Abstract
Background
Human-resident microbes can influence both health and disease. Investigating the microbiome using next-generation sequencing technology has revealed examples of mutualism and conflict between microbes and humans. Comparing to bacteria, the viral component of the microbiome (i.e., the “virome”) is understudied. Somatic tissues of healthy individuals are usually inaccessible for the virome sampling; therefore, there is limited understanding of the presence and distribution of viruses in tissues in healthy individuals and how virus infection associates with human gene expression and perturbs immunological homeostasis.
Results
To characterize the human virome in a tissue-specific manner, here we performed meta-transcriptomic analysis using the RNA-sequencing dataset from the Genotype-Tissue Expression (GTEx) Project. We analyzed the 8991 RNA-sequencing data obtained from 51 somatic tissues from 547 individuals and successfully detected 39 viral species in at least one tissue. We then investigated associations between virus infection and human gene expression and human disease onset. We detected some expected relationships; for instance, hepatitis C virus infection in the liver was strongly associated with interferon-stimulated gene upregulation and pathological findings of chronic hepatitis. The presence of herpes simplex virus type 1 in one subject’s brain strongly associated with immune gene expression. While torque teno virus was detected in a broad range of human tissues, it was not associated with interferon responses. Being notable in light of its association with lymphoproliferative disorders, Epstein-Barr virus infection in the spleen and blood was associated with an increase in plasma cells in healthy subjects. Human herpesvirus 7 was often detected in the stomach; intriguingly, it associated with the proportion of human leukocytes in the stomach as well as digestive gene expression. Moreover, virus infections in the local tissues associated with systemic immune responses in circulating blood.
Conclusions
To our knowledge, this study is the first comprehensive investigation of the human virome in a variety of tissues in healthy individuals through meta-transcriptomic analysis. Further investigation of the associations described here, and application of this analytical pipeline to additional datasets, will be useful to reveal the impact of viral infections on human health.
Keywords: Human virome, Transcriptome, GTEx, Human gene expression, Microbiome
Introduction
Advances in next-generation sequencing (NGS) methods in recent decades have made comprehensive surveys of a variety of microorganisms possible. Metagenomic analyses have explored microorganisms, including bacteria, phages, and viruses, in a variety of places, such as the oceans [1–4] and soils [5–7] and on Earth [8]. Perhaps, the most deeply surveyed microbiome is that of humans. The Human Microbiome Project aims to characterize bacteria, viruses, and other microorganisms in the human body [9, 10]. As they often live outside human cells, the human bacterial microbiome has been well described in multiple organs and samples via non-destructive sampling: the bacterial microbiome of the skin [11, 12], oral cavity [13], and gastrointestinal tract (including feces) [14] are well described. A major theme emerging from these studies is that, while certain microbial species are associated with pathology, many components of the microbiome likely play a symbiotic role in maintaining human health (reviewed in [15–18]).
Many viruses are clearly human pathogens: human immunodeficiency virus (HIV) and influenza virus are the causative agents of human diseases, and Epstein-Barr virus (EBV; also known as human herpesvirus 4 [HHV-4]) [19] and hepatitis C virus (HCV) [20] can drive oncogenesis. On the other hand, similar to the cases of the human bacterial microbiome, some viruses can chronically infect a broad range of human tissues without overt pathology. Previous studies have suggested that these viruses nonetheless have detrimental effects. For example, human respiratory syncytial virus and human rhinoviruses may play an important role in the inceptions of childhood asthma and atopic asthma, respectively [21, 22]. On the other hand, there are a few examples of viruses that exhibit protective effects. For example, GB virus C (GBV-C, also known as hepatitis G virus [HGV]) infection can be protective against HIV infection and improve survival [23]. In experiments using animal models, latent infection with murine gammaherpesvirus, which is genetically related to EBV in humans, confers symbiotic protection from bacterial infection [24]. Thus, virus infections are associated with multiple aspects of human health, and revealing the human “virome” would be beneficial for understanding the hidden mutualism and/or conflict between humans and viruses [25]. In the largest study of the human virome to date, Simon et al. performed meta-transcriptome analysis using more than 17,000 human RNA-sequencing (RNA-Seq) datasets to reveal the human virome [26]. However, the dataset used in this previous study is related to human diseases. Moustafa et al. used DNA-sequencing data obtained after whole-genome sequencing of more than 8000 “healthy” subjects using blood samples. The authors detected 19 human viruses in the blood; however notably, their approach was “blind” to RNA viruses [27]. Previous studies of the human virome have used specimens that are relatively easy to access in healthy individuals (e.g., blood or skin). In other words, it is technically difficult to obtain the somatic tissues from inside the human body (e.g., brain and internal organs) of healthy individuals for virome investigation. Moreover, it remains unclear (1) what kind of viruses infect in various tissues in healthy individuals and (2) how these virus infections influence human gene expression and perturb the homeostasis of these tissues.
To characterize the virome in the human body, we performed meta-transcriptomic analysis using RNA-Seq dataset provided by the Genotype-Tissue Expression (GTEx) Project [28]. We detected 39 viruses in a variety of human tissues, revealing both expected and unexpected associations between viral infections and human gene expression and human disease.
Results
The human virome across tissues
To characterize the virome across the human body, we analyzed the 8991 RNA-Seq data obtained from 51 somatic tissues of 547 individuals provided by the GTEx Project [28] (Additional file 1: Table S1). Using a new analytical pipeline (Fig. 1), we screened for viral RNAs derived from 5139 viruses and measured their abundances in the RNA-Seq dataset (see the “Methods” section). We detected 39 viral species (Fig. 2 and Additional file 2: Table S2 and Additional file 3: Table S3). We detected human herpesviruses, including EBV, herpes simplex virus type 1 (HSV-1; also known as human herpes virus 1 [HHV-1]), varicella-zoster virus (VZV; also known as human herpes virus 3 [HHV-3]), cytomegalovirus (CMV; also known as human herpes virus 5 [HHV-5]), human herpesvirus 6A (HHV-6A) and 6B (HHV-6B), human herpesvirus 7 (HHV-7), and other human viruses such as HCV and torque teno virus (TTV) (Fig. 2). Consistent with a previous study [27], we also detected non-human viruses, including insect viruses (e.g., deformed wing virus) and a plant virus (e.g., tomato spotted wilt virus). These non-human viruses may reflect “contamination” at the tissue level (e.g., plant matter in the gastrointestinal tract), during library preparation/sequencing or potentially, unexpected tropism of these or related viruses.
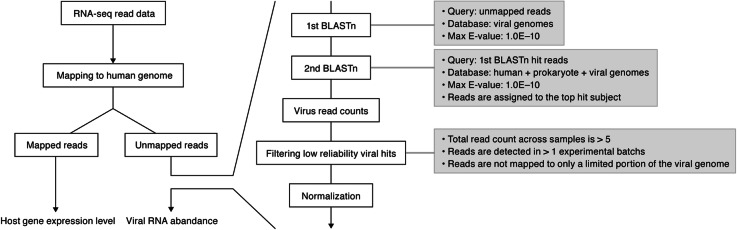
Fig. 1.
Workflow of this study. Flow diagrams of the meta-transcriptomic analysis pipeline constructed in this study. A detailed description is provided in the “Methods” section
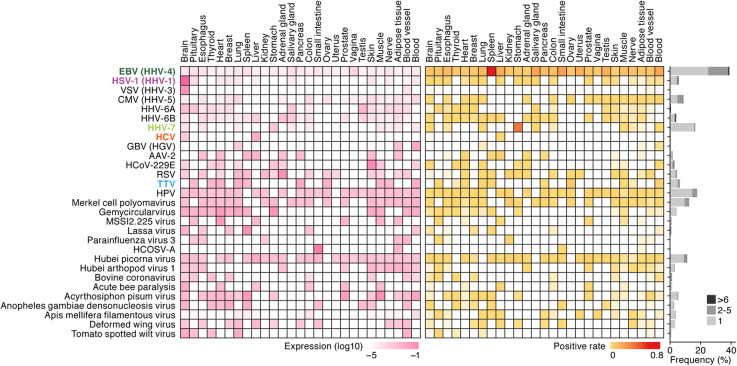
Fig. 2.
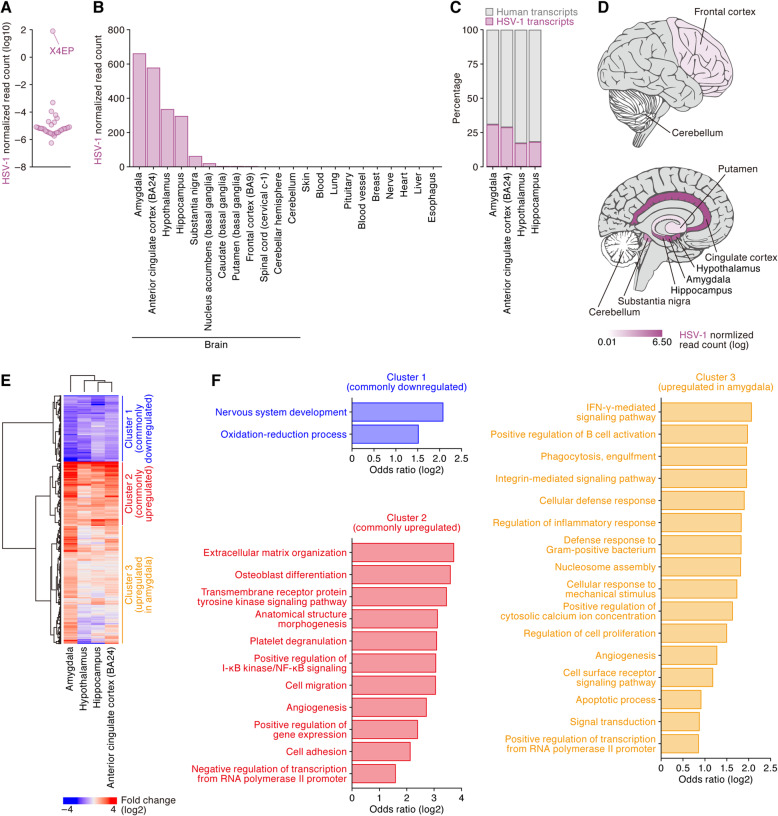
Landscape of the healthy human virome. The viruses detected in the GTEx dataset are summarized. The results of various subtypes of TTVs, HPVs, and Hubei picornaviruses detected were unified. The viruses for further investigations in Figs. 3, 4, 5, 6, and 7 (EBV, HSV-1, HHV-7, HCV, and TTV) are indicated in bold and colored. The alternative name of each virus is indicated in parentheses. (Left) The level of the transcript of each virus in each human tissue. The averages in the positive samples are shown as a heatmap. (Middle) The positive rate for each virus in each human tissue. (Right) The frequency of the individuals positive for respective viruses is summarized in bar graphs. The numbers of tissues positive for the respective viruses (1, 2–5, or more than 6) are also shown. AAV, adeno-associated virus; HCoV, human coronavirus; RSV, respiratory syncytial virus
Some human viruses (e.g., EBV and human papilloma virus [HPV]) were detected in a broad range of tissues in positive individuals, while the other viruses, including HCV and HHV-7, were detected in a tissue-specific manner (Fig. 2, right; for additional detail, see the subsequent sections). While we detected 16 RNA viruses, most detected human viruses were DNA viruses, including herpesviruses. Overrepresentation of DNA viruses in the human virome has previously been considered to result from sampling bias, as a previous study of the human virome has used DNA-sequencing [30]. However, our results suggest that mainly DNA viruses shape the healthy human virome even at the transcriptome level. We cannot fully exclude the possibility that the detection sensitivity of RNA viruses is lower than that of DNA viruses, perhaps due to the lower sequence conservation of RNA viruses compared to DNA viruses [31], or the acute nature of many RNA virus infections.
Another possibility for the abundant detection of human herpesviruses (e.g., EBV, HHV-6, and HHV-7) is that reads were dominantly mapped to telomeric/repetitive sequences of these viruses [19, 32]. Although the telomeric/repetitive regions were not covered by the masked regions completely, only a limited portion of viral-assigned reads were mapped to these regions (Additional file 4: Figure S1 and Additional file 5: Table S4). Therefore, we concluded that the presence of viral telomeric/repetitive regions does not affect our computational pipeline to measure viral abundance and that the human herpesviruses detected are authentic.
Association of HCV infection with robust interferon responses in the liver and the onset of hepatitis
Importantly, our approach allows us to quantify both viral RNA and host gene expression in the same sample (Fig. 1). We thus analyzed these two quantities to explore the effect of each virus on human gene expression patterns, comparing virus-positive samples to virus-negative samples in the same tissue. We first focused on HCV, which was specifically detected in liver (Fig. 2). HCV is classified into the family Flaviviridae, genus Hepacivirus and possesses a positive-sense single-stranded RNA (~ 10 kb) genome [20]. HCV is a causative agent of human hepatitis, and chronic infection with HCV can lead to severe illnesses including liver cirrhosis and hepatocellular carcinoma [20]. HCV was detected almost only in the liver (Fig. 2) and was found in three (GTEx sample IDs: ZAB4, 13SLX, and 139TS; see also Additional file 6: Table S5) out of 136 subjects with liver sampled (Fig. 3a). In histopathological examination, all three HCV-positive hepatic tissues showed portal tract expansion with fibrosis, bile duct reactive change, and lymphocyte infiltration and aggregation (Fig. 3b, left). Interface hepatitis and bridging fibrosis were also observed in two (13SLX and 139TS) and one (13SLX) case(s), respectively (Fig. 3b, left). These histological findings are compatible with hepatitis. On the other hand, two HCV-negative liver samples did not show these morphologic features suggesting hepatitis (Fig. 3b, right). Although hepatic disease did not cause their death, our findings suggest that HCV infection contributed to undiagnosed hepatitis in these three individuals.
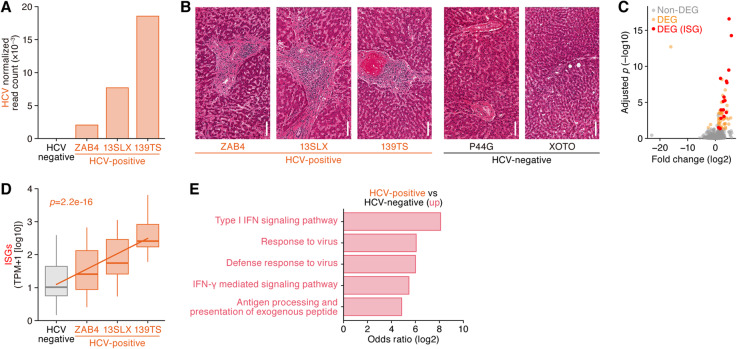
Fig. 3.
HCV in the livers with chronic hepatitis. a Detection of three individuals positive for HCV transcripts in the liver. The normalized read counts of HCV in the respective samples (sample IDs: ZAB4, 13SLX, and 139TS) are shown. b Histological observations in the liver of the three HCV-positive individuals. The photos of hematoxylin and eosin staining are provided from the GTEx dataset [29]. Two HCV-negative livers (sample IDs: P44G and XOTO) are shown as the negative controls. c Differential expression of genes between HCV-positive and HCV-negative livers. The X-axis indicates the fold change score, and the Y-axis indicates the statistical score. The positive and negative fold change scores indicate the up- and downregulations in the HCV-positive livers, respectively. The DEGs are indicated in pink dots, and ISGs are indicated in red with each gene symbol. d Expression levels of the upregulated ISGs in the three HCV-positive livers. Statistical significance was evaluated by linear regression test. e GO enrichment analysis of the upregulated genes in HCV-positive livers. The statistical summary of distribution is shown as a box plot. The terms with statistical significance (FDR < 0.05) are shown
HCV infection is sensed by cellular pathogen recognition receptors (PRRs) and can cause interferon (IFN) production. IFN-mediated signaling leads to expression of IFN-stimulated genes (ISGs), many of which exhibit antiviral effects (reviewed in [33, 34]). To address whether HCV infection is associated with IFN responses, including ISG upregulation in this dataset, we assessed the expression levels of ISGs in the liver. As shown in Fig. 3c, 18 out of the 62 genes significantly upregulated in HCV-positive compared to HCV-negative livers were ISGs. Moreover, the abundance of HCV RNA (Fig. 3a) clearly correlated with the expression levels of these 18 ISGs (Fig. 3d; p = 2.2e−16 by linear regression test). Moreover, gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) between HCV-positive and HCV-negative livers indicated that the genes associated with “type I interferon signaling pathway,” “defense response to virus,” and “viral process” were highly upregulated in the HCV-positive livers (Fig. 3e). Altogether, these findings are consistent with previous findings [33, 34] and verify the biological significance of the results by our analytic pipeline.
Based on the following two hallmarks, histological observations suggesting hepatitis (Fig. 3b) and upregulation of ISGs (Fig. 3c and d), the detection of HCV reads is a good chance to validate the specificity and sensitivity of our analytical pipeline. To validate the performance of our computational pipeline, we compared ours with the other three computational pipelines, Kraken [35], CLARK [36], and Kaiju [37], which have been previously reported as computational pipelines to identify viral sequences from NGS data. As shown in Additional file 7: Figure S2, these three pipelines detected HCV reads from the three HCV-positive samples (sample IDs: ZAB4, 13SLX and 139TS) identified by our pipeline. However, CLARK and Kraken detected tremendous amounts of HCV reads from all liver samples (Additional file 7: Figure S2). Taken together with the fact that the prevalence of HCV infection worldwide is less than 5% [38], these results suggest that the results obtained by CLARK and Kraken contain many false-positive hits, and these pipelines are relatively less specific for at least HCV in the dataset used in this study. On the other hand, the result obtained by Kaiju was similar to that by our pipeline (Additional file 7: Figure S2). Collectively, these results strongly support the validity of our analytical pipeline.
EBV infection in human plasma cells
We next focused on EBV, the most broadly detected virus in multiple individuals (Fig. 2, right). EBV is classified into the family Herpesviridae, genus Lymphocryptovirus, and possesses a double-stranded circular DNA (~ 172 kb) genome. EBV is known as the causative agent of infectious mononucleosis and some malignant diseases such as Burkitt lymphoma and post-transplant lymphoproliferative disorder. However, more than 90% of adults are positive for EBV, leading to the concept that EBV chronically infects humans without causing serious disease [19]. Although EBV preferentially infects human leukocytes, particularly B cells [19], EBV was broadly detected in various tissues (Fig. 2, left). This result may be attributed to the residual blood in each tissue even after the perfusion process, or to tissue-resident cells from the B cell lineage [28].
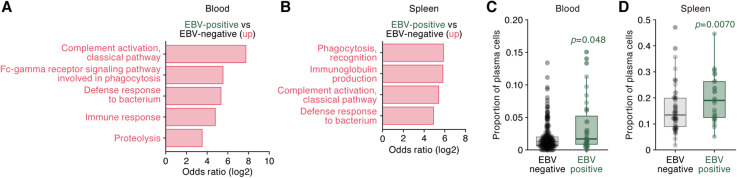
To address the biological impact of EBV infection, we particularly focused on the spleen and peripheral blood, where leukocytes, including B cells, naturally reside or circulate in high abundance. The spleen was the tissue with the highest proportion of EBV-positive samples (Fig. 2, middle). We detected DEGs that were upregulated in EBV-positive samples compared to EBV-negative samples and performed GO enrichment analysis. In both the blood (Fig. 4a) and spleen (Fig. 4b), genes encoding a variety of immunoglobulins were upregulated, leading to enrichment of GO terms such as “complement activation.” This suggested that the abundance of B cells may be increased in EBV-positive samples. To further address this point, we performed deconvolution analysis [39], which estimates the proportion of different immune cell types from bulk RNA-Seq data. The proportion of plasma cells, which produce antibodies in high abundance [40, 41], was significantly increased in EBV-positive samples compared to EBV-negative samples in both the blood (Fig. 4c; see also Additional file 8: Figure S3) and spleen (Fig. 4d; see also Additional file 9: Figure S4). It has been reported that productive EBV replication (also known as “lytic infection”) is initiated during B cell differentiation into plasma cells [42–44]. Moreover, a recent study suggested that EBV infection reprograms the gene expression pattern of infected B cells to resemble plasmablasts and early plasma cells [45]. Our findings correspond well to these experimental observations [42–45] further suggesting the biological validity of our investigations. Moreover, our results raise the possibility that the “healthy” human virome, in particular EBV, may influence the spectrum of B cell lymphoproliferative disorders such as monoclonal gammopathy of unknown significance [46, 47] in different individuals.
Fig. 4.
EBV in blood and spleen. a, b GO enrichment analysis of the upregulated genes in EBV-positive samples in blood (a) and spleen (b). The terms with statistical significance (FDR < 0.05) are shown. c, d Increase in the proportion of plasma cells in EBV-positive samples. The results of deconvolution analysis in the blood (c) and spleen (d) are shown. Each dot indicates the result from respective samples, and the statistical summary of distribution is shown as a box plot. Statistical significance was evaluated by Welch’s t test. See also Additional file 8: Figure S3 and Additional file 9: Figure S4
Unrecognized limbic encephalitis associated with HSV-1
HSV-1 is classified into the family Herpesviridae, genus Simplexvirus, and possesses a double-stranded circular DNA (~ 150 kb) genome. HSV-1 infects a broad range of cell types and tissues and causes cold sores and genital herpes infections [48]. After primary infection, HSV-1 latently infects nerve cells. It sporadically reactivates, leading to recurrent symptoms [48].
HSV-1 encephalitis is a severe and often fatal condition caused by HSV-1 infection [49]. Surprisingly, we detected high levels of HSV-1 transcripts in the brain (Fig. 2, left) of one individual (sample ID: X4EP) (Fig. 5a). As shown in Fig. 5b, HSV-1 transcripts were highly detected in certain brain regions, such as the amygdala, anterior cingulate cortex (also known as Brodmann’s area [BA] 24), hypothalamus and hippocampus, but were faintly detected in other regions, such as the basal ganglia and spinal cord. Reads assigned to HSV-1 accounted more than 20% of the total RNA-Seq reads in these four brain regions (Fig. 5c), which comprises the limbic system (Fig. 5d). Site-specific HSV-1 expression in the brain is consistent with anatomically restricted HSV-1 replication in the brain of patients who exhibit HSV-1 encephalitis [50, 51]. Notably, this subject died a slow death, and ischemic changes are noted on the brain biopsy. It is thus unclear whether the HSV-1 infection present in this subject reflects peri- or even port-mortem reactivation [52], or clinically unrecognized HSV-1 encephalitis present at the time of or even contributing to this subject’s death.
Fig. 5.
HSV-1 in the brain of an individual. a Detection of an individual (sample ID: X4EP) with severe HSV-1 infection. Each dot indicates the normalized read count of HSV-1 in each individual (n = 32). Note that HSV-1 reads were undetectable in 515 individuals. b HSV-1 transcript abundance across tissues in the individual (sample ID: X4EP). The normalized read counts of HSV-1 are shown. Only the brain was subdivided into each region. BA, Brodmann’s area. c Proportion of HSV-1 reads in the total assigned reads. The percentages in the respective brain regions of the individual (sample ID: X4EP) are shown. d Anatomical distribution of HSV-1 transcripts in the brain. The normalized expression levels of HSV-1 in the respective brain regions of the individual (sample ID: X4EP) are shown as a brain-shaped heatmap. The regions without RNA-Seq data are shown in gray. e Differential gene expression in the brain. DESeq2 was used for feature selection of genes toward downstream analyses. The heatmap contains 1242 DEGs (X4EP sample versus other samples) that were detected in any of the four brain regions (amygdala, hypothalamus, hippocampus, and anterior cingulate cortex). The DEGs were classified into three clusters: cluster 1 (blue), cluster 2 (red), and cluster 3 (orange), mainly consisting of commonly downregulated genes, commonly upregulated genes, and the genes upregulated specifically in the amygdala, respectively. f GO enrichment analysis of the three gene clusters. The terms with statistical significance (FDR < 0.05) are shown
To assess the impact of HSV-1 gene expression in these four brain regions, we compared the expression levels of human genes to those from these same regions in subjects with no HSV-1 expression. DEGs were classified into three clusters: commonly downregulated genes (cluster 1), commonly upregulated genes (cluster 2), and the genes upregulated in the amygdala (cluster 3) (Fig. 5e). GO analysis revealed that neuronal genes (GO term “nervous system development”) were commonly downregulated, while the genes associated with cell activation, inflammation (e.g., GO terms “positive regulation of I-κB kinase/NF-κB signaling” in cluster 2 and “regulation of inflammatory response” in cluster 3), and immune responses (e.g., “platelet degranulation” in cluster 2 and “positive regulation of B cell activation” in cluster 3) were commonly upregulated (Fig. 5f). In particular, the GO term “IFN-γ-mediated signaling pathway” was the highest ranked in cluster 3, and this ontology included ISGs (Fig. 5f). This suggests that HSV-1 expression was present in this subject long enough prior to death to allow for subsequent immune responses. The GTEx annotation for this subject indicates that the cause of death was a hepatic disorder. However, our data may suggest limbic encephalitis due to HSV-1 infection as an alternative cause of death in this subject.
TTV infection in many human tissues without inducing an IFN response
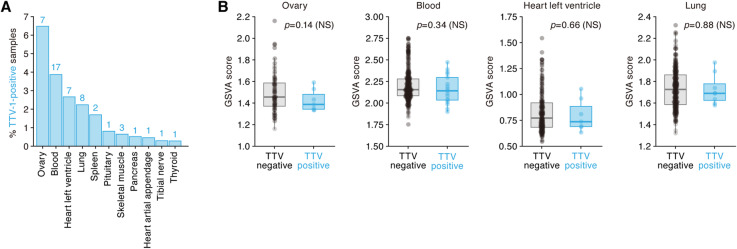
Torque teno virus (TTV) was recently classified into the family Circoviridae, genus Anellovirus, by the International Committee on Taxonomy of Viruses (ICTV) in 2011 [53]. TTV is the first recognized human virus with a single-stranded circular DNA (~ 3.8 kb) genome [54]. Although this virus was first found in a patient with non-A to G hepatitis in Japan [55], its association with diseases is poorly understood [54]. As shown in Fig. 6a, TTV was detected in a variety of tissues in multiple individuals. TTV is a single-stranded DNA virus, and human Toll-like receptor 9 is a PRR that senses single-stranded DNA and induces IFN production [56, 57]. Therefore, we hypothesized that TTV infection is associated with increased ISG expression, as observed for HCV (Fig. 3) and HSV-1 (Fig. 5). To address this hypothesis, we chose four tissues (ovary, blood, heart [left ventricle], and lung) with a high percentage of TTV-positive samples (Fig. 6a). However, upregulation of ISGs in association with TTV infection was not observed (Fig. 6b). Our findings expand on those from a DNA-based virome survey [30] to suggest that TTV not only infects, but is transcribed in a variety of tissues in a large fraction of people. Moreover, we show that it does so without triggering IFN production. In light of the known association with increased TTV viremia after transplantation and immunosuppression [58], these results raise the possibility that an immune pathway other than IFN may be responsible for the control of this virus. Although it remains unclear whether TTV infection potentially associates with certain diseases, our findings suggest that TTV is a “commensal” virus that is easily detectable at the level of RNA expression in a variety of human tissues with relatively high prevalence.
Fig. 6.
TTV in a variety of tissues in multiple individuals. a Percentages of the samples positive for TTV in each tissue. The numbers on the bar graphs indicate the number of samples positive for TTV. b The gene set-level expression score (GSVA score) of ISGs in the four tissues of TTV-positive and TTV-negative samples. Each dot indicates the score of each sample. Statistical significance is evaluated by Welch’s t test. NS, no statistical significance
Strong association of a human gene expression pattern with the presence of HHV-7 transcripts in the stomach
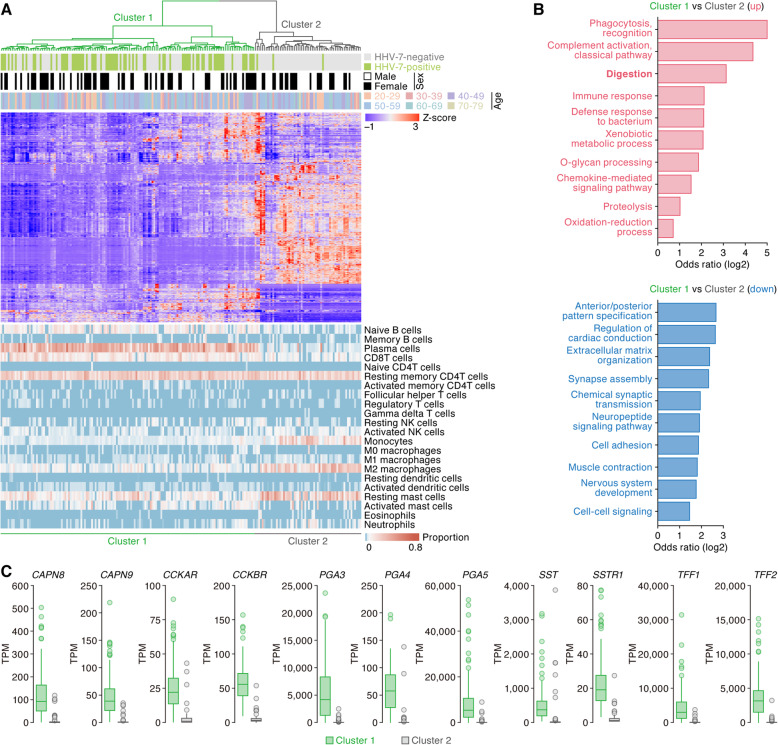
HHV-7 is classified into the family Herpesviridae, genus Roseolovirus, and possesses a double-stranded circular DNA (~ 153 kb) genome [32]. This virus is known as one of the causative agents of roseola infantum (also known as exanthema subitum), a usually mild illness of infants [32]. Surprisingly, we detected HHV-7 transcripts specifically in the stomach (Fig. 2, left), and 37.4% (76 out of the 203 samples) of the stomach samples were positive for HHV-7 (Fig. 2, middle and right). Our findings expand on those from a recent DNA-based virome survey [59] and suggest that HHV-7, a member of roseolovirus, not only infects, but is transcribed in the stomach. Using unsupervised clustering of human gene expression patterns, stomach samples were classified into two clusters according to the human gene expression patterns (Fig. 7a, top). HHV-7-positive samples were strikingly enriched in cluster 1 (Fig. 7a, middle; 73 out of the 76 HHV-7-positive samples; 96.1%). These results suggest that the HHV-7 transcription status is strongly associated with the global human gene expression pattern in the stomach. The difference of global human transcriptome between clusters 1 and 2 may be due to the different anatomical regions, and HHV-7 may predominantly infect the stomach region categorized as cluster 1.
Fig. 7.
Potential effect of HHV-7 infection on human gene expression in the stomach. a Association of the human gene expression pattern with HHV-7 infection in the stomach. Stomach samples (n = 203) were classified into the two clusters based on the 1000 most highly expressed human genes. (Top) The relative expression levels of human genes are shown as a heatmap. (Middle) Distribution of the samples positive for HHV-7 (n = 78). Note that HHV-7-positive samples accumulated in cluster 1. (Bottom) Deconvolution analysis of human leukocytes residing in the stomach. The proportions of the respective human leukocytes are shown as a heatmap. b GO enrichment analysis of the DEGs between clusters 1 and 2. The top 10 terms significantly upregulated (top) and downregulated (bottom) in cluster 1 compared to those in cluster 2 are shown. c Differential expression of the genes associated with the digestion process. The expression levels of 11 genes associated with the digestion process (CAPN8, CAPN9, CCKAR, CCKBR, PGA3-5, SST, SSTR1, TFF1, and TFF2) in clusters 1 and 2 are shown as TPM
HHV-7 preferentially infects CD4+ T cells [32]. The results of deconvolution analysis [39] of the transcriptome of both HHV-7-positive and HHV-7-negative samples were consistent with the presence of resting memory CD4+ T cells in the stomach (Fig. 7a, bottom). Additionally, transcripts attributable to plasma cells were relatively abundant in cluster 1, while those attributable to myeloid cells (monocytes and M2 macrophages) and mast cells were abundant in cluster 2 (Fig. 7a, bottom). These findings suggest that HHV-7 infection is associated with the pattern of leukocytes residing in the stomach. We further performed GO analysis on the DEGs between these two clusters. In addition to the GO terms associated with tissue-resident leukocytes (e.g., “phagocytosis” and “immune response”), GO terms such as “digestion” were highly ranked in the upregulated genes in cluster 1 compared to those in cluster 2 (Fig. 7b). In fact, the expression levels of some genes encoding enzymes and proteins that play critical roles in digestion in cluster 1 were significantly higher than those in cluster 2 (Fig. 7c). For instance, calpains (CAPN8 and CAPN9) [60] and pepsinogens (PGA3–5) [61] digest proteins and peptides, and the signals mediated by cholecystokinin receptors (CCKAR and CCKBR) help the digestion of proteins and lipids [62]. Somatostatin (SST) and its receptor (SSTR1) control the secretion of gastric acids [63], and trefoil factors (TFF1 and TFF2) help protect and repair the gastrointestinal mucosa [64]. These genes are expressed in stomach cells (i.e., secretory epithelial cells) but not in leukocytes. Thus, our results suggest that HHV-7 infection may be associated with increased expression of the transcripts important in the function of the stomach (Fig. 7b). Another possibility is that HHV-7 is highly transcribed in the stomach region where digestive genes are highly expressed. More study of the potentially mutualistic relationship between HHV-7 and humans influencing digestive function will be needed.
Effect of local virus infections on systemic antiviral immunological homeostasis
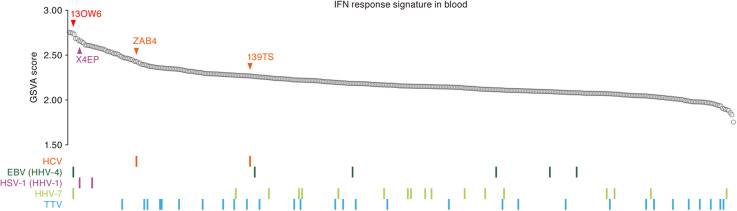
In Fig. 2, we summarized the tissue-level tropism of 39 viruses present in the human virome. We found upregulation of ISGs, a hallmark of the human responses against virus infections, in certain tissues with evidence of viral transcription, including HCV in the liver (Fig. 3) and HSV-1 in the brain (Fig. 5). However, it was unclear whether abundant viral transcription and likely productive viral infection we observed localized to certain tissues (e.g., liver and brain) affected systemic immunological homeostasis in the human body. To investigate this issue, we evaluated the magnitude of IFN responses in the blood, circulating throughout the human body, as the gene set-based expression score of ISGs [65]. As shown in Fig. 8, the magnitude of ISG expression in the blood varied in the respective individuals. Interestingly, the individuals that exhibited HCV infection in the liver (Fig. 3) and HSV-1 in the brain (Fig. 5) exhibited relatively increased levels of ISG expression even in the blood, where the transcription of these viruses was hardly detected. These results suggest that local tissue-specific expression of these viruses may have triggered an IFN response in the blood, perturbing systemic immunological homeostasis. On the other hand, the sole infection of EBV, HHV-7, and TTV was not associated with the magnitude of ISG expression in the blood (Fig. 8). These findings suggest that the potential to trigger innate immune responses (e.g., IFN production) may be dependent on the type of virus or the chronicity of viral infection. However, a sample positive for both EBV and HHV-7 (sample ID: 13OW6) exhibited a very high GSVA score of ISGs in the blood (Fig. 8), which suggests the possibility that co-infection of these viruses may synergistically trigger an IFN response in the blood.
Fig. 8.
Effect of virus infections on IFN responses in body-circulating blood. (Top) The gene set-level expression score (GSVA score) of ISGs in the blood. The colored arrows with sample IDs indicate the positions of the samples positive for HCV (orange) and HSV-1 (purple). The sample positive for both EBV and HHV-7 (sample ID: 13OW6) is indicated by a red arrow. (Bottom) Individuals positive for respective viruses. “Positive” means that the virus abundance is more than the upper quartile
Discussion
In this study, we comprehensively addressed the human virome in a variety of human tissues through meta-transcriptomic analysis. We found that the human virome includes several viruses “hidden” by expression/replication in tissues inside the human body without being abundant in the peripheral blood. As expected, different individuals and different tissues harbor distinct virome. We described some expected (e.g., the upregulation of ISGs by HCV infection) and unexpected (e.g., the association of HHV-7 infection with higher expression of digestive genes in the stomach) associations with the presence of viral RNAs. Collectively, these findings strongly suggest that our analytical pipeline is useful to reveal the potential impacts of viral infections on human health. Moreover, in our recent study [66], we used the same analytical pipeline and detected the expression of inherited chromosomally integrated HHV-6 from the GTEx subjects, which were positive for this integrated virus reported in another study [67]. This further strengthens the confidence of our approach. However, some improvements to the analytical pipeline could be beneficial for subsequent work to define the virome. First, since we employed a quantification-oriented analytical pipeline, we probably failed to detect viruses that are unknown or highly divergent from the reference genome sequences. To find such viruses in silico, de novo assembly techniques followed by sequence similarity searches on translated nucleotide databases (i.e., tBLASTn) should be employed. Second, here we excluded retroviruses, including HIV, from investigation because abundant sequences derived from endogenous retroviruses in the human genome were detected, and it was difficult to distinguish them from authentic exogenous retroviruses. If retroviruses, including HIV, are targeted, the analytical pipeline should be modified. Third, as implied in a previous report [27], relatively low abundant sequences might be attributed to the contamination from commercial reagents and the environment or mistakes in the demultiplexing of NGS reads. To exclude the effect of cross-contamination, our analytical pipeline included several filters (see Fig. 1 and the “Methods” section). Nevertheless, we detected a low number of reads mapped to Lassa virus in some samples (Fig. 2). Lassa virus is the causative agent of the often fatal Lassa hemorrhagic fever and is prevalent only in Africa (reviewed in [68]); the GTEx samples were collected in the USA. We are unable to completely exclude the possibility that the GTEx samples positive for Lassa virus transcripts came from subjects asymptomatically infected with this virus. However, these reads mapped only one (L segment) out of the viral two segments (see the “Methods” section and Additional file 10: Figure S5). Moreover, although Lassa virus infection strongly induces IFN responses [69], ISGs were not upregulated in Lassa virus-positive specimens (data not shown). Since we did not find supportive biological results suggesting that the reads of Lassa virus we detected are authentic, we did not deeply focus on this virus in this study. To deeply survey the human virome with high accuracy, experimental verification of the presence of viruses suggested by reads at very low is required.
Conclusion
Here we performed integrative analyses on the association of virus infections with the human transcriptome at tissue-level resolution. Our results suggest that the human virome potentially influences human health, not only disease. While our results document the healthy human virome in new detail, they will surely motivate even more granular investigations of this virome in the future, both as described above and at the resolution of single cells. This could allow screening for new viral pathogens and simultaneous definition of viral tropism. Future investigations using NGS data obtained from both disease-affected and healthy subjects will thus enable detection of currently hidden associations between the human virome and human health and disease.
Methods
Dataset
Pair-ended, poly A-enriched RNA-Seq data provided by GTEx (version 7.p2) were analyzed. Information of the analyzed RNA-Seq dataset is summarized in Additional file 1: Table S1. The raw fastq files were downloaded from the Sequence Read Archive (SRA) using SRA Toolkit (version 2.8.2-1). Only data with the expected sequencing length (i.e., “AvgSpotLen” column in Additional file 1: Table S1) less than 200 bp were analyzed (i.e., long-read sequencing data were excluded).
Reference genome sequences of viruses registered in the NCBI Viral Genomes Resource [70] were downloaded via Batch Entrez, accessed 13 January 2019. Only viruses whose hosts are registered as invertebrates, vertebrates, and humans were analyzed. Information on the viral genome sequences analyzed in the present study is summarized in Additional file 11: Table S6.
Sequences of prokaryotic representative genomes were downloaded from the NCBI RefSeq Database [71], accessed 14 February 2019. Information of the prokaryotic genome sequences used in the present study is summarized in Additional file 12: Table S7.
Image data were downloaded manually from Histology Viewer on GTEx Portal [29]. Then, representative digital images of each data were collected using Aperio Imagescope software (version 12.3.3.5048, Leica Biosystems).
As sources of functional gene sets, “GO biological process,” “GO cellular component,” “MSigDB canonical pathway,” and “InterPro” were used. “GO biological process” and “GO cellular component” were obtained from the GO Consortium [72, 73] (GO validation date: 30 August 2017), “canonical pathway” was from MSigDB [74, 75] (version 6.1), and “InterPro” was from BioMart on the Ensembl website [76] on 13 February 2018.
Construction of custom sequence databases
A viral genomic sequence database was constructed as follows. First, of the viral sequences, sequence regions that highly resemble the human or bacterial genomes were masked (i.e., replaced by the sequence “NNN …”). The sequence regions to be masked were determined by a local sequence similarity search using BLASTn (in BLAST+ version 3.9.0) [77]. The word size and E value parameters were set at 11 and 1.0e−3, respectively. As sources of the human and bacterial genome sequences, the human reference genome (GRCh38/hg38) and the prokaryotic representative genomes were used, respectively. Second, redundant viral sequences (i.e., sequences disclosing > 90% global sequence identity with each other) were concatenated. The sequence identity was calculated by Stretcher software (in EMBOSS version 6.6.0.0) [78]. The viral sequences included in the viral sequence database are summarized in Additional file 11: Table S6. In addition, a sequence database comprising (1) the human reference genome, (2) the prokaryotic representative genomes, and (3) the custom viral genomes prepared above was also constructed.
Quantification of human gene expression
Low-quality sequences in RNA-Seq fragments (i.e., a set of paired RNA-Seq reads) were trimmed using Trimmomatic (version 0.36) [79] with the option “SLIDINGWINDOW:4:20.” RNA-Seq fragments were mapped to the human reference genome (GRCh38/hg38) using STAR (version 2.6.0c) [80] with the GENCODE gene annotation (version 22) [81]. STAR was run using the same options and parameters as those used in the GDC mRNA Analysis Pipeline. RNA-Seq fragments mapped on the exons of genes were counted using Subread featureCounts (version 1.6.3) [82] with GENCODE gene annotation. The option “fracOverlap” was set at 0.25. RNA-Seq fragments assigned to multiple features were not counted. Among the GENCODE gene annotations, only the genes assigned to any biological features (i.e., the genes that are registered in any of the functional gene sets [described in the above section]) were used for analysis. The expression level of human genes was normalized as transcripts per million (TPM) [83].
Extraction of RNA-Seq fragments disclosing similarity to viral sequences
The analytical pipeline is illustrated in Fig. 1. First, RNA-Seq fragments that were not properly mapped to the human reference genome were extracted using the “samtools view” [84] command with the options “-f 12 -F 256.” Second, a 1st BLASTn search of the unmapped RNA-Seq reads was performed on the viral genome sequence database prepared above. The word size and E value parameters were set at 11 and 1.0e−10, respectively. Regarding the BLAST-hit reads, a 2nd BLASTn search was performed on the database comprising the human, bacterial, and viral genome sequences prepared above. RNA-Seq fragments were assigned to the top-hit feature according to the bit score. In the case of a tie, the reads were discarded. If a set of paired reads was assigned to the distinct features, the reads were discarded. RNA-Seq fragments assigned to respective viruses were counted in each RNA-Seq dataset, and a count matrix of the viral RNA-Seq fragments was generated.
Filtering low-reliability viral hits
From the count matrix of the viral RNA-Seq fragments, viruses in which the total count was less than 5 were excluded. Viruses that were detected only in a single experimental batch (SMGEBTCH; Additional file 1: Table S1) were also excluded. In addition, we excluded viruses for which only a limited portion of the viral genome was covered by the RNA-Seq fragment hits. We divided equally a viral genome sequence into 10 regions, and we excluded the virus if RNA-Seq fragments hit only < 5 out of the 10 regions in the viral genome. For example, Lassa virus satisfied this filtering criteria, but Pepper chlorotic spot virus did not because of only limited fraction assigned with reads (Additional file 10: Figure S5). Finally, we manually curated the list of detected viruses. Retroviridae viruses were removed because there is a possibility of cross-contamination from endogenous retroviral sequences. “Autographa californica nucleopolyhedro virus (accession no. NC_001623.1)” was removed because there is a possibility of cross-contamination from baculovirus vectors. “Chimpanzee alpha-1 herpesvirus strain 105640” (accession no. NC_023676.1) was also removed because of the suspicion of misshit of HSV-1 transcripts: “Chimpanzee alpha-1 herpesvirus strain 105640” was detected only in one individual who held an extremely large amount of HSV-1 reads (sample ID: X4EP, in Fig. 5a). The final set of detected viruses, consisting of 39 viral species, is listed in Additional file 2: Table S2. For illustration and analysis, various subtypes of detected TTVs, human papilloma viruses (HPVs), and Hubei picornavirus were unified as shown in Additional file 2: Table S2.
GTEx provides RNA-Seq data of lymphocytes immortalized by EBV infection (i.e., lymphoblastoid cells [LCLs]). Since it is suspected that there is read contamination from LCL samples in the sequencing step, we did not measure the EBV transcript abundance in the samples that had the same experimental batch (SMGEBTCH) as the LCL samples. As a result, the EBV transcript abundance was measured only in 3031 out of the 8991 samples. Additionally, as shown in Fig. 5a, extremely high levels of HSV-1 transcripts were detected in the samples of the subject “X4EP.” Therefore, we did not measure the HSV-1 transcript abundance in the samples that had the same experimental batch as the X4EP samples. As a result, the HSV-1 transcript abundance was measured only in 8879 of the 8991 samples.
Quantification of viral transcript abundances
To normalize viral transcript abundance, the number of RNA-Seq fragments assigned to a certain virus was divided by the total number of RNA-Seq fragments assigned to any human genes, the length of the human genome sequence, and the length of the viral genome sequence, as described in a previous study [27]. The RefSeq annotation recodes many subtypes of TTV, HPV, and Hubei picornavirus. In each virus species, viral RNA abundances of the subtypes were summed, and the total viral abundance was used for analyses.
Comparison of the performance of analytical pipelines
To evaluate the performance of our pipeline, we used three published pipelines: Kraken (version 2.0.8) [35], CLARK (version 1.2.6.1) [36], and Kaiju (version 1.7.3) [37]. As the viral sequence database, NCBI Viral Genomes Resource [70] was downloaded through the command implemented in each pipeline (downloaded 24 February 2020). From 136 liver samples, the number of reads assigned to HCV taxonomy (taxid 11103 or 41856) was quantified by three pipelines with default parameters and compared to our pipeline result (Additional file 7: Figure S2).
Repetitive sequence annotation in EBV, HHV-6, and HHV-7
To annotate telomeric/repetitive regions on herpesvirus genomes, we used a web-based RepeatMasker software (version open-4.0.9) [85]. From the search results, genomic regions annotated as “Simple_repeat” class were regarded as repetitive regions. Of these, regions annotated as “(CTAACC)n” were regarded as telomeric regions (Additional file 4: Figure S1).
Differential gene expression analysis and functional annotation
Differential expression analysis was performed by DESeq2 (version 1.24.0) [86]. Differentially expressed genes were detected according to the following criteria: false discovery rate (FDR) < 0.05 and the absolute value of log2-transformed fold change > 1. Gene ontology enrichment analysis (Figs. 3e, 4a, b, 5f, and 7b) was performed by an overlapping-based method with Fisher’s exact test. FDR correction was performed using the Benjamini-Hochberg method. Redundant gene sets were summarized using REViGO [87]. Only the results for the GO biological process are shown. Immune cell composition in a “bulk” tissue sample (Figs. 4c, d, 7a, Additional file 8: Figure S3, and Additional file 9: Figure S4) was estimated from the gene expression data using CIBERSORT (version 1.06) [39]. The TPM-normalized gene expression matrix and LIM-22 dataset in CIBERSORT were used as mixture and signature matrices, respectively. As the gene set-level expression score of ISGs, that of the “type I IFN signaling pathway (GO: 0060337)” calculated by Gene Set Variation Analysis (GSVA) software [65] was used (Figs. 6b and 8).
Visualization
All heatmaps were drawn by the R package ComplexHeatmap (version 1.17.1) [88]. HSV-1 expression annotation to brain regions (Fig. 5d) was performed by the R package cerebroViz (version 1.0) [89].
Supplementary information
Additional file 1: Table S1 Information on the RNA-Seq dataset used in this study.
Additional file 2: Table S2 List of the 39 viral species detected in this study.
Additional file 3: Table S3 Read count of the respective 39 viral species detected in this study.
Additional file 4: Figure S1 Read coverage on EBV, HHV-6 and HHV-7 genomes.
Additional file 5: Table S4 List of the genomic positions of the masked regions in each viral genome.
Additional file 6: Table S5 Information on the GTEx samples.
Additional file 7: Figure S2 Comparison of our analytical pipeline with the other pipelines.
Additional file 8: Figure S3 Deconvolution analysis in the blood of EBV-positive and -negative subjects.
Additional file 9: Figure S4 Deconvolution analysis in the spleen of EBV-positive and -negative subjects.
Additional file 10: Figure S5 Read mapping to Lassa virus segment L and Pepper chlorotic spot virus segment L.
Additional file 11: Table S6 Information on the viral genome sequences used in this study.
Additional file 12: Table S7. Information on the prokaryotic genome sequences used in this study.
Acknowledgements
We would like to thank Mai Suganami (Division of Systems Virology, Institute for Medical Science, the University of Tokyo, Tokyo, Japan) for technical support and Nicholas Parrish (RIKEN Center for Integrative Medical Sciences, Kanagawa, Japan) for kind proofreading of the manuscript. The super-computing resource, SHIROKANE, was provided by Human Genome Center, The Institute of Medical Science, The University of Tokyo, Japan. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Abbreviations
- AAV
Adeno-associated virus
- AIDS
Acquired immunodeficiency syndrome
- BA
Brodmann’s area
- CMV
Cytomegalovirus
- dbGaP
Database of Genotypes and Phenotypes
- DEG
Differentially expressed gene
- EBV
Epstein-Barr virus
- FDR
False discovery rate
- GBV-C
GB virus C
- GO
Gene ontology
- GSVA
Gene Set Variation Analysis
- GTEx
Genotype-Tissue Expression
- HCoV
Human coronavirus
- HCV
Hepatitis C virus
- HCV
Hepatitis G virus
- HHV-1
Human herpesvirus 1
- HHV-3
Human herpesvirus 3
- HHV-4
Human herpesvirus 4
- HHV-5
Human herpesvirus 5
- HHV-6A
Human herpesvirus 6A
- HHV-6B
Human herpesvirus 6B
- HHV-7
Human herpesvirus 7
- HIV
Human immunodeficiency virus
- HPV
Human papilloma virus
- HSV-1
Herpes simplex virus type 1
- ICTV
International Committee on Taxonomy of Viruses
- IFN
Interferon
- ISG
Interferon-stimulated gene
- LCL
Lymphoblastoid cell
- NGS
Next-generation sequencing
- NS
No statistical significance
- PRR
Pathogen recognition receptor
- RNA-Seq
RNA-sequencing
- RSV
Respiratory syncytial virus
- TPM
Transcripts per million
- TTV
Torque teno virus
- VZV
Varicella-zoster virus
Authors’ contributions
RK and JI designed the project and performed the bioinformatics analysis. KT and TS conducted the pathohistological analysis. KS supervised the project and wrote the manuscript. All authors edited, reviewed, and approved the manuscript.
Funding
This study was supported in part by JST CREST (to KS) including AIP challenge program (to RK); AMED J-PRIDE JP19fm0208006 (to KS); AMED Research Program on HIV/AIDS JP19fk0410014 (to KS) and JP19fk0410019 (to KS); AMED Emerging/Re-emerging Infectious Diseases 20fk0108146h0001 (to KS) and JP19fk0108104 (to TS); KAKENHI Scientific Research B JP18H02662 (to KS); KAKENHI Scientific Research on Innovative Areas JP16H06429 (to KS), JP16K21723 (to KS), JP17H05813 (to KS), and JP19H04826 (to KS); KAKENHI Early-Career Scientists 20 K15767 (to JI); JSPS Research Fellow PD JP19J01713 (to JI); Ichiro Kanehara Foundation (to KS); Lotte Foundation (to KS); Mochida Memorial Foundation for Medical and Pharmaceutical Research (to KS); Sumitomo Foundation (to KS); Daiichi Sankyo Foundation of Life Science (to KS); and Uehara Foundation (to KS).
Availability of data and materials
The data, associated protocols, code, and materials in this study are available at reference [91].
Ethics approval and consent to participate
The utilization of the GTEx dataset was authorized through the Database of Genotypes and Phenotypes (dbGaP) [90] for the following project: “Screening of subclinical viral infections in healthy human tissues (#19481).”
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ryuichi Kumata, Email: ryuichi.kumata.study@gmail.com.
Jumpei Ito, Email: jampei0513@yahoo.co.jp.
Kenta Takahashi, Email: tkenta@nih.go.jp.
Tadaki Suzuki, Email: tksuzuki@nih.go.jp.
Kei Sato, Email: ksato@ims.u-tokyo.ac.jp.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12915-020-00785-5.
References
- 1.Breitbart M, Salamon P, Andresen B, Mahaffy JM, Segall AM, Mead D, Azam F, Rohwer F. Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci U S A. 2002;99(22):14250–14255. doi: 10.1073/pnas.202488399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory AC, Zayed AA, Conceicao-Neto N, Temperton B, Bolduc B, Alberti A, Ardyna M, Arkhipova K, Carmichael M, Cruaud C, et al. Marine DNA viral macro- and microdiversity from pole to pole. Cell. 2019;177(5):1109–1123. doi: 10.1016/j.cell.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roux S, Brum JR, Dutilh BE, Sunagawa S, Duhaime MB, Loy A, Poulos BT, Solonenko N, Lara E, Poulain J, et al. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature. 2016;537(7622):689–693. doi: 10.1038/nature19366. [DOI] [PubMed] [Google Scholar]
- 4.Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304(5667):66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 5.Jin M, Guo X, Zhang R, Qu W, Gao B, Zeng R. Diversities and potential biogeochemical impacts of mangrove soil viruses. Microbiome. 2019;7(1):58. doi: 10.1186/s40168-019-0675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15(10):579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasiah S, Lovett J, Ghosh D, Roy K, Fuhrmann JJ, Radosevich M, Wommack KE. Dynamics of autochthonous soil viral communities parallels dynamics of host communities under nutrient stimulation. FEMS Microbiol 866 Ecol. 2015;91(7):fiv063. [DOI] [PubMed]
- 8.Paez-Espino D, Eloe-Fadrosh EA, Pavlopoulos GA, Thomas AD, Huntemann M, Mikhailova N, Rubin E, Ivanova NN, Kyrpides NC. Uncovering Earth’s virome. Nature. 2016;536(7617):425–430. doi: 10.1038/nature19094. [DOI] [PubMed] [Google Scholar]
- 9.Zou S, Caler L, Colombini-Hatch S, Glynn S, Srinivas P. Research on the human virome: where are we and what is next. Microbiome. 2016;4(1):32. doi: 10.1186/s40168-016-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Program NCS, Bouffard GG, Blakesley RW, Murray PR, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: moving beyond associations. Cell Host Microbe. 2017;22(5):589–599. doi: 10.1016/j.chom.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes. 2017;8(6):607–615. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virgin HW. The virome in mammalian physiology and disease. Cell. 2014;157(1):142–150. doi: 10.1016/j.cell.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delwart E. A roadmap to the human virome. PLoS Pathog. 2013;9(2):e1003146. doi: 10.1371/journal.ppat.1003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longnecker RM, Kieff E, Cohen JI. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology, vol. 2, 6th edn. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1898–1959. [Google Scholar]
- 20.Ray SC, Bailey JR, Thomas DL. Hepatitis C virus. In: Knipe DM, Howley PM, editors. Fields Virology, vol. 1, 6th edn. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 795–824. [Google Scholar]
- 21.Piedimonte G. Respiratory syncytial virus and asthma: speed-dating or long-term relationship? Curr Opin Pediatr. 2013;25(3):344–349. doi: 10.1097/MOP.0b013e328360bd2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, Ruuskanen O. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10(6):1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang J, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345(10):707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 24.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 25.Pennisi E. Microbiology. Going viral: exploring the role of viruses in our bodies. Science. 2011;331(6024):1513. doi: 10.1126/science.331.6024.1513. [DOI] [PubMed] [Google Scholar]
- 26.Simon LM, Karg S, Westermann AJ, Engel M, Elbehery AHA, Hense B, Heinig 915 M, Deng L, Theis FJ. MetaMap: an atlas of metatranscriptomic reads in human disease-related RNA-seq data. Gigascience. 2018;7(6):giy070. [DOI] [PMC free article] [PubMed]
- 27.Moustafa A, Xie C, Kirkness E, Biggs W, Wong E, Turpaz Y, Bloom K, Delwart E, Nelson KE, Venter JC, et al. The blood DNA virome in 8,000 humans. PLoS Pathog. 2017;13(3):e1006292. doi: 10.1371/journal.ppat.1006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Histology Viewer on GTEx Portal. https://www.gtexportal.org/home/histologyPage. Accessed March 9, 2020.
- 30.Popgeorgiev N, Temmam S, Raoult D, Desnues C. Describing the silent human virome with an emphasis on giant viruses. Intervirology. 2013;56(6):395–412. doi: 10.1159/000354561. [DOI] [PubMed] [Google Scholar]
- 31.Woolhouse MEJ, Adair K. The diversity of human RNA viruses. Future Virol. 2013;8(2):159–171. doi: 10.2217/fvl.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanishi K, Mori Y, Pellett PE. Human herpesviruses 6 and 7. In: Knipe DM, Howley PM, editors. Fields Virologyvol. 2, 6th edn. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 2058–2079. [Google Scholar]
- 33.Wieland S, Makowska Z, Campana B, Calabrese D, Dill MT, Chung J, Chisari FV, Heim MH. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology. 2014;59(6):2121–2130. doi: 10.1002/hep.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihm S, Frese M, Meier V, Wietzke-Braun P, Scharf JG, Bartenschlager R, Ramadori G. Interferon type I gene expression in chronic hepatitis C. Lab Investig. 2004;84(9):1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- 35.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ounit R, Wanamaker S, Close TJ, Lonardi S. CLARK: fast and accurate classification of metagenomic and genomic sequences using discriminative k-mers. BMC Genomics. 2015;16:236. doi: 10.1186/s12864-015-1419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7:11257. doi: 10.1038/ncomms11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol. 2017;45:97–102. doi: 10.1016/j.coi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–971. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Al Tabaa Y, Tuaillon E, Bollore K, Foulongne V, Petitjean G, Seigneurin JM, Duperray C, Desgranges C, Vendrell JP. Functional Epstein-Barr virus reservoir in plasma cells derived from infected peripheral blood memory B cells. Blood. 2009;113(3):604–611. doi: 10.1182/blood-2008-02-136903. [DOI] [PubMed] [Google Scholar]
- 43.Sun CC, Thorley-Lawson DA. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J Virol. 2007;81(24):13566–13577. doi: 10.1128/JVI.01055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 45.Mrozek-Gorska P, Buschle A, Pich D, Schwarzmayr T, Fechtner R, Scialdone A, Hammerschmidt W. Epstein-Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc Natl Acad Sci U S A. 2019;116(32):16046–16055. doi: 10.1073/pnas.1901314116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nael A, Wu WW, Siddiqi I, Zhao X, Kahlon KS, Rezk SA. Epstein - Barr virus association with plasma cell neoplasms. Histol Histopathol. 2019;34(6):655–662. doi: 10.14670/HH-18-066. [DOI] [PubMed] [Google Scholar]
- 47.Li R, Du J, Hou J. Identification of the potential risk factors for monoclonal gammopathy of undetermined significance of progression. Hematology. 2015;20(1):11–17. doi: 10.1179/1607845414Y.0000000163. [DOI] [PubMed] [Google Scholar]
- 48.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virologyvol. 2, 6th edn. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1823–1897. [Google Scholar]
- 49.Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359(9305):507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy PG, Chaudhuri A. Herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 2002;73(3):237–238. doi: 10.1136/jnnp.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esiri MM. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci. 1982;54(2):209–226. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- 52.Ptaszynska-Sarosiek I, Dunaj J, Zajkowska A, Niemcunowicz-Janica A, Krol M, Pancewicz S, Zajkowska J. Post-mortem detection of six human herpesviruses (HSV-1, HSV-2, VZV, EBV, CMV, HHV-6) in trigeminal and facial nerve ganglia by PCR. PeerJ. 2019;6:e6095. doi: 10.7717/peerj.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King AMQ, Adams MJ, Lefkowitz EJ. Virus taxonomy: ninth report of the international committee on taxonomy of viruses. San Diego: Elsevier; 2011.
- 54.Hino S, Miyata H. Torque teno virus (TTV): current status. Rev Med Virol. 2007;17(1):45–57. doi: 10.1002/rmv.524. [DOI] [PubMed] [Google Scholar]
- 55.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241(1):92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 56.Ohto U, Shibata T, Tanji H, Ishida H, Krayukhina E, Uchiyama S, Miyake K, Shimizu T. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature. 2015;520(7549):702–705. doi: 10.1038/nature14138. [DOI] [PubMed] [Google Scholar]
- 57.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 58.Focosi D, Macera L, Pistello M, Maggi F. Torque Teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J Infect Dis. 2014;210(4):667–668. doi: 10.1093/infdis/jiu209. [DOI] [PubMed] [Google Scholar]
- 59.Zapatka M, Borozan I, Brewer DS, Iskar M, Grundhoff A, Alawi M, Desai N, Sultmann H, Moch H, Pathogens P, et al. The landscape of viral associations in human cancers. Nat Genet. 2020;52(3):320–330. doi: 10.1038/s41588-019-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7(8):355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 61.Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28(6):587–593. doi: 10.1097/MOG.0b013e328358e5cc. [DOI] [PubMed] [Google Scholar]
- 62.Reidelberger RD. Cholecystokinin and control of food intake. J Nutr. 1994;124(8 Suppl):1327S–1333S. doi: 10.1093/jn/124.suppl_8.1327S. [DOI] [PubMed] [Google Scholar]
- 63.Schubert ML. Gastric acid secretion. Curr Opin Gastroenterol. 2016;32(6):452–460. doi: 10.1097/MOG.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62(24):2932–2938. doi: 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumata R, Ito J, Sato K. Inherited chromosomally integrated HHV-6 possibly modulates human gene expression. Virus Genes. 10.1007/s11262-020-01745-5. [DOI] [PubMed]
- 67.Peddu V, Dubuc I, Gravel A, Xie H, Huang ML, Tenenbaum D, Jerome KR, Tardif JC, Dube MP, Flamand L, et al. Inherited chromosomally integrated human herpesvirus 6 demonstrates tissue-specific RNA expression in vivo that correlates with an increased antibody immune response. J Virol. 2019;94(1):e01418–e01419. doi: 10.1128/JVI.01418-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Happi AN, Happi CT, Schoepp RJ. Lassa fever diagnostics: past, present, and future. Curr Opin Virol. 2019;37:132–138. doi: 10.1016/j.coviro.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaeffer J, Carnec X, Reynard S, Mateo M, Picard C, Pietrosemoli N, Dillies MA, Baize S. Lassa virus activates myeloid dendritic cells but suppresses their ability to stimulate T cells. PLoS Pathog. 2018;14(11):e1007430. doi: 10.1371/journal.ppat.1007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brister JR, Ako-Adjei D, Bao Y, Blinkova O. NCBI viral genomes resource. Nucleic Acids Res. 2015;43(Database issue):D571–D577. doi: 10.1093/nar/gku1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44(D1):D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The Gene Ontology C The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47(D1):D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cunningham F, Achuthan P, Akanni W, Allen J, Amode MR, Armean IM, Bennett R, Bhai J, Billis K, Boddu S, et al. Ensembl 2019. Nucleic Acids Res. 2019;47(D1):D745–D751. doi: 10.1093/nar/gky1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- 79.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47(D1):D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 83.Wagner GP, Kin K, Lynch VJ. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012;131(4):281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.RepeatMasker. http://www.repeatmasker.org. Accessed 26 February 2020.
- 86.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6(7):e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin L, Jin Z, Tan H, Xu Q, Peng T, Li H. Atypical ubiquitination by E3 ligase WWP1 inhibits the proteasome-mediated degradation of mutant huntingtin. Brain Res. 2016;1643:103–112. doi: 10.1016/j.brainres.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 89.Bahl E, Koomar T, Michaelson JJ. cerebroViz: an R package for anatomical visualization of spatiotemporal brain data. Bioinformatics. 2017;33(5):762–763. doi: 10.1093/bioinformatics/btw726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, Hao L, Kiang A, Paschall J, Phan L, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39(10):1181–1186. doi: 10.1038/ng1007-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.The Sato Lab: Human Virome Analysis Github Repository. doi: 10.5281/zenodo.3750497.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Information on the RNA-Seq dataset used in this study.
Additional file 2: Table S2 List of the 39 viral species detected in this study.
Additional file 3: Table S3 Read count of the respective 39 viral species detected in this study.
Additional file 4: Figure S1 Read coverage on EBV, HHV-6 and HHV-7 genomes.
Additional file 5: Table S4 List of the genomic positions of the masked regions in each viral genome.
Additional file 6: Table S5 Information on the GTEx samples.
Additional file 7: Figure S2 Comparison of our analytical pipeline with the other pipelines.
Additional file 8: Figure S3 Deconvolution analysis in the blood of EBV-positive and -negative subjects.
Additional file 9: Figure S4 Deconvolution analysis in the spleen of EBV-positive and -negative subjects.
Additional file 10: Figure S5 Read mapping to Lassa virus segment L and Pepper chlorotic spot virus segment L.
Additional file 11: Table S6 Information on the viral genome sequences used in this study.
Additional file 12: Table S7. Information on the prokaryotic genome sequences used in this study.
Data Availability Statement
The data, associated protocols, code, and materials in this study are available at reference [91].