Abstract
The prevalence of antimicrobial resistance among many common bacterial pathogens is increasing. The emergence and global dissemination of these antibiotic-resistant bacteria (ARB) is fuelled by antibiotic selection pressure, inter-organism transmission of resistance determinants, suboptimal infection prevention practices and increasing ease and frequency of international travel, among other factors. Patients with chronic kidney disease, particularly those with end-stage renal disease who require dialysis and/or kidney transplantation, have some of the highest rates of colonization and infection with ARB worldwide. These ARB include methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp. and several multidrug-resistant Gram-negative organisms. Antimicrobial resistance limits treatment options and increases the risk of infection-related morbidity and mortality. Several new antibiotic agents with activity against some of the most common ARB have been developed, but resistance to these agents is already emerging and highlights the dire need for new treatment options as well as consistent implementation and improvement of basic infection prevention practices. Clinicians involved in the care of patients with renal disease must be familiar with the local epidemiology of ARB, remain vigilant for the emergence of novel resistance patterns and adhere strictly to practices proven to prevent transmission of ARB and other pathogens.
Antibiotic resistance among common human bacterial pathogens is on the rise worldwide and is estimated to cause at least 700,000 deaths every year1. Compared with infections caused by antibiotic-susceptible bacteria of the same species, those caused by antibiotic-resistant bacteria (ARB) are associated with significantly higher mortality, prolonged hospitalization and greater health-care costs and economic burden. These poor outcomes are thought to be due to the severity of the underlying illness, delays in the initiation of effective antimicrobial therapy and/or toxic effects associated with some of the antibiotics used to treat infections caused by ARB. Crucially, optimal antimicrobial treatment regimens have not been defined for many ARB and treatment options are extremely limited for some. The emergence of clinical isolates of common pathogens (such as Klebsiella pneumoniae and Acinetobacter spp.) that are resistant to all currently available antimicrobial agents has caused some experts to predict a ‘post-antibiotic’ era2. In fact, some have predicted that infection due to ARB will be the most common cause of death worldwide by the year 2050, causing up to 10 million deaths annually1.
Currently, substantial variability exists in clinical practice and the scientific literature with regard to the use of terms such as ‘multidrug resistant’ (MDR) to describe pathogens that are resistant to multiple antimicrobial agents. This inconsistency hinders the direct comparison of the epidemiology and clinical outcomes associated with ARB that are reported for different populations, and makes it challenging to accurately describe the global burden of specific antimicrobial resistance profiles. To address this important issue, an international panel of experts proposed standardized definitions for several common ARB on the basis of the number of specific antimicrobial categories to which the pathogen demonstrates non-susceptibility3. The defined terms include MDR, extensively drug resistant and pandrug resistant. However, these terms and definitions have not yet been widely adopted in the literature.
Infection is second only to cardiovascular disease as the leading cause of death among patients with end-stage renal disease (ESRD) and those who have undergone renal transplantation4. The growing problem of antimicrobial resistance is particularly relevant to patients with chronic kidney disease (CKD), as they are disproportionally affected by antimicrobial resistance when compared with the general population. Chronic dialysis, the presence of indwelling vascular and urinary catheters, renal transplantation, treatment with antibiotics and other health-care exposures have been identified as factors associated with an increased risk of colonization and infection with ARB5. However, data are somewhat limited regarding the prevalence of ARB colonization and infection among patients with CKD. Of note, the prevalence of many ARB varies substantially, both globally and regionally. Therefore, clinicians should be familiar with the local epidemiology of antimicrobial resistance and remain vigilant for the emergence of new resistance mechanisms and phenotypes within their region of practice.
Antimicrobial agents exert their antibacterial effect through various modes of action (FIG. 1a), but some of these microorganisms have, in turn, developed a variety of resistance mechanisms that can counter the effects of those drugs (FIG. 1b). These mechanisms, which vary by drug and organism, include the enzymatic destruction of the antibiotic, alteration of the antibiotic’s target site, and seclusion or elimination of the antibiotic from the bacterial cell6. Acquired resistance refers to a resistance determinant that is not inherent to an organism but is attained, for example, through bacterial conjugation, mutation, transformation and transduction. Of note, bacteria often possess more than one mechanism of resistance. In this Review, we discuss the epidemiology, prevention strategies and treatment of infections caused by ARB, as well as future directions in the fight against antimicrobial resistance.
Fig. 1 |. Mechanisms of action of antibacterial agents and of antibacterial resistance.
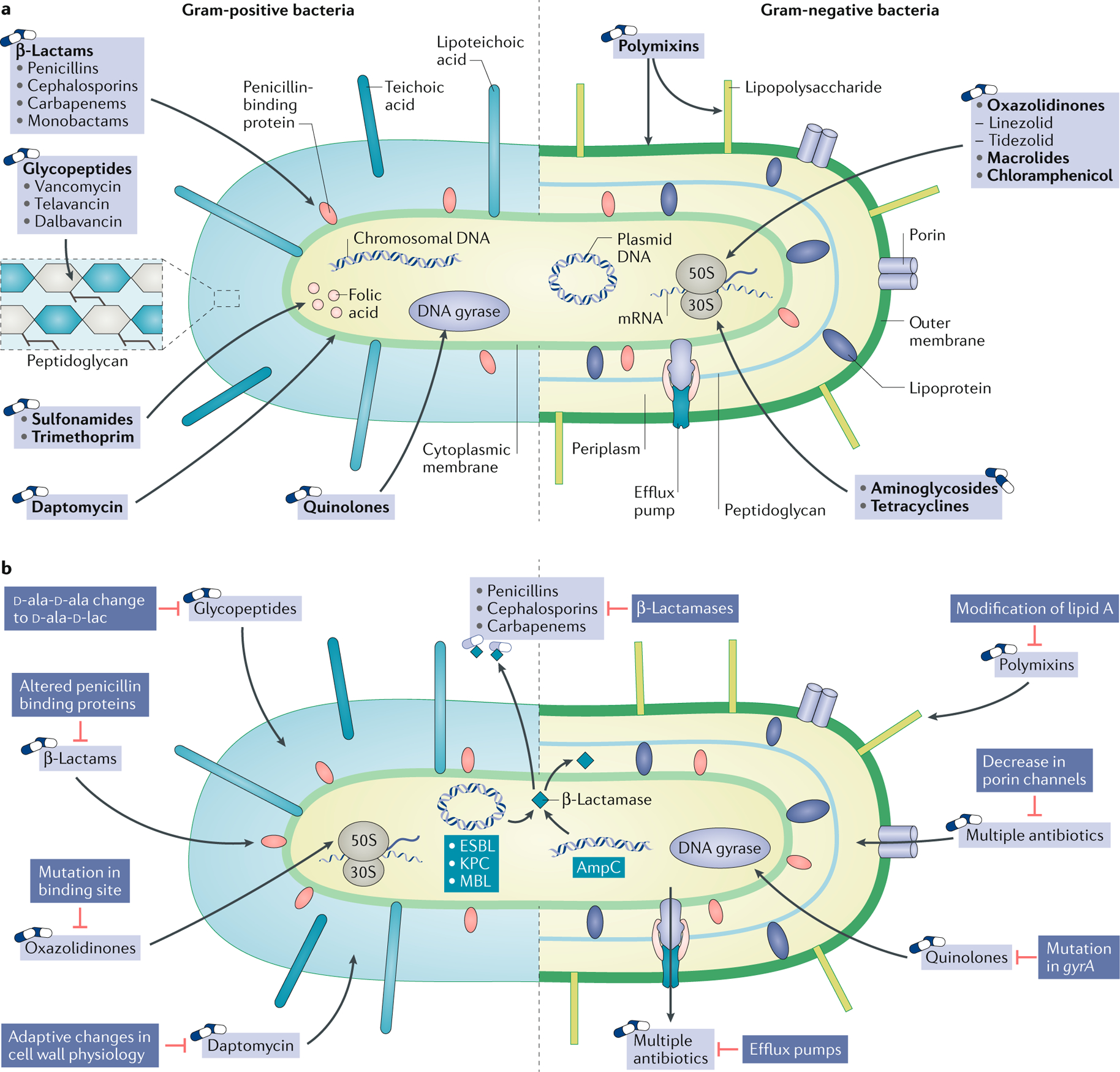
Structures of Gram-positive (left) and Gram-negative bacteria (right). a | Sites and mechanisms of actions of antibacterial drugs. Bacterial cell wall synthesis is inhibited by β-lactam antibiotics and glycopeptides. Daptomycin and polymyxin disrupt the bacterial cell membrane. Bacterial protein synthesis is inhibited by oxazolidinones, macrolides and chloramphenicol, which bind to the 50S ribosomal subunit, and by tetracyclines and aminoglycosides, which bind to the 30S ribosomal subunit. Bacterial DNA synthesis is inhibited by fluoroquinolones via inhibition of DNA gyrase and topoisomerase IV. Bacterial folic acid synthesis, which is required for nucleic acid synthesis, is inhibited by sulfonamides and trimethoprim. b | Mechanisms of resistance to antibacterial drugs. Common mechanisms of resistance include destruction of the antibiotic via enzymes encoded by chromosomal genes or by plasmids, modification of the target of the antimicrobial agent, decreased penetration of the antimicrobial agent into the bacterial cell through alterations in the structure or reductions in the number of functional porin channels, and enhanced elimination of the antimicrobial agent from the bacterial cell via drug efflux pumps.
ESBL, extended-spectrum β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-β-lactamase.
Gram-positive organisms
Staphylococcus aureus
β-Lactam antibiotics interrupt the formation of the bacterial cell wall by binding to penicillin-binding proteins (PBPs) involved in peptidoglycan synthesis, resulting in cell death. The development of resistance to β-lactam antibiotics by Staphylococcus aureus has been an ongoing clinical challenge since penicillin was first introduced into clinical use7,8. The resistance of S. aureus to penicillin was initially due to the production of penicillinases, which are β-lactamases that can degrade penicillin in the extracellular space by hydrolysing the β-lactam ring, a core structural component of β-lactam antibiotics (FIG. 1b). On the other hand, resistance to methicillin and other anti-staphylococcal penicillins, which were developed in response to the emergence and dissemination of penicillin-resistant strains, is due to the production of an altered PBP (PBP2a), encoded by mecA6 (FIG. 1b). The production of PBP2a not only results in resistance to methicillin but also renders bacteria resistant to all other β-lactam antibiotics, which bind to PBP2a, with the exception of the more recently developed cephalosporin, ceftaroline, which has a much higher binding affinity for PBP2a9. Methicillin-resistant S. aureus (MRSA) was initially regarded as a health-care-associated pathogen, but community-associated strains of MRSA have since emerged and spread in many parts of the world since the 1990s, complicating its epidemiology10. Although data from some national surveillance programmes, including those in the United States and Europe, suggest that the rates of MRSA infection are declining in some areas, the global burden of MRSA remains substantial, with rates of resistance above 80% in some countries2,11,12.
Compared with the general population, patients with renal disease are more likely to be affected by infection with both methicillin-susceptible and methicillin-resistant strains of S. aureus, which is associated with substantial morbidity and mortality. Reported rates of MRSA colonization in patients receiving haemodialysis range from 2.3% to 27.3%13, and up to 35% of colonized patients subsequently develop MRSA infections within 1 year14. Data from the US Centers for Disease Control and Prevention (CDC) showed that invasive MRSA infections affect more than 4 out of every 100 dialysis patients, which is more than 100 times the incidence observed in the general population15. Among kidney transplant recipients, MRSA colonization ranges from 1.2% to 12.5%16, and pre-transplant colonization with MRSA has been identified as an independent risk factor for graft failure17.
The glycopeptide vancomycin, which inhibits bacterial cell wall synthesis by preventing peptidoglycan elongation and crosslinking, has long served as a first-line option for the treatment of serious MRSA infections. Thus, the emergence of strains with reduced susceptibility and even high-level resistance to vancomycin is concerning. The first case of infection with vancomycin-intermediate S. aureus (VISA) was reported in 1997 (REF.18), and since then, the number of new cases reported in the United States and around the world has continued to increase19. Heteroresistant or heterogeneous ViSA (hVISA) has also been implicated in cases of vancomycin treatment failure and persistent infection20. The reduced susceptibility to vancomycin in both VISA and hVISA strains results from thickening of the cell wall and mutations in or downregulation of a number of genes, including the accessory gene regulator (agr) operon21; these changes are thought to be driven by prolonged exposure to vancomycin22,23. S. aureus with full resistance to vancomycin (VRSA) has also been described and was first reported in the United States in 2002, notably in a patient with multiple comorbidities that included haemodialysis-requiring ESRD24. Since 2002, 14 persons with VRSA colonization and/or infection have been identified in the United States; several of these patients were also affected by CKD25. Among the US isolates, the MRSA strain developed resistance to vancomycin by acquiring the vanA gene cluster from vancomycin-resistant Enterococcus (VRE) (see Enterococcus section below). Additional cases of VRSA have been sporadically reported around the world, including in Portugal, Iran, India and Pakistan26,27. In addition to dialysis, common risk factors for VRSA include prolonged exposure to health-care settings, previous treatment with vancomycin, chronic skin wounds and co-colonization with MRSA and VRE28. Thus far, VRSA is rare and the clinical impact of MRSA remains substantially higher.
Enterococcus species
Among the enterococcal species, Enterococcus faecalis and Enterococcus faecium are the most common human pathogens29. In the 1980s, acquired vancomycin resistance emerged in health-care-associated enterococcal strains, particularly E. faecium29. This resistance is due to the acquisition of gene clusters, which include genes such as vanA and vanB, that lead to the synthesis of an altered cell wall terminal peptide, d-alanyl-d-lactate, that replaces the normal d-alanyl-d-alanine peptide and prevents the binding of vancomycin30 (FIG. 1b). The global prevalence of VRE varies widely31 (FIG. 2). As with MRSA, VRE colonization and infection are relatively common among patients with CKD. Studies of patients on dialysis from around the world show that rates of VRE colonization range from 2.8% to 10.8%; previous antibiotic use and recent hospitalization are factors associated with colonization32. Among kidney transplant recipients, VRE colonization was detected in 13.6% of patients at a transplant centre in Brazil33. Enterococcus spp. were the second most common pathogen recovered from patients with bloodstream infections in US haemodialysis facilities in 2014 and 11.4% of those isolates were resistant to vancomycin34. In the early 2000s, the introduction of linezolid, an oxazolidinone antibiotic, and daptomycin, a lipopeptide antibiotic, offered greatly needed alternatives for the treatment of VRE and MRSA infections (FIG. 1a). However, reports of treatment failure with linezolid and daptomycin, as well as demonstration of in vitro resistance, are increasingly frequent, which is of particular concern for patients with CKD, in whom MRSA and VRE infections are common. VRE isolates resistant to both linezolid and daptomycin have also been described35. Linezolid inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit, which includes the 5S and 23S rRNA subunits. Resistance to linezolid is thought to result from the cumulative effect of multiple mutations in 23S rRNA genes and has been associated with prolonged use of this antibiotic36. Although resistance is well described, one study reported that the rates of resistance detected in a large surveillance programme that involved 33 countries, excluding the United States, remained less than 1%37. Daptomycin penetrates the bacterial cell wall and interacts with the cell membrane phospholipid phosphatidylglycerol, which allows it to disrupt cell division and cause membrane depolarization. The Daptomycin Surveillance Programme Worldwide reported that daptomycin resistance across all geographic regions remained extremely low in both staphylococci (0.05% for S. aureus) and enterococci (0.18% for E. faecium) clinical isolates collected between 2005 and 2012 (REF.38). However, daptomycin resistance in enterococci is increasing39 owing to genetic mutations that cause stepwise adaptive changes in the physiology of the bacterial cell wall and cytoplasmic membrane40. Although resistance can emerge after prolonged daptomycin exposure, it has also been reported in patients not previously treated with daptomycin41. In fact, resistance can also be associated with vancomycin exposure and is thought to be caused by an adaptive thickening of the cell wall, which leads to reduced susceptibility to both daptomycin and vancomycin40. This risk factor may be particularly relevant to patients with ESRD as they have fairly high rates of exposure to vancomycin.
Fig. 2 |. Global prevalence of resistance of Enterococcus faecium to vancomycin.
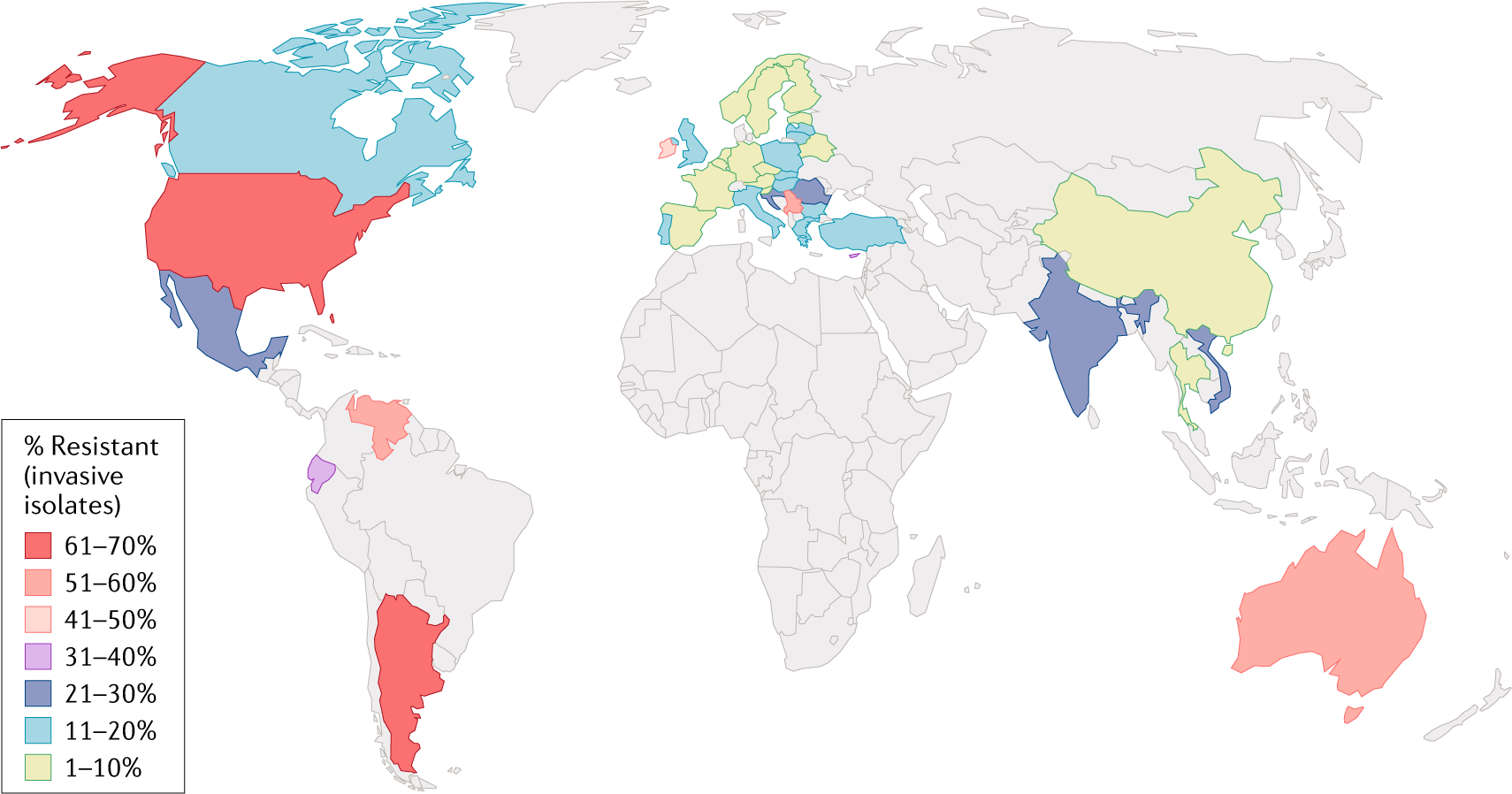
World map displaying the frequency of invasive (that is, isolated from blood and/or cerebrospinal fluid) E. faecium non-susceptible to vancomycin.
Non-susceptible isolates include those that are resistant or are of intermediate susceptibility. Data from REF.31.
Gram-negative organisms
Despite the lack of large epidemiological studies of colonization and infection with antimicrobial-resistant Gram-negative organisms in patients with CKD, the frequent exposure of these patients to health-care settings and antibiotics increases their risk of colonization with these ARB. In fact, available data suggest that these organisms are relatively common among dialysis patients. One prospective study conducted in an ambulatory haemodialysis facility in the United States showed that 28% of patients were colonized with a Gram-negative organism resistant to at least three of six antimicrobial agents tested. An additional 20% of patients acquired one of these MDR Gram-negative pathogens during a 6-month follow-up period42.
Enterobacteriaceae
The Enterobacteriaceae family comprises several bacterial genera, including Escherichia coli, Klebsiella spp. and Enterobacter spp., all of which have important roles in community-associated and health-care-associated infections. Antimicrobial resistance among the Enterobacteriaceae continues to evolve as new mechanisms of resistance emerge, which present substantial patient safety concerns and therapeutic challenges43,44. In addition to nosocomial infections and outbreaks caused by MDR Enterobacteriaceae, there is a growing number of reports of MDR Enterobacteriaceae in the community. Resistant organisms have been isolated from rivers, sewage, wild animals, food-producing animals and companion animals45–47. The following sections discuss some of the most common and concerning resistance determinants among the Enterobacteriaceae.
Extended-spectrum β-lactamases.
Extended-spectrum β-lactamases (ESBLs) are a heterogeneous family of primarily plasmid-mediated enzymes that inactivate β-lactam antibiotics by cleaving the β-lactam ring; common ESBLs include the TEM, SHV and CTX-M enzyme families. These enzymes confer resistance to penicillins and many cephalosporins including oxyimino-β-lactams (for example, cefotaxime, ceftazidime and ceftriaxone) but do not act against cephamycins or carbapenems, which include cefoxitin and meropenem, respectively. ESBLs are generally susceptible to β-lactamase inhibitors in vitro. However, a randomized trial reported in 2018 found that treatment with piperacillin–tazobactam, a β-lactam–β-lactamase inhibitor combination antibiotic, did not result in non-inferior 30-day mortality compared with the use of meropenem for the treatment of patients with ceftriaxone-resistant but piperacillin–tazobactam-susceptible E. coli or K. pneumoniae bloodstream infection48. Although approximately 16% of the study participants had moderate or severe renal dysfunction, the study did not specifically provide outcome data for patients with ESRD. Community and hospital-acquired ESBL-producing organisms are globally widespread and are a growing problem, particularly in developing countries49 (FIG. 3).
Fig. 3 |. Estimated global prevalence of eSBl-producing Escherichia coli.
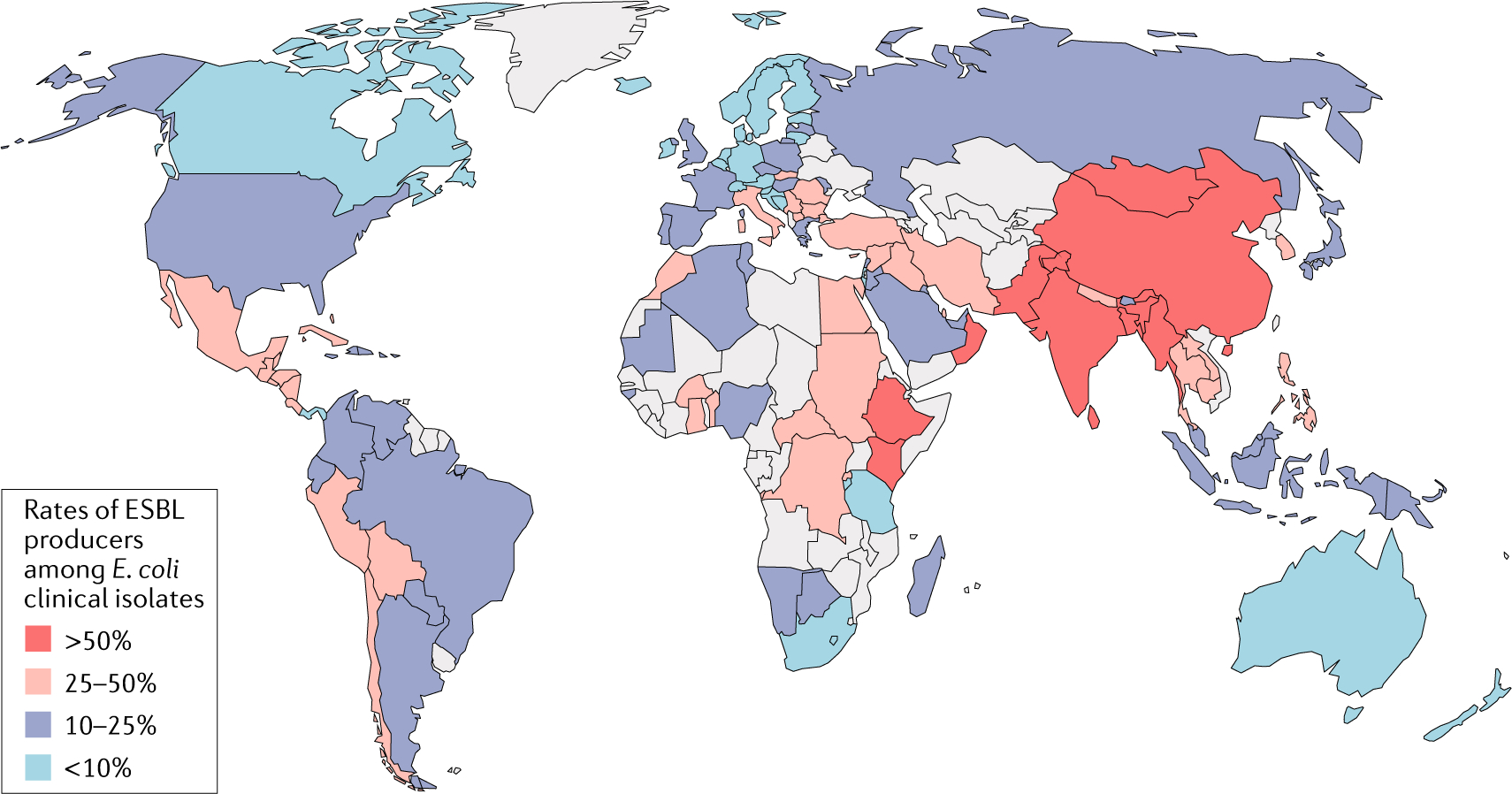
World map displaying the frequency of extended-spectrum β-lactamase (ESBL) production among clinical E. coli isolates. Grey shading indicates insufficient data. Data from REF.49.
AmpC β-lactamases.
Similar to ESBLs, AmpC β-lactamases are enzymes that lead to oxyimino-β-lactam resistance50. However, in contrast to ESBLs, AmpC enzymes hydrolyse cephamycins and are not inhibited by most β-lactamase inhibitors. ‘SPICE’ organisms (that is, Serratia, Providencia, indole-positive Proteus, Citrobacter and Enterobacter spp.) are the primary producers of AmpC enzymes, although they are also found in other Enterobacteriaceae and several other Gram-negative organisms50. In most Enterobacteriaceae, AmpC enzymes are not constitutively expressed but their expression is induced by exposure to β-lactam antibiotics, which can thus create resistance in an apparently susceptible isolate following exposure to β-lactams. This particular feature of AmpC enzyme expression raises many challenges in the laboratory detection of AmpC-producing organisms. For example, whereas an isolate that is shown to be resistant to cefoxitin and oxyimino-β-lactams in vitro is likely to be an AmpC-producing organism, strains in which the AmpC enzyme can be produced but have not yet been induced often test as susceptible to third-generation cephalosporins. Thus, an organism that appears to be susceptible to a third-generation cephalosporin might quickly become resistant to that drug during the course of therapy and lead to treatment failure.
AmpC genes are most commonly encoded in bacterial chromosomes but, since their discovery in 1989, the prevalence of plasmid-mediated AmpC genes has risen around the world, increasing the epidemiological risk of transmission of this resistance determinant among and between different bacterial species50. In addition to previous exposure to β-lactam antibiotics51, other potential risk factors for the acquisition of AmpC β-lactamase-producing organisms include the presence of external biliary or urinary drains, anatomic urinary abnormalities, urinary tract infection (UTI) in the past year and organ transplantation.
Carbapenemases.
In the past 30 years, several classes of carbapenem-hydrolysing β-lactamases, also known as carbapenemases, have emerged (FIG. 4). Most carbapenemases hydrolyse all β-lactam antibiotics and they are not generally inactivated by β-lactamase inhibitors. In addition, carbapenemase-producing organisms usually carry determinants of resistance to other antimicrobial drug classes, which renders these organisms resistant to most antibiotics. In the United States, K. pneumoniae carbapenemases (KPCs) are the most commonly encountered carbapenemases among Enterobacteriaceae isolates. Although first discovered in K. pneumoniae, the blaKPC gene, which encodes these Ambler class A serine carbapenemases, is found on plasmids that are transmissible from Klebsiella to other genera. Following the first reported case of KPC-producing Enterobacteriaceae in North Carolina in 1996 (REF.52), new cases have been reported from nearly every state in the United States and around the globe. Plasmid-mediated Ambler class B metallo-β-lactamases (MBLs), named for their dependence on zinc for the hydrolysis of β-lactams, were first reported in Japan in 1991 (REF.53) but have since become a worldwide concern. Of the MBLs, the New Delhi MBL (NDM) has gained attention owing to the mobility of its encoding gene and its complex epidemiology54 – whereas KPC remains largely associated with health-care exposure, NDM is also present in community-associated strains. Other MBLs identified among Enterobacteriaceae include active on imipenem MBL (IMP) and Verona integron-encoded MBL (VIM). The Ambler class D carbapenemases, such as oxacillinase-type carbapenem-hydrolysing β-lactamase 48 (OXA-48), are most commonly found in the Middle East and North Africa55 (FIG. 4). Although substantial geographic variability exists in the prevalence of the various carbapenemases55, frequent international travel, transfers between health-care facilities and ‘medical tourism’ have increased the rapidity by which these and other MDR organisms are globally disseminated56.
Fig. 4 |. Geographic distribution of carbapenemases in enterobacteriaceae.
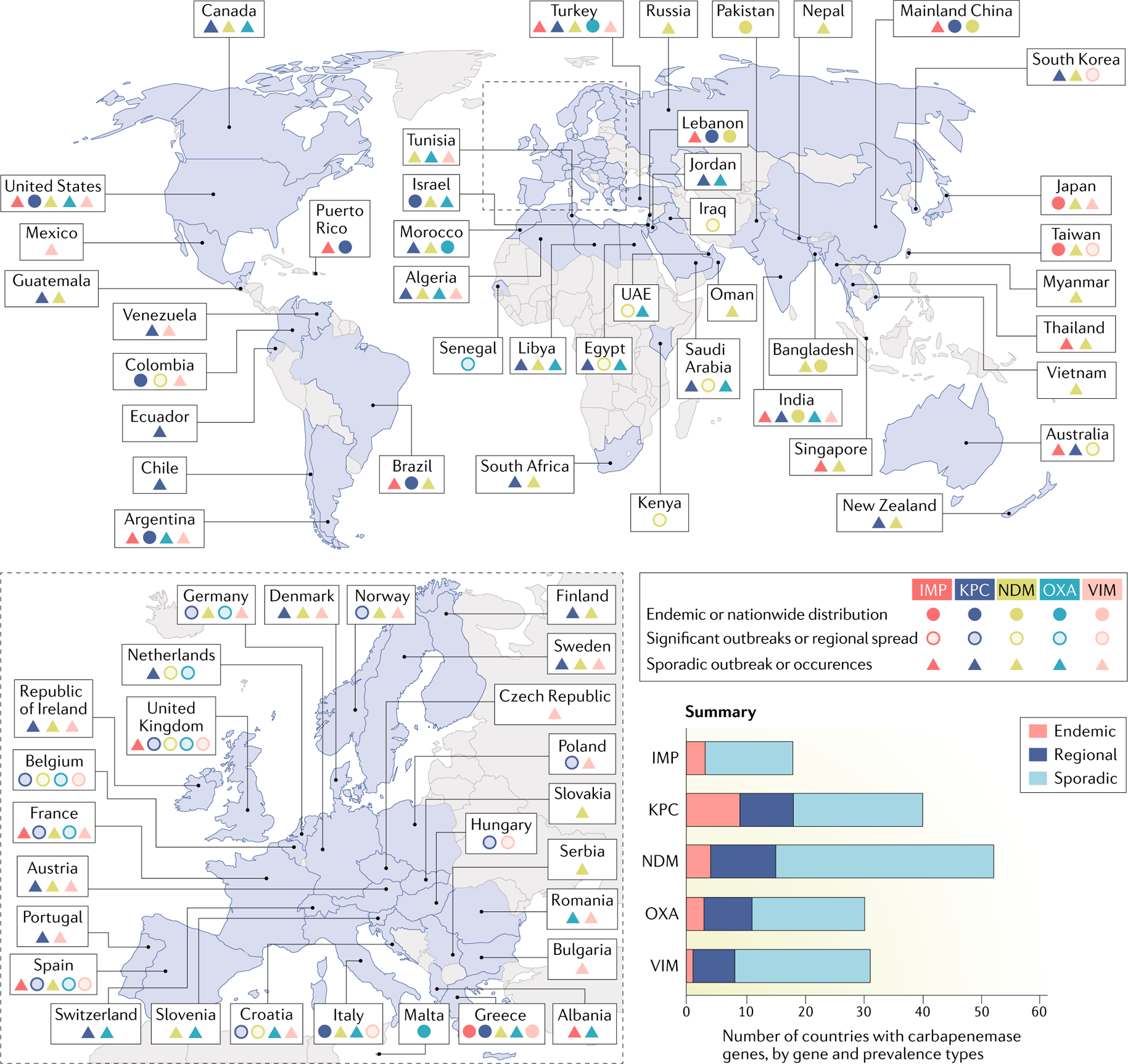
World map displaying the global distribution of carbapenemases among Enterobacteriaceae. The colour of the marker indicates a specific carbapenemase. The shape of the marker (solid circle, open circle or triangle) refers to the prevalence of the carbapenemases (endemic, significant outbreaks or regional spread and sporadic outbreak and occurrences, respectively). Owing to lack of available data, information cannot be provided for the countries shaded in grey. IMP, active on imipenem metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; OXA, oxacillinase-type carbapenem-hydrolysing β-lactamase (OXA largely refers to OXA-48, except in India, where it refers to OXA-181); VIM, Verona integron-encoded metallo-β-lactamase. Data from REF.55.
Although carbapenemase-producing Enterobacteriaceae (CPE) are less prevalent than ESBL and AmpC-producing Enterobacteriaceae in most geographic regions, they have become a substantial worldwide public health concern as treatment options for infections with CPE are severely limited and these infections are associated with substantial morbidity and mortality. On the basis of data from 2013, the CDC estimated that carbapenem-resistant Enterobacteriaceae (CRE) are responsible for 7,900 infections and 520 deaths in the United States each year57. Moreover, in 2014, 10.9% of central-line associated bloodstream infections caused by Klebsiella spp., 1.9% of those caused by E. coli and 3.4% of those caused by Enterobacter spp., were resistant to carbapenems58. The European Centre for Disease Prevention and Control (ECDC) reported that in 2017, 7.2% of invasive K. pneumoniae isolates identified in 30 European countries were carbapenem resistant11. On the other hand, data from 703 intensive care units in developing countries showed that between 2010 and 2015 carbapenem resistance was detected in 43% of K. pneumoniae bloodstream isolates; the report included data from countries in Latin America, Europe, the eastern Mediterranean, Southeast Asia and the western Pacific that participate in the International Nosocomial Infection Control Consortium (INICC)12. It should be noted that not all carbapenem resistance among Gram-negative organisms, including Enterobacteriaceae, is due to the production of carbapenemases. For example, a carbapenemase was detected in only 32% of more than 4,000 CRE isolates submitted to the CDC for testing in 2017 (REF.56). Other mechanisms of carbapenem resistance include AmpC hyperproduction in combination with porin mutations and drug efflux pumps (FIG. 1b). As with other resistance mechanisms, the use of broad-spectrum antibiotics and prolonged antibiotic exposure are risk factors for CPE and other CRE; however, they are not essential55. Other factors associated with CRE colonization and infection include prolonged hospitalization, organ transplantation, use of immunosuppressive therapies and indwelling urinary or vascular catheters.
Other mechanisms of resistance.
Quinolone antibiotics inhibit bacterial DNA synthesis by interacting with DNA gyrase and topoisomerase IV, which modulate the topology of DNA during replication59. However, quinolone resistance among clinical isolates of Enterobacteriaceae is increasingly common. Indeed, fluoroquinolone non-susceptibility was detected in 34% of the nearly 300,000 urinary tract E. coli isolates identified by the US Veterans Affairs Health Care System between 2009 and 2013 (REF.60). Resistance commonly occurs owing to chromosomal mutations in genes such as gyrA and parC, which encode the quinolone targets. In addition, downregulation of porin channels, chromosomal mutations in the genes that encode porin proteins and upregulation of chromosomally encoded drug efflux pumps can result in quinolone resistance59 (FIG. 1b). Plasmid-encoded genes that mediate resistance include qnr genes, which encode proteins that protect DNA gyrase and topoisomerase IV from the action of quinolones, as well as genes that encode drug efflux pumps.
Aminoglycoside resistance, which can be either intrinsic or acquired, is typically mediated by aminoglycoside-modifying enzymes61. Of note, cross-resistance between different aminoglycoside antibiotics is often incomplete and in vitro testing for each individual agent is typically recommended62. In the case of acquired aminoglycoside resistance, the modifying enzymes are encoded on plasmids that might also include genes for determinants of resistance to other antibiotics, such as KPCs or ESBLs63, and the risk factors that predispose to acquisition of resistance to other antibiotics apply similarly to aminoglycosides. However, unlike other antibiotic classes, development of resistance while on therapy is rare for aminoglycosides and overall rates of resistance have remained stable and relatively low61. Despite their known risk of nephrotoxicity, which may be particularly problematic for patients with underlying renal disease, the use of polymyxins such as polymyxin B and polymyxin E (also known as colistin) has increased in recent years owing to their activity against many Enterobacteriaceae isolates, including CRE that are resistant to multiple antibiotic classes64. Unfortunately, resistance to the polymyxins is also emerging and is an independent risk factor for mortality65. Resistance is thought to be secondary to post-translational modifications of the polymyxin binding target, the lipid A component of outer membrane lipopolysaccharides66. Several studies have identified polymyxin exposure as the only independent risk factor for both colonization and subsequent infection with a polymyxin-resistant organism67. Polymyxin resistance is typically mediated by genes encoded in bacterial chromosomes, but the recent discovery of one of these genes (mcr1) in bacterial plasmids raised concerns over the potential rapid dissemination of polymyxin resistance46. This gene was first encountered in E. coli isolated from food-producing animals in China between 2011 and 2014, later found in hospitalized patients with Enterobacteriaceae infections and subsequently seen in animal and human isolates from multiple countries around the world68.
Pseudomonas aeruginosa
Pseudomonas aeruginosa possesses intrinsic resistance to many antibiotics and can acquire resistance to additional antibiotic classes69. Mechanisms of resistance frequently found in P. aeruginosa isolates include AmpC β-lactamases, ESBLs, MBLs, downregulation of the porin protein outer membrane porin D (OprD) and multidrug efflux pumps (FIG. 1b). The emergence of resistance during therapy is well documented and is associated with higher morbidity, mortality and health-care costs70. A CDC report based on health-care-associated infection data collected in US hospitals in 2014 showed that P. aeruginosa was the sixth most commonly reported pathogen in cases of device-associated and surgery-associated infections. In these isolates, rates of resistance to fluoroquinolones, piperacillin–tazobactam and carbapenems were 11.5–32.6%, 7.4–18% and 7.7–25.8%, respectively58. ECDC data from 2017 showed that 30.8% of P. aeruginosa isolates were resistant to at least one anti-pseudomonal antibiotic11. The rate of multidrug resistance, defined as non-susceptibility to at least one drug in three of five drug classes, ranged from 4.3% to 17.9%. Antimicrobial-resistant P. aeruginosa is a globally relevant problem, and countries with limited resources are heavily affected12. In fact, 46.3% and 44.4% of Pseudomonas bloodstream isolates from intensive care units in 50 resource-limited countries were resistant to cefepime (a cephalosporin) and carbapenems, respectively12.
Acinetobacter species
Acinetobacter spp. are ubiquitous organisms found in water and soil that gained attention as an emerging nosocomial pathogen in the 1970s71. Since then, Acinetobacter spp., particularly Acinetobacter baumannii, have achieved global recognition for their ability to develop extensive drug resistance and to cause nosocomial outbreaks, particularly in intensive care units; AmpC β-lactamases are intrinsically present in all A. baumannii72,73. Acquired resistance mechanisms include MBLs, cell wall porin mutations and antibiotic efflux pumps (FIG. 1b); Ambler class D β-lactamases, also called OXA carbapenemases, have also been increasingly reported in Acinetobacter spp.71. Among Acinetobacter isolates from device-associated and surgery-associated infections reported to the US CDC in 2014, rates of carbapenem resistance ranged from 33% to 64% and rates of multidrug resistance ranged from 33% to 69%58. In Europe, the population-weighted mean percentage for combined resistance to fluoroquinolones, aminoglycosides and carbapenems was 28.4%, with substantial intercountry variation11. Among bloodstream isolates reported to INICC, 90.2% were resistant to carbapenems12.
ARB infections in patients with CKD
This section discusses the epidemiology of ARB among infections commonly encountered in patients with CKD. When available, incidence and/or prevalence data specific to these patients are provided, otherwise data from a broader health-care setting are provided.
UTIs and pyelonephritis
The prevalence of antimicrobial resistance among organisms that cause UTIs varies by country, region and patient-level risk factors. Risk factors associated with ARB UTIs include health-care facility exposure (including nursing homes and long-term care facilities), previous antibiotic use, indwelling urinary catheters, prior history of ARB UTI and travel to or residence in a country or region with a high prevalence of ARB. Polycystic kidney disease (PKD) is associated with abnormal kidney anatomy and patients with PKD have an increased risk of recurrent UTIs, particularly pyelonephritis. Multiple exposures of these patients to antibiotics are likely to increase their risk of colonization and infection with ARB, but data for this population are limited.
In the general population, an analysis of 1,438 urinary tract E. coli isolates collected in hospitals in Canada and the United States between 2013 and 2014 showed that 12.6% of the Canadian isolates and 16.8% of the US isolates were ESBL positive74. Among the US isolates, those from health-care-associated infections were significantly more likely to be ESBL positive than isolates from community-associated infections (21.8% versus 14.9%; P < 0.05); rates of fluoroquinolone resistance ranged from 28.6% to 35%. Antimicrobial resistance was even more prevalent among catheter-associated UTIs (CAUTIs) reported by US hospitals in 2014 (REF.58). For example, among E. coli isolates, the most commonly reported CAUTI pathogen, 16.1% were resistant to extended-spectrum cephalosporins and 34.8% were resistant to fluoroquinolones. Carbapenem resistance was reported in 4% of Enterobacteriaceae isolates and was even more common among P. aeruginosa (23.9%) and Acinetobacter spp. (64%) isolates. The percentage of MDR Gram-negative organisms was 8% for E. coli, 11.2% for Enterobacter spp., 14.6% for Klebsiella spp., 17.7% for P. aeruginosa and 69.1% for Acinetobacter spp. Among Gram-positive pathogens, 85% of E. faecium isolates were vancomycin resistant. In many developing countries, antimicrobial resistance among health-care-associated UTI pathogens is even greater. A 2016 report from the INICC described rates of resistance to third-generation cephalosporins of 64% and 77% for E. coli and K. pneumoniae, respectively, whereas resistance to carbapenems was detected in 6.6% of E. coli and 33.7% of K. pneumoniae isolates12.
Dialysis-associated infections
Bacterial infections, including bloodstream infections associated with haemodialysis and peritonitis associated with peritoneal dialysis, are a leading cause of morbidity and mortality in patients with ESRD75,76. Complications such as endocarditis, disseminated infection (for example, haematogenous osteomyelitis) and limitation or loss of vascular and peritoneal access options contribute to these adverse outcomes. Moreover, pathogens that cause dialysis-associated infections often carry determinants of antimicrobial resistance.
Haemodialysis.
In 2014, outpatient haemodialysis facilities in the United States reported 29,516 bloodstream infections in patients receiving chronic haemodialysis; 76% of these infections were related to vascular access34. S. aureus was the most commonly reported pathogen, accounting for 30.6% of episodes; 39.5% of isolates were methicillin resistant. Among Enterococcus spp., which accounted for 5.5% of the reported bloodstream infections, 11.5% were vancomycin resistant. Antimicrobial resistance was also relatively common among Gram-negative pathogens, such as E. coli, E. cloacae, K. pneumoniae and P. aeruginosa, which collectively accounted for approximately 15% of bloodstream infection isolates. Among E. coli isolates, 17.8% were resistant to third-generation cephalosporins (for example, ESBL-producing organisms), 14.6% of Klebsiella spp. isolates were resistant to cephalosporins and 4.8% of Enterobacter spp. isolates were resistant to carbapenems. It should be noted that these reported infections include only those diagnosed in outpatient dialysis facilities or within 1 calendar day after a hospital admission. Thus, bloodstream infections that occur or are diagnosed more than 1 day after hospital admission are not included in the reported data. In addition, one study found substantial underreporting of events, particularly for events that are diagnosed during the first day of hospitalization77. Consequently, these data likely underestimate the incidence of bloodstream infections, and therefore the incidence of bloodstream infections with ARB, among patients on haemodialysis in the United States.
The distribution and antibiotic resistance patterns of pathogens linked to haemodialysis-associated bloodstream infections vary substantially among geographic regions. Reported rates of methicillin resistance among S. aureus bloodstream infection isolates range from 0% in a study from Denmark to 100% in a single-centre study conducted in Algeria78–81. Rates of antimicrobial resistance among enterococci and Gram-negative pathogens also vary widely and are very high in some regions. The above-mentioned Algerian study reported that 100% of K. pneumoniae isolates produced ESBLs80, whereas a retrospective review of haemodialysis-associated bloodstream infections detected over 7 years at a single centre in Greece reported that 38% of Gram-negative organisms were resistant to fourth-generation cephalosporins and 24% were resistant to carbapenems79.
Peritoneal dialysis.
Peritoneal-dialysis-related infections include exit-site infections, tunnel infections and peritonitis; peritoneal-dialysis-associated peritonitis is most commonly caused by Gram-positive organisms, such as coagulase-negative staphylococci and S. aureus, but a substantial minority of cases are due to Gram-negative organisms and other pathogens82. The available data are limited but suggest that, as in haemodialysis-associated infections, antimicrobial resistance is a growing problem among peritoneal-dialysis-associated infections and that the prevalence of antimicrobial resistance among these pathogens varies geographically. For instance, a review of cases of dialysis-associated peritonitis diagnosed between 2002 and 2011 at a single centre in northern India showed high rates of resistance among multiple pathogens83. In that study, 28.6% of S. aureus isolates were methicillin resistant; 15.4% of Enterococcus isolates were vancomycin resistant; 54% and 76% of Enterobacteriaceae isolates were resistant to third-generation cephalosporins and fluoroquinolones, respectively; and 11.5% and 23.5% of P. aeruginosa and Acinetobacter spp., respectively, were resistant to carbapenems owing to MBL production. Mortality was significantly higher among patients with peritonitis caused by VRE and by Gram-negative organisms that produce ESBL and MBL. However, other regions report lower levels of resistance. In Western Australia, resistance to fluoroquinolones, later-generation cephalosporins and carbapenems was detected in 6%, 18% and 0%, respectively, of Gram-negative pathogens that caused peritonitis between 2008 and 2013 (REF.84). In a single-centre study in Germany, rates of MRSA, VRE and third-generation cephalosporin-resistant Gram-negative pathogens were fairly low but increased over a 32-year study period (1974–2014)85.
Infections after renal transplantation
Infection is an important cause of morbidity and mortality after renal transplantation. In the first month after transplantation, health-care-associated infections, including surgery-related infections and donor-derived infections, are the most common.86 Two to six months post-transplantation, immunosuppression-related infections are most frequent and include both opportunistic infections and infections due to viral reactivation. After 6 months, immunosuppression is typically reduced and infections are primarily due to environmental or community exposures but patients remain at risk of opportunistic infections such as Cryptococcus and Pneumocystis jirovecii86. UTIs are the most common bacterial infections in kidney transplant recipients, and various single centres have reported an incidence of 20–50%87–90. Most patients present with uncomplicated cystitis, but serious infections such as transplant pyelonephritis also occur91. Recurrent UTI, defined as more than three episodes of infection in 1 year, is also common in renal transplant recipients, as are other health-care-associated infections, including surgical-site infections, bloodstream infections and pneumonia.
Infections with ARB such as VRE, ESBL-producing Enterobacteriaceae and CRE and other Gram-negative pathogens are becoming increasingly common after renal transplantation. Patients acquire these ARB through a variety of mechanisms, including health-care-associated transmission via contaminated medical equipment, environmental surfaces and health-care workers’ hands as well as direct transmission via the transplanted organ or within the community (for example, in the case of community-associated MRSA). Post-surgical anatomic abnormalities, including vesicoureteral reflux, ureterovesical junction stenosis or neurogenic bladder, might predispose transplant recipients to recurrent UTIs and increase the risk of acquiring or developing ARB due to repeated exposure to antibiotics and health-care settings88. Other risk factors include advanced age, surgical re-intervention, kidney–pancreas transplant, post-transplant requirement of renal replacement therapy, post-transplant nephrostomy, previous antibiotic exposure, urinary tract obstruction or instrumentation and the presence of a central venous catheter89,92–94.
The prevalence of multidrug resistance among bacteria isolated from renal transplant recipients varies greatly and has been reported to range from 8% to 46%, depending on the type of infection, organism and region87,92,95. In one prospective study performed at a Spanish centre between 2003 and 2006, 58 (14%) of 416 renal transplant recipients developed a post-transplantation ARB infection94. The most commonly identified ARB were E. coli, K. pneumoniae and P. aeruginosa. In a retrospective study of renal transplant recipients in Brazil, the incidence of ESBL-producing Enterobacteriaceae among UTI isolates increased from 23.8% to 37.5% between 2003–2005 and 2006–2008 (REF.96). The reported incidence of CRE infection after renal transplantation ranged between 1.1% and 26%97. In another study of 280 kidney transplant recipients followed at a Brazilian centre between 2001 and 2003, the prevalence of VRE faecal colonization was 13.6%33.
Treatment of infections caused by ARB
Empirical treatment of a suspected bacterial infection should be based on the anticipated pathogens, the suspected infection site, local antimicrobial resistance patterns and the individual patient’s medical history, including prior colonization or infection with ARB (TABLE 1). In patients with renal dysfunction, issues such as vascular access requirements, the potential for nephrotoxicity and the need for dose adjustment of antibiotics that undergo renal clearance are important factors that need to be taken into account when selecting antimicrobial agents. In renal transplant recipients, knowledge of the donor’s prior colonization and infection status should also be considered. Antimicrobial therapy should be adjusted on the basis of the results of antimicrobial susceptibility tests performed on the pathogen or pathogens isolated from the site of infection (TABLE 1). In addition to antimicrobial therapy, other basic principles of treatment, such as source control, which includes drainage of abscesses and removal of infected foreign bodies, have a critical role in optimizing the outcome of ARB infections98. Early consultation with an infectious diseases physician should also be considered99. Antimicrobial treatment of ARB infections is a complex and rapidly evolving topic that is beyond the scope of this article. However, antibiotics that have recently been approved for use and that have activity against one or more ARB are reviewed below.
Table 1 |.
Antimicrobial agents with potential in vitro activity against common and emerging mechanisms of resistance
| Organism | Common and emerging mechanism(s) of resistance | Resistance phenotype | Antimicrobial agents with potential in vitro activity against resistant organismsa,b |
|---|---|---|---|
| Staphylococcus aureus | PBP2a (mecA) | Methicillin resistance | |
| Alterations in cell wall peptidoglycan (vanA) | Reduced susceptibility or full resistance to vancomycin | ||
| Enterococcus species | Alterations in cell wall peptidoglycan (vanA and van B) | Vancomycin resistance | |
| Enterobacteriaceae (for example, Escherichia coli, Klebsiella pneumoniae and Enterobacter spp.) | ESBL | Resistance to penicillins, oxyiminocephalosporins and monobactams with retained susceptibility to cephamycins, β- lactam–β-lactamase inhibitor combinations and carbapenems | |
| Gram- negative ‘SPICE’ organisms (for example, Serratia, Pseudomonas, Providencia, indole-positive Proteus, Citrobacter and Enterobacter spp.) and Acinetobacter | AmpC β-lactamases | Resistance to oxyiminocephalosporins (for example, cefotaxime, ceftazidime and ceftriaxone), cephamycins (for example, cefoxitin) and many β-lactam-β-lactamase inhibitor combinations | |
| Enterobacteriaceae (for example, Proteus, E. coli, and Klebsiella spp.) | Carbapenemasesf
|
Carbapenem resistance and resistance to other β-lactam antibiotics | |
| AmpC β-lactamases plus cell wall porin mutations | Resistance to most β-lactam antibiotics, often including carbapenems | ||
|
Fluoroquinolone resistance | Variable | |
| Drug efflux pumps | Resistance to various antibiotics | Variable | |
|
Polymyxin resistance | Variable | |
| Pseudomonas aeruginosa | ESBL | Resistance to penicillins, oxyiminocephalosporins and monobactams, with retained susceptibility to cephamycins,β-lactam and/or (β-lactamase inhibitor combinations and carbapenems | |
| AmpC β-lactamases | Resistance to oxyiminocephalosporins (for example, cefotaxime, ceftazidime and ceftriaxone), cephamycins (for example, cefoxitin) and many β-lactam and/or β-lactamase inhibitor combinations | ||
| Carbapenemases (for example, OXA-48)f | Carbapenem resistance and resistance to other β-lactam antibiotics | ||
| Gene mutations affecting outer membrane protein (porin) mutations (for example, OprD) | OprD confers only carbapenem resistance; other porin mutations may affect the other β-lactams | ||
| Gene mutations affecting DNA gyrase and topoisomerase (gyrA and parC) | Quinolone resistance | Variable | |
| Drug efflux pumps | Variable: can actively expel β-lactams, quinolonesand aminoglycosides | Variable | |
| Acinetobacter spp. | ESBL | Resistance to penicillins, oxyiminocephalosporins and monobactams, with retained susceptibility to cephamycins, β-lactam and/or (β-lactamase inhibitor combinations and carbapenems | |
| AmpC β-lactamases | Resistance to oxyiminocephalosporins (for example, cefotaxime, ceftazidime and ceftriaxone), cephamycins (for example, cefoxitin) and many β-lactam and/or β-lactamase inhibitor combinations | ||
|
Carbapenem resistance and resistance to some β-lactam antibiotics | ||
| Outer membrane protein (porin) mutations | Carbapenem resistance and resistance to some β-lactam antibiotics | Variable | |
| Aminoglycoside-modifying enzymes (acetylating, adenylating and phosphorylating) | Aminoglycosides | Variable | |
| gyrA and parC | Quinolone resistance | Variable | |
| Drug efflux pumps | Varies: can actively expel β-lactams, quinolonesand aminoglycosides | Variable |
ESBL, extended-spectrum β-lactamase; IMP, active on imipenem metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; OprD, outer membrane porin D; OXA, oxacillinase-type carbapenem-hydrolysing β-lactamase (OXA largely refers to OXA-48, except in India, where it refers to OXA-181); PBP2a, penicillin-binding protein 2a; VIM, Verona integron-encoded metallo-β-lactamase.
Selection of empirical therapy for an individual patient should be based on the patient’s history, epidemiological risk factors and local susceptibility patterns. Definitive antimicrobial therapy should be guided by the results of antimicrobial susceptibility testing.
These antibiotics frequently demonstrate in vitro activity against the specific organism and resistance mechanism. However, susceptibility should be confirmed as bacteria may harbour multiple mechanisms of resistance that can render them resistant to the listed antibiotic.
May require dose adjustment in patients with reduced creatinine clearance and/or dialysis dependence.
Potential for nephrotoxicity.
Contraindicated for patients with renal impairment.
Emerging mechanism of resistance.
Ertapenem does not possess activity against P. aeruginosa.
Available in the United States only as ampicillin–sulbactam.
New antibiotics for ARB infections
β-Lactams.
Ceftaroline, often referred to as a fifth-generation cephalosporin, has a greater affinity for PBP2a than other β-lactam antibiotics and is effective against organisms such as MRSA, which produce this altered PBP. In vitro, ceftaroline has bactericidal activity against MRSA, VISA and VRSA100, including some strains with reduced susceptibility to linezolid, daptomycin and/or vancomycin101. Ceftaroline has been used alone and in combination with other antibiotics to treat a variety of infections caused by susceptible organisms but is currently approved by the US Food and Drug Administration (FDA) only for the treatment of skin and soft tissue infections, including those caused by MRSA, and community-acquired pneumonia, excluding cases caused by MRSA.
Ceftolozane–tazobactam combines the novel cephalosporin, ceftolozane, with the β-lactamase inhibitor tazobactam to produce an antibiotic with activity against many ESBL-producing Enterobacteriaceae isolates and MDR P. aeruginosa isolates with chromosomal AmpC production, loss of outer membrane porins and/or upregulation of drug efflux pumps; however, this antibiotic is not active against serine carbapenemases or MBLs. International multicentre studies found that ceftolozane–tazobactam is active in vitro against more than 90% of MDR Pseudomonas and non-CRE ESBL-producing Enterobacteriaceae clinical isolates102,103. A randomized clinical trial demonstrated that ceftolozane–tazobactam plus metronidazole is non-inferior to meropenem in the treatment of intra-abdominal infections caused by ESBL-producing organisms104. Moreover, several retrospective clinical studies suggest a role for ceftolozane–tazobactam in the treatment of infections caused by susceptible strains of MDR P. aeruginosa, although the emergence of resistance during therapy has also been described105,106.
Ceftazidime with avibactam is another β-lactam–β-lactamase inhibitor combination107. Avibactam has activity against Ambler class A and C β-lactamases, including ESBLs, AmpC and KPC, which means that it is effective against many resistant Gram-negative bacteria. Of note, ceftazidime–avibactam is not active against Ambler class B MBL-producing organisms or Acinetobacter spp. In a randomized clinical trial, treatment with ceftazidime–avibactam provided outcomes similar to the best-available therapy when used for the treatment of complicated UTIs and intra-abdominal infections caused by ceftazidime-resistant Enterobacteriaceae and P. aeruginosa108. Another trial found that ceftazidime–avibactam used in combination with metronidazole had a similar efficacy to meropenem for the treatment of complicated intra-abdominal infections caused by both ceftazidime-susceptible and ceftazidime-resistant pathogens109. Thus far, no randomized clinical trials have assessed the use of ceftazidime–avibactam for the treatment of CRE infections. However, a small prospective multicentre observational study reported better clinical outcomes for patients with infections caused by KPC-producing CRE who were treated with ceftazidime–avibactam than for those treated with colistin110. Although these results are encouraging, further evaluation of this antibiotic for the treatment of KPC-producing CRE is needed to validate these findings.
Meropenem–vaborbactam combines meropenem with a non-β-lactam, boronic-acid-based β-lactamase inhibitor. In vitro and in vivo studies showed that this antibiotic combination is effective against Enterobacteriaceae that produce AmpC, ESBL and KPC111. Meropenem–vaborbactam has limited activity against other classes of carbapenemases, including MBLs such as NDM-1 and VIM, and OXA-type carbapenemases. Clinical studies that evaluate the clinical efficacy of meropenem–vaborbactam are limited. In one study, this combination antibiotic was found to be non-inferior to piperacillin–tazobactam for the treatment of complicated UTIs112. More relevant to this discussion of ARB, a small randomized, controlled and non-blinded clinical trial found that monotherapy with meropenem–vaborbactam was associated with increased clinical cure rates, decreased mortality and reduced nephrotoxicity when compared with best-available therapy for treatment of infections caused by CRE113. Meropenem–vaborbactam is currently approved by the FDA for the treatment of complicated UTIs caused by susceptible strains of E. coli, K. pneumoniae and Enterobacter cloacae.
Lipoglycopeptides.
The lipoglycopeptides dalbavancin, oritavancin and telavancin have in vitro activity against many staphylococci, including MRSA, as well as streptococci and enterococci, including many VRE isolates114,115. Unlike vancomycin, therapeutic drug monitoring is not required for these agents. Dalbavancin and oritavancin have long half-lives that allow for prolonged interval dosing or single-dose therapy, respectively. Whereas dalbavancin can be used in patients with renal failure and those being treated with intermittent haemodialysis116, the need for dose adjustments of oritavancin in the setting of renal impairment has not been studied. In contrast to dalbavancin, telavancin has been associated with worsening renal function, which may limit its use in patients with renal disease117. These three lipoglycopeptide agents are currently approved by the FDA for the treatment of acute bacterial skin and soft tissue infections as data on their efficacy in the treatment of more serious infections are lacking.
Other novel antibiotics.
Plazomicin is a new aminoglycoside that remains active in the presence of many aminoglycoside-modifying enzymes, which gives it greater in vitro activity than other aminoglycosides against many MDR Enterobacteriaceae, including isolates that produce ESBL and carbapenemase, and other MDR Gram-negative organisms118. Plazomicin might also be associated with lower toxicity rates, including reduced nephrotoxicity, ototoxicity and vestibular toxicity, than other aminoglycosides119. A phase II study provided evidence for the microbiological and clinical efficacy of plazomicin in the treatment of complicated UTIs120, for which the FDA approved the use of plazomicin in June 2018.
Eravacycline is a synthetic fluorocycline that is broadly active against many Gram-positive and Gram-negative bacteria, including several ARB121. Eravacycline is active against MRSA, VRE and many Gram-negative organisms that produce ESBLs and several carbapenemases, including KPC, NDM and OXA121,122. In a randomized clinical trial, eravacycline was found to be non-inferior to ertapenem for the treatment of complicated intra-abdominal infections123; the FDA approved its use for the treatment of these types of infection.
Delafloxacin is a new fluoroquinolone with activity against MRSA; its Gram-negative spectrum of activity is similar to other fluoroquinolones. A randomized clinical study found that delafloxacin was non-inferior to vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections124; delafloxacin is approved by the FDA for the treatment of acute skin and subcutaneous tissue infections. Of note, delafloxacin is not recommended for use in patients with severe renal dysfunction and ESRD owing to insufficient data to inform appropriate dosing.
Prevention and control of ARB infection
Given the high rates of infection-related morbidity and mortality observed in patients with CKD, preventing pathogen transmission and the development of clinical infection is of critical importance.
Basic infection control practices
Consistent implementation of basic infection prevention strategies is a crucial element in the effort to prevent transmission of and infection by ARB. These basic practices, which include hand hygiene, standard precautions and cleaning and disinfection of the health-care environment and medical equipment, are often referred to as ‘horizontal’ strategies because they reduce the risk of pathogen transmission and infection, including ARB. Public health agencies and professional societies have developed evidence-based guidelines that summarize best practices related to the insertion, use and maintenance of invasive devices (such as urinary catheters, central venous catheters and other forms of haemodialysis vascular access); the prevention of surgical-site infections and of infection among patients receiving chronic haemodialysis125; and other aspects of care for patients with renal disease. However, despite the availability of guidelines and the evidence showing that consistent implementation of the recommended practices is associated with reduced rates of infection, substantial evidence indicates that these practices are not consistently implemented.
Infection prevention in haemodialysis facilities.
Although relatively infrequent, outbreaks of infection, including infections caused by ARB, continue to occur among patients receiving care in haemodialysis facilities. Sources of such outbreaks have included contaminated tap water, reverse osmosis water storage tanks, antiseptic solutions and environmental surfaces, or have involved inadequate reprocessing and disinfection of dialysis equipment, and patient-to-patient transmission through health-care personnel126–128. Most of these outbreaks occur owing to lapses in the implementation of recommended infection prevention practices, and considerable evidence substantiates the need for improvement of basic policies and practices for the prevention of infection in haemodialysis facilities. One survey carried out in 37 hospital-based haemodialysis units in Quebec, Canada between December 2011 and March 2012 identified several opportunities to improve local protocols related to the insertion, use and maintenance of catheters by adding recommended evidence-based infection control practices129. For example, only 79% of protocols from the surveyed facilities called for the use of a full-body drape during catheter insertion, only 44% called for application of an antimicrobial ointment at the catheter insertion site and only 67% required the use of a recommended chlorhexidine-alcohol antiseptic solution for skin preparation. In addition, most facilities (69%) did not conduct audits to assess compliance with written protocols for catheter insertion or maintenance.
An observational study in 34 US haemodialysis facilities conducted between 2011 and 2012 by independent evaluators also found multiple areas for improvement in infection prevention practices130. These included procedures for environment and equipment disinfection, hand hygiene, dialysis catheter maintenance, dialysis initiation and termination, and medication preparation and administration. For example, overall compliance with recommended hand hygiene practices was only 72%, appropriate disinfection of surfaces and non-disposable items occurred in only 18–41% of observed opportunities and disinfecting of the external and internal hubs of central venous catheters was observed in only 45% and 34% of indicated situations, respectively. The findings of these two studies are important and actionable, as every lapse in infection prevention practice increases the risk of pathogen transmission and clinical infection. Conversely, improved adherence to evidence-based prevention strategies has been associated with reductions in infections such as bloodstream infections associated with haemodialysis access131,132. In addition to assessing compliance, identifying the reasons for non-compliance, which might include factors related to individuals, organizations and the external environment, as well as addressing any facility-specific reasons for non-compliance might help to optimize adherence to evidence-based guidelines and minimize the risk of infection133.
Another strategy to prevent bloodstream infections among haemodialysis patients is to select the vascular access option with the lowest associated risk of infection. The use of intravascular catheters, when compared with arteriovenous fistulas (AVFs), is associated with a higher risk of infection34 and a higher rate of antibiotic exposures, including non-indicated antibiotic exposures134. Early AVF creation is thus recommended for patients who require chronic haemodialysis, but challenges to this strategy include patient preference and lack of access to a vascular surgeon or surgical facility129. Identifying and addressing patient-specific and facility-specific barriers might be helpful in reducing the prevalence of catheter-based haemodialysis135.
The previously described ‘horizontal’ interventions prevent the transmission of and infection with a wide variety of potential pathogens, whereas ‘vertical’ strategies target one or more specific pathogens. Vertical strategies include the use of contact precautions for patients colonized or infected with ARB, the use of active surveillance testing to detect patients who are asymptomatically colonized with one or more ARB (for example, MRSA) and decolonization of MRSA carriage with the use of topical antiseptics with or without antibiotics136. The routine use of vertical strategies outside of outbreak situations remains controversial in many regions.
Antimicrobial stewardship
One of the major risk factors for propagation of antimicrobial resistance and the development of ARB infection is exposure to antibiotics, mainly due to antibiotic selection pressure. Although antibiotic therapy can be life-saving and the benefits of antibiotics often outweigh the potential risk of development of antimicrobial resistance, inappropriate and unnecessary use of antimicrobial agents increases this risk without any benefit to the patient. Of note, antimicrobial use and, more importantly, misuse are common among patients with CKD. According to data submitted to the CDC’s National Healthcare Safety Network (NHSN) in 2014, 149,722 patients receiving treatment in 6,005 outpatient haemodialysis facilities were started on antibiotics34. This equates to 3.27 antibiotic starts per 100 patient-months, ranging from 2.07 for patients with AVFs to 7.91 for patients with catheters. Studies conducted in haemodialysis facilities in the United States and Australia reported that 32–55% of patients received one or more doses of antibiotics in a time period that ranged from 6 to 12 months and that 22–35% of antimicrobial doses were inappropriate134,137,138. In these studies, commonly identified examples of inappropriate prescribing included administration of antibiotics in the absence of evidence of infection, use of antimicrobials with an inappropriate spectrum of activity, incorrect dosing and dosing frequency, and incorrect duration of therapy.
These data demonstrate that substantial opportunities exist to improve antimicrobial prescribing among patients with renal disease in order to optimize treatment outcomes and minimize adverse consequences, including propagation of antimicrobial resistance. Implementation of antimicrobial stewardship programmes in hospitals and ambulatory care settings is associated with improvements in the appropriateness of antimicrobial use, patient outcomes and antimicrobial susceptibility among targeted pathogens139. Thus, antimicrobial stewardship programmes (ASPs) are considered an important part of efforts to combat antimicrobial resistance. A 2016 model predicted that implementation of ASPs in outpatient dialysis facilities in the United States would result in 2,182 fewer ARB and Clostridioides difficile (formerly known as Clostridium difficile) infections, 629 fewer deaths and cost savings of $106 million per year140. Guidelines for the implementation of antimicrobial stewardship programmes in health-care facilities have been published141 and specific strategies for developing and implementing such programmes in outpatient dialysis facilities have been reviewed142.
Decolonization therapy
In many cases, ARB infection is preceded by acquisition of the organism followed by asymptomatic colonization; therefore, eliminating established colonization could reduce the risk of subsequent infection. Decolonization therapy refers to the application of antibiotic or antiseptic agents to suppress or eliminate carriage of an organism; this approach is most often used to eliminate S. aureus, including MRSA. Typical decolonization regimens include intranasal mupirocin with or without topical antiseptics such as chlorhexidine, but other agents have also been used. Several studies demonstrated that decolonization therapy reduced S. aureus infections among colonized patients on peritoneal dialysis143 or haemodialysis144. It should be noted, however, that the optimal regimen and duration of treatment are not defined, and that current strategies for decolonization rely on antiseptics and/or antimicrobial agents, with a potential risk of resistance and collateral damage due to alterations of the patient’s microbiome. In fact, some centres have reported the emergence of mupirocin resistance in association with the widespread use of mupirocin-based decolonization therapy145. Elimination or suppression of antibiotic-resistant Gram-negative bacteria carried in the gastrointestinal tract has proven to be more challenging146–148.
Novel strategies and future directions
Although the importance of adherence to basic infection prevention practices to reduce the risk of person-to-person transmission and decrease the risk of infection among carriers of an organism cannot be overstated, even high levels of compliance with these strategies are unlikely to be sufficient to address the growing problem of antimicrobial resistance. Similarly, although the development of several antibiotics with activity against some ARB, such as MRSA and CRE, is a much welcomed advance, the pipeline of new traditional antibiotics is unlikely to keep pace with the continued evolution of antimicrobial resistance. In addition, the use of traditional antibiotics will continue to exert collateral damage on the healthy human microbiota, further contributing to antibiotic selection pressure and the risk of emergence and propagation of antimicrobial resistance. Thus, novel approaches to prevent and treat bacterial infections that are more effective, that are less prone to the development of resistance and/or that avoid or reduce collateral damage are needed. Fortunately, several such strategies are under active investigation. A few examples are provided here (and reviewed elsewhere149).
Phage lysins are enzymes produced by bacteriophages that hydrolyse the bacterial cell wall peptidoglycan, resulting in a bactericidal effect with a high barrier to resistance150. The activity of each lysin is specific to certain bacterial species, thereby reducing the risk of significant microbiome disruption. Lysins are under active investigation for use in the eradication of important bacterial pathogens in both asymptomatic carriers and patients with infection. For example, animal models have demonstrated that lysins can reduce the burden of pathogens such as Streptococcus pneumoniae, Streptococcus pyogenes and S. aureus, including MRSA, and even eliminate bacterial colonization when administered topically151–153. Systemic administration of lysins contributed to the successful treatment of invasive infections caused by pathogens such as S. aureus and A. baumannii154,155. The use of lysins for the treatment of bacterial infections in humans is currently under investigation.
Other natural products are similarly being investigated for their potential role in the treatment and/or prevention of human infection. For example, the common human commensal organism Staphylococcus lugdunensis produces lugdunin, a thiazolidine-containing cyclic peptide antibiotic that has activity against S. aureus and other Gram-positive bacteria156. In vitro and in vivo experiments suggested that, compared with current decolonization agents, this compound might prevent S. aureus colonization and subsequent infection with less risk of bacterial resistance. Additional novel sources of natural anti-infective products, such as deep sea actinobacteria157, are also being actively sought and studied.
Although the development of more effective treatment options for individuals infected with ARB is critical, better strategies for prevention of pathogen acquisition and infection could reduce the need for new treatments and abate the morbidity and mortality associated with infection. Such prevention strategies could include vaccination and interventions that enhance resistance to colonization or infection with pathogenic bacteria. Vaccination has long been used for the prevention of communicable diseases. The pneumococcal vaccine exemplifies the ability of vaccines to reduce antimicrobial resistance among clinical isolates of common bacterial pathogens by providing protection against antimicrobial-resistant strains158,159. Compared with classic communicable diseases, the use of vaccines to prevent infection caused by endogenous pathogens is a relatively new concept, but it could serve an important role in efforts to control ARB160. For example, development of an effective S. aureus vaccine could dramatically reduce morbidity and mortality among patients on haemodialysis. Over the past two decades, several S. aureus vaccines have been developed and studied in vitro and in vivo. However, although some of these candidate vaccines demonstrated immunogenicity, they failed to protect against infection. This failure is exemplified by a vaccine containing S. aureus type 5 and 8 capsular polysaccharides that was immunogenic but did not prevent S. aureus bacteraemia when administered to patients with ESRD161. Another vaccine containing the S. aureus iron surface determinant B protein was immunogenic in adults with ESRD162. However, in patients undergoing cardiothoracic surgery, the same vaccine not only failed to reduce the rate of serious S. aureus infections but was also associated with increased mortality among vaccinated patients who later developed S. aureus infection163. Subsequent investigations suggested that inclusion of multiple antigens that target different virulence mechanisms might be needed for a vaccine to provide protection from clinical infection164. One study demonstrated that a vaccine containing four S. aureus antigens — polysaccharide conjugates of serotypes 5 and 8, recombinant surface protein clumping factor A and recombinant manganese transporter protein C — was immunogenic164; it is currently being tested in a phase II clinical trial. The NDV-3 vaccine contains the amino-terminal portion of the Candida albicans agglutinin-like sequence 3 protein (Als3p), which acts as an adhesin and invasin and has both sequence and structural homology with S. aureus surface proteins165,166. The ability of the NDV-3 vaccine to prevent S. aureus colonization is currently being tested in clinical trials.
Exposure to antibiotics that alter the normal microbiota can reduce colonization resistance and thus, microbiota transplantation, particularly faecal microbiota transplantation (FMT), has been explored as a potential approach to reduce the antibiotic-driven dysbiosis that increases the risk of colonization and infection with ARB. FMT might exert a beneficial effect by increasing bacterial diversity and/or altering local and/or systemic immune responses167. Although prevention of recurrent C. difficile infection (rCDI) is the most commonly studied indication for FMT, evidence suggests that FMT could offer protection against colonization with ARB. For example, FMT for rCDI has been associated with the loss of antimicrobial resistance genes in faecal samples168–170. Several case reports171 and small, uncontrolled case series have assessed the ability of FMT to eradicate gastrointestinal carriage of ARB. In one study, FMT was associated with increased faecal bacterial diversity and loss of ESBL-producing Enterobacteriaceae in 3 of 15 (20%) carriers 1 month after the first FMT, and in an additional 3 of 7 (40%) patients who underwent a second FMT after failing to respond to the first FMT172. In another study, 20 patients with blood disorders and ARB gastrointestinal colonization that included VRE, ESBL-producing Enterobacteriaceae, CRE and carbapenem-resistant P. aeruginosa were treated with 25 FMT procedures. Decolonization was documented after 15 procedures (60%) at the 1-month follow-up assessment and in 13 of 14 procedures (93%) for which a 6-month follow-up was performed. Of note, recurrence or relapse was detected in 20% of patients173. These data suggest that FMT is potentially promising for ARB decolonization and might reverse the gut dysbiosis that predisposes patients to colonization with ARB. Several clinical trials of this strategy are currently in progress, including studies in patients who have received kidney and other solid-organ transplants.
A potential alternative or complementary strategy to microbiota-driven colonization resistance is the enhancement of pathogen tolerance. One study reports that secreted antigen A (SagA), a bacterial peptidoglycan hydrolase produced by E. faecium, protects Caenorhabditis elegans from Salmonella typhimurium pathogenesis in a colonization-independent manner, possibly by enhancing the integrity of the intestinal epithelial barrier174. The potential role of such a strategy in preventing human infection remains to be explored.
Conclusions
The emergence of antibiotic resistance among many of the most common bacterial causes of human infection and the global dissemination of these ARB represent a major public health risk; patients with CKD are disproportionately affected. The lack of prevalence and outcomes data related to ARB colonization and infection, particularly for antibiotic-resistant Gram-negative pathogens, among patients with CKD represents an important gap in research and a public health need. The growing burden of multidrug resistance limits therapeutic options and increases the risk of infection-related morbidity and mortality. Thus, clinicians providing care to patients with renal disease must be familiar with local antimicrobial resistance patterns in order to select appropriate empirical antimicrobial therapy and to recognize the appearance of novel resistance profiles within the community. Although several new antibiotics with activity against one or more of these MDR pathogens have now been approved for clinical use, an ongoing and critical need exists for improved adherence to basic infection control practices, antimicrobial stewardship and the development of more effective prevention and treatment strategies.
Key points.
Infections caused by antibiotic-resistant bacteria (ARB) are associated with higher mortality, longer hospitalization and a greater economic burden than those caused by antibiotic-susceptible bacteria of the same species.
The growing global burden of antimicrobial resistance is particularly relevant to patients with chronic kidney disease who are disproportionally affected by antimicrobial resistance when compared with the general population.
Consistent implementation of basic infection prevention strategies is a crucial element in the effort to prevent transmission of and infection by ARB.
Critical infection prevention practices include hand hygiene, cleaning and disinfection of the environment and medical equipment, and use of evidence-based practices for insertion, use and maintenance of invasive devices.
Novel mechanisms of antimicrobial resistance continue to emerge and spread, leading to infections that are difficult to treat and highlighting the need for development of new antimicrobial agents.
Antimicrobial treatment of ARB infections is a complex and evolving topic. Consultation with an infectious disease specialist should be considered in order to optimize antimicrobial agent selection and patient outcomes.
Infections.
Disease states produced by a microorganism that may be symptomatic or asymptomatic.
Multidrug resistant.
(MDR). Non-susceptibility to at least one agent in three or more antimicrobial categories to which an organism does not possess intrinsic resistance.
Extensively drug resistant.
Non-susceptibility to at least one agent in all but two or fewer antimicrobial categories to which an organism does not possess intrinsic resistance.
Pandrug resistant.
Non-susceptibility to all agents in all antimicrobial categories to which an organism does not possess intrinsic resistance.
Colonization.
The asymptomatic presence of a microorganism on or within the body.
Conjugation.
Direct transfer of genetic material between bacterial cells.
Transformation.
Acquisition of new genetic material (DNA) via uptake from the environment.
Transduction.
Transfer of bacterial DNA from one bacterium to another via a viral vector.
Invasive MRSA infections.
MRSA infections within a normally sterile body site, such as the blood.
Vancomycin-intermediate S. aureus.
(VISA). An isolate of Staphylococcus aureus that exhibits an elevated minimum inhibitory concentration (MIC) for vancomycin but that does not reach the MIC considered to represent full resistance to vancomycin.
Heteroresistant or heterogeneous VISA.
(hVISA). Subpopulations of Staphylococcus aureus with reduced susceptibility present among a larger population of fully susceptible organisms.
Drug efflux pumps.
Proteins in the bacterial cell membrane that transport a drug, such as an antibiotic, out of the cell.
Haematogenous osteomyelitis.
Infection of bone that results from inoculation of the bone by microorganisms present in the bloodstream.
Vesicoureteral reflux.
Abnormal retrograde flow of urine from the urinary bladder into the ureter and, possibly, the kidney.
Ureterovesical junction stenosis.
Narrowing at the site where the ureter enters the urinary bladder that may obstruct the flow of urine from the kidney into the bladder.
Neurogenic bladder.
Dysfunction of the urinary bladder due to neurological damage.
Therapeutic drug monitoring.
Measurement of medication concentrations in blood at specified time intervals in order to optimize treatment effectiveness and/or minimize toxicity.
Arteriovenous fistulas.
(AVFs). Surgically created connections between an artery and a vein used for vascular access for haemodialysis.
Contact precautions.
interventions used to reduce the risk of transmission of organisms transmitted by contact with the affected patient and their environment.
Antibiotic selection pressure.
Reduction or elimination of bacteria that are susceptible to an administered antibiotic, allowing antimicrobial-resistant bacterial populations to gain a survival advantage and thus become predominant members of the microbiota.
Antimicrobial stewardship.
A set of coordinated strategies to improve the use of antimicrobial medications with the goal of enhancing patient health outcomes, reducing resistance to antibiotics and decreasing unnecessary costs.
Endogenous pathogens.
Organisms that are part of the normal microbiota but that in some circumstances can cause symptomatic infection.
Colonization resistance.
The ability of the body’s microbiota, such as commensal gut bacteria, to prevent colonization and infection with pathogenic organisms.
Acknowledgements
T.Z.W. was supported by US National Institutes of Health, National Institute of Allergy and Infectious Disease grant T32 A1007613.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Nephrology thanks P. Tambyah and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: final report and recommendations. amr-review https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (2016).
- 2.World Health Organization. Antimicrobial resistance: global report on surveillance 2014. WHO; http://www.who.int/drugresistance/documents/surveillancereport/en (2014). [Google Scholar]
- 3.Magiorakos AP et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect 18, 268–281 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Saran R et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis 71, A7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calfee DP Multidrug-resistant organisms in dialysis patients. Semin. Dial 26, 447–456 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Blair JM, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJ Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Barber M & Rozwadowska-Dowzenko M Infection by penicillin-resistant staphylococci. Lancet 2, 641–644 (1948). [DOI] [PubMed] [Google Scholar]
- 8.Barber M Methicillin-resistant staphylococci. J. Clin. Pathol 14, 385–393 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villegas-Estrada A, Lee M, Hesek D, Vakulenko SB & Mobashery S Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA beta-lactam antibiotics. J. Am. Chem. Soc 130, 9212–9213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deleo F, Otto M, Kreiswirth B & Chambers H Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375, 1557–1568 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2017. ECDC; https://ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017 (2018). [Google Scholar]
- 12.Rosenthal VD et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am. J. Infect. Control 44, 1495–1504 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Zacharioudakis IM, Zervou FN, Ziakas PD & Mylonakis E Meta-analysis of methicillin-resistant Staphylococcus aureus colonization and risk of infection in dialysis patients. J. Am. Soc. Nephrol 25, 2131–2141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P et al. Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol. Dial. Transplant 23, 1659–1665 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Nguyen DB et al. Invasive methicillin-resistant Staphylococcus aureus infections among chronic dialysis patients in the United States, 2005–2011. Clin. Infect. Dis 57, 1392–1400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giarola LB, Dos Santos RR, Tognim MC, Borelli SD & Bedendo J Carriage frequency, phenotypic and genotypic characteristics of Staphylococcus aureus isolated from dialysis and kidney tranplant patients at a hosptial in northern Paraná. Braz. J. Microbiol 43, 923–930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore C et al. Colonisation with methicillin-resistant Staphylococcus aureus prior to renal transplantation is associated with long-term renal allograft failure. Transpl. Int 27, 926–930 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu K et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother 40, 135–136 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Sun X, Chang W, Dai Y & Ma X Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLOS ONE 10, e0136082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae IG et al. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis 200, 1355–1366 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes DM, Ward KE & LaPlante KL Clinical implications of vancomycin heteroresistant and intermediately susceptible Staphylococcus aureus. Pharmacotherapy 35, 424–432 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Howden BP, Peleg AY & Stinear TP The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect. Genet. Evol 21, 575–582 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Smith TL et al. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N. Engl. J. Med 340, 493–501 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—-United States, 2002. MMWR Morb. Mortal. Wkly Rep 51, 565–567 (2002). [PubMed] [Google Scholar]
- 25.Walters MS et al. Vancomycin-resistant Staphylococcus aureus -Delaware, 2015. MMWR Morb. Mortal. Wkly Rep 64, 1056 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Melo-Cristino J, Resina C, Manuel V, Lito L & Ramirez M First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. Lancet 382, 205 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Moravvej Z et al. Update on the global number of vancomycin-resistant Staphylococcus aureus (VRSA) strains. Int. J. Antimicrob. Agents 42, 370–371 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Centers Disease Control and Prevention. VISA/VRSA in healthcare settings. CDC; http://www.cdc.gov/hai/organisms/visa_vrsa/visa_vrsa.html (updated 21 Jul 2015). [Google Scholar]
- 29.Murray BE The life and times of the Enterococcus. Clin. Microbiol. Rev 3, 46–65 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster TJ Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev 41, 430–449 (2017). [DOI] [PubMed] [Google Scholar]
- 31.The Center for Disease Dynamics Economics & Policy. ResistanceMap: antibiotic resistance. CDDEP; https://resistancemap.cddep.org/AntibioticResistance.php (2018). [Google Scholar]
- 32.Zacharioudakis IM, Zervou FN, Ziakas PD, Rice LB & Mylonakis E Vancomycin-resistant enterococci colonization among dialysis patients: a meta-analysis of prevalence, risk factors, and significance. Am. J. Kidney Dis 65, 88–97 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Freitas MC et al. Prevalence of vancomycin-resistant Enterococcus fecal colonization among kidney transplant patients. BMC Infect. Dis 6, 133 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen DB et al. National Healthcare Safety Network (NHSN) Dialysis Event Surveillance Report for 2014. Clin. J. Am. Soc. Nephrol 12, 1139–1146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene MH et al. Risk factors and outcomes associated with acquisition of daptomycin and linezolid-nonsusceptible vancomycin-resistant Enterococcus. Open Forum Infect. Dis 5, ofy185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall SH, Donskey CJ, Hutton-Thomas R, Salata RA & Rice LB Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother 46, 3334–3336 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendes RE, Hogan PA, Jones RN, Sader HS & Flamm RK Surveillance for linezolid resistance via the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): evolving resistance mechanisms with stable susceptibility rates. J. Antimicrob. Chemother 71, 1860–1865 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Sader HS, Farrell DJ, Flamm RK & Jones RN Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int. J. Antimicrob. Agents 43, 465–469 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Kamboj M et al. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect. Control Hosp. Epidemiol 32, 391–394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TT, Munita JM & Arias CA Mechanisms of drug resistance: daptomycin resistance. Ann. NY Acad. Sci 1354, 32–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelesidis T, Humphries R, Uslan DZ & Pegues D De novo daptomycin-nonsusceptible enterococcal infections. Emerg. Infect. Dis 18, 674–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pop-Vicas A, Strom J, Stanley K & D’Agata EM Multidrug-resistant gram-negative bacteria among patients who require chronic hemodialysis. Clin. J. Am. Soc. Nephrol 3, 752–758 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterson DL & Lipman J Returning to the pre-antibiotic era in the critically ill: the XDR problem. Crit. Care Med 35, 1789–1791 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Patel G & Bonomo RA “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol 4, 48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomi R et al. Occurrence of clinically important lineages, including the sequence type 131 C1-M27 subclone, among extended-spectrum-β-lactamase-producing Escherichia coli in wastewater. Antimicrob. Agents Chemother 61, e00564–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu YY et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis 16, 161–168 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Lübbert C et al. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection 45, 479–491 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Harris P et al. Effect of piperacillin-tazobactam versus meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance. JAMA 320, 984–994 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doi Y, Iovleva A & Bonomo RA The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J. Travel Med 24, S44–S51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacoby GA AmpC beta-lactamases. Clin. Microbiol. Rev 22, 161–182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascual V et al. Epidemiology and risk factors for infections due to AmpC β-lactamase-producing Escherichia coli. J. Antimicrob. Chemother 70, 899–904 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Yigit H et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother 45, 1151–1161 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe M, Iyobe S, Inoue M & Mitsuhashi S Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother 35, 147–151 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson AP & Woodford N Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol 62, 499–513 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Logan LK & Weinstein RA The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J. Infect. Dis 215, S28–S36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodworth KR et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms -United States, 2006–2017. MMWR Morb. Mortal. Wkly Rep 67, 396–401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC; https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (2013). [Google Scholar]
- 58.Weiner LM et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol 37, 1288–1301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldred KJ, Kerns RJ & Osheroff N Mechanism of quinolone action and resistance. Biochemistry 53, 1565–1574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrill HJ et al. Antimicrobial resistance of Escherichia coli urinary isolates in the Veterans Affairs Health Care System. Antimicrob. Agents Chemother 61, e02236–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doi Y, Wachino JI & Arakawa Y Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect. Dis. Clin. North Am 30, 523–537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leclercq R et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect 19, 141–160 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Haidar G et al. Association between the presence of aminoglycoside-modifying enzymes and in vitro activity of gentamicin, tobramycin, amikacin, and plazomicin against Klebsiella pneumoniae carbapenemase-and extended-spectrum-β-lactamase-producing Enterobacter species. Antimicrob. Agents Chemother 60, 5208–5214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein EY et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA 115, E3463–E3470 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rojas LJ et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin. Infect. Dis 64, 711–718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron S, Hadjadj L, Rolain JM & Olaitan AO Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int. J. Antimicrob. Agents 48, 583–591 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Giamarellou H Epidemiology of infections caused by polymyxin-resistant pathogens. Int. J. Antimicrob. Agents 48, 614–621 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Al-Tawfiq JA, Laxminarayan R & Mendelson M How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int. J. Infect. Dis 54, 77–84 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Pang Z, Raudonis R, Glick BR, Lin TJ & Cheng Z Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv 37, 177–192 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Yusuf E et al. Emergence of antimicrobial resistance to Pseudomonas aeruginosa in the intensive care unit: association with the duration of antibiotic exposure and mode of administration. Ann. Intensive Care 7, 72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong D et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin. Microbiol. Rev 30, 409–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Towner KJ Acinetobacter: an old friend, but a new enemy. J. Hosp. Infect 73, 355–363 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Morgan DJ et al. Multidrug-resistant Acinetobacter baumannii in New York City −10 years into the epidemic. Infect. Control Hosp. Epidemiol 30, 196–197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lob SH et al. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn. Microbiol. Infect. Dis 85, 459–465 (2016). [DOI] [PubMed] [Google Scholar]
- 75.United States Renal Data System. 2018 Annual Data Report. USRDS; http://www.usrds.org/adr.aspx (2018). [Google Scholar]
- 76.ANZDATA Registry. 40th Annual ANZDATA Report (2017). ANZDATA; www.anzdata.org.au/v1/report_2017.html (updated 18 Aug 2018). [Google Scholar]
- 77.Nguyen DB et al. Completeness of methicillin-resistant Staphylococcus aureus bloodstream infection reporting from outpatient hemodialysis facilities to the National Healthcare Safety Network, 2013. Infect. Control Hosp. Epidemiol 37, 205–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalgaard L et al. Risk and prognosis of bloodstream infections among patients on chronic hemodialysis: a population-based cohort study. PLOS ONE 10, e0124547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fysaraki M et al. Incidence, clinical, microbiological features and outcome of bloodstream infections in patients undergoing hemodialysis. Int. J. Med. Sci 10, 1632–1638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahli F, Feidjel R & Laalaoui R Hemodialysis catheter-related infection: rates, risk factors and pathogens. J. Infect. Public Health 10, 403–408 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Worth LJ et al. Epidemiology of infections and antimicrobial use in Australian haemodialysis outpatients: findings from a Victorian surveillance network, 2008–2015. J. Hosp. Infect 97, 93–98 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Akoh JA Peritoneal dialysis associated infections: an update on diagnosis and management. World J. Nephrol 1, 106–122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prasad KN et al. Microbiology and outcomes of peritonitis in northern India. Perit. Dial. Int 34, 188–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGuire AL, Carson CF, Inglis TJ & Chakera A Effects of a statewide protocol for the management of peritoneal dialysis-related peritonitis on microbial profiles and antimicrobial susceptibilities: a retrospective five-year review. Perit. Dial. Int 35, 722–728 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitterer D, Latus J, Pöhlmann C, Alscher MD & Kimmel M Microbiological surveillance of peritoneal dialysis associated peritonitis: antimicrobial susceptibility profiles of a referral center in GERMANY over 32 years. PLOS ONE 10, e0135969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fishman JA Infection in organ transplantation. Am. J. Transplant 17, 856–879 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Aguado JM et al. Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant. Rev. (Orlando) 32, 36–57 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Ariza-Heredia EJ et al. Urinary tract infections in kidney transplant recipients: role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann. Transplant 18, 195–204 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Chuang P, Parikh CR & Langone A Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin. Transplant 19, 230–235 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Takai K, Tollemar J, Wilczek HE & Groth CG Urinary tract infections following renal transplantation. Clin. Transplant 12, 19–23 (1998). [PubMed] [Google Scholar]
- 91.Cervera C et al. Multidrug-resistant bacteria in solid organ transplant recipients. Clin. Microbiol. Infect 20 (Suppl. 7), 49–73 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Bodro M et al. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 96, 843–849 (2013). [DOI] [PubMed] [Google Scholar]
- 93.van Duin D, van Delden C & AST Infectious Diseases Community of Practice. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation. Am. J. Transplant 13 (Suppl. 4), 31–41 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Linares L et al. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transplant. Proc 39, 2222–2224 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Lee JR et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation 96, 732–738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pinheiro HS, Mituiassu AM, Carminatti M, Braga AM & Bastos MG Urinary tract infection caused by extended-spectrum beta-lactamase-producing bacteria in kidney transplant patients. Transplant. Proc 42, 486–487 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Pouch SM & Satlin MJ Carbapenem-resistant Enterobacteriaceae in special populations: solid organ transplant recipients, stem cell transplant recipients, and patients with hematologic malignancies. Virulence 8, 391–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel G, Huprikar S, Factor SH, Jenkins SG & Calfee DP Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol 29, 1099–1106 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Burnham JP et al. Infectious diseases consultation reduces 30-day and 1-year all-cause mortality for multidrug-resistant organism infections. Open Forum Infect. Dis 5, ofy026 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhanel GG et al. Ceftaroline pharmacodynamic activity versus community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus, heteroresistant vancomycin-intermediate S. aureus, vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus using an in vitro model. J. Antimicrob. Chemother 66, 1301–1305 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Sader HS, Flamm RK & Jones RN Antimicrobial activity of ceftaroline tested against staphylococci with reduced susceptibility to linezolid, daptomycin, or vancomycin from U. S. hospitals, 2008 to 2011. Antimicrob. Agents Chemother 57, 3178–3181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pfaller MA, Shortridge D, Sader HS, Flamm RK & Castanheira M Ceftolozane-tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing healthcare-associated infections in Australia and New Zealand: report from an Antimicrobial Surveillance Program (2013–2015). J. Glob. Antimicrob. Resist 10, 186–194 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Sader HS, Farrell DJ, Flamm RK & Jones RN Ceftolozane/tazobactam activity tested against aerobic gram-negative organisms isolated from intra-abdominal and urinary tract infections in European and United States hospitals (2012). J. Infect 69, 266–277 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Solomkin J et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin. Infect. Dis 60, 1462–1471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haidar G et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin. Infect. Dis 65, 110–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munita JM et al. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin. Infect. Dis 65, 158–161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Falcone M & Paterson D Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J. Antimicrob. Chemother 71, 2713–2722 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Carmeli Y et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect. Dis 16, 661–673 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Mazuski JE et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin. Infect. Dis 62, 1380–1389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Duin D et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin. Infect. Dis 66, 163–171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castanheira M, Huband MD, Mendes RE & Flamm RK Meropenem-vaborbactam tested against contemporary gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob. Agents Chemother 61, e00567–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaye KS et al. Effect of meropenem-vaborbactam versus piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 319, 788–799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wunderink RG et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect. Dis. Ther 7, 439–455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duncan LR, Sader HS, Smart JI, Flamm RK & Mendes RE Telavancin activity in vitro tested against a worldwide collection of gram-positive clinical isolates (2014). J. Glob. Antimicrob. Resist 10, 271–276 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Pfaller MA, Sader HS, Castanheira M, Flamm RK & Mendes RE Antimicrobial activity of oritavancin and comparator agents when tested against Gram-positive bacterial isolates causing infections in cancer patients (2014–2016). J. Antimicrob. Chemother 73, 916–922 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Smith JR, Roberts KD & Rybak MJ Dalbavancin: a novel lipoglycopeptide antibiotic with extended activity against gram-positive infections. Infect. Dis. Ther 4, 245–258 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rubinstein E et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin. Infect. Dis 52, 31–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castanheira M et al. Activity of plazomicin against gram-negative and gram-positive isolates collected from U. S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob. Agents Chemother 62, e00313–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdul-Mutakabbir JC, Kebriaei R, Jorgensen SCJ & Rybak MJ Teaching an old class new tricks: a novel semi-synthetic aminoglycoside, plazomicin. Infect. Dis. Ther 10.1007/s40121-019-0239-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Connolly LE, Riddle V, Cebrik D, Armstrong ES & Miller LG A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob. Agents Chemother 62, e01989–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhanel GG et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 76, 567–588 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Lin X & Bush K In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE) to eravacycline. J. Antibiot 69, 600–604 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Solomkin J et al. Assessing the efficacy and safety of eravacycline versus ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 152, 224–232 (2017). [DOI] [PubMed] [Google Scholar]
- 124.O’Riordan W et al. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin. Infect. Dis 67, 657–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm. Rep 50, 1–43 (2001). [PubMed] [Google Scholar]
- 126.Coulliette AD & Arduino MJ Hemodialysis and water quality. Semin. Dial 26, 427–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merino JL et al. Serratia marcescens bacteraemia outbreak in haemodialysis patients with tunnelled catheters due to colonisation of antiseptic solution. Experience at 4 hospitals. Nefrologia 36, 667–673 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Edens C et al. Hemodialyzer reuse and gram-negative bloodstream infections. Am. J. Kidney Dis 69, 726–733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trépanier P et al. Survey of infection control practices in hemodialysis units: preventing vascular access-associated bloodstream infections. Infect. Control Hosp. Epidemiol 35, 833–838 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Chenoweth CE et al. Variation in infection prevention practices in dialysis facilities: results from the national opportunity to improve infection control in ESRD (End-Stage Renal Disease) project. Infect. Control Hosp. Epidemiol 36, 802–806 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Patel PR et al. Bloodstream infection rates in outpatient hemodialysis facilities participating in a collaborative prevention effort: a quality improvement report. Am. J. Kidney Dis 62, 322–330 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Yi SH et al. Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effort. Infect. Control Hosp. Epidemiol 37, 863–866 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Lewis VR, Clark L, Benda N & Hardwick MJ Reducing healthcare-associated infections in an ambulatory dialysis unit: identification and alignment of work system factors. Am. J. Infect. Control 42, S284–290 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Snyder GM et al. Factors associated with the receipt of antimicrobials among chronic hemodialysis patients. Am. J. Infect. Control 44, 1269–1274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vassalotti JA et al. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin. Dial 25, 303–310 (2012). [DOI] [PubMed] [Google Scholar]
- 136.Septimus E, Weinstein RA, Perl TM, Goldmann DA & Yokoe DS Approaches for preventing healthcare-associated infections: go long or go wide? Infect. Control Hosp. Epidemiol 35, 797–801 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Snyder GM et al. Antimicrobial use in outpatient hemodialysis units. Infect. Control Hosp. Epidemiol 34, 349–357 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Hui K et al. Patterns of use and appropriateness of antibiotics prescribed to patients receiving haemodialysis: an observational study. BMC Nephrol. 18, 156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wagner B et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect. Control Hosp. Epidemiol 35, 1209–1228 (2014). [DOI] [PubMed] [Google Scholar]
- 140.D’Agata EMC, Tran D, Bautista J, Shemin D & Grima D Clinical and economic benefits of antimicrobial stewardship programs in hemodialysis facilities: a decision analytic model. Clin. J. Am. Soc. Nephrol 13, 1389–1397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barlam TF et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis 62, e51–e77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cunha CB & D’Agata EM Implementing an antimicrobial stewardship program in out-patient dialysis units. Curr. Opin. Nephrol. Hypertens 25, 551–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grothe C, Taminato M, Belasco A, Sesso R & Barbosa D Prophylactic treatment of chronic renal disease in patients undergoing peritoneal dialysis and colonized by Staphylococcus aureus: a systematic review and meta-analysis. BMC Nephrol. 17, 115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grothe C, Taminato M, Belasco A, Sesso R & Barbosa D Screening and treatment for Staphylococcus aureus in patients undergoing hemodialysis: a systematic review and meta-analysis. BMC Nephrol. 15, 202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miller MA, Dascal A, Portnoy J & Mendelson J Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect. Control Hosp. Epidemiol 17, 811–813 (1996). [DOI] [PubMed] [Google Scholar]
- 146.Saidel-Odes L et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect. Control Hosp. Epidemiol 33, 14–19 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Halaby T, Al Naiemi N, Kluytmans J, van der Palen J & Vandenbroucke-Grauls CM Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob. Agents Chemother 57, 3224–3229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rieg S et al. Intestinal decolonization of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBL): a retrospective observational study in patients at risk for infection and a brief review of the literature. BMC Infect. Dis 15, 475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Czaplewski L et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis 16, 239–251 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Fischetti VA Development of phage lysins as novel therapeutics: a historical perspective. Viruses 10, 310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corsini B et al. Chemotherapy with phage lysins reduces pneumococcal colonization of the respiratory tract. Antimicrob. Agents Chemother 62, e02212–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nelson D, Loomis L & Fischetti VA Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl Acad. Sci. USA 98, 4107–4112 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng M et al. An ointment consisting of the phage lysin LysGH15 and apigenin for decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses 10, 244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schuch R et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis 209, 1469–1478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lood R et al. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother 59, 1983–1991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zipperer A et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535, 511–516 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Xu D et al. Bioprospecting deep-sea actinobacteria for novel anti-infective natural products. Front. Microbiol 9, 787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.von Gottberg A et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N. Engl. J. Med 371, 1889–1899 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Schroeder MR et al. A population-based assessment of the impact of 7-and 13-valent pneumococcal conjugate vaccines on macrolide-resistant invasive pneumococcal disease: emergence and decline of Streptococcus pneumoniae serotype 19A (CC320) with dual macrolide resistance mechanisms. Clin. Infect. Dis 65, 990–998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rappuoli R, Bloom DE & Black S Deploy vaccines to fight superbugs. Nature 552, 165–167 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Fattom A et al. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum. Vaccin. Immunother 11, 632–641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moustafa M et al. Phase IIa study of the immunogenicity and safety of the novel Staphylococcus aureus vaccine V710 in adults with end-stage renal disease receiving hemodialysis. Clin. Vaccine Immunol 19, 1509–1516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fowler VG et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309, 1368–1378 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Begier E et al. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine 35, 1132–1139 (2017). [DOI] [PubMed] [Google Scholar]
- 165.Schmidt CS et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 30, 7594–7600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Edwards JE et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-a phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis 66, 1928–1936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hudson LE, Anderson SE, Corbett AH & Lamb TJ Gleaning insights from fecal microbiota transplantation and probiotic studies for the rational design of combination microbial therapies. Clin. Microbiol. Rev 30, 191–231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Millan B et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin. Infect. Dis 62, 1479–1486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jouhten H, Mattila E, Arkkila P & Satokari R Reduction of antibiotic resistance genes in intestinal microbiota of patients with recurrent Clostridium difficile infection after fecal microbiota transplantation. Clin. Infect. Dis 63, 710–711 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Leung V, Vincent C, Edens TJ, Miller M & Manges AR Antimicrobial resistance gene acquisition and depletion following fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Infect. Dis 66, 456–457 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Manges AR, Steiner TS & Wright AJ Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect. Dis. (Lond.) 48, 587–592 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Singh R et al. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing enterobacteriaceae: a proof of principle study. BMC Res. Notes 11, 190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bilinski J et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin. Infect. Dis 65, 364–370 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Rangan KJ et al. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science 353, 1434–1437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


