The continuous spread of antimalarial drug resistance is a threat to current chemotherapy efficacy. Therefore, characterizing the genetic diversity of drug resistance markers is needed to follow treatment effectiveness and further update control strategies. Here, we genotyped Plasmodium falciparum resistance gene markers associated with sulfadoxine-pyrimethamine (SP) and artemisinin-based combination therapy (ACT) in isolates from pregnant women in Ghana. The prevalence of the septuple IRNI-A/FGKGS/T pfdhfr/pfdhps haplotypes, including the pfdhps A581G and A613S/T mutations, was high at delivery among post-SP treatment isolates (18.
KEYWORDS: Plasmodium falciparum, pfdhfr, pfdhps, pfk13, sulfadoxine-pyrimethamine, artemisinin, drug resistance
ABSTRACT
The continuous spread of antimalarial drug resistance is a threat to current chemotherapy efficacy. Therefore, characterizing the genetic diversity of drug resistance markers is needed to follow treatment effectiveness and further update control strategies. Here, we genotyped Plasmodium falciparum resistance gene markers associated with sulfadoxine-pyrimethamine (SP) and artemisinin-based combination therapy (ACT) in isolates from pregnant women in Ghana. The prevalence of the septuple IRNI-A/FGKGS/T pfdhfr/pfdhps haplotypes, including the pfdhps A581G and A613S/T mutations, was high at delivery among post-SP treatment isolates (18.2%) compared to those of first antenatal care (before initiation of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine [IPTp-SP]; 6.1%; P = 0.03). Regarding the pfk13 marker gene, two nonsynonymous mutations (N458D and A481C) were detected at positions previously related to artemisinin resistance in isolates from Southeast Asia. These mutations were predicted in silico to alter the stability of the pfk13 propeller-encoding domain. Overall, these findings highlight the need for intensified monitoring and surveillance of additional mutations associated with increased SP resistance as well as emergence of resistance against artemisinin derivatives.
INTRODUCTION
In sub-Saharan Africa, Plasmodium falciparum infection represents one of the major causes of low birth weight (1–3). As such, expanded efforts to diminish the burden of the disease on affected populations have been increasingly prioritized over the last two decades (4). Intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) has been the principal intervention toward that and has been successful across different transmission settings (5–7). However, growing concern over the rise and spread of SP resistance and its impact on the effectiveness of IPTp is rising, especially in eastern and southern Africa, where levels of parasite resistance to SP are high. Regardless, no alternative has been identified that effects positively on birth weight and is well tolerated (8–10). As such, the World Health Organization (WHO) updated its IPTp-SP policy from at least 2 doses to a monthly dose starting from the second trimester (11). This increase in SP pressure in pregnant women has likely favored the selection of parasites carrying mutant haplotypes in the pfdhfr/pfdhps genes that confer increased resistance to SP, for example, pfdhps K540E, A581G, and A613S/T mutations, which have been previously correlated with high levels of SP resistance (12). However, the prevalence of these mutations is lower in west and central Africa compared to eastern and southern Africa. Notwithstanding this, SP-resistant parasites are susceptible to artemisinin (ART)-based combination therapy (ACT), which is the first-line treatment in the management of uncomplicated malaria (13). However, since its inception, only a few studies have been conducted to determine the spread of resistance to these drugs in Ghana. In this study, we aimed to characterize the genetic diversity of SP and ART resistance markers among parasites from pregnant women in southern Ghana.
RESULTS
Study population characteristics.
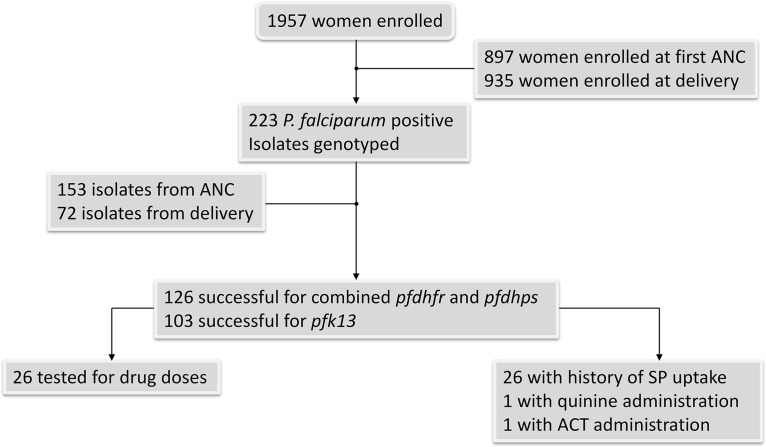
The general characteristics of pregnant women have been described elsewhere (14). In total, 223 isolates were evaluated, consisting of 151 isolates from pregnant women enrolled during their first antenatal clinic (ANC) visit and 72 isolates from pregnant women enrolled during delivery (Fig. 1). Of these, 68.6% (153/223), 63.2% (141/223), and 46.2% (103/223) of samples were successfully amplified, sequenced, and genotyped, respectively, for the pfdhfr and pfdhps genes and the pfk13 propeller-encoding gene fragment. At ANC, 64.7% (99/153), 65.2% (92/141), and 50.5% (52/103) isolates were successfully genotyped, respectively, for pfdhfr, pfdhps, and pfk13. At delivery, 37.2% (57/153), 32.6% (46/141), and 49.5% (51/103) were also genotyped for pfdhfr, pfdhps, and pfk13, respectively.
FIG 1.
Study flow chart. ATS, acidic terminal segment of Pf var gene; pfdhfr, dihydrofolate reductase; pfdhps, dihydropterate synthase; pfk13, kelch13.
Prevalence of pfdhfr and pfdhps mutations.
Mutant pfdhfr alleles were predominant at codons 108 (S108N, 93.5%, 112/153), 59 (C59R, 90.8%, 111/153); and 51 (N51I, 85%, 130/153) (Table 1). The high prevalence of these mutant pfdhfr alleles was observed at both time points (Table 2). In the pfdhps gene associated with sulfadoxine resistance, the prevalence of mutant alleles was high at codons 436 (S436A/F, 95.0%, 134/141) and 437 (A437G, 96.4%, 133/141), but very low at codon 540 (K540E, 0.7%; 1/141). Mutations at codons 581 (A581G) and 613 (A613S/T) were, respectively, found in 12.8% (18/141) and 17.7% (25/141) of the isolates (Table 1). These proportions were similar at both time points (data not shown), except for mutant alleles at codon 581 (A581G), which were preferentially found in post-IPTp-SP treatment isolates (ratio of first ANC to delivery of 1:1.7) (Table 2).
TABLE 1.
Prevalence of pfdhfr, pfdhps, and pfk13 SNPs among study isolates
| Gene (total no.) | SNP | No. (%) of wild-type isolates | No. (%) of mutations | No. (%) of mixed-type isolates |
|---|---|---|---|---|
| pfdhfr (153)a | N51I | 23 (15.0) | 130 (85.0) | 0 (0) |
| C59R | 14 (9.2) | 111 (90.8) | 0 (0) | |
| S108N | 10 (6.5) | 112 (93.5) | 0 (0) | |
| I164L | 153 (100) | 0 (0) | 0 (0) | |
| pfdhps (141)a | S436A/F | 2 (1.4) | 134 (95.0) | 5 (36) |
| A437G | 5 (3.6) | 133 (96.4) | 0 (0) | |
| K540E | 140 (99.3) | 1 (0.7) | 0 (0) | |
| A581G | 123 (87.2) | 18 (12.8) | 0 (0) | |
| A613S/T | 116 (82.63) | 25 (17.7) | 0 (0) | |
| pfk13 (103)a | E455G | 101 (98.1) | 2 (1.9) | |
| N458D | 101 (98.1) | 2 (1.9) | ||
| A481C | 102 (99.0) | 1 (1.0) | ||
| T535A | 102 (99.0) | 1 (1.0) | ||
| Y616S | 102 (99.0) | 1 (1.0) | ||
| L618V | 101 (98.1) | 2 (1.9) | ||
| A621G | 102 (99.0) | 1 (1.0) | ||
| L663I | 102 (99.0) | 1 (1.0) | ||
| N672I | 101 (98.1) | 2 (1.9) |
pfdhfr, dihydrofolate reductase; pfdhps, dihydropteroate synthase; pfk13, kelch13 propeller-encoding gene.
TABLE 2.
Distribution of pfdhfr and pfdhps at first ANC and delivery among study isolates
| Gene | SNP | Data from first ANC visit (n = 99) |
Data from time of delivery (n = 57) |
||||
|---|---|---|---|---|---|---|---|
| No. (%) of wild-type isolates | No. (%) of mutations | No. (%) of mixed-type isolates | No. (%) of wild-type isolates | No. (%) of mutations | No. (%) of mixed-type isolates | ||
| pfdhfra | N51I | 15 (15.2) | 82 (82.2) | 0 (0) | 8 (13.6) | 48 (81.4) | 0 (0) |
| C59R | 8 (8.1) | 89 (89.9) | 0 (0) | 6 (10.2) | 50 (84.8) | 0 (0) | |
| S108N | 5 (5.1) | 92 (92.9) | 0 (0) | 5 (8.5) | 51 (86.4) | 0 (0) | |
| pfdhpsa | S436A/F | 1 (1.0) | 91 (91.9) | 2 (2.0) | 1 (1.8) | 43 (75.4) | 3 (5.3) |
| A437G | 2 (2.0) | 90 (90.9) | 0 (0) | 0 (0.0) | 46 (80.7) | 0 (0) | |
| K540E | 91 (91.9) | 1 (1.0) | 0 (0) | 44 (77.2) | 0 (0.0) | 0 (0) | |
| A581G | 83 (83.8) | 9 (9.1) | 0 (0) | 35 (61.4) | 9 (15.8) | 0 (0) | |
| A613S/T | 77 (77.8) | 15 (15.2) | 0 (0) | 34 (59.6) | 10 (17.5) | 0 (0) | |
pfdhfr, dihydrofolate reductase; pfdhps, dihydropteroate synthase.
Prevalence of pfdhfr and pfdhps haplotypes.
Five and nine different haplotypes were detected for pfdhfr and pfdhps genes, respectively.
For pfdhfr, 6.5% (10/153) of isolates carried the wild-type (NCSI) haplotype, while 79.7% (96/153) carried the triple IRNI mutant haplotype. Double mutant haplotypes occurred at a low prevalence: 7.8% (12/153) and 2.0% (3/153) for NRNI and ICNI, respectively. A single mutant haplotype NCNI was observed once (0.7%; 1/153).
For pfdhps, two haplotypes predominated: the double mutant A/FGKAA and quadruple mutant A/FGKGS/T haplotypes that were present in 71.6% (101/141) and 9.9% (14/141) of isolates, respectively. Of the remaining isolates, 7.8% (11/141), 2.8% (4/141), and 0.7% (1/141) carried the triple mutant A/FGKAS/T, A/FGKGA, and A/FGEAA haplotypes, respectively. Overall, 2.1% (3/141) and 1.4% (2/141) of isolates carried a single mutant haplotype SGKAA or A/FAKAA, while 3.6% (5/141) isolates carried a mixed haplotype (Table 3).
TABLE 3.
Prevalence of pfdhfr and pfdhps haplotypes
| Gene (total no.) | Category | Haplotype | No. (%) |
|---|---|---|---|
| pfdhfr (153)a | Wild type | NCSI | 10 (6.5) |
| Single | NCNI | 1 (0.7) | |
| Double | ICNI | 3 (2.0) | |
| NRNI | 12 (7.8) | ||
| Triple | IRNI | 96 (79.7) | |
| pfdhps (141)a | Single | A/FAKAA | 2 (1.4) |
| SGKAA | 3 (2.1) | ||
| Double | A/FGKAA | 101 (71.6) | |
| Triple | A/FGEAA | 1 (0.7) | |
| A/FGKGA | 4 (2.8) | ||
| A/FGKAS/T | 11 (7.8) | ||
| Quadruple | A/FGKGS/T | 14 (9.9) | |
| Mixed type | S/AAKAA | 2 (1.4) | |
| S/AGKAA | 3 (2.1) |
pfdhfr, dihydrofolate reductase; pfdhps, dihydropterate synthase.
Combined pfdhfr-pfdhps haplotypes.
Of the successfully sequenced isolates, 126 were analyzed for combined pfdhfr and pfdhps haplotypes. Ten distinct haplotypes were observed. A quintuple mutant haplotype, consisting of triple IRNI and double A/FGKAA mutations, was the most common combined pfdhfr/pfdhps haplotype (63.5%; 80/126), followed by the septuple combined haplotype IRNI + A/FGKGS/T (10.3%; 13/126). The prevalence of other combined haplotypes was low. Only one isolate was wild type at both pfdhfr and pfdhps loci. The combined quintuple mutant haplotype was observed at similar proportions at both the first ANC (63.4%, 52/82) and delivery (63.6%, 28/44). However, there was a high prevalence of septuple mutant haplotypes observed at delivery (18.2%) compared to the first ANC (6.1%) (P = 0.03; chi-squared test) (Table 4).
TABLE 4.
Combined pfdhfr/pfdhps haplotypes based on sample time point and study site
| Gene (n) | Category | Haplotype | No. (%) at first ANC visit (total n = 82) | No. (%) at delivery (total n = 44) |
|---|---|---|---|---|
| pfdhfr/pfdhps (126) | Wild type | NCSI/SAKAA | 1 (1.2) | 0 (0.0) |
| Double | NCSI-A/FGKAA | 3 (3.7) | 4 (9.1) | |
| Triple | NCNI-A/FGKAA | 1 (1.2) | 0 (0.0) | |
| Quadruple | ICNI-A/FGKAA | 2 (2.4) | 0 (0.0) | |
| NRNI-A/FGKAA | 8 (9.8) | 1 (2.3) | ||
| IRNI-A/FAKAA | 1 (1.2) | 0 (0.0) | ||
| Quintuple | IRNI-A/FGKAA | 52 (63.4) | 28 (63.6) | |
| Sextuple | IRNI-A/FGEAA | 1 (1.2) | 0 (0.0) | |
| IRNI-A/FGKAS/T | 8 (9.8) | 3 (6.8) | ||
| Septuple | IRNI-A/FGKGS/T | 5 (6.1) | 8 (18.2) |
Mutation frequency in the pfk13 propeller-encoding domain.
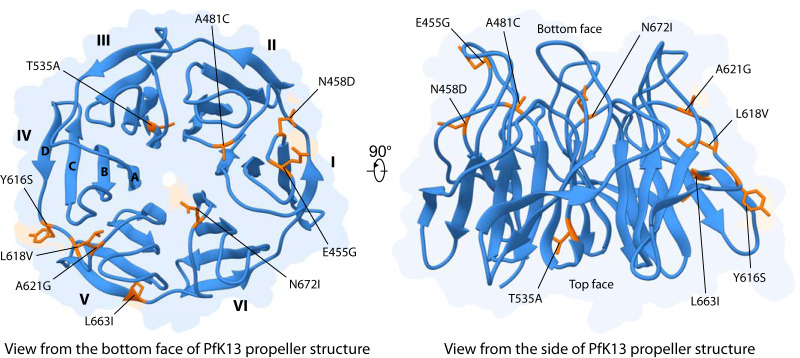
The pfk13 propeller-encoding domain was sequenced for a total of 113 P. falciparum isolates from both time points. Ten isolates failed to generate high-quality sequences and were discarded for further analysis. Among the 103 remaining sequences, 10 (9.7%) harbored nonsynonymous mutations only found in one (A481C, T535A, Y616S, A621G, L663I) or two (E455G, N458D, L618V, N672I) isolates. Interestingly, the nonsynonymous mutations N458D and A481C are related to positions at which ART resistance mutations (N458Y and A481V) were previously described in isolates from Southeast Asia (15). Another mutation (T535A) was observed at a position found mutated in Southeast Asia (T535M), although its relationship with ART resistance remains uncharacterized. The location of these pfk13-nonsynonymous mutations was spotted on the propeller tertiary structure (Fig. 2). Those were mainly located on the loops connecting blades (Table 5), except the mutation T535A, which was located on the inner strand (strand A) lining the central channel of the domain. Finally, the mutation N672I was localized at the shallow pocket, predicted as a putative propeller interaction surface (16).
FIG 2.
Location of amino acids associated with pfk13 mutations observed in southern Ghana on the PfK13 propeller tertiary structure. The propeller domain is shown as cartoon from the bottom face (left) and from the side (right). Positions associated with pfk13 mutations are colored in orange and shown as stick. On the left structure, blade number (I to VI) and strand label (A to D) are indicated.
TABLE 5.
Prediction of structural damages and structure stability of the mutant propeller domains
| Mutation | Bladea | Locationb | Statec | Result from Missence 3D | Result from DynaMutd |
|---|---|---|---|---|---|
| E455G | I | BC loop | Exposed | Not damaging | +0.140 |
| N458D | I | BC loop | Exposed | Not damaging | −0.664 |
| A481C | II | DA loop | Buried | Not damaging | −0.230 |
| T535A | III | Strand A | Exposed | Not damaging | +0.116 |
| Y616S | V | DA loop | Exposed | Not damaging | −3.063 |
| L618V | V | DA loop | Buried | Not damaging | −0.865 |
| A621G | V | DA loop | Exposed | Not damaging | −0.148 |
| L663I | V | DA loop | Exposed | Not damaging | +0.073 |
| N672I | VI | DA loop | Buried | Not damaging | +0.180 |
Blade refers to the six Kelch-repeat motifs of the PfK13 propeller domain, labeled I to VI.
Mutations are either located on strands (innermost, strand A; outermost, strand D) (Fig. 2). Each loop connects two strands; for example, the DA loop connects strand D with the strand A of the next blade.
A position is supposed to be exposed to the solvent when it exhibited relative solvent accessibility ≥ 16%.
Values correspond to the folding free energy (ΔΔG), expressed in kcal/mol. Negative and positive values suggest destabilizing and stabilizing effects, respectively.
The Missence 3D tool was used to predict structural damages caused by nonsynonymous mutations. None were predicted to be damaging for the propeller tertiary structure (Table 5). However, the DynaMut server predicted that five mutations may decrease the propeller domain stability, including mutations N458D and A481C.
Relationships between the number of IPTp doses taken, sulfadoxine-pyrimethamine plasma levels, and prevalence of pfdhfr/pfdhps mutations.
Based on the declaration and verification on the ANC booklet, 9.1% (4/44) of pregnant women reported taking one dose of IPTp-SP, and 9.1% (4/44) reported taking two doses, while 63.6% (28/44) of pregnant women took three or more SP doses. Five pregnant women did not receive any SP during their pregnancy.
To explore the relationship between plasma SP levels and the prevalence of drug resistance mutations, we measured SP and ACT residual levels in peripheral plasma at delivery. SP and ACT residual levels were measured in 31 plasma samples, with sulfadoxine metabolites being detected in 77.4% (24/31) of samples at a mean concentration of 3,467 ± 5,543.1 μg/ml, while pyrimethamine metabolites were detected in 16.1% (5/31) of samples at a mean concentration of 4.0 ± 10.2 ng/ml. Women in whom pyrimethamine was detected were also sulfadoxine positive; however, sulfadoxine was detected in more women. Therefore, we considered the presence of sulfadoxine as a proxy for SP in the analyses.
There was no association between the number of IPTp-SP doses taken and the frequency of observed mutant alleles or combined haplotypes among women at delivery. When the analysis was carried out with the SP level in the blood taken as a quantitative variable between women whose parasites were mutated or not, A581G and A613S/T were associated with increased SP levels (both P = 0.05; Welch t test). This significant difference was, however, lost when the pfdhfr and pfdhps haplotypes were combined. Finally, we were unable to determine an association between either ACT treatment or level of ACT metabolites and pfk13 mutations because only one pregnant woman had taken ACT during pregnancy.
DISCUSSION
Gains achieved toward the fight against malaria are now dwindling, especially in sub-Saharan Africa, where most of the burden is felt. This is attributable to the high transmission rate and the low coverage of intervention strategies. In regard to these, the WHO is now recommending an increased administration of SP as part of IPTp to correct the coverage deficit (11). Thus, understanding the effect of the increased drug pressure on parasite populations is crucial to the sustainability of the IPTp program, particularly in western Africa, where quintuple mutations (triple pfdhfr with double pfdhps mutations) are low (drugresistancemaps.org).
In this study, we characterized parasites obtained from pregnant women coming for their first contact with antenatal care and those of women at delivery who participated in ANCs and have been exposed to several doses of IPTp-SP. Ghana, like other African countries, has adopted and is implementing the new IPTp-SP policy, which advocates an increase in the number of treatment doses, and we have recently reported that this new policy was very well implemented in Ghana (14). This study is one of the first to assess the SP-resistant genetic variation among parasite populations in pregnant women in the context of increased IPTp-SP pressure. One noteworthy observation here is the high prevalence of highly mutated pfdhfr/pfdhps haplotypes observed among posttreatment isolates compared to the first ANC. The observation of a clear tendency toward the increase of highly mutated parasites in women still infected at delivery draws attention to a possible selection of these mutant parasites by treatment. Overall, the prevalence of mutant pfdhfr and pfdhps haplotypes conferring SP resistance was high among the parasite isolates in the study participants at both the first ANC visit and at delivery, respectively, with 63% and 64% of isolates carrying the quintuple pfdhfr/pfdhps mutant haplotype (IRNIA/FGKAA). This is in agreement with Mouchenhaupt et al. (17) and other studies in Africa (18–21) where the prevalence of the quadruple pfdhfr/pfdhps mutant haplotype (IRNIA/FGKAA) had been reported as very high with almost fixation of the triple pfdhfr mutant haplotype. This predominance of triple pfdhfr mutant parasites could be due to the transmission advantage that SP resistant parasites have since acquired in the context of continuous use of the drug (22). When looking specifically at the pfdhps gene, additional critical mutations capable of further impacting resistance to SP, like mutations at codons 581 (A581G) and 613 (A613S/T) of the pfdhps gene, have been observed at frequencies close to those reported among children in northern Ghana (23). However, in this study, we especially noted that these proportions slightly increased from 9% and 15% at first ANC (that is, before the initiation of IPTp-SP) to 16% and 18% at delivery, respectively. The fact that parasites carrying these mutations are found more in women at delivery with higher SP concentrations in their blood suggests that these parasites are better able to withstand these conditions and therefore to withstand SP treatment. When considering these observations, and adding to the fact that parasites carrying the K540E mutation are also present in the study area as recently reported in one isolate from Bioko Island in Equatorial Guinea (24), one may wonder whether the conditions necessary for the emergence of full resistance to SP in West Africa will not be gradually favored with the current IPTp-SP strategy. Whether the successful implementation of IPTp in Ghana raises fears in this regard remains a topical issue. These findings warn of the impact of increasing uptake of IPTp-SP on the rising level of resistance among circulating parasites if these few mutants come to disseminate and become the dominant population. In a meta-analysis study assessing IPTp-SP effectiveness, which includes 42,394 births, a mutation at codon 540 (K540E) was associated with a high prevalence of low birth weight (25). Therefore, it would certainly be necessary to study the impact on the pregnancy outcome of the pfdhps A581G and A613S/T mutant parasites that seem to be favored in the context of IPTp-SP.
The polymorphism data generated on the pfk13 gene revealed two nonsynonymous mutations related to positions known to confer artemisinin resistance in Southeast Asia (26–29). However, the changes in amino acid observed differ from those described in Asia, and further investigations are needed to check whether they confer a survival advantage to parasites pressured by ART. Importantly, one Singaporean returning from Ghana presented with a P. falciparum infection exhibiting the N458D mutation and was not effectively cured by ACT treatment (30). These findings, in addition to those reported by Ocan et al. from Uganda (31), Bayih et al. in northwest Ethiopia (32), and Ouattara et al. from Mali (33), are suggestive indications of possible ART resistance emergence in Africa. However, several studies have shown that increased parasite survival under ART pressure may also be caused by mutations at other loci such as pfap2-mu, pfcoronin, falcipain 2a, and pfubp1 mutations (34–36) that were not investigated here.
In conclusion, this study shows the high prevalence of quintuple-pfdhfr/pfdhps mutations in parasite isolates from two Ghanaian study sites with the development of additional mutations that confer higher resistance to SP. The spread of these new mutations could be facilitated by the continued pressure of IPTp-SP and may impact negatively on its effectiveness. Thus, other interventions to better protect pregnancy from malaria infection and its damaging effects on mother and child should be evaluated now and not wait until the efficacy of SP is completely eroded. On the other hand, the description of mutations on ART resistance-related positions of the pfk13 gene in this study also resonates as an alert for more surveillance on the emergence of resistance against ART derivatives, currently part of the recommended treatment of uncomplicated malaria cases during pregnancy.
MATERIALS AND METHODS
Ethics statement.
Ethical clearance was obtained from the Institutional Ethics Review Committee of the Noguchi Memorial Institute for Medical Research (NMIMR) and the Ethical Review Committee of the Ghana Health Service. Written informed consent was obtained from each participant.
Study area and design.
The study was a cross-sectional hospital-based survey conducted from December 2015 to May 2017 in two distinct communities in southern Ghana: Kpone on-Sea, a peri-urban community, and Maamobi, an urban community, both within the Greater Accra region in Ghana. Malaria transmission is perennial, with two peaks, one from April to July and the other from September to November. A two-parallel cross-sectional enrollment was carried out among pregnant women attending the first antenatal clinic (ANC) and at delivery. A detailed description of the study sites and design is reported elsewhere (14).
PCR assays for the detection and amplification of pfdhfr, pfdhps, and pfk13 genes.
DNA extraction was carried out on total blood from the peripheral circulation or placenta at delivery using the QIAamp DNA blood minikit (Qiagen, France) according to the manufacturer’s recommendation. The presence of P. falciparum parasites was tested in duplicate by real-time quantitative PCR (qPCR) targeting the 18S rRNA of P. falciparum (37). Subsequently, pfdhfr and pfdhps genes were amplified by nested PCR, and the conditions for amplification were as previously described (38), while that of pfk13 was amplified as previously described in reference 26. Mutations of the pfdhfr, pfdhps, and pfk13 genes in the amplified nested PCR products were purified and detected subsequently by Sanger sequencing (GATC, Cologne, Germany). All sequences generated were analyzed with the Chromas software (Technelysium Pty Ltd.) and then aligned using MEGA 5.2 (39) and compared with reference genes of the P. falciparum 3D7 genome.
Effect(s) of pfk13 nonsynonymous mutations on the structure and stability of the PfK13 propeller domain.
The tools Missence3D (40) and DynaMut (41) were used to predict, respectively, the structural damages and structure stability alterations caused by amino acid changes in the propeller domain of the PfK13 protein. We used the X-ray crystallographic tertiary structure determined at a resolution of 1.5 Å (PDB ID 4YY8, chain A) (42). Beforehand, all the missing atoms were added with Swiss PDB Viewer (43).
Antimalarial drug level measurements.
Plasma sulfadoxine and pyrimethamine levels were measured by liquid chromatography coupled to tandem mass spectrometry (TSQ Quantum Ultra; Thermo Fisher, France) as previously reported (44). Briefly, using Oase 96-well microplates (Waters, France), 100 μl of plasma was mixed with 300 μl of acetonitrile containing quinidine-d3 (50 ng/ml) as an internal standard. Phospholipids were eliminated by positive pressure (20 lb/in2 during 1 min), and eluents were evaporated at room temperature. Dry residues were dissolved in 20 mM ammonium formate buffer with formic acid (0.5% vol/vol) before 10 μl was injected into the system. This was used to measure metabolites of sulfadoxine, pyrimethamine, amodiaquine, lumefantrine, primaquine, artemisinin, and quinine. Homemade and external controls obtained from the Worldwide Antimalarial Resistance Network (WWARN) were used as controls.
Statistical analysis.
All data were analyzed with R programming. The chi-squared test was used to determine the association between the single nucleotide polymorphisms (SNPs) of the pfdhfr and pfdhps genes and the number of doses of SP taken. Association between mutations observed and the level of SP in the peripheral plasma were tested using the Welch t test or logistic regression where appropriate. P values of less than 0.05 indicated significance.
ACKNOWLEDGMENTS
We are grateful to all the women who participated in the study. We thank the staff of the different study sites for their contribution to the collection of samples and Emmanuel Kakra Dickson for his technical assistance.
B.T. was supported by a joint ARTS Ph.D. fellowship from IRD (Institut de recherche pour le développement) and a SCAC (Service de Coopération et d'Action Culturelle) fellowship from the French Embassy in Ghana.
The project was funded by the Canal 1 grant from Initiative 5% and Expertise France (grant no. 14SANIN163).
We declare no conflicts of interest.
N.T.N., I.Q., M.F.O., K.A.K., and A.K.A. conceived and designed the study. B.T., N.C., and A.M. carried out the sample collections. B.T. performed the parasite genotyping. B.T. and P.H. carried out the SP level measurements. B.T., R.T., and B.A. performed the sequence analysis and statistical analysis. B.T. and R.C. investigated structural alterations induced by pfk13 mutations. B.T., R.C., R.T., and N.T.N. wrote the paper. J.C. and P.D. reviewed the manuscript.
REFERENCES
- 1.Parise M, Nahlen B, Menendez C, Steketee R. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt HL, Snow RW. 2004. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev 17:760–769. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2000. The Abuja declaration and the plan of action. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/handle/10665/67816/WHO_CDS_RBM_2003.46.pdf;jsessionid=5F7931C4093B96E712B4825865CEF7F8?sequence=1. [Google Scholar]
- 5.ter Kuile FO, van Eijk AM, Filler SJ. 2007. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 6.Mpogoro FJ, Matovelo D, Dosani A, Ngallaba S, Mugono M, Mazigo HD. 2014. Uptake of intermittent preventive treatment with sulphadoxine-pyrimethamine for malaria during pregnancy and pregnancy outcomes: a cross-sectional study in Geita district, North-Western Tanzania. Malar J 13:455. doi: 10.1186/1475-2875-13-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutu EO, Lawson B, Browne E. 2011. The effectiveness and perception of the use of sulphadoxine-pyrimethamine in intermittent preventive treatment of malaria in pregnancy programme in Offinso district of Ashanti region, Ghana. Malar J 10:385. doi: 10.1186/1475-2875-10-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerk CA, Bruce J, Affipunguh PK, Mensah N, Hodgson A, Greenwood B, Chandramohan D. 2008. A randomized, controlled trial of intermittent preventive treatment with sulfadoxine-pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis 198:1202–1211. doi: 10.1086/591944. [DOI] [PubMed] [Google Scholar]
- 9.Briand V, Bottero J, Noël H, Masse V, Cordel H, Guerra J, Kossou H, Fayomi B, Ayemonna P, Fievet N, Massougbodji A, Cot M. 2009. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis 200:991–1001. doi: 10.1086/605474. [DOI] [PubMed] [Google Scholar]
- 10.Kimani J, Phiri K, Kamiza S, Duparc S, Ayoub A, Rojo R, Robbins J, Orrico R, Vandenbroucke P. 2016. Efficacy and safety of azithromycin-chloroquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of Plasmodium falciparum malaria infection in pregnant women in Africa: an open-label, randomized trial. PLoS One 11:e0157045. doi: 10.1371/journal.pone.0157045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.van Lenthe M, van der Meulen R, Lassovski M, Ouabo A, Bakula E, Badio C, Cibenda D, Okell L, Piriou E, Grignard L, Lanke K, Rao B, Bousema T, Roper C. 2019. Markers of sulfadoxine–pyrimethamine resistance in Eastern Democratic Republic of Congo; implications for malaria chemoprevention. Malar J 18:430. doi: 10.1186/s12936-019-3057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2018. World malaria report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.Quakyi I, Tornyigah B, Houze P, Kusi KA, Coleman N, Escriou G, Laar A, Cot M, Fobil J, Asare GQ, Deloron P, Anang AK, Cottrell G, Ofori MF, Ndam NT. 2019. High uptake of intermittent preventive treatment of malaria in pregnancy is associated with improved birth weight among pregnant women in Ghana. Sci Rep 9:1–8. doi: 10.1038/s41598-019-55046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2018. Status report on artemisinin resistance and ACT efficacy. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16.Coppée R, Jeffares DC, Miteva MA, Sabbagh A, Clain J. 2019. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci Rep 9:10675. doi: 10.1038/s41598-019-47034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Hommerich L, Holmberg V, von Oertzen C, Bienzle U. 2008. Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis 198:1545–1549. doi: 10.1086/592455. [DOI] [PubMed] [Google Scholar]
- 18.Duah NO, Quashie NB, Abuaku BK, Sebeny PJ, Kronmann KC, Koram KA. 2012. Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg 87:996–1003. doi: 10.4269/ajtmh.2012.12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussiliou A, De Tove Y-S, Doritchamou J, Luty AJ, Massougbodji A, Alifrangis M, Deloron P, Ndam NT. 2013. High rates of parasite recrudescence following intermittent preventive treatment with sulphadoxine-pyrimethamine during pregnancy in Benin. Malar J 12:195. doi: 10.1186/1475-2875-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruizendaal E, Tahita MC, Geskus RB, Versteeg I, Scott S, d'Alessandro U, Lompo P, Derra K, Traore-Coulibaly M, de Jong MD, Schallig HDFH, Tinto H, Mens PF. 2017. Increase in the prevalence of mutations associated with sulfadoxine–pyrimethamine resistance in Plasmodium falciparum isolates collected from early to late pregnancy in Nanoro, Burkina Faso. Malar J 16:179. doi: 10.1186/s12936-017-1831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cisse M, Awandare GA, Soulama A, Tinto H, Hayette M-P, Guiguemdé RT. 2017. Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar J 16:38. doi: 10.1186/s12936-017-1695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins W, White NJ. 2008. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in Falciparum malaria. J Infect Dis 197:1605–1613. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- 23.Mockenhaupt FP, Teun Bousema J, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, Otchwemah RN, Sauerwein RW, Bienzle U. 2005. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop Med Int Health 10:901–908. doi: 10.1111/j.1365-3156.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang T, Chen J, Fu H, Wu K, Yao Y, Eyi JUM, Matesa RA, Obono MMO, Du W, Tan H, Lin M, Li J. 2019. High prevalence of Pfdhfr–Pfdhps quadruple mutations associated with sulfadoxine–pyrimethamine resistance in Plasmodium falciparum isolates from Bioko Island, Equatorial Guinea. Malar J 18:101. doi: 10.1186/s12936-019-2734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Eijk AM, Larsen DA, Kayentao K, Koshy G, Slaughter DEC, Roper C, Okell LC, Desai M, Gutman J, Khairallah C, Rogerson SJ, Hopkins Sibley C, Meshnick SR, Taylor SM, Ter Kuile FO. 2019. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis 19:546–556. doi: 10.1016/S1473-3099(18)30732-1. [DOI] [PubMed] [Google Scholar]
- 26.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale J-C, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ménard D, Khim N, Beghain J, Adegnika A, Shafiul-Alam M, Amodu O, Rahim-Awab G, Barnadas C, Berry A, Boum Y, Bustos M, Cao J, Chen J-H, Collet L, Cui L, Thakur G-D, Dieye A, Djallé D, Dorkenoo M, Eboumbou-Moukoko C, Espino F-E-C, Fandeur T, Ferreira-da-Cruz M-F, Fola A, Fuehrer H-P, Hassan A, Herrera S, Hongvanthong B, Houzé S, Ibrahim M, Jahirul-Karim M, Jiang L, Kano S, Ali-Khan W, Khanthavong M, Kremsner P, Lacerda M, Leang R, Leelawong M, Li M, Lin K, Mazarati J-B, Ménard S, Morlais I, Muhindo-Mavoko H, Musset L, Na-Bangchang K, Nambozi M, Niaré K, Noedl H, et al. . 2016. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyunt MH, Hlaing T, Oo HW, Tin-Oo L-L, Phway HP, Wang B, Zaw NN, Han SS, Tun T, San KK, Kyaw MP, Han E-T. 2015. Molecular assessment of artemisinin resistance markers, polymorphisms in the K13 propeller, and a multidrug-resistance gene in the eastern and western border areas of Myanmar. Clin Infect Dis 60:1208–1215. doi: 10.1093/cid/ciu1160. [DOI] [PubMed] [Google Scholar]
- 29.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, Dhorda M, Cheah PY, Pukrittayakamee S, Ashley EA, Anderson TJC, Nair S, McDew-White M, Flegg JA, Grist EPM, Guerin P, Maude RJ, Smithuis F, Dondorp AM, Day NPJ, Nosten F, White NJ, Woodrow CJ. 2015. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis 15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang EX, Chavatte J-M, Yi CSX, Tow C, Ying WJ, Khan K, Oh OSH, Chin SNM, Xin KW, Said Z, James L, Cutter J, Ho M, Tey J. 2019. Assessment of the risk posed to Singapore by the emergence of artemisinin-resistant malaria in the Greater Mekong subregion. West Pac Surveill Response 10:6–13. doi: 10.5365/wpsar.2018.9.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocan M, Bwanga F, Okeng A, Katabazi F, Kigozi E, Kyobe S, Ogwal-Okeng J, Obua C. 2016. Prevalence of K13-propeller gene polymorphisms among Plasmodium falciparum parasites isolated from adult symptomatic patients in northern Uganda. BMC Infect Dis 16:428. doi: 10.1186/s12879-016-1777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayih AG, Getnet G, Alemu A, Getie S, Mohon AN, Pillai DR. 2016. A unique Plasmodium falciparum K13 gene mutation in northwest Ethiopia. Am J Trop Med Hyg 94:132–135. doi: 10.4269/ajtmh.15-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouattara A, Kone A, Adams M, Fofana B, Maiga AW, Hampton S, Coulibaly D, Thera MA, Diallo N, Dara A, Sagara I, Gil JP, Bjorkman A, Takala-Harrison S, Doumbo OK, Plowe CV, Djimde AA. 2015. Polymorphisms in the K13-propeller gene in artemisinin-susceptible Plasmodium falciparum parasites from Bougoula-Hameau and Bandiagara, Mali. Am J Trop Med Hyg 92:1202–1206. doi: 10.4269/ajtmh.14-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henriques G, Hallett RL, Beshir KB, Gadalla NB, Johnson RE, Burrow R, van Schalkwyk DA, Sawa P, Omar SA, Clark TG, Bousema T, Sutherland CJ. 2014. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis 210:2001–2008. doi: 10.1093/infdis/jiu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda M, Kaneko M, Tachibana S-I, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, Takahashi N, Yamauchi M, Semihard M, Hashimoto M, Kaptur OT, Olio A, Lowboy PS, Aura MA, Any war DA, Odongo-Aginya EI, Okello-One J, Hirai M, Ohashi J, Palapas NMQ, Kataoka M, Tubo T, Kimura E, Horii T, Mitta T. 2018. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis 24:718–726. doi: 10.3201/eid2404.170141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutherland CJ, Langdell P, Sanders M, Mwangi J, van Schalkwyk DA, Kaur H, Nolde D, Tucker J, Bennett HM, Otto TD, Berriman M, Patel Ta Lynn R, Gkrania-Klotsas E, Chiodini PL. 2017. pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother 61:e02382-16. doi: 10.1128/AAC.02382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diallo A, Ndam NT, Moussiliou A, Dos Santos S, Ndonky A, Borderon M, Oliveau S, Lalou R, Le Hesran J-Y. 2012. Asymptomatic carriage of Plasmodium in urban Dakar: the risk of malaria should not be underestimated. PLoS One 7:e31100. doi: 10.1371/journal.pone.0031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce RJ, Drakeley C, Chandramohan D, Mosha F, Roper C. 2003. Molecular determination of point mutation haplotypes in the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum in three districts of northern Tanzania. Antimicrob Agents Chemother 47:1347–1354. doi: 10.1128/aac.47.4.1347-1354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ittisoponpisan S, Islam SA, Khanna T, Alhuzimi E, David A, Sternberg M. 2019. Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J Mol Biol 431:2197–2212. doi: 10.1016/j.jmb.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues CH, Pires DE, Ascher DB. 2018. DynaMut: predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res 46:W350–W355. doi: 10.1093/nar/gky300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang DQ, Tempel W, Loppnau P, Graslund S, He H, Ravichandran M, Seitova A, Arrowsmith CH, Edwards AM, Bountra C, El Bakkouri M, Senisterra G, Osman KT, Lovato DV, Hui R, Hutchinson A, Lin YH. 2015. Crystal structure analysis of Kelch protein from Plasmodium falciparum. PDB https://www.rcsb.org/structure/4ZGC (accession no. 4ZGC).
- 43.Guex N, Peitsch MC. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 44.Ramiole C, D'Hayer B, Boudy V, Legagneux J, Fonsart J, Houzé P. 2017. Determination of ketamine and its main metabolites by liquid chromatography coupled to tandem mass spectrometry in pig plasma: comparison of extraction methods. J Pharm Biomed Anal 146:369–377. doi: 10.1016/j.jpba.2017.09.001. [DOI] [PubMed] [Google Scholar]