ABSTRACT
Background
In low-resource settings, urbanization may contribute to the individual-level double burden of malnutrition (DBM), whereby under- and overnutrition co-occur within the same individuals.
Objective
We described DBM prevalence among Malawian women by urban-rural residence, examined whether urban residence was associated with DBM, and assessed whether DBM prevalence was greater than the prevalence expected by chance given population levels of under- and overnutrition, which would suggest DBM is a distinct phenomenon associated with specific factors.
Methods
We analyzed nationally representative data of 723 nonpregnant women aged 15–49 y from the 2015–2016 Malawi Micronutrient Survey. DBM was defined as co-occurring overweight or obesity (OWOB) and ≥1 micronutrient deficiency or anemia. We used Poisson regression models to examine the association between urban residence and DBM and its components. The Rao-Scott modified chi-square test compared the observed and expected DBM prevalence.
Results
Nationally, 10.8% (95% CI: 7.0, 14.5) of women had co-occurring OWOB and any micronutrient deficiency and 3.4% (95% CI: 1.3, 5.5) had co-occurring OWOB and anemia. The prevalence of co-occurring OWOB and any micronutrient deficiency was 2 times higher among urban women than rural women [urban 32.6 (24.1, 41.2) compared with rural 8.6 (5.2, 11.9), adjusted prevalence ratio: 2.0 (1.1, 3.5)]. Co-occurring OWOB and anemia prevalence did not significantly differ by residence [urban 6.9 (0.6, 13.2) compared with rural 3.0 (0.8, 5.3)]. There were no statistically significant differences in observed and expected prevalence estimates of DBM.
Conclusions
This analysis shows that co-occurring OWOB and any micronutrient deficiency was higher among women in urban Malawi compared with rural areas. However, our finding that co-occurring OWOB and any micronutrient deficiency or anemia may be due to chance suggests that there may not be common causes driving DBM in Malawian women. Thus, there may not be a need to design and target interventions specifically for women with DBM.
Keywords: double burden, malnutrition, overweight, obesity, anemia, micronutrient deficiency, women, Africa, Malawi
Introduction
Low- and middle-income countries (LMICs) are experiencing a rapid increase in overweight, obesity, and diet-related noncommunicable diseases in the face of persisting undernutrition (1–4). The coexistence of under- and overnutrition, termed the ‘double burden of malnutrition’ (DBM), can occur in populations (e.g. high prevalence of undernutrition and overweight or noncommunicable diseases in a country), households (e.g. an overweight adult with a stunted child), and individuals (e.g. simultaneous overweight and micronutrient deficiencies in a person) (5). Addressing the DBM is a high priority on the UN global health agenda, given the adverse health consequences of malnutrition and negative impact on developing economies due to lost economic productivity (1, 6). An essential first step toward reducing DBM is to understand the prevalence and causes of DBM at every level, which in turn, can inform the development and targeting of interventions. Yet, many previous studies have investigated DBM at the population (2, 4, 7) or household levels (8–11), whereas comparatively little research has focused on individual-level DBM, especially in sub-Saharan Africa (12).
Urbanization may be a key driver of DBM within individuals in LMICs (13). Urbanization and attendant economic growth are thought to expand access to processed foods, increase exposure to food marketing, and reduce physical activity through more motorized transportation and sedentary occupations (13–16). These macrolevel changes in environment and lifestyle are believed to lead to a nutrition transition characterized by decreased energy expenditure and shifts away from diets composed of legumes, coarse grains, and vegetables and toward energy-dense, nutrient-poor diets composed of foods high in refined carbohydrates and added sugars. This nutrition transition is viewed as a major contributor to the rise in prevalence of overweight, obesity, and noncommunicable diseases (13, 14). At the same time, it has been posited that diets associated with the nutrition transition play a role in the continued problem of micronutrient deficiencies in LMICs, since processed and packaged foods are often low in micronutrients (16, 17). Thus, exposure to an urban environment may shape lifestyle behaviors, such as high consumption of energy-dense, processed and packaged foods, which may contribute to the co-occurrence of overweight or obesity (OWOB) and micronutrient deficiencies or anemia within individuals. Given the potential role of urbanization in DBM, many empirical studies have investigated the prevalence of conditions of under- and overnutrition among people who reside in urban and rural areas (2, 15). However, since these are ecological studies, their findings cannot be used to make inferences about the co-occurrence of under- and overnutrition within the same individual. Furthermore, few studies have considered that this co-occurrence may be due to chance, with the prevalence of DBM reflecting underlying levels of under- and overnutrition in the population.
Due to the scarcity of micronutrient status data (18–20), previous studies of DBM at the individual level have predominately examined co-occurring OWOB and anemia (12, 17, 21–24), with anemia used to reflect micronutrient deficiencies. Solely assessing co-occurring OWOB and anemia may not provide a clear understanding of individual-level DBM, because anemia is not only associated with micronutrient deficiencies but also nonnutritional factors such as malaria and other infections, inflammation, and hemoglobinopathies (25, 26). As such, analyses of co-occurring OWOB and micronutrient deficiencies are needed to further elucidate the issue of individual-level DBM, particularly in LMICs with a high infection burden (25).
We used nationally representative data based on a sample of nonpregnant women of reproductive age to investigate individual-level DBM in Malawi, an urbanizing sub-Saharan African country with unresolved undernutrition and problems of OWOB (2, 27). Most studies of individual-level DBM have been conducted in middle-income countries, where the nutrition transition is in advanced stages (12). Malawi is a low-income country in the early stages of the nutrition transition (28, 29), and thus offers a different context in which to investigate DBM in individual women. Our objectives were 3-fold. First, we estimated the prevalence of 2 characterizations of DBM at the individual level in Malawian women (1) co-occurring OWOB and anemia, 2) co-occurring OWOB and any micronutrient deficiency) by urban-rural residence. Second, we examined the association between individual-level DBM and residence in urban or rural areas. Finally, we tested whether the prevalence of DBM would differ from that expected by chance.
Methods
Data source and study population
We analyzed nationally representative data from the 2015–2016 Malawi Micronutrient Survey (MNS), which was conducted in co-ordination with the 2015–2016 Malawi Demographic and Health Survey (MDHS) (30, 31). The MDHS used the 2008 Malawi Population and Housing Census and employed a 2-stage cluster sampling design in which clusters were selected using a probability proportional to population size approach and then 30 households per urban cluster and 33 households per rural cluster were selected by applying an equal probability systematic selection method. Additional details of the MDHS sampling methodology are presented elsewhere (31). For the MNS, a subsample of MDHS clusters were randomly selected, and 20 households per urban cluster and 22 per rural cluster were included (30). In each household, eligible participants (defined as usual members of the household who spent the night in that household before the survey) were invited to participate. Of 830 sampled women of reproductive age (15–49 y), we excluded women who were pregnant (n = 34), women for whom pregnancy status was not available (n = 18), and women who had missing (n = 10) or biologically implausible BMI values (beyond 5 SD from reference means; n = 3) (15), missing values on anemia or micronutrient status (n = 41), or missing values on demographic variables used in our analysis (n = 1). Our final analytic sample consisted of 723 nonpregnant women. The proportion of missing or biologically implausible BMI values was 1.8%, indicating good anthropometric data quality (32). All available sociodemographic characteristics (age, wealth, education, residence) among women in our analytic sample and those who were excluded from this analysis were similar. Ethical approval for the MDHS and MNS was granted by The National Health Sciences Research Committee in Malawi. Community leaders also granted consent of MDHS and MNS activities.
Characterizations of DBM at the individual level
We examined 2 characterizations of DBM at the individual level in Malawian women: 1) co-occurring OWOB and anemia and 2) co-occurring OWOB and any micronutrient deficiency. For analyses examining any micronutrient deficiency in women, we created a micronutrient deficiency index that was a composite measure of a woman's micronutrient status. The micronutrient index was comprised of 5 micronutrients (zinc, iron, folate, vitamin B-12, vitamin A), and women with 1 or more deficiency in these micronutrients were categorized as having ≥1 micronutrient deficiency. In subanalyses, we examined co-occurring OWOB and single micronutrient deficiencies. Given that in some cases cut-offs above those used to define deficiency of micronutrients are clinically meaningful, we also examined co-occurring OWOB and folate insufficiency (which is associated with risk of neural tube defects), co-occurring OWOB and vitamin A insufficiency, and co-occurring OWOB and vitamin B-12 depletion.
Measurement of OWOB, anemia, and micronutrient deficiencies
Weight was measured with a digital floor scale (SECA brand) to the nearest 0.1 kg, and a wooden stadiometer [ShorrBoard® (Weigh and Measure, LLC)] was used to measure height to the nearest 0.1 cm. We used weight and height measurements to calculate BMI and classify women into 3 weight categories, using the WHO recommended cut-off points: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9), and OWOB (BMI 25–29.9 [overweight], ≥30 [obesity]) (33). A BMI ≥25 indicates excess weight and has been shown to be associated with increased risk of adverse health outcomes, making it appropriate to collapse BMI categories of OWOB (17).
We measured deficiencies in zinc, iron, folate, vitamin B-12, and vitamin A using serum from venous blood samples, which were collected from consenting participants, using venipuncture into 1 trace element-free and a second EDTA-containing tube. Anemia was tested in the field using the HemoCue® 301 (UNICEF Supply catalogue) and defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL in nonpregnant women (34). Zinc deficiency was defined as a serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples (35). Iron deficiency was defined as inflammation-adjusted ferritin <15 μg/L. We adjusted ferritin concentrations for C-reactive protein (CRP) and α-1-acid glycoprotein (AGP) concentrations using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia internal (country-specific) regression-corrected approach (36, 37). Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L (38). Vitamin B-12 deficiency was defined as serum vitamin B-12 concentration <150 pmol/L (39). Vitamin A deficiency was defined as retinol-binding protein (RBP) <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L using regression analysis (40, 41). Folate insufficiency was defined as RBC folate <748 nmol/L (42). Vitamin B-12 depletion was defined as serum vitamin B-12 concentration <220 pmol/L (39). Vitamin A insufficiency was defined as RBP ≥0.46 to <0.86 μmol/L calibrated to equal retinol ≥0.7 to <1.05 μmol/L using regression analysis (41).
Statistical analysis
All analyses were conducted in SAS 9.4 (SAS Institute, Inc.) and used survey weights to generate results representative of women in Malawi. Survey procedures in SAS were used to account for the complex sampling design (strata and clusters). We generated descriptive statistics for sociodemographic characteristics of women (residence, education level, household wealth index, age).
We calculated the prevalence of OWOB, anemia, any micronutrient deficiency, co-occurring OWOB and anemia, and co-occurring OWOB and any micronutrient deficiency. In a bivariate analysis, we examined the association between residence and OWOB, using the Rao-Scott modified chi-square test. We then calculated unadjusted and adjusted (for household wealth index, education level, continuous age in years) prevalence ratios to examine the association between residence and OWOB, using a log-binomial regression model performed with PROC GENMOD (43). We evaluated multicollinearity among our independent variables by assessing variance inflation factors. We conducted similar analyses to assess the association between residence and each of the following outcomes: anemia, any micronutrient deficiency, co-occurring OWOB and anemia, and co-occurring OWOB and any micronutrient deficiency.
The Rao-Scott modified chi-square test was used to compare the observed and expected prevalence of co-occurring OWOB and anemia. The expected prevalence of co-occurring OWOB and anemia was estimated as the product of OWOB prevalence and anemia prevalence. We also compared the observed and expected prevalence of co-occurring OWOB and anemia stratified by residence. We carried out a similar analysis to compare the observed and expected prevalence of co-occurring OWOB and any micronutrient deficiency. For these analyses, underweight women were excluded, and normal weight women served as the reference category.
In subanalyses, we repeated the analyses described above to examine the co-occurrence of OWOB and deficiencies in single micronutrients (i.e. OWOB and zinc deficiency, OWOB and iron deficiency, OWOB and folate deficiency, OWOB and vitamin B-12 deficiency, OWOB and vitamin A deficiency). We also examined the co-occurrence of OWOB and single micronutrient insufficiencies (i.e. OWOB and folate insufficiency, OWOB and vitamin B-12 depletion, OWOB and vitamin A insufficiency). In addition, we estimated the prevalence of micronutrient deficiencies and insufficiencies in women who were underweight, normal weight, and OWOB.
Results
Sociodemographic characteristics of women are provided in Table 1. 79.0% had less than a secondary education, and the largest age category of women was aged 20–29 y with 38.6%. The proportion of women residing in urban areas and rural areas was 9.1% and 90.9%, respectively.
TABLE 1.
Sociodemographic characteristics of nonpregnant women of reproductive age in Malawi1
| Total (n = 723) | Urban (n = 117) | Rural (n = 606) | |
|---|---|---|---|
| Residence | |||
| Urban | 9.1 (1.5, 16.8) | — | — |
| Rural | 90.9 (83.2, 98.5) | — | — |
| Education | |||
| <Secondary | 79.0 (73.2, 84.8) | 35.6 (18.0, 53.2) | 83.3 (79.0, 87.6) |
| ≥Secondary | 21.0 (15.2, 26.8) | 64.4 (46.8, 82.0) | 16.7 (12.4, 21.0) |
| Wealth tertile2 | |||
| Low | 35.8 (28.7, 42.9) | 10.8 (0.0, 27.7) | 38.3 (31.0, 45.6) |
| Middle | 32.2 (27.2, 37.3) | 9.7 (3.7, 15.7) | 34.5 (29.4, 39.5) |
| High | 32.0 (24.4, 39.6) | 79.5 (66.3, 92.7) | 27.2 (20.3, 34.1) |
| Age, y | |||
| 15–19 | 20.2 (16.3, 24.0) | 14.4 (7.2, 21.6) | 20.7 (16.7, 24.8) |
| 20–29 | 38.6 (32.7, 44.5) | 57.0 (34.9, 79.0) | 36.8 (31.0, 42.5) |
| 30–39 | 24.7 (20.4, 29.0) | 14.2 (6.6, 21.9) | 25.8 (21.2, 30.3) |
| 40–49 | 16.5 (13.5, 19.6) | 14.4 (2.2, 26.6) | 16.7 (13.6, 19.9) |
| Weight status | |||
| Underweight3 | 9.2 (6.9, 11.5) | 13.7 (4.1, 23.3) | 8.7 (6.4, 11.1) |
| Normal4 | 76.3 (71.7, 81.0) | 51.9 (39.5, 64.4) | 78.8 (74.4, 83.1) |
| Overweight5 | 10.7 (7.3, 14.1) | 20.8 (7.8, 33.7) | 9.7 (6.4, 13.0) |
| Obesity6 | 3.7 (1.8, 5.6) | 13.6 (3.7, 23.5) | 2.8 (1.1, 4.4) |
Based on data from the 2015–2016 Malawi Micronutrient Survey.
Data are weighted percent and 95% CIs.
Prevalence estimates accounted for the complex sampling design.
Wealth tertiles were created from a wealth index, a variable that is derived from principal component analysis and serves as an indicator of household wealth.
Underweight defined as BMI <18.5 kg/m2.
Normal weight defined as BMI 18.5–24.9 kg/m2.
Overweight defined as BMI 25–29.9 kg/m2.
Obesity defined as ≥30 kg/m2.
Prevalence of OWOB, anemia, and micronutrient deficiencies
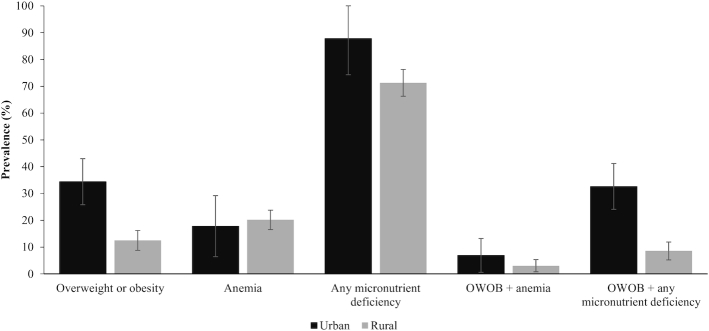
The national prevalence of OWOB, anemia, and any micronutrient deficiency was 14.5%, 19.9%, and 72.8%, respectively (Table 2). Table 2 and Figure 1 display the prevalence of OWOB, anemia, and any micronutrient deficiency stratified by residence. In bivariate analyses, the prevalence of OWOB in women was higher in urban areas than in rural areas [urban: 34.4% (95% CI: 25.8, 43.0), rural: 12.5% (95% CI: 8.8, 16.2)] (Table 2). The prevalence of anemia among women in urban and rural areas was 17.8% (95% CI: 6.4, 29.2) and 20.2% (95% CI: 16.5, 23.8), respectively. The prevalence of any micronutrient deficiency was high in both urban and rural areas [urban: 87.8% (95% CI: 74.3, 100.0), rural: 71.3% (95% CI: 66.3, 76.3)]. In adjusted regression analyses, OWOB was not statistically more prevalent among women in urban areas compared with women in rural areas [adjusted prevalence ratio (aPR): 1.5 (95% CI: 0.9, 2.5)] (Table 3). The prevalence of anemia did not significantly differ among women in urban areas and those in rural areas [aPR: 0.7 (95% CI: 0.4, 1.4)]; however, there was a higher prevalence of any micronutrient deficiency among women in urban areas compared with women in rural areas [aPR: 1.3 (95% CI: 1.0, 1.5)].
TABLE 2.
Weighted prevalence estimates of single conditions of malnutrition and co-occurring overweight or obesity and anemia or micronutrient deficiencies among nonpregnant women of reproductive age in Malawi, according to residence (n = 723)
| Total | Urban (n = 117) | Rural (n = 606) | P value1 | |
|---|---|---|---|---|
| Single malnutrition conditions | ||||
| Overweight2 | 10.7 (7.3, 14.1) | 20.8 (7.8, 33.7) | 9.7 (6.4, 13.0) | 0.15 |
| Obesity2 | 3.7 (1.8, 5.6) | 13.6 (3.7, 23.5) | 2.8 (1.1, 4.4) | 0.09 |
| OWOB2 | 14.5 (10.6, 18.4) | 34.4 (25.8, 43.0) | 12.5 (8.8, 16.2) | 0.02 |
| Anemia3 | 19.9 (16.5, 23.4) | 17.8 (6.4, 29.2) | 20.2 (16.5, 23.8) | 0.69 |
| Micronutrient deficiencies | ||||
| ≥1 micronutrient deficiency4 | 72.8 (67.7, 77.8) | 87.8 (74.3, 100.0) | 71.3 (66.3, 76.3) | 0.15 |
| Zinc deficiency5 | 61.8 (54.8, 68.9) | 82.8 (66.2, 99.5) | 59.7 (52.7, 66.8) | 0.15 |
| Iron deficiency6 | 16.0 (12.6, 19.4) | 16.2 (7.5, 24.9) | 16.0 (12.3, 19.7) | 0.96 |
| Folate deficiency7 | 7.6 (4.7, 10.5) | 4.5 (0.0, 9.3) | 7.9 (4.8, 11.1) | 0.36 |
| Vitamin B-12 deficiency8 | 12.4 (8.7, 16.2) | 0.4 (0.0, 1.0) | 13.6 (9.6, 17.6) | 0.02 |
| Vitamin A deficiency9 | 0.3 (0.0, 0.8) | — | 0.4 (0.0, 0.9) | — |
| Micronutrient insufficiencies | ||||
| Folate insufficiency10 | 80.9 (75.3, 86.6) | 98.3 (96.0, 100.0) | 79.2 (73.3, 85.1) | 0.02 |
| Vitamin B-12 depletion11 | 39.6 (33.2, 46.0) | 19.6 (6.7, 32.4) | 41.6 (34.8, 48.5) | 0.05 |
| Vitamin A insufficiency12 | 5.0 (2.8, 7.2) | 1.0 (0.0, 2.4) | 5.4 (3.1, 7.8) | 0.07 |
| Co-occurring OWOB and anemia | ||||
| OWOB + anemia | 3.4 (1.3, 5.5) | 6.9 (0.6, 13.2) | 3.0 (0.8, 5.3) | 0.36 |
| Co-occurring OWOB and micronutrient deficiencies | ||||
| OWOB + ≥1 micronutrient deficiency | 10.8 (7.0, 14.5) | 32.6 (24.1, 41.2) | 8.6 (5.2, 11.9) | 0.02 |
| OWOB + zinc deficiency | 9.8 (6.1, 13.6) | 31.3 (22.1, 40.4) | 7.7 (4.4, 11.0) | 0.03 |
| OWOB + iron deficiency | 2.6 (1.0, 4.1) | 2.5 (0.0, 5.3) | 2.6 (0.9, 4.3) | 0.97 |
| OWOB + folate deficiency | 1.8 (0.8, 2.9) | 1.5 (0.0, 3.2) | 1.8 (0.7, 3.0) | 0.73 |
| OWOB + vitamin B-12 deficiency | 0.8 (0.2, 1.5) | — | 0.9 (0.2, 1.7) | — |
| OWOB + vitamin A deficiency | 0.1 (0.0, 0.3) | — | 0.1 (0.0, 0.3) | — |
| Co-occurring OWOB and micronutrient insufficiencies | ||||
| OWOB + folate insufficiency | 12.5 (8.9, 16.0) | 34.2 (25.7, 42.8) | 10.3 (7.1, 13.5) | 0.02 |
| OWOB + vitamin B-12 depletion | 4.4 (2.0, 6.7) | 1.6 (0.0, 3.8) | 4.6 (2.0, 7.2) | 0.21 |
| OWOB + vitamin A insufficiency | 0.0 (0.0, 0.1) | — | 0.0 (0.0, 0.1) | — |
Based on data from the 2015–2016 Malawi Micronutrient Survey.
Data are weighted proportions and 95% CIs.
Prevalence estimates accounted for the complex sampling design.
—, For prevalence estimates with 0 unweighted cases. For P value that cannot be calculated.
Based on Rao-Scott modified chi-square test.
Overweight defined as BMI 25–29.9 kg/m2. Obesity defined as ≥30 kg/m2. Overweight or obesity defined as BMI ≥25.0 kg/m2.
Anemia defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL for nonpregnant women.
≥1 micronutrient deficiency defined as a deficiency in 1 or more of the following micronutrients: zinc, iron, folate, vitamin B-12, vitamin A.
Zinc deficiency defined as serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples.
Iron deficiency defined as inflammation-corrected ferritin <15 μg/L.
Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L.
Vitamin B-12 deficiency defined as serum vitamin B-12 concentration <150 pmol/L.
Vitamin A deficiency defined as retinol-binding protein <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L using regression analysis.
Folate insufficiency defined as RBC folate concentration <748 nmol/L.
Vitamin B-12 depletion defined as serum vitamin B-12 concentration <220 pmol/L.
Vitamin A insufficiency defined as RBP ≥0.46 to <0.86 μmol/L calibrated to equal retinol ≥0.7 to <1.05 μmol/L using regression analysis.
OWOB, overweight or obesity.
FIGURE 1.
Prevalence of overweight or obesity, anemia, and any micronutrient deficiency and 2 characterizations of the individual-level double burden of malnutrition by residence (n = 723). Individual level-double burden of malnutrition defined 2 ways: co-occurring overweight or obesity and anemia, and co-occurring overweight or obesity and any micronutrient deficiency. Based on data from the 2015–2016 Malawi Micronutrient Survey. Urban (n = 117), Rural (n = 606). Prevalence estimates accounted for the complex sampling design. Survey weights were used to calculate nationally representative prevalence estimates. Overweight or obesity defined as BMI ≥25.0 kg/m2. Anemia defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL for nonpregnant women. Any micronutrient deficiency defined as a deficiency in 1 or more of the following micronutrients: zinc, iron, folate, vitamin B-12, vitamin A. Zinc deficiency defined as serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples. Iron deficiency defined as inflammation-corrected ferritin <15 μg/L. Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L. Vitamin B-12 deficiency defined as serum vitamin B-12 concentration <150 pmol/L. Vitamin A deficiency defined as retinol-binding protein <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L using regression analysis. OWOB, overweight or obesity.
TABLE 3.
Associations between urban residence and single conditions of malnutrition, co-occurring overweight or obesity and micronutrient deficiencies, and co-occurring overweight or obesity and micronutrient insufficiency among nonpregnant women of reproductive age in Malawi (n = 723)
| Unadjusted PR (95% CI) (ref = rural) | P value | Adjusted PR (95% CI)1 (ref = rural) | P value | |
|---|---|---|---|---|
| Single malnutrition conditions | ||||
| OWOB 2 | 2.8 (1.9, 4.0) | <0.0001 | 1.5 (0.9, 2.5) | 0.10 |
| Anemia3 | 0.9 (0.5, 1.7) | 0.71 | 0.7 (0.4, 1.4) | 0.31 |
| ≥1 micronutrient deficiency4 | 1.2 (1.0, 1.5) | 0.01 | 1.3 (1.0, 1.5) | 0.02 |
| Zinc deficiency4 | 1.4 (1.1, 1.7) | <0.01 | 1.5 (1.1, 1.9) | <0.01 |
| Folate insufficiency5 | 1.2 (1.1, 1.3) | <0.0001 | 1.2 (1.1, 1.3) | <0.0001 |
| Co-occurring OWOB and micronutrient deficiencies | ||||
| OWOB + ≥1 micronutrient deficiency | 3.8 (2.4, 6.1) | <0.0001 | 2.0 (1.1, 3.5) | 0.02 |
| OWOB + zinc deficiency | 4.1 (2.4, 6.8) | <0.0001 | 2.0 (1.1, 3.8) | 0.03 |
| Co-occurring OWOB and micronutrient insufficiency | ||||
| OWOB + folate insufficiency | 3.3 (2.2, 4.9) | <0.0001 | 1.9 (1.2, 3.0) | 0.01 |
Based on data from the 2015–2016 Malawi Micronutrient Survey.
Data are prevalence ratios (PRs) (95% CI). Reference for PRs is rural residence.
Adjusted for education (<secondary, ≥secondary), household wealth index (low, middle, high), and age in years (continuous).
Overweight or obesity defined as BMI ≥25.0 kg/m2.
Anemia defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL for nonpregnant women.
≥1 micronutrient deficiency defined as a deficiency in 1 or more of the following micronutrients: zinc, iron, folate, vitamin B-12, vitamin A.
Zinc deficiency defined as serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples.
Iron deficiency defined as inflammation-corrected ferritin <15 μg/L.
Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L.
Vitamin B-12 deficiency defined as serum vitamin B-12 concentration <150 pmol/L.
Vitamin A deficiency defined as retinol-binding protein <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L using regression analysis.
Folate insufficiency defined as RBC folate concentration <748 nmol/L.
OWOB, overweight or obesity.
The prevalence of single micronutrient deficiencies was highly variable (Table 2). The prevalence of zinc deficiency was highest (61.8%), followed by inflammation-adjusted iron deficiency (16.0%), vitamin B-12 deficiency (12.4%), folate deficiency (7.6%), and vitamin A deficiency (0.3%). In bivariate analyses, the prevalence of vitamin B-12 deficiency in women was lower in urban areas than in rural areas [urban: 0.4% (95% CI: 0.0, 1.0), rural: 13.6% (95% CI: 9.6, 17.6)]. Zinc deficiency prevalence was high in both urban and rural areas, and in adjusted analysis, zinc deficiency prevalence was slightly higher in women in urban areas compared with those in rural areas [aPR: 1.5 (95% CI: 1.1, 1.9)]. The prevalence of iron deficiency was similar in urban [16.2% (95% CI: 7.5, 24.9)] and rural [16.0% (95% CI: 12.3, 19.7)] areas. Folate deficiency prevalence was 4.5% (95% CI: 0.0, 9.3) in urban areas and 7.9% (95% CI: 4.8, 11.1) in rural areas. As the prevalence of vitamin A deficiency was very low, vitamin A deficiency prevalence in urban compared with rural areas could not be evaluated.
Prevalence estimates of folate insufficiency, vitamin B-12 depletion, and vitamin A insufficiency were 80.9%, 39.6%, and 5.0%, respectively. The prevalence of folate insufficiency in women was higher in urban areas than in rural areas [urban: 98.3% (95% CI: 96.0, 100.0), rural 79.2% (95% CI: 73.3, 85.1)]. Prevalence estimates of vitamin B-12 depletion and vitamin A insufficiency were not significantly different between urban and rural areas [vitamin B-12 depletion: urban: 19.6% (95% CI: 6.7, 32.4), rural: 41.6% (95% CI: 34.8, 48.5); vitamin A insufficiency: urban: 1.0% (95% CI: 0.0, 2.4), rural: 5.4% (95% CI: 3.1, 7.8)]. In adjusted regression analysis, women in urban areas had a slightly higher prevalence of folate insufficiency than women in rural areas [aPR: 1.2 (95% CI: 1.1, 1.3)] (Table 3).
Prevalence of co-occurring OWOB and anemia or any micronutrient deficiency
The prevalence of DBM among Malawian women, defined as co-occurring OWOB and anemia was 3.4% (95% CI: 1.3, 5.5), and defined as co-occurring OWOB and any micronutrient deficiency was 10.8% (95% CI: 7.0, 14.5). The prevalence of co-occurring OWOB and anemia in women was 6.9% (95% CI: 0.6, 13.2) in urban areas and 3.0% (95% CI: 0.8, 5.3) in rural areas. The prevalence of co-occurring OWOB and any micronutrient deficiency was higher among women in urban areas than in rural areas [urban: 32.6% (95% CI: 24.1, 41.2), rural: 8.6% (95% CI: 5.2, 11.9)] (Table 2, Figure 1).
Unadjusted analysis indicated that women in urban areas had a higher prevalence of co-occurring OWOB and any micronutrient deficiency compared with women in rural areas [PR: 3.8 (95% CI: 2.4, 6.1)] (Table 3). The strength of this association was attenuated in an adjusted model but remained statistically significant; co-occurring OWOB and any micronutrient deficiency was twice as prevalent for women in urban areas compared with rural areas [aPR: 2.0 (95% CI: 1.1, 3.5)]. Low prevalence of co-occurring OWOB and anemia prevented modeling its association between urban and rural residence.
In subanalyses of single micronutrient deficiencies and OWOB, the prevalence estimates were as follows: 9.8% for co-occurring OWOB and zinc deficiency; 2.6% for co-occurring OWOB and iron deficiency; 1.8% for co-occurring OWOB and folate deficiency; 0.8% for co-occurring OWOB and vitamin B-12 deficiency; and 0.1% for co-occurring OWOB and vitamin A deficiency (Table 2). The prevalence estimates of co-occurring OWOB and folate insufficiency, co-occurring OWOB and vitamin B-12 depletion, and co-occurring OWOB and vitamin A insufficiency in women were 12.5%, 4.4%, and 0.0%, respectively. In bivariate analyses, the prevalence of co-occurring OWOB and zinc deficiency was higher among women in urban areas than rural areas [urban: 31.3% (95% CI: 22.1, 40.4), rural: 7.7% (95% CI: 4.4, 11.0)]. Similarly, the prevalence of co-occurring OWOB and folate insufficiency was higher among women in urban areas than rural areas [urban: 34.2% (95% CI: 25.7, 42.8), rural: 10.3% (95% CI: 7.1, 13.5)]. The prevalence estimates of co-occurring OWOB with other single micronutrient deficiencies (iron deficiency, folate deficiency) did not differ significantly among women according to residence (Table 2). Similarly, the prevalence of co-occurring OWOB and vitamin B-12 depletion did not differ significantly among women in urban areas and women in rural areas (Table 2). As such, differences in the prevalence estimates of co-occurring OWOB with these single micronutrient deficiencies and insufficiencies were not assessed further. Differences in the prevalence estimates of co-occurring OWOB and vitamin B-12 deficiency, co-occurring OWOB and vitamin A deficiency, and co-occurring OWOB and vitamin A insufficiency were also not assessed in unadjusted and adjusted analyses due to no unweighted cases with these co-occurring forms of malnutrition in urban areas. The higher prevalence of co-occurring OWOB and zinc deficiency [PR: 4.1 (95% CI: 2.4, 6.8)] and co-occurring OWOB and folate insufficiency [PR: 3.3 (95% CI: 2.2, 4.9)] in urban areas compared with rural areas persisted in adjusted analyses [co-occurring OWOB and zinc deficiency: aPR: 2.0 (95% CI: 1.1, 3.8), co-occurring OWOB and folate insufficiency: aPR: 1.9 (95% CI: 1.2, 3.0)].
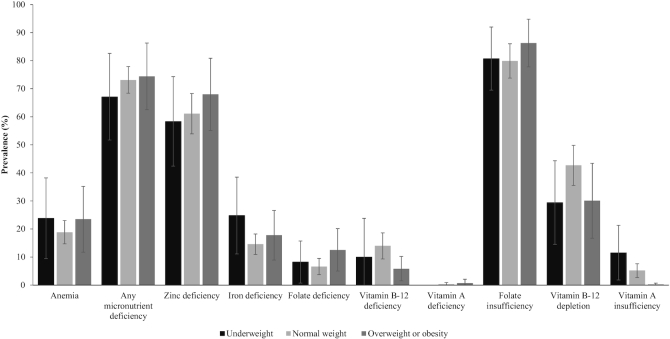
In secondary analyses to determine if the prevalence of anemia and micronutrient deficiencies or insufficiencies differed according to weight category (underweight, normal weight, and OWOB), there were no apparent differences (Figure 2, Supplemental Table 1). The exception was vitamin A insufficiency, which was highest in underweight women [underweight: 11.5% (95% CI: 1.8, 21.3), normal weight: 5.2% (95% CI: 2.7, 7.6), OWOB: 0.2% (95% CI: 0.0, 0.7)].
FIGURE 2.
Anemia and micronutrient deficiencies and insufficiencies according to weight status (underweight, normal weight, overweight or obesity) in Malawian women of reproductive age (n = 723). Based on data from the 2015–2016 Malawi Micronutrient Survey. Urban (n = 117), Rural (n = 606). Prevalence estimates accounted for the complex sampling design. Survey weights were used to calculate nationally representative prevalence estimates. Overweight or obesity defined as BMI ≥25.0 kg/m2. Anemia defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL for nonpregnant women. Any micronutrient deficiency defined as a deficiency in 1 or more of the following micronutrients: zinc, iron, folate, vitamin B-12, vitamin A. Zinc deficiency defined as serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples. Iron deficiency defined as inflammation-corrected ferritin <15 μg/L. Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L. Vitamin B-12 deficiency defined as serum vitamin B-12 concentration <150 pmol/L. Vitamin A deficiency defined as retinol-binding protein <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L using regression analysis. Folate insufficiency defined as RBC folate concentration <748 nmol/L. Vitamin B-12 depletion defined as serum vitamin B-12 concentration <220 pmol/L. Vitamin A insufficiency defined as retinol-binding protein ≥0.46 to <0.86 μmol/L calibrated to equal retinol ≥0.7 to <1.05 μmol/L.
Observed versus expected prevalence of co-occurring OWOB and anemia or any micronutrient deficiency
We investigated whether the prevalence of DBM would differ from that expected by chance, assuming the conditions were independent. The observed prevalence of co-occurring OWOB and anemia was 3.7%, which did not differ significantly from the expected prevalence (3.1%, P = 0.49). The observed and expected prevalence of co-occurring OWOB and any micronutrient deficiency was 11.9% and 11.7%, respectively (P = 0.82). There were no statistically significant differences in observed and expected prevalence estimates of DBM, by either definition (co-occurring OWOB and anemia or co-occurring OWOB and any micronutrient deficiency), or in analyses stratified by residence (Table 4).
TABLE 4.
Comparison of the observed prevalence of co-occurring overweight or obesity and anemia and co-occurring overweight or obesity and micronutrient deficiencies with the prevalence expected by chance among nonpregnant women of reproductive age in Malawi (n = 660)
| Observed co-occurrence (%) | Expected co-occurrence (%) | P value1 | |
|---|---|---|---|
| Co-occurring OWOB and anemia | |||
| OWOB + anemia2, 3 | 3.7 | 3.1 | 0.49 |
| Urban | 8.0 | 5.5 | 0.45 |
| Rural | 3.3 | 2.7 | 0.52 |
| Co-occurring OWOB and micronutrient deficiencies | |||
| OWOB + ≥1 micronutrient deficiency4 | 11.9 | 11.7 | 0.82 |
| Urban | 37.8 | 37.0 | 0.54 |
| Rural | 9.4 | 9.8 | 0.65 |
| OWOB + zinc deficiency5 | 10.8 | 9.9 | 0.30 |
| Urban | 36.2 | 35.0 | 0.40 |
| Rural | 8.4 | 8.2 | 0.77 |
| OWOB + iron deficiency6 | 2.8 | 2.4 | 0.48 |
| Urban | 2.9 | 4.8 | 0.26 |
| Rural | 2.8 | 2.1 | 0.26 |
| OWOB + folate deficiency7 | 2.0 | 1.2 | 0.07 |
| Urban | 1.7 | 2.0 | 0.66 |
| Rural | 2.0 | 1.1 | 0.05 |
| OWOB + vitamin B-12 deficiency8 | 0.9 | 2.0 | 0.01 |
| Urban | — | 0.2 | — |
| Rural | 1.0 | 1.9 | 0.04 |
| OWOB + vitamin A deficiency9 | 0.1 | 0.1 | 0.58 |
| Urban | — | — | — |
| Rural | 0.1 | 0.1 | 0.53 |
| Co-occurring OWOB and micronutrient insufficiencies | |||
| OWOB + folate insufficiency10 | 13.8 | 12.9 | 0.18 |
| Urban | 39.7 | 39.0 | 0.22 |
| Rural | 11.3 | 10.8 | 0.49 |
| OWOB + vitamin B-12 depletion11 | 4.8 | 6.5 | 0.09 |
| Urban | 1.9 | 9.0 | 0.05 |
| Rural | 5.1 | 5.8 | 0.43 |
| OWOB + vitamin A insufficiency12 | 0.04 | 0.7 | <0.001 |
| Urban | — | 0.2 | — |
| Rural | 0.04 | 0.6 | 0.001 |
Based on data from the 2015–2016 Malawi Micronutrient Survey.
Data are weighted proportions.
Prevalence estimates accounted for the complex sampling design.
Women who were underweight were excluded from the analyses.
—, for prevalence estimates, 0 unweighted cases. For chi-square test statistic and P value, cannot be calculated.
Based on Rao-Scott modified chi-square test.
Overweight or obesity defined as BMI ≥25.0 kg/m2.
Anemia defined as hemoglobin (adjusted for altitude and smoking) <12.0 g/dL in pregnant women.
≥1 micronutrient deficiency defined as a deficiency in 1 or more of the following micronutrients: zinc, iron, folate, vitamin B-12, vitamin A.
Zinc deficiency defined as serum zinc concentration <70 μg/dL for morning fasted samples, <66 μg/dL for morning nonfasting samples, and <59 μg/dL for afternoon nonfasting samples.
Iron deficiency defined as inflammation-corrected ferritin <15 μg/L.
Folate deficiency was based on risk of megaloblastic anemia defined as serum folate concentration <6.8 nmol/L.
Vitamin B-12 deficiency defined as serum vitamin B-12 concentration <150 pmol/L.
Vitamin A deficiency defined as retinol-binding protein <0.46 μmol/L calibrated to equal retinol <0.7 μmol/L.
Folate insufficiency defined as RBC folate concentration <748 nmol/L.
Vitamin B-12 depletion defined as serum vitamin B-12 concentration <220 pmol/L.
Vitamin A insufficiency defined as retinol-binding protein ≥0.46 to <0.86 μmol/L calibrated to equal retinol ≥0.7 to <1.05 μmol/L.
OWOB, overweight or obesity.
In subanalyses, the observed prevalence estimates were lower than the expected prevalence estimates for co-occurring OWOB and vitamin B-12 deficiency and co-occurring OWOB and vitamin A insufficiency (P = 0.01 and P <0.001, respectively). In analyses stratified by residence, these findings held among women in rural areas (P = 0.04 and P = 0.001, respectively). The observed prevalence of co-occurring OWOB and folate deficiency was slightly higher than the expected prevalence among women in rural areas (P = 0.05). We did not find statistically significant differences in observed and expected prevalence estimates of other co-occurring OWOB and single micronutrient deficiencies and insufficiencies.
Discussion
Nationally, the prevalence of co-occurring OWOB and anemia was very low, but >1 in 10 women had co-occurring OWOB and any micronutrient deficiency. The prevalence of co-occurring OWOB and anemia did not significantly differ by residence, whereas the prevalence of co-occurring OWOB and any micronutrient deficiency was 2-fold higher among women in urban areas compared with women in rural areas. The larger prevalence of co-occurring OWOB and any micronutrient deficiency in urban areas may be driven by the larger prevalence of OWOB and micronutrient deficiencies among urban women, given that we did not detect women with OWOB to be more or less likely to have micronutrient deficiencies in tests of independence, and based on the observation that the majority of micronutrient deficiencies affected women across all weight categories equally.
The prevalence of co-occurring OWOB and anemia in Malawian women was lower than the prevalence observed in other LMICs. For example, 1 study found that 9% of women in a region of southern India had co-occurring OWOB and anemia, though the results are not directly comparable since BMI cut-offs for Asian populations were used (24). Studies from Latin American countries have found 12% (Guatemala) to 14% (Brazil) of women with co-occurring OWOB and anemia (23). It is not surprising that co-occurring OWOB and anemia is higher in Guatemala and Brazil than that observed in Malawi, given the very high prevalence of both OWOB and anemia in these Latin American countries: in Guatemala, 40.6% of women have OWOB and 24.5% of women have anemia; in Brazil 64.9% have OWOB and 30.6% have anemia (23). The level of co-occurring OWOB and anemia observed in a given country may be related to its stage within the nutrition transition; the nutrition transition is thought to be advanced in some countries in Latin America (22, 44) and in the early stages in sub-Saharan African countries like Malawi (28, 29).
The prevalence of co-occurring OWOB and any micronutrient deficiency was nearly 3 times that of co-occurring OWOB and anemia. Notably, this finding was largely due to the high prevalence of zinc deficiency. In previous studies on DBM, anemia has been used to reflect micronutrient deficiencies, such as deficiencies of iron, folate, and vitamin A (17, 24). Although research supports the role of micronutrient deficiencies in the etiology of anemia, nonnutritional factors, such as chronic disease, genetic blood diseases, malaria, and inflammation are not only known to be associated with anemia, but may contribute even more to the development of anemia than nutritional causes in some settings (25, 26, 45, 46). Furthermore, there is now strong evidence that the contribution of micronutrient deficiencies to anemia varies by setting (25, 45, 46). Context-specific determinants of anemia need to be understood to assess whether anemia is an appropriate proxy for micronutrient deficiencies, as well as to better understand estimates of co-occurring OWOB and anemia. Moreover, given that anemia does not reflect micronutrient deficiencies uniformly across contexts, making direct comparisons of prevalence estimates of co-occurring OWOB and anemia across LMICs is likely problematic. Additional studies that examine co-occurring OWOB and anemia or micronutrient deficiencies in other LMICs are needed to explore the extent to which the prevalence estimates of these 2 characterizations of DBM may differ by setting.
We found that the prevalence of co-occurring OWOB and micronutrient deficiencies in women largely consisted of the prevalence of co-occurring OWOB and zinc deficiency, as the prevalence of other single micronutrient deficiencies (iron, folate, vitamin B-12, vitamin A) was relatively low. Our results are consistent with a large, cross-sectional analysis from Vietnam, where the prevalence of co-occurring OWOB and zinc deficiency (12.2%) was highest, and the prevalence of co-occurring OWOB and iron deficiency and prevalence of co-occurring OWOB and vitamin B-12 deficiency were low (2.3% and 3.0%, respectively) (47). Similar to the high levels of zinc deficiency found in women in Vietnam (67%), Malawian women bear a high burden of zinc deficiency (48). Research investigating co-occurring OWOB and micronutrient deficiencies in other LMICs is warranted. In LMICs such as Mexico and Cameroon, for example, there may be even higher levels of co-occurring OWOB and micronutrient deficiencies in women than that found in Malawian women, as both Mexico and Cameroon have larger burdens of OWOB (11, 27) than Malawi and high prevalence of micronutrient deficiencies (25).
Overall, the patterning of indicators of malnutrition by residence observed in our study is consistent with previous studies. Sub-Saharan Africa, along with South Asia, are the only regions in the world where OWOB remains concentrated in urban areas (16, 49). Simultaneously, cross-sectional studies have shown that intakes of micronutrients, such as vitamin B-12 and folate, are low among urban women in sub-Saharan Africa (50). Constrained potentially by the survey design, we did not observe a significant association between urban residence and co-occurring OWOB and anemia in Malawian women. By contrast, a pooled analysis of nationally representative data for 30 sub-Saharan African countries found that women in periurban and urban areas had significantly higher odds of concurrent OWOB and anemia than women in rural areas (periurban, OR: 1.18; urban, OR: 1.43) (12).
Perhaps 1 of the most salient findings of our analysis was that the national prevalence of individual-level DBM observed in Malawi was consistent with chance association, and this relationship persisted when examined by residence. Other studies in LMICs to evaluate OWOB and anemia in women have reported similar results (23, 24, 51). To our knowledge, few studies have tested the independence of OWOB and micronutrient deficiencies in women. The statistical independence of over- and undernutrition at the individual level among Malawian women is consistent with results examining the interplay of over- and undernutrition at the household level. For example, using 121 datasets from 54 countries, Dieffenbach and Stein found that households with children aged 2–5 y with stunting were not more or less likely to have mothers with OWOB, leading them to conclude that this characterization of household-level DBM was a “statistical artifact,” rather than a distinct entity (52). Subsequent research in Latin America found that in 5 of 6 countries (Brazil, Colombia, Ecuador, Guatemala, and Mexico), the observed prevalence of a child with stunting aged under 5 y and a woman with OWOB within the same household was significantly lower than the prevalence expected by chance, but all absolute differences were small and not considered to be practically meaningful (23).
Collectively, these findings suggest that there may not be shared drivers of over- and undernutrition, which can inform health and nutrition programming and policy in Malawi that is targeted at reducing DBM among women. Although Malawi has experienced steep declines in anemia from 2001 to 2015–2016, multiple forms of malnutrition still exist at the population level. The country is faced with the complex challenge of addressing OWOB, while continuing to combat micronutrient deficiencies and anemia. Accordingly, evidence-based approaches to reducing OWOB that are tailored to the Malawian context may be more consistent with current programming and investments in health and nutrition in Malawi than programs focused specifically on DBM. Reducing either component of DBM will in essence reduce DBM prevalence. Since the national prevalence of OWOB among women in Malawi is still relatively low compared with other LMICs (4, 53), the Malawi government has the opportunity to focus health resources on the prevention of OWOB alongside evidence-based interventions to reduce the current burden of OWOB. An emphasis on OWOB prevention is essential if Malawi is to avoid increases in the prevalence of OWOB and its consequences such as type 2 diabetes and cardiovascular diseases, which are costly to individuals, health care systems, and economies (3). Programming to reduce anemia and iron deficiency is also necessary, and this applies across the weight spectrum. With the optimism for double-duty actions to simultaneously reduce both undernutrition and OWOB and diet-related noncommunicable diseases (54, 55), Malawi may be an appropriate context to test the efficacy of these actions before making national program shifts without an adequate evidence base.
A major strength of this study was its examination of co-occurring OWOB and micronutrient deficiencies, and being able to contrast it with co-occurring OWOB and anemia. Since micronutrient status is not routinely assessed in population-based surveys (19, 20, 25), due to the complexity and expense of collecting and analyzing specimens for micronutrient biomarkers (20), evaluations of co-occurring OWOB and micronutrient deficiencies are rare. By including micronutrient deficiencies in the definition of individual-level DBM, we broaden the undernutrition component of the DBM definition and add to the evidence base that choice of DBM definition will substantially affect the prevalence estimates of DBM (56). A related strength was that we examined 5 different micronutrient deficiencies. Another strength of this study was that we evaluated the statistical independence of conditions of malnutrition, which yielded policy-relevant insights that can inform nutrition programming in the context of DBM.
There were also limitations of the analysis. Although all prevalence estimates are representative at the national level, the MNS was not designed to be representative at the residence level (urban-rural). Indeed, the lack of micronutrient status data that are representative at the residence level in Malawi, as well as most LMICs, limits assessment of urban-rural differences in DBM. Still, examining the individual-level DBM by residence using available data did highlight important findings, such as higher deficiencies of some micronutrients in urban areas that may be due to changes in diet patterns in urban versus rural areas. When micronutrient status data that are representative at the residence level become available, similar analyses can be done to corroborate (or contradict) this evidence. Another limitation was the inability to statistically detect small, but potentially meaningful, differences in DBM and its components across urban and rural areas. Therefore, we urge readers to notice differences in the prevalence estimates and recognize that with 80% power, a design effect of 2, and α = 0.05, the median minimal detectable difference between urban and rural prevalence estimates was 15 percentage points. In addition, although there are gradations of urbanicity (57), our analysis used a dichotomous urban-rural measure for residence. Research using measures with improved ability to capture degrees of urbanization is warranted, particularly given the growth of periurban areas in LMICs (12, 58). Another challenge of this study was that estimates for co-occurring OWOB and vitamin A deficiency and co-occurring OWOB and vitamin A insufficiency were based on a small number of unweighted cases, and thus, must be interpreted with caution. Furthermore, we used RBP as an indicator of vitamin A status, although the WHO recommends retinol (41). In some scenarios RBP is well correlated with plasma retinol (40), but as RBP can be influenced by adiposity and protein energy malnutrition it is not a reliable vitamin A biomarker (59). Finally, dietary data for women were not available to explore the relationship between dietary patterns and women's nutritional status. Analyses of food consumption data may help explain differences (or similarities) in women's nutritional status according to residence. For example, such analyses could uncover reasons why zinc deficiency was higher in women in urban areas compared with their rural counterparts, such as differential intakes of total zinc, lower consumption of higher bioavailable animal-source zinc, or higher intake of phytate, which inhibits zinc absorption (60–62).
In conclusion, this study is 1 of the few to investigate DBM at the individual level in women in sub-Saharan Africa (12) and to include micronutrient deficiencies as part of the DBM definition, which is critical for understanding and addressing DBM. Our results provide evidence for public health practitioners and policymakers, which suggests that individual-level DBM may not require its own set of interventions. Instead, scaling up evidence-based interventions that address OWOB, micronutrient deficiencies, and anemia separately could help reduce the burden of DBM among women in Malawi.
Supplementary Material
Acknowledgments
The 2015–2016 Malawi Micronutrient Survey (MNS) was implemented by the Malawi National Statistical Office (NSO), Community Health Services Unit (CHSU) of the Ministry of Health, and Department of Nutrition, HIV and AIDS (DNHA) with funding from Irish Aid, World Bank, and Emory Global Health Institute, coordination from UNICEF, and technical assistance from the CDC, ICF, and Emory University. We thank all the survey team members for conducting the 2015–2016 MNS and Malawian women for their participation. Finally, we acknowledge the Nutritional Biomarkers Branch of the CDC, Division of Laboratory Sciences in the National Center for Environmental Health for laboratory technical assistance, analysis of biological specimens, and review of this manuscript.
The authors’ contributions were as follows—ECR, PSS, and AMW: designed the research; ECR: analyzed the data; PSS, AMW, and KMVN: supervised the data analysis; ECR: wrote the first draft of the manuscript; PSS, KMVN, SC, MBW, KT, CM, UR, MH, and AMW: contributed to the final draft of the manuscript; and all authors provided critical input and read and approved the final manuscript.
Notes
Financial support for the Malawi Micronutrient Survey was provided by the Government of Malawi, United States Agency for International Development (USAID), United Nations Children's Fund(UNICEF), Irish Aid, World Bank, and Emory Global Health Institute. Financial support for analysis of biological specimens to assess vitamin B-12 and folate status was provided by the National Center on Birth Defects and Developmental Disabilities of the CDC. ECR received salary support from the NIH National Heart, Lung, and Blood Institute (grant number R01HL125442) and NIH Fogarty International Center (grant number R25Tw09337). MBW was supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30DK111024).
Author disclosures: The authors report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: aPR, adjusted prevalence ratio; DBM, double burden of malnutrition; LMICs, low- and middle-income countries; MDHS, Malawi Demographic and Health Survey; MNS, Malawi Micronutrient Survey; OWOB, overweight or obesity; RBP, retinol-binding protein.
References
- 1. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R et al.. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 2. Jaacks LM, Slining MM, Popkin BM. Recent underweight and overweight trends by rural-urban residence among women in low- and middle-income countries. J Nutr. 2015;145(2):352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atun R, Davies JI, Gale EAM, Barnighausen T, Beran D, Kengne AP, Levitt NS, Mangugu FW, Nyirenda MJ, Ogle GD et al.. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017;5(8):622–67. [DOI] [PubMed] [Google Scholar]
- 4. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. The Double Burden of Malnutrition: Policy Brief. [Internet] Geneva; 2017. [Accessed 2019 Nov 13]. Available from: https://www.who.int/nutrition/publications/doubleburdenmalnutrition-policybrief/en/. [Google Scholar]
- 6. Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370(9603):1929–38. [DOI] [PubMed] [Google Scholar]
- 7. Jaacks LM, Slining MM, Popkin BM. Recent trends in the prevalence of under- and overweight among adolescent girls in low- and middle-income countries. Pediatr Obes. 2015;10(6):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes (Lond). 2005;29(1):129–36. [DOI] [PubMed] [Google Scholar]
- 9. Wojcicki JM. The double burden household in sub-Saharan Africa: maternal overweight and obesity and childhood undernutrition from the year 2000: results from World Health Organization Data (WHO) and Demographic Health Surveys (DHS). BMC Public Health. 2014;14:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freire WB, Silva-Jaramillo KM, Ramirez-Luzuriaga MJ, Belmont P, Waters WF. The double burden of undernutrition and excess body weight in Ecuador. Am J Clin Nutr. 2014;100(6):1636s–43s. [DOI] [PubMed] [Google Scholar]
- 11. Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS, Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr. 2014;100(6):1652s–8s. [DOI] [PubMed] [Google Scholar]
- 12. Jones AD, Acharya Y, Galway LP. Urbanicity gradients are associated with the household- and individual-level double burden of malnutrition in sub-Saharan Africa. J Nutr. 2016;146(6):1257–67. [DOI] [PubMed] [Google Scholar]
- 13. Popkin BM. Nutrition transition and the global diabetes epidemic. Curr Diab Rep. 2015;15(9):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nandi A, Sweet E, Kawachi I, Heymann J, Galea S. Associations between macrolevel economic factors and weight distributions in low- and middle-income countries: a multilevel analysis of 200,000 adults in 40 countries. Am J Public Health. 2014;104(2):e162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel SA, Narayan KM, Cunningham SA. Unhealthy weight among children and adults in India: urbanicity and the crossover in underweight and overweight. Ann Epidemiol. 2015;25(5):336–41..e2. [DOI] [PubMed] [Google Scholar]
- 16. Ford ND, Patel SA, Narayan KM. Obesity in low- and middle-income countries: burden, drivers, and emerging challenges. Annu Rev Public Health. 2017;38:145–64. [DOI] [PubMed] [Google Scholar]
- 17. Eckhardt CL, Torheim LE, Monterrubio E, Barquera S, Ruel MT. The overlap of overweight and anaemia among women in three countries undergoing the nutrition transition. Eur J Clin Nutr. 2008;62(2):238–46. [DOI] [PubMed] [Google Scholar]
- 18. Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha SR, Rogers LM, Namaste SM. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr. 2017;106(Suppl 6):1626s–33s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, Garrett GS, Klemm RD, Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients. 2017;9(3):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wessells KR, Brown KH.. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kordas K, Fonseca Centeno ZY, Pachon H, Jimenez Soto AZ. Being overweight or obese is associated with lower prevalence of anemia among Colombian women of reproductive age. J Nutr. 2013;143(2):175–81. [DOI] [PubMed] [Google Scholar]
- 22. Jones AD, Mundo-Rosas V, Cantoral A, Levy TS. Household food insecurity in Mexico is associated with the co-occurrence of overweight and anemia among women of reproductive age, but not female adolescents. Matern Child Nutr. 2017;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rivera JA, Pedraza LS, Martorell R, Gil A. Introduction to the double burden of undernutrition and excess weight in Latin America. Am J Clin Nutr. 2014;100(6):1613s–6s. [DOI] [PubMed] [Google Scholar]
- 24. Jones AD, Hayter AK, Baker CP, Prabhakaran P, Gupta V, Kulkarni B, Smith GD, Ben-Shlomo Y, Krishna KV, Kumar PU et al.. The co-occurrence of anemia and cardiometabolic disease risk demonstrates sex-specific sociodemographic patterning in an urbanizing rural region of southern India. Eur J Clin Nutr. 2016;70(3):364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, Macdonald B, Suchdev PS, Rohner F, Aaron GJ. Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):416s–27s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):333s–47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amugsi DA, Dimbuene ZT, Mberu B, Muthuri S, Ezeh AC. Prevalence and time trends in overweight and obesity among urban women: an analysis of demographic and health surveys data from 24 African countries, 1991–2014. BMJ Open. 2017;7(10):e017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abrahams Z, McHiza Z, Steyn NP. Diet and mortality rates in sub-Saharan Africa: stages in the nutrition transition. BMC Public Health. 2011;11:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steyn NP, McHiza ZJ. Obesity and the nutrition transition in sub-Saharan Africa. Ann N Y Acad Sci. 2014;1311:88–101. [DOI] [PubMed] [Google Scholar]
- 30. National Statistical Office, Community Health Sciences Unit, Centers for Disease Control and Prevention, & Emory University. Malawi Micronutrient Survey 2015–16. Atlanta (GA) USA; 2017. [Google Scholar]
- 31. National Statistical Office, & ICF. Malawi Demographic and Health Survey 2015–16. Zomba (Malawi) and Rockville (MD) USA; 2017. [Google Scholar]
- 32. Corsi DJ, Perkins JM, Subramanian SV. Child anthropometry data quality from Demographic and Health Surveys, Multiple Indicator Cluster Surveys, and National Nutrition Surveys in the West Central Africa region: are we comparing apples and oranges?. Global Health Action. 2018;11(1):1444115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization and Food and Agriculture Organization. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. Geneva (Switzerland: ): World Health Organization; 2003. [Google Scholar]
- 34. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. [Internet] Geneva (Switzerland): World Health Organization; 2011. [Accessed 2019 Nov 13]. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 35. King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of Nutrition for Development (BOND) – zinc review. J Nutr. 2016;146(4):858S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ et al.. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):359s–71s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Adv Nutr. 2016;7(2):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and mineral nutrition information system. [Internet] Geneva (Switzerland): World Health Organization; 2012. [Accessed 2019 Nov 13]. Available from:https://apps.who.int/iris/handle/10665/75584. [Google Scholar]
- 39. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238–44. [DOI] [PubMed] [Google Scholar]
- 40. Engle-Stone R, Haskell MJ, Ndjebayi AO, Nankap M, Erhardt JG, Gimou MM, Brown KH. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J Nutr. 2011;141(12):2233–41. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Vitamin and Mineral Nutrition Information System. [Internet]. Geneva: World Health Organization; 2011. [Accessed 2019 Nov 13]. Available from: http://www.who.int/vmnis/indicators/retinol.pdf. [Google Scholar]
- 42. World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. [Internet]. Geneva: World Health Organization; 2015. [Accessed 2019 Nov 13]. Available from: https://apps.who.int/iris/handle/10665/162114. [Google Scholar]
- 43. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 44. Rivera JA, Barquera S, Gonzalez-Cossio T, Olaiz G, Sepulveda J. Nutrition transition in Mexico and in other Latin American countries. Nutr Rev. 2004;62(7 Pt 2):S149–57. [DOI] [PubMed] [Google Scholar]
- 45. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP et al.. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients. 2016;8(11):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laillou A, Yakes E, Le TH, Wieringa FT, Le BM, Moench-Pfanner R, Berger J. Intra-individual double burden of overweight and micronutrient deficiencies among Vietnamese women. PLoS One. 2014;9(10):e110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laillou A, Pham TV, Tran NT, Le HT, Wieringa F, Rohner F, Fortin S, Le MB, Tran do T, Moench-Pfanner R et al.. Micronutrient deficits are still public health issues among women and young children in Vietnam. PLoS One. 2012;7(4):e34906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Popkin BM, Slining MM. New dynamics in global obesity facing low- and middle-income countries. Obes Rev. 2013;14(Suppl 2):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Becquey E, Martin-Prevel Y. Micronutrient adequacy of women's diet in urban Burkina Faso is low. J Nutr. 2010;140(11):2079s–85s. [DOI] [PubMed] [Google Scholar]
- 51. Gosdin L, Martorell R, Bartolini RM, Mehta R, Srikantiah S, Young MF. The co-occurrence of anaemia and stunting in young children. Matern Child Nutr. 2018;14(3):e12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dieffenbach S, Stein AD. Stunted child/overweight mother pairs represent a statistical artifact, not a distinct entity. J Nutr. 2012;142(4):771–3. [DOI] [PubMed] [Google Scholar]
- 53. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hawkes C, Demaio AR, Branca F. Double-duty actions for ending malnutrition within a decade. Lancet Glob Health. 2017;5(8):e745–e6. [DOI] [PubMed] [Google Scholar]
- 55. Hawkes C, Ruel MT, Salm L, Sinclair B, Branca F. Double-duty actions: seizing programme and policy opportunities to address malnutrition in all its forms. Lancet. 2020;395(10218):142–55. [DOI] [PubMed] [Google Scholar]
- 56. Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395(10217):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jones-Smith JC, Popkin BM.. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med. 2010;71(8):1436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Novak NL, Allender S, Scarborough P, West D. The development and validation of an urbanicity scale in a multi-country study. BMC Public Health. 2012;12:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND) – vitamin A review. J Nutr. 2016;146(9):1816s–48s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ma G, Li Y, Jin Y, Zhai F, Kok FJ, Yang X. Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. Eur J Clin Nutr. 2007;61(3):368–74. [DOI] [PubMed] [Google Scholar]
- 61. Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130(5S Suppl):1378s–83s. [DOI] [PubMed] [Google Scholar]
- 62. Rahman S, Ahmed T, Rahman AS, Alam N, Ahmed AM, Ireen S, Chowdhury IA, Chowdhury FP, Rahman SM. Status of zinc nutrition in Bangladesh: the underlying associations. J Nutri Sci. 2016;5:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.