Abstract
Objective:
The aim of this study was to compare the health of vaccinated versus unvaccinated pediatric populations.
Methods:
Using data from three medical practices in the United States with children born between November 2005 and June 2015, vaccinated children were compared to unvaccinated children during the first year of life for later incidence of developmental delays, asthma, ear infections and gastrointestinal disorders. All diagnoses utilized International Classification of Diseases–9 and International Classification of Diseases–10 codes through medical chart review. Subjects were a minimum of 3 years of age, stratified based on medical practice, year of birth and gender and compared using a logistic regression model.
Results:
Vaccination before 1 year of age was associated with increased odds of developmental delays (OR = 2.18, 95% CI 1.47–3.24), asthma (OR = 4.49, 95% CI 2.04–9.88) and ear infections (OR = 2.13, 95% CI 1.63–2.78). In a quartile analysis, subjects were grouped by number of vaccine doses received in the first year of life. Higher odds ratios were observed in Quartiles 3 and 4 (where more vaccine doses were received) for all four health conditions considered, as compared to Quartile 1. In a temporal analysis, developmental delays showed a linear increase as the age cut-offs increased from 6 to 12 to 18 to 24 months of age (ORs = 1.95, 2.18, 2.92 and 3.51, respectively). Slightly higher ORs were also observed for all four health conditions when time permitted for a diagnosis was extended from ⩾ 3 years of age to ⩾ 5 years of age.
Conclusion:
In this study, which only allowed for the calculation of unadjusted observational associations, higher ORs were observed within the vaccinated versus unvaccinated group for developmental delays, asthma and ear infections. Further study is necessary to understand the full spectrum of health effects associated with childhood vaccination.
Keywords: Vaccination, developmental delays, asthma, ear infections, gastrointestinal disorders
Introduction
Vaccination is considered to be one of the most important advances in modern public health.1 Currently, children between birth and 6 years of age receive up to 36 vaccine doses to protect against 14 different diseases, according to the Centers for Disease Control and Prevention’s (CDC) recommended schedule.2 By ages 1 and 2 years, the CDC recommends approximately 21 and 28 such vaccination doses, respectively. The number of vaccine doses received by infants and children has increased most notably since the early 1990s, when the hepatitis B and Haemophilus influenzae type B vaccines were introduced. Currently, children in the United States are vaccinated for hepatitis A and B, Haemophilus influenzae type B, diphtheria, pertussis, tetanus, polio, measles, mumps, rubella, rotavirus, pneumococcal pneumonia, influenza and varicella.
Although short-term clinical testing is completed on individual vaccines (with limited longer-term follow-up for specific vaccine adverse events) prior to approval by the US Food and Drug Administration (FDA), the health outcomes related to these vaccines and the vaccination schedule as a whole are largely unknown.3 For instance, Kuter et al.4 detailed 23 different post-licensing trials conducted on the measles, mumps and rubella (MMR)-II vaccine and in no instance were the patients followed for more than 42 days post-vaccination. In 2011, the Institute of Medicine (IOM)5 published the report “Adverse Effects of Vaccines: Evidence and Causality” where the relationships between specific vaccines and different adverse health effects were considered. Based on the current scientific literature, the IOM committee found inadequate evidence to accept or reject a causal relationship between 135 of 158 relationships between vaccines and adverse events. Among the remaining 23 adverse events, 18 were found to be associated with vaccination and 5 were not.
The medical community does in general acknowledge that vaccination is not without health risks, including death.6 However, it is widely purported that these side effects or “adverse events” are extremely rare and justified compared to the overall benefit of vaccination.7 There have been very few studies reported where health effects of the US infant and childhood vaccination schedule have been assessed. This is in part based on ethical concerns of withholding vaccination from an unvaccinated control group within such a study.8 Indeed, this precludes the use of double-blinded placebo studies on vaccine health effects, and even in clinical trials an earlier version of the same vaccine is often used as the placebo control for the newly tested vaccine.
One study, published by Mawson et al.,3 was based on a convenience sample of homeschooled children where a significant portion of the sample (39%) was unvaccinated. In this small sample, vaccinated children showed higher odds of being diagnosed with pneumonia, otitis media, allergies and neurodevelopmental disorders. In addition, preterm birth coupled with vaccination significantly increased the odds of a neurodevelopmental disorder diagnosis. This study was unique in the inclusion of entirely unvaccinated populations to provide a comparison to partially vaccinated and fully vaccinated children. However, the risk of bias is high when comparing vaccinated versus unvaccinated children. Also, health outcomes were based on parental survey, not confirmed by medical chart review, and may be subject to recall bias, and the small size of the sample (666 patients) made it difficult to analyze for rare disorders.
Between 2001 and 2004, the IOM9 Immunization Safety Review Committee rejected a relationship between multiple vaccinations and sudden infant death syndrome (SIDS) but could not rule out a relationship with other types of “sudden unexpected infant death.” This included the neonatal hepatitis B vaccine as well as the diphtheria and tetanus toxoids and whole-cell pertussis (DTwP) vaccine, which was strongly associated with anaphylaxis but is no longer given in the United States. A relationship between multiple vaccines and type 1 diabetes was ruled out, but evidence was inadequate to accept or reject a relationship with asthma.10 In addition, the committee rejected a relationship between multiple vaccines and increased “heterologous” infections, such as bacterial infections unrelated to vaccine-preventable diseases, although recent studies have provided evidence of both beneficial and detrimental non-specific effects associated with several vaccines.11–13 The remainder of the IOM Immunization Safety Review Committee focused on single types of vaccines and specific adverse events as recommended by the CDC who commissioned these studies.
In the study presented here, children from three different pediatric medical practices in the United States were used as a convenience sample for comparing patients vaccinated and unvaccinated within the first year of life. Vaccination records were based on data within each practice’s electronic medical records (EMRs) system. Four different diagnoses were evaluated, along with one control diagnosis presumed not to correlate with vaccination status. To allow time for a diagnosis to be made, children were a minimum of 3 years of age for each analysis completed (except for Table 9, where the minimum age was extended).
Table 9.
Vaccinated versus unvaccinated (during the first year of life), stratified based on medical practice, gender and year of birth (child ⩾ 5 years of age).
| Diagnosis | Vaccinated Cases/total |
Unvaccinated Cases/total |
Odds ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Developmental delay | 83/800 (10.4%) |
14/272 (5.1%) |
2.36 (1.29–4.31) | 0.0051 |
| Asthma | 45/803 (5.6%) |
4/273 (1.5%) |
4.93 (1.75–13.9) | 0.0026 |
| Ear infection | 168/648 (25.9%) |
40/235 (17.0%) |
2.49 (1.65–3.76) | <0.0001 |
| Gastrointestinal disorder | 37/776 (6.5%) |
6/268 (2.2%) |
2.48 (1.02–6.02) | 0.045 |
| Head injury | 63/797 (7.9%) |
16/270 (5.9%) |
1.58 (0.89–2.81) | 0.12 |
CI: confidence interval.
Materials and methods
Source of data
Patient data were obtained from EMR systems from three pediatric practices in the United States. All data used directly for the study were first de-identified such that specific patient identification could not be made from the source files used in statistical programming. The Institutional Review Board at Simpson University for research with human subjects reviewed and authorized this analysis independent of the researchers.
Patients in the study were a minimum of 3 years of age and continuously enrolled in their medical practice from birth to June 2018. All patients were born after November 2005. The process of cohort selection is shown in Figure 1. Vaccination date, age at the time of vaccination and type (when available) were obtained from practice EMRs and tabulated in a separate, de-identified data file. All diagnoses considered were based on appropriate International Classification of Diseases (ICD)-9 and ICD-10 codes. Diagnoses considered included developmental delays, asthma, ear infections and gastrointestinal disorders. Head injury was included as a negative control outcome, or control diagnosis, presumed not to be associated with vaccination status. Other diagnoses, including autism and ADD/ADHD, were considered for assessment. However, insufficient numbers of cases existed among the practices to complete a rigorous statistical analysis.
Figure 1.
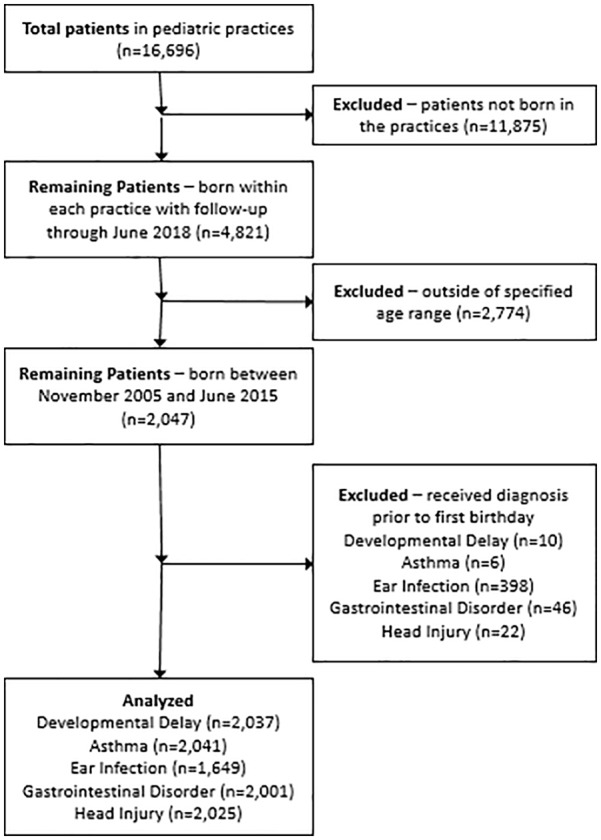
Creation of study cohorts for each analysis.
Diagnosis codes (ICD-9 and ICD-10) used for each condition are shown in Table 1. Truncated codes, for example, ICD-9 code 315 (specific delays in development) as a broad category for developmental delays, include all codes under that classification. An ICD-9 code of 315.9 (unspecified delay in development) would, therefore, be counted as a case in the category “developmental delay.” Also, in some instances, such as “gastrointestinal disorders,” a range of ICD-9 and ICD-10 codes was used to determine cases. Specifically for gastrointestinal disorders, only non-infective enteritis and colitis were considered.
Table 1.
Diagnosis codes used.
| Diagnosis | ICD-9 code(s) | ICD-10 code(s) | Description |
|---|---|---|---|
| Developmental delay | 315 | F80–F82 | Specific delays in development |
| Asthma | 493 | J45 | Asthma, excludes wheezing, not otherwise specified |
| Ear infection | 382 | H66, H67 | Suppurative and unspecified otitis media |
| Gastrointestinal disorder | 555–558 | K50–K52 | Non-infective enteritis or colitis |
| Head injury | 959.01 | S00–S09 | Head injury (non-specific) |
ICD: International Classification of Diseases.
Since ear infections may occur more than once in the same child, cases were identified as children who received the diagnosis code in at least one medical provider visit. Thus, for example, children who had one ear infection or multiple ear infections were counted as cases and children with no reported ear infections were counted as non-cases.
Patients in the “vaccinated” category received a minimum of one vaccine dose prior to their first birthday plus 15 days to capture 1-year vaccines as recommended in the CDC schedule, whereas “unvaccinated” patients had no vaccine doses on record prior to their first birthday plus 15 days. Number of vaccine doses received prior to 1 year of age was calculated as the number of times an ICD-9 or ICD-10 code for vaccination was recorded in the patient’s EMR. This age cut-off was used because the largest proportion of vaccines given based on the US CDC infant and child vaccination schedule is administered prior to 1 year of age (21 vaccine doses from birth to 1 year of age versus 33 vaccine doses from 1 to 18 years of age). This also accounted for multiple vaccine doses given in a single visit to the medical provider. (Tetanus–diphtheria–acellular pertussis (TdaP) and MMR, among other combination vaccinations, were counted as one vaccine, although they consist of three vaccines in a single injection.) Due to differences in recording practices among the participating pediatricians, no attempts were made in this study to differentiate between the types of vaccines administered to these infants. In addition, due to unavailability of the type of vaccine given in each visit in one of the medical practices, temporal relationships between specific vaccines and diagnoses were not taken into account.
Analysis method
This study employed a cohort study design with strata for medical practice, year of birth and gender. Cases were evaluated against non-cases for an association between vaccination status and the different health conditions considered using a conditional logistic regression model. SAS® University Edition was used for statistical analyses with relationships deemed significant at p < 0.05 without correction for the number of statistical tests performed. In general, with a sample size of approximately 2000 subjects, the study was designed to have a power of 80% to detect odds ratios of 1.8 (α = 0.05 and a confidence level of 0.95), but because of some more rare diagnoses, 80% power in select instances was only sufficient to detect odds ratios of 2.4 and above. No covariates were considered in this model due to the lack of availability of relevant maternal and birth data.
In the primary analysis (Table 4), outcomes for “vaccinated children” were compared directly to those for “unvaccinated children.” Children who received no vaccines during the first year of life (plus 15 days) were considered as “unvaccinated” regardless of vaccines that might have been received after their first birthday. The unvaccinated group consisted of 83.7% children unvaccinated within their entire EMR and 16.3% children who received their first vaccine after 1 year of age, based on the 3-year-old and above total cohort. This analysis was completed on all children as well as males and females separately (Tables 5 and 6). Diagnoses were considered for both vaccinated and unvaccinated subjects only when they occurred after the first birthday (plus 15 days). Children receiving diagnoses prior to their first birthday (plus 15 days) were excluded from each specific analysis.
Table 4.
Vaccinated versus unvaccinated (during the first year of life), stratified based on medical practice, gender and year of birth (child ⩾ 3 years of age).
| Diagnosis | Vaccinated Cases/total |
Unvaccinated Cases/total |
Odds ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Developmental delay | 153/1407 (10.9%) |
34/630 (5.4%) |
2.18 (1.47–3.24) | 0.0001 |
| Asthma | 67/1412 (4.7%) |
7/629 (1.1%) |
4.49 (2.04–9.88) | 0.0002 |
| Ear infection | 324/1116 (29.0%) |
104/533 (19.5%) |
2.13 (1.63–2.78) | <0.0001 |
| Gastrointestinal disorder | 55/1382 (4.0%) |
18/619 (2.9%) |
1.47 (0.84–2.57) | 0.17 |
| Head injury | 93/1398 (6.7%) |
31/627 (4.9%) |
1.26 (0.82–1.94) | 0.29 |
CI: confidence interval.
Table 5.
Males only, vaccinated versus unvaccinated (during the first year of life), stratified based on medical practice and year of birth (child ⩾ 3 years of age).
| Diagnosis | Vaccinated Cases/total |
Unvaccinated Cases/total |
Odds ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Developmental delay | 107/714 (15.0%) |
27/343 (7.9%) |
1.92 (1.21–3.04) | 0.0054 |
| Asthma | 40/716 (5.6%) |
3/342 (0.9%) |
6.89 (2.10–22.6) | 0.0015 |
| Ear infection | 170/554 (30.7%) |
62/290 (21.4%) |
2.07 (1.45–2.57) | <0.0001 |
| Gastrointestinal disorder | 29/701 (4.1%) |
10/337 (3.0%) |
1.51 (0.70–3.23) | 0.29 |
| Head injury | 51/710 (7.2%) |
21/342 (6.1%) |
1.05 (0.61–1.80) | 0.87 |
CI: confidence interval.
Table 6.
Females only, vaccinated versus unvaccinated (during the first year of life), stratified based on medical practice, gender and year of birth (child ⩾ 3 years of age).
| Diagnosis | Vaccinated Cases/total |
Unvaccinated Cases/total |
Odds ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Developmental delay | 46/693 (6.6%) |
7/287 (2.4%) |
3.10 (1.37–7.01) | 0.0068 |
| Asthma | 27/696 (3.9%) |
4/287 (1.4%) |
2.70 (0.93–7.87) | 0.068 |
| Ear infection | 154/562 (27.4%) |
42/243 (17.3%) |
2.20 (1.48–3.26) | <0.0001 |
| Gastrointestinal disorder | 26/681 (3.8%) |
8/282 (2.8%) |
1.44 (0.64–3.25) | 0.39 |
| Head injury | 42/688 (6.1%) |
10/285 (3.5%) |
1.69 (0.83–3.43) | 0.15 |
CI: confidence interval.
In the second analysis (Table 7), subjects were separated into quartiles based on the number of vaccine doses received within the first year of life (plus 15 days) calculated based on the distribution among the sample with a median of nine vaccine doses. The first quartile included children who received 1–5 vaccine doses (n = 353), the second included children who received 6–10 vaccine doses (n = 390), the third included children who received 11–12 vaccine doses (n = 417) and the fourth included children who received 13–21 vaccine doses (n = 254). Diagnoses for conditions within this analysis were considered only if they were made after each child’s first birthday (plus 15 days). This analysis was limited to vaccine doses received in the first year of life to capture a significant portion of the diagnoses that may occur early in life, including ear infections and gastrointestinal disorders.
Table 7.
Quartile analysis, vaccinated versus unvaccinated (during the first year of life), stratified based on medical practice, year of birth and gender (child ⩾ 3 years of age).
| Diagnosis | Quartile 1 1–5 vaccines (95% CI) |
Quartile 2 6–10 vaccines (95% CI) |
Quartile 3 11–12 vaccines (95% CI) |
Quartile 4 13–21 vaccines (95% CI) |
|---|---|---|---|---|
| Developmental delay | 1.36 (0.53–3.48) | 2.54 (1.30–4.96) | 3.22 (1.70–6.09) | 2.42 (1.17–4.99) |
| Asthma | 1.94 (0.59–6.40) | 6.48 (2.64–15.9) | 3.66 (1.42–9.46) | 4.62 (1.68–12.7) |
| Ear infection | 1.43 (0.98–2.07) | 2.48 (1.72–3.60) | 2.26 (1.53–3.33) | 2.81 (1.80–4.40) |
| Gastrointestinal disorder | 0.49 (0.19–1.31) | 1.61 (0.68–3.84) | 3.77 (1.65–8.59) | 4.03 (1.57–10.3) |
| Head injury | 0.68 (0.32–1.44) | 1.56 (0.93–2.62) | 1.12 (0.65–1.94) | 1.37 (0.73–2.56) |
CI: confidence interval.
In the third analysis (Table 8), vaccination status was considered at separate age intervals from birth to 6 months, 1 year, 18 months and 2 years in four separate analyses. Diagnoses were considered only after the age interval of vaccination. A fourth analysis (Table 9) was also completed which was identical to the first analysis (considering vaccination status up to the first birthday). However, the age cut-off for the cohort was 5 years and above, rather than 3 years and above, to give additional time for children to be diagnosed with the conditions considered.
Table 8.
Temporal analysis, vaccinated versus unvaccinated (during 6, 12, 18 and 24 months of life), stratified based on medical practice, year of birth and gender (child ⩾ 3 years of age).
| Diagnosis | 6 months (95% CI) |
12 months (95% CI) |
18 months (95% CI) |
24 months (95% CI) |
|---|---|---|---|---|
| Developmental delay | 1.95 (1.35–2.84) | 2.18 (1.47–3.24) | 2.92 (1.81–4.72) | 3.51 (1.94–6.35) |
| Asthma | 3.10 (1.64–5.85) | 4.49 (2.04–9.88) | 3.74 (1.69–8.28) | 5.99 (2.15–16.7) |
| Ear infection | 1.97 (1.58–2.46) | 2.13 (1.63–2.78) | 2.22 (1.61–3.05) | 2.08 (1.42–3.04) |
| Gastrointestinal disorder | 2.02 (1.23–3.33) | 1.48 (0.84–2.57) | 1.45 (0.74–2.82) | 1.25 (0.60–1.45) |
| Head injury | 1.32 (0.88–1.99) | 1.26 (0.82–1.94) | 1.77 (1.04–3.01) | 1.29 (0.73–2.29) |
CI: confidence interval.
Results
Demographic data
Demographic data for the study sample are shown in Table 2. The overall sample size, including children under 3 years of age, is 4821, of which 44.5% were unvaccinated, while 55.5% were vaccinated. Among the 3797 children over 1 year of age, 37.6% were unvaccinated and 62.4% were vaccinated. Considering children with continuous follow-up who were over 3 years of age reduced the sample to 2047 patients, with 52% males. Unvaccinated children by 1 year of age comprised 30.9% of the sample as compared to vaccinated children (69.1%). The most prevalent diagnosis was ear infection.
Table 2.
Demographic data.
| Category | Male | Female | Total |
|---|---|---|---|
| Total sample | 2483 | 2338 | 4821 |
| Over 3 years of age | 1063 | 984 | 2047 |
| Unvaccinated by age 1 year | 345 | 288 | 633 (30.9%) |
| Vaccinated by age 1 year | 718 | 696 | 1414 (69.1%) |
| First vaccine after age 1 year | 64 | 39 | 103 (16.3%)a |
| Developmental delay | 140 | 57 | 197 (9.6%) |
| Asthma | 48 | 32 | 80 (3.9%) |
| Ear infection | 451 | 375 | 826 (40.4%) |
| Gastrointestinal disorder | 64 | 55 | 119 (5.8%) |
| Head injury | 83 | 63 | 146 (7.1%) |
Percentage of unvaccinated sample by age 1 year.
Additional demographic data in Table 3 include the number of vaccines administered prior to each child’s first birthday (range = 1–21), the age of first vaccination in days (mean = 102, or 3.3 months) and the age of the children at the conclusion of the study period (mean = 5.6 years). Finally, the ages of the first diagnosis for each of the conditions considered in the analyses are included. While diagnoses, such as developmental delays, asthma and head injury, occurred generally after the 1-year cut-off age for the analyses, a significant number of ear infection (48.2%) and gastrointestinal disorder (38.7%) diagnoses were made prior to the first birthday.
Table 3.
Additional demographic data (children aged 3 years and above).
| Variable | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|
| Number of vaccines (vaccinated by age 1 year) | 8.9 | 4.1 | 1 | 21 |
| Age of first vaccine (days, vaccinated by age 1 year) | 102 | 65 | 2 | 380 |
| Age as of June 2018 (years) | 5.6 | 2.2 | 3 | 12.8 |
| Age of developmental delay diagnosis (days/years) | 775/2.1 | 458/1.3 | 113/0.31 | 2284/6.3 |
| Age of asthma diagnosis (days/years) | 1156/3.2 | 608/1.7 | 274/0.75 | 2616/7.2 |
| Age of ear infection diagnosis (days/years) | 520/1.4 | 464/1.3 | 3/0.01 | 4393/12.0 |
| Age of gastrointestinal disorder diagnosis (days/years) | 647/1.8 | 556/1.5 | 27/0.07 | 4073/11.2 |
| Age of head injury diagnosis (days/years) | 1034/2.8 | 767/2.1 | 33/0.09 | 3714/10.2 |
Statistical analysis
Table 4 shows results when cases were compared to non-cases in vaccinated versus unvaccinated categories (3 years of age and above with diagnoses considered only after the first birthday). Vaccination before 1 year of age was associated with increased odds of developmental delays (odds ratio, OR = 2.18, 95% CI 1.47–3.24), asthma (OR = 4.49, 95% CI 2.04–9.88) and ear infections (OR = 2.13, 95% CI 1.63–2.78). No relationship was observed for gastrointestinal disorders and head injuries (the control diagnosis). Similar results were observed for males only (Table 5) with a sharp increase in the OR for asthma (6.89, 95% CI 2.10–22.6, p = 0.0015). In females only (Table 6), an increase in OR was observed for developmental delays (OR = 3.10, 95% CI 1.37–7.01, p = 0.0068). Confidence intervals for this relationship are consistent with overall and “males only” results. Also for females only, the result for asthma fell below the level of significance (p = 0.068). The remainder of the conditions studied showed responses consistent with previous results for males and the entire sample.
Results from the quartile analysis, assessing number of vaccine doses received over the first year of life compared to unvaccinated children, are shown in Table 7. Higher ORs were observed in Quartiles 3 and 4 (where more vaccine doses were received) for all four health conditions considered, as compared to Quartile 1. A consistent linear increase in ORs with increasing vaccine doses is observed for gastrointestinal disorders, although the relationship is only significant in the third and fourth quartiles (OR = 3.77, 95% CI 1.65–8.59 and OR = 4.03, 95% CI 1.57–10.3, respectively). Relationships for asthma and developmental delay are non-significant for the first quartile only but ORs peak within the second quartile for asthma and within the third quartile for developmental delay, followed by a decline—although still highly significant—within subsequent quartiles. The control diagnosis does not show a relationship in any of the quartiles.
Within the temporal analysis (results shown in Table 8), vaccines were considered to the cut-off ages (6, 12, 18 and 24 months) and diagnoses were included only after those cut-off ages. Thus, the 6-month cut-off would help to account for early diagnoses, especially of ear infections and gastrointestinal disorders which were diagnosed often within the first year of life. The unvaccinated group was comprised of children receiving their first vaccines only after each age cut-off. A consistent linear increase in ORs was observed for developmental delays as the age cut-offs increased from 6 to 12 to 18 to 24 months of age (ORs = 1.95, 2.18, 2.92 and 3.51, respectively). All results for developmental delays were statistically significant as were all results for asthma and ear infections. Asthma, which was associated with the highest mean age of diagnosis of all conditions studied, showed the highest OR at the 24-month cut-off (OR = 5.99, 95% CI 2.15–16.7), similar to the result for developmental delays. However, the increase observed between the 6-month and 24-month cut-offs was not consistent. The ORs for ear infections were nearly constant at all age cut-offs while the relationship for gastrointestinal disorders was highest and significant only at the 6-month cut-off (OR = 2.02, 95% CI 1.23–3.33). A single significant relationship was seen for the head injury control diagnosis at the 18-month vaccination cut-off.
A final analysis was completed similar to the analysis presented in Table 4 but with children in the sample who were 5 years and above prior to the cut-off date of June 2018. Results for this group (Table 9) are consistent with those observed previously. When the time permitted for a diagnosis was extended from children ⩾ 3 years of age to children ⩾ 5 years of age, slightly higher ORs were detected for all four health conditions: developmental delays (OR = 2.36, 95% CI 1.29–4.31), asthma (OR = 4.93, 95% CI 1.75–13.9), ear infections (OR = 2.49, 95% CI 1.65–3.76) and gastrointestinal disorders (OR = 2.48, 95% CI 1.02–6.02).
Discussion
Within this study, the number of vaccines received and vaccination status early in life are related to different acute and chronic conditions. The strongest relationships observed for vaccination status were for asthma, developmental delays and ear infections (Table 4). Although the association between vaccinations and asthma in males was elevated (Table 5), it should be noted that there were only three asthma cases in the unvaccinated group. No association between vaccinations and asthma in females was found (Table 6); this may also be due to just four asthma cases in the unvaccinated group. Although some studies were unable to find correlations between vaccines and asthma,14,15 a relationship between vaccination and allergy/atopy incidence (including asthma) has been reported.16–18 In a study involving Korean children who were all vaccinated against hepatitis B, a significantly higher asthma incidence was seen among children who had actually seroconverted to produce anti-HepB.16 In addition, Hurwitz and Morgenstern17 reported an association between diphtheria–tetanus–pertussis (DTP) and tetanus toxoid vaccination and allergy symptoms and could not rule out a relationship with asthma. In an animal study, mice vaccinated according to the Chinese infant vaccine schedule showed airway hyperresponsiveness at a significantly higher rate than unvaccinated mice.18
The IOM19 Immunization Safety Review Committee conducted an evaluation regarding thimerosal-containing vaccines and concluded that “the hypothesis that exposure to thimerosal-containing vaccines could be associated with neurodevelopment disorders” was biologically plausible. Mawson et al.3 found a relationship between vaccination status and learning disability and neurodevelopmental disorders. Delong20 also reported a significant relationship to neurodevelopmental disorders (autism and speech and language delay) when looking at the proportions of vaccine uptake in US children. Other research, focused more on the uptake of specific vaccines, has elucidated such relationships. Gallagher and Goodman21 saw a greater number of boys receiving special education services if they had received the entire hepatitis B vaccine series in infancy. Geier et al.22–24 also documented a link between neurodevelopmental disorders and thimerosal-containing vaccines. (Although thimerosal has been phased out of most vaccines administered in the United States, it still remains in some formulations of the influenza vaccine given to pregnant women and infants.)
Mawson et al.3 reported a significant relationship between vaccination status and ear infections. Wilson et al.25 found that for both males and females, top reasons for emergency room visits and/or hospital admissions after their 12-month vaccinations included ear infections and non-infective gastroenteritis or colitis. Prior to the RotaTeq rotavirus vaccine achieving FDA approval, 71,725 infants were evaluated in three placebo-controlled clinical trials. Otitis media (middle ear infection) occurred at a statistically higher incidence (p < 0.05) within 6 weeks of any dose among the recipients of RotaTeq as compared with the recipients of placebo.26
Within the quartile analysis (Table 7), asthma was non-significant in the first quartile, peaked in the second quartile (OR = 6.48, 95% CI 2.64–15.9), then decreased in the third and fourth quartiles but maintained significance (OR = 3.66, 95% CI 1.42–9.46 and OR = 4.62, 95% CI 1.68–12.7, respectively). Developmental delays followed a similar pattern, although the peak occurred in the third quartile. This may indicate the presence of “healthy user bias” within the overall sample where healthy subjects continue to vaccinate but subjects with health issues limit or curtail further vaccination, as defined previously by Fine and Chen.27 These authors discussed the phenomenon where avoidance or delay of vaccination is associated with an increased risk of vaccine adverse events. In other words, healthier vaccinated children are more likely to stay “up-to-date” with vaccinations, whereas children showing health issues may opt for a delayed schedule or to skip specific vaccines. In the context of their article, Fine and Chen pointed out that this may confound analyses of risks associated with vaccinated versus unvaccinated children leading to an under-ascertainment of risk. However, in the analysis presented in this article, the number of vaccine doses was compared (through quartiles) directly to fully unvaccinated children to minimize such bias. In contrast to asthma and developmental delays, higher ORs were observed in Quartiles 3 and 4 for all four health conditions considered, as compared to Quartile 1, which may indicate a cumulative effect of vaccine doses.
The temporal analysis (Table 8) allowed different cut-off ages of vaccination status and diagnosis. For example, at 6 months, only vaccine doses between birth and 6 months were counted and diagnoses were considered only after 6 months of age. The earlier cut-off of 6 months allowed the accounting of more diagnoses of ear infections and gastrointestinal disorders which possess an earlier mean diagnosis age. However, this resulted in a trade-off whereby fewer vaccinated children were available to assess. Conversely, at 24 months, a greater number of vaccinated children were accounted for but at the expense of diagnoses prior to that age cut-off. Interestingly, developmental delays, which possessed a higher mean age of diagnosis showed a linear increase in ORs with increasing cut-off age. Asthma, which possessed the highest mean age of diagnosis of all conditions studied also showed the highest OR at the 24-month cut-off. However, the increase observed between the 6-month and 24-month cut-offs was not consistent and may reflect the low number of asthma cases in the overall sample. The OR for gastrointestinal disorders was highest and significant only at the 6-month cut-off, which may suggest a connection with earlier vaccination in children. A single significant relationship was seen for the head injury control diagnosis at the 18-month vaccination cut-off, which may be indicative of differences in healthcare-seeking behavior among families of vaccinated versus unvaccinated children. This might also be an artifact of the small number of injuries overall in the analysis group which could introduce granularity within analyses involving subgroups of vaccinated subjects (Tables 7 and 8). This limits our ability to see potential confounding and bias within this study.
In the final analysis (Table 9), higher ORs were detected for all four health conditions when the time permitted for a diagnosis was extended from children ⩾ 3 years of age to children ⩾ 5 years of age. This higher age requirement allowed additional time for children to receive diagnoses, which is important especially for developmental delays and asthma which are diagnosed later within the sample (Table 3). However, this also resulted in fewer children overall, including only four children with an asthma diagnosis in the unvaccinated group.
Statistical significance was seen for gastrointestinal disorders when considering the third and fourth quartiles of vaccine doses, at the 6-month cut-off age in the temporal analysis, and when additional time was permitted for a diagnosis. The remaining analyses did not show a relationship. Although Wilson et al.25 found an association between 12-month vaccinations and emergency room visits for non-infective gastroenteritis, there is a paucity of research elsewhere regarding gastroenteritis following vaccination, with the majority focused on intussusception following the rotavirus vaccine.28–31 Other reports have attributed gastrointestinal disorders as adverse events following the oral polio vaccine32 and the human papillomavirus vaccine.33
Study strengths
One of the main strengths of this study is that the data are based directly on patient chart records and diagnosis codes. Practitioners making these diagnoses were also directly available for consultation on how specific diagnosis codes were applied. In addition, vaccination records were based on patient chart data, although coding practices for vaccination varied among the three different pediatric practices. To account for any differences in diagnosing among the three different practices, cases and non-cases were stratified based on medical practice. Thus, no “cross comparisons” were made among two or more medical practices. To account for differences in likelihood of particular diagnoses based on the age and gender of the patient, cases and non-cases were stratified based on the year of birth and gender.
It is possible that diagnoses may have been missed or information regarding vaccines administered could have been incorrectly recorded leading to exposure misclassification, which might explain the high rates of unvaccinated children in the cohort. However, all children considered in the study were enrolled in their medical practice from birth and followed up continuously to minimum age cut-offs of 3 years (Tables 4–8) and 5 years (Table 9). This minimized the risk of missing vaccination doses or diagnoses associated with tracking patients with multiple practitioners. This also eliminated recall bias associated with studies focused on parental surveys. The high proportion of unvaccinated children is most likely indicative of pediatric practices which accepted unvaccinated and partially vaccinated children into their case load.
Also, cut-off dates (e.g. 1 year plus 15 days) established clear boundaries between the time when a child’s vaccination status could be determined and when diagnoses would be considered. Any vaccines received by the child were tallied prior to the cut-off and diagnoses were considered only after the cut-off. Any child receiving a diagnosis prior to the age cut-off was eliminated from that portion of the analysis. In this respect, this study focuses more on vaccines received earlier in life rather than those received after 1 and 2 years of age. For the 1-year and 2-year cut-offs, 83.7% and 91.1% of individuals were by definition “completely unvaccinated,” respectively (calculated based on the entire unvaccinated sample for each cut-off), whereas the remainder received their first vaccines after the cut-off age. This would tend to exert bias toward the null hypothesis as diagnoses in the “unvaccinated” group could instead be those in the vaccinated group.
Finally, effect estimates in this article were generally above 2.0. Thus, for some confounder to explain this association, it would need to be twice as frequent in vaccinated children.34
Potential limitations
The main weakness of this study is the use of a convenience sample of three different pediatric practices. In addition, the size of the sample, although sufficient for some diagnoses, such as the five main conditions studied, was too small for analysis of conditions with lower prevalence, such as autism. Also, this sample may not accurately represent a cross-section of US children given the low incidence of autism (0.5%) and ADD/ADHD (0.7%) compared to incidences observed nationwide (at 1.7%35 and between 5% and 9%,36 respectively). In addition, vaccine uptake, which is approximately 95% nationwide, is rather low in these practices and may reflect demographic differences between the study sample and the general population. Also, due to different coding practices among the three caseloads studied, we were unable to differentiate between the types of vaccinations given. This limited the analysis to counting the number of vaccinations received by 1 year of age.
The low level of vaccine uptake overall in these practices (mean = 8.9 vaccines by 1 year of age) obviates our ability to do a comparison between fully vaccinated and unvaccinated children within this cohort. Also, the median age at first vaccine dose in the cohort was 81 days (just under 3 months) as compared to the hepatitis B vaccine that is recommended within 24 h of birth. Medical chart records did not include specific demographic factors that may be associated with health outcomes, including socioeconomic status, maternal education, gestational age at birth, Appearance, Pulse, Grimace, Activity and Respiration (APGAR) score, type of birth and duration of breastfeeding, among others. The “hygiene hypothesis” has shown relationships between type of birth/breastfeeding and allergies, asthma and eczema.37,38 There are undoubtedly demographic differences within the two groups studied (vaccinated versus unvaccinated), especially regarding socioeconomic status and maternal education. According to Smith et al.,39 mothers in families where vaccines were delayed and refused tended to have higher levels of college education and families were more affluent. Although there are no direct studies on gestational age at birth in vaccinating versus non-vaccinating families, Zerbo et al.40 indicated that children born to women receiving the influenza vaccine during pregnancy had significantly higher gestational age. Dueker et al.41 showed that each week of gestational age beyond 35–41 weeks significantly decreased developmental delays in infants. In addition, children born prematurely (34–37 weeks) also showed a higher rate of hospitalizations for asthma.42
It was also difficult to discern healthcare-seeking behavior among families of vaccinated versus unvaccinated children outside of assessment of the control diagnosis, head injury, which showed significance only within one group in the temporal analysis. The three participating medical practices recommended that all children go to well-child visits regardless of whether they were receiving vaccines. However, none of the practices kept data on the frequency of visits. If more vaccinated than unvaccinated children showed up at these check-ups, this would be indicative of a difference in healthcare-seeking behavior and could lead to more diagnoses in the group that was seen by the practitioner more often. There was a higher proportion of unvaccinated children in the overall sample as compared to those who were included in the main analysis, which could be indicative of divergent healthcare-seeking behavior. However, the overall sample included children who were excluded from the main analysis because they were younger than the study permitted (Figure 1). Many of these children were classified as unvaccinated prior to their exclusion although their true vaccination status was indeterminate as they had not yet achieved 1 year (and 15 days) of age. This had the effect of artificially inflating the proportion of unvaccinated children in the overall sample.
Glanz et al.43 reported that undervaccinated children showed significantly lower rates of outpatient medical provider visits (incidence risk ratio = 0.89, 95% CI 0.89–0.90) within a large retrospectively matched cohort study involving the CDC’s Vaccine Safety Datalink. However, in this study, consistent relationships were observed within three of the health conditions considered as compared to marginal significance seen for head injury in only one analysis involving a subgroup of the cohort. Homeschooling families have been shown to have lower vaccination rates44 which may also contribute to differences in healthcare-seeking behavior given that homeschooled children could be underdiagnosed. This type of demographic data was not available for the analysis.
Recent studies have shown that some vaccines have non-specific effects that either increase or decrease susceptibility to infectious diseases not targeted by the vaccine. The most recent vaccine administered exerts the greatest effect. Live vaccines, such as measles, MMR and Bacillus Calmette–Guérin (BCG), tend to lower risk (providing a protective influence), while non-live vaccines, such as hepatitis B,11 DTP and inactivated polio (IPV), tend to increase risk. For example, Bardenheier et al.12 found a lower risk of non-targeted infectious disease hospitalizations among children whose last vaccine received was live compared with inactivated vaccine (hazard ratio (HR) = 0.50, 95% CI 0.43–0.57). In a recent meta-analysis conducted by Aaby et al.,13 girls who received an inactivated vaccine after receiving a measles vaccine were significantly more likely to die from other causes compared with girls who received an inactivated vaccine before receiving a measles vaccine (mortality rate ratio (MRR) = 1.89, 95% CI 1.27–2.80). Although this current study did not consider non-specific effects (due to differences in how the three pediatricians recorded patient data), it is possible that the most recent vaccine administered could have influenced the results.
No effort was made to assess children who may have lost diagnoses for chronic disorders, such as developmental delay and asthma. However, according to the CDC, developmental disabilities “usually last throughout a person’s lifetime.”45 Asthma is normally a lifelong chronic condition as well.46 Since losing these diagnoses is rare, this is unlikely to have affected the results.
Conclusion
In this study, based on a convenience sample of children born into one of three distinct pediatric medical practices, higher ORs were observed within the vaccinated versus unvaccinated group for developmental delays, asthma and ear infections. No association was found for gastrointestinal disorders in the primary analysis, but a significant relationship was detected in the third and fourth quartiles (where more vaccine doses were administered), at the 6-month cut-off in the temporal analysis, and when time permitted for a diagnosis was extended from children ⩾ 3 years of age to children ⩾ 5 years of age. Similar results have been observed in earlier studies by Mawson et al.3 and Delong.20 The findings in this study must be weighed against the strengths and limitations of the available data and study design, which only allowed for the calculation of unadjusted observational associations. Additional research utilizing a larger sample from a variety of pediatric medical practices will yield greater certainty in results and allow for the investigation of health conditions with lower prevalence, such as autism. A thorough evaluation of vaccinated versus unvaccinated populations is essential to understanding the full spectrum of health effects associated with specific vaccines and the childhood vaccine schedule in totality.
Acknowledgments
The authors thank Dr David Rice III (Assistant Professor of Biology, Simpson University) for his expert assistance in preliminary setup of the patient database. They also thank Dr Beatrice Golomb (Professor of Medicine, University of California, San Diego) for her critical review of the original study design.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Hooker is a paid scientific advisor and serves on the advisory board for Focus for Health (formerly Focus Autism). He also serves on the Board of Trustees for Children’s Health Defense (formerly World Mercury Project) and is a paid independent contractor of Children’s Health Defense as well. Dr Hooker is the father of a 22-year old male who has been diagnosed with autism and developmental delays. Mr Miller is the director of Thinktwice Global Vaccine Institute and was a paid consultant to Physicians for Informed Consent.
Ethical approval: Ethical approval for this study was waived by the Simpson University Institutional Review Board because the above referenced research project meets the conditions for exemption under 45 CFR 46.101(b)(4). All of the data are in existence as of 1 June 2018 and the information will be recorded in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. The authors have also confirmed that the results of this study will not be submitted to the Food and Drug Administration (FDA) for marketing approval.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not sought for this study because all of the data are in existence as of 1 June 2018 and the information was recorded in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
ORCID iD: Brian S Hooker  https://orcid.org/0000-0003-2010-1899
https://orcid.org/0000-0003-2010-1899
References
- 1. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States 1900-1999. Morb Mortal Wkly Rep 1999; 48: 241–243. [PubMed] [Google Scholar]
- 2. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or young—United States. Morb Mortal Wkly Rep 2018; 67: 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mawson AR, Ray BD, Bhuiyan AR, et al. Pilot comparative study on the health of vaccinated and unvaccinated 6- to 12-year-old U.S. children. J Transl Sci 2017; 3: 1–12. [Google Scholar]
- 4. Kuter BJ, Brown M, Wiedmann RT, et al. Safety and immunogenicity of M-M-RII (Combination Measles-Mumps-Rubella Vaccine) in clinical trials of healthy children conducted between 1988 and 2009. Pediatr Infect Dis J 2016; 35(9): 1011–1020. [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 6. Gold MS, Balakrishnan MR, Amarasinghe A, et al. An approach to death as an adverse event following immunization. Vaccine 2016; 34(2): 212–217. [DOI] [PubMed] [Google Scholar]
- 7. Pollard AJ. Childhood immunisation: what is the future? Arch Dis Child 2007; 92: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Institute of Medicine. The childhood immunization schedule and safety: stakeholder concerns, scientific evidence, and future studies. Washington, DC: The National Academies Press, 2013. [PubMed] [Google Scholar]
- 9. Institute of Medicine. Immunization safety review: vaccinations and sudden unexpected death in infancy. Washington, DC: The National Academies Press, 2003. [PubMed] [Google Scholar]
- 10. Institute of Medicine. Immunization safety review: multiple immunizations and immune dysfunction. Washington, DC: The National Academies Press, 2002. [PubMed] [Google Scholar]
- 11. Garly ML, Jensen H, Martins CL, et al. Hepatitis B vaccination associated with higher female than male mortality in Guinea-Bissau: an observational study. Pediatr Infect Dis J 2004; 23(12): 1086–1092. [PubMed] [Google Scholar]
- 12. Bardenheier BH, McNeil MM, Wodi AP, et al. Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005-2014. Clin Infect Dis 2017; 65(5): 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aaby P, Ravn H, Benn CS, et al. Randomized trials comparing inactivated vaccine after medium- or high-titer measles vaccine with standard titer measles vaccine after inactivated vaccine: a meta-analysis. Pediatr Infect Dis J 2016; 35(11): 1232–1241. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson L, Kjellman NI, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Arch Pediatr Adolesc Med 1998; 152(8): 734–738. [DOI] [PubMed] [Google Scholar]
- 15. Anderson HR, Poloniecki JD, Strachan DP, et al. Immunization and symptoms of atopic disease in children: results from the International Study of Asthma and Allergies in Childhood. Am J Public Health 2001; 91(7): 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yon DK, Ha EK, Lee S, et al. Hepatitis B immunogenicity after a primary vaccination course associated with childhood asthma, allergic rhinitis, and allergen sensitization. Pediatr Allergy Immunol 2018; 29(2): 221–224. [DOI] [PubMed] [Google Scholar]
- 17. Hurwitz EL, Morgenstern H. Effects of diphtheria-tetanus-pertussis or tetanus vaccination on allergies and allergy-related respiratory symptoms among children and adolescents in the United States. J Manipulative Physiol Ther 2000; 23: 81–90. [PubMed] [Google Scholar]
- 18. Zhang JL, Ma Z, Sun WW, et al. Programmed vaccination may increase the prevalence of asthma and allergic diseases. Am J Rhinol Allergy 2016; 30(4): 113–117. [DOI] [PubMed] [Google Scholar]
- 19. Institute of Medicine. Immunization safety review: thimerosal-containing vaccines and neurodevelopmental disorders. Washington, DC: The National Academies Press, 2001. [PubMed] [Google Scholar]
- 20. Delong G. A positive association found between autism prevalence and childhood vaccination uptake across the U.S. population. J Toxicol Environ Health A 2011; 74: 903–916. [DOI] [PubMed] [Google Scholar]
- 21. Gallagher C, Goodman M. Hepatitis B triple series vaccine and developmental disability in U.S. children aged 1-9 years. Toxicol Environ Chem 2008; 90: 997–1008. [Google Scholar]
- 22. Geier DA, Kern JK, Homme KG, et al. Abnormal brain connectivity spectrum disorders following thimerosal administration: a prospective longitudinal case-control assessment of medical records in the vaccine safety datalink. Dose Response 2017; 15(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geier DA, Kern SK, Hooker BS, et al. A longitudinal cohort study of the relationship between thimerosal-containing hepatitis b vaccination and specific delays in development in the United States: assessment of attributable risk and lifetime care costs. J Epidemiol Glob Health 2016; 6(2): 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geier DA, Kern JK, King PG, et al. The risk of neurodevelopmental disorders following a Thimerosal-preserved DTaP formulation in comparison to its Thimerosal-reduced formulation in the Vaccine Adverse Event Reporting System (VAERS). J Biochem Pharmacol Res 2014; 2(2): 64–73. [Google Scholar]
- 25. Wilson K, Ducharme R, Ward B, et al. Increased emergency room visits or hospital admissions in females after 12-month MMR vaccination, but no difference after vaccinations given at a younger age. Vaccine 2014; 32: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 26. Merck & Co., Inc. RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent) (Product insert 2017; Table 5: 6). Whitehouse Station, NJ: Merck & Co., Inc. [Google Scholar]
- 27. Fine PE, Chen RT. Confounding in studies of adverse reactions to vaccines. Am J Epidemiol 1992; 136: 121–135. [DOI] [PubMed] [Google Scholar]
- 28. Haber P, Parashar UD, Haber M, et al. Intussusception after monovalent rotavirus vaccine—United States, vaccine adverse event reporting system (VAERS), 2008-2014. Vaccine 2015; 33(38): 4873–4877. [DOI] [PubMed] [Google Scholar]
- 29. Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 2014; 370(6): 503–512. [DOI] [PubMed] [Google Scholar]
- 30. Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis 2013; 57(10): 1427–1434. [DOI] [PubMed] [Google Scholar]
- 31. Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014; 370(6): 513–519. [DOI] [PubMed] [Google Scholar]
- 32. Nzolo D, Ntetani Aloni M, Mpiempie Ngamasata T, et al. Adverse events following immunization with oral poliovirus in Kinshasa, Democratic Republic of Congo: preliminary results. Pathog Glob Health 2013; 107(7): 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geier DA, Geier MR. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: a case-control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res 2017; 65(1): 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cornfield J, Haenszel W, Hammond EC, et al. Smoking and lung cancer: recent evidence and a discussion of some questions. J Natl Cancer Inst 1959; 22(1): 173–203. [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 2018; 67(6): 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition: DSM-5. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 37. Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol 2011; 38(2): 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson CC, Ownby DR. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl Res 2017; 179: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith PJ, Humiston SG, Marcuse EK, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the health belief model. Public Health Rep 2011; 126(Suppl. 2): 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zerbo O, Qian Y, Yoshida C, et al. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr 2017; 171(1): e163609. [DOI] [PubMed] [Google Scholar]
- 41. Dueker G, Chen J, Cowling C, et al. Early developmental outcomes predicted by gestational age from 35 to 41 weeks. Early Hum Dev 2016; 103: 85–90. [DOI] [PubMed] [Google Scholar]
- 42. Leung JY, Lam HS, Leung GM, et al. Gestational age, birthweight for gestational age, and childhood hospitalisations for asthma and other wheezing disorders. Paediatr Perinat Epidemiol 2016; 30(2): 149–159. [DOI] [PubMed] [Google Scholar]
- 43. Glanz JM, Newcomer SR, Narwaney KJ, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr 2013; 167(3): 274–281. [DOI] [PubMed] [Google Scholar]
- 44. Mohanty S, Joyce CM, Delamater PL, et al. Homeschooling parents in California: attitudes, beliefs and behaviors associated with child’s vaccination status. Vaccine 2020; 38: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention (CDC). Facts about developmental disabilities, https://www.cdc.gov/ncbddd/developmentaldisabilities/facts.html (accessed 30 March 2020).
- 46. Centers for Disease Control and Prevention (CDC). You can control your asthma, https://www.cdc.gov/nceh/features/asthmaawareness/ (accessed 30 March 2020).


