Golgi are emerging as key regulators of acentrosomal microtubule networks. In neurons, the role of dendrite-specific Golgi outposts in creating or maintaining the unique organization of the dendritic cytoskeleton is an open question...
Keywords: Drosophila, Golgi, microtubules, neuron
Abstract
Microtubule-organizing centers often play a central role in organizing the cellular microtubule networks that underlie cell function. In neurons, microtubules in axons and dendrites have distinct polarities. Dendrite-specific Golgi “outposts,” in particular multicompartment outposts, have emerged as regulators of acentrosomal microtubule growth, raising the question of whether outposts contribute to establishing or maintaining the overall polarity of the dendritic microtubule cytoskeleton. Using a combination of genetic approaches and live imaging in a Drosophila model, we found that dendritic microtubule polarity is unaffected by eliminating known regulators of Golgi-dependent microtubule organization including the cis-Golgi matrix protein GM130, the fly AKAP450 ortholog pericentrin-like protein, and centrosomin. This indicates that Golgi outposts are not essential for the formation or maintenance of a dendrite-specific cytoskeleton. However, the overexpression of GM130, which promotes the formation of ectopic multicompartment units, is sufficient to alter dendritic microtubule polarity. Axonal microtubule polarity is similarly disrupted by the presence of ectopic multicompartment Golgi outposts. Notably, multicompartment outposts alter microtubule polarity independently of microtubule nucleation mediated by the γ-tubulin ring complex. Thus, although Golgi outposts are not essential to dendritic microtubule polarity, altering their organization correlates with changes to microtubule polarity. Based on these data, we propose that the organization of Golgi outposts is carefully regulated to ensure proper dendritic microtubule polarity.
PROPER neuronal structure and function depends on the underlying microtubule cytoskeleton, which is uniquely organized in axons and dendrites. The compartment-specific orientation of microtubules is thought to contribute to the specific morphologies and functions of these compartments. In axons, microtubules are uniformly oriented with their plus-ends positioned distal to the cell body. In contrast, microtubule polarity in dendrites is mixed to varying degrees, and the percentage of microtubules of a particular orientation differs locally within the dendritic arbor. How the distinctly organized cytoskeletons in axons and dendrites are created and maintained remains an open question. Microtubules are often generated at and organized by cellular structures called microtubule-organizing centers (MTOCs), which anchor and stabilize microtubules and support microtubule nucleation (Sanchez and Feldman 2017; Wu and Akhmanova 2017). The centrosome is one example of a well-studied MTOC. Although neurons have a centrosome, recent work indicates that the neuronal centrosome does not have a major role in either generating or anchoring dendritic or axonal microtubules (Stiess et al. 2010; Nguyen et al. 2011; Sánchez-Huertas et al. 2016). However, the centrosome is not the only organelle that functions as an MTOC (Sanchez and Feldman 2017; Wu and Akhmanova 2017). The Golgi apparatus and nonconventional Golgi structures such as Golgi elements and Golgi outposts have emerged as potential MTOCs in several cell types, including epithelia, muscles, and neurons (Rios 2014; Sanders and Kaverina 2015; Martin and Akhmanova 2018). Unlike centrosomes, which support the nucleation of microtubules radially, Golgi-based microtubule nucleation can create asymmetric microtubule arrays (Efimov et al. 2007; Zhu and Kaverina 2013). Such asymmetric Golgi-derived microtubule arrays have also been shown to contribute to cell polarity (Zhu and Kaverina 2013; Rios 2014). Thus, Golgi in neurons have the potential to shape the polarity of the microtubule cytoskeleton, and influence neuronal polarity, by selectively seeding or stabilizing microtubules in a particular orientation.
In developing flies and mammals, many neurons have Golgi in the form of mini stacks called outposts that localize specifically to dendrites and have a different structure than the somatic Golgi (Gardiol et al. 1999; Pierce et al. 2001; Horton and Ehlers 2003; Ye et al. 2007; Liu et al. 2017; Rao et al. 2018; Tann and Moore 2019). The term Golgi outposts is used to refer generally to a heterogenous population of dendritic Golgi composed of one or more compartments. In studies using fluorescently tagged End-binding 1 (EB1::GFP), which marks growing microtubule ends, Golgi outposts have been shown to correlate with microtubule growth initiation sites in developing dendrites (Ori-McKenney et al. 2012; Zhou et al. 2014; Yalgin et al. 2015). Decreasing the levels of proteins involved in microtubule nucleation, such as γ-tubulin and its interactors centrosomin (cnn, the fly ortholog of CDK5RAP2) and pericentrin-like protein (plp, the Drosophila ortholog of AKAP450), disrupts the correlation between microtubule growth initiation sites and outposts, and perturbs dendrite branch growth (Ori-McKenney et al. 2012; Yalgin et al. 2015). This correlation has led to the model that Golgi outposts function as MTOCs that support acentrosomal nucleation and the directional growth of microtubules during dendrite branch formation (Delandre et al. 2016). These studies have focused on the connection between Golgi outposts, microtubule growth, and dendrite growth, leaving unanswered the broader question of whether outposts have a role in creating and/or maintaining the distinct polarity of the dendritic microtubule cytoskeleton. Notably, the link between microtubule growth and Golgi outposts relies on the compartmental organization of outposts: multicompartment dendritic Golgi outposts are more likely than single-compartment outposts to correlate with microtubule growth start sites (Zhou et al. 2014), and dragging individual Golgi compartments into the axon is not sufficient to alter axonal microtubule organization (Nguyen et al. 2014). The dendrite-specific localization of Golgi outposts and their MTOC potential raises the possibility that Golgi outposts, and in particular multicompartment outposts, may play a role in establishing and/or maintaining the unique polarity of the dendritic microtubule cytoskeleton. However, this idea has not been tested.
Here, we investigated whether Golgi outposts are necessary for the compartment-specific orientation of dendritic microtubules. In particular, we asked whether outposts are necessary for the formation of the minus-end-distal microtubules that are specific to the dendritic cytoskeleton. To test this notion, we leveraged a combination of genetics and live imaging, and used the class IV dendritic arborization (da) neurons in Drosophila as a model. In the class IV da neurons, dendritic microtubules are predominately oriented with their minus-ends distal to the cell body, providing an advantageous paradigm to detect changes in microtubule orientation. If Golgi outposts regulate the overall polarity of the dendritic microtubule cytoskeleton, then blocking the MTOC activity of Golgi should disrupt the stereotyped minus-ends-distal orientation of dendritic microtubules. We targeted the cis-Golgi matrix protein GM130, which has two key roles. First, work done in mammalian cells has shown that GM130 recruits AKAP450, which in turn recruits protein complexes that nucleate, tether, and stabilize microtubules (Rivero et al. 2009; Hurtado et al. 2011; Roubin et al. 2013; Wu and Akhmanova 2017). Second, GM130 is needed for proper Golgi structure and connects Golgi compartments to form multicompartment units, including outposts (Nakamura et al. 1995; Barr et al. 1997; Kondylis et al. 2005; Zhou et al. 2014; Liu et al. 2017; Lowe 2019). We found that the global orientation of dendritic microtubules is unaffected by the loss of GM130 or the fly AKAP450 ortholog plp. Cnn, which is proposed to orient Golgi outpost-associated microtubule growth (Yalgin et al. 2015), is also dispensable. This suggests that Golgi outposts do not have an essential role in establishing the overall polarity of the dendritic microtubule cytoskeleton. However, our studies of GM130 overexpression reveal that inducing the formation of ectopic multicompartment units correlates with a disruption in microtubule polarity. This suggests that compartment connectedness is critical to the microtubule organization capacity of outposts. Interestingly, the ability of multicompartment outposts to organize microtubules is independent of γ-tubulin ring complex (γ-TuRC)-mediated microtubule nucleation, which suggests that outposts can regulate microtubule polarity via microtubule anchoring or stabilizing.
Materials and Methods
Fly stocks
The following alleles and transgenic fly strains from the Bloomington Drosophila Stock Center (BDSC) and individual laboratories were used as follows: cnnHK21 (Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999) (BDSC 5039), which produces a drastically truncated ∼106 amino acid-long protein that is not detectable by western blot, and Df(2R)BSC306 (BDSC 23689) were used to eliminate cnn; GM130Δ23, a protein null allele (Zhou et al. 2014) (BDSC 65255), and Df(2R)Exel7170 (BDSC 7901) were used to eliminate GM130; UAS-GM130::eBFP (Zhou et al. 2014) (BDSC 65254) and ppk-Gal4 (BDSC 32079) were used to overexpress GM130; dGrip75175 (Schnorrer et al. 2002) (Conduit laboratory, University of Cambridge and Raff laboratory, University of Oxford) and Df(2L)Exel7048 (BDSC 7999) were used to eliminate dGrip75; KhcE177K (Kelliher et al. 2018) was used in trans to the null allele Khc27 (Brendza et al. 1999) (Saxton laboratory, University of California, Santa Cruz); nudE39A (Wainman et al. 2009) (Goldberg laboratory, Cornell University), a protein null allele, and Df(3L)BSC673 (BDSC 26525) were used eliminate nudE; plp5 (BDSC 9567) (Martinez-Campos et al. 2004), an EMS-induced loss-of-function allele that strongly reduces plp levels, and Df(3L)Brd15, pp (BDSC 5354) were used to eliminate plp activity; ppk-ManII::GFP (Jenkins et al. 2017) and UAS-GalNacT2::TagRFP (Zhou et al. 2014) (Ye laboratory, University of Michigan) were used to label medial and trans Golgi compartments, respectively; ppk-CD4::GFP (BDSC 35842 and BDSC 35843) and ppk-CD4::tdTom (Han et al. 2011) (BDSC 35844 and BDSC 35845) were used to visualize neuron morphology; and ppk-EB1::GFP (Arthur et al. 2015) was used to analyze microtubule polarity and dynamics.
Live imaging
Fly crosses for live imaging were set up using five to eight virgin females and four to six young males; larvae were collected in 12-hr intervals and aged to the desired developmental stage (larvae produced in the first 24–48 hr after mating were not used). Larvae of the desired genotype were washed with 1× phosphate-buffered saline (PBS), mounted in a 50:50 1 × PBS:glycerol solution on a slide between two strips of vacuum grease, and immobilized by pressing on a coverslip mounted on top of the larva and vacuum-grease spacers. The dorsal class IV ddaC neurons within abdominal segments 2–4 were imaged with a 40 × 1.3 numerical aperture oil immersion objective. All imaging was performed on a Leica SP5 (Leica Microsystems) using hybrid detector (HyD) photodetectors.
Microtubule polarity and growth analysis
Microtubule polarity and growth were analyzed by scoring EB1::GFP comets within 150 µm of the cell body in dendrites or axons. EB1::GFP comet trajectories were captured at a resolution of 1024 × 512 pixels and a rate of 0.86 sec per frame for 5–7 min. One or two ddaC neurons per larva were imaged at 96–120 hr after egg laying (AEL). Videos were stabilized with the stabilizer plugin in FIJI ImageJ (ImageJ; National Institutes of Health); kymographs were generated in Metamorph (Molecular Devices). Movies that did not contain at least two comets were excluded. Comet trajectories were manually traced, and the position and time coordinates were recorded to calculate comet direction (microtubule orientation) and frequency. A comet trajectory was included only if it could be clearly traced in at least 12 continuous frames (∼11 sec). Anterograde comets traveled away from the cell body whereas retrograde comets traveled toward the cell body. The frequency of EB1::GFP comets was calculated as the number of comets present in a 100-µm segment (axons) per minute. To calculate microtubule polarity in dendrites, EB1::GFP comets were scored in a segment ≥ 30 µm between two branch points. To identify the origin of anterograde EB1::GFP comets in dendrites, dendrite segments within 100 µm of the cell body that contained at least one comet were selected for analysis. Comet trajectories were identified in kymographs, and any anterograde comets were then traced back to their origin in the corresponding movie.
Axon branching analysis
Axon morphology was visualized at late third instar (120–144 hr AEL) using CD4::GFP and CD4::Tomato. Images were captured at 1024 × 1024 resolution and 1-µm z-steps (5–15 steps in total). Analysis was performed on axons within 150 µm of the cell body. Z-stacks 5–15-µm thick were maximally projected for analysis. Axon branching was assessed manually by determining whether an axon had split into one or more branches.
Golgi compartment analysis
Images of ManII::GFP and GalNacT2::TagRFP puncta in the dendrites and axons of neurons in 96–120 hr AEL larvae were captured at a resolution of 1024 × 1024 pixels and 0.75-µm z-steps over 5–15 µm. Analysis of Golgi outposts in axons included outposts within 100 µm of the cell body. For dendrite analysis, Golgi outposts throughout the entire arbors were included and split into two groups: (1) those within a radius of 95 µm of the cell body and (2) those outside this radius. One to two ddaC neurons were imaged per larva. Analysis was performed on the maximally projected images. Signals outside the regions of interest (ROI) in axons and dendrites were masked. Masked images were subjected to a threshold gray value of 100 for segmentation. The segmented signals were quantified with the ImageJ particle analysis function with the size cutoff of 0.10–15 µm2. Puncta outlines were saved as ROIs. The resulting particle numbers and sizes were exported to Excel for analysis. For multicompartment analysis, overlapping compartments were manually scored by overlaying the puncta outlines from each channel, namely ManII::GFP and GalNacT2::TagRFP. Golgi units were scored as multicompartment when the ManII::GFP and GalNacT2::TagRFP signals overlapped.
Statistical analysis
Statistical analyses were performed using GraphPad Prism8. The Anderson–Darling and Shapiro–Wilk tests were used to determine whether data were normally distributed. For normally distributed data, Student’s unpaired t-tests were used to compare two groups; one-way ANOVA with a post hoc Tukey test was used for multiple comparisons. For non-normally distributed data, a Mann–Whitney U-test was used to compare two groups and a Kruskal–Wallis test followed by a post hoc Dunn test was used for multiple comparisons. Fisher’s exact test was used for comparing proportions. P = 0.05 was used as a cutoff for significance. Significance levels are represented as: *P = 0.05–0.01, **P = 0.01–0.001, ***P = 0.001–0.0001, and ****P < 0.0001. n.s. indicates not significant.
Data availability
All strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are represented fully within the article and its figures.
Results
Golgi outposts are not essential to the overall polarity of the dendritic microtubule cytoskeleton
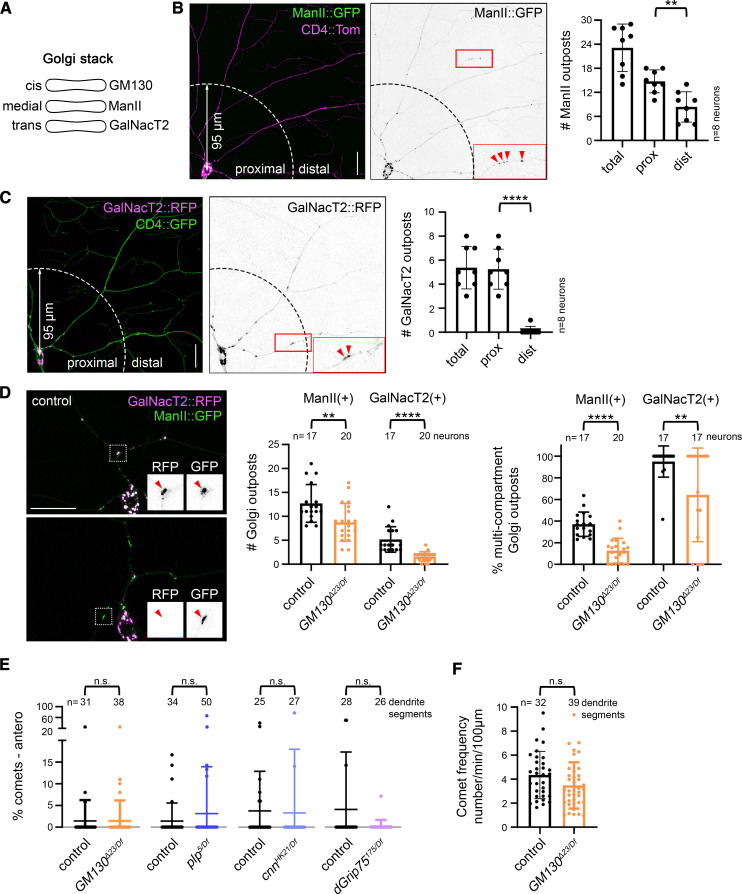
To determine whether Golgi outposts play a critical role in determining the unique polarity of the dendritic microtubule cytoskeleton, we turned to the class IV da neurons in Drosophila as a model. The da neurons are an ideal model as they are easily accessible for live imaging, the orientation of microtubules in the dendritic microtubule cytoskeleton is well defined, and there is a wealth of tools for labeling and manipulating Golgi, microtubules, and microtubule regulators. In the class IV da neurons, Golgi compartments are present in the cell body and throughout the dendritic arbor, but most are found close to the cell body (Figure 1, A–C). Indeed, GalNacT2-positive trans Golgi compartments are seldom present beyond 95 µm of the cell body. Next, we quantified the number of multicompartment outposts in the arbor. Consistent with previous work, we defined multicompartment Golgi outposts as units with at least two compartments (Zhou et al. 2014). Here, we used markers of the medial and trans Golgi, namely ManII::GFP and GalNacT2::TagRFP, respectively. Fewer than one-half of the ManII-positive Golgi outposts colocalized with GalNacT2, indicating ManII::GFP predominantly labels single-compartment Golgi outposts (Figure 1D). In contrast, the majority of GalNacT2-positive outposts colocalized with ManII::GFP (Figure 1D), which suggests that most multicompartment Golgi outposts cluster relatively close to the cell body. Given that Golgi-associated microtubule growth correlates predominantly with multicompartment outposts (Zhou et al. 2014), this suggests that outposts in the proximal arbor may be most likely to regulate microtubule organization.
Figure 1.
Global dendritic microtubule polarity does not depend on Golgi outposts. (A) Cartoon showing the compartmental distribution of GM130, ManII, and GalNacT2 in a Golgi stack. (B–D) ManII::GFP-positive outposts are present throughout the class IV da neuron dendritic arbor, but fewer than one-half of these outposts are multicompartment units (B and D). Multicompartment outposts are defined as those that have overlapping ManII::GFP and GalNacT2::TagRFP signals. In contrast to ManII::GFP-positive outposts, GalNacT2::TagRFP-positive outposts cluster in the proximal arbor (prox) and nearly all GalNacT2::TagRFP-positive outposts are multicompartment (C and D). Eliminating GM130 reduces the overall number of Golgi outposts and the percentage of outposts that are multicompartment in the prox. The prox encompasses a radius of 95 µm from the cell body; distal (dist) is beyond this radius. Red arrowheads indicate Golgi outposts. The left side of each graph represents the fraction of ManII::GFP-positive outposts that are multicompartment and the right side of each graph represents GalNacT2::TagRFP-positive outposts that are multicompartment (D). **P = 0.01–0.001, and ****P < 0.0001; Student’s unpaired t-tests. (E) Dendritic microtubule polarity is not affected by the loss of GM130, plp, cnn, or dGrip75. Mann–Whitney U-test. (F) EB1::GFP comet frequency is normal in the dendrites of neurons lacking GM130. Student’s unpaired t-test. Microtubule polarity and EB1::GFP comet frequency were quantified in the proximal dendritic arbor, which contained the majority of multicompartment outposts. Scale bars, 25 µm. All data are mean ± SD. Antero, anterograde; cnn, centrosomin; n.s., not significant; plp, pericentrin-like protein; RFP, red fluorescent protein.
To determine whether Golgi outposts function to create or maintain the dendrite-specific orientation of microtubules, we analyzed microtubule polarity in neurons in which we eliminated the cis-Golgi matrix protein GM130. GM130 both recruits the protein machinery for MTOC activities (microtubule nucleation, anchoring, and stabilization) and contributes to forming multicompartment Golgi units (Nakamura et al. 1995; Barr et al. 1997; Kondylis et al. 2005; Zhou et al. 2014; Sanders and Kaverina 2015; Liu et al. 2017; Martin and Akhmanova 2018; Lowe 2019). We found that the percentage of multicompartment outposts decreases when GM130 is absent, which supports the model that GM130 participates in connecting Golgi compartments in neurons (Figure 1D) (Zhou et al. 2014). The loss of GM130 also reduces the number of ManII- and GalNacT2-positive Golgi outposts, consistent with previous reports (Liu et al. 2017). To readout microtubule orientation, we used EB1::GFP, whose binding to growing microtubule ends produces a comet-like trajectory. The majority of microtubule growth occurs at plus-ends, which also grow faster than minus-ends, enabling a clear distinction of plus- and minus-end growth and thus microtubule polarity (Feng et al. 2019). As previously reported, in control neurons dendritic microtubules are oriented predominantly minus-ends-distal (Figure 1E). Strikingly, eliminating GM130 had no effect on the overall polarity of microtubules within the proximal dendritic arbor where multicompartment Golgi outposts clustered (Figure 1E). The overall frequency of microtubule growth was also unaffected by the loss of GM130 (Figure 1F). In mammalian cells, GM130 affects microtubule organization through the recruitment of AKAP450, whose fly ortholog is plp (Martinez-Campos et al. 2004; Rios 2014; Sanders and Kaverina 2015; Martin and Akhmanova 2018). Plp in fly neurons has likewise been implicated in the MTOC activity of Golgi outposts (Ori-McKenney et al. 2012). Similar to the loss of GM130, eliminating plp had no effect on dendritic microtubule polarity (Figure 1E). Plp is proposed to regulate microtubule growth at Golgi outposts in conjunction with cnn and γ-tubulin, the latter of whose activity is controlled by the γ-TuRC (Ori-McKenney et al. 2012; Yalgin et al. 2015). Eliminating either cnn or the γ-TuRC component dGrip75 does not alter dendritic microtubule polarity (Figure 1E). Thus, the results of our experiments provide compelling evidence that Golgi outposts do not play an essential role in creating or maintaining the overall polarity of the dendritic microtubule cytoskeleton.
Loss of GM130 significantly reduces misoriented microtubules in nudE− axons
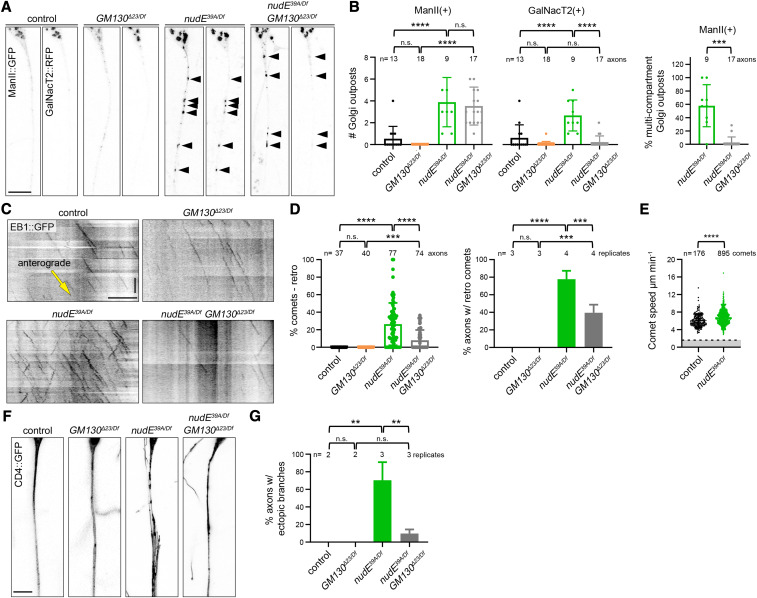
The results of our GM130 loss-of-function experiments in da neurons suggest that the overall polarity of the dendritic microtubule cytoskeleton does not depend on Golgi outposts. However, microtubule polarity varies within the dendritic arbors of da neurons (Stone et al. 2008), and it is possible that Golgi outposts are nevertheless sufficient to locally influence microtubule polarity as previously reported (Ori-McKenney et al. 2012; Yalgin et al. 2015; Delandre et al. 2016). To test the idea that outposts have the capacity to affect microtubule polarity, we used a paradigm in which Golgi outposts are ectopically localized to axons, a compartment from which outposts are normally excluded and in which microtubules are uniformly oriented with their plus-ends distal. If Golgi outposts are capable of regulating microtubule organization, we reasoned that their ectopic presence in axons should disrupt the uniform plus-ends-distal array of axonal microtubules. To ectopically localize Golgi outposts to axons, we relied on mutations that disrupt the activity of the molecular motors dynein and kinesin-1, which transport outposts (Ye et al. 2007; Zheng et al. 2008; Arthur et al. 2015; Lin et al. 2015; Kelliher et al. 2019). While disrupting the activity of either motor results in Golgi outposts invading axons, we and others have previously shown that these ectopic outposts do not always correlate with a change in axonal microtubule polarity (Ye et al. 2007; Nguyen et al. 2014; Kelliher et al. 2019). Analyzing these different motor mutants and the outposts in their axons enables us to determine whether Golgi outposts are sufficient to affect microtubule polarity, and to then identify the factors that are essential for this activity. The loss of dynein activity alters both Golgi outpost localization and axonal microtubule polarity (Zheng et al. 2008; Arthur et al. 2015; del Castillo et al. 2015; Klinman et al. 2017; Rao et al. 2017). We first asked whether the misoriented microtubules in the axons of dynein loss-of-function neurons depend on the ectopic Golgi outposts. The multisubunit dynein motor complex has several cofactors that are important for its activity (Reck-Peterson et al. 2018); in Drosophila neurons, this includes the conserved cofactor nudE (Arthur et al. 2015). In the absence of nudE, axons are infiltrated by multicompartment Golgi outposts (Figure 2, A and B) (Arthur et al. 2015). In the axons of these nudE39A/Df mutant neurons, EB1::GFP comets travel both anterograde and retrograde, which indicates a disruption in axonal microtubule polarity (Figure 2, C and D). Although EB1::GFP can also bind to slowly growing microtubule minus-ends (Feng et al. 2019), the speed of the retrograde comets in the nudE39A/Df mutant axons is indicative of microtubule plus-end growth (Figure 2E). Thus, the ectopic axonal Golgi outposts in the nudE39A/Df mutant neurons correlate with a change in axonal microtubule polarity.
Figure 2.
Misoriented microtubules in nudE mutant axons are significantly reduced when GM130 is eliminated. (A and B) Golgi outposts (marked by ManII::GFP and GalNacT2::RFP; arrowheads) mislocalize to axons in nudE39A/Df mutant neurons. In nudE39A/Df mutant neurons, the loss of GM130 does not affect the mislocalization of ManII-positive Golgi outposts, but suppresses the mislocalization of GalNacT2-positive outposts; as a result, the percentage of multicompartment ManII-positive outposts is dramatically reduced. ***P = 0.001–0.0001 and ****P < 0.0001; one-way ANOVA with Tukey’s post hoc analysis. Scale bar, 10 µm. (C and D) In the absence of nudE, axonal microtubule polarity is perturbed. Eliminating GM130 reduces the number of misoriented microtubules in nudE39A/Df mutant axons. Cell body is to the left; yellow arrow indicates the direction of anterograde comet movement. ***P = 0.001–0.0001, and ****P < 0.0001; Kruskal–Wallis test with post hoc Dunn’s multiple comparison analysis (% retro comets) and one-way ANOVA with Tukey’s post hoc analysis (% axons). Scale bars, 10 µm (x-axis) and 30 sec (y-axis). (E) The speed of EB1::GFP comets in control and nudE39A/Df mutant axons is consistent with microtubule plus-end growth, indicating nudE39A/Df mutant axons indeed contain microtubules with mixed polarity (comets moving at speeds below the dotted line would be consistent with microtubule minus-end growth). ****P < 0.0001, Mann–Whitney U-test. (F and G) The ectopic branches that sprout from nudE39A/Df mutant axons are suppressed by removing GM130. n = 16 (control), 27 (GM130Δ23/Df), 59 (nudE39A/Df), and 41 (GM130Δ23/Df; nudE39A/Df) axons in replicates as indicated (G). **P = 0.01–0.001; one-way ANOVA with Tukey’s post hoc analysis. Scale bar, 10 µm. All data are mean ± SD. Antero, anterograde; n.s., not significant; retro, retrograde; RFP, red fluorescent protein.
To determine whether ectopic Golgi outposts might contribute to the alteration in axonal microtubule polarity in nudE39A/Df mutant axons, we eliminated GM130. By itself, the loss of GM130 did not affect either the orientation of axonal microtubules or the localization of Golgi outposts (Figure 2, A–D). However, eliminating GM130 significantly reduced the number of misoriented minus-end-distal microtubules in nudE39A/Df mutant axons (Figure 2, C and D). ManII-positive Golgi were still present in the nudE39A/Df GM130Δ23/Df double-mutant axons, but, strikingly, the loss of GM130 suppressed the axonal mislocalization of GalNacT2-positive outposts in nudE39A/Df mutant neurons (Figure 2, A and B). A little over one-half of the ManII-positive outposts in the nudE39A/Df mutant axons were multicompartment, but there were virtually no multicompartment outposts in the axons of nudE39A/Df GM130Δ23/Df double-mutant neurons, likely because the loss of GM130 suppressed the axonal mislocalization of the GalNacT2-positive compartments. These data suggest that microtubule polarity may be affected by multicompartment, but not single-compartment, Golgi outposts.
Notably, the loss of GM130 did not completely suppress the appearance of misoriented microtubules. This may be due to the “microtubule gatekeeper” role that dynein is proposed to play in maintaining axonal microtubule polarity. In addition to carrying cargo, dynein also transports, or slides, microtubules (Rao and Baas 2018). Dynein anchored in the proximal axon translocates microtubules into or out of the axon, and prevents the entry of minus-end-distal microtubules (del Castillo et al. 2015; Rao et al. 2017; Rao and Baas 2018). Our data suggest that dynein also maintains axonal microtubule polarity by excluding Golgi outposts that have the capacity to induce changes in microtubule organization.
The presence of ectopic Golgi outposts in the nudE39A/Df mutant axons also correlates with the formation of ectopic axonal branches (Arthur et al. 2015). The axons of neurons lacking nudE develop multiple fine branches that run parallel to the main axon but terminate before reaching the ventral nerve cord; occasionally ectopic branches even extend back toward the cell body and dendrites (Figure 2, F and G). Since dendritic Golgi outposts have been correlated with dendrite branch formation, and stability and loss of GM130 decreases branch number (Ye et al. 2007; Ori-McKenney et al. 2012; Zhou et al. 2014; Yalgin et al. 2015; Liu et al. 2017), we tested whether Golgi outposts might be implicated in the formation of ectopic branches that sprout from the nudE39A/Df mutant axons. We found that loss of GM130 suppressed the axonal morphology defects of the nudE39A/Df mutant axons, implicating the mislocalized Golgi outposts in the growth of ectopic axonal branches (Figure 2, F and G). Together, these data suggest that the ectopic Golgi outposts may be a key contributing factor to both the cytoskeletal and morphological defects of the nudE39A/Df mutant axons. Thus, Golgi outposts are likely capable of inducing changes both in the microtubule cytoskeleton and neurite branching.
Golgi outposts affect microtubule polarity independently of γ-TuRC-mediated microtubule nucleation
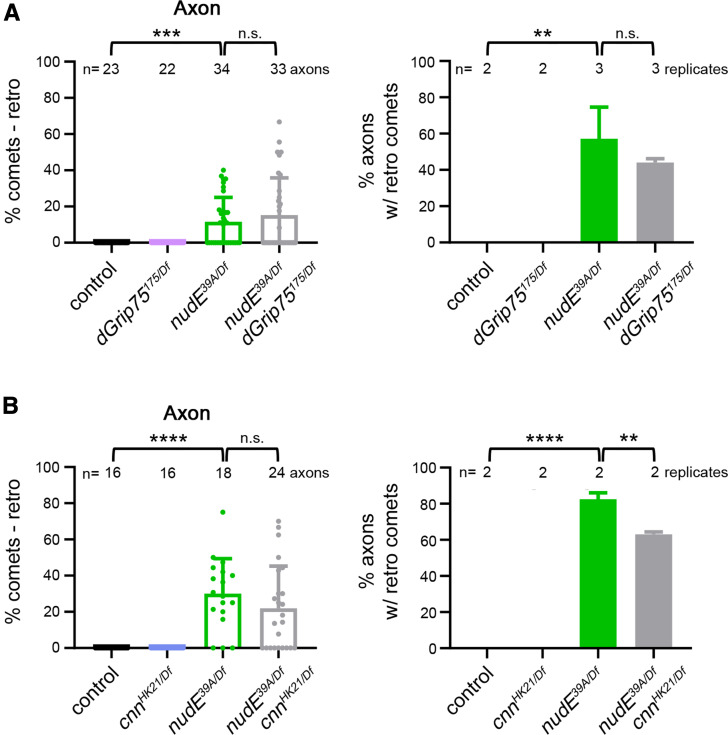
Dendritic Golgi outposts have been reported to serve as platforms for oriented microtubule growth during dendrite branch extension (Ori-McKenney et al. 2012; Yalgin et al. 2015). This suggests that Golgi outposts might influence microtubule polarity by controlling microtubule nucleation. Therefore, we tested whether the misoriented microtubules in the nudE39A/Df mutant axons resulted from ectopic nucleation at Golgi outposts. Microtubule nucleation at Golgi membranes is templated by γ-tubulin whose nucleation activity is regulated by additional proteins, including cnn and γ-TuRC components (Sanders and Kaverina 2015; Martin and Akhmanova 2018; Tann and Moore 2019). In dendrites, cnn has been implicated in regulating the directional growth of Golgi-derived microtubules during branching (Yalgin et al. 2015). Thus, we focused on whether γ-TuRC-mediated microtubule nucleation might mediate the Golgi-induced change in microtubule polarity in nudE39A/Df mutant axons.
We have previously shown that reducing γ-tubulin does not suppress the appearance of minus-end-distal microtubules in nudE39A/Df mutant axons (Arthur et al. 2015). Nonetheless, we followed up our earlier findings by testing the γ-tubulin regulator cnn and the γ-TuRC component GCP4, known as dGrip75 in Drosophila (Vérollet et al. 2006). Consistent with our prior report, we found that eliminating either cnn (cnnHK21/Df) or dGrip75 (dGrip75175/Df) does not suppress the formation of misoriented microtubules in nudE39A/Df mutant axons (Figure 3, A and B). Altogether, our results suggest that the ectopic Golgi outposts have the capacity to affect microtubule polarity but do so through a pathway that is independent of γ-TuRC-mediated microtubule nucleation.
Figure 3.
Appearance of misoriented microtubules in nudE mutant axons does not depend on microtubule nucleation machinery. (A and B) Eliminating either dGrip75 (A) or cnn (B) does not affect the microtubule polarity phenotype of nudE39A/Df mutant axons. n = 23 (control), 22 (dGrip75175/Df), 34 (nudE39A/Df), and 33 (dGrip75175/Df; nudE39A/Df) axons (A) and n = 16 (control), 16 (cnnHK21/Df), 18 (nudE39A/Df), and 24 (cnnHK21/Df; nudE39A/Df) axons (B) in replicates as indicated. **P = 0.01–0.001 ***P = 0.001–0.0001, and ****P<0.0001; Kruskal–Wallis test with post hoc Dunn’s multiple comparison analysis. ANOVA with Tukey’s post-hoc anaylsis. All data are mean ± SD. cnn, centrosomin; n.s., not significant.
Elevating GM130 levels increases the number of multicompartment Golgi and alters microtubule polarity
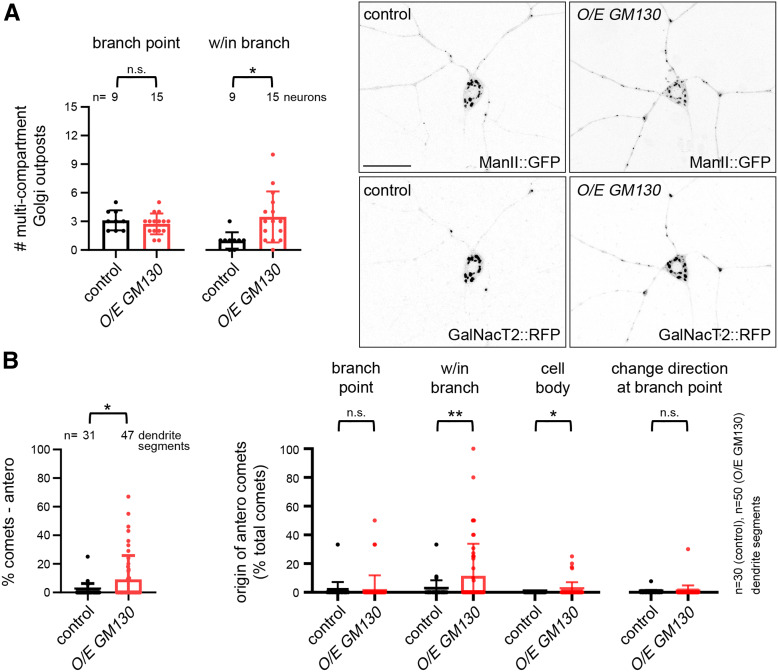
Our manipulations of GM130 in nudE39A/Df mutant neurons indicate that ectopic multicompartment, but not single-compartment, Golgi outposts disrupt axonal microtubule polarity (Figure 2, A-D). As previously mentioned, GM130 is implicated in both the MTOC activity of Golgi and in promoting the formation of multicompartment Golgi units (Zhou et al. 2014; Martin and Akhmanova 2018). Connectedness between Golgi compartments may be important to the coordinated regulation of microtubules by protein complexes that are present on the cis- and trans-Golgi compartments (Rios 2014; Sanders and Kaverina 2015). Thus, our results and the work of others suggest that the ability of Golgi to regulate microtubule polarity may depend on the formation of multicompartment units. We asked whether increasing GM130 levels would be sufficient to increase the number of multicompartment Golgi outposts and to alter microtubule polarity in dendrites. We found that GM130 overexpression increased the number of multicompartment outposts in dendrites as previously reported (Zhou et al. 2014) and that these additional outposts were more prevalent in dendrite branches than branch points (Figure 4A). Thus, our results provide additional support to the idea that GM130 is integral to the connectedness of Golgi compartments in neurons (Zhou et al. 2014; Liu et al. 2017); this is significant given that the role of GM130 in Golgi stack formation in other cell types has been debated (Kondylis and Rabouille 2003; Puthenveedu et al. 2006; Marra et al. 2007; Baschieri et al. 2014; Tormanen et al. 2019). Notably, this increase in multicompartment units within branches correlated with an increase in anterograde EB1::GFP comets that originated within branches (there was also a mild but significant increase in the anterograde comets that originated from the cell body; Figure 4B). There was no significant increase in anterograde comets that originated at branch points and the frequency of comets that changed direction at branch points was also unaffected (Figure 4B). Others have suggested that multicompartment Golgi outposts are prime sites of EB1::GFP comet initiation (Zhou et al. 2014), leading us to propose that the additional anterograde EB1::GFP comets we observe in the neurons overexpressing GM130 originate from ectopic multicompartment outposts. Altogether, these data are consistent with the model that the formation of ectopic multicompartment outposts is sufficient to alter microtubule polarity in dendrites.
Figure 4.
Multicompartment Golgi are sufficient to alter microtubule polarity. (A) The overexpression of GM130 increases the number of multicompartment Golgi outposts within dendrite branches but not branch points. Scale bar, 25 µm. *P = 0.05–0.01; Student’s unpaired t-tests. (B) The percentage of anterograde comets increases in the dendrites of neurons overexpressing GM130. The percentage of anterograde comets that originate from within branches increases, paralleling the increase in multicompartment outposts within branches. The graph on the right represents the number of anterograde comets that fall into a designated category divided by the total number of comets in the dendrite segment (% total comets). *P = 0.05–0.01 and **P = 0.01–0.001; Mann–Whitney U-test. All data are mean ± SD. n.s., not significant; O/E, overexpressed; RFP, red fluorescent protein.
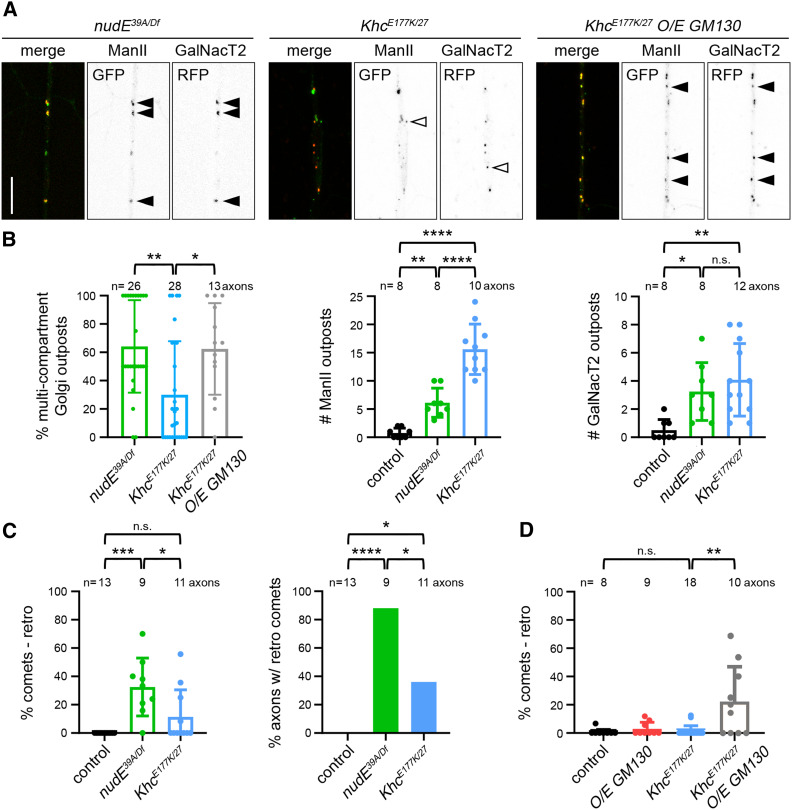
To further test the idea that Golgi compartmentalization correlates with an effect on microtubule polarity, we turned to a mutation in Kinesin heavy chain (KhcE177K) that enhances kinesin-1 activity by disrupting motor autoinhibition (Kelliher et al. 2018). Golgi outposts mislocalize to KhcE177K mutant axons; however, unlike in nudE39A/Df mutant axons, the polarity of axonal microtubules is not significantly affected (Kelliher et al. 2018). Our results indicate that the compartmental organization of Golgi outposts is key to their ability to affect microtubule polarity in dendrites. This led us to characterize the compartmental organization of the ectopic Golgi outposts in the KhcE177K and nudE39A/Df mutant axons to determine whether the differences in axonal microtubule polarity in the two mutants might correlate with differences in Golgi compartmentalization. More specifically, we reasoned that there may be a higher number of multicompartment Golgi outposts in nudE39A/Df mutant axons, which have altered axonal microtubule polarity, than in the KhcE177K mutant axons, which do not.
We analyzed the distribution of ManII::GFP and GalNacT2::TagRFP in KhcE177K and nudE39A/Df mutant axons. Consistent with the idea that compartment connectedness enables Golgi to influence microtubule polarity, we found that there was a higher percentage of multicompartment Golgi outposts in the nudE39A/Df mutant axons than the KhcE177K/27 mutant axons (Figure 5, A and B). Notably, KhcE177K/27 mutant axons contained equal numbers of GalNacT2-positive outposts and more ManII-positive outposts than nudE39A/Df mutant axons (Figure 5B). This indicates that the KhcE177K/27 mutant axons have just as many Golgi compartments as the nudE39A/Df mutant axons, but that the compartments are not as connected. Correspondingly, microtubule polarity is largely normal in KhcE177K/27 mutant axons, which is in contrast to the nudE39A/Df mutant axons (Figure 5C). Combined, these results suggest that nudE (and dynein activity) are needed for the proper localization, but not the formation, of multicompartment Golgi outposts. In contrast, enhancing kinesin-1 activity both perturbs Golgi localization and antagonizes the connectedness of Golgi compartments.
Figure 5.
Inducing the formation of multicompartment Golgi alters microtubule polarity in the axons of Khc mutant neurons. (A and B) Axons of KhcE177K/27 mutant neurons contain fewer multicompartment Golgi outposts than nudE39A/Df mutants, despite have similar or more numbers of ManII- and GalNacT2-positive compartments. Overexpressing GM130 increases the percentage of multicompartment outposts in KhcE177K/27 mutant axons. *P = 0.05–0.01, **P = 0.01–0.001 and ****P < 0.0001; Kruskal–Wallis test with post hoc Dunn’s multiple comparison analysis (% multicompartment Golgi outposts) and one-way ANOVA with Tukey’s post hoc analysis (# outposts). Scale bar, 10 µm. Closed arrowheads indicate multicompartment outposts and open arrowheads indicate single compartments. (C) In contrast to nudE39A/Df mutants, KhcE177K/27 mutant axons have normal microtubule polarity. *P = 0.05–0.01, ***P = 0.001–0.0001, and ****P < 0.0001; Kruskal–Wallis test with post hoc Dunn’s multiple comparison analysis (% comets); Fisher’s Exact test (% axons). (D) The increase in multicompartment Golgi outposts in KhcE177K/27 mutant axons that results from the overexpression of GM130 is accompanied by an increase in misoriented axonal microtubules. **P = 0.01–0.001; Kruskal–Wallis test with post hoc Dunn’s multiple comparison analysis. All data are mean ± SD. n.s., not significant; RFP, red fluorescent protein.
We then asked whether increasing GM130 might alter the polarity of axonal microtubules in the KhcE177K/27 mutant axons, which contain predominantly single-compartment Golgi outposts. In control neurons, the overexpression of GM130 alone did not affect axonal microtubule polarity (Figure 5D). The overexpression of GM130 in KhcE177K/27 mutant neurons both increased the percentage of multicompartment outposts and resulted in the appearance of ectopic minus-end-distal microtubules (Figure 5, A and D). Altogether, our data support the idea that multicompartment Golgi outposts have the capacity to remodel microtubule polarity locally even if they are not essential to the overall polarity of the dendritic cytoskeleton.
Discussion
Microtubules in axons and dendrites have distinct polarities. In a variety of cell types microtubule orientation is regulated by MTOCs, raising the question of whether neurons have MTOCs that carry out a similar function. In dendrites, Golgi outposts are likely candidates (Ori-McKenney et al. 2012; Zhou et al. 2014; Yalgin et al. 2015). The compartmental organization of Golgi outposts and their correlation with microtubule growth initiation sites were recently shown to depend on the cis-Golgi matrix protein GM130, the fly AKAP450 ortholog plp, and cnn (Ori-McKenney et al. 2012; Zhou et al. 2014; Yalgin et al. 2015). We found that the elimination of these factors does not affect the predominantly minus-end-distal orientation of microtubules in class IV da neuron dendrites, suggesting that Golgi outposts are not necessary for the unique polarity of the dendritic microtubule network. This raises the question of whether Golgi outposts might have any capacity to affect microtubule orientation. Our analysis of outposts in dendrites and outposts mislocalized to axons suggests that ectopic multicompartment Golgi outposts are sufficient to alter microtubule polarity, and likely do so independently of microtubule nucleation. We propose that the unique polarity of the dendritic microtubule cytoskeleton is established and maintained independently of Golgi outposts, but that multicompartment Golgi outposts may have the capacity to locally influence microtubule polarity during events such as dendrite branch extension.
Studies carried out in mammalian cells have shown that Golgi serve as platforms for microtubule nucleation, anchoring, and stabilization (Sanders and Kaverina 2015; Martin and Akhmanova 2018; Fu et al. 2019). These activities arise from distinct protein complexes, whose recruitment to the cis-Golgi depends on GM130. Golgi are generally thought to anchor and stabilize microtubules that have been generated at Golgi membranes, although the molecular mechanism by which Golgi would selectively capture these microtubules is unclear (Zhu and Kaverina 2013; Martin and Akhmanova 2018). Our studies indicate that γ-TuRC-mediated microtubule nucleation is dispensable for Golgi outposts to affect microtubule polarity. Consistently, a new study indicates that γ-tubulin only rarely associates with outposts (Mukherjee et al., 2019 preprint). One possibility is that microtubules are nucleated at Golgi independently of γ-tubulin. For example, it was recently reported that the mammalian tubulin polymerization promoting protein (TPPP) nucleates microtubules at Golgi outposts independently of γ-tubulin; however, TPPP is enriched in glia cells, not neurons, in the mammalian nervous system (Fu et al. 2019). Another possibility is that Golgi outposts may be able to capture and stabilize microtubules that are growing in the vicinity of outposts in the relatively confined spaces of dendrites (and axons). For example, in the nudE mutant axons, ectopic multicompartment Golgi outposts may alter microtubule polarity by stabilizing nearby misoriented microtubules that would otherwise be eliminated. Thus, outposts may influence microtubule polarity by tethering and stabilizing microtubules that are not generated at Golgi.
Our results raise the question of what molecular players may organize microtubules at Golgi outposts in neurons. A key component of the complexes that tether and stabilize microtubules on Golgi in vertebrate cells is myomegalin (Roubin et al. 2013; Wang et al. 2014; Wu et al. 2016). However, there is no clear Drosophila ortholog of myomegalin, making it difficult to directly test this model. The most closely related family member in flies is cnn, which is implicated in activating γ-tubulin-templated microtubule assembly (Choi et al. 2010; Roubin et al. 2013) and which we have shown is not necessary for Golgi outposts to alter axonal microtubule polarity. Moreover, new findings call into question whether cnn strongly associates with Golgi in fly neurons (Mukherjee et al., 2019 preprint). Another component of the Golgi-associated complex that anchors and stabilizes microtubules is the microtubule minus-end-binding protein CAMSAP2, whose fly ortholog is Patronin. Recent studies using da neurons have shown that Patronin is needed for minus-end-distal microtubules in dendrites, and likely acts by antagonizing the kinesin-13 microtubule depolymerase Klp10A (Feng et al. 2019; Wang et al. 2019). However, our preliminary analysis of Patronin localization makes it unclear whether Patronin localizes to or functions at Golgi outposts. In work using mitotic mammalian cells, GM130 has also been implicated in the stabilization of microtubules on Golgi through a mechanism that depends on the microtubule-associated protein TPX2; however, TPX2 structure and function are likely not conserved between mammals and flies (Goshima 2011; Hayward et al. 2014; Wei et al. 2015). Thus, additional studies are needed to identify the molecular players that tether and stabilize microtubules on Golgi in Drosophila neurons.
The organization of the Golgi apparatus into a multicompartment stack gives it morphological and functional polarity. Correspondingly, microtubules associated with Golgi are proposed to be oriented in a particular direction relative to the Golgi compartments (Efimov et al. 2007; Zhu and Kaverina 2013; Martin and Akhmanova 2018). In da neurons, the correlation between microtubule polarity and Golgi compartment organization is supported by findings that microtubule growth typically initiates in a single direction from an outpost (Ori-McKenney et al. 2012; Yalgin et al. 2015). This suggests a relationship between the polarity of the Golgi stack and the associated microtubules. Thus, the relative orientation of the Golgi outpost stack likely influences the polarity of Golgi-associated microtubules (Delandre et al. 2016). Our finding that elevated GM130 levels altered microtubule polarity in dendrites may suggest that GM130 instigated the formation of misoriented Golgi stacks that in turn stabilized misoriented microtubules. Given the potential of multicompartment outposts to influence microtubule polarity, it will be interesting to determine how Golgi outpost compartmentalization in dendrites is controlled to ensure proper dendritic microtubule polarity.
Acknowledgments
We thank the following laboratories and stock centers for generously sharing Drosophila strains: Paul Conduit (University of Cambridge) and Jordan Raff (University of Oxford) for dGrip75 flies, Bing Ye (University of Michigan) for UAS-GalNacT2::TagRFP flies, and the Bloomington Drosophila Stock Center [supported by National Institutes of Health (NIH) grant P40 OD-018537]; Erik Dent and Mary Halloran (University of Wisconsin–Madison), Adrian Moore (the Riken Center for Brain Science), Paul Conduit (University of Cambridge), and members of the Wildonger laboratory for insightful discussions and feedback; and Lindsay Mosher and Dena Johnson-Schlitz for technical assistance. This work is supported by NIH grant R01 NS-102385.
Footnotes
Communicating editor: H. Bellen
Literature Cited
- Arthur A. L., Yang S. Z., Abellaneda A. M., and Wildonger J., 2015. Dendrite arborization requires the dynein cofactor NudE. J. Cell Sci. 128: 2191–2201. 10.1242/jcs.170316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F. A., Puype M., Vandekerckhove J., and Warren G., 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91: 253–262. 10.1016/S0092-8674(00)80407-9 [DOI] [PubMed] [Google Scholar]
- Baschieri F., Confalonieri S., Bertalot G., Di Fiore P. P., Dietmaier W. et al. , 2014. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat. Commun. 5: 4839 10.1038/ncomms5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza K. M., Rose D. J., Gilbert S. P., and Saxton W. M., 1999. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J. Biol. Chem. 274: 31506–31514. 10.1074/jbc.274.44.31506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. K., Liu P., Sze S. K., Dai C., and Qi R. Z., 2010. CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J. Cell Biol. 191: 1089–1095. 10.1083/jcb.201007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo U., Winding M., Lu W., and Gelfand V. I., 2015. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. Elife 4: e10140 10.7554/eLife.10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delandre C., Amikura R., and Moore A. W., 2016. Microtubule nucleation and organization in dendrites. Cell Cycle 15: 1685–1692. 10.1080/15384101.2016.1172158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M. et al. , 2007. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12: 917–930. 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Thyagarajan P., Shorey M., Seebold D. Y., Weiner A. T. et al. , 2019. Patronin-mediated minus end growth is required for dendritic microtubule polarity. J. Cell Biol. 218: 2309–2328. 10.1083/jcb.201810155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M. M., McAlear T. S., Nguyen H., Oses-Prieto J. A., Valenzuela A. et al. , 2019. The Golgi outpost protein TPPP nucleates microtubules and is critical for myelination. Cell 179: 132–146.e14. 10.1016/j.cell.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol A., Racca C., and Triller A., 1999. Dendritic and postsynaptic protein synthetic machinery. J. Neurosci. 19: 168–179. 10.1523/JNEUROSCI.19-01-00168.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., 2011. Identification of a TPX2-like microtubule-associated protein in Drosophila. PLoS One 6: e28120 10.1371/journal.pone.0028120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Jan L. Y., and Jan Y. N., 2011. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc. Natl. Acad. Sci. USA 108: 9673–9678. 10.1073/pnas.1106386108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward D., Metz J., Pellacani C., and Wakefield J. G., 2014. Synergy between multiple microtubule-generating pathways confers robustness to centrosome-driven mitotic spindle formation. Dev. Cell 28: 81–93. 10.1016/j.devcel.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C., and Ehlers M. D., 2003. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 23: 6188–6199. 10.1523/JNEUROSCI.23-15-06188.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado L., Caballero C., Gavilan M. P., Cardenas J., Bornens M. et al. , 2011. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J. Cell Biol. 193: 917–933. 10.1083/jcb.201011014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B. V., Saunders H. A. J., Record H. L., Johnson-Schlitz D. M., and Wildonger J., 2017. Effects of mutating α-tubulin lysine 40 on sensory dendrite development. J. Cell Sci. 130: 4120–4131. 10.1242/jcs.210203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. T., Yue Y., Ng A., Kamiyama D., Huang B. et al. , 2018. Autoinhibition of kinesin-1 is essential to the dendrite-specific localization of Golgi outposts. J. Cell Biol. 217: 2531–2547. 10.1083/jcb.201708096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. T., Saunders H. A., and Wildonger J., 2019. Microtubule control of functional architecture in neurons. Curr. Opin. Neurobiol. 57: 39–45. 10.1016/j.conb.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman E., Tokito M., and Holzbaur E. L. F., 2017. CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons. Traffic 18: 808–824. 10.1111/tra.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis V., and Rabouille C., 2003. A novel role for dp115 in the organization of tER sites in Drosophila. J. Cell Biol. 162: 185–198. 10.1083/jcb.200301136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis V., Spoorendonk K. M., and Rabouille C., 2005. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol. Biol. Cell 16: 4061–4072. 10.1091/mbc.e04-10-0938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Li H., Lee Y. N., Cheng Y. J., Wu R. M. et al. , 2015. Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J. Cell Biol. 210: 471–483. 10.1083/jcb.201411033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Mei M., Li Q., Roboti P., Pang Q. et al. , 2017. Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. Proc. Natl. Acad. Sci. USA 114: 346–351. 10.1073/pnas.1608576114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M., 2019. The physiological functions of the golgin vesicle tethering proteins. Front. Cell Dev. Biol. 7: 94 10.3389/fcell.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P., Salvatore L., Mironov A. Jr., Di Campli A., Di Tullio G. et al. , 2007. The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol. Biol. Cell 18: 1595–1608. 10.1091/mbc.e06-10-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., and Akhmanova A., 2018. Coming into focus: mechanisms of microtubule minus-end organization. Trends Cell Biol. 28: 574–588. 10.1016/j.tcb.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., and Raff J. W., 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165: 673–683. 10.1083/jcb.200402130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw T. L., Li K., Kao L. R., and Kaufman T. C., 1999. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development 126: 2829–2839. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Brooks P., Bernard F., Guichet A., and Conduit P. T., 2019. The Somatic Golgi nucleates microtubules that are directed by Kinesin-2 to maintain microtubule polarity within neurons. bioRxiv. (Preprint posted January 8, 2020). 10.1101/846832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N. et al. , 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131: 1715–1726. 10.1083/jcb.131.6.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. M., Stone M. C., and Rolls M. M., 2011. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 6: 38 10.1186/1749-8104-6-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. M., McCracken C. J., Milner E. S., Goetschius D. J., Weiner A. T. et al. , 2014. Gamma-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol. Biol. Cell 25: 2039–2050. 10.1091/mbc.e13-09-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K. M., Jan L. Y., and Jan Y. N., 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76: 921–930. 10.1016/j.neuron.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. P., Mayer T., and McCarthy J. B., 2001. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr. Biol. 11: 351–355. 10.1016/S0960-9822(01)00077-X [DOI] [PubMed] [Google Scholar]
- Puthenveedu M. A., Bachert C., Puri S., Lanni F., and Linstedt A. D., 2006. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 8: 238–248. 10.1038/ncb1366 [DOI] [PubMed] [Google Scholar]
- Rao A. N., and Baas P. W., 2018. Polarity sorting of microtubules in the axon. Trends Neurosci. 41: 77–88. 10.1016/j.tins.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. N., Patil A., Black M. M., Craig E. M., Myers K. A. et al. , 2017. Cytoplasmic dynein transports axonal microtubules in a polarity-sorting manner. Cell Rep. 19: 2210–2219. 10.1016/j.celrep.2017.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Kirschen G. W., Szczurkowska J., Di Antonio A., Wang J. et al. , 2018. Repositioning of somatic Golgi apparatus is essential for the dendritic establishment of adult-born hippocampal neurons. J. Neurosci. 38: 631–647. 10.1523/JNEUROSCI.1217-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson S. L., Redwine W. B., Vale R. D., and Carter A. P., 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 19: 382–398 (erratum: Nat. Rev. Mol. Cell Biol. 19: 479). 10.1038/s41580-018-0004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios R. M., 2014. The centrosome-Golgi apparatus nexus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369: 20130462. 10.1098/rstb.2013.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero S., Cardenas J., Bornens M., and Rios R. M., 2009. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 28: 1016–1028. 10.1038/emboj.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubin R., Acquaviva C., Chevrier V., Sedjai F., Zyss D. et al. , 2013. Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol. Open 2: 238–250. 10.1242/bio.20123392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A. D., and Feldman J. L., 2017. Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 44: 93–101. 10.1016/j.ceb.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Huertas C., Freixo F., Viais R., Lacasa C., Soriano E. et al. , 2016. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 7: 12187 10.1038/ncomms12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A. A., and Kaverina I., 2015. Nucleation and dynamics of Golgi-derived microtubules. Front. Neurosci. 9: 431 10.3389/fnins.2015.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer F., Luschnig S., Koch I., and Nusslein-Volhard C., 2002. Gamma-tubulin37C and gamma-tubulin ring complex protein 75 are essential for bicoid RNA localization during drosophila oogenesis. Dev. Cell 3: 685–696. 10.1016/S1534-5807(02)00301-5 [DOI] [PubMed] [Google Scholar]
- Stiess M., Maghelli N., Kapitein L. C., Gomis-Ruth S., Wilsch-Brauninger M. et al. , 2010. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327: 704–707. 10.1126/science.1182179 [DOI] [PubMed] [Google Scholar]
- Stone M. C., Roegiers F., and Rolls M. M., 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell 19: 4122–4129. 10.1091/mbc.e07-10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tann J. Y., and Moore A. W., 2019. MTOC organization and competition during neuron differentiation. Results Probl. Cell Differ. 67: 337–357. 10.1007/978-3-030-23173-6_14 [DOI] [PubMed] [Google Scholar]
- Tormanen K., Ton C., Waring B. M., Wang K., and Sutterlin C., 2019. Function of Golgi-centrosome proximity in RPE-1 cells. PLoS One 14: e0215215 10.1371/journal.pone.0215215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaizel-Ohayon D., and Schejter E. D., 1999. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 9: 889–898. 10.1016/S0960-9822(99)80393-5 [DOI] [PubMed] [Google Scholar]
- Vérollet C., Colombié N., Daubon T., Bourbon H. M., Wright M. et al. , 2006. Drosophila melanogaster gamma-TuRC is dispensable for targeting gamma-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 172: 517–528. 10.1083/jcb.200511071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainman A., Creque J., Williams B., Williams E. V., Bonaccorsi S. et al. , 2009. Roles of the Drosophila NudE protein in kinetochore function and centrosome migration. J. Cell Sci. 122: 1747–1758. 10.1242/jcs.041798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang C., and Qi R. Z., 2014. A newly identified myomegalin isoform functions in Golgi microtubule organization and ER-Golgi transport. J. Cell Sci. 127: 4904–4917. 10.1242/jcs.155408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Rui M., Tang Q., Bu S., and Yu F., 2019. Patronin governs minus-end-out orientation of dendritic microtubules to promote dendrite pruning in Drosophila. Elife 8: e39964. 10.7554/eLife.39964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. H., Zhang Z. C., Wynn R. M., and Seemann J., 2015. GM130 regulates Golgi-derived spindle assembly by activating TPX2 and capturing microtubules. Cell 162: 287–299. 10.1016/j.cell.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., and Akhmanova A., 2017. Microtubule-organizing centers. Annu. Rev. Cell Dev. Biol. 33: 51–75. 10.1146/annurev-cellbio-100616-060615 [DOI] [PubMed] [Google Scholar]
- Wu J., de Heus C., Liu Q., Bouchet B. P., Noordstra I. et al. , 2016. Molecular pathway of microtubule organization at the Golgi apparatus. Dev. Cell 39: 44–60. 10.1016/j.devcel.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Yalgin C., Ebrahimi S., Delandre C., Yoong L. F., Akimoto S. et al. , 2015. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat. Neurosci. 18: 1437–1445. 10.1038/nn.4099 [DOI] [PubMed] [Google Scholar]
- Ye B., Zhang Y., Song W., Younger S. H., Jan L. Y. et al. , 2007. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130: 717–729. 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wildonger J., Ye B., Zhang Y., Kita A. et al. , 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10: 1172–1180. 10.1038/ncb1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Chang J., Wang X., Savelieff M. G., Zhao Y. et al. , 2014. GM130 is required for compartmental organization of dendritic Golgi outposts. Curr. Biol. 24: 1227–1233. 10.1016/j.cub.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., and Kaverina I., 2013. Golgi as an MTOC: making microtubules for its own good. Histochem. Cell Biol. 140: 361–367. 10.1007/s00418-013-1119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are represented fully within the article and its figures.