To the Editor:
Shortages of mechanical ventilators during the COVID-19 pandemic have prompted clear messaging about the hazards of ventilating multiple patients with a single ventilator.1 Nonetheless, some hospitals are forced to undertake the practice. A protocol using pressure control ventilation for well-matched patients under deep sedation and neuromuscular blockade2 and novel solutions for some limitations of ventilator splitting have been published.3–5 These recommendations mitigate some concerns about ventilator settings and monitoring.1 Adequate matching of ventilator parameters (driving pressure, respiratory rate, and positive end-expiratory pressure [PEEP]) and continuous or frequent monitoring for each individual patient (oxygen saturation measured by pulse oximetry, end-tidal carbon dioxide, pH, and PCO2) are complimented by monitoring of shared ventilator parameters (e.g., driving pressure, PEEP, total tidal volume [VT], and dynamic compliance), with alarms set for deviations from initial values.2
However, several potential situations deserve further thought to improve safety in an inherently unsafe technique:
-
(1)
Changes in one patient affecting the other: Potential issues include respiratory compliance changes, saturation of airway filters (increasing resistance, which may be imbalanced across circuits), pneumothorax, or obstruction in the circuit or airway. In pressure control ventilation, none of these scenarios would lead to significantly changed ventilation in the shared patient(s), but clearly risks hypoventilation for the affected patient. We found that even seemingly minor obstructions like failure to fully retract a closed suction catheter can decrease VT for that circuit in test lungs with shared ventilation. Similarly, secretions that saturate heat and moisture exchange filters or obstruct the airway could impact VT unequally. Compliance changes or obstruction should be detected with individual patient monitoring and ventilator alarms set for small deviations from expected total VT. Although total VT is an inherently inaccurate reflection of patient ventilation, a trended change should prompt evaluation for changes in individual patient VT. Options for earlier detection include (a) individual patient VT monitoring (as close to the patient as possible, on the ventilator side of the distal filter) and/or (b) continuous side stream measurement of distal circuit airway pressures, with peak and trough (PEEP) alarms set (fig. 1). A pressure transducer can be attached to a sampling port as near the patient’s airway; this can be directly connected without tubing and used dry, to avoid introduction of fluid that may saturate filters.
-
(2)
Importance of deep sedation and neuromuscular blockade: Patient–ventilator interactions (and impact on shared patient[s]) are largely mitigated by maximized ventilator trigger thresholds and deep sedation/neuromuscular blockade. Added protection can be provided by one-way check valves distal to the splitter for each patient’s inspiratory and expiratory circuit limbs, preventing flow of expiratory gas from a coughing patient into shared patients’ circuits. Still, coughing would pause ventilation for both patients. Thus, early detection of inadequate sedation/neuromuscular blockade and impending patient–ventilator dyssynchrony can be facilitated by continuous measurement of distal airway pressures, with alarms sensitive to high or negative airway pressures (this can be monitored remotely).
-
(3)
Matching of patients throughout shared ventilation: Ideally, patients with divergent VTs or compliances should not share ventilation. Even if initially matched, deterioration or recovery may occur differentially, resulting in compliance mismatching. Adding inspiratory limb flow restriction for the patient needing a lowered VT has been proposed.3–5 In pressure control ventilation mode, the addition of flow restriction to one circuit would not significantly change VT for the “unrestricted” patient circuit; however, hypoventilation of the “restricted” patient is a concern. Also, in pressure control ventilation, the effect of flow restriction on VT is heavily dependent on the inspiratory time (VT = flow × time, where flow = pressure/resistance; VT across resistance increases with longer inspiratory time). Flow restriction that adequately balances two patients may have a very different effect when settings are titrated, or if compliance changes. The drop in total (shared) VT roughly indicates a decreased VT for the flow-restricted patient, but due to uncertainty in compliance compensation, shared VT should be interpreted cautiously, and individual patient VTs should also be measured whenever possible. Distal airway pressures can measure the effect of flow restriction on the driving pressure (peak – trough [PEEP] pressure) actually seen by each patient. Others have suggested adding dead space2; this should not be done unless single-patient VT is monitored and is within suggested limits for lung protection.
Fig. 1.
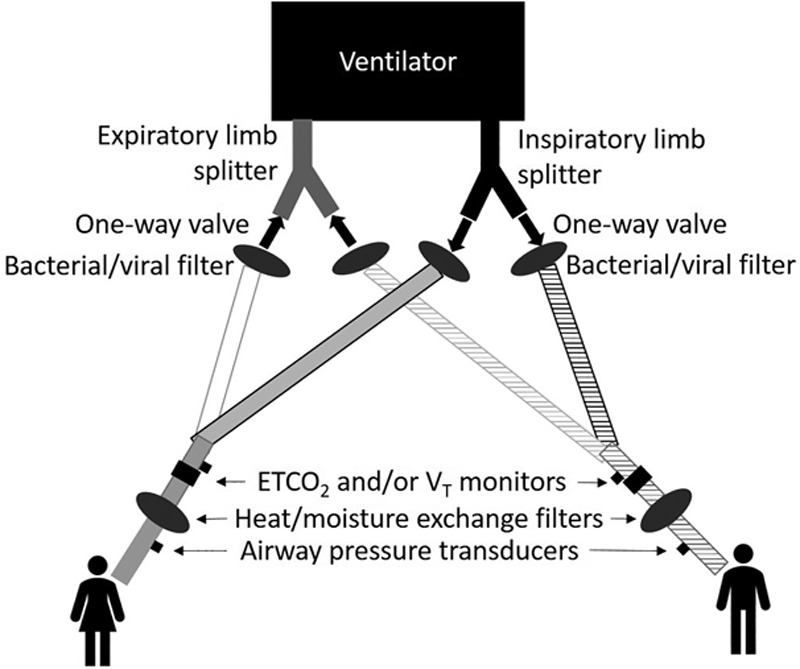
Shared ventilation circuit diagram. Selected circuit components highlighting inclusion of, for each circuit: (1) inspiratory and expiratory limb one-way valves, (2) bacterial/viral filters (protection for ventilator and shared patient), (3) end-tidal carbon dioxide (ETco2) and/or tidal volume (VT) monitoring on patient side of circuit wye, (4) heat and moisture exchange filters, and (5) airway pressure monitoring, using a dry pressure transducer directly connected (no tubing) to a side-stream Luer lock port (gas sampling port) on patient side of heat and moisture exchange filters, if possible, which allows detection of diminished airway pressures if heat and moisture exchange filters saturate and add resistance.
Finally, these safety considerations do not address all hazards:
-
(1)
Prolonged deep sedation and neuromuscular blockade may be difficult with drug shortages, add risk in critically ill patients, and delay assessment for weaning from mechanical ventilation.
-
(2)
Despite use of microbial filters, the risk of patient cross-contamination remains.
-
(3)
One-way valves in individual patient circuits will not prevent immediate loss of all ventilation if any circuit is disconnected without capping.
-
(4)
Staff with expertise to perform shared ventilation (and equipment) may also be a limited resource.
Research Support
Dr. Bishawi has received research funding from Abbott Labs (Abbott Park, Illinois) and Medtronic Inc. (Minneapolis, Minnesota) for research unrelated to this manuscript. Dr. Bishawi is supported by National Institutes of Health (Bethesda, Maryland) grant No. 1R38HL143612-01.
Competing Interests
The authors declare no competing interests.
References
- 1.SCCM, AARC, ASA, APSF, AACN, and CHEST: Joint Statement on Multiple Patients Per Ventilator, American Society of Anesthesiologists. March 26, 2020. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2020/03/joint-statement-on-multiple-patients-per-ventilator. Accessed April 11, 2020.
- 2.Beitler JR, Kallet R, Robert K, Branson RB, Brodie D, Mittel AM, Olson M, Hill LLH, Hess D, Thompson BT. NewYork-Presbyterian Hospital’s Working Protocol for Supporting Two Patients with a Single Ventilator, Greater New York Hospital Association. March 26, 2020. Available at: https://www.gnyha.org/news/working-protocol-for-supporting-two-patients-with-a-single-ventilator. Accessed April 11, 2020.
- 3.Lai BK, Erian JL, Pew SH, Eckmann MS. Emergency open-source three-dimensional printable ventilator circuit splitter and flow regulator during the COVID-19 pandemic.. Anesthesiology. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke AL, Stephens AF, Liao S, Byrne TJ, Gregory SD. Coping with COVID-19: Ventilator splitting with differential driving pressures using standard hospital equipment.. Anaesthesia. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke AL. Three-dimensional printed circuit splitter and flow restriction devices for multiple patient lung ventilation using one anaesthesia workstation or ventilator.. Anaesthesia. 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


