Abstract
Aggregations of β-amyloid (Aβ) and α-synuclein (αS) into oligomeric and fibrillar assemblies are the pathological hallmarks of Alzheimer’s and Parkinson’s diseases, respectively. Although Aβ and αS affect different regions of the brain and are separated at the cellular level, there is evidence of their eventual interaction in the pathology of both disorders. Characterization of interactions of Aβ and αS at various stages of their aggregation pathways could reveal mechanisms and therapeutic targets for the prevention and cure of these neurodegenerative diseases. In this study, we comprehensively examined the interactions and their molecular manifestations using an array of characterization tools. We show for the first time that αS monomers and oligomers, but not αS fibrils, inhibit Aβ fibrillization while promoting oligomerization of Aβ monomers and stabilizing preformed Aβ oligomers via co-assembly, as judged by Thioflavin T fluorescence, transmission electron microscopy and SDS- and native-PAGE with fluorescently labeled peptides/proteins. In contrast, soluble Aβ species, such as monomers and oligomers, aggregate into fibrils, when incubated alone under the otherwise same condition. Our study provides evidence that the interactions with αS soluble species, responsible for the effects, are mediated primarily by the C-terminus of Aβ, when judged by competitive immunoassays using antibodies recognizing various fragments of Aβ. We also show that the C-terminus of Aβ is a primary site for its interaction with αS fibrils. Collectively, these data demonstrate aggregation state-specific interactions between αS and Aβ, and offer insight into a molecular basis of synergistic biological effects between the two polypeptides.
Keywords: alpha-synuclein, aggregation, beta-amyloid, oligomer, protein-protein interaction
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by the loss of hippocampal neurons and forebrain cholinergic neurons.1, 2 AD pathology is caused by the aggregation of the 40 or 42 residue peptide, β-amyloid (Aβ), leading to the formation of the characteristic extracellular amyloid plaques found in the brains of AD patients.3, 4 Aβ is generated from the proteolytic degradation of the transmembrane protein, amyloid precursor protein (APP), resulting in accumulation of Aβ peptides predominantly in the brain extracellular fluid space.3, 4 The wild-type Aβ sequence consists of alternating hydrophilic and hydrophobic regions: the hydrophilic N-terminus (Aβ1-16), the hydrophobic Aβ17-21, the hydrophilic Aβ22-30, and the hydrophobic C-terminus (Aβ31-40 or Aβ31-42), where the hydrophobic sequences are thought to drive Aβ aggregation.5, 6 Monomeric Aβ spontaneously self-assembles into “soluble” oligomeric species, such as globular aggregates and protofibrils, which then further aggregate to form mature, “insoluble” fibrils.7-15 It has been suggested that the accumulation of soluble oligomeric species, rather than the fibrils, disrupt neuronal activity at the synapse, initiating the degeneration in AD.13, 16-19
Parkinson’s disease (PD) is the second most common neurodegenerative disease after AD and the most common movement disorder.20-23 PD is characterized by the loss of substantia nigra dopamine neurons and the presence of Lewy bodies (LB) with intracellular protein inclusions that contain a 140 residue-protein, α-synuclein (αS).24, 25 αS consists of three distinct regions: the N-terminus (αS1-60) is amphipathic, while the central domain (αS61-95) is hydrophobic and the C-terminus (αS96-140) is acidic.26 Following similar pathways to Aβ, the aggregation of αS is the hallmark event in the pathology of PD.20, 21 Similar to AD, αS oligomers, particularly those rich in β-sheets, are thought to be the major toxic species in PD, for example, by forming pores across cell membranes, compared to monomeric and fibrillar conformers.27-30
Although AD pathology predominates in the cerebral cortex and hippocampus,1 and PD pathology mostly affects the substantia nigra,22, 31 there is overlap in the symptoms and pathologies of the two diseases. Many (~50%) patients with AD develop signs of PD (e.g., LB pathology),32 and conversely, PD patients can be diagnosed with dementia (PDD), and diffuse Lewy body disease (DLB) is a neurodegenerative disorder characterized by dementia and parkinsonism.32-34 Up to 50% of PDD patients build up Aβ plaques,24 and PDD patients typically exhibit more pronounced cognitive dysfunction than AD patients.32, 35 Consistent with these observations, cognitive decline in transgenic mice and pathological features in cultured neurons are accelerated in models with both AD and PD pathology.36-38 These findings have raised questions about pathological synergy between Aβ and αS.24, 39 Expression levels of αS are high in brain regions where AD lesions are abundant,40 and αS load is associated with Aβ plaques in cortical areas.41 In addition, the hydrophobic region of αS (i.e., αS61-95), known as the non-amyloid component (NAC), was one of the first constituents of amyloid plaques to be discovered.42 Recently, localized proteomics also revealed the presence of full-length αS within Aβ plaques, which likely plays a role in rapidly progressive AD.43 Although Aβ is primarily extracellular, a growing body of evidence indicates that Aβ can also be accumulated intraneuronally.44 Likewise, αS initially produced intracellularly can be excreted from cells.45 Thus, Aβ-αS interactions can occur both intracellularly and extracellularly. Given their potential roles in the pathology of AD, PD and PDD, it is important to define these interactions, which have not been properly addressed by anti-amyloid drugs, and thereby illuminate new paths for the development of effective therapeutic strategies.24
Several studies have demonstrated Aβ-αS interactions.37, 40, 46-52 For example, Aβ has been shown to enhance αS aggregation and Lewy body inclusions in transgenic mice and cultured neurons.37, 38 Analogously, αS can promote Aβ aggregation both in vivo and in vitro.36, 40 Aβ aggregates can seed αS aggregation, and vice versa.51, 53, 54 Unfortunately, aggregation has often been poorly defined in these previous studies, with no distinction between oligomerization and fibrilization. Moreover, these previous studies have provided limited insight into Aβ-αS interactions and how they lead to amyloid aggregation − including the impact on distinct amyloid assembly states (monomers, oligomers, and fibrils), and the interplay among these Aβ and αS species. Such comprehensive examination is critical to understand outcomes of Aβ-αS interactions, including the origin of toxic synergistic effects in AD and PD. Surprisingly, experimental, rather than computational, evidence of co-assembled oligomerization between Aβ and αS in formation of potentially toxic oligomeric conformers is lacking. Moreover, in previous studies, amyloid aggregation was often characterized under denaturing conditions (e.g., in the presence of SDS), which could introduce undesired artifacts during amyloid aggregation,49, 55 and/or in the excess of Aβ, which is not physiologically relevant.50, 51
In the present study, we experimentally examined amyloid assembly resulting from interactions between Aβ and αS in their respective monomeric, oligomeric, and fibrillar forms under aqueous conditions in excess αS. Our comprehensive characterization demonstrates that αS monomers and oligomers, but not fibrils, inhibit Aβ fibrillization. Moreover, the soluble αS species promote Aβ oligomerization and stabilize pre-formed Aβ oligomers by co-assembly with Aβ. We also provide evidence that the Aβ-αS interactions are mediated primarily by the Aβ C-terminus. Overall, these data reveal a novel molecular mechanism by which synergistic toxic effects can be generated through Aβ-αS interactions.
RESULTS
We examined amyloid assembly in samples containing Aβ only and αS only, and their mixtures. The Aβ isoform containing 40 rather than 42 amino acids was chosen because (i) isoform 40 is 10-fold more abundant than isoform 42,56 and (ii) the plaque level of the shorter Aβ isoform was greater with αS aggregates.57 Given that αS is more abundant than Aβ,58, 59 αS in excess was co-incubated with Aβ in mixture samples, unless otherwise mentioned. A concentration of 70 μM Aβ was chosen to facilitate aggregation within a reasonable timeframe under static incubation at 37 °C. Note that the in vitro concentrations used in this study are higher than apparent physiological concentrations of Aβ and αS (≤ low nM for Aβ60 and ≤ low μM for αS61), at which levels concentration-dependent aggregation may be disfavored kinetically and thermodynamically.62, 63 However, in vivo, Aβ and αS can be concentrated locally in various ways by several orders of magnitude, which facilitates aggregation.60, 64-71. Moreover, any aggregates formed as a result of interactions between Aβ and αS could act as seeds to facilitate aggregation, even at low concentrations. Therefore, despite the difference between overall Aβ and αS concentrations in vivo and those required to monitor aggregation in vitro, our investigations provide valuable information on how Aβ and αS interact and consequences thereof.
For comprehensive examination of interactions between Aβ and αS, samples containing Aβ only, αS only or a mixture of Aβ and αS in their three representative aggregation states (i.e., monomers, oligomers and fibrils) were prepared and characterized for assembly. For specific monitoring of Aβ and αS by fluorescence in their mixtures, samples containing HiLyte Fluor 488-labeled Aβ or Alexa Fluor 647-labeled αS were also prepared. Similar N-terminal labeling did not affect aggregation properties of Aβ or αS during their fibrillizations, as judged by Thioflavin T (ThT) fluorescence and size exclusion chromatography26, 72-75. Furthermore, our samples containing the labeled polypeptides exhibited electrophoretic mobility as expected according to identity and aggregation state of the samples (Figs. S1A and S1B). Aggregation behaviors of our samples containing the labeled Aβ and exclusively unlabeled Aβ were similar during oligomerization (Fig. S2), further conforming the lack of significant effect of the labeling on aggregation.
Aβ only and αS only
As described in detail in the Supporting Text and Figs. S3-S6, Aβ monomers and oligomers readily aggregated into insoluble fibrils and Aβ fibrils remained fibrillar during the 7-day incubation. The results are summarized in Table 1. In contrast, all three αS only samples remained relatively stable during the incubation (see Supporting Text and Fig. S4-S8).
Table 1.
Summary of the effects of αS on the aggregation of Aβ
| Sample | The major aggregate species observed after 7 day incubation |
|
|---|---|---|
| Aβ M | only | Insoluble fibrils |
| + αS M | Soluble oligomers | |
| + αS O | Soluble oligomers | |
| + αS F | Insoluble fibrils | |
| Aβ O | only | Insoluble fibrils |
| + αS M | Soluble oligomers | |
| + αS O | Soluble oligomers | |
| + αS F | Insoluble fibrils | |
| Aβ F | only | Insoluble fibrils |
| + αS M | Insoluble fibrils | |
| + αS O | Insoluble fibrils | |
| + αS F | Insoluble fibrils | |
M, O and F represent monomers, oligomers and fibrils, respectively.
Aβ monomers and αS monomers
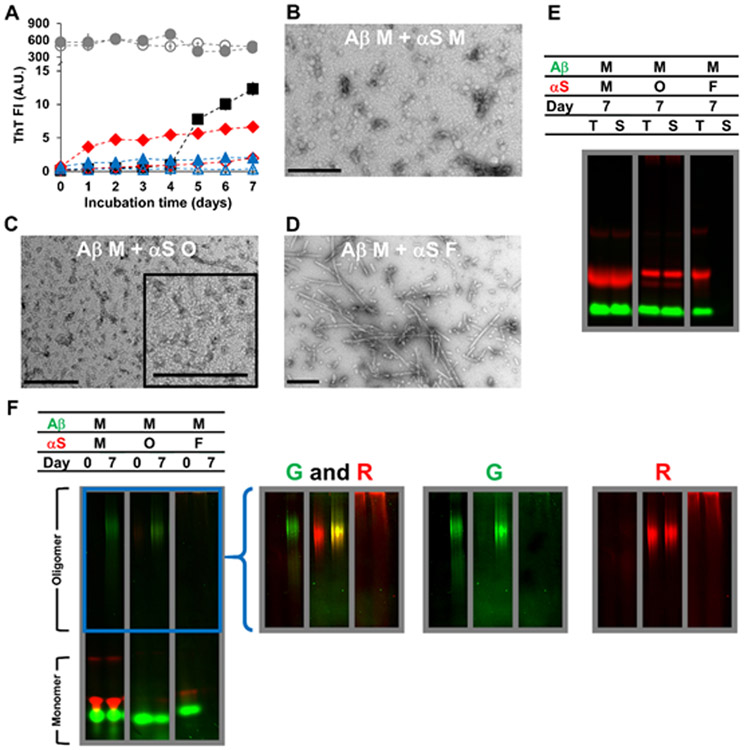
The effect of αS on the aggregation of Aβ monomers (70 μM) was investigated by co-incubation (Fig. 1A-F). The co-incubation with αS monomers (350 μM) reduced the lag phase to <1 day (Fig. 1A) with lower ThT fluorescence intensity of the mixture compared to the Aβ monomer only samples after the 7-day incubation. TEM reveals that the aggregates of the mixture included mostly globular oligomeric assemblies, with no fibrillar aggregates (Fig. 1B). The inhibition of fibrillization from Aβ monomers by αS monomers, together with the ThT data, indicates a direct interaction between the two species that alters the aggregation pathway of Aβ.
Figure 1.
Characterizations of Aβ monomers (M) mixed with αS monomers (M), αS oligomers (O) or αS fibrils (F) incubated for 7 days at 37 °C, as examined by (A) ThT fluorescence, (B-D) TEM, and (E-F) in-gel fluorescence imaging of (E) SDS-PAGE and (F) native-PAGE. In (A-F), the concentration of Aβ monomers was 70 μM. The concentrations of αS monomers and αS fibrils were 350 μM and that of αS oligomers was 17 μM. Concentrations of oligomers and fibrils were monomer-equivalent concentrations. In (A), the data on Aβ monomers mixed with αS monomers (filled red diamonds), αS oligomers (filled blue triangles) and αS fibrils (filled gray circles) are shown along with those on αS monomers alone (empty red diamonds), αS oligomers alone (empty blue triangles) and αS fibrils alone (empty gray circles) taken from Figure S7A. The data on Aβ monomers alone (filled black squares) taken from Figure S3A are also shown for comparison. Error bars: 1 standard deviation of triplicates. In (B-D), representative TEM images of mixtures containing (B) Aβ monomers and αS monomers, (C) Aβ monomers and αS oligomers and (D) Aβ monomers and αS fibrils are shown with scale bars of 200 nm. In (E-F), samples contained HiLyte Fluor 488-labeled Aβ (green) and Alexa Fluor 647-labeled αS (red). Each panel was taken from a bigger gel image (see Figure S5) and reassembled for better presentation. In (E), T: total fraction and S: soluble fraction. In (F), the images in the right provide brighter upper portions of the native-PAGE gels – enclosed by a blue box in the left – obtained by renormalizing the brightness from the monomer to the oligomer bands, which often contained lower percentages of fluorophores. G: green channel and R: red channel.
During incubation of the mixture, most αS species remained soluble and monomeric (Figs. 1E-F; left panels), as seen with the aforementioned αS only samples. However, in contrast to Aβ only samples, there was no noticeable loss of Aβ to insoluble material in the presence of αS (Fig. 1E; left panel). Instead, formation of soluble oligomeric Aβ was observed as a smeared band in the upper portion of the native-PAGE gel, while major fractions of Aβ and αS remained monomeric (Fig. 1F; left panel). Note that the relative positions of the major bands on both SDS- and native-PAGE gels appeared consistent across all individual and mixture samples of Aβ and αS (Fig. S5). Collectively, αS monomers inhibited Aβ aggregation into fibrils while promoting formation of ThT-positive soluble oligomeric aggregates (Table 1).
The aggregation-modulatory effect was weakened with decreasing αS concentrations after 7 day incubation reflecting: (i) greater conversion of monomeric Aβ to insoluble aggregates and reduced formation of oligomeric Aβ with ≤ 70 μM of αS (Fig. S9); and (ii) aggregation of monomeric αS at 7 μM to insoluble materials, possibly facilitated by Aβ fibrils, during co-incubation with Aβ (Fig. S9). Note that the total mass of labeled αS was kept constant for all three concentrations tested (7, 70, and 350 μM) in the mixtures, while the fraction of unlabeled (i.e., non-fluorescent) αS was modified. As such, the total fluorescence intensity of αS at the three concentrations before the incubation would be relatively similar with slight positional differences of bands on SDS-PAGE depending on the amount of total protein loaded (Fig. S9A).
Aβ monomers and αS oligomers
The effects of αS oligomers on the aggregation of Aβ monomers were also examined. Similar to αS monomers at 350 μM, mixing with αS oligomers at 17 μM significantly hindered aggregation of Aβ monomers into insoluble fibrils, while promoting the formation of ThT-positive soluble oligomeric assemblies when judged by ThT fluorescence (Fig. 1A), TEM (Fig. 1C), SDS-PAGE and native-PAGE (Figs. 1E-F; middle panels). Note that such aggregation-modulatory effects of αS monomers were significantly weakened when αS concentrations dropped to 70 μM, as described above. Thus, αS oligomers appear to be more potent modulators than αS monomers, based on the relative αS concentrations of each required for these effects. The oligomeric band on the native-PAGE indicates the presence of each fluorophore at the same location (Fig. 1F; middle panel), which may result from co-assembly of Aβ and αS.
Aβ monomers and αS fibrils
To study the aggregation state-dependency of the modulatory effects of αS further, we examined whether αS fibrils also interfere with aggregation of Aβ monomers. When Aβ monomers were mixed with αS fibrils, ThT fluorescence and morphology were similar to those of the αS fibril only samples (Fig. 1A and Fig. 1D; compare to Fig. S7B). Similar to results with the Aβ monomer only samples, Aβ monomers in the mixture were lost to insoluble aggregates after the incubation period (Figs. 1E-F; right panels). A majority of αS remained insoluble in the presence of Aβ monomers (Fig. 1E; right panel).
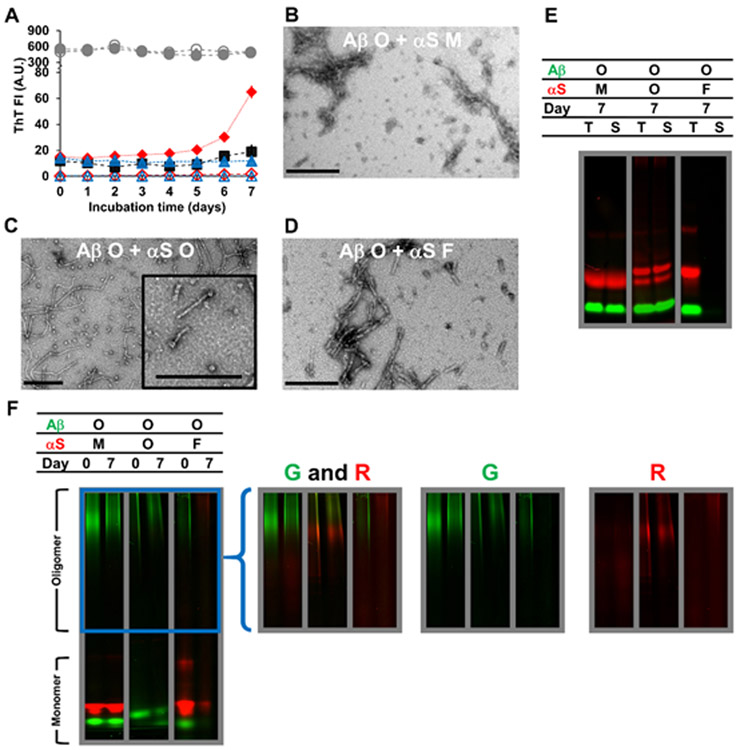
Aβ oligomers and αS monomers
The results described above demonstrate that soluble (i.e., monomeric and oligomeric) rather than fibrillar αS promotes oligomerization of Aβ monomers and prevents Aβ fibrillization. Next, we examined the effects of αS on pre-formed Aβ oligomers (Fig. 2A-F). During incubation, the mixtures of Aβ oligomers and αS monomers displayed a significant increase in ThT fluorescence, greater than the mathematical sum of Aβ and αS only samples (Fig. 2A). The result demonstrates interactions between the Aβ and αS species, which is also supported by TEM imaging, where it appears that protofibrils are surrounded by clumps of globular aggregates (Fig. 2B). SDS-PAGE and native-PAGE analyses revealed that αS monomers stabilized most soluble Aβ species (e.g., oligomers) and inhibited Aβ fibrilization (Fig. 2E-F; left panels). There was an overlap of oligomeric bands after 7-day incubation (Fig. 2F; left panel), which may result from co-assembly of Aβ and αS.
Figure 2.
Characterizations of Aβ oligomers (O) mixed with αS monomers (M), αS oligomers (O) or αS fibrils (F) incubated for 7 days at 37 °C, as examined by (A) ThT fluorescence, (B-D) TEM, and (E-F) in-gel fluorescence imaging of (E) SDS-PAGE and (F) native-PAGE. In (A-F), the concentration of Aβ oligomers was 70 μM. The concentrations of αS monomers and αS fibrils were 350 μM and that of αS oligomers was 17 μM. Concentrations of oligomers and fibrils were monomer-equivalent concentrations. In (A), the data on Aβ oligomers mixed with αS monomers (filled red diamonds), αS oligomers (filled blue triangles) and αS fibrils (filled gray circles) are shown along with those on αS monomers alone (empty red diamonds), αS oligomers alone (empty blue triangles) and αS fibrils alone (empty gray circles) taken from Figure S7A. The data on Aβ oligomers alone (filled black squares) taken from Figure S3A are also shown for comparison. Error bars: 1 standard deviation of triplicates. In (B-D), representative TEM images of mixtures containing (B) Aβ oligomers and αS monomers, (C) Aβ oligomers and αS oligomers and (D) Aβ oligomers and αS fibrils are shown with scale bars of 200 nm. In (E-F), samples contained HiLyte Fluor 488-labeled Aβ (green) and Alexa Fluor 647-labeled αS (red). Each panel was taken from a bigger gel image (see Figure S5) and reassembled for better presentation. In (E), T: total fraction and S: soluble fraction. In (F), the images in the right provide brighter upper portions of the native-PAGE gels – enclosed by a blue box in the left – obtained by renormalizing the brightness from the monomer to the oligomer bands, which often contained lower percentages of fluorophores. G: green channel and R: red channel.
Aβ oligomers and αS oligomers
When Aβ and αS oligomers were co-incubated at 70 and 17 μM, respectively, ThT fluorescence of the mixture samples remained relatively constant during 7-day incubation, in contrast to the 50% increase with the Aβ oligomer only samples (Fig. 2A). Thus, Aβ oligomers were stabilized by αS oligomers, which was confirmed with TEM images (Fig. 2C). This analysis revealed the presence of globular and annular oligomers (similar to αS oligomers shown in Fig. S7B), as well as protofibrils (similar to fresh Aβ oligomers shown in Fig. S3B, top middle panel) rather than mature fibrils. The formation of aggregates, presumably co-assemblies of Aβ and αS (see below), with hydrodynamic diameter being ~300 nm was detected after mixing samples of Aβ and αS oligomers (Fig. S6C). The mixture of Aβ and αS oligomers was A11-positive, similar to samples of Aβ oligomer only and αS oligomer only, implying that their conformations remained mostly intact upon mixing (Fig. S6D). Enhanced stability of Aβ oligomers in the presence of αS oligomers was also confirmed with in-gel fluorescence imaging of SDS-and native-PAGE (Fig. 2E-F; middle panels). On native-PAGE, Aβ and αS oligomer bands appeared highly overlapped (Fig. 2F; middle panel), which is likely due to co-assembly of the two species, possibly exemplified by a small fraction of (presumably Aβ) protofibrils being “capped” at one end with (presumably αS) globular or annular oligomers (Fig. 2C; inset). After the 7-day incubation of the Aβ and αS mixture, two bands were seen on the native-PAGE gel indicating non-oligomeric, low molecular weight Aβ species (Fig. 2F; lower portion of the middle panel). The two bands presumably reflect formation of two different monomeric Aβ conformers or the establishment of an Aβ monomer-dimer relationship, as suggested by others.63, 76
Aβ oligomers and αS fibrils
No noticeable effect on Aβ oligomers was observed when the samples were incubated with αS fibrils, similar to the results when Aβ monomers were mixed with αS fibrils, assessed by ThT fluorescence (Fig. 2A) and in-gel fluorescence imaging of SDS-PAGE and native-PAGE (Fig. 2E-F; right panels). Additionally, TEM of the mixture after the incubation shows fibrils (Fig. 2D), similar to αS fibrils in morphology (Fig. S7B). During incubation, most αS fibrils remained insoluble regardless of the presence or absence of Aβ (Fig. 2E; right panel).
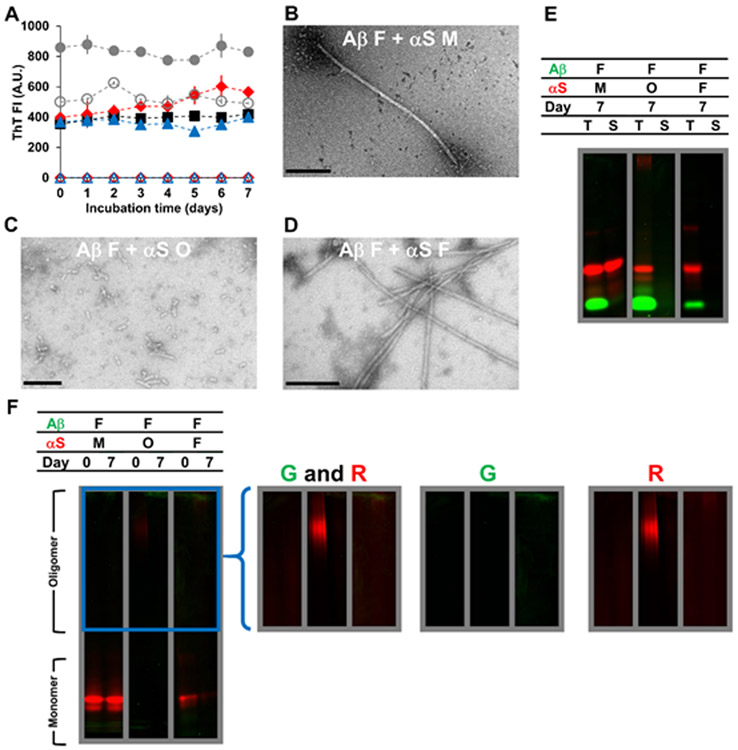
Aβ fibrils and αS in varying aggregation states
The effect of αS on preformed Aβ fibrils was also investigated (Fig. 3A-F). Upon addition of αS monomers (350 μM), oligomers (17 μM), and fibrils (350 μM), Aβ fibrils remained ThT-positive at a level of corresponding theoretical controls (i.e. a mathematical sum of fluorescence intensities of Aβ and αS; Fig. 3A) and insoluble without any significant dissociation (Fig. 3E-F). Fibrils were found in all the mixture samples though their morphologies appeared different depending on aggregation states of added αS (Fig. 3B-D). Clumping of non-fibrillar species at the tip of fibrils was observed when Aβ fibrils and αS monomers were mixed (Fig. 3B). Aggregates in samples of Aβ fibrils mixed with αS oligomers or fibrils were not similar in morphology to Aβ (Fig. 3C-D). This observation might reflect either αS-induced morphology changes and/or the low abundance of Aβ fibrils on TEM. Interestingly, unlike the αS oligomer only samples, αS oligomers were lost to insoluble aggregates in the presence of Aβ fibrils during the incubation (Fig. 3E-F; middle panel), suggesting that Aβ fibrils induced fibrillization of αS oligomers.
Figure 3.
Characterizations of Aβ fibrils (F) mixed with αS monomers (M), αS oligomers (O) or αS fibrils (F) incubated for 7 days at 37 °C, as examined by (A) ThT fluorescence, (B-D) TEM, and (E-F) in-gel fluorescence imaging of (E) SDS-PAGE and (F) native-PAGE. In (A-F), the concentration of Aβ fibrils was 70 μM. The concentrations of αS monomers and αS fibrils were 350 μM and that of αS oligomers was 17 μM. Concentrations of oligomers and fibrils were monomer-equivalent concentrations. In (A), the data on Aβ fibrils mixed with αS monomers (filled red diamonds), αS oligomers (filled blue triangles) and αS fibrils (filled gray circles) are shown along with those on αS monomers alone (empty red diamonds), αS oligomers alone (empty blue triangles) and αS fibrils alone (empty gray circles) taken from Figure S7A. The data on Aβ fibrils alone (filled black squares) taken from Figure S3A are also shown for comparison. Error bars: 1 standard deviation of triplicates. In (B-D), representative TEM images of mixtures containing (B) Aβ fibrils and αS monomers, (C) Aβ fibrils and αS oligomers and (D) Aβ fibrils and αS fibrils are shown with scale bars of 200 nm. In (E-F), samples contained HiLyte Fluor 488-labeled Aβ (green) and Alexa Fluor 647-labeled αS (red). Each panel was taken from a bigger gel image (see Figure S5) and reassembled for better presentation. In (E), T: total fraction and S: soluble fraction. In (F), the images in the right provide brighter upper portions of the native-PAGE gels – enclosed by a blue box in the left – obtained by renormalizing the brightness from the monomer to the oligomer bands, which often contained lower percentages of fluorophores. G: green channel and R: red channel.
Data on the overall impact of αS on Aβ aggregation are summarized in Table 1.
Binding between Aβ and αS
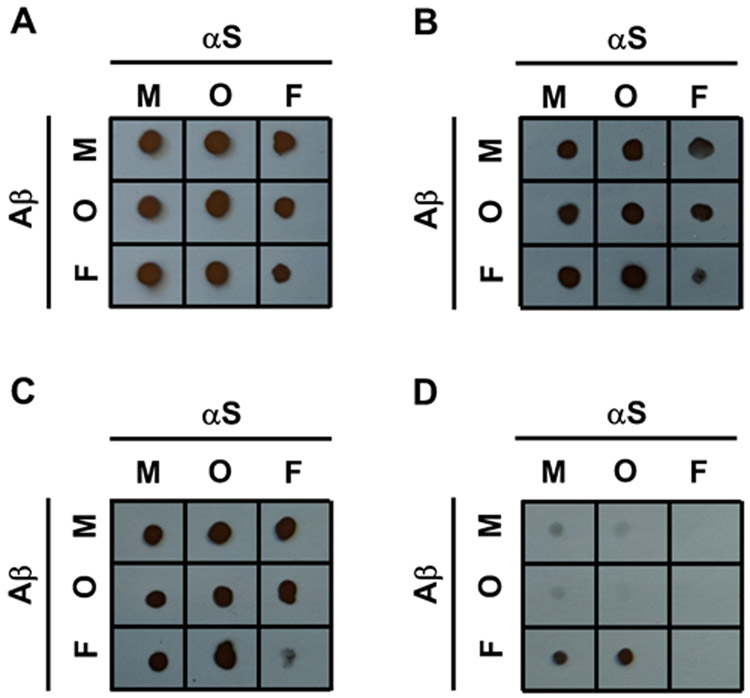
To decipher the Aβ region that might interact directly with αS, a competitive binding dot blot assay with Aβ sequence-specific antibodies was performed (see Figs. 4 and S10). In this experiment, Aβ was mixed with excess αS at a 1:10 ratio to ensure that most of the Aβ is bound to αS, if there is an interaction. Aβ, αS, and the mixtures of their respective aggregation states were dotted on membranes, and then incubated with Aβ sequence-specific antibodies to varying epitopes on Aβ. The panel of antibodies was carefully chosen to comprehensively evaluate parts of the Aβ sequence involved in αS binding, and included 6E10 (for Aβ1-16), 4G8 (for Aβ17-22), anti-Aβ (22-35) (for Aβ22-35) and 5C3 (for Aβ32-40). If αS binds to Aβ in a given location, the antibody will be hindered from binding, leading to a reduced or no signal on the membrane. As desired, the four antibodies were bound to Aβ, but not to αS (Fig. S10). While the antibody 4G8 was previously shown to bind to αS fibrils prepared by incubation of αS in water,77 no such binding was observed in our study, suggesting that the discrepancy might result from different incubation conditions to prepare αS fibrils.
Figure 4.
Competitive binding dot blot assay using Aβ sequence-specific antibodies of (A) 6E10 recognizing Aβ1-16, (B) 4G8 recognizing Aβ17-22, (C) Anti-Aβ (22-35) recognizing Aβ22-35, and (D) 5C3 recognizing Aβ32-40 of Aβ monomers (top row), Aβ oligomers (middle row), and Aβ fibrils (bottom row) mixed with αS monomers (left column), αS oligomers (middle column), and αS fibrils (right column). M, O and F represent monomers, oligomers and fibrils, respectively.
By analyzing the membranes, most of the interactions with αS were found to occur at Aβ32-40, which is the C-terminus of Aβ, rather than the other parts of Aβ (Fig. 4A-D). Specifically, our competitive binding assay results indicate that binding of antibody 5C3 to the C-terminus of Aβ in monomeric or oligomeric states was significantly weakened with αS monomers or αS oligomers present (see intersections between the first two columns and rows in Fig. 4D); in contrast, there was no such interference for 6E10, 4G8 and anti-Aβ (22-35) antibodies (see intersections between the first two columns and rows in Fig. 4A-C).
Thus, binding interactions occurring at the Aβ C-terminus mediate promotion of Aβ oligomerization and stabilization of Aβ oligomers by soluble αS (i.e., monomers and oligomers) as described above. The detected binding between Aβ and αS soluble species (i.e., monomers and oligomers) is consistent with a view that the overlapping oligomeric bands on native-PAGE (Figs. 1F and 2F; left and middle panels), another manifestation of the interactions, represent co-assembled oligomers consisting of Aβ and αS.
The competitive binding assay also provides evidence for binding of Aβ in all three aggregation forms to αS fibrils via the Aβ C-terminus (Fig. 4D). Note no notable change in ThT fluorescence and/or morphology of αS fibrils when mixed with Aβ monomers and oligomers (Figs. S7B, 1A, 1D, 2A and 2D). This is presumably because most αS molecules are buried inside in a fibrillar state, leaving only a small fraction available for interaction with Aβ soluble species. Interactions between Aβ and αS fibrils also occurs primarily via the Aβ C-terminus (Fig. 4D), but a part of Aβ22-35 is also likely involved, as judged by the competitive binding assay (Fig. 4C).
Interestingly, no direct binding between fibrillar Aβ and oligomeric αS species was detected in the competitive binding assay (Fig. 4), despite the observed facilitation of αS oligomer aggregation by Aβ fibrils (Fig. 3E-F; middle panel). One possible explanation for this seeming inconsistency is that interaction of αS oligomers with Aβ fibrils may occur across multiple Aβ molecules constituting fibrils, as is the case for other small Aβ fibril binders,78 rather than consecutive amino acids in a single Aβ chain. Alternatively, Aβ fibrils might induce conversion of αS oligomers to insoluble aggregates as a catalytic surface without strong binding, as they do with Aβ oligomers.79 Our observation that the Aβ C-terminus was primarily utilized for interaction between αS fibrils and soluble (i.e., monomeric and oligomeric) Aβ species, but not between Aβ fibrils and soluble αS species, might be related to a difference in cross-seeding effects of the two fibrils.51
We also carried out similar competitive binding assays with mixtures at a high concentration ratio of Aβ to αS using αS sequence-specific antibodies, which recognizes αS2-24, αS61-95, αS121-125 and αS112-140, respectively. Though no significant binding inhibition was observed from the experiments (data not shown), the study was limited by the rather long antibody epitopes or incomplete coverage of αS. We also attempted to examine interactions between soluble species of Aβ and αS using CD; however, the CD spectra of the mixtures were similar to sum of those for unmixed Aβ and αS with our incubation condition and concentrations (data not shown), and so were not analyzed further.
DISCUSSION
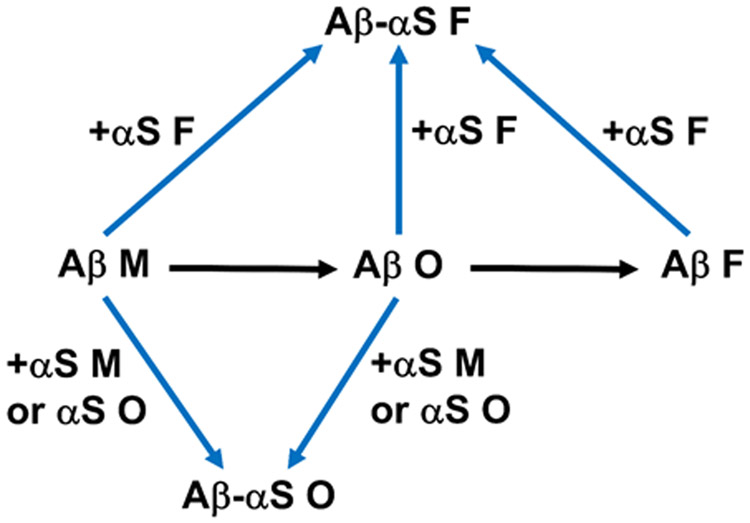
These studies show for the first time that soluble αS species, including αS monomers and oligomers, inhibit the fibrillization of Aβ monomers and oligomers. Additionally, the data indicate that the αS species promote Aβ oligomerization and stabilized pre-formed Aβ oligomers by co-assembly with Aβ. By contrast, when αS fibrils were mixed with the Aβ species, no such effect was realized. Indeed, no significant dissociation of Aβ fibrils to oligomers was observed in the presence of αS monomers and oligomers, indicating that the co-oligomerization requires both Aβ and αS to be soluble. Although our study does not provide direct evidence of strong binding between Aβ fibrils and αS soluble species (Fig. 4), we did find interactions between the two for αS oligomers (Fig. 3E-F; middle panels), and previous studies have provided evidence for αS monomers.51 Indeed, Aβ in all three forms, including fibrils, can be incorporated into αS fibrils, indicated by results from competitive binding immunoassays. It is interesting to note that incorporation of Aβ into αS fibrils and vice versa, when determined by ThT fluorescence, was relatively minor or slow compared to co-assembled oligomerization under most circumstances in our study. This finding may be associated with a difference in concentrations of monomeric units, depending on their aggregation states, exposed and available for the co-assembly. The implication is that cross-interaction might occur more drastically for formation of small, soluble rather than large, insoluble aggregates, as is the case with co-assembly of two different Aβ isoforms.80 Effects of αS on Aβ aggregation are summarized in Fig. 5.
Figure 5.
Summary of experimental findings described in this study. Aβ monomers self-assemble into Aβ oligomers, which then further aggregate to form Aβ fibrils. Addition of αS soluble species (i.e., monomers and oligomers) promotes oligomerization of Aβ monomers and stabilizes pre-formed Aβ oligomers via co-assembly. Monomeric, oligomeric and fibrillar forms of Aβ can be incorporated to αS fibrils. M, O and F represent monomers, oligomers and fibrils, respectively.
According to our competitive binding assay, interactions between Aβ and αS, including those inhibiting Aβ fibrilization, are mediated primarily through the C-terminus of Aβ. The result is consistent with previous data showing that soluble αS can bind to Aβ1-38, but not Aβ1-28.46 Evidence in the literature also suggests that the C-terminus is critical in fibrillization of Aβ and Aβ fragments.46, 52, 81 Our immunoassay data indicate the C-terminus is exposed in all three forms of Aβ for binding to antibodies (Fig. S10D), although possibly to different extents. The Aβ C-terminus may readily serve as the site for formation of fibrillar nuclei, as well as additional Aβ binding during fibrillization. Thus, αS binding at the C-terminus of Aβ may interfere with Aβ fibril formation. In addition, the Aβ C-terminus could be directly involved in αS binding, which leads to co-assembly into stable oligomers, likely those kinetically trapped from further aggregation into fibrils. While exact locations on αS for interaction with the Aβ C-terminus are currently unknown, the αS sites are likely to be hydrophobic given that the Aβ C-terminus contains primarily hydrophobic residues without any charged ones. Computational modeling has also identified hydrophobic interactions important in association between Aβ and αS.48 In addition, the Aβ C-terminus might interact with at least a portion of αS N- or C-termini,49 because these Aβ and αS fragments display high probabilistic sequence consistency.39 In particular, Aβ binding to the C-terminus of αS may have direct biological consequences in PD, as this αS region is a key domain in modulation of αS aggregation-related pathology.82 We did not attempt to determine Aβ binding sites on αS using αS fragments as competitive inhibitors because of previously reported differences between the molecular behavior of αS and its fragments on Aβ aggregation.52
The data reported here demonstrate that αS must be in a soluble form to exert modulatory effects on Aβ aggregation. Interestingly, αS oligomers appeared to be more effective modulators than were αS monomers. The difference may be, at least in part, of structural origin. For example, the Aβ and αS oligomers tested were β-sheet structured, whereas their monomers were structurally disordered (Fig. S4). As such, interaction with Aβ oligomers might be energetically favored for αS oligomers over αS monomers. Alternatively, the presence of multiple αS chains in close proximity in oligomeric states (i.e., multivalency) might make αS oligomers more effective than monomers for modulation of Aβ aggregation. Multivalent interactions collectively can be stronger than the corresponding monovalent interactions.83 In particular, multivalent rather than monovalent interactions should be preferred for binding to Aβ multimers, such as oligomers, due to the high degeneracy of bound states.83 While both Aβ and αS oligomers are rich in β-sheets, they differ in terms of ThT fluorescence. In the present studies, Aβ oligomers displayed ThT fluorescence intensity more than 10 times greater than αS oligomers, when the data (Figs. S3A and S7A) were normalized by monomer-equivalent concentration, suggesting a difference in local structure and amino acid composition of their ThT binding sites. Notably, ThT fluorescence of mixtures containing Aβ and αS oligomers remained at a similar level during incubation as fresh Aβ oligomers (Fig. 2A), suggesting that structural features of Aβ oligomers might be kept mostly intact upon interaction with αS oligomers, which was also supported by the A11 dot blot assay result (Fig. S6D).
Our results on Aβ-αS interactions has significant biological implications in the pathology of AD and PD as well as DLB and PDD, in which overlapping symptoms of AD and PD have been identified.32-34 In particular, promotion of Aβ oligomerization and stabilization of Aβ oligomers by soluble αS species – a synergistic mechanism to produce oligomeric agents – could have common biological consequences in AD, PD, DLB and PDD, given that oligomers of both species, particularly those rich in β-sheets, are neurotoxic.13, 16-18, 27-29 This mechanism could explain the observed acceleration of AD-related cognitive decline by αS without enhancing the extracellular deposition of Aβ fibrillar plaques.37 In particular, we show that αS oligomers at 17 μM (close to physiological concentrations) were effective for inducing Aβ to form oligomers in vitro, indicating that preformed αS oligomers might act as seeds to facilitate oligomerization of Aβ at low concentrations in vivo. Once formed, oligomers are kinetically stable even at sub-micromolar concentrations.63 Compared to individual oligomers, co-assembled oligomers may more readily escape from cellular clearance machinery by shielding proteolytic sites and, thus, make proteolysis less efficient.53 Assuming that co-assembled oligomers rather than fibrils are toxic, interactions between Aβ and αS may play either a toxic or protective role depending on their respective aggregation states before binding. Recent evidence supports both intracellular and extracellular Aβ-αS interactions.44 In particular, αS can be taken up by or secreted from both neuronal and non-neuronal cells.45 It would be interesting, therefore, to study whether αS soluble species might promote Aβ oligomerization, and also serve as a carrier for the resulting oligomers in a biological context. Due to concentrating effects,60 intracellular rather than extracellular Aβ-αS interactions (e.g., those in vesicles rather than extracellular fluid) can promote oligomerization more drastically, which may otherwise be kinetically and thermodynamically unfavorable at low concentrations.62 An additional study with Aβ and αS variants in the presence of membranes will further elucidate the impact of sequence variation and local environment on Aβ-αS interactions. Lastly, the pathological synergy between Aβ and αS could be applicable for other amyloid proteins, including tau and islet amyloid polypeptide, which are also associated with AD and PD,36, 84 thereby extending the potential impact of the interactions reported here.
Supplementary Material
ACKNOWLEDGMENT
The authors thank support from the NIH/NIA Grant R21AG049137 (J.R.K and M.E.R) and New York University (J.R.K and M.E.R).
Funding Sources
Research reported in this article was supported by the NIH/NIA Grant R21AG049137 (J.R.K and M.E.R) and New York University (J.R.K and M.E.R).
ABBREVIATIONS
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- αS
α-synuclein
- CD
circular dichroism
- CF
correction factor
- DLB
dementia with Lewy body disease
- DOL
degree of labeling
- HFIP
hexafluoroisopropanol
- IPTG
isopropyl-β-ᴅ−1-galactopyranoside
- LB
Lewy bodies
- NAC
non-amyloid component
- PAGE
polyacrylamide gel electrophoresis
- PBSA
phosphate buffered saline with azide
- PD
Parkinson’s disease
- PDD
Parkinson’s disease with dementia
- SDS
sodium dodecyl sulfate
- TEM
transmission electron microscopy
- ThT
Thioflavin T
Footnotes
Accession Codes. β-amyloid containing 40 residues (Aβ; NCBI Accession ID: 2M9S_A); α-synuclein (αS; UniProtKB ID: P37840)
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
REFERENCES
- (1).Dallaire-Theroux C, Callahan BL, Potvin O, Saikali S, and Duchesne S (2017) Radiological-Pathological Correlation in Alzheimer's Disease: Systematic Review of Antemortem Magnetic Resonance Imaging Findings. J. Alzheimers Dis 57, 575–601. [DOI] [PubMed] [Google Scholar]
- (2).Schirinzi T, Di Lorenzo F, Sancesario GM, Di Lazzaro G, Ponzo V, Pisani A, Mercuri NB, Koch G, and Martorana A (2018) Amyloid-Mediated Cholinergic Dysfunction in Motor Impairment Related to Alzheimer's Disease. J. Alzheimers Dis 64, 525–532. [DOI] [PubMed] [Google Scholar]
- (3).Aguzzi A, and O'Connor T (2010) Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov 9, 237–248. [DOI] [PubMed] [Google Scholar]
- (4).Hardy JA, and Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- (5).Christopeit T, Hortschansky P, Schroeckh V, Guhrs K, Zandomeneghi G, and Fandrich M (2005) Mutagenic analysis of the nucleation propensity of oxidized Alzheimer's beta-amyloid peptide. Protein Sci. 14, 2125–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bolognesi B, Kumita JR, Barros TP, Esbjorner EK, Luheshi LM, Crowther DC, Wilson MR, Dobson CM, Favrin G, and Yerbury JJ (2010) ANS binding reveals common features of cytotoxic amyloid species. ACS Chem. Biol 5, 735–740. [DOI] [PubMed] [Google Scholar]
- (7).Marshall KE, Marchante R, Xue W-F, and Serpell LC (2014) The relationship between amyloid structure and cytotoxicity. Prion 8, 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, and Tycko R (2002) A structural model for Alzheimer's beta -amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. USA 99, 16742–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hu Y, Su B, Kim CS, Hernandez M, Rostagno A, Ghiso J, and Kim JR (2010) A strategy for designing a peptide probe for detection of beta-amyloid oligomers. Chembiochem 11, 2409–2418. [DOI] [PubMed] [Google Scholar]
- (10).Garai K, and Frieden C (2013) Quantitative analysis of the time course of Abeta oligomerization and subsequent growth steps using tetramethylrhodamine-labeled Abeta. Proc. Natl. Acad. Sci. USA 110, 3321–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Huang D, Zimmerman MI, Martin PK, Nix AJ, Rosenberry TL, and Paravastu AK (2015) Antiparallel beta-Sheet Structure within the C-Terminal Region of 42-Residue Alzheimer's Amyloid-beta Peptides When They Form 150-kDa Oligomers. J. Mol. Biol 427, 2319–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Harper JD, Wong SS, Lieber CM, and Lansbury PT (1997) Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem. Biol 4, 119–125. [DOI] [PubMed] [Google Scholar]
- (13).Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, and Smith SO (2010) Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol 17, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Riek R, and Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539, 227–235. [DOI] [PubMed] [Google Scholar]
- (15).Bemporad F, and Chiti F (2012) Protein misfolded oligomers: experimental approaches, mechanism of formation, and structure-toxicity relationships. Chem. Biol 19, 315–327. [DOI] [PubMed] [Google Scholar]
- (16).Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, and Klein WL (2003) Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. USA 100, 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Haass C, and Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol 8, 101–112. [DOI] [PubMed] [Google Scholar]
- (18).Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, and Hillen H (2005) Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J. Neurochem 95, 834–847. [DOI] [PubMed] [Google Scholar]
- (19).Ladiwala AR, Dordick JS, and Tessier PM (2011) Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J. Biol. Chem. 286, 3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Surmeier DJ, Obeso JA, and Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci 18, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Johnson ME, Stecher B, Labrie V, Brundin L, and Brundin P (2019) Triggers, Facilitators, and Aggravators: Redefining Parkinson's Disease Pathogenesis. Trends Neurosci. 42, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Albin RL, Young AB, and Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. [DOI] [PubMed] [Google Scholar]
- (23).Wang W, Nguyen LT, Burlak C, Chegini F, Guo F, Chataway T, Ju S, Fisher OS, Miller DW, Datta D, Wu F, Wu CX, Landeru A, Wells JA, Cookson MR, Boxer MB, Thomas CJ, Gai WP, Ringe D, Petsko GA, and Hoang QQ (2016) Caspase-1 causes truncation and aggregation of the Parkinson's disease-associated protein alpha-synuclein. Proc. Natl. Acad. Sci. USA 113, 9587–9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Irwin DJ, Lee VM, and Trojanowski JQ (2013) Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci 14, 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Breydo L, Wu JW, and Uversky VN (2012) Alpha-synuclein misfolding and Parkinson's disease. Biochim. Biophys. Acta 1822, 261–285. [DOI] [PubMed] [Google Scholar]
- (26).Hernandez M, Golbert S, Zhang LG, and Kim JR (2011) Creation of aggregation-defective alpha-synuclein variants by engineering the sequence connecting beta-strand-forming domains. Chembiochem 12, 2630–2639. [DOI] [PubMed] [Google Scholar]
- (27).Giehm L, Svergun DI, Otzen DE, and Vestergaard B (2011) Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proc. Natl. Acad. Sci. USA 108, 3246–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kim HY, Cho MK, Kumar A, Maier E, Siebenhaar C, Becker S, Fernandez CO, Lashuel HA, Benz R, Lange A, and Zweckstetter M (2009) Structural properties of pore-forming oligomers of alpha-synuclein. J. Am. Chem. Soc 131, 17482–17489. [DOI] [PubMed] [Google Scholar]
- (29).Chen SW, Drakulic S, Deas E, Ouberai M, Aprile FA, Arranz R, Ness S, Roodveldt C, Guilliams T, De-Genst EJ, Klenerman D, Wood NW, Knowles TP, Alfonso C, Rivas G, Abramov AY, Valpuesta JM, Dobson CM, and Cremades N (2015) Structural characterization of toxic oligomers that are kinetically trapped during alpha-synuclein fibril formation. Proc. Natl. Acad. Sci. USA 112, E1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Snead D, and Eliezer D (2014) Alpha-synuclein function and dysfunction on cellular membranes. Exp. Neurobiol 23, 292–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Jucker M, and Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hamilton RL (2000) Lewy Bodies in Alzheimer's Disease: A Neuropathological Review of 145 Cases Using α-Synuclein Immunohistochemistry. Brain Pathol. 10, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, de Vos RAI, Wilcock GK, Jellinger KA, and Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 47, 1113–1124. [DOI] [PubMed] [Google Scholar]
- (34).Wirths O (2003) α-Synuclein, Aβ and Alzheimer's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 103–108. [DOI] [PubMed] [Google Scholar]
- (35).Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M, Rice V, Butters N, and Alford M (1990) The Lewy body variant of Alzheimer's disease: A clinical and pathologic entity. Neurology 40, 1–8. [DOI] [PubMed] [Google Scholar]
- (36).Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, and LaFerla FM (2010) Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci 30, 7281–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, and Mucke L (2001) beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc. Natl. Acad. Sci. USA 98, 12245–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Bate C, Gentleman S, and Williams A (2010) alpha-synuclein induced synapse damage is enhanced by amyloid-beta1-42. Mol. Neurodegener 5, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Luo J, Warmlander SK, Graslund A, and Abrahams JP (2016) Cross-interactions between the Alzheimer Disease Amyloid-beta Peptide and Other Amyloid Proteins: A Further Aspect of the Amyloid Cascade Hypothesis. J. Biol. Chem 291, 16485–16493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, and Mandal R (2006) Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochem. Res 31, 1153–1162. [DOI] [PubMed] [Google Scholar]
- (41).Lashley T, Holton JL, Gray E, Kirkham K, O'Sullivan SS, Hilbig A, Wood NW, Lees AJ, and Revesz T (2008) Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol. 115, 417–425. [DOI] [PubMed] [Google Scholar]
- (42).Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, and Saitoh T (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 90, 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Drummond E, Nayak S, Faustin A, Pires G, Hickman RA, Askenazi M, Cohen M, Haldiman T, Kim C, Han X, Shao Y, Safar JG, Ueberheide B, and Wisniewski T (2017) Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer's disease. Acta Neuropathol. 133, 933–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).LaFerla FM, Green KN, and Oddo S (2007) Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci 8, 499–509. [DOI] [PubMed] [Google Scholar]
- (45).Lee HJ, Patel S, and Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci 25, 6016–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Yoshimoto M, Iwai A, Kang D, Otero DA, Xia Y, and Saitoh T (1995) NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer disease amyloid, binds A beta and stimulates A beta aggregation. Proc. Natl. Acad. Sci. USA 92, 9141–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Atsmon-Raz Y, and Miller Y (2016) Non-Amyloid-beta Component of Human alpha-Synuclein Oligomers Induces Formation of New Abeta Oligomers: Insight into the Mechanisms That Link Parkinson's and Alzheimer's Diseases. ACS Chem. Neurosci 7, 46–55. [DOI] [PubMed] [Google Scholar]
- (48).Jose JC, Chatterjee P, and Sengupta N (2014) Cross dimerization of amyloid-beta and alphasynuclein proteins in aqueous environment: a molecular dynamics simulations study. PLoS One 9, e106883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Jensen PH, Hojrup P, Hager H, Nielsen MS, Jacobsen L, Olesen OF, Gliemann J, and Jakes R (1997) Binding of Abeta to alpha-and beta-synucleins: identification of segments in alpha-synuclein/NAC precursor that bind Abeta and NAC. Biochem. J 323, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, and Masliah E (2008) Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PLoS One 3, e3135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (51).Ono K, Takahashi R, Ikeda T, and Yamada M (2012) Cross-seeding effects of amyloid beta-protein and alpha-synuclein. J. Neurochem 122, 883–890. [DOI] [PubMed] [Google Scholar]
- (52).Paik SR, Lee J-H, Kim D-H, Chang C-S, and Kim Y-S (1998) Self-oligomerization of NACP, the precursor protein of the non-amyloid β/A4 protein (Aβ) component of Alzheimer's disease amyloid, observed in the presence of a C-terminal Aβ fragment (residues 25-35). FEBS Lett. 421, 73–76. [DOI] [PubMed] [Google Scholar]
- (53).Morales R, Moreno-Gonzalez I, and Soto C (2013) Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 9, e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chia S, Flagmeier P, Habchi J, Lattanzi V, Linse S, Dobson CM, Knowles TPJ, and Vendruscolo M (2017) Monomeric and fibrillar alpha-synuclein exert opposite effects on the catalytic cycle that promotes the proliferation of Abeta42 aggregates. Proc. Natl. Acad. Sci. USA 114, 8005–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Breydo L, and Uversky VN (2015) Structural, morphological, and functional diversity of amyloid oligomers. FEBS Lett. 589, 2640–2648. [DOI] [PubMed] [Google Scholar]
- (56).Hellstrom-Lindahl E, Viitanen M, and Marutle A (2009) Comparison of Abeta levels in the brain of familial and sporadic Alzheimer's disease. Neurochem. Int 55, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lippa SM, Lippa CF, and Mori H (2005) Alpha-Synuclein aggregation in pathological aging and Alzheimer's disease: the impact of beta-amyloid plaque level. Am. J. Alzheimers Dis. Other Demen 20, 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).LeVine H 3rd. (2004) Alzheimer's beta-peptide oligomer formation at physiologic concentrations. Anal. Biochem 335, 81–90. [DOI] [PubMed] [Google Scholar]
- (59).Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH, Lee S, Suh SW, and Suh YH (2002) Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 16, 1826–1828. [DOI] [PubMed] [Google Scholar]
- (60).Hu X, Crick SL, Bu G, Frieden C, Pappu RV, and Lee JM (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc. Natl. Acad. Sci. USA 106, 20324–20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schafer C, Rammner B, Koo SJ, Classen GA, Krauss M, Haucke V, Urlaub H, and Rizzoli SO (2014) Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028. [DOI] [PubMed] [Google Scholar]
- (62).Sengupta P, Garai K, Sahoo B, Shi Y, Callaway DJ, and Maiti S (2003) The amyloid beta peptide (Abeta(1-40)) is thermodynamically soluble at physiological concentrations. Biochemistry 42, 10506–10513. [DOI] [PubMed] [Google Scholar]
- (63).Nag S, Sarkar B, Bandyopadhyay A, Sahoo B, Sreenivasan VK, Kombrabail M, Muralidharan C, and Maiti S (2011) Nature of the amyloid-beta monomer and the monomer-oligomer equilibrium. J. Biol. Chem 286, 13827–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Munishkina LA, Cooper EM, Uversky VN, and Fink AL (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J. Mol. Recognit 17, 456–464. [DOI] [PubMed] [Google Scholar]
- (65).Castillo GM, Ngo C, Cummings J, Wight TN, and Snow AD (1997) Perlecan binds to the beta-amyloid proteins (A beta) of Alzheimer's disease, accelerates A beta fibril formation, and maintains A beta fibril stability. J. Neurochem 69, 2452–2465. [DOI] [PubMed] [Google Scholar]
- (66).Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, and Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, and Bateman RJ (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann. Neurol 76, 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Banerjee S, Hashemi M, Lv Z, Maity S, Rochet JC, and Lyubchenko YL (2017) A novel pathway for amyloids self-assembly in aggregates at nanomolar concentration mediated by the interaction with surfaces. Sci. Rep 7, 45592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Novo M, Freire S, and Al-Soufi W (2018) Critical aggregation concentration for the formation of early Amyloid-beta (1-42) oligomers. Sci. Rep 8, 1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Galvagnion C, Buell AK, Meisl G, Michaels TC, Vendruscolo M, Knowles TP, and Dobson CM (2015) Lipid vesicles trigger alpha-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol 11, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Owen MC, Gnutt D, Gao M, Warmlander S, Jarvet J, Graslund A, Winter R, Ebbinghaus S, and Strodel B (2019) Effects of in vivo conditions on amyloid aggregation. Chem. Soc. Rev 48, 3946–3996. [DOI] [PubMed] [Google Scholar]
- (72).Luk KC, Hyde EG, Trojanowski JQ, and Lee VM (2007) Sensitive fluorescence polarization technique for rapid screening of alpha-synuclein oligomerization/fibrillization inhibitors. Biochemistry 46, 12522–12529. [DOI] [PubMed] [Google Scholar]
- (73).Nath S, Meuvis J, Hendrix J, Carl SA, and Engelborghs Y (2010) Early aggregation steps in alpha-synuclein as measured by FCS and FRET: evidence for a contagious conformational change. Biophys. J 98, 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Hernandez M, Hu Y, and Kim JR (2013) A conformation-switching fluorescent protein probe for detection of alpha synuclein oligomers. Chem. Commun. (Camb) 49, 10712–10714. [DOI] [PubMed] [Google Scholar]
- (75).Hu Y, Zheng H, Su B, Hernandez M, and Kim JR (2012) Modulation of beta-amyloid aggregation by engineering the sequence connecting beta-strand forming domains. Biochim. Biophys. Acta 1824, 1069–1079. [DOI] [PubMed] [Google Scholar]
- (76).Garzon-Rodriguez W, Sepulveda-Becerra M, Milton S, and Glabe CG (1997) Soluble Amyloid Aβ-(1–40) Exists as a Stable Dimer at Low Concentrations. J. Biol. Chem 272, 21037–21044. [DOI] [PubMed] [Google Scholar]
- (77).Hatami A, Monjazeb S, and Glabe C (2016) The Anti-Amyloid-beta Monoclonal Antibody 4G8 Recognizes a Generic Sequence-Independent Epitope Associated with alpha-Synuclein and Islet Amyloid Polypeptide Amyloid Fibrils. J. Alzheimers Dis 50, 517–525. [DOI] [PubMed] [Google Scholar]
- (78).Wu C, Scott J, and Shea JE (2012) Binding of Congo red to amyloid protofibrils of the Alzheimer Abeta(9-40) peptide probed by molecular dynamics simulations. Biophys. J 103, 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Cohen SI, Arosio P, Presto J, Kurudenkandy FR, Biverstal H, Dolfe L, Dunning C, Yang X, Frohm B, Vendruscolo M, Johansson J, Dobson CM, Fisahn A, Knowles TP, and Linse S (2015) A molecular chaperone breaks the catalytic cycle that generates toxic Abeta oligomers. Nat. Struct. Mol. Biol 22, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Heo CE, Choi TS, and Kim HI (2018) Competitive homo- and hetero- self-assembly of amyloid- β 1–42 and 1–40 in the early stage of fibrillation. Int. J. Mass Spectrom 428, 15–21. [Google Scholar]
- (81).Misra P, Kodali R, Chemuru S, Kar K, and Wetzel R (2016) Rapid alpha-oligomer formation mediated by the Abeta C terminus initiates an amyloid assembly pathway. Nat. Commun 7, 12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Sorrentino ZA, Vijayaraghavan N, Gorion KM, Riffe CJ, Strang KH, Caldwell J, and Giasson BI (2018) Physiological C-terminal truncation of alpha-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem 293, 18914–18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Kitov PI, and Bundle DR (2003) On the nature of the multivalency effect: a thermodynamic model. Journal of the American Chemical Society 125, 16271–16284. [DOI] [PubMed] [Google Scholar]
- (84).Wijesekara N, Ahrens R, Sabale M, Wu L, Ha K, Verdile G, and Fraser PE (2017) Amyloid-beta and islet amyloid pathologies link Alzheimer's disease and type 2 diabetes in a transgenic model. FASEB J. 31, 5409–5418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.