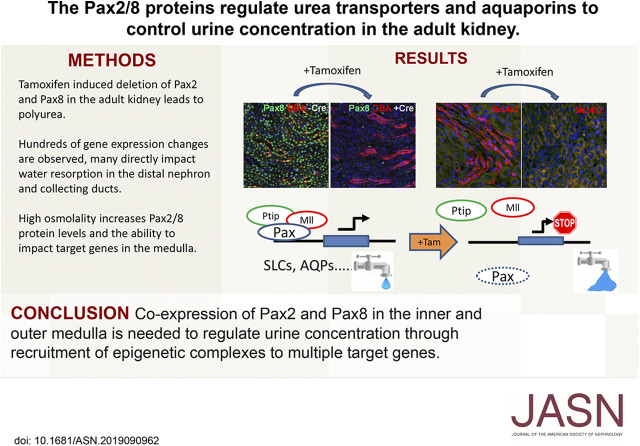
Significance Statement
Pax2 plays an essential role in kidney development, and although subsets of epithelial cells in the adult kidney continue to express Pax2 and the related Pax8 protein, their function in adult kidneys has not been defined. The authors examined phenotypes and altered gene expression patterns in adult mice lacking Pax2, Pax8, or both, showing that Pax2 and Pax8 regulate multiple transmembrane ion and water channels in the adult renal medulla, including aquaporins and urea transporters. Inner medullary collecting duct cells respond to high-salt levels by upregulating Pax8, leading to increased activation of such transporters through specific methylation of histones, defining a mechanism for regulating urine concentration. These findings point to a novel and redundant role for Pax proteins in regulating salt and water homeostasis in the adult kidney.
Keywords: Cell & Transport Physiology, diabetes insipidus, genetics and development, osmolality, renal epithelial cell, water channels
Visual Abstract
Abstract
Background
As the glomerular filtrate passes through the nephron and into the renal medulla, electrolytes, water, and urea are reabsorbed through the concerted actions of solute carrier channels and aquaporins at various positions along the nephron and in the outer and inner medulla. Proliferating stem cells expressing the nuclear transcription factor Pax2 give rise to renal epithelial cells. Pax2 expression ends once the epithelial cells differentiate into mature proximal and distal tubules, whereas expression of the related Pax8 protein continues. The collecting tubules and renal medulla are derived from Pax2-positive ureteric bud epithelia that continue to express Pax2 and Pax8 in adult kidneys. Despite the crucial role of Pax2 in renal development, functions for Pax2 or Pax8 in adult renal epithelia have not been established.
Methods
To examine the roles of Pax2 and Pax8 in the adult mouse kidney, we deleted either Pax2, Pax8, or both genes in adult mice and examined the resulting phenotypes and changes in gene expression patterns. We also explored the mechanism of Pax8-mediated activation of potential target genes in inner medullary collecting duct cells.
Results
Mice with induced deletions of both Pax2 and Pax8 exhibit severe polyuria that can be attributed to significant changes in the expression of solute carriers, such as the urea transporters encoded by Slc14a2, as well as aquaporins within the inner and outer medulla. Furthermore, Pax8 expression is induced by high-salt levels in collecting duct cells and activates the Slc14a2 gene by recruiting a histone methyltransferase complex to the promoter.
Conclusions
These data reveal novel functions for Pax proteins in adult renal epithelia that are essential for retaining water and concentrating urine.
The kidney regulates salt and water homeostasis and BP while continuously filtering waste products from the bloodstream into the urine. Reabsorption of water from the glomerular filtrate is essential for urine concentration and maintaining proper hydration in land-dwelling vertebrates. Failure to concentrate urine results in a diabetes insipidus phenotype characterized by excessive urination, electrolyte imbalance, volume depletion, and excessive thirst. The regulation of water reabsorption is controlled by families of aquaporins (AQPs) and urea transporters (UTs), located at various points along the nephron and the collecting ducts.1 AQP2 and Arginine Vasopressin receptor (AVPr) are among the most well studied proteins that regulate water reabsorption within the inner medullary collecting duct (IMCD) epithelia. UTs have also been implicated in urine concentration. The Tonicity enhancer binding protein TonEBP can regulate the expression of AQP2 and UTA1 (solute carrier family 14 member 2, Slc14a2) in response to water deprivation,2,3 but the mechanisms of action and its effects on other transporters remains unknown. In addition to TonEBP, nuclear transcription factors expressed in the renal medulla include the Pax2 and Pax8 proteins, whose roles in development are well characterized but whose functions in adult renal epithelia are unclear.
The paired-box containing Pax genes were first identified as regulators of segmentation in Drosophila, but have since been associated with mammalian development, congenital abnormalities, and cancer.4,5 The Pax2/5/8 subfamily of regulatory genes is expressed during embryonic development of a variety of tissues, including the kidney, the eye, the ear, the thyroid, the central nervous system, and B lymphocytes. Pax2, 5, and 8 proteins share an identical DNA-binding paired domain and have significant homology in the carboxy-terminal octapeptide, the partial homeodomain, and the activation domains, suggesting that all of these genes were derived from duplication of an ancestral precursor.6 Genetic mutations in mouse and humans indicate an essential requirement for Pax2 in the development of the kidney, the otic vesicle, and the optic nerve,7,8 whereas Pax8 is required for thyroid development,9 and Pax5 is required for B cell differentiation.10 Thus, although there is significant overlap in tissue-specific gene expression patterns, individual Pax2, 5, or 8 mutants have distinct phenotypes that reflect unique functions in a subset of tissues that express the proteins.
Kidney development is initiated by two cell types within the intermediate mesoderm, the metanephric mesenchyme and the ureteric bud epithelium, both of which express Pax2. The invasion of metanephric mesenchyme by the ureteric bud epithelia initiates an inductive interaction that promotes aggregation and proliferation of Six Homeobox 2–positive nephron progenitor cells around the ureteric bud tips.11,12 These nephron progenitors also express high levels of Pax2, which becomes downregulated as the glomerular, proximal tubule, and distal tubule epithelia matures. The differentiating renal epithelia now begins to express Pax8, which persists into adult kidneys. Pax2 germline-null mice have complete renal agenesis, as the initial inductive interactions required for kidney development never occur.7,13,14 However, Pax8 mutants do not have any overt renal phenotypes, presumably because of the redundancy with Pax2 at early times in development.15 In adults, Pax2 expression remains in the ureteric bud derived epithelia of the collecting ducts and the inner and outer medulla, whereas it is off in the proximal and distal tubules. Pax8 remains on in most all renal epithelial cells of the adult kidney. Although the necessity for Pax2 in development is well established, the need for continued expression of Pax2 or Pax8 in adult kidneys has not been examined.
In this report, we examined the functions of Pax2 and Pax8 in the adult mouse kidney using conditional mutant alleles and a tamoxifen-inducible Cre recombinase driver. Deletion of either Pax2 or Pax8 did not produce a discernible phenotype in healthy adult mice, whereas a double knockout (KO) of both Pax2 and Pax8 generated mice with severe polyuria and dehydration, consistent with the overlapping expression patterns of these two Pax proteins in the inner and outer renal medulla. The histology of the renal epithelium from cortex to papilla did not exhibit obvious structural abnormalities; however, gene expression analyses revealed significant differences in families of transporters needed for water reabsorption and urine concentration. Among the most affected proteins were AQPs and UTs, which were directly activated by Pax proteins in cell culture and contained Pax binding elements in the transcriptional promoter regions. Our data point to a novel and redundant role for Pax proteins in regulating salt and water homeostasis in the adult kidney.
Methods
Animals
The mouse embryonic stem cells carrying a conditional Pax2 floxed allele were obtained from the University of California, Davis Knockout Mouse Project repository (clone EPD0847_2_B03). Mice were rederived by blastocyst injection and germline transmitters were crossed to Flip recombinase expressing mice to delete the lacZ marker and the neomycin cassette. The Pax8 floxed allele was kindly provided by Dr. M. De Felice16 and deletes exons 3 and 4 of the paired domain to generate a null allele. The Rosa26-CreER allele was from The Jackson Laboratory (no. 008463, B6.129-Gt(ROSA)26Sortm(Cre.ERT2)TyiIJ). Mice were bred to homozygosity for Pax2 and Pax8 floxed alleles and were heterozygous for the CreER allele. For each experiment, cohorts of six adult mice at 8–10 weeks of age were injected intraperitoneally with 50 mg/kg of tamoxifen in corn oil for three consecutive days and again within 4 weeks. Both male and female mice were used and kept according to National Institutes of Health guidelines. For water and urine measurements, mice were housed blindly in metabolic cages for 24 hours at the Michigan Mouse Metabolic Phenotyping Center. All procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Antibodies
Rabbit antibodies to Pax2 and PTIP have been previously validated.17,18 Rabbit IgG (011–000–003) was from Jackson ImmunoResearch (West Grove, PA), anti–β-actin (A-1978) was from Sigma (St. Louis, MO), anti-H3K4me3 (ab8580) and anti-Pol II CTD (ab5408) were from Abcam (Cambridge, UK), anti–Ash2-like (Ash2L, A300–107A) and anti–retinoblastoma-binding protein 5 (RBBP5, A300–109A) were from Bethyl Laboratories (Montgomery, TX); and anti-Pax8 antibodies (sc-81353 mouse and sc-16279 goat) were from Santa Cruz Biotechnology (Dallas, TX). Antibodies used for immunostaining were Pax8, 1:300 (10336–1-AP; Proteintech, Rosemont IL); SLC14a2, 1:50 (ab95365; Abcam); AQP1, 1:100 (AQP-001; Alomone Labs, Jerusalem, Israel); AQP2, 1:100 (AQP-002; Alomone Labs); AQP3, 1:100 (AQP-003; Alomone Labs); and AQP4, 1:100 (AQP-004; Alomone Labs).
Gene Expression Analyses
Total RNA was extracted from three independent adult kidneys of each genotype using TRIzol reagent (Life Technologies) and RNeasy Mini Kit (Qiagen). RNAs were numbered and submitted blindly for microarray expression analysis by the University of Michigan Comprehensive Cancer Center Affymetrix and Microarray Core Facility using Affymetrix Mouse Gene 2.1 ST Arrays, as described.19 The microarray data were analyzed using Bioconductor packages in R version 3.4.0. Probe sets were limited to those defined as “main” by the Affymetrix. Robust multiarray average was used to fit log2 expression values.20 We further removed probe sets that had a variance across all samples <0.025 because they are, by definition, uninteresting to us and likely unexpressed genes in the tissue. We then used weighted21 linear models22 similar to fitting t-statistics to each probe set through the limma package of bioconductor. From there we selected probe sets with a ≥1.5-fold change and an adjusted P value of ≤0.05, using the Benjamini–Hochberg technique.23 Expression values for each gene was calculated using the robust multiarray average method and fitted to weighted linear models in R using the Affymetrix package of bioconductor.24 The complete excel file with all probe sets is available in Supplemental Table 1 and can be accessed at the Gene Expression Omnibus (accession number GSE141455). Gene Ontology and functional expression analyses of the genes whose expression were altered in Pax2-null embryos, compared with controls, was carried out using Toppgene.25 For transcription factor motif analyses, we utilized the gene to promoter function in the Genomatix Genome Analyzer (Genomatix Software GmbH) and extracted 1000 bp upstream and 100 bp downstream from all transcription start sites of the top 100 genes downregulated. These sequences were searched for common represented motifs using default parameter in an unbiased manner.
Immunostaining
After euthanasia with CO2, kidneys were collected, fixed in 4% formaldehyde or Carnoy fixative, paraffin embedded, and sectioned 5-µm thick. Slides were numbered and selected for similar areas of the kidney. Sections were permeabilized with TBST (5% Triton X-100 in Tris-buffered saline) and blocked with 1% BSA (50–109–0681; Proliant Biologicals, Boone, IA) in TBST. The primary antibodies, as listed above, were diluted in TBST and incubated overnight at 4°C. Secondary antibodies conjugated to Alexa Fluor 594 (A-11012 or R37121; Invitrogen, Carlsbad, CA) were diluted 1:500 in TBST with 1% horse serum containing fluorescein-labeled lotus tetragonolobus lectin (LTL) or dolichos biflorus agglutinin (DBA) at 1:500 (FL-1321 or FL-1031; Vector Laboratories, Burlingame, CA) and sections were incubated for 1 hour at room temperature. Nuclei were labeled with 4′6-diamidino-2- phenylindole (D1306; Life Technologies, Carlsbad, CA). Images were taken at 40×, 20×, or 10× on an Olympus IX73 inverted microscope fitted with an Olympus DP80 digital camera.
For frozen sections, tissues from adult animals were fixed with 4% PFA for 3 hours at 4°C, followed by sucrose substitution, and embedded in tissue-Tek O.C.T. compound (Sakura Finetek). Blocks were sectioned using a cryostat (6 µm). Sections were treated with 0.1% Triton X-100 for 10 minutes at room temperature, and subsequently blocked with 5% normal goat serum in PBS-0.05% Tween for 1 hour at room temperature.
Cell Culture and Transfection
HEK293 cells were cultured in DMEM (450 mg/dl glucose) supplemented with 10% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin in 5% CO2/95% air at 37°C. Then, 10-cm plates were transfected with 1–2 μg of DNA and 12 μl of Fugene 6 as described (Roche Molecular Biochemicals). At 40 hours after transfection, cells were harvested, and chromatin or whole-cell lysates were prepared as described.26,27 Mouse mIMCD3 cells (American Type Culture Collection, Manassas, VA) were cultured as recommended by the supplier. Cells were grown in 300 mOsm/kg control medium until confluent, and treated at various times (4–24 hours) with experimental medium hyperosmotic (500 mOsm/kg) by the addition of NaCl or urea.
Real-Time RT-PCR
RNA was prepared from cells using the TRIzol RNA Isolation system (Life Technologies). Briefly, 1 μg of total RNA was reverse-transcribed into complementary DNA using random hexamers and the SuperScript First Strand kit (Life Technologies) as described by the manufacturer. Complementary DNA was diluted 20-fold and quantitative real-time PCR performed with the iTaq SYBR Green master mix (Bio-Rad) in a 7900HT Real-Time PCR system (Applied Biosystems, Foster City, CA). Each reaction was performed in triplicate. β-Actin was used as an endogenous control to normalize values. Primers pairs for PCR for β-actin (Life Technologies), and AQPs and UTs are listed in Supplemental Table 2. At least one primer for Pax2 or Pax8 RT-PCR maps to the deleted domains in the mutants. Annealing temperature was 60°C. Results were analyzed with the Applied Biosystems Prism 7900 system software and mRNA expression levels were calculated using the ΔΔCT method, as performed previously.28 The normalized expression of each transcript in cells grown in 300 mOsm/kg media was set to 1.0 and expression in the other cells is relative to each respective control.
Chromatin Immunoprecipitation and Real-Time PCR
Mouse mIMCD3 cells in triplicate were treated with control media or media supplemented with NaCl to raise osmolality to 500 mOsm/kg for 24 hours. DNA was crosslinked for 10 minutes by the addition of formaldehyde to a final concentration of 1%, followed by quenching in 0.125 M glycine for 5 minutes. Chromatin was prepared and chromatin immunoprecipitation (ChIP) was performed as described previously.27,28 The precipitated DNA was reconstituted in sterile water and real-time PCR quantitation of precipitated genomic DNA relative to inputs was performed in triplicate using IQ SYBR GREEN with ROX mastermix (Bio-Rad) in a Prism 7900 Sequence Detection system (Applied Biosystems). Eleven primer sets within the SLC14A2 gene, spanning 2 kb upstream of ATG to 1 kb downstream, were used (Supplemental Table 3). ChIP data are presented as percent input after subtraction of nonspecific binding to rabbit IgG. In all experiments, nonspecific binding was <0.005% of input.
Results
Generation of Pax2 and Pax8 KOs in Adult Kidneys
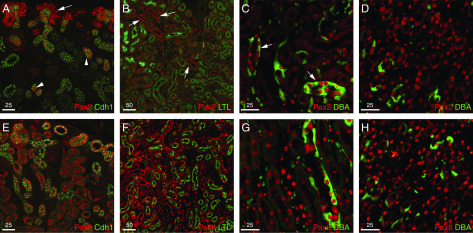
Although Pax2 expression in the kidney has been well described in development, a direct comparison of Pax2 and Pax8 in adults was lacking. Thus, we compared the expression of Pax2 and Pax8 in newborn, developing kidneys and in 8-week-old adults (Figure 1). As published previously, Pax2 protein expression is easily detected in the nephrogenic zone, in Six Homeobox 2–positive progenitor cells, also known as cap mesenchyme, surrounding the Pax2-positive ureteric bud tips (Figure 1A). As the nephron develops further, Pax2 is downregulated in more proximal and distal, but remains on in the ureteric bud–derived collecting ducts. In contrast, nuclear Pax8 protein is observed in tubular epithelial cells in the kidneys of newborn mice (Figure 1E). In adult kidneys, Pax2 is still easily detected in the DBA-positive cortical collecting ducts, and in the outer and inner medulla, where the collecting ducts converge to form the renal papilla (Figure 1, B–D). Nuclear Pax2 protein is low to undetectable in the LTL-positive proximal tubules and in the parietal epithelia of the glomeruli. Pax8 continues to be expressed in the LTL-positive proximal tubules as well as the DBA lectin–positive collecting ducts and the renal papilla (Figure 1, F–H). Thus, Pax2 and Pax8 are coexpressed in the nuclei of collecting ducts and in the inner and outer medulla, suggesting some potential for redundancy, whereas only Pax8 remains highly expressed in proximal tubules.
Figure 1.
Expression of Pax2 and Pax8 in newborn and adult kidneys. Immunostaining for Pax2 (red [A–D]) and Pax8 (red [E and F]) is shown with either cadherin1 (Cdh1), LTL, or DBA in green. Newborn kidneys show strong Pax2 in the nephrogenic zone ([A] arrows) and in collecting ducts (arrowheads), whereas Pax8 is in all proximal, distal, and collecting tubules (E). The adult cortex is negative for Pax2 in LTL-positive proximal tubules (B) but remains Pax2-positive in LTL-negative collecting ducts ([B] arrow), whereas LTL-positive epithelia exhibit strong Pax8 expression (F). The outer medulla shows strong Pax2 in DBA-positive collecting ducts ([C] arrows), whereas Pax8 is strong in DBA-positive and DBA-negative epithelial cells of the outer medulla (G). Cells in the renal papilla show strong Pax2-positive (D) and Pax8-positive (H) nuclei in all epithelial nuclei.
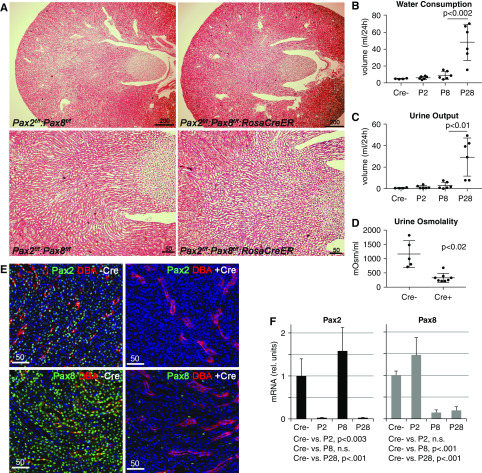
To study the function of Pax2 and Pax8 in adult kidneys, we obtained embryonic stem cells carrying a conditional floxed alleles of Pax2 from the University of California, Davis Knockout Mouse Project repository29 and rederived germline chimeras. The LacZ and Neo cassettes were then deleted by crossing mice to a Flip recombinase expressing strain. Activation of Cre recombinase will delete exons 2 and 3 of the Pax2 mRNA, which encodes most of the DNA-binding paired domain. The Pax8 floxed allele was obtained from Marotta et al.16 and deletes exons 3 and 4 of the DNA-binding paired domain, but leaves the rest of the mRNA intact. We utilized a Rosa26-CreEr driver to delete the Pax2 and Pax8 conditional floxed alleles after tamoxifen injection. Given the potential for redundancy between Pax2 and Pax8, we first deleted the genes individually and also generated double mutants that were Pax2fl/fl:Pax8fl/fl:Rosa26CreER with tamoxifen at 8–10 weeks of age. Control cohorts also receive tamoxifen but did not carry the Rosa26CreER allele. Adult mice that had deleted either Pax2 or Pax8 appeared healthy; however, within days of receiving tamoxifen, the Pax2/8 double mutants (KOs) exhibit severe polyuria, as evidenced by excessive urination and thirst. Histologic sections did not reveal any remarkable gross abnormalities in the Pax2/8 KOs (Figure 2A), with intact epithelial tubules in the cortex and medulla and no evidence of fibrosis or other pathologies. Slight increase in the dilation of IMCDs could be observed and likely reflects the increased urine output measured in the Pax2/8 KOs. Additional representative histologic sections and higher-powered magnifications are shown in the supplemental data (Supplemental Figure 1, A and B). Water consumption and urine excretion was measured overnight in metabolic cages for controls, single Pax2 or Pax8 KOs, and Pax2/8 double KOs (Figure 2, B and C). Only the Pax2/8 KOs exhibited a significant five- to ten-fold increase in water consumption and a ten-fold increase in urine production compared with controls or single mutants. Urine osmolality was also significantly reduced in the Pax2/8 KOs, indicating a severe urine concentration defect (Figure 2D).
Figure 2.
Gross phenotypes of Pax2/8 double mutant kidneys. (A) Histology of adult kidneys with the indicated genotypes after tamoxifen administration shows no major structural abnormalities of pathology. Slight dilation of IMCDs is seen in the Pax2/8 KOs (right lower panel), which may reflect increased urine output. (B) Water consumption as measured in single-housed metabolic cages of mice with no Pax2/8 deletion (Cre−) or deletions of either Pax2 (P2), Pax8 (P8), or both genes (P28). A significant increase in water consumption was observed in Pax2/8 double KOs (*P<0.001; n=6 per genotype). (C) Urine outputs as collected from metabolic cages over 24-hour periods shows significant increase in urine excretion only in Pax2/8 double KOs (*P<0.001; n=6 per genotype). (D) Osmolality was measured in urines from Pax2/8 double KOs (Cre+) and controls (Cre−). (E) Immunostaining for Pax2 or Pax8 (green) in kidneys from Pax2fl/fl;Pax8fl/fl mice with or without RosaCreEr after tamoxifen administration. (F) Quantitative RT-PCR for Pax2 or Pax8 mRNA from three total kidney RNAs per cohort after tamoxifen treatment of control mice (Cre−), Pax2, Pax8, or Pax2/8 double KOs.
Because Cre recombination only deletes selected exons, we also examined the efficiency of Cre-Lox–mediated Pax gene excision by immunostaining for Pax2 or Pax8 in kidneys from mice carrying both floxed alleles with or without tamoxifen (Figure 2E). Nuclear Pax2 or Pax8 staining was nearly completely absent throughout the kidneys of mice carrying all four floxed alleles when given tamoxifen. Using primers that map to the deleted domains, quantitative RT-PCR for Pax2 or Pax8 in controls, single, and double KOs also confirmed the efficient deletion of Pax2 and/or Pax8 mRNAs (Figure 2F). Thus, although the ROSA26Cre driver is reported to exhibit some leakiness, varied efficiency, and potential off-target effects, the fact that our single Pax2 or Pax8 mutants did not show a robust phenotype upon Cre activation indicates that the double Pax2/8 KO phenotypes were specific and not attributable to off-target effects.
Gene Expression Analyses in Pax2/8 Double KOs
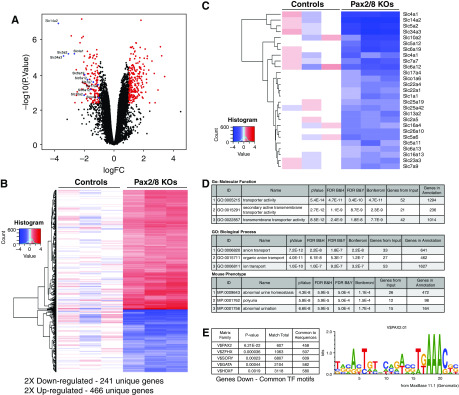
To more precisely define the genes and proteins affected in the Pax2//8 KOs, we isolated RNA from whole kidneys and compared relative gene expression by Affymetrix microarrays (Figure 3). The complete data set is available from the Gene Expression Omnibus (accession number GSE141455). Principal component analyses showed good separation for controls and Pax2/8 KOs, with >680 genes significantly altered at least two-fold (Figure 3, A and B, Supplemental Table 1). Among the genes whose levels were significantly reduced in the Pax2/8 KOs were the solute carriers (Slcs; Figure 3C), which encode transmembrane ion and UTs. Gene Ontology analyses confirmed that the number one molecular function downregulated in Pax2/8 KOs was ion transport, with abnormal urine homeostasis or polyuria the most significant phenotypic correlation. For the top 100 genes downregulated in Pax2/8 mutants, we used Genomatix software to examine transcription start sites for common transcription factor binding motifs and found the number one motif was the Pax2/5/8 consensus binding site (V$Pax2; P<6.21E-22). The second most common identified motif was for the zinc finger homeobox family of proteins (V$ZFHX), yet this motif had a much lower statistical P value of 3.6E-5. For genes whose expression was higher in the Pax2/8 KOs, no correlation with polyuria or transmembrane ion channels was observed, nor did the transcription start sites for genes upregulated contain significant Pax binding motifs. These data suggest that many of the affected transmembrane ion channels whose expression is reduced may be direct targets activated by Pax2, Pax8, or most likely both proteins, because they share an identical DNA-binding paired domain. Note that the Affymetrix probe set for Pax8 still hybridized to the remaining mRNA sequences downstream of the deletion encompassing exons 3 and 4. Also of note, there were no changes in the expression levels of arginine vasopressin (Avp), the receptors Avpr1a and Avpr1b, or the AVP-induced gene Avpi1 in kidney RNAs isolated from control or Pax2/8 double KOs (Supplemental Table 3). However, we did see a two-fold decrease in the Avpr2 receptor upon loss of Pax2 and Pax8.
Figure 3.
Gene expression analysis of Pax2/8 mutant kidneys. (A) Volcano plot of Affymetrix gene array comparisons between control (n=3) and Pax2/8 KOs (n=3) using total kidney RNA. (B) Heat map and clustering of 241 genes downregulated (blue) and 466 genes upregulated (red) in Pax2/8 KOs. Gene symbols and fold change are available in Supplemental Table 1. (C) Heat map of genes encoding the solute carrier family of transmembrane transporters that are downregulated in Pax2/8 KOs. (D) Gene Ontology analyses of genes downregulated in Pax2/8 KOs, using the Toppgene suite.25 The gene list reduced in KOs shows strong correlation with ion transporters and urine concentration. (E) Common transcription factor motifs (TFs) found in the genes downregulated in Pax2/8 KOs and in genes upregulated in Pax2/8 KOs. The top 5 TFs are shown, the most significant by far being the Pax2 family found in genes downregulated. The Pax2 DNA-binding matrix common to most genes downregulated in the mutants is shown. V$IRFF, interferon regulatory factors; V$MYT1, C2H2 zinc finger proteins; V$SORY, Sox/Sry HMG Box proteins; V$SP1F, GC-Box factor Sp1; V$ZFHX, Zinc Finger homeodomain.
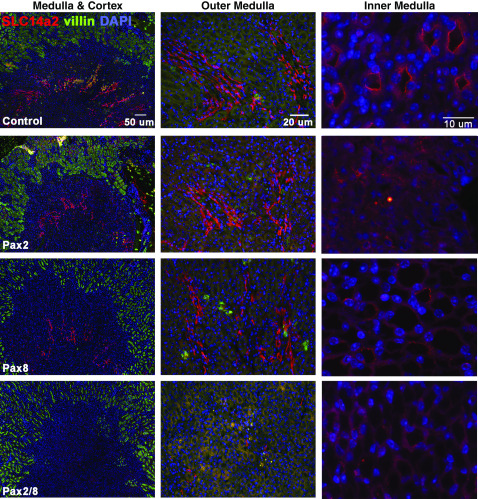
To confirm some of the observations obtained from differential gene expression analyses, we performed quantitative RT-PCR for several genes and immunostaining for Slc14a2 and selected AQPs. Affymetrix arrays tend to underestimate the fold change, as confirmed by quantitative RT-PCR for genes using total RNAs isolated from the Pax2/8 double KOs and single mutants (Supplemental Figure 2). The Slc14a2 gene encodes multiple splice forms that generate different UT proteins. The SLc14a2 antibody (ab95365) recognizes the carboxy-terminal exon that is common to UTA1 and UTA2 proteins encoded by Slc14a2 (Figure 4). In control animals, strong Slc14a2 staining is seen in thin epithelial tubules of the inner medulla, which is likely to represent UTA2 in the thin descending limb. Weaker staining was observed along the apical surface of cells in the inner medulla, which would likely correspond to UTA1. The single Pax2 and Pax8 KOs exhibited similar UTA2 staining as controls in the outer medulla, but had reduced apical staining in the inner medulla (Figure 4). The Pax2/8 double KOs did not show UTA2 staining in the outer medulla and had no detectable UTA1 staining in the inner medulla, consistent with the polyuria phenotype observed.
Figure 4.
Regulation of UTA1 and UTA2 in Pax2/8 mutants. Adult kidney sections from control mice or mice carrying floxed alleles of either Pax2, Pax8, or Pax2 and Pax8 after tamoxifen treatment were immunostained with antibodies against Slc14a2 (red) or the brush border protein villin (green). Low-power images on the left show staining in the outer medulla (UTA2) of controls and single mutants, but not in the Pax2/8 double KOs. Villin marks the proximal tubule rich cortex. Higher-powered images through the inner medulla are shown on the right, with little detectable apical UTA1 in the Pax2/8 double KOs. Representative sections from three independently derived kidneys are shown for each genotype.
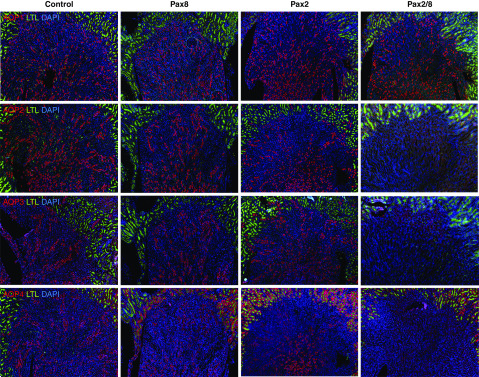
We also observed reduction in expression of select AQP proteins in the renal medulla and in subsets of tubules (Figure 5). The Pax2/8 double KOs had a clear reduction of AQPs 2–4 expression in the medulla, whereas AQP1 was not affected. Additional staining for AQPs 1–4 confirmed localization of AQP1 and AQP2 to apical surfaces in controls, whereas Pax2/8 double KOs had a complete loss of AQP2 staining in the inner and outer medulla (Supplemental Figure 3). However, AQP3 and AQP4 could still be detected on apical surfaces of inner medullary epithelial cells in Pax2/8 double KOs, although this was clearly reduced compared with the controls (Supplemental Figure 4). Nevertheless, the clear reduction of AQPs 2–4 and UTA1 and UTA2 implicates these proteins in the urine concentration defect.
Figure 5.
Differential regulation of AQPs in Pax mutants. Immunostaining for AQPs 1–4 as indicated (red) and LTL (green) in adult kidneys from mice with the indicated Pax gene deletions. Expression of AQP1 protein appears unaffected in all mutants, whereas Pax2/8 double KOs exhibit decreased expression of AQPs 2–4 in the inner and outer medulla. Representative sections are shown from immunostainings done with three independent kidneys for each genotype.
Mechanism of Osmoregulation by Pax8 in IMCD Cells
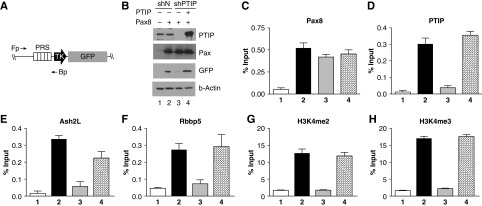
The Pax2 and Pax8 proteins have identical DNA-binding paired domains and show significant homology within the carboxy terminus. Genetic studies also suggest that the Pax2/5/8 family have redundancy in functions and can, at least in part, substitute for one another.30 Previous work indicated Pax2 recruits the adaptor protein PTIP, which is part of an Mll3/4 histone methyltransferase complex.27 Given the widespread expression of Pax8 in adult renal epithelia, it was critical to determine whether Pax8 functions similarly to Pax2. Thus, we transfected Pax8 into HEK293 cells that carried an integrated Pax responsive GFP reporter gene. Because HEK293 cells express neither Pax2 or Pax8, this enabled us to examine precisely what happens at the Pax-responsive element that binds the paired domain (Figure 6). We examined expression of the reporter in the presence or absence of Pax8 and PTIP, using short hairpin RNAs against PTIP. ChIP revealed that Pax8 can bind to the PRS sequence regardless of PTIP. However, PTIP was necessary to recruit elements of the MLL complex (Ash2L and Rbbp5) and for high levels of H3K4 di- and trimethylation (Figure 6, C–H). Thus, Pax8-mediated reporter gene activation was very similar to that described for Pax2, consistent with the conservation of the DNA-binding and transactivation domains.
Figure 6.
Mechanism of Pax8-activated gene expression. Human HEK293 cells carrying an integrated Pax reporter gene (A) driven by Pax Responsive Sequences (PRS) were used to characterize Pax8-mediated transcription activation. (B) Cells were treated with negative control short hairpin RNA(shN) or PTIP KO short hairpin RNA (shPTIP) and transfected with Pax8 or an shPTIP-resistant rescue plasmid (lane 4). Note that PTIP KOs fail to activate the GFP reporter gene in the presence of Pax8. (C–H) Chromatin from cells transfected as in (B) was immunoprecipitated with antibodies against the indicated proteins. Quantitative PCR was done with primers against the PRS sequence to determine protein occupancy. Note that Pax8 binds regardless of PTIP KOs. However, Pax8 recruits PTIP and components of the Mll3/4 complex (Ash2L and Rbbp5) to imprint high levels of H3K4me2 and H3K4me3. All ChIP assays were performed in triplicate, with error bars representing one SD from the mean.
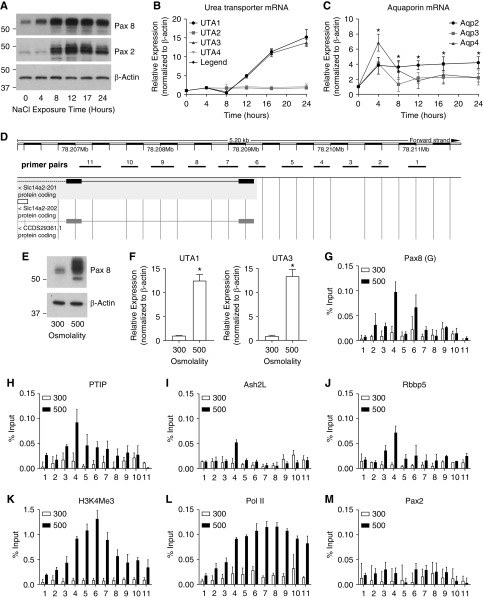
For a more physiologically relevant cell culture model, we next utilized IMCD cells that do express both Pax2 and Pax8. Prior work suggested that Pax2 was necessary to protect IMCD cells from the high-osmolality environment that these cells are exposed to during urine concentration.31 We noted that culturing mouse IMCD cells in 500 mM NaCl strongly increased the amounts of Pax2 and Pax8 proteins within 4–8 hours (Figure 7A). High-salt conditions also increased the expression of UT mRNAs UTA1 and UTA3 more than ten-fold after 16 hours (Figure 7B). However, AQPs 2–4 increased approximately four-fold within 4 hours after high-salt culture (Figure 7C), which preceded the strong increase of Pax2 and Pax8 mRNA and suggests activation by alternative mechanisms. Transfection of Pax8 into IMCD cells under normal salt conditions also strongly activated the UTA1 and UTA3 mRNAs, but not the AQPs (Supplemental Figure 5), again suggesting an alternate mechanism of activation in response to high-salt conditions in this mouse cell line. Surprisingly, Pax2 did not activate the UTA1 and UTA3 mRNAs in IMCD cells (Supplemental Figure 5), which suggests the Pax2 and 8 proteins are not entirely redundant.
Figure 7.
Pax8-dependent osmoregulation of Slc14a2. (A) Protein lysates from IMCD cells cultured with 500 mM NaCl were isolated at various times after salt addition and blotted for Pax8 and Pax2. (B) Expression of UTs was measured post salt addition. (C) As in (B), AQP mRNAs were measured in response to high-salt addition in IMCD cells. (D) A schematic of exons 1 and 2 of the Slc14a2 gene on mouse chromosome 18 is shown with the position of primer pairs used for ChIP indicated. (E and F) IMCD cells cultured under 300 or 500 mM NaCl show increase in Pax8 protein and increased UTA1 and UTA3 expression (*P<0.01). (G–M) ChIP experiments using chromatin form IMCD cells cultured with 300 or 500 mM NaCl and immunoprecipitated with the indicated antibodies. Note binding of Pax8 near primer pairs 4 and 6, the recruitment of PTIP, Ash2L, and Rbbp5, and the increase in H3K4me2 and RNA Pol II. ChIP PCR reactions were run in triplicate for two independent chromatin sets. Error bars are one SD from the mean.
We next examined the promoter region of Slc14a2 around the transcription start site for UTA1 and UTA3 (Figure 7D) by ChIP, using IMCD cells cultured under normal and high-salt conditions (Figure 7, E–M). Primer pairs spanning the Slc14a2 transcriptional start site were tested for binding to various proteins of interest. As predicted, 500 mM NaCl strongly activated Pax8 above baseline and resulted in increased UTA1 and UTA3 expression (Figure 7, E and F). ChIP experiments showed increased Pax8 binding to primer pair 4 and 6 under high-salt conditions (Figure 7G). Furthermore, increased Pax8 expression resulted in the recruitment of PTIP, Ash2L, and Rbbp5, which led to increased H3K4me3 and RNA Pol II (Figure 7, H–L). However, we did not detect Pax2 binding by ChIP to the Slc14a2 promoter in this mIMCD cell line, consistent with the inability of Pax2 to activate Slc14a2 by transfection. Taken together, the data show that Pax8 directly binds to the promoter region of Slc14a2 and regulates expression in response to high-salt conditions to maintain salt and water homeostasis.
Discussion
In this report, we demonstrate overlapping expression of Pax2 and Pax8 within the cortical collecting ducts and in the inner and outer medulla of the adult kidney. Given the identical DNA-binding paired domains and significant homologies throughout the carboxy-terminal transactivation domain, we proposed that these two proteins have similar biochemical properties and functions. This was confirmed in cell culture models and genetically by creating adult loss-of-function mutations with conditional alleles and tamoxifen-induced Cre recombinase in mice. The most striking phenotype was the inability to concentrate urine, resulting in a diabetes insipidus–like phenotype in the Pax2/8 double KOs. Nephrogenic diabetes insipidus (NDI) is characterized by the insensitivity of the water channel AQP2 to the antidiuretic hormone Avp in the collecting ducts. Upon water deprivation, vasopressin promotes post-translational modifications and translocation of AQP2 to the apical membrane of principal cells to promote osmotic reabsorption of water.32,33 Congenital NDI is often because of mutations in the AVPR or AQP2 genes and can be managed.34 Coincidentally, Avpr2 is downregulated more than two-fold in the Pax2/8 double KOs (see Supplemental Table 3), but RNA levels of Avp or the Avpr1a and Avpr1b receptors are unchanged. Thus, the loss of AQP and UT protein expression is most likely not because of a lack of Avp signaling.
NDI is also associated with lithium use,35 routinely prescribed and effective for bipolar disorders. Lithium is also a potent inhibitor of GSK3, which activates NFAT5 (TonEBP), a positive regulator of UTs and AQPs.2,3 More recently, molecular changes linked to lithium use have been described, which include cell cycle progression arrest, altered inositol and PG signaling,36 and increased NF-κB signaling.37 The Pax2/8 KO mice also exhibit an increase in NF-κB target transcripts, such as Ccl2, 12, 17, and Cxcl10, suggesting that NF-κB signaling may be upregulated in our mutants. There is an increase in Nfkb2 (NF-κB, subunit 2) but not in Nfkb1 in the Pax2/8 double KOs. NF-κB can bind to and repress the AQP2 promoter,38 which could contribute to the loss of AQP2 expression, but seems unlikely to be the sole reason. The data with Pax2/8 double mutants show an absence or reduction of AQP2–4 proteins in the outer and medulla, which had not been previously linked to Pax proteins. Thus, in addition to defects in apical localization, the AQPs may thus be affected by mutations or loss of Pax transcription factors.
The single Pax2 or Pax8 KOs appeared generally healthy, although the Pax8 KOs did show slight increases in water consumption and urine output, which did not approach statistical significance. Because the single mutants were subject to tamoxifen-induced Cre recombinase activation, they also served as controls for nonspecific effects of the recombinase compared with the double KOs. Hundreds of genes were altered in the Pax2/8 double KOs, many of which were transmembrane ion transporters or channels. Immunostaining of control and mutant sections confirmed the reduced levels of the UT UTA1, whose loss had been previously correlated with urine concentration defects.39 We also noted the loss of select AQPs 2–4, but surprisingly not AQP1. Of these, mutations in the vasopressin regulated AQP2 water channel have the most severe urinary concentration defect.40 However, mice carrying AQP3 mutations survive but also have increased fluid consumption, decreased urine osmolality, and could respond to vasopressin.41
In addition to the downregulation of many solute carriers, loss of Pax2/8 increases expression of cell cycle control genes, such as cyclins A2, B1, B2, and G1, and cell cycle checkpoint kinases including Cdk1, Bub1 and 1b, Chek1, and Plk1. Furthermore, transcripts for proliferation markers such as MKI67 and Sox9 are also increased. Because renal proliferation is rare in normal kidneys,42 it is possible the cells may be dedifferentiating or responding to some sort of disruption to epithelial homeostasis. Similarly, there is an increase in the kidney injury marker Lcn2 (or NGAL) and in the immediate early response genes Fos, Jun, and Nr4a1, which can also cause dedifferentiation and may indicate cellular stress such as hypoxia.43 Additionally, TGF-β, TGFβ-induced, and gremlin are elevated suggesting potential upregulation of TGF-β signaling, which is associated with AQP2 and AQP3 downregulation in the cortical collecting ducts.44,45 The upregulated genes suggest more complex changes in response to the loss of Pax2/8 that are likely not direct targets, activated or repressed by Pax proteins at promoters or enhancers. Given the severe dehydration phenotype, mice were euthanized within a few weeks after Pax deletion. Thus, if they were kept alive longer then additional pathology could develop.
Despite many Pax2 and Pax8 mutations described in the adult population, urine concentration defects have not been specifically associated with Pax2/8 loss of function. Numerous loss-of-function mutations in the DNA-binding paired domain of Pax2 have been described and are associated with congenital abnormalities, often described as papillorenal syndrome, which consists of ocular colobomas, renal dysplasia or hypoplasia, reflux, and progressive renal failure.8 These are most likely attributable to abnormal kidney development because of Pax2 haploinsufficiency. Given the expression of Pax2 in the progenitor cells of the nephron and in the branching ureteric buds, reduced Pax2 gene dosage could affect the number of nephrons formed or the branching pattern of the ureteric bud. Nevertheless, patients that are heterozygous for Pax2 are likely homozygous for wild-type Pax8, with no defects in urine concentration described to date. Similarly, for Pax8 loss of function, no renal defects have been described in most patients, presumably because of heterozygosity and redundancy of function with Pax2. Pax8 heterozygous individuals do have congenital hypothyroidism, which is not complemented by Pax2 because of the lack of Pax2 expression in the developing thyroid.9,46,47 Thus, our mouse genetics and conditional deletion of Pax2/8 revealed a novel regulatory function in the adult kidney that was not evident from either germline-null mouse mutations or clinically identified Pax2/8 heterozygous mutations. The direct regulation of UTA1 by Pax8 was confirmed in cultured IMCD cells, which respond to increased osmolality by upregulating Pax8 levels. Pax8 bound near the transcription start site of the UTA1 (Slc14a2) gene and recruited the histone methyltransferase complex via the adaptor protein PTIP, a mechanism similar to that of Pax2.27,28
In summary, despite the clear need for Pax2 in kidney development, the function of Pax proteins in the adult kidney have not been examined to date. Our data shows a critical need for Pax2 or Pax8 in regulating selective transporters in the renal medulla that are responsible for maintaining salt and water balance. Increased expression of Pax genes is responsive to high osmolality and may help to maintain the viability of renal epithelial cells in a high-salt environment.31
Disclosures
Dr. Dressler reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, during the conduct of the study. All other authors have nothing to disclose.
Funding
This work was supported in part by National Institutes of Health grants DK073722 and DK054740 (to Dr. Dressler), and a Young Scientist Fellowship of the Japan Society for the Promotion of Science (to Dr. Higashi).
Supplementary Material
Acknowledgments
We thank the University of Michigan Transgenic Animal Core for blastocyst injections and rederivation of Pax2 floxed mice, the University of Michigan Cancer Center microarray core facility for Affymetrix analyses, Jeff Sands for antibodies against UTA1, and Nathan Qi and the Michigan Mouse Metabolic Phenotyping Center.
Dr. Laszczyk designed and executed experiments and prepared figures. Dr. Higashi bred mice, designed and executed experiments, and collected and prepared tissues. Dr. Patel designed cell culture assays and prepared figures. Dr. Johnson did the Affymetrix analyses and figures. Dr. Soofi managed mouse breeding, genotyping, and histology. Dr. Abraham executed cell culture and chromatin immunoprecipitation experiments. Dr. Abraham planned experiments, prepared figures, analyzed data, and wrote the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019090962/-/DCSupplemental.
Supplemental Figure 1. (A) Histological sections from control and Pax2/8 double KOs. (B) Higher magnifications of histological sections from control and Pax2/8 double KOs.
Supplemental Figure 2. Quantitative RT-PCR for selected genes identified by Affymetrix arrays.
Supplemental Figure 3. Immunostaining of control and Pax2/8 double KOs with antibodies against AQP1 and AQP2.
Supplemental Figure 4. Immunostaining of control and Pax2/8 double KOs with antibodies against AQP3 and AQP4.
Supplemental Figure 5. Transfection of Pax8 or Pax2 in IMCD cells.
Supplemental Table 1. Excel spreadsheet of gene expression changes identified by Affymetrix arrays.
Supplemental Table 2. List of PCR primer pairs.
Supplemental Table 3. Relative expression levels of AVP and receptors.
References
- 1.Nawata CM, Pannabecker TL: Mammalian urine concentration: A review of renal medullary architecture and membrane transporters. J Comp Physiol B 188: 899–918, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Féraille E, et al.: Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM: Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A 96: 2538–2542, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimley E, Dressler GR: Are Pax proteins potential therapeutic targets in kidney disease and cancer? Kidney Int 94: 259–267, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan P, Leavey PJ, Galindo RL: PAX genes in childhood oncogenesis: Developmental biology gone awry? Oncogene 34: 2681–2689, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Underhill DA: PAX proteins and fables of their reconstruction. Crit Rev Eukaryot Gene Expr 22: 161–177, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Torres M, Gómez-Pardo E, Dressler GR, Gruss P: Pax-2 controls multiple steps of urogenital development. Development 121: 4057–4065, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, et al.: Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat 33: 457–466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al.: PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet 19: 83–86, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Urbánek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M: Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79: 901–912, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dressler GR: Advances in early kidney specification, development and patterning. Development 136: 3863–3874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranghini EJ, Dressler GR: Evidence for intermediate mesoderm and kidney progenitor cell specification by Pax2 and PTIP dependent mechanisms. Dev Biol 399: 296–305, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soofi A, Levitan I, Dressler GR: Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys. Dev Biol 365: 241–250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M: Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: 2958–2970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marotta P, Amendola E, Scarfò M, De Luca P, Zoppoli P, Amoresano A, et al.: The paired box transcription factor Pax8 is essential for function and survival of adult thyroid cells. Mol Cell Endocrinol 396: 26–36, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Dressler GR, Douglass EC: Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci U S A 89: 1179–1183, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechner MS, Levitan I, Dressler GR: PTIP, a novel BRCT domain-containing protein interacts with Pax2 and is associated with active chromatin. Nucleic Acids Res 28: 2741–2751, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Cai Y, Soofi A, Dressler GR: Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J Biol Chem 287: 21290–21302, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al.: Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie ME, Diyagama D, Neilson J, van Laar R, Dobrovic A, Holloway A, et al.: Empirical array quality weights in the analysis of microarray data. BMC Bioinformatics 7: 261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth GK: Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Y, Benjamini Y: More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Irizarry RA, Wu Z, Jaffee HA: Comparison of Affymetrix GeneChip expression measures. Bioinformatics 22: 789–794, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Bardes EE, Aronow BJ, Jegga AG: ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305–W311, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SR, Dressler GR: Expression of Pax2 in the intermediate mesoderm is regulated by YY1. Dev Biol 267: 505–516, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Patel SR, Kim D, Levitan I, Dressler GR: The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell 13: 580–592, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SR, Bhumbra SS, Paknikar RS, Dressler GR: Epigenetic mechanisms of Groucho/Grg/TLE mediated transcriptional repression. Mol Cell 45: 185–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, et al.: A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474: 337–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchard M, Pfeffer P, Busslinger M: Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development 127: 3703–3713, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Cai Q, Dmitrieva NI, Ferraris JD, Brooks HL, van Balkom BW, Burg M: Pax2 expression occurs in renal medullary epithelial cells in vivo and in cell culture, is osmoregulated, and promotes osmotic tolerance. Proc Natl Acad Sci U S A 102: 503–508, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moeller HB, Praetorius J, Rützler MR, Fenton RA: Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107: 424–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olesen ET, Fenton RA: Aquaporin-2 membrane targeting: Still a conundrum. Am J Physiol Renal Physiol 312: F744–F747, 2017. [DOI] [PubMed] [Google Scholar]
- 34.Bockenhauer D, Bichet DG: Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11: 576–588, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Davis J, Desmond M, Berk M: Lithium and nephrotoxicity: Unravelling the complex pathophysiological threads of the lightest metal. Nephrology (Carlton) 23: 897–903, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Pop IL, Carlson NG, Kishore BK: Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302: F70–F77, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Sung CC, Chen L, Limbutara K, Jung HJ, Gilmer GG, Yang CR, et al.: RNA-Seq and protein mass spectrometry in microdissected kidney tubules reveal signaling processes initiating lithium-induced nephrogenic diabetes insipidus. Kidney Int 96: 363–377, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, et al.: NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283: 28095–28105, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA: Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S: Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A 103: 6037–6042, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, et al.: Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A 97: 4386–4391, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 111: 1527–1532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koltsova SV, Shilov B, Birulina JG, Akimova OA, Haloui M, Kapilevich LV, et al.: Transcriptomic changes triggered by hypoxia: Evidence for HIF-1α-independent, [Na+]i/[K+]i-mediated, excitation-transcription coupling. PLoS One 9: e110597, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JW, Alsady M, Chou CL, de Groot T, Deen PMT, Knepper MA, et al.: Single-tubule RNA-Seq uncovers signaling mechanisms that defend against hyponatremia in SIADH. Kidney Int 93: 128–146, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Ariunbold U, Suhaimi N, Sunn N, Guo J, McMahon JA, et al.: Collecting duct-derived cells display mesenchymal stem cell properties and retain selective in vitro and in vivo epithelial capacity. J Am Soc Nephrol 26: 81–94, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho A, Hermanns P, Rodrigues AL, Sousa I, Anselmo J, Bikker H, et al.: A new PAX8 mutation causing congenital hypothyroidism in three generations of a family is associated with abnormalities in the urogenital tract. Thyroid 23: 1074–1078, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Hermanns P, Grasberger H, Cohen R, Freiberg C, Dörr HG, Refetoff S, et al.: Two cases of thyroid dysgenesis caused by different novel PAX8 mutations in the DNA-binding region: In vitro studies reveal different pathogenic mechanisms. Thyroid 23: 791–796, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.