106 | 9/10 | −9 | 45 | 24 | 4.05 |
| Anterior cingulate cortex | L/R | |||||||
| Contralateral FCD variance | ASD < TC | |||||||
| Cluster 1 | Fusiform gyrus | R | 81 | 20 | 33 | −3 | −39 | −3.54 |
| Inferior temporal gyrus | ||||||||
| ASD > TC | ||||||||
| Cluster 1 | Medial prefrontal cortex | L/R | 107 | 9/10 | 12 | 48 | 3 | 4.07 |
| Anterior cingulate cortex | ||||||||
| Ipsilateral FCD variance | ASD < TC | |||||||
| Cluster 1 | Fusiform gyrus | R | 208 | 20/38 | 33 | −3 | −42 | −4.63 |
| Inferior temporal gyrus | R | |||||||
| Temporal pole: middle | R | |||||||
| Cluster 2 | Precentral gyrus | R | 92 | 4 | 21 | −27 | 72 | −3.64 |
| Postcentral gyrus | R | |||||||
| ASD > TC | ||||||||
| Cluster 1 | Medial prefrontal cortex | L/R | 238 | 9/10 | −3 | 54 | 0 | 4.16 |
| Anterior cingulate cortex | L/R | |||||||
Abbreviations: ASD, autism spectrum disorder; BA, Brodmann Area; Hemi, hemisphere; FCD, functional connectivity density; MNI, montreal neurological institute; L, left; R, right; TC, typical‐developing controls.
In order to exclude the potential effects of head motion on the observed group differences on dFCD variability, we performed the Pearson correlation between mean FD values and dFCD variability in brain regions identified in group comparisons. No significant relationship was observed.
3.2. Relationships with autism symptom severity
To investigate the relationship between dFCD variance abnormalities and autism symptom severity, multivariate regression model and LOOCV were used followed by nonparametric permutation test. Aberrant temporal variability of the contralateral dFCD predicted scores on the communication domain of the ADOS (r = .36, p = .006; Figure 3). No significant relationships were observed between the contralateral dFCD variance and other domains of the ADOS or between the ipsilateral dFCD variance and any domain of the ADOS.
Figure 3.
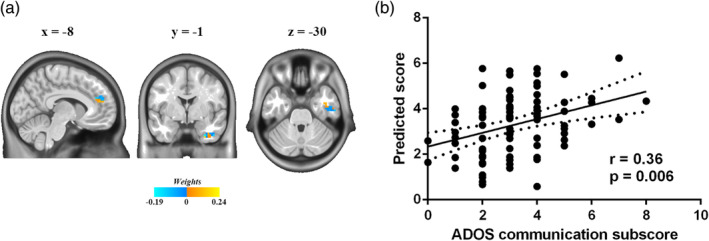
Relationships between abnormal contralateral dynamic functional connectivity density (dFCD) variance and autism symptom severity. (a) Voxel‐level weightings in prediction analysis of Autism Diagnostic Observation Schedule (ADOS) communication subscore. (b) Relationships between ADOS communication subscore and predicted scores using abnormal contralateral dFCD variance (r = .36, p = .006) [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Taking advantage of FCD mapping and sliding‐window analysis, this study investigated the temporal variability alterations of intra‐ and interhemisphere intrinsic functional connectivity patterns in ASD compared with TC children. Both intra‐ and interhemispheric comparisons showed aberrant dFCD variability in the social brain regions including the ACC/mPFC and FG/ITG in autistic children compared with TC children. Reduced intrahemispheric dFCD variability were also found in sensorimotor regions including the precentral/postcentral gyrus in autistic children. Importantly, alterations in contralateral dFCD variability predicted the severity of social communication impairments in autistic children. From a dynamic perspective, these findings deepen our understanding of functional connectivity abnormalities in autistic brain.
Previous studies showing static functional connectivity abnormalities at intra‐ and interhemispheric levels in ASD implied the importance of examining intra‐ and interhemispheric functional connectivity pathways individually in autistic brain (Hahamy et al., 2015; Lee et al., 2016). Our results further supported these findings by showing discrepant intra‐ and interhemispheric patterns of dFCD variance alterations in children with ASD. It is thus imperative to consider topological location and anatomical distance effects on functional connectivity in future studies exploring brain network abnormalities in ASD (Anderson et al., 2011; Long, Duan, Mantini, & Chen, 2016). More importantly, the present study revealed time‐varying abnormalities of intrinsic functional connectivity patterns in children with ASD, which is consistent with previous reports of altered communication dynamics of brain networks in ASD (Chen et al., 2017; Falahpour et al., 2016). Therefore, dynamic functional connectome analyses or a combination of static and dynamic functional connectome approaches may serve as a promising way for future research to advance our understanding of this condition (Liao et al., 2018).
Analogous to global FCD, ipsilateral and contralateral FCD reflect intra‐ and interhemispheric functional hubs in brain networks (Tomasi & Volkow, 2010, 2011). Our results showed both intra‐ and interhemispheric dFCD variability alterations in the ACC/mPFC and FG/ITG in autistic children compared with TC children. Particularly, these identified regions with impaired dFCD variability are critical brain areas of the social brain network, a neural circuitry involved in social perception and cognition (Adolphs, 2009). Functional connectivity and activations alterations of social brain network have been proposed as the potential candidates for the social impairments of ASD (Gotts et al., 2012; Patriquin, DeRamus, Libero, Laird, & Kana, 2016). Our findings of intrinsic functional connectivity abnormalities across time possibly reveal the globally damaged functional integration dynamics of information processing between social brain network and other large‐scale brain networks (Li, Wang, et al., 2019). Individuals with ASD were reported to have decreased static functional connectivity between the ACC/mPFC and other DMN regions in ASD (Michal et al., 2010; Weng et al., 2010). Decreased intrahemispheric FCD was also observed in the mPFC in a previous static functional connectivity study of ASD (Lee et al., 2016). As to the FG/ITG, decreased interhemispheric static functional connectivity between homologous voxels was reported in the FG in ASD (Anderson et al., 2011). Abnormalities in this region have also been identified as neurofunctional markers for social impairments of ASD (Patriquin et al., 2016). The present dFCD findings complement our understanding of the functional organization of the autistic brain from a dynamic perspective and provide more nuanced views of the temporal changes of the functional connectivity hubs that may be masked in conventional static studies (Guo et al., 2019). Together with our results, these findings consistently highlight the potential role of the neural circuits associated with the ACC/mPFC and FG/ITG in the pathophysiological mechanisms underlying ASD.
Relative to TC children, autistic children showed decreased intrahemispheric dFCD variability in sensorimotor regions including the precentral/postcentral gyrus. Aberrant sensory and motor behaviors are common features of ASD, such as clumsiness, motor coordination impairments, hyper‐ and hyposensitivities (Whyatt & Craig, 2013). Impaired functional synchronization between visual and sensory‐motor network has been demonstrated in previous static functional connectivity studies of ASD (Nebel et al., 2016; Oldehinkel et al., 2019). Decreased interhemispheric functional connectivity was also identified in the sensorimotor cortex in individuals with ASD (Anderson et al., 2011). In addition, the investigation of static functional hubs distribution showed significantly decreased FCD in the precentral and postcentral gyrus in ASD (Lee et al., 2016). Consistent with these findings, invariable intrahemispheric dFCD in the sensorimotor regions observed in the current study further emphasized abnormal dynamic functional coordination of the sensorimotor network in autistic brain. Such functional connectivity abnormalities may contribute to the aberrant sensory and motor processing in ASD (Oldehinkel et al., 2019). Future studies with integrated clinical assessment of sensorimotor behaviors are thus needed to ascertain the latent relationship between intrahemispheric functional connectivity alterations and sensorimotor impairments in ASD.
Brain‐behavior analysis revealed the potential links between abnormal interhemispheric dFCD variability and social communication deficits in children with ASD. Altered contralateral dFCD variability in the ACC/mPFC and FG/ITG predicted the severity of social communication impairments in autistic children. These abnormalities in temporal dynamics of the interhemispheric functional connectivity of the social brain areas, which may reflect aberrant integration of interhemispheric brain regions, may lead to behavioral manifestation in social communicative domain of children with ASD. This brain–behavior relationship also stresses the potential significance of interhemispheric functional connectivity abnormalities in ASD. Our findings are in accordance with previous reports that elucidated potential associations between functional connectivity abnormalities associated with social brain network and atypical social processing in ASD (Gotts et al., 2012; Guo et al., 2019).
Several limitations should be noted when interpreting our findings. The current study included only male children due to the relatively limited number of female participants in ABIDE database. Yet, there is considerable evidence that biological sex contributes to the heterogeneity of ASD on functional connectivity (Alaerts, Swinnen, & Wenderoth, 2016; Kozhemiako et al., 2019), neuroanatomy (Lai et al., 2013) and genetics (Jeste & Geschwind, 2014). How sex heterogeneity interacts with intra‐ and interhemispheric functional connectivity dynamics should be addressed in future studies to advance our understanding the sex differences in ASD. In addition, it remains unknown how temporal dynamics of dFCD develop in other developmental stages, such as adolescent and adulthood. Future longitudinal studies are needed to examine the developmental trajectory of dFCD variability in ASD.
5. CONCLUSIONS
The current study demonstrates global alterations in dFCD variability in social brain areas including the ACC/mPFC and FG/ITG in children with ASD compared with TC children. Furthermore, atypical temporal dynamics of interhemispheric dFCD may contribute to social communicative impairments in autistic children. Overall, these findings offer new insights into functional interaction between brain networks in ASD from a dynamic perspective and suggest the potential role of interhemispheric functional connectivity dynamics in neural mechanisms underlying atypical social processing in autistic children.
CONFLICT OF INTEREST
None declared.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in Autism Brain Imaging Data Exchange (ABIDE, http://fcon_1000.projects.nitrc.org/indi/abide/) database.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (Nos. 61533006,61673089, 81871432, 81771919, and U1808204), Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120185110028), Fundamental Research Funds for the Central Universities (Nos. ZYGX2016J187, 2672018ZYGX2018J079 and ZYGX2019Z017) and Sichuan Science and Technology Program (2018TJPT0016, 19YYJC0051). H.C. was supported by the Science and Technology Plan Project of Guizhou Province of China ([2018]5781) and the 2018 Talent Research Program of Guizhou University (702570183301). Funding sources for the datasets comprising the 1000 Functional Connectome Project are listed at http://fcon_1000.projects.nitrc.org/fcpClassic/FcpTable.html. Funding sources for the ABIDE dataset are listed at http://fcon_1000.projects.nitrc.org/indi/abide/.
Guo X, Duan X, Chen H, et al. Altered inter‐ and intrahemispheric functional connectivity dynamics in autistic children. Hum Brain Mapp. 2020;41:419–428. 10.1002/hbm.24812
Funding information 2018 Talent Research Program of Guizhou University, Grant/Award Number: 702570183301; Fundamental Research Funds for the Central Universities, Grant/Award Numbers: 2672018ZYGX2018J079, ZYGX2016J187, ZYGX2019Z017; National Natural Science Foundation of China, Grant/Award Numbers: 61533006, 61673089, 81771919, 81871432, U1808204; Science and Technology Plan Project of Guizhou Province of China, Grant/Award Number: [2018]5781; Sichuan Science and Technology Program, Grant/Award Numbers: 19YYJC0051, 2018TJPT0016; Specialized Research Fund for the Doctoral Program of Higher Education of China, Grant/Award Number: 20120185110028
Contributor Information
Xujun Duan, Email: duanxujun@uestc.edu.cn.
Huafu Chen, Email: chenhf@uestc.edu.cn.
REFERENCES
- Adolphs, R. (2009). The social brain: Neural basis of social knowledge. Annual Review of Psychology, 60(1), 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts, K. , Swinnen, S. P. , & Wenderoth, N. (2016). Sex differences in autism: A resting‐state fMRI investigation of functional brain connectivity in males and females. Social Cognitive and Affective Neuroscience, 11(6), 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24(3), 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). Arlington, VA: American Psychiatric Pub. [Google Scholar]
- Anderson, J. S. , Druzgal, T. J. , Froehlich, A. , DuBray, M. B. , Lange, N. , Alexander, A. L. , … Lainhart, J. E. (2011). Decreased interhemispheric functional connectivity in autism. Cerebral Cortex, 21(5), 1134–1146. 10.1093/cercor/bhq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena‐Koenigsberger, A. , Misic, B. , & Sporns, O. (2018). Communication dynamics in complex brain networks. Nature Reviews Neuroscience, 19(1), 17. [DOI] [PubMed] [Google Scholar]
- Baio, J. , Wiggins, L. , Christensen, D. L. , Maenner, M. J. , Daniels, J. , Warren, Z. , … White, T. (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte, M. K. , Allen, G. , Beckel‐Mitchener, A. , Boulanger, L. M. , Carper, R. A. , & Webb, S. J. (2004). Autism and abnormal development of brain connectivity. Journal of Neuroscience, 24(42), 9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186. [DOI] [PubMed] [Google Scholar]
- Cerliani, L. , Mennes, M. , Thomas, R. M. , Di Martino, A. , Thioux, M. , & Keysers, C. (2015). Increased functional connectivity between subcortical and cortical resting‐state networks in autism spectrum disorder. JAMA Psychiatry, 72(8), 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. C. , & Lin, C. J. (2011). LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems & Technology, 2(3), 27. [Google Scholar]
- Chen, H. , Nomi, J. S. , Uddin, L. Q. , Duan, X. , & Chen, H. (2017). Intrinsic functional connectivity variance and state‐specific under‐connectivity in autism. Human Brain Mapping, 38(11), 5740–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Uddin, L. Q. , Guo, X. , Wang, J. , Wang, R. , Wang, X. , … Chen, H. (2019). Parsing brain structural heterogeneity in males with autism spectrum disorder reveals distinct clinical subtypes. Human Brain Mapping, 40(2), 628–637. 10.1002/hbm.24400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne, E. , Pierce, K. , Schumann, C. M. , Redcay, E. , Buckwalter, J. A. , Kennedy, D. P. , & Morgan, J. (2007). Mapping early brain development in autism. Neuron, 56(2), 399–413. 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Damaraju, E. , Allen, E. A. , Belger, A. , Ford, J. M. , Mcewen, S. , Mathalon, D. H. , … Preda, A. (2013). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clinical, 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damarla, S. R. , Keller, T. A. , Kana, R. K. , Cherkassky, V. L. , Williams, D. L. , Minshew, N. J. , & Just, M. A. (2010). Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Research, 3(5), 273–279. 10.1002/aur.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , O'Connor, D. , Chen, B. , Alaerts, K. , Anderson, J. S. , Assaf, M. , … Milham, M. P. (2017). Data descriptor: Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Scientific Data, 4, 170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Yan, C.‐G. , Li, Q. , Denio, E. , Castellanos, F. X. , Alaerts, K. , … Dapretto, M. (2014). The autism brain imaging data exchange: Towards a large‐scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry, 19(6), 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein, I. , Pierce, K. , Eyler, L. , Solso, S. , Malach, R. , Behrmann, M. , & Courchesne, E. (2011). Disrupted neural synchronization in toddlers with autism. Neuron, 70(6), 1218–1225. 10.1016/j.neuron.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker, H. , Burges, C. J. C. , Kaufman, L. , Smola, A. J. , & Vapnik, V. (1996). Support vector regression machines. Advances in Neural Information Processing Systems, 28(7), 779–784. [Google Scholar]
- Duan, X. , Chen, H. , He, C. , Long, Z. , Guo, X. , Zhou, Y. , … Chen, H. (2017). Resting‐state functional under‐connectivity within and between large‐scale cortical networks across three low‐frequency bands in adolescents with autism. Progress in Neuropsychopharmacology Biological Psychiatry, 79(Pt B), 434–441. 10.1016/j.pnpbp.2017.07.027 [DOI] [PubMed] [Google Scholar]
- Falahpour, M. , Thompson, W. K. , Abbott, A. E. , Jahedi, A. , Mulvey, M. E. , Datko, M. , … Muller, R. A. (2016). Underconnected, but not broken? Dynamic functional connectivity MRI shows underconnectivity in autism is linked to increased intra‐individual variability across time. Brain Connectivity, 6(5), 403–414. 10.1089/brain.2015.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Golland, P. , & Fischl, B. (2003). Permutation Tests for Classification: Towards Statistical Significance in Image‐Based Studies. Paper presented at the Information Processing in Medical Imaging: Conference. [DOI] [PubMed]
- Gotts, S. J. , Simmons, W. K. , Milbury, L. A. , Wallace, G. L. , Cox, R. W. , & Martin, A. (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain, 135(Pt 9), 2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Chen, H. , Long, Z. , Duan, X. , Zhang, Y. , & Chen, H. (2017). Atypical developmental trajectory of local spontaneous brain activity in autism spectrum disorder. Scientific Reports, 7, 39822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Duan, X. , Suckling, J. , Chen, H. , Liao, W. , Cui, Q. , & Chen, H. (2019). Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Human Brain Mapping, 40(4), 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen, E. A. H. V. D. , Stoyanova, R. S. , Baron‐Cohen, S. , & Calder, A. J. (2012). Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social Cognitive & Affective Neuroscience, 8(6), 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy, A. , Behrmann, M. , & Malach, R. (2015). The idiosyncratic brain: Distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature Neuroscience, 18(2), 302. [DOI] [PubMed] [Google Scholar]
- He, C. , Chen, Y. , Jian, T. , Chen, H. , Guo, X. , Wang, J. , … Duan, X. (2018). Dynamic functional connectivity analysis reveals decreased variability of the default‐mode network in developing autistic brain. Autism Research, 11(11), 1479–1493. 10.1002/aur.2020 [DOI] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , … Gonzalezcastillo, J. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage, 80(1), 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste, S. S. , & Geschwind, D. H. (2014). Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature Reviews Neurology, 10(2), 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just, M. A. , Cherkassky, V. L. , Keller, T. A. , Kana, R. K. , & Minshew, N. J. (2007). Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex, 17(4), 951–961. 10.1093/cercor/bhl006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just, M. A. , Cherkassky, V. L. , Keller, T. A. , & Minshew, N. J. (2004). Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of underconnectivity. Brain, 127(Pt 8), 1811–1821. 10.1093/brain/awh199 [DOI] [PubMed] [Google Scholar]
- Just, M. A. , Keller, T. A. , Malave, V. L. , Kana, R. K. , & Varma, S. (2012). Autism as a neural systems disorder: A theory of frontal‐posterior underconnectivity. Neuroscience & Biobehavioral Reviews, 36(4), 1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana, R. K. , Libero, L. E. , & Moore, M. S. (2011). Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Physics of Life Reviews, 8(4), 410–437. [DOI] [PubMed] [Google Scholar]
- Kennedy, D. P. , & Courchesne, E. (2008). The intrinsic functional organization of the brain is altered in autism. NeuroImage, 39(4), 1877–1885. 10.1016/j.neuroimage.2007.10.052 [DOI] [PubMed] [Google Scholar]
- Kozhemiako, N. , Vakorin, V. , Nunes, A. S. , Iarocci, G. , Ribary, U. , & Doesburg, S. M. (2019). Extreme male developmental trajectories of homotopic brain connectivity in autism. Human Brain Mapping, 40(3), 987–1000. 10.1002/hbm.24427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. C. , Lombardo, M. V. , Suckling, J. , Ruigrok, A. N. , Chakrabarti, B. , Ecker, C. , … Bullmore, E. T. (2013). Biological sex affects the neurobiology of autism. Brain, 136(9), 2799–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. M. , Kyeong, S. , Kim, E. , & Cheon, K. A. (2016). Abnormalities of inter‐ and intra‐hemispheric functional connectivity in autism spectrum disorders: A study using the autism brain imaging data exchange database. Frontiers in Neuroscience, 10, 191 10.3389/fnins.2016.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, N. , & Van De Ville, D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage, 104, 430–436. 10.1016/j.neuroimage.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Li, R. , Liao, W. , Yu, Y. , Chen, H. , Guo, X. , Tang, Y. L. , & Chen, H. (2018). Differential patterns of dynamic functional connectivity variability of striato–cortical circuitry in children with benign epilepsy with centrotemporal spikes. Human Brain Mapping, 39(3), 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Wang, L. , Chen, H. , Guo, X. , Liao, W. , Tang, Y. L. , & Chen, H. (2019). Abnormal dynamics of functional connectivity density in children with benign epilepsy with centrotemporal spikes. Brain Imaging and Behavior, 13(4), 985–994. 10.1007/s11682-018-9914-0 [DOI] [PubMed] [Google Scholar]
- Liao, W. , Li, J. , Duan, X. J. , Cui, Q. , Chen, H. , & Chen, H. F. (2018). Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Human Brain Mapping, 39(10), 4105–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Guo, W. , Fouche, J. P. , Wang, Y. , Wang, W. , Ding, J. , … Zhang, W. (2013). Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Structure & Function, 220(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Long, Z. , Duan, X. , Mantini, D. , & Chen, H. (2016). Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Scientific Reports, 6, 26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L. , Cook, E. H., Jr. , Leventhal, B. L. , DiLavore, P. C. , … Rutter, M. (2000). The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Marusak, H. A. , Calhoun, V. D. , Brown, S. , Crespo, L. M. , Sala‐Hamrick, K. , Gotlib, I. H. , & Thomason, M. E. (2017). Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping, 38(1), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal, A. , Kanchana, J. , Calhoun, V. D. , Laura, M. , Stevens, M. C. , Robert, S. , … Pearlson, G. D. (2010). Abnormal functional connectivity of default mode sub‐networks in autism spectrum disorder patients. NeuroImage, 53(1), 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew, N. J. , & Williams, D. L. (2007). The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology, 64(7), 945–950. 10.1001/archneur.64.7.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, S. , Jao Keehn, R. J. , Berkebile, M. M. , Maximo, J. O. , Witkowska, N. , & Muller, R. A. (2018). Local resting state functional connectivity in autism: Site and cohort variability and the effect of eye status. Brain Imaging and Behavior, 12(1), 168–179. 10.1007/s11682-017-9678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel, M. B. , Eloyan, A. , Nettles, C. A. , Sweeney, K. L. , Ament, K. , Ward, R. E. , … Mostofsky, S. H. (2016). Intrinsic visual‐motor synchrony correlates with social deficits in autism. Biological Psychiatry, 79(8), 633–641. 10.1016/j.biopsych.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi, J. S. , Farrant, K. , Damaraju, E. , Rachakonda, S. , Calhoun, V. D. , & Uddin, L. Q. (2016). Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Human Brain Mapping, 37(5), 1770–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel, M. , Mennes, M. , Marquand, A. , Charman, T. , Tillmann, J. , Ecker, C. , … group, E.‐A. L . (2019). Altered connectivity between cerebellum, visual, and sensory‐motor networks in autism Spectrum disorder: Results from the EU‐AIMS longitudinal European autism project. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 260–270. 10.1016/j.bpsc.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Patriat, R. , Molloy, E. K. , Meier, T. B. , Kirk, G. R. , Nair, V. A. , Meyerand, M. E. , … Birn, R. M. (2013). The effect of resting condition on resting‐state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage, 78, 463–473. 10.1016/j.neuroimage.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin, M. A. , DeRamus, T. , Libero, L. E. , Laird, A. , & Kana, R. K. (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Human Brain Mapping, 37(11), 3957–3978. 10.1002/hbm.23288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti, M. G. , Bolton, T. A. , & Ville, D. V. D. (2016). The dynamic functional connectome: State‐of‐the‐art and perspectives. NeuroImage, 160, 41–54. [DOI] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Wolf, D. H. , Loughead, J. , Ruparel, K. , Elliott, M. A. , Hakonarson, H. , … Gur, R. E. (2012). Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkopf, B. , & Smola, A. J. (2002). Learning with kernels : Support vector machines, regularization, optimization, and beyond. Cambridge: MIT Press. [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America, 107(21), 9885–9890. 10.1073/pnas.1001414107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2011). Functional connectivity hubs in the human brain. NeuroImage, 57(3), 908–917. 10.1016/j.neuroimage.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Abnormal functional connectivity in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 71(5), 443–450. 10.1016/j.biopsych.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Weng, S.‐J. , Wiggins, J. L. , Peltier, S. J. , Carrasco, M. , Risi, S. , Lord, C. , & Monk, C. S. (2010). Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research, 1313, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt, C. , & Craig, C. (2013). Sensory‐motor problems in autism. Frontiers in Integrative Neuroscience, 7, 51 10.3389/fnint.2013.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , Cheung, B. , Kelly, C. , Colcombe, S. , Craddock, R. C. , Di Martino, A. , … Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , & Zang, Y.‐F. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenkoyan, K. , Grigoryan, A. , Fereshetyan, K. , & Yepremyan, D. (2017). Advances in understanding the pathophysiology of autism spectrum disorders. Behavioural Brain Research, 331, 92–101. 10.1016/j.bbr.2017.04.038 [DOI] [PubMed] [Google Scholar]
- Zou, Q. , Long, X. , Zuo, X. , Yan, C. , Zhu, C. , Yang, Y. , … Zang, Y. (2009). Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: A resting‐state fMRI study. Human Brain Mapping, 30(9), 3066–3078. 10.1002/hbm.20728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available in Autism Brain Imaging Data Exchange (ABIDE, http://fcon_1000.projects.nitrc.org/indi/abide/) database.


