OBJECTIVES:
Chronic idiopathic constipation (CIC) is characterized by unsatisfactory defecation and difficult or infrequent stools. CIC affects 9%–20% of adults in the United States, and although prevalent, gaps in knowledge remain regarding CIC healthcare seeking and medication use in the community. We recruited a population-based sample to determine the prevalence and predictors of (i) individuals having discussed their constipation symptoms with a healthcare provider and (ii) the use of constipation therapies.
METHODS:
We recruited a representative sample of Americans aged 18 years or older who had experienced constipation. Those who met the Rome IV criteria for irritable bowel syndrome and opioid-induced constipation were excluded. The survey included questions on constipation severity, healthcare seeking, and the use of constipation medications. We used multivariable regression methods to adjust for confounders.
RESULTS:
Overall, 4,702 participants had experienced constipation (24.0% met the Rome IV CIC criteria). Among all respondents with previous constipation, 37.6% discussed their symptoms with a clinician (primary care provider 87.6%, gastroenterologist 26.0%, and urgent care/emergency room physician 7.7%). Age, sex, race/ethnicity, marital status, employment status, having a source of usual care, insurance status, comorbidities, locus of control, and constipation severity were associated with seeking care (P < 0.05). Overall, 47.8% of respondents were taking medication to manage their constipation: over-the-counter medication(s) only, 93.5%; prescription medication(s) only, 1.3%; and both over-the-counter medication(s) and prescription medication(s), 5.2%.
DISCUSSION:
We found that 3 of 5 Americans with constipation have never discussed their symptoms with a healthcare provider. Furthermore, the use of prescription medications for managing constipation symptoms is low because individuals mainly rely on over-the-counter therapies.
INTRODUCTION
Chronic idiopathic constipation (CIC) is a functional gastrointestinal (GI) disorder that leads to difficult, infrequent, or incomplete defecation (1). CIC affects daily life, increases psychological distress, and impairs health-related quality of life (2). Moreover, CIC is common in the community and previous reports estimate its prevalence to range from 9% to 20% in the United States (3).
As a highly prevalent disease, CIC imparts a significant burden on the healthcare system. In 2010, 2.8 million ambulatory and emergency room visits centered on the evaluation and management of constipation (4). CIC also imposes a substantial economic burden; the annual direct cost ranges from $1,900 to $12,000 per patient (5–7). Patients also report that constipation symptoms interfere with 4 of 30 days and decrease productivity by 25%, which further increases the overall financial and societal costs associated with the disorder (5,8).
Despite the high prevalence and large economic burden associated with CIC, important gaps in knowledge remain. For example, it remains unclear what drives individuals with chronic constipation to seek or not seek professional medical care for management of their symptoms. There have also been few efforts to examine the use of over-the-counter (OTC) and prescription medications among individuals in the general community. In this descriptive study, we aimed to address these gaps in knowledge by surveying a large representative sample of Americans with chronic constipation to determine (i) the prevalence and predictors of individuals who have sought medical care for their constipation symptoms and (ii) the use of and satisfaction with OTC and prescription medicines for treating constipation.
METHODS
Study design and participant recruitment
We performed a cross-sectional, US population-based, online survey of individuals with chronic constipation between March 21 and March 28, 2018. Participants completed an online, self-administered questionnaire that was estimated to take 10–20 minutes to complete. This study was approved by the Cedars-Sinai Institutional Review Board (Pro47804).
To recruit a representative sample of Americans, we collaborated with Cint (www.cint.com), a survey research firm that partners with research panels across the United States. They use quotas for age, sex, and region of the country (Northeast, South, Midwest, and West) based on the latest US Census data to support recruitment of a population-based sample. Cint's platform also uses a reward system based on marketplace points, which we describe in detail elsewhere (9,10). Participants who fully completed our survey received points worth between $0.40 and $1.45; the actual amount of points awarded to each respondent was determined by the research panel's incentive policy.
Panelists who met the Cint US quota criteria were sent an email through the Cint research panels inviting them to complete an online survey. The email included a link to the survey along with the following text: “Based on the information stored in your [research panel] profile, we believe we have a survey that you will qualify & earn from. The survey takes approximately 10–20 minutes and if you successfully complete it, your account will be credited with [incentive].” Users who clicked the link were then brought to our survey home page which was labeled as a “National Health Survey.”
Study population
All respondents who accessed the survey were first asked which of the following GI symptoms they had ever experienced (presented in random order): constipation, abdominal pain, bloat/gas, bowel incontinence, diarrhea, dysphagia, heartburn/reflux, nausea/vomiting, or none of the above. Only those who noted previous constipation, which was described as “hard, lumpy, or infrequent stools; straining,” were allowed to continue with the survey. By having a “blinded” screening question naming 8 GI symptoms, we aimed to maximize the likelihood that respondents had, in fact, experienced constipation in the past and were not simply seeking compensation by participating in a survey.
Respondents who reported previous constipation were guided through the Rome IV irritable bowel syndrome (IBS), CIC, and opioid-induced constipation (OIC) questionnaires (1). Those who met the IBS or OIC criteria were ineligible for the study because the Rome IV states that individuals with CIC cannot have a concomitant diagnosis of IBS or OIC (1). Of note, we allowed individuals who did not meet the Rome IV CIC criteria to complete the study because those currently taking OTC or prescription medicines may not have met these criteria at the time of the survey; they could have been Rome IV-positive before starting such therapies. This allowed us to assess the global landscape for constipation healthcare seeking and medication use in the United States. However, we conducted an a priori subgroup analysis among those who met the Rome IV criteria for CIC.
Survey instrument
Eligible individuals who had experienced constipation and did not meet the Rome IV criteria for IBS or OIC proceeded through the remaining survey items; see Supplemental File 1, Supplementary Digital Content 1, http://links.lww.com/AJG/B499, for the survey instrument. They next completed questions on their constipation onset and severity as measured by the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaire (11). The survey also assessed the extent that respondents believed constipation to be a severe health problem, locus of control, and perceived cause of constipation as determined through a free-text response.
Next, we examined whether individuals sought medical care for evaluation and management of their constipation symptoms. For those who reported seeking health care, we asked which provider(s) they met with to discuss their symptoms. We also assessed which diagnostic tests were performed specifically to evaluate their constipation. Afterward, the respondents reported which medications they were currently taking for the management of their constipation (see Outcomes section for details). For each therapy, the participants completed the abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) (12) and were asked whether they experienced any issues with each medicine. The survey concluded with demographic, socioeconomic, and medical, neurologic, and psychiatric comorbidity questions.
Outcomes
Our primary outcome was whether an individual sought health care for his or her constipation. This was determined by identifying those who answered “Yes” to “Have you ever discussed your constipation with a healthcare provider?” Our secondary outcome was the use of medications for managing constipation. All respondents were asked to “Select all treatments that you are currently using for your constipation”; answer options included OTC medicines (bisacodyl, docusate, fiber supplement, magnesium, polyethylene glycol 3350, and senna), prescription therapies (lactulose, linaclotide, lubiprostone, and plecanatide), “other,” “unsure,” and “none of the above.”
Statistical analyses
All statistical analyses were performed in Stata 15.1 (StataCorp LP, College Station, TX), and a 2-tailed P value of less than 0.05 was considered statistically significant. We used bivariate analyses to compare individuals with chronic constipation who did vs did not seek health care for their symptoms and those who were vs were not taking medications. Specifically, we compared normally distributed continuous, non-normally distributed continuous, and categorical variables between the groups using the Student t tests, Mann-Whitney U tests, and χ2 tests, respectively. For multivariable analyses, we used logistic regression models to identify predictive factors and to calculate odds ratios (ORs) and 95% confidence intervals. The regression analyses were performed on our outcomes of seeking health care for constipation and the current use of constipation medications. Both models adjusted for constipation-related factors, cognitions, sociodemographics, and medical, neurologic, and psychiatric comorbidity covariates collected through the survey.
Qualitative analyses.
The survey included an open-ended question that enabled respondents to share their thoughts regarding the perceived etiology of their constipation: “In your own words, what do you think is the cause(s) of your constipation?” A summative content analysis was adopted to examine their perceived constipation etiologies and to assess the presence of each trigger in the data (13). We coded the free texts (word, sentence, and paragraph) and organized the codes into categories and subcategories.
RESULTS
Study population
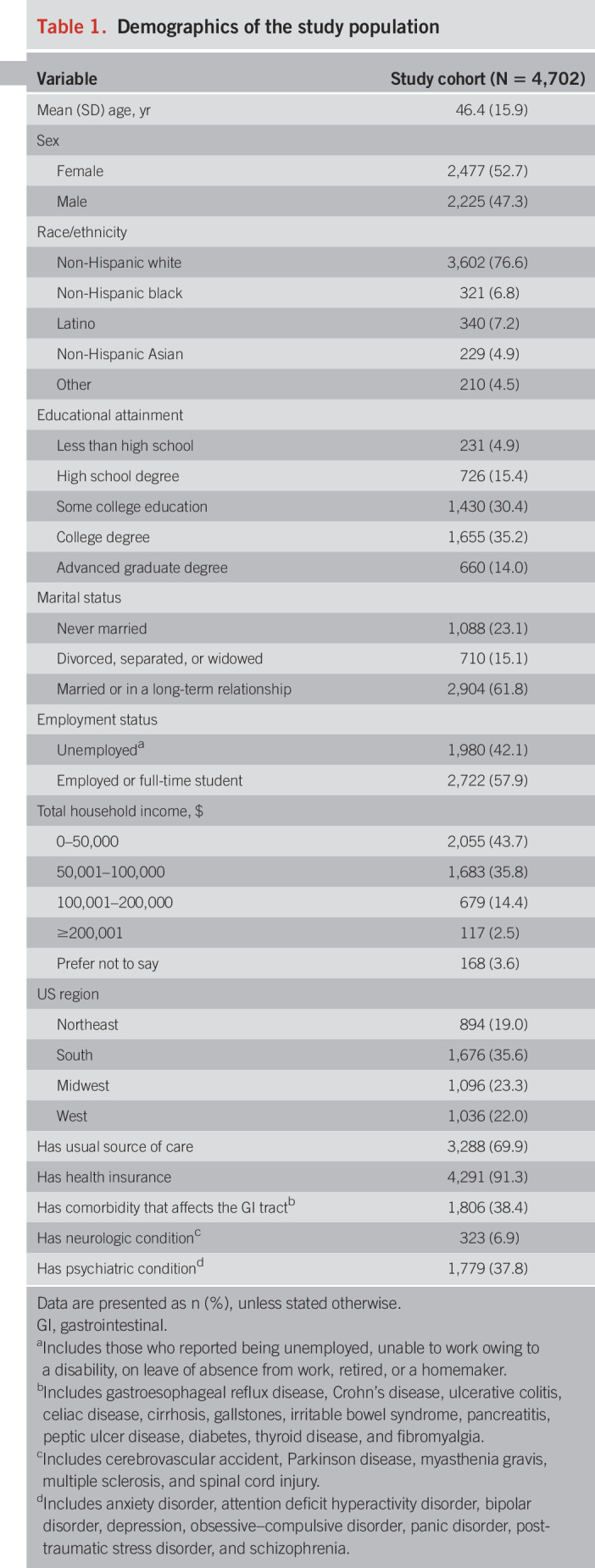
A total of 66,279 individuals were invited through Cint to complete the survey, of whom 16,053 accessed the survey. We excluded those who did not provide consent (529, 3.3%), did not experience previous constipation (8,423, 52.5%), met the Rome IV criteria for IBS (1,430, 8.9%) or OIC (305, 1.9%), had missing data (390, 2.4%), or finished the survey too quickly (274, 1.7%). Therefore, the analytic sample included 4,702 respondents with chronic constipation. The demographics of the study cohort are listed in Table 1; comparison to the US population is shown in Table 1, Supplementary Digital Content 2, http://links.lww.com/AJG/B494.
Table 1.
Demographics of the study population
Constipation characteristics and perceived etiologies
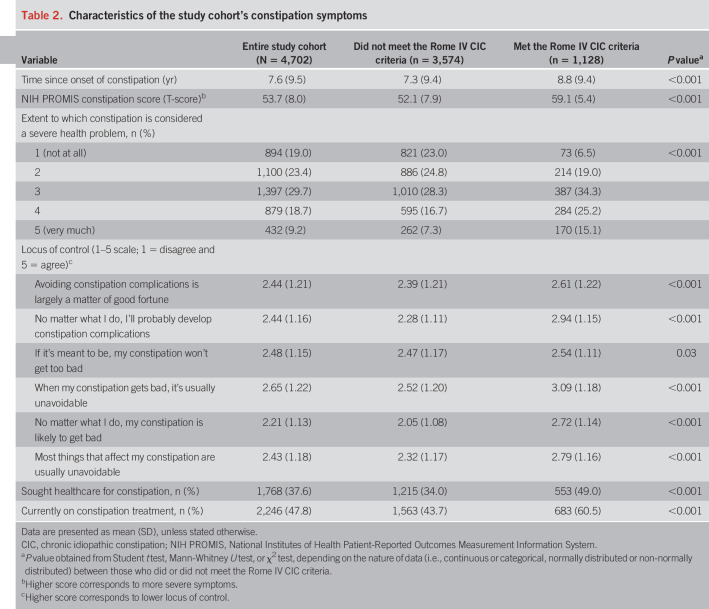
Overall, the average time since onset of constipation was 7.6 years, and we found that 4,372 participants (93.0%) experienced constipation symptoms within the past week, as measured by the NIH PROMIS. The average NIH PROMIS severity score on a T-scale was 53.7 ± 8.0. Subjectively, 19.0% reported that constipation was not a severe health issue at all, whereas 9.2% stated it was a very severe problem. We noted that 1,128 respondents (24.0%) met the Rome IV CIC criteria; their demographics are shown in Table 2, Supplementary Digital Content 3, http://links.lww.com/AJG/B495.
Table 2 shows the descriptions of the study cohort's constipation symptoms, stratified by the Rome IV CIC status. Those who met the Rome IV criteria for CIC had longer duration of symptoms, higher PROMIS constipation scores, lower locus of control, and were more likely to consider constipation to be a severe health problem when compared with those who did not meet the criteria.
Table 2.
Characteristics of the study cohort's constipation symptoms
Figure 1, Supplementary Digital Content 4, http://links.lww.com/AJG/B493, shows a word cloud generated from the qualitative content analysis detailing the various constipation triggers reported by respondents. Food intake was the most common cause: “I don't get enough fiber and I eat too much junk food.” Lack of fluid intake was also cited as a main trigger (e.g., “not drinking enough water”). Medication, particularly those used for pain, was the next most prevalent etiology: “This is mostly caused by the pain medication I take to treat my chronic pain” (note: those who met the Rome IV OIC criteria were excluded but those on pain medicines who did not meet such criteria remained eligible). Many respondents believed that their constipation was caused by multiple factors such as food, dehydration, medications, stress, and lack of exercise: “Generally I am pretty aware and do things I should do like eating right and exercise. I walk three miles every day but sometimes the schedule gets thrown off and that's when I notice changes.”
Healthcare seeking behavior for constipation
Of the 4,702 participants with chronic constipation, 1,768 (37.6%) had ever discussed their constipation with a healthcare provider. Respondents had consulted at any point with primary care providers (87.6%), gastroenterologists (26.0%), and urgent care/emergency room physicians (7.7%) regarding their symptoms.
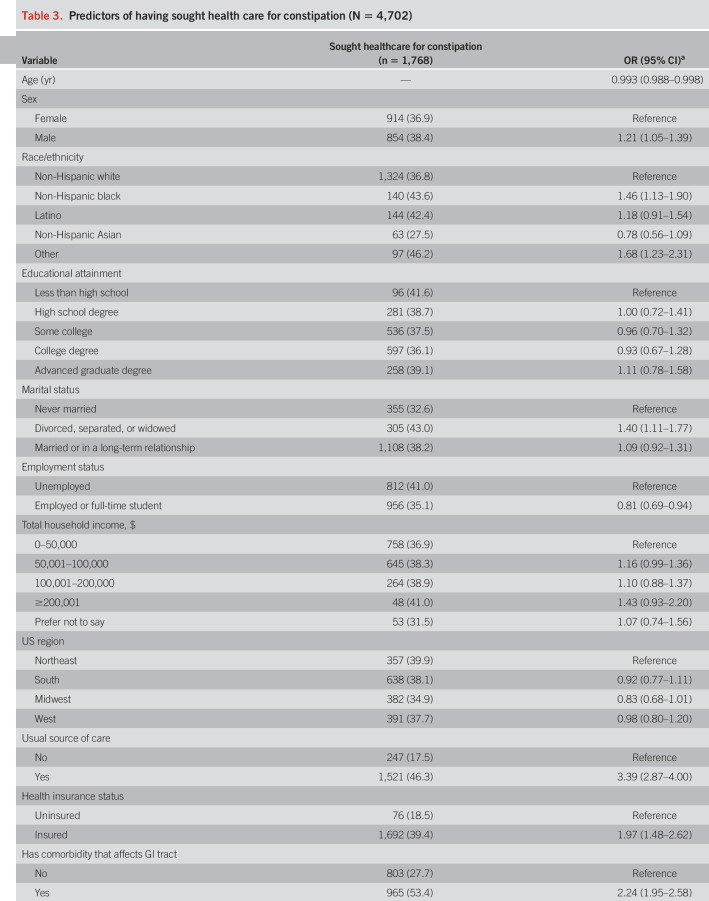
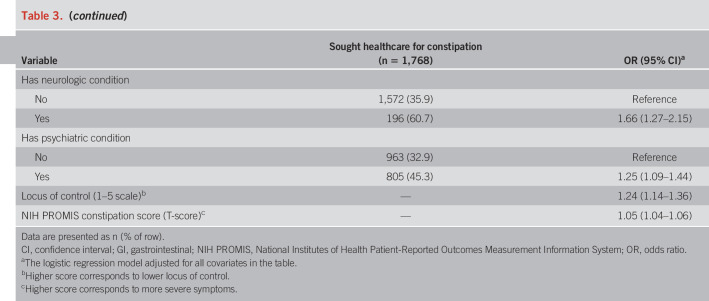
Table 3 shows the findings from the regression analysis on healthcare seeking for constipation. Those who were male (OR 1.21), non-Hispanic black (OR 1.46), other race/ethnicity (OR 1.68), and divorced, separated, or widowed (OR 1.40) were more likely to have sought health care for their symptoms. Individuals with a usual source of care (OR 3.39), health insurance (OR 1.97), medical (OR 2.24), neurologic (OR 1.66), or psychiatric comorbidities (OR 1.25), lower locus of control (OR 1.24), and more severe constipation symptoms (OR 1.05) also had higher odds for seeking care. Conversely, those who were older (OR 0.993) or employed or a full-time student (OR 0.81) were significantly less likely to have sought care. We found no associations with education level, total household income, or the US region.
Table 3.
Predictors of having sought health care for constipation (N = 4,702)
Diagnostic studies.
Among those who sought health care for their constipation (n = 1,768), 946 (53.5%) underwent diagnostic procedures to evaluate their symptoms. Colonoscopy (806, 45.6%) was the most common test; 72.0% (n = 331) and 36.3% (n = 475) of those who did (n = 460) or did not (n = 1,308) consult with a gastroenterologist, respectively, reported undergoing a colonoscopy. This was followed by barium enema (184, 10.4%) and flexible sigmoidoscopy (145, 8.2%). MRI defecography (69, 3.9%), defecogram (53, 3.0%), SmartPill (43, 2.4%), anorectal manometry (34, 1.9%), and Sitzmarks study (27, 1.5%) were ordered less often.
Subgroup analyses—individuals with Rome IV-positive CIC.
Among the 1,128 participants who met the Rome IV criteria for CIC, 553 (49.0%) had discussed their constipation with a healthcare provider. In Table 3, Supplementary Digital Content 5, http://links.lww.com/AJG/B496, we present the results from the regression analysis conducted among this subgroup.
Use of constipation treatments
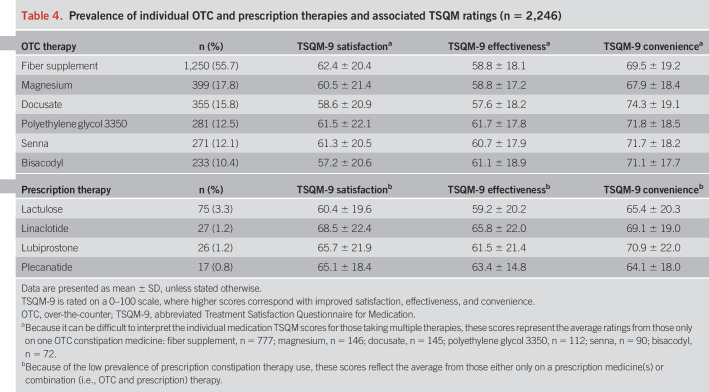
In the overall cohort, 2,246 (47.8%) indicated that they were currently taking medication for their constipation (note: 221 did not know the name of the treatment or were on other treatments). Among those on a known treatment (n = 2,025), 1,893 (93.5%) were only on OTC medicine(s), 27 (1.3%) were only taking a prescription medicine(s), and 105 (5.2%) were on both OTC and prescription therapies. Table 4 shows the proportion of respondents taking each individual medicine. Fiber supplements were the most commonly used therapy, followed by magnesium, docusate, polyethylene glycol 3350, senna, and bisacodyl. Lactulose was the most commonly prescribed medication, followed by linaclotide, lubiprostone, and plecanatide.
Table 4.
Prevalence of individual OTC and prescription therapies and associated TSQM ratings (n = 2,246)
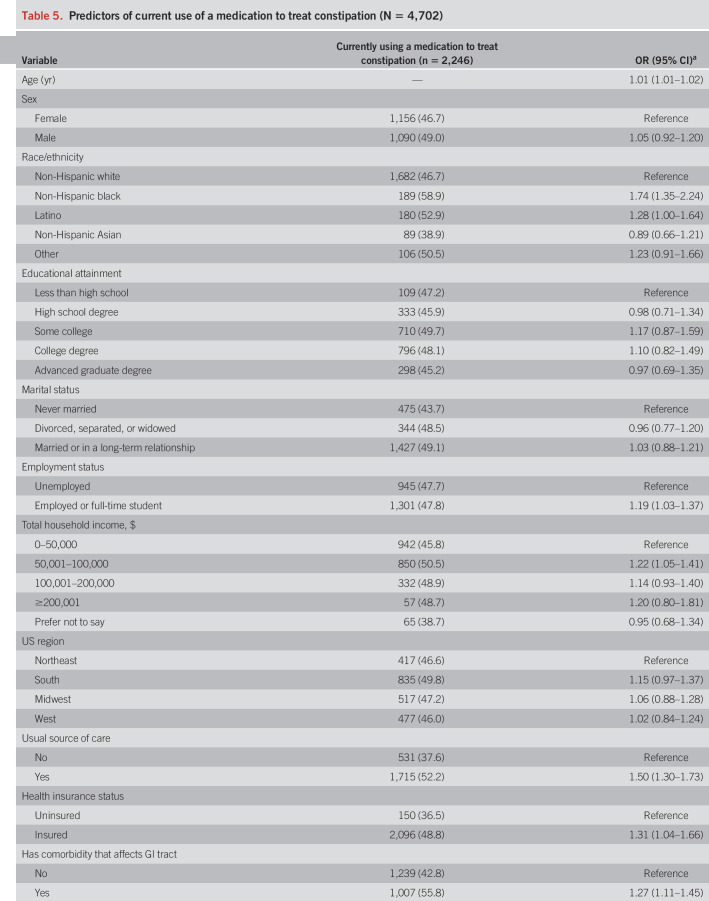
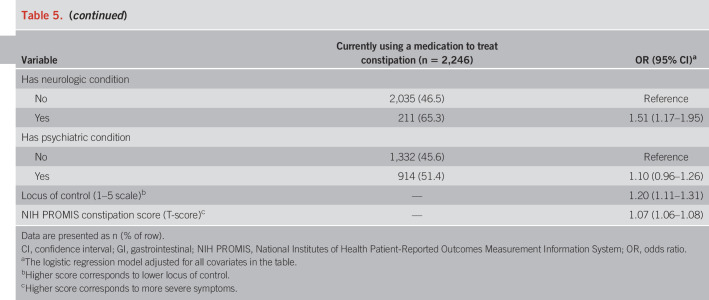
Several variables were associated with participants taking medication (Table 5). Older individuals (OR 1.01), non-Hispanic black (OR 1.74), and Latino individuals (OR 1.28), as well as those who were employed or full-time students (OR 1.19), were more likely to be on treatment. Those with a usual source of care (OR 1.50), insurance (OR 1.31), medical (OR 1.27) or neurologic (OR 1.51) comorbidity, lower locus of control (OR 1.20), and more severe constipation symptoms (OR 1.07) also had higher odds of currently taking medication. On the whole, we did not see associations with sex, education level, marital status, household income, US region, and psychological comorbidities.
Table 5.
Predictors of current use of a medication to treat constipation (N = 4,702)
Subgroup analyses—individuals with Rome IV-positive CIC.
Among the 1,128 participants who met the Rome IV criteria for CIC, 683 (60.5%) were taking a medicine to treat their constipation (note: 66 did not know the name of the treatment or were on other treatments). Of those on a known treatment (n = 617), 572 (92.7%) were only on OTC medicine(s), 7 (1.1%) were only taking a prescription medicine(s), and 38 (6.2%) were on both OTC and prescription therapies. Individuals with Rome IV-positive CIC reported taking the following medicines: fiber supplements (384, 62.2%), magnesium (138, 22.4%), docusate (122, 19.8%), polyethylene glycol 3350 (100, 16.2%), senna (88, 14.3%), bisacodyl (83, 13.5%), lactulose (23, 3.7%), linaclotide (12, 1.9%), lubiprostone (9, 1.5%), and plecanatide (6, 1.0%). Table 4, Supplementary Digital Content 6, http://links.lww.com/AJG/B497, shows the results from the regression analysis on pharmacotherapy use.
Satisfaction with constipation treatments
Overall, when comparing the average TSQM-9 scores between those only on OTC medications with those taking prescription therapies, we found no differences in their ratings for satisfaction (OTC 60.8 ± 21.3 vs prescription 63.3 ± 20.5; P = 0.21), effectiveness (OTC 59.6 ± 18.6 vs prescription 62.3 ± 18.5; P = 0.12), and convenience (OTC 69.9 ± 19.2 vs prescription 67.9 ± 19.2; P = 0.26). Table 4 and Table 5, Supplementary Digital Content 7, http://links.lww.com/AJG/B498, list the TSQM ratings and issues (does not treat symptoms, takes too long to work, high out-of-pocket costs, causes bothersome diarrhea, and causes sudden urge to defecate) reported by respondents, respectively, for each individual medicine.
DISCUSSION
In this large national survey, we found that nearly two-thirds of community-based individuals with constipation have never discussed their symptoms with a healthcare provider. Moreover, only half of individuals are currently taking a therapy for their constipation and most rely on OTC options.
In our study, only 38% of respondents reported ever discussing their constipation symptoms with a healthcare provider, consistent with the range of 22%–43% seen in other studies (14–18). Not surprisingly, having a usual source of care, insurance, comorbidities, and more severe symptoms are significant predictors of healthcare seeking (14,15,18). Men are also 21% more likely to seek care for constipation than women. This finding possibly reflects differential willingness between men and women to discuss constipation symptoms with a provider. Of note, this is in contrast to studies conducted outside of the United States that found that women are more likely than men to seek such care (15,17,18). We also noted differential healthcare seeking among the racial/ethnic groups. When compared with non-Hispanic white people, non-Hispanic black individuals and those in the other group are 46% and 68% more likely to seek care for constipation, respectively. The reasons behind these differences are unclear but likely reflect cultural factors in reporting specific symptoms to healthcare providers.
We found that the locus of control—the extent to which individuals believe they can control events that affect them (19,20)—is associated with constipation healthcare seeking. Namely, those with lower locus of control (i.e., believe symptoms are driven by others, chance, or fate) are more likely to consult with providers regarding their symptoms. However, individuals experiencing this maladaptive cognition may be resistant to both undergoing indicated diagnostic testing and accepting and adhering to treatments, thereby undercutting treatment success and reducing patient satisfaction. Although there are limited data examining the impact of this GI cognition on CIC outcomes, Lackner et al. (21) found that patients with IBS with lower locus of control are less likely to rapidly respond to cognitive behavioral therapy. Therefore, for providers to engage in an optimized, tailored approach with each patient, it is important to screen for this maladaptive cognition, which can be done by asking “How much control do you think you have over your constipation?” (20,22,23). On the other hand, those with higher locus of control are less likely to seek medical care for their constipation. These individuals may be comfortable self-managing their symptoms using OTC therapies, lifestyle modifications, advice from pharmacists, insurance company nurse call lines, or the Internet. Additional research examining why constipated individuals choose not to seek medical care and other sources of information they are leveraging is warranted.
Among those who sought care, 54% reported previous diagnostic testing. Colonoscopy was the most commonly performed test; 46% of health seekers specifically underwent the procedure to evaluate their constipation. Although we did not ask the respondents about alarm features or have access to their medical records to confirm the “true” indication for the procedure, this suggests potential overuse of endoscopy in the evaluation of constipation. This is an issue because the diagnostic yield of colonoscopy for constipation is limited (24,25). Pepin and Ladabaum noted that in 234 individuals undergoing lower endoscopy solely for constipation, no cancers were found and only 3% had advanced lesions (25). The American Society for Gastrointestinal Endoscopy states that colonoscopy should not be performed in the initial evaluation of constipated patients without alarm features or suspicion of organic disease (26). The high usage of endoscopy and other tests seen in our study, in combination with the high prevalence of constipation (3), further reinforces the significant impact of constipation on population health and healthcare costs and emphasizes that efforts to reduce unnecessary testing are needed.
Regarding constipation treatments, nearly half of the respondents are currently taking a medicine to treat their symptoms, which falls within the range previously reported (14%–72%) (15,18,27–30). However, because 93% of our overall cohort reported constipation symptoms in the past week, this suggests that there are numerous individuals who are symptomatic but not on treatment. There are many potential reasons for this finding. For example, some individuals, particularly those with intermittent constipation symptoms, may consider constipation a “nuisance” that does not require pharmacotherapy. In fact, 19% of our cohort reported that their constipation was not at all a severe health problem. In addition, some individuals may not be aware of all the effective OTC and prescription therapies available for managing constipation. The low use of medications could also be related to our finding that participants most often reported food and decreased water intake as the etiologies for their symptoms; many individuals may choose to engage solely in lifestyle changes rather than use pharmacotherapy. Further studies systematically assessing how and the degree to which individuals engage in lifestyle modifications for managing their constipation are needed.
Of those on pharmacotherapy, the majority are only taking OTC medicines (94%). Fiber supplements are the most commonly used product by a large margin, followed by magnesium and docusate. Previous studies have also shown that fiber is the most frequently used therapy (27,28), whereas others have differed (29). For example, Müller-Lissner et al. (29) conducted a survey in 10 European countries and found that bisacodyl is the most commonly used therapy, followed by polyethylene glycol and senna. As for prescription therapies, very few respondents are taking these medicines (6%), with lactulose being the most common (3.3%), followed by linaclotide (1.2%), lubiprostone (1.2%), and plecanatide (0.8%). This is lower than the rates found in the BURDEN-CIC study by Harris et al. (27), who reported that 16% of those with CIC are on prescription treatments. However, they included polyethylene glycol 3350 and senna in the prescription category along with lubiprostone and linaclotide (note: plecanatide was not available at the time of the study) and they did not comment on the prevalence for each medicine (27). To our knowledge, our study is the first in the United States to determine the use of each individual pharmacotherapy option for CIC and the first to include plecanatide.
The reason for the low rate of use of prescription medications in the management of CIC noted in our study is likely multifactorial. From the patient perspective, many may view constipation as a symptom that can be solely managed by OTC medication and they may be reticent to escalate to prescription therapies. Supporting this notion is the finding by Harris et al. (27) that almost half of all patients with CIC use an average of 3 OTC products before seeking help from a healthcare provider. From the providers' side, there may be a lack of knowledge regarding the available medicines, especially the newer prescription options. Some providers, particularly those in primary care, may be hesitant to recommend prescription medicines for constipation, instead preferring to defer to gastroenterologists. This issue, combined with our finding that only 26% of those who sought care have seen a gastroenterologist for their symptoms, may be contributing to the low use of prescription therapies. Given these missed opportunities for using evidence-based, effective therapies for managing constipation, further research to better understand the barriers faced by both patients and healthcare providers as well as continued efforts for improving awareness and education are warranted.
Our study has several strengths. First, this is one of the largest population-based studies evaluating people with chronic constipation. By gathering data on over 4,700 participants, more than 1,100 of whom met the Rome IV CIC criteria, we shed light on the demographics and healthcare utilization of those with chronic constipation in the community setting. Second, we systematically evaluated the current use of and satisfaction with the available OTC and prescription medications used to treat constipation. Unlike studies that focus on data from claims databases or patients presenting to healthcare systems, we analyzed the use and efficacy of these medications among those in the general population, many of whom are self-treating and have not consulted with a healthcare provider.
There are limitations to our survey. First, there is risk of recall bias because the symptom, healthcare seeking, and medication data were self-reported. However, the recall period for the PROMIS constipation questionnaire is only one week and it has been widely validated as part of an NIH consortium (11,31–33). Recall bias is also less of a concern for the medication questions because we asked about participants' current use of the various therapies. Previous research found high concordance between medicines documented in ambulatory medical records and patient survey data (34). As for healthcare seeking, previous studies note that patients tend to underreport their healthcare utilization (35–37); therefore, we may have underestimated the rates of healthcare seeking for constipation and diagnostic testing. Second, given our study's cross-sectional design, we could not quantify the improvement in constipation symptoms experienced by those on therapy; this is an area worthy of further study, particularly in the community setting. Third, there are potential issues related to generalizability. The survey was only administered online, and our results may not extend to those who do not possess basic computing skills or lack regular access to the Internet. However, at the time of the survey in 2018, nearly 90% of Americans were actively using the Internet (38). Finally, participation bias is possible because Cint provided a small financial incentive for those who completed the survey. However, previous research reveals that incentives do not affect the quality of survey responses (39–42) and the demographics of our study cohort largely match those of the US population.
In conclusion, 3 of 5 community-dwelling Americans with chronic constipation have never discussed their symptoms with a healthcare provider. Yet, among those who sought care, we noted that nearly half reported undergoing colonoscopy to evaluate their symptoms, despite its limited diagnostic yield. We also found that most individuals rely on OTC therapies for managing their symptoms and that the use of prescription medicines is very low. Because chronic constipation is highly prevalent with a significant economic burden, further research is needed to better understand why most individuals do not seek care, despite having a treatable condition. Additional research is also needed to determine the patient- and provider-related barriers to using evidence-based, effective prescription medicines for treating constipation.
CONFLICTS OF INTEREST
Guarantor of the article: Christopher V. Almario, MD, MSHPM.
Specific author contributions: S.J.O., G.F., W.S., A.N., B.M.R.S., and C.V.A.: substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published. D.P. and C.K.: substantial contributions to the design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published.
Financial support: This study was funded by the Shire Human Genetic Therapies, a member of the Takeda group of companies. The Cedars-Sinai Center for Outcomes Research and Education (CS-CORE) is supported by The Marc and Sheri Rapaport Fund for Digital Health Sciences & Precision Health. C.V.A. is supported by a career development award from the American College of Gastroenterology. C.V.A. and B.M.R.S. are supported by a CTSI grant from the NIH/NCATS UL1TR001881-01.
Potential competing interests: W.S. and A.N. are employees of Shire, a member of the Takeda group of companies, and stockholders of Takeda. B.M.R.S. has the following relevant disclosures: grant support from AstraZeneca and Takeda Pharmaceuticals; consulting fees from Ironwood Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. C.V.A. has the following relevant disclosures: speaker's fee from Takeda Pharmaceuticals; consulting fees from Bayer U.S. and Synergy Pharmaceuticals. The remaining authors do not have any relevant disclosures.
Previous presentation: This work was presented at the American College of Gastroenterology Annual Scientific Meeting; October 5-10, 2018; Philadelphia, Pennsylvania.
Writing assistance: No medical writing support was received. All content for the manuscript was written and developed by the authors.
Study Highlights.
WHAT IS KNOWN
✓ Chronic constipation is a common and costly disorder that significantly affects health-related quality of life.
✓ Comparatively less is known about healthcare seeking behaviors in relation to constipation and the use of medicines for treating constipation among the general population.
WHAT IS NEW HERE
✓ In this population-based study of community-dwelling Americans, we found that only 37.6% of those with constipation have discussed their symptoms with a healthcare provider.
✓ We also found that 47.8% of respondents are currently taking medications to manage their constipation, most of whom rely on OTC therapies.
Supplementary Material
ACKNOWLEDGMENTS
Proofreading and data checking of the manuscript were provided by PharmaGenesis London, who received funding from Shire, a member of the Takeda group of companies.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B499, http://links.lww.com/AJG/B494, http://links.lww.com/AJG/B495, http://links.lww.com/AJG/B493, http://links.lww.com/AJG/B496, http://links.lww.com/AJG/B497, http://links.lww.com/AJG/B498
REFERENCES
- 1.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016;150(6):1393–407. [DOI] [PubMed] [Google Scholar]
- 2.Rao SS, Seaton K, Miller MJ, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosom Res 2007;63(4):441–9. [DOI] [PubMed] [Google Scholar]
- 3.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am J Gastroenterol 2011;106(9):1582–91; quiz 1, 92. [DOI] [PubMed] [Google Scholar]
- 4.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149(7):1731–41.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013;19(9):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Buono JL, Spalding WM, et al. Healthcare costs among patients with chronic constipation: A retrospective claims analysis in a commercially insured population. J Med Econ 2014;17(2):148–58. [DOI] [PubMed] [Google Scholar]
- 7.Herrick LM, Spalding WM, Saito YA, et al. A case-control comparison of direct healthcare-provider medical costs of chronic idiopathic constipation and irritable bowel syndrome with constipation in a community-based cohort. J Med Econ 2017;20(3):273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neri L, Basilisco G, Corazziari E, et al. Constipation severity is associated with productivity losses and healthcare utilization in patients with chronic constipation. United Eur Gastroenterol J 2014;2(2):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menees SB, Almario CV, Spiegel BMR, et al. Prevalence of and factors associated with fecal incontinence: Results from a population-based survey. Gastroenterology 2018;154(6):1672–81.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almario CV, Ballal ML, Chey WD, et al. Burden of gastrointestinal symptoms in the United States: Results of a nationally representative survey of over 71,000 Americans. Am J Gastroenterol 2018;113(11):1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel BM, Hays RD, Bolus R, et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol 2014;109(11):1804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009;7:36 Those seeking information regarding or permission to use the TSQM are directed to IQVIA at www.iqvia.com/TSQM or TSQM@iqvia.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15(9):1277–88. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38(9):1569–80. [DOI] [PubMed] [Google Scholar]
- 15.Galvez C, Garrigues V, Ortiz V, et al. Healthcare seeking for constipation: A population-based survey in the Mediterranean area of Spain. Aliment Pharmacol Ther 2006;24(2):421–8. [DOI] [PubMed] [Google Scholar]
- 16.Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol 2015;110(4):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: Focus on constipation and resource utilization. Am J Gastroenterol 2002;97(8):1986–93. [DOI] [PubMed] [Google Scholar]
- 18.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: Definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol 2001;96(11):3130–7. [DOI] [PubMed] [Google Scholar]
- 19.Hobbis IC, Turpin G, Read NW. Abnormal illness behaviour and locus of control in patients with functional bowel disorders. Br J Health Psychol 2003;8(Pt 4):393–408. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel BM, Khanna D, Bolus R, et al. Understanding gastrointestinal distress: A framework for clinical practice. Am J Gastroenterol 2011;106(3):380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackner JM, Gudleski GD, Keefer L, et al. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2010;8(5):426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain 1983;17(1):33–44. [DOI] [PubMed] [Google Scholar]
- 23.Wallston BS, Wallston KA, Kaplan GD, et al. Development and validation of the health locus of control (HLC) scale. J Consult Clin Psychol 1976;44(4):580–5. [DOI] [PubMed] [Google Scholar]
- 24.Gupta M, Holub J, Knigge K, et al. Constipation is not associated with an increased rate of findings on colonoscopy: Results from a national endoscopy consortium. Endoscopy 2010;42(3):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepin C, Ladabaum U. The yield of lower endoscopy in patients with constipation: Survey of a university hospital, a public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56(3):325–32. [DOI] [PubMed] [Google Scholar]
- 26.Cash BD, Acosta RD, Chandrasekhara V, et al. The role of endoscopy in the management of constipation. Gastrointest Endosc 2014;80(4):563–5. [DOI] [PubMed] [Google Scholar]
- 27.Harris LA, Horn J, Kissous-Hunt M, et al. The Better Understanding and Recognition of the Disconnects, Experiences, and Needs of Patients with Chronic Idiopathic Constipation (BURDEN-CIC) Study: Results of an online questionnaire. Adv Ther 2017;34(12):2661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanson JF, Kralstein J. Chronic constipation: A survey of the patient perspective. Aliment Pharmacol Ther 2007;25(5):599–608. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Lissner S, Tack J, Feng Y, et al. Levels of satisfaction with current chronic constipation treatment options in Europe—An internet survey. Aliment Pharmacol Ther 2013;37(1):137–45. [DOI] [PubMed] [Google Scholar]
- 30.Wald A, Mueller-Lissner S, Kamm MA, et al. Survey of laxative use by adults with self-defined constipation in South America and Asia: A comparison of six countries. Aliment Pharmacol Ther 2010;31(2):274–84. [DOI] [PubMed] [Google Scholar]
- 31.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochar B, Martin CF, Kappelman MD, et al. Evaluation of gastrointestinal Patient-Reported Outcomes Measurement Information System (GI-PROMIS) symptom scales in subjects with inflammatory bowel diseases. Am J Gastroenterol 2018;113(1):72–9. [DOI] [PubMed] [Google Scholar]
- 34.Tisnado DM, Adams JL, Liu H, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care 2006;44(2):132–40. [DOI] [PubMed] [Google Scholar]
- 35.Ritter PL, Stewart AL, Kaymaz H, et al. Self-reports of health care utilization compared to provider records. J Clin Epidemiol 2001;54(2):136–41. [DOI] [PubMed] [Google Scholar]
- 36.Roberts RO, Bergstralh EJ, Schmidt L, et al. Comparison of self-reported and medical record health care utilization measures. J Clin Epidemiol 1996;49(9):989–95. [DOI] [PubMed] [Google Scholar]
- 37.Short ME, Goetzel RZ, Pei X, et al. How accurate are self-reports? An analysis of self-reported healthcare utilization and absence when compared to administrative data. J Occup Environ Med 2009;51(7):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pew Research Center. Internet/broadband fact sheet (http://www.pewinternet.org/fact-sheet/internet-broadband/) (2018). Accessed June 18, 2019.
- 39.Bonke J, Fallesen P. The impact of incentives and interview methods on response quantity and quality in diary- and booklet-based surveys. Surv Res Methods 2010;4(2):91–101. [Google Scholar]
- 40.Deutskens E, De Ruyter K, Wetzels M, et al. Response rate and response quality of internet-based surveys: An experimental study. Mark Lett 2004;15(1):21–36. [Google Scholar]
- 41.Medway R. Beyond response rates: The effect of prepaid incentives on measurement error. (https://drum.lib.umd.edu/handle/1903/13646) (2012). Accessed December 17, 2019.
- 42.Medway RL, Tourangeau R. Response quality in telephone surveys: Do prepaid cash incentives make a difference? Public Opin Q 2015;79(2):524–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.