ABSTRACT
Background
Although greater flavonoid intake is associated with a reduced risk of Alzheimer's disease (AD) and related dementias (ADRD), evidence relating dietary flavonoid intake to brain health based on MRI is lacking.
Objective
The objective of this study was to explore the association between dietary flavonoid intake and MRI measures of brain health, including total brain tissue volume (TBV), white matter hyperintensities volume (WMHV), and hippocampal volume (HV).
Methods
Eligible subjects included members of the Framingham Heart Study Offspring Cohort who were free of stroke at exam 7 and had at least 1 valid food frequency questionnaire from exams 5, 6, or 7 (n = 2086; mean age at exam 7, 60.6 y). Flavonoid intakes represented the cumulative mean of intakes across the 3 exams and were categorized based on quartiles categories of intake. TBV, WMHV, and HV were assessed at exam 7. Multiple linear regression models were used to examine the cross-sectional association between total and the 6 classes of flavonoids and the 3 aforementioned MRI measures.
Results
The mean (95% CI) of the WMHV of subjects in the highest quartile category of flavan-3-ols [0.56 (0.52, 0.61)] and flavonoid polymers [0.57 (0.52, 0.61)] intake was significantly smaller relative to that of subjects in the lowest quartile category of flavan-3-ols [0.65 (0.60, 0.71)] and flavonoid polymers [0.66 (0.60, 0.71)] after accounting for important demographic, lifestyle, and clinical factors. Inverse trend associations with WMHV were also seen for flavan-3-ols (P = 0.01) and flavonoid polymers (P = 0.01) as well as for total flavonoids (P = 0.01). TBV and HV were not associated with dietary flavonoid intake following the adjustment for potential confounders.
Conclusions
Our results contribute to the literature on flavonoids and ADRD as they suggest that higher flavonoid intakes may affect ADRD risk in middle-aged and older adults by reducing WMHV, a marker strongly associated with ADRD.
Keywords: Alzheimer's disease and related dementias, brain imaging, brain aging, polyphenols, flavonoids
Introduction
Alzheimer's disease (AD), the most common form of Alzheimer's disease and related dementias (ADRD), represents one of the unresolved global public health problems in the aging population (1). There are about 50 million people who are living with ADRD worldwide, with nearly 10 million new cases diagnosed every year (2). AD is characterized by cellular dysfunction and death, as measured by loss of total and hippocampal brain volume (3). Another sign of neurodegeneration and risk factor for ADRD is the presence of white matter hyperintensities, lesions that appear as areas of increased brightness when visualized by MRI of the brain (4–6).
With rates of ADRD reaching global epidemic levels (1, 7), it is of paramount importance to identify strategies that could help reduce or reverse this epidemic. One potential promising avenue is diet. The Mediterranean dietary pattern (MedDiet), which is rich in polyphenol-containing foods, has been shown to be associated with a reduced the risk of cognitive decline and ADRD (8, 9). Studies on the relationship between the MedDiet and brain MRI measures have also shown that a higher adherence to the MedDiet is associated with healthier brain structures including greater total, gray and white matter, and hippocampal volumes, and cortical thickness (10–13), and less volumes of white matter hyperintensities (14).
Reports from our group and others on flavonoids, a class of polyphenols, have demonstrated that higher intakes of these phytochemicals are associated with a reduced risk of ADRD (15–17). However, there are no studies that have examined the association between flavonoids and MRI measures of brain health. Therefore, we sought to address this gap in the literature and explore the association between dietary flavonoid intake and 3 brain MRI structure measures that are associated with ADRD, total brain tissue volume (TBV) and hippocampal volume (HV) as markers of brain tissue atrophy and white matter hyperintensities volume (WMHV) as a radiological correlate of cognitive decline. We hypothesize that flavonoid intakes will be positively associated with TBV and HV and inversely associated with WMHV.
Methods
Study population
We used data from the Framingham Heart Study (FHS) offspring cohort, which was established in 1970 and includes 5124 men and women who were the offspring of the FHS original cohort and their spouses (18). The cohort is described in greater detail elsewhere (19, 20). Briefly, about every 4 y, the cohort fills out a series of questionnaires, completes laboratory and cardiovascular tests, and undergoes a physical examination. It is also continuously monitored for various incident diseases including ADRD.
In order to conduct this cross-sectional study, we used exposure (dietary) data derived from Offspring Cohort exams 5 (1991–1995), 6 (1995–1998), and 7 (1998–2001). Figure 1 illustrates the flow of our study population. Eligible subjects had to meet the following crateria: 1) have available MRI data at exam 7; 2) have at least 1 valid FFQ (as described below) at any of the 3 above mentioned exams, and 3) be stroke free at exam 7. Of the 2197 participants with available brain MRI data at exam 7, 34 did not have any valid dietary data across the 3 exams and 31 had stroke at exam 7, resulting in a total of 2132 participants. Of those, 1 participant was excluded due to missing information on BMI (in kg/m2), prevalent diabetes and hypercholesterolemia, and an additional 45 were excluded due to missing information on total intracranial vault volume, resulting in a final total sample of 2086 participants.
FIGURE 1.
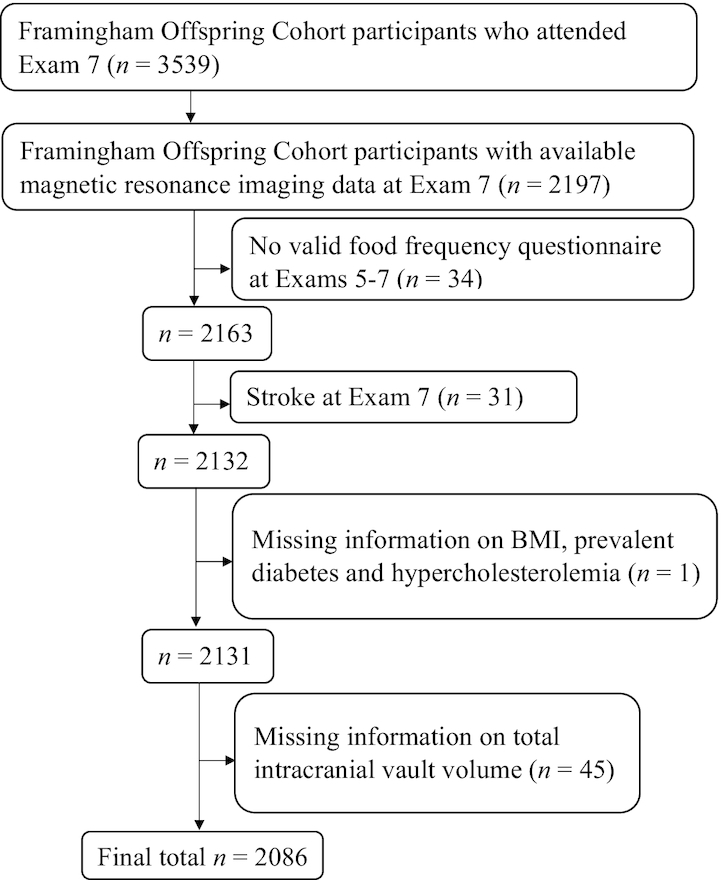
Flowchart of the Framingham Offspring cohort study participants included in the study.
The original data collection protocols were approved by the institutional review board at Boston University Medical Center, and written informed consent was obtained from all participants. The present study protocol was reviewed and approved by the Tufts University Health Sciences Institutional Review Board.
Flavonoid exposures
Dietary assessment tool
Using a validated semiquantitative FFQ, the dietary intakes of subjects were assessed at Offspring Cohort exam cycles 5 through 7 (21). The FFQs were mailed to free-living subjects prior to each Offspring Cohort exam cycle, with instructions on how to complete the questionnaire. The FFQ records subjects’ frequency of foods consumed over the past year. It consists of a list of 126 foods with a standard serving size and a selection of 9 frequency categories ranging from “never or <1 serving/month” to “≥6 servings/d.” It also allows subjects to add up to 4 extra food items that are essential components of their diets but missing from the food list. Other dietary information such as the use of vitamin and mineral supplements, type of breakfast cereal most commonly consumed, types of fats and oils typically used, and frequency of consumption of fried foods are also captured. Nutrient and non-nutrient intakes of food components were calculated by multiplying the frequency of consumption of each food item by the nutrient content of the specified portions.
Validity of FFQ
An FFQ was considered invalid if reported total energy intakes were <600 kcal/d or >4000 kcal/d for women, and >4200 kcal/d for men, respectively, or if >12 food items were left blank. In addition, due to the concern regarding the accuracy of diet reporting of subjects with cognitive impairment or ADRD, an FFQ was also judged as invalid for individuals with cognitive impairment or ADRD based on the timing between cognitive impairment onset date or ADRD date and the exam date at which dietary data were collected (as described below). This approach resulted in the exclusion of 2 subjects with ADRD.
Characterization of flavonoid intakes
We considered as our exposure the usual intakes of the total and each of the 6 flavonoid classes typically consumed in the US diet, including flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, and flavonoid polymers, using the flavonoid classification of Cassidy et al (22). To derive flavonoid intake information, we used the USDA flavonoid content of foods and the proanthocyanidin databases (23). The sum of intakes of the 6 flavonoid classes were used to calculate total flavonoid intake. Given the low consumption of isoflavones in the US diet (24, 25), we did not incorporate this flavonoid class in our analyses.
The validity of flavonoid intake from the FFQ used in the present study has not been directly assessed. However, the validity of food intake measurements based on a comparison between the FFQ and two 7-d diet records collected during the year time interval covered by the FFQ has been previously reported (26). This report revealed rather high correlation levels between intakes from the FFQ and 7-d diet records for important dietary sources of flavonoids in the Framingham Offspring cohort, including red wine (0.83), orange juice (0.78), tea (0.77), oranges (0.76), apples/pears (0.70), and strawberries (0.38).
Brain MRI measures
Brain MRI techniques used in the FHS have been described previously (27). The brain MRI scan data used in this study were collected as part of an ancillary study which was performed on a subset of the Offspring cohort contemporaneous with Offspring exam 7. Our primary outcomes, which were established a priori, included the following MRI measures: TBV, WMHV, and HV. TBV was derived as the sum of the total white and gray matter volumes and WMHV. All images were read masked to demographic and clinical information using a custom-designed image analysis package (27). Interrater reliability values for agreement among more >10 raters were generally good and ranged above 0.90 (27).
Covariates
The following nondietary covariates were included as potential confounders in our analyses: age, sex, education (<college graduate, ≥college graduate), total energy intake (TEI, kcal/d), apoE ε4 allele (having at least 1 apoE ε4 allele), BMI (kg/m2), total intracranial vault volume (cubic centimeters), physical activity index (PAI) expressed in metabolic equivalents (28), smoking status (never, current, or former), prevalent hypercholesterolemia, hypertension, and diabetes. Hypercholesterolemia was defined based on either use of cholesterol-lowering medication or total cholesterol concentrations ≥200 mg/dL. Similarly, hypertension was defined by either use of blood pressure–lowering medication or systolic and/or diastolic blood pressure readings of ≥130 mmHg and ≥80 mmHg, respectively. Diabetes was assessed by any of the following criteria: 1) taking oral hypoglycemic medication, 2) insulin use, 3) fasting blood glucose concentrations ≥126 mg/dL, and 4) nonfasting blood glucose concentrations ≥200 mg/dL.
In addition, due to evidence on the relation between various dietary factors and ADRD/cognitive function (29–31), we also considered the following dietary covariates as potential confounders: overall diet quality, as assessed by the 2010 Dietary Guidelines Adherence Index (DGAI) (32), vitamin and mineral supplement use, and dietary intakes of the ω-3 fatty acids EPA and DHA (grams per day), lutein and zeaxanthin (micrograms per day), and alcohol (grams per day).
Finally, given the correlation between our 3 MRI measures of interest and head size, it is necessary to correct for head size in order to more accurately assess the relation between intake of flavonoids and these MRI measures. Thus, we included the variable total intracranial vault volume, a measure of head size, as a covariate in our models.
Statistical analyses
Main analyses
SAS software (version 9.4; SAS Institute) was used to perform all analyses, all of which were determined a priori. Flavonoid intakes were categorized into quartile categories of intake and were updated at each exam by using the cumulative mean of intakes from exams 5 through 7. For subjects with missing dietary intake data at 1 or 2 of the 3 exams, the cumulative mean of their flavonoid intake was based on available intake data. We used exam 7 data for the categorical covariates. Cumulative mean data from exams 5 through 7 were used for the continuous covariates, with the exception of total intracranial vault volume as it was only available at exam 7. The update of the continuous covariate data followed the same cumulative mean approach as the flavonoid intake data. In order to minimize subject losses due to missing categorical covariate data, we included an additional missing category. This was applied to the following categorical covariates: education, apoEε4 allele, smoking status, diabetes, hypertension, and hypercholesterolemia. Our total sample consisted of 2086 subjects who had complete data on HV and were missing data on TBV and WMHV (n missing = 26), PAI (n missing = 46) and DGAI (n missing = 86) (Table 1).
TABLE 1.
Age- and sex-adjusted characteristics of participants based on total sample and extreme quartiles of total flavonoid intakes1
| Total flavonoid intake | ||||
|---|---|---|---|---|
| Characteristic | Total sample (n = 2086) | Quartile 1 (n = 521) | Quartile 4 (n = 521) | P-trend2 |
| Total flavonoid intake, mg/d | 238 (19.4–2323) | 109 (19.4–150) | 525 (388–2323) | |
| Total brain tissue volume,3 cm3 | 1087 (1083, 1091) | 1081 (1073, 1088) | 1089 (1081, 1096) | 0.42 |
| WMHV,3 cm3 | 0.62 (0.59, 0.64) | 0.66 (0.61, 0.72) | 0.57 (0.53, 0.62) | 0.007 |
| Hippocampal volume, cm3 | 6.65 (6.62, 6.67) | 6.62 (6.56, 6.67) | 6.66 (6.61, 6.72) | 0.42 |
| Age, y | 60.6 (60.2, 61.1) | 59.7 (58.9, 60.5) | 61.1 (60.3, 61.9) | 0.01 |
| Female, % | 53.7 (51.5, 55.8) | 48.4 (44.2, 52.7) | 66.7 (62.4, 70.9) | <0.001 |
| Education ≥ college degree,3 % | 69.9 (68.0, 71.9) | 62.3 (58.4, 66.1) | 74.8 (70.9, 78.7) | <0.001 |
| apoE ε4 allele,3 % | 22.9 (21.1, 24.7) | 21.5 (17.9, 25.2) | 27.0 (23.3, 30.7) | 0.01 |
| Total energy intake, kcal/d | 1893 (1870, 1916) | 1607 (1564, 1650) | 2073 (2029, 2116) | <0.001 |
| PAI,3 MET | 36.1 (35.9, 36.3) | 35.8 (35.4, 36.2) | 36.3 (35.9, 36.7) | 0.09 |
| Former/current smoker,3 % | 64.0 (62.0, 66.1) | 71.5 (67.4, 75.6) | 59.2 (55.1, 63.4) | <0.001 |
| BMI, kg/m2 | 27.7 (27.5, 27.9) | 28.3 (27.9, 28.7) | 26.8 (26.4, 27.3) | <0.001 |
| Diabetes,3 % | 10.4 (9.1, 11.7) | 10.9 (8.4, 13.5) | 6.8 (4.2, 9.5) | 0.06 |
| Hypertension,3 % | 58.1 (56.0, 60.1) | 58.7 (54.6, 62.8) | 55.4 (51.3, 59.6) | 0.29 |
| Hypercholesterolemia,3 % | 62.5 (60.5, 64.6) | 63.5 (59.3, 67.6) | 61.1 (56.9, 65.3) | 0.33 |
| DGAI3 | 60.0 (59.6, 60.5) | 54.2 (53.3, 55.0) | 62.9 (62.1, 63.8) | <0.001 |
| ω-3 fatty acid (EPA and DHA) intake, g/d | 0.27 (0.26, 0.28) | 0.21 (0.19, 0.23) | 0.31 (0.29, 0.32) | <0.001 |
| Lutein and zeaxanthin intake, mg/d | 3.3 (3.2, 3.4) | 2.3 (2.1, 2.5) | 3.9 (3.7, 4.1) | <0.001 |
| Alcohol intake, g/d | 10.7 (10.1, 11.3) | 10.1 (8.9, 11.3) | 10.6 (9.4, 11.8) | 1.00 |
| Multivitamin and mineral supplement use, % | 47.1 (45.0, 49.3) | 40.2 (36.0,44.5) | 55.8 (51.5, 60.1) | <0.001 |
Unless otherwise indicated, values represent cumulative means (95% CIs) from up to 3 exams (exams 5 through 7) for continuous variables or exam 7 values for categorical variables and are age- and sex-adjusted least-squares arithmetic means or least-squares percentages, with the exceptions of WMHVs, which are age- and sex-adjusted geometric means (95% CIs), and total flavonoid intake data, which are medians (ranges). apoE ε4, apolipoprotein E ε4; BMI, body mass index; DGAI, dietary guidelines adherence index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MET, metabolic equivalents; PAI, physical activity index; WMHV, white matter hyperintensities volume.
P values for the test of linear trend across extreme quartile categories of total flavonoid intake were based on linear regression models, with the median intake of each extreme quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Missing from total: total brain tissue volume, n = 26; WMHV, n = 26; education, n = 41; apoE ε4, n = 49; PAI, n = 46; former/current smoking, n = 3; diabetes, n = 34; hypertension, n = 3; hypercholesterolemia, n = 27; DGAI, n = 86.
The distribution of the outcome WMHV was severely skewed and was handled by natural log transformation. This was followed by a Winsorization procedure due to the presence of 2 lower and 1 upper extreme outlier. Similarly, the outcome TBV included 2 upper extreme outliers and HV included 1 lower and 1 upper extreme outlier, which were also dealt with by a Winsorization procedure. We applied a 3-y cutoff for the handling and update of dietary intake and covariate data of subjects with ADRD, i.e., if the time difference between a subject's ADRD diagnosis date and the exam prior to ADRD diagnosis was ≥3 y, we stopped the update at the exam prior to which ADRD was diagnosed. Conversely, if the time difference was <3 y, we stopped the update at 2 exams prior to which ADRD was diagnosed. For the subject with cognitive impairment, we stopped updating his/her dietary and covariate information at the exam prior to cognitive impairment onset.
We used multiple linear regression models to assess the cross-sectional association between total and 6 classes of flavonoid intake and the 3 brain MRI measures that were assessed at exam 7, including TBV, WMHV, and HV. We performed linear trend tests across quartile categories of flavonoid intake by using the median value of intake of each quartile category treated as a continuous variable. A P value of 0.05 was used to identify significant trends. We used Dunnett's correction for multiple comparisons to examine any significant differences between means in the extreme quartile categories of flavonoid intake for the 3 MRI outcomes (P < 0.05). We used 3 cumulative models in our analyses, each accounting for important potential confounders: 1) Model 1 (basic model): adjusted for sex, age, education, TEI, apoE ε4 allele, and total intracranial vault volume. 2) Model 2 (lifestyle and clinical): adjusted for model 1 covariates and PAI, smoking status, BMI, prevalent diabetes, hypertension, and hypercholesterolemia. 3) Model 3 (dietary model): adjusted for model 1 and model 2 covariates and overall dietary quality (DGAI), multivitamin and mineral supplement use, and dietary intakes of alcohol, ω-3 fatty acids (EPA and DHA), and lutein and zeaxanthin.
Results
The participant characteristics, including their brain MRI measures and demographic, clinical, lifestyle, and key dietary intake information, are presented in Table 1. Participants’ mean age was 60.6 y and ∼54.0% were females. On average, subjects were highly educated, ∼70% with ≥college degree), overweight, and had low physical activity and moderate DGAI scores. They had a high prevalence of smoking, hypertension, and hypercholesterolemia but a low prevalence of diabetes. Relative to subjects in the lowest quartile category of total flavonoid intake, those in the highest quartile category were older, were women and educated, had a higher prevalence of the apoE ε4 allele, and consumed higher-quality diets, with more calories, ω-3 fatty acids (EPA and DHA), lutein and zeaxanthin, and multivitamin and mineral supplements. Additionally, these subjects had lower WMHV, BMI, and prevalence of smoking. There were only 5 subjects with ADRD and 1 subject with cognitive impairment.
Findings from multiple linear regression models showed significant trends toward smaller WMHV with higher intakes total flavonoids, flavan-3-ols, and flavonoid polymers (Table 2). These trends persisted after taking into account important lifestyle and clinical covariates, as well as overall diet quality and key dietary components. The mean WMHV of those in the highest quartile category of flavan-3-ols and flavonoid polymer intake was significantly smaller relative to those in the lowest quartile category of flavan-3-ol and flavonoid polymer intake (Table 2, models 1 and 2). However, after controlling for overall diet quality and other key dietary components, the differences in the means between the 2 extreme quartile categories were no longer significant (Table 2, model 3). Similarly, a significant trend towards larger TBV was only seen with higher intakes of anthocyanins with significant differences in the means of TBV in the extreme quartile categories of anthocyanin intake (Table 3, model 1). Additionally, we observed as significant difference between the means of TBV in the extreme quartile categories of total flavonoid intake (Table 3, model 1). However, after accounting for additional clinical, lifestyle, or dietary factors, these associations were no longer present (Table 3, models 2 and 3). We did not observe any significant trends between total flavonoids and the 6 flavonoid classes and HV (Table 4).
TABLE 2.
Mean WMHV across quartiles of total and 6 classes of flavonoid intake in members of the Framingham Offspring cohort, men and women with mean age 60.6 y1
| Mean WMHV across flavonoid intake quartiles, cm3 | |||||
|---|---|---|---|---|---|
| Flavonoid class | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend2 |
| Total flavonoids (median intake 237.3 mg) | (n = 514)3 | (n = 517) | (n = 514) | (n = 515) | |
| Model 14 | 0.66 (0.60, 0.71) | 0.64 (0.59, 0.69) | 0.61 (0.56, 0.66) | 0.58 (0.53, 0.63) | 0.02 |
| Model 25 | 0.65 (0.60, 0.71) | 0.64 (0.59, 0.69) | 0.61 (0.56, 0.66) | 0.57 (0.52, 0.61) | 0.01 |
| Model 36 | 0.65 (0.60, 0.71) | 0.64 (0.59, 0.69) | 0.62 (0.57, 0.67) | 0.57 (0.53, 0.62) | 0.03 |
| Flavonols (median intake 11.6 mg) | (n = 513) | (n = 519) | (n = 513) | (n = 515) | |
| Model 1 | 0.65 (0.60, 0.70) | 0.64 (0.59, 0.69) | 0.60 (0.55, 0.65) | 0.60 (0.55, 0.65) | 0.14 |
| Model 2 | 0.64 (0.59, 0.70) | 0.64 (0.59, 0.69) | 0.60 (0.55, 0.64) | 0.59 (0.55, 0.64) | 0.14 |
| Model 3 | 0.65 (0.59, 0.71) | 0.64 (0.59, 0.69) | 0.60 (0.56, 0.65) | 0.59 (0.54, 0.64) | 0.11 |
| Flavones (median intake 1.8 mg) | (n = 516) | (n = 514) | (n = 516) | (n = 514) | |
| Model 1 | 0.61 (0.57, 0.66) | 0.59 (0.55, 0.64) | 0.64 (0.60, 0.70) | 0.63 (0.58, 0.68) | 0.38 |
| Model 2 | 0.61 (0.56, 0.66) | 0.59 (0.55, 0.64) | 0.64 (0.59, 0.70) | 0.62 (0.57, 0.68) | 0.48 |
| Model 3 | 0.61 (0.56, 0.66) | 0.59 (0.55, 0.64) | 0.65 (0.60, 0.70) | 0.63 (0.58, 0.69) | 0.34 |
| Flavanones (median intake 39.9 mg) | (n = 513) | (n = 518) | (n = 517) | (n = 512) | |
| Model 1 | 0.62 (0.57, 0.67) | 0.60 (0.55, 0.64) | 0.66 (0.61, 0.71) | 0.61 (0.56, 0.66) | 0.77 |
| Model 2 | 0.62 (0.57, 0.67) | 0.60 (0.55, 0.64) | 0.65 (0.60, 0.71) | 0.60 (0.56, 0.65) | 0.91 |
| Model 3 | 0.62 (0.57, 0.67) | 0.59 (0.55, 0.64) | 0.66 (0.61, 0.71) | 0.61 (0.56, 0.66) | 0.79 |
| Flavan-3-ols (median intake 27.1) | (n = 513) | (n = 516) | (n = 516) | (n = 515) | |
| Model 1 | 0.65 (0.60, 0.71) | 0.63 (0.58, 0.68) | 0.63 (0.58, 0.68) | 0.57* (0.53, 0.62) | 0.01 |
| Model 2 | 0.65 (0.60, 0.71) | 0.63 (0.58, 0.68) | 0.63 (0.58, 0.68) | 0.56* (0.52, 0.61) | 0.01 |
| Model 3 | 0.64 (0.59, 0.70) | 0.64 (0.59, 0.69) | 0.63 (0.59, 0.69) | 0.56 (0.52, 0.61) | 0.01 |
| Anthocyanins (median intake 11.2 mg) | (n = 511) | (n = 516) | (n = 516) | (n = 517) | |
| Model 1 | 0.63 (0.58, 0.68) | 0.62 (0.57, 0.67) | 0.63 (0.58, 0.68) | 0.60 (0.56, 0.65) | 0.48 |
| Model 2 | 0.62 (0.57, 0.68) | 0.62 (0.58, 0.67) | 0.62 (0.57, 0.67) | 0.60 (0.56, 0.66) | 0.58 |
| Model 3 | 0.62 (0.57, 0.68) | 0.62 (0.57, 0.67) | 0.63 (0.58, 0.68) | 0.61 (0.56, 0.66) | 0.78 |
| Flavonoid polymers (median intake 134.4 mg) | (n = 513) | (n = 520) | (n = 512) | (n = 515) | |
| Model 1 | 0.66 (0.61, 0.72) | 0.63 (0.58, 0.68) | 0.62 (0.57, 0.67) | 0.57* (0.53, 0.62) | 0.01 |
| Model 2 | 0.66 (0.60, 0.71) | 0.63 (0.59, 0.68) | 0.62 (0.57, 0.67) | 0.57* (0.52, 0.61) | 0.01 |
| Model 3 | 0.65 (0.60, 0.71) | 0.63 (0.58, 0.68) | 0.63 (0.58, 0.68) | 0.57 (0.52, 0.62) | 0.03 |
Values are cross-sectional geometric means (95% CIs) of WMHVs across quartiles of total and 6 classes of flavonoid intake, unless otherwise indicated. WMHV, white matter hyperintensities volume.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models. *Significantly different from quartile 1 by Dunnett's test, P < 0.05.
The n values are numbers of participants per quartile category for model 1.
Adjusted for age, sex, education, total energy intake, apoE ε4, and total intracranial vault volume; of the total 2086 observations, model 1 included 2060 observations.
Adjusted for model 1 + BMI, physical activity index, smoking, prevalent hypercholesterolemia, hypertension, and diabetes; of the total 2086 observations, model 2 included 2015 observations.
Adjusted for model 1 covariates + overall dietary quality, vitamin and mineral supplement use, and dietary intakes of alcohol, ω-3 fatty acids EPA and DHA, and lutein and zeaxanthin; of the total 2086 observations, model 3 included 1976 observations.
TABLE 3.
Mean total brain tissue volume across quartiles of total and 6 classes of flavonoid intake in members of the Framingham Offspring cohort, men and women with mean age 60.6 y1
| Mean total brain tissue volume, cm3 across flavonoid quartiles | |||||
|---|---|---|---|---|---|
| Flavonoid class | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend2 |
| Total flavonoids (median intake 237.3 mg) | (n = 514)3 | (n = 517) | (n = 514) | (n = 515) | |
| Model 14 | 1085 (1082, 1087) | 1089 (1086, 1091) | 1086 (1084, 1089) | 1090* (1087, 1092) | 0.06 |
| Model 25 | 1085 (1082, 1088) | 1088 (1085, 1090) | 1086 (1083, 1088) | 1089 (1086, 1091) | 0.16 |
| Model 36 | 1086 (1083, 1089) | 1089 (1086, 1091) | 1085 (1083, 1088) | 1089 (1086, 1091) | 0.37 |
| Flavonols (median intake 11.6 mg) | (n = 513) | (n = 519) | (n = 513) | (n = 515) | |
| Model 1 | 1086 (1083, 1089) | 1087 (1085, 1090) | 1087 (1085, 1090) | 1088 (1086, 1091) | 0.25 |
| Model 2 | 1086 (1083, 1088) | 1087 (1084, 1089) | 1087 (1084, 1090) | 1088 (1085, 1091) | 0.29 |
| Model 3 | 1085 (1082, 1088) | 1087 (1084, 1089) | 1087 (1085, 1090) | 1089 (1086, 1092) | 0.07 |
| Flavones (median intake 1.8 mg) | (n = 516) | (n = 514) | (n = 516) | (n = 514) | |
| Model 1 | 1086 (1083, 1089) | 1087 (1084, 1089) | 1089 (1087, 1092) | 1087 (1084, 1090) | 0.55 |
| Model 2 | 1086 (1083, 1089) | 1086 (1084, 1089) | 1089 (1086, 1092) | 1086 (1083, 1089) | 0.90 |
| Model 3 | 1086 (1084, 1089) | 1086 (1084, 1089) | 1089 (1086, 1091) | 1087 (1084, 1090) | 0.63 |
| Flavanones (median intake 39.9 mg) | (n = 513) | (n = 518) | (n = 517) | (n = 512) | |
| Model 1 | 1086 (1083, 1088) | 1088 (1085, 1091) | 1088 (1085, 1090) | 1088 (1085, 1090) | 0.40 |
| Model 2 | 1086 (1083, 1089) | 1087 (1085, 1090) | 1087 (1084, 1090) | 1087 (1084, 1090) | 0.62 |
| Model 3 | 1086 (1083, 1089) | 1088 (1085, 1090) | 1087 (1085, 1090) | 1087 (1085, 1090) | 0.67 |
| Flavan-3-ols (median intake 27.1 mg) | (n = 513) | (n = 516) | (n = 516) | (n = 515) | |
| Model 1 | 1087 (1084, 1090) | 1087 (1084, 1090) | 1088 (1085, 1091) | 1087 (1085, 1090) | 0.79 |
| Model 2 | 1087 (1084, 1089) | 1087 (1084, 1089) | 1087 (1085, 1090) | 1087 (1084, 1089) | 0.98 |
| Model 3 | 1087 (1084, 1089) | 1086 (1084, 1089) | 1088 (1086, 1091) | 1087 (1084, 1090) | 0.79 |
| Anthocyanins (median intake 11.2 mg) | (n = 511) | (n = 516) | (n = 516) | (n = 517) | |
| Model 1 | 1085 (1082, 1088) | 1088 (1085, 1090) | 1087 (1084, 1089) | 1090* (1087, 1093) | 0.02 |
| Model 2 | 1085 (1083, 1088) | 1087 (1084, 1090) | 1087 (1084, 1089) | 1088 (1086, 1091) | 0.14 |
| Model 3 | 1086 (1083, 1089) | 1087 (1084, 1090) | 1086 (1084, 1089) | 1089 (1086, 1092) | 0.15 |
| Flavonoid polymers (median intake 134.4 mg) | (n = 513) | (n = 520) | (n = 512) | (n = 515) | |
| Model 1 | 1086 (1083, 1088) | 1088 (1085, 1091) | 1086 (1084, 1089) | 1089 (1086, 1092) | 0.17 |
| Model 2 | 1086 (1083, 1089) | 1087 (1085, 1090) | 1086 (1083, 1088) | 1088 (1086, 1091) | 0.30 |
| Model 3 | 1087 (1084, 1090) | 1088 (1086, 1091) | 1085 (1083, 1088) | 1088 (1085, 1090) | 0.90 |
Values are cross-sectional means (95% CIs) of total brain tissue volumes across quartiles of total and 6 classes of flavonoid intakes, unless otherwise indicated.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models. *Significantly different from quartile 1 by Dunnett's test, P < 0.05.
The n values are numbers of participants per quartile category for model 1.
Adjusted for age, sex, education, total energy intake, apoE ε4, and total intracranial vault volume; of the total 2086 observations, model 1 included 2060 observations.
Adjusted for model 1 + BMI, physical activity index, smoking, and prevalent hypercholesterolemia, hypertension, and diabetes; of the total 2086 observations, model 2 included 2015 observations.
Adjusted for model 1 covariates + overall dietary quality, vitamin and mineral supplement use, and dietary intakes of alcohol, ω-3 fatty acids EPA and DHA, and lutein and zeaxanthin; of the total 2086 observations, model 3 included 1976 observations.
TABLE 4.
Mean hippocampal volume across quartiles of total and 6 classes of flavonoid intake in members of the Framingham Offspring cohort, men and women with mean age 60.6 y1
| Mean hippocampal volume across flavonoid intake quartiles, cm3 | |||||
|---|---|---|---|---|---|
| Flavonoid class | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend2 |
| Total flavonoids (median intake 237.5 mg) | (n = 521)3 | (n = 522) | (n = 522) | (n = 521) | |
| Model 14 | 6.63 (6.58, 6.68) | 6.65 (6.61, 6.70) | 6.63 (6.58, 6.68) | 6.67 (6.62, 6.72) | 0.36 |
| Model 25 | 6.63 (6.58, 6.68) | 6.65 (6.60, 6.70) | 6.63 (6.58, 6.68) | 6.67 (6.62, 6.71) | 0.41 |
| Model 36 | 6.64 (6.59, 6.69) | 6.66 (6.61, 6.70) | 6.63 (6.58, 6.68) | 6.66 (6.61, 6.71) | 0.69 |
| Flavonols (median intake 11.6 mg) | (n = 521) | (n = 522) | (n = 522) | (n = 521) | |
| Model 1 | 6.65 (6.60, 6.70) | 6.65 (6.60, 6.69) | 6.65 (6.61, 6.70) | 6.64 (6.59, 6.69) | 0.77 |
| Model 2 | 6.64 (6.59, 6.69) | 6.64 (6.60, 6.69) | 6.66 (6.61, 6.70) | 6.64 (6.59, 6.69) | 0.96 |
| Model 3 | 6.64 (6.59, 6.69) | 6.65 (6.60, 6.69) | 6.65 (6.60, 6.70) | 6.64 (6.59, 6.69) | 0.96 |
| Flavones (median intake 1.8 mg) | (n = 522) | (n = 521) | (n = 521) | (n = 522) | |
| Model 1 | 6.66 (6.61, 6.71) | 6.64 (6.59, 6.69) | 6.67 (6.62, 6.71) | 6.62 (6.57, 6.66) | 0.27 |
| Model 2 | 6.66 (6.61, 6.71) | 6.64 (6.59, 6.69) | 6.66 (6.62, 6.71) | 6.61 (6.57, 6.66) | 0.29 |
| Model 3 | 6.67 (6.62, 6.72) | 6.64 (6.59, 6.69) | 6.66 (6.61, 6.71) | 6.61 (6.56, 6.66) | 0.21 |
| Flavanones (median intake 40.0 mg) | (n = 521) | (n = 522) | (n = 522) | (n = 521) | |
| Model 1 | 6.66 (6.61, 6.71) | 6.64 (6.59, 6.68) | 6.66 (6.61, 6.70) | 6.63 (6.59, 6.68) | 0.61 |
| Model 2 | 6.66 (6.61, 6.71) | 6.63 (6.59, 6.68) | 6.66 (6.61, 6.70) | 6.63 (6.58, 6.68) | 0.48 |
| Model 3 | 6.67 (6.62, 6.72) | 6.64 (6.59, 6.69) | 6.65 (6.60, 6.70) | 6.62 (6.57, 6.67) | 0.32 |
| Flavan-3-ols (median intake 27.0 mg) | (n = 521) | (n = 522) | (n = 522) | (n = 521) | |
| Model 1 | 6.65 (6.60, 6.70) | 6.67 (6.62, 6.71) | 6.61 (6.57, 6.66) | 6.66 (6.61, 6.70) | 0.95 |
| Model 2 | 6.64 (6.59, 6.69) | 6.67 (6.62, 6.71) | 6.62 (6.57, 6.66) | 6.65 (6.60, 6.70) | 0.97 |
| Model 3 | 6.65 (6.59, 6.70) | 6.66 (6.62, 6.71) | 6.63 (6.58, 6.67) | 6.65 (6.60, 6.70) | 0.90 |
| Anthocyanins (median intake 11.2 mg) | (n = 521) | (n = 522) | (n = 522) | (n = 521) | |
| Model 1 | 6.62 (6.58, 6.67) | 6.64 (6.60, 6.69) | 6.65 (6.60, 6.69) | 6.67 (6.62, 6.72) | 0.18 |
| Model 2 | 6.62 (6.57, 6.67) | 6.64 (6.60, 6.69) | 6.65 (6.60, 6.70) | 6.67 (6.62, 6.71) | 0.21 |
| Model 3 | 6.62 (6.57, 6.68) | 6.64 (6.60, 6.69) | 6.65 (6.60, 6.70) | 6.66 (6.61, 6.71) | 0.34 |
| Flavonoid polymers (median intake 134.6 mg) | (n = 521) | (n = 522) | (n = 522) | (n = 521) | |
| Model 1 | 6.63 (6.58, 6.68) | 6.63 (6.59, 6.68) | 6.65 (6.61, 6.70) | 6.67 (6.62, 6.71) | 0.28 |
| Model 2 | 6.63 (6.58, 6.68) | 6.63 (6.58, 6.68) | 6.66 (6.61, 6.71) | 6.66 (6.61, 6.71) | 0.29 |
| Model 3 | 6.64 (6.59, 6.69) | 6.63 (6.59, 6.68) | 6.66 (6.61, 6.71) | 6.65 (6.60, 6.70) | 0.58 |
Values are cross-sectional means (95% CIs) of hippocampal volumes across quartiles of total and 6 classes of flavonoid intake, unless otherwise indicated.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
The n values are numbers of participants per quartile category for model 1.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and total intracranial vault volume; of the total 2086 observations, model 1 included 2086 observations.
Adjusted for model 1 + BMI, physical activity index, smoking, and prevalent hypercholesterolemia, hypertension, and diabetes; of the total 2086 observations, model 2 included 2040 observations.
Adjusted for model 1 covariates + overall dietary quality, vitamin and mineral supplement use, and dietary intakes of alcohol, ω-3 fatty acids EPA and DHA, and lutein and zeaxanthin; of the total 2086 observations, model 3 included 2000 observations.
Discussion
There appear to be no studies to date that have explored the association between flavonoid intake and brain MRI measures that are associated with ADRD. Findings from the current study offer evidence that intakes of flavan-3-ols and flavonoid polymers are inversely associated with WMHV after taking into account important demographic, lifestyle, and clinical factors. Similar results were not seen with either TBV or HV, which in turn may suggest that flavonoids may possess unique white matter neuroprotective effects that are independent of general measures of brain atrophy.
The outcomes of interest considered in this study are of clinical importance and have strong associations with ADRD. White matter hyperintensities, also referred to as leukoaraiosis, are areas of myelin injury in the brain of older patients (4). Clinically, white matter hyperintensities are typically considered indices of vascular damage in the brain as well as the connection between vascular risk and AD (6). A plethora of evidence suggest that the presence of white matter hyperintensities is significantly associated with cognitive impairment and cortical atrophy in subjects with AD, and double the risk of ADRD (33–37). ADRD is characterized by neuronal injury and brain atrophy as well structural connectivity (3, 38, 39). As such, TBV is an important MRI measure associated with the severity of ADRD. Conversely, the hippocampus is somewhat more specific to AD pathology (40–42), often associated with the early memory impairment accompanying AD (43).
A number of studies from the current population and others on the relationship between flavonoid intake and risk of ADRD suggest that higher intake of flavonoids may offer protection against ADRD (15–17). In addition, several studies on the MedDiet, a dietary pattern that is rich in flavonoids, and ADRD risk have also shown that individuals with higher adherence to the MedDiet have a reduced risk of ADRD (8). More recent research on the relationship between the MedDiet and brain MRI measures has similarly revealed promising findings. Three cross-sectional studies conducted in the United States investigated the relation between adherence to the MedDiet and different structural brain MRI measures, all of which produced promising findings (11, 12, 44). The first study was conducted on 707 elderly participants (aged ≥65 y) of the Washington Heights/Inwood Columbia Aging Project. It examined the association of increasing adherence to the MedDiet, based on 3 groups (low, middle, and high tertiles), with presence of infarcts on MRI. The results of this study, after controlling for basic demographic and clinical risk factors, showed that compared to individuals in the low-adherence group, individuals in the moderate- and high-adherence groups had significantly lower odds of having an infarct. In addition, they did not find an association between the MedDiet and white matter hyperintensities (44). The other 2 studies were conducted on individuals who participated in the brain MRI imaging studies at New York University (11, 12). In the first study, which included 52 cognitively normal individuals (aged 25–72 y), Mosconi et al. demonstrated that individuals with higher adherence to the MedDiet showed greater thickness of AD-vulnerable regions of interest relative to those with lower adherence (11). In the second study, data from 116 young to middle-aged individuals (age 30–60 y) showed that adherence to the MedDiet and insulin sensitivity were both positively associated with MRI-based cortical thickness, further supporting the notion that diet is potentially an important lifestyle factor that could guard against brain aging and AD (12). A longitudinal study on the aforementioned population assessed the effects of higher compared with lower adherence to the MedDiet on the 3-y change in brain MRI structure of 70 cognitively normal subjects (aged 30–60 y) (45). Unlike the positive findings seen with the cross-sectional studies, this study did not observe any significant changes between the 2 groups in terms of change in their brain MRI structure over the 3-y period. An additional 3 cross-sectional studies conducted in the United States on different populations examined the association between adherence to the MedDiet and brain MRI structures, all of which have also revealed positive findings (10, 14, 46). Briefly, Gu et al. examined the association between the MedDiet and structural MRI-assessed brain volume and cortical thickness among 674 ADRD-free elderly participants (mean age 80.1 y) of a community-based multiethnic cohort, the Washington Heights/Hamilton Heights Inwood Columbia Aging Project (46). Results from their study indicated that relative to lower adherence to the MedDiet, higher adherence was associated with larger volumes of the total brain, as well as the white and gray matters (46). Staubo et al. explored associations of the MedDiet score and its components with MRI measures of cortical thickness for the mean lobar and 4 lobes separately in 672 cognitively normal older subjects (mean age 79.9 y) of the Mayo Clinic Study on Aging (10). Their findings showed that higher MedDiet score was associated with larger frontal, parietal, occipital, and mean lobar cortical thickness, with higher legume and fish intakes being the key dietary components associated with larger cortical thickness. Lastly, Gardener et al. examined the association between the MedDiet score and brain MRI WMHV on 966 cognitively normal subjects (mean age 72 y) of the Northern Manhattan Study (14). Higher scores on the MedDiet were found to be associated with lower WMHV. Similar promising findings were also seen in a cross-sectional study conducted in France, which explored whether higher adherence to the MedDiet was associated with higher white and gray matter volumes assessed 9 y after the dietary evaluation on a sample of 146 older (mean age 73.0 y at time of dietary assessment) participants of the Bordeaux Three-City study (47). Their results demonstrated significantly higher white matter volumes in individuals with a higher adherence to the MedDiet, while this was not seen for gray matter volume. On the other hand, findings from a Swedish study examining the association between dietary habits and brain volumes in 194 cognitively healthy older individuals (mean age 70 y) who participated in the Prospective Investigation of the Vasculature in Uppsala Seniors cohort showed no associations between the MedDiet score and volumes of gray matter, white matter, or their sum (TBV) (48). Finally, a second longitudinal study conducted in Scotland on 562 older age participants (age 73–76 y) of the Lothian Birth Cohort examined the association between the MedDiet and change in brain MRI volumetric measures (TBV and gray matter volume) and mean cortical thicknesses over a 3-y time period (13). They demonstrated that greater adherence to the MedDiet was associated with lower total brain atrophy over time, but not with lower atrophy of gray matter volume or cortical thickness.
The present study has a number of strengths. Unlike the abovementioned studies, which only assessed diet at 1 point in time, we used 3 repeated assessments of diet over a time period of ∼7 y to measure the cumulative mean of our subjects’ dietary intakes, thereby reducing measurement error and providing a more accurate estimate of usual flavonoid intake in relation to the brain MRI measures of interest. Also, using the cumulative mean of flavonoid intakes minimizes the potential for flavonoid intakes to be impacted by changes in diet and behavior resulting from possible cognitive impairment associated with brain atrophy, a potential bias present in cross-sectional studies of diet and brain health. We made additional efforts to limit the impact of cognitive decline on dietary assessment of subjects with cognitive impairment and ADRD by stopping the update of their dietary data based on the noted time cutoffs. Other strengths of our study include the large sample size and the use of a well-characterized and established cohort.
In addition to the above noted strengths, our study also had some limitations. While most of the studies discussed above included older adults, our study included relatively younger subjects (mean age 60.6 y) who are less likely to show clear signs of brain atrophy. However, despite the relatively young age of the subjects, we were still able to observe significant inverse associations with WMHV, which is an MRI measure of clinical importance given its strong relation to ADRD (34). Furthermore, due to the many comparisons tested, the associations seen between flavan-3-ols, flavonoid polymers, and total flavonoids with WMHV may be a result of chance. Other limitations of the current work include the cross-sectional design, which prevents the establishment of a temporal relationship; the use of more global measures of brain structure rather than more specific measures of regions of interest, which might account for the lack of findings on measures of TBV and HV; the lack of validity of data regarding estimating flavonoid intake from the FFQ used in this study; and the inability to generalize our results to populations with different races and ethnic backgrounds, as essentially all subjects of the Framingham Offspring Cohort are white and of European origin.
In conclusion, our results add value to the literature that flavonoids may reduce the risk of ADRD by eliciting protective effects on brain health through decreasing the development of white matter hyperintensities, a strong predictor of neuropsychological impairment and ADRD. In light of the ever-growing prevalence of ADRD, flavonoids represent an alternative dietary strategy that holds significant promise in combating age-related cognitive decline. Longitudinal studies assessing change in these brain MRI measures with relatively long follow-up periods in older populations are warranted to confirm these findings.
Acknowledgments
We acknowledge Ting Fang Alvin Ang from the Brain Health Research Lab/Neuropsychology Group at the Framingham Heart Study for his help with MRI data clarification and interpretation.
The authors’ responsibilities were as follows—ES, PFJ, RA, and JBB: designed research; ES: conducted research, analyzed data and wrote the paper; GTR: assisted in data programming and guided the statistical analyses; CDC: provided essential MRI data; PFJ: provided guidance on data interpretation and analysis and had primary responsibility for final content; all authors: contributed to the interpretation of the study findings and editing of the final manuscript; and all authors: read and approved the final manuscript.
Notes
Supported in part by the US Department of Agriculture–Agricultural Research Service (58-1950-4-003), the National Heart Lung and Blood Institute (HHSN2682015000011), the National Institute on Aging (AG008122, AG016495, and AG062109), the National Institute of Neurological Disorders and Stroke (NS017950), and the Embassy of the State of Kuwait (ES). The views expressed in this article are of those of the authors and do not necessarily represent the views of the funding organizations.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AD, Alzheimer's disease; ADRD, Alzheimer's disease and related dementias; DGAI, dietary guidelines adherence index; HV, hippocampal volume; MedDiet, the Mediterranean dietary pattern; MRF, Markov Random Field; PAI, physical activity index; TBV, total brain tissue volume; TEI, total energy intake; WMHV, white matter hyperintensities volume.
References
- 1. Global challenge. World Dementia Council [Internet]. [Cited 2019 Sep 19]. Available from: https://worlddementiacouncil.org/global-challenge. [Google Scholar]
- 2. Dementia [Internet]. [Cited 2019 Sep 19]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. [Google Scholar]
- 3. What happens to the brain in Alzheimer's disease? [Internet]. National Institute on Aging. [Cited 2019 Sep 19]. Available from: https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease. [Google Scholar]
- 4. Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;; 4::001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kandel BM, Avants BB, Gee JC, McMillan CT, Erus G, Doshi J, Davatzikos C, Wolk DA. White matter hyperintensities are more highly associated with preclinical Alzheimer's disease than imaging and cognitive markers of neurodegeneration. Alzheimers Dement (Amst). 2016;4:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alber J, Alladi S, Bae H-J, Barton DA, Beckett LA, Bell JM, Berman SE, Biessels GJ, Black SE, Bos I et al.. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement (N Y). 2019;5:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Global challenge. Global Alzheimer's & Dementia Action Alliance (GADAA) [Internet]. [Cited 2020 Feb 6]. Available from: https://www.gadaalliance.org/global-challenge/. [Google Scholar]
- 8. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence123. Adv Nutr. 2016;7:889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479. [DOI] [PubMed] [Google Scholar]
- 10. Staubo SC, Aakre JA, Vemuri P, Syrjanen JA, Mielke MM, Geda YE, Kremers WK, Machulda MM, Knopman DS, Petersen RC et al.. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. 2017;13:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mosconi L, Murray J, Tsui WH, Li Y, Davies M, Williams S, Pirraglia E, Spector N, Osorio RS, Glodzik L et al.. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for alzheimer's disease. J Prev Alzheimers Dis. 2014;1:23–32. [PMC free article] [PubMed] [Google Scholar]
- 12. Mosconi L, Walters M, Sterling J, Quinn C, McHugh P, Andrews RE, Matthews DC, Ganzer C, Osorio RS, Isaacson RS et al.. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer's disease: a cross-sectional study of middle-aged adults from the broader New York City area. BMJ Open. 2018;8:e019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luciano M, Corley J, Cox SR, Valdés Hernández MC, Craig LCA, Dickie DA, Karama S, McNeill GM, Bastin ME, Wardlaw JM et al.. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017;88:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardener H, Scarmeas N, Gu Y, Boden-Albala B, Elkind MSV, Sacco RL, DeCarli C, Wright CB. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan study. Arch Neurol. 2012;69:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–63. [DOI] [PubMed] [Google Scholar]
- 16. Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–9. [DOI] [PubMed] [Google Scholar]
- 17. Shishtar E. Dietary flavonoid intake and cognitive health. Boston (MA): Tufts University; 2019. [Google Scholar]
- 18. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 19. Framingham Heart Study (FHS). National Heart, Lung, and Blood Institute (NHLBI) [Internet]. [Cited 2020 Jan 28]. Available from: https://www.nhlbi.nih.gov/science/framingham-heart-study-fhs. [Google Scholar]
- 20. Tsao CW, Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26.; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 22. Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. USDA Special Interest Databases on Flavonoids: USDA ARS [Internet]. [Cited 2019 May 18]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-special-interest-databases-on-flavonoids/. [Google Scholar]
- 24. Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–54. [DOI] [PubMed] [Google Scholar]
- 25. de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1-4). J Nutr. 2001;131:1826–32. [DOI] [PubMed] [Google Scholar]
- 26. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 27. DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 28. Kannel WB, Sorlie P.. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–61. [PubMed] [Google Scholar]
- 29. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–26. [DOI] [PubMed] [Google Scholar]
- 30. Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, Pilotto A. Diet and Alzheimer's disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11:677–708. [DOI] [PubMed] [Google Scholar]
- 31. Yaffe K. Modifiable risk factors and prevention of dementia: what is the latest evidence?. JAMA Intern Med. 2018;178:281–2. [DOI] [PubMed] [Google Scholar]
- 32. Sauder KA, Proctor DN, Chow M, Troy LM, Wang N, Vita JA, Vasan RS, Mitchell GF, Jacques PF, Hamburg NM et al.. Endothelial function, arterial stiffness and adherence to the 2010 Dietary Guidelines for Americans: a cross-sectional analysis. Br J Nutr. 2015;113:1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–65. [DOI] [PubMed] [Google Scholar]
- 34. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kloppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E. Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology. 2014;82:2127–38. [DOI] [PubMed] [Google Scholar]
- 36. Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Capizzano AA, Ación L, Bekinschtein T, Furman M, Gomila H, Martínez A, Mizrahi R, Starkstein SE. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mapping brain shrinkage in dementia—Atlas of Science [Internet]. [Cited 2019 Sep 19]. Available from: https://atlasofscience.org/mapping-brain-shrinkage-in-dementia/. [Google Scholar]
- 39. Brain changes speak volumes about normal aging and dementia | ALZFORUM [Internet]. [Cited 2019 Sep 19]. Available from: https://www.alzforum.org/news/research-news/brain-changes-speak-volumes-about-normal-aging-and-dementia. [Google Scholar]
- 40. Size of hippocampus may indicate early dementia [Internet]. ScienceDaily. [Cited 2019 Sep 19]. Available from: https://www.sciencedaily.com/releases/2010/11/101116072756.htm. [Google Scholar]
- 41. Shrinking hippocampus signals early Alzheimer's [Internet]. Fisher Center for Alzheimer's Research Foundation; 2009; [cited 2019 Sep 19]. Available from: https://www.alzinfo.org/articles/shrinking-hippocampus-signals-early-alzheimers/. [Google Scholar]
- 42. Vijayakumar A, Vijayakumar A. Comparison of hippocampal volume in dementia subtypes. ISRN Radiol 2013 [Internet]. [cited 2019 Sep 19]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4045526/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. What Are the Signs of Alzheimer's Disease? [Internet]. National Institute on Aging; [cited 2019 Sep 19]. Available from: https://www.nia.nih.gov/health/what-are-signs-alzheimers-disease [Google Scholar]
- 44. Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, Brown T, Brickman AM. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berti V, Walters M, Sterling J, Quinn CG, Logue M, Andrews R, Matthews DC, Osorio RS, Pupi A, Vallabhajosula S et al.. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90:e1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, Scarmeas N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015;85:1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pelletier A, Barul C, Féart C, Helmer C, Bernard C, Periot O, Dilharreguy B, Dartigues J-F, Allard M, Barberger-Gateau P et al.. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement. 2015;11:1023–31. [DOI] [PubMed] [Google Scholar]
- 48. Titova OE, Ax E, Brooks SJ, Sjögren P, Cederholm T, Kilander L, Kullberg J, Larsson E-M, Johansson L, Åhlström H et al.. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol. 2013;48:1443–8. [DOI] [PubMed] [Google Scholar]


