Abstract
Mitochondria are crucial cellular organelles. Under extracellular stimulations, mitochondria undergo constant fusion and fission dynamics to meet different cellular demands. Mitochondrial dynamics is regulated by specialized proteins and lipids. Dysregulated mitochondrial dynamics has been linked to the initiation and progression of diverse human cancers, affecting aspects such as cancer metastasis, drug resistance and cancer stem cell survival, suggesting that targeting mitochondrial dynamics is a potential therapeutic strategy. In the present review, we summarize the molecular mechanisms underlying fusion and fission dynamics and discuss the effects of mitochondrial dynamics on the development of human cancers.
Keywords: Mitochondrial dynamics, human cancers, lipids, cancer stem cells
Introduction
Mitochondria are semiautonomous intracellular organelles vital to cellular physiological activity. As the main site of oxidative respiration to produce ATP, mitochondria are not only the cellular ‘powerhouse’ but also the site where many crucial metabolic processes take place [1]. Because of their functional diversity, mitochondria play important roles in cell proliferation, apoptosis, calcium ion storage and reactive oxygen species (ROS) generation [2,3]. Furthermore, mitochondria play an important role in immunity and inflammation [4].
In most cells, mitochondria are highly dynamic and, through fusion and fission, undergo constant changes in number and morphology in response to metabolic and extracellular insults [5]. These fission and fusion events determine the shape of mitochondria and further influence their function.
Mitochondrial dynamics contributes to the genesis and progression of various kinds of human cancers [6]. Elucidating the role of mitochondrial dynamics in human cancers is of great importance, as this understanding will offer new insights into related treatments.
After presenting a detailed description of protein mediators and lipids that have been acknowledged to regulate mitochondrial fusion and fission, this review focuses on summarizing fundamental cellular functions impacted by unbalanced fusion and fission. In addition, an overview of cancers involving dysregulated mitochondrial dynamics is presented in the third section.
Regulation of mitochondrial fusion and fission
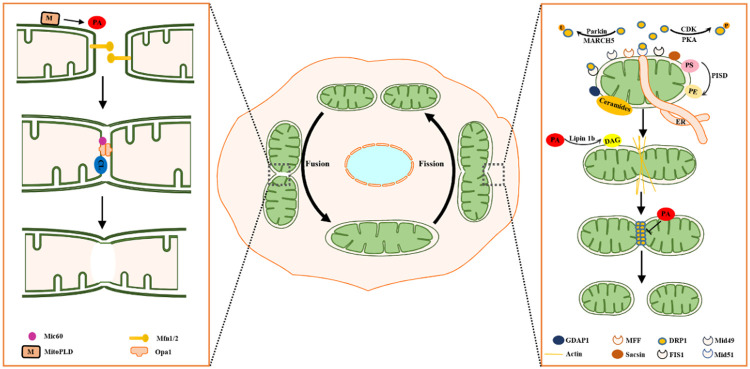
Mitochondrial dynamics is exquisitely regulated by proteins and lipids (Figure 1).
Figure 1.
A schematic diagram of mitochondrial dynamics. Human mitochondria undergo constant fusion and fission dynamics. The fusion of the OMM is mediated by Mfn1/2. PA generated by MitoPLD promotes fusion of the OMM. CL and Opa1 coordinate IMM fusion. Mic60 also interacts with Opa1. ER tubules mark sites of fission. During mitochondrial fission, Drp1 is recruited to mitochondria by receptors such as Fis1, Mid49, Mid51 and Mff. GDAP1 and sacsin are two additional proteins localized in the OMM that facilitate fission. Drp1 activity is regulated by posttranslational modifications such as phosphorylation and ubiquitination. The preconstriction process is completed by actin filaments, and Drp1 performs mitochondrial scission. Lipids, including ceramides, PA and DAG, participate in mitochondrial fission. PA, phosphatidic acid; OMM, outer mitochondrial membrane; CL, cardiolipin; Drp1, dynamin-related protein 1; DAG, diacylglycerol; ER, endoplasmic reticulum.
Proteins involved in mitochondrial fusion
In mammalian cells, the fusion machinery includes three essential GTPases, mitofusin (Mfn) 1 and 2 on the outer mitochondrial membrane (OMM) and optic atrophy protein 1 (Opa1) on the inner mitochondrial membrane (IMM) [7,8].
Mfn1 and Mfn2 coordinate OMM fusion. The c-terminal heptad repeats of Mfn1 and Mfn2 have been shown to form an intermolecular antiparallel coiled coil via which adjacent mitochondria may be drawn together and initiate mixing of their lipid bilayers, leading to fusion of the OMM [9].
Opa1 drives IMM fusion [10]. Opa1 is localized to the mitochondrial intermembrane space and the IMM. OPa1 has 8 isoforms, and the steady-state morphology of mitochondria depends on the balance of the long and short Opa1 isoforms [11]. Opa1 is likely to interact with Mfns to form intermembrane protein complexes that couple OMM fusion to IMM fusion [12].
The mitochondrial structural protein Mic60, also called mitofilin, appears to be a key player in regulating mitochondrial shape [13]. Increased levels of Mic60 suppress mitochondrial fission in neurites, producing elongated neuritic mitochondria [14]. Mic60 also interacts with Opa1 [15]. MitoPLD belongs to the phospholipase D superfamily of signaling enzymes that generate phosphatidic acid (PA). MitoPLD is anchored to the mitochondrial surface [16], and MitoPLD-generated PA facilitates mitochondrial fusion [17].
Mitochondrial fission proteins
Endoplasmic reticulum (ER) tubules contact mitochondria and mark the sites of mitochondrial division [18]. Several outer membrane proteins, including Mff, Fis1, Mid49 and Mid51, have been identified as dynamin-related protein 1 (Drp1) receptors [19-21]. Drp1 is recruited to the mitochondrial surface via its transmembrane receptors and assembles into oligomeric complexes. Before the scission of mitochondria by Drp1, preconstriction is completed by actin and nonmuscle myosin II. A study reported that myosin II induced stochastic deformations of the interstitial actin network and exerted pressure on the mitochondrial surface, promoting mitochondrial fission [22].
The activity of Drp1 is regulated by posttranslational modifications. Several different posttranslational modifications of Drp1, including phosphorylation, ubiquitination and sumoylation, regulate its activity, thus influencing the fission process [23-26].
Moreover, human Fis1 was reported to regulate mitochondrial fission in the absence of Drp1. Fis1 binds to Mfn1, Mfn2 and Opa1, thus inhibiting their GTPase activity and the fusion machinery [27].
GDAP1 is another protein involved in mitochondrial fission events. This protein is localized in the OMM [28]. In animal models, neurons from GDAP1-knockout mice show large and defective mitochondria [29].
Sacsin localizes to the OMM in a variety of cell lines [30]. Loss of sacsin induces the generation of hyperfused mitochondria. The results of a coimmunoprecipitation assay showed that sacsin interacts directly with Drp1 [31].
Syntaphilin (SNPH) has been identified to inhibit mitochondrial trafficking in neurons [32]. SNPH can be ubiquitinated at Lys111 and Lys153 in the microtubule-binding domain by the E3 ligase CHIP. SNPH ubiquitination results in anchoring of SNPH to tubulin, which inhibits the movement of mitochondria. Perturbation of SNPH ubiquitination causes recruitment of Drp1 to mitochondria [33].
Lipids involved in regulating mitochondrial dynamics
Apart from proteins, accumulating evidence implies the striking role of lipids in the governance of mitochondrial dynamics.
Phospholipids are the primary lipid components of the mitochondrial membrane. Cardiolipin (CL) is a mitochondria-specific phospholipid [34]. It is synthesized from PA in the IMM [35]. CL is related to the assembly of Opa1. Liposomes containing CL bind to purified Opa1 and Mgm1, stimulating their assembly into liposomes [36]. In addition, CL has been shown to stimulate the oligomerization of Drp1, which induces its tubulation and sequential mitochondrial fission [37,38].
PA is produced mainly in the ER and is then transported to the OMM through an ER-mitochondria contact site [39]. PA promotes mitochondrial fusion possibly by creating a negative curvature in the opposing OMM [40]. PA also regulates mitochondrial fission by interacting with Drp1. After its oligomerization on mitochondria, Drp1 is blocked by PA and the saturated acyl chains of phospholipids, which makes mitochondria resistant to the division induced by mitochondrial stress [41,42].
Phosphatidylethanolamine (PE) and phosphatidylserine (PS) are two other mitochondrial membrane components. PS is well known for its role in apoptosis. PE is produced from PS by phosphatidylserine decarboxylase (PISD) [43]. A novel tumor suppressor, LACTB, reduces the levels of mitochondrial PISD possibly through its proteolytic activity, thus altering the generation of PE. A reduction in the PE/PS ratio shifts mitochondrial dynamics toward fission and exerts an anticancer effect [44].
Diacylglycerol (DAG) triggers mitochondrial fission [45]. DAG may drive actin filament polymerization by activating RhoA at the sites of ER constriction, which could shrink mitochondria to an appropriate diameter for Drp1 to encircle and cleave [46,47].
Ceramides are synthesized in both the OMM and IMM [48]. Ceramides stimulate mitochondrial fission in cardiomyocytes [49]. Human choriocarcinoma cells treated with ceramides 16:0 exhibited mitochondrial fragmentation through increases in the p-Drp1/Drp1 ratio and Mfn2 expression [50].
Biological functions of mitochondrial dynamics
The continuous fusion and division processes of mitochondria are crucial for essential physiological functions of cells (Figure 2). The main cellular functions involved in the link between mitochondrial dynamics and cancer development are summarized herein.
Figure 2.
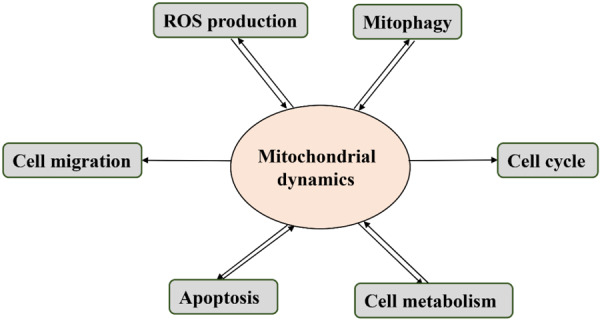
Cellular physiological activities affected by mitochondrial dynamics. Mitochondrial dynamics is tightly linked to the cell cycle, apoptosis, cell migration, mitophagy, apoptosis and ROS production.
Mitochondrial dynamics and ROS production
ROS, short-lived molecules consisting of unpaired electrons, are considered byproducts of cellular metabolism. The mitochondrial electron transport chain is a major contributor to ROS production in cancer cells [51]. Accumulating evidence demonstrates that mitochondrial morphology and ROS levels are closely related. For example, parallel changes in mitochondrial morphology and ROS levels were found in patient primary fibroblasts [52]. Besides, genetic ablation of Mfn1 or Mfn2 led to elevated ROS levels, and mitochondrial fission promoted ROS production [53,54]. In turn, experimental results revealed that overproduced mitochondrial ROS altered posttranslational modifications of Drp1 and affected other mediators, such as Opa1 and Mfns, causing mitochondrial dysfunction [55-57].
ROS homeostasis is required for cell survival. Due to metabolic activity, gene mutation and hypoxia, cancer cells yield high levels of ROS. Depending on enhanced antioxidant activity, cancer cells managed to maintain moderate levels of ROS, which facilitate tumor initiation and progression. ROS participate in various cell signaling pathways, such as the Ras/MAPK/ERK, PI3K/Akt and NF-κB pathways [58]. These pathways have been linked to cellular transformation, cancer cell proliferation, apoptosis resistance, cancer stem cells (CSCs) maintenance and cancer metastasis [59].
In general, aberrant mitochondrial dynamics might enhance ROS production, and high concentrations of ROS can modify fusion and fission effectors, forming a feedback loop.
Cell migration
Metastasis accounts for most cases of cancer progression, resulting in failure of clinal therapy and death of the patients. Cancer cells enter blood or lymphatic vessels through intravasation. After extravasation from these vessels, they form cloning lesions in distant organs [60]. Cell migration is a crucial step in metastasis. Accumulating data suggest the role of mitochondria in the dissemination of cancer cells. For example, mitochondrial dynamics regulates the migration and invasion of breast cancer cells; researchers found higher expression of Drp1 and lower expression of Mfn1 in metastatic breast cancer cells than in their nonmetastatic counterparts [61]. Recently, additional evidence indicating the regulatory role of mitochondrial dynamics in cancer metastasis has emerged [33,62-64].
Mitochondrial fusion and fission influence mitochondrial transportation in lymphocytes as well as in cancer cells. Mitochondria are redistributed during lymphocyte migration. Experimental results show that mitochondria accumulate at the uropod of polarized lymphocytes in a manner dependent on unperturbed mitochondrial fission. Moreover, dysregulation of mitochondrial fusion/fission suppresses lymphocyte polarization and migration [65].
Researchers have investigated the connection between mitochondrial dynamics and T cell metabolism. In contrast to memory T cells, activated effector T cells maintain a fused mitochondrial network. Memory T cells require mitochondrial fusion for development and survival, and forced fusion promotes the generation of memory-like T cells regardless of the presence of activating signals [66]. In animal models, T cell mitochondrial fusion was shown to enhance antitumor immune responses [67].
Mitophagy
mtDNA is frequently exposed to ROS and liable to mutate and damage. Accumulation of mtDNA mutations gradually leads to functional impairment of respiratory chain complexes and finally reduces the bioenergetic capacity. Damaged mitochondria are degraded through a specialized form of macroautophagy called mitophagy [68]. The molecular pathway of mitophagy is divided into two pathways according to differential dependency on Parkin: the phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1)-Parkin pathway and the Parkin-independent pathway.
Mitochondrial dynamics has been found to be intertwined with mitophagy. Mitochondrial fission plays an important role, as mitophagy is preceded by mitochondrial division, after which fragmented mitochondria are encapsulated by autophagosomes [69]. Accordingly, overexpression of Fis1 triggers mitophagy, and depletion of Fis1 or overexpression of the dominant-negative Drp1 K38A mutant attenuates mitophagy [70].
Researchers found that the mammalian mitophagy receptor FUNDC1 interacts with and recruits LC3 to mitochondria for mitophagy. FUNDC1 interacts with both DNM1L/Drp1 and Opa1 to coordinate mitochondrial dynamics and mitophagy [71]. Another study revealed that PINK1 impairs the anti-fission machinery to ensure segregation of damaged mitochondria. PINK1 indirectly interacts with Drp1 and enhances Drp1 activity [72]. In addition, the SNARE protein Syntaxin 17 (STX17) was recently identified to interact with Fis1. Fis1 loss triggers abnormal STX17 accumulation on mitochondria, which promotes self-oligomerization of STX17 and mitophagy [73].
Mitochondrial dynamics and cell metabolism
A major characteristic of cancer cells is their reduction in mitochondrial respiration and their predilection to use glycolysis to obtain energy even under aerobic conditions [74]. However, many types of cancer cells obtain energy via oxidative phosphorylation [75]. Metabolic reprogramming of cancer cells has been identified to occur during tumorigenesis and highlights the role of mitochondria during oncogenesis. Cellular metabolic changes impact mitochondrial dynamics, and mitochondrial dynamics in turn alter the state of cell metabolism.
Mitochondrial dynamics has been linked to the balance between energy demand and nutrient supply. Fusion is positively associated with increased ATP production, while inhibition of fusion results in oxidative phosphorylation impairment, mtDNA depletion, and ROS production [76]. In skeletal muscle, the liver and the pancreas, manipulation of key fusion and fission proteins causes metabolic variations [77]. Similarly, in cancer cells, mitochondrial dynamics plays a pivotal role in metabolic reprogramming. In prostate cancer cells, Drp1 upregulation is required for metabolic reprogramming because it controls the mitochondrial pyruvate transport complex [78]. Furthermore, Mfn1 regulates metastasis in hepatocellular carcinoma (HCC) by shifting cell metabolism from glycolysis to oxidative phosphorylation [79]. Similar experimental results have also been reported in other human cancers [64,80-82].
On the other hand, metabolic changes influence the amount and activity of mitochondria-shaping mediators. For example, serine deprivation affects mitochondrial function and inhibits colorectal cancer cell proliferation through ceramide metabolism. Supplementation of C 16:0-ceramide was shown to partially restore mitochondrial fragmentation [83]. Another study reported that glucose starvation causes KAP1 phosphorylation on Ser473, which limits mitochondrial hyperfusion through a reduction in the Mfn2 level and favors breast cancer cell survival [84]. Similar types of interplay has been studied in several kinds of immune cells [85].
Apoptosis
Mitochondria play a critical role in cell apoptosis. An important step during apoptosis is mitochondrial outer membrane permeabilization (MOMP), which releases cytochrome c and other proapoptotic factors from the intermembrane space into the cytosol [86]. MOMP is mediated by translocation of cytosolic BAX and BAK to mitochondria, where a pore is formed in the OMM, allowing the release of proapoptotic proteins.
Multiple lines of evidence show that Drp1 is crucial in cell apoptosis. Drp1 activity is required for cytochrome c release and subsequent apoptotic events. For instance, SUMOylation of Drp1 stabilizes ER/mitochondrial contact sites that are important for remodeling of cristae and release of cytochrome c [87]. Mitochondrial fission appears to be an upstream event of apoptosis [88]. However, increased mitochondrial fission does not necessarily correlate with apoptosis activation [89]. Mfn1 and Mfn2 also control cell apoptosis by interacting with Bak or by triggering an influx of Ca2+ into mitochondria [90,91].
Opa1 protects cells from apoptosis by preventing cytochrome c release. Opa1 sustains the tightness of cristae junctions, a feature that likely regulates the mobilization of cytochrome c [92]. Similarly, another report found that Opa1 blunts cytochrome c release in hepatocellular cells [93].
In addition, Bax and Bak have been reported to regulate mitochondrial dynamics in healthy cells. Bax can induce mitochondrial fusion by promoting the assembly of Mfn2 [94].
Mitochondrial dynamics in the cell cycle
Mitochondrial dynamics is critical during cell cycle regulation. In G1 and G2 phases, mitochondria form an interconnected network [95,96]. However, mitochondria become fragmented during S phase and mitosis [97]. Mediators of mitochondrial dynamics, including Drp1, Fis1, Opa1 and mfn proteins, are linked to cell cycle phase transition [98-100]. For instance, inhibition of Drp1 causes cell cycle arrest in G1 phase by affecting cyclin E accumulation [101], and Mfn2 overexpression in VSMCs causes G0/G1 phase arrest [102].
The serine/threonine kinase Aurora A (AURKA) is overexpressed in several cancers [103,104]. AURKA was reported to promote mitochondrial fission through phosphorylation of RALA in the cytosol [105]. In 2018, a study showed that at endogenous levels, AURKA induces mitochondrial fragmentation but enhances mitochondrial fusion when overexpressed [106].
Imbalanced mitochondrial dynamics in human cancers
Dysregulation of mitochondrial dynamics has been frequently reported to drive malignant phenotypes of cancer (Figure 3). Various evidence implicating mitochondrial dynamics in the onset and progression of cancer has emerged (Table 1). We summarize the publications from the past five years below.
Figure 3.
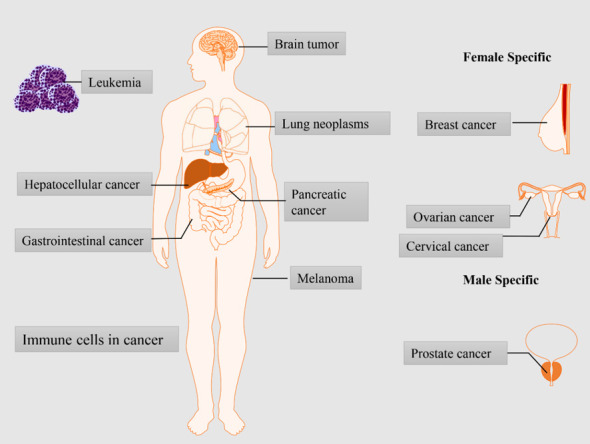
Human cancers connected with dysregulation of mitochondrial dynamics.
Table 1.
A list of recent research results involving mitochondrial dynamics and cancer
| Cancer type | Mitochondrial dynamics | Malignant property | Reference |
|---|---|---|---|
| HCC | Dynamin-1-like protein upregulation and Mfn1 downregulation | Enhancement of cell migration | [62] |
| HCC | Mfn1 downregulation | Depletion of Mfn1 modulated cancer metastasis via a metabolic shift | [79] |
| HCC | Increased DNM1L/MFN1 expression ratio | Cell survival | [108] |
| HCC | Drp1 upregulation | Infiltration of tumor-associated macrophages | [107] |
| HCC | Starvation-induced Drp1 Ser637 phosphorylation and suppression of its mitochondrial translocation | Metabolic reprogramming | [80] |
| HCC | Excessive fusion | Metabolic alteration | [81] |
| Promotion of cell growth | |||
| Ovarian cancer | Drp1 phosphorylation on Ser616 promoted by SIK2 | Support of cell growth and metastasis | [64] |
| Epithelial ovarian cancer | Correlation of Drp1 with cell cycle genes | Mitotic transition and | [110] |
| chemosensitivity | |||
| Ovarian cancer | Hypoxia-induced increase in mitochondrial fission | Cisplatin resistance | [111] |
| Breast cancer | Enhanced fusion driven by myc | YAP/TAZ suppression | [112] |
| Breast cancer | Mfn2 downregulation | Cancer cell survival under metabolic stress | [84] |
| Breast cancer | Drp1 upregulation | Cancer cell survival | [113] |
| Mfn1 downregulation | |||
| Breast cancer | DRP1 phosphorylation on Ser637 | Tamoxifen resistance | [114] |
| Breast cancer | Liensinine treatment induced fission via DNM1L activation | Cellular apoptosis | [115] |
| Pancreatic cancer | Increased Drp1 phosphorylation | Tumor growth | [23] |
| Pancreatic ductal adenocarcinoma | Acquisition of a myoferlin-induced branched mitochondrial structure | Mitochondrial fission inhibited cancer cell proliferation | [119] |
| Pancreatic ductal adenocarcinoma | Enhanced mitochondrial fission | Cell proliferation and invasion | [118] |
| NSCLC | Mfn2 upregulation | Decreased ROS production | [121] |
| NSCLC | Inhibition of Drp1 phosphorylation by sirt4 | Cell cycle arrest | [120] |
| Repressed invasion | |||
| NSCLC | PIM1-inhibition induced Drp1 upregulation | Chemosensitivity | [122] |
| Lung cancer | Enhanced Drp1 phosphorylation caused by MDM2 depletion | Suppressed cell migration and invasion | [123] |
| Glioblastoma | Drp1 upregulation in BITCs | Tumor growth | [124] |
| Glioma | Promotion of Drp1-dependent fission by NF-κB-inducing kinase | Cell invasion | [63] |
| Cervical cancer | Mfn2 activation | - | [125] |
| Melanoma | Drp1 upregulation | Tumor growth | [127] |
| T-ALL | Drp1 phosphorylation on Ser616 | Drug resistance | [130] |
| AML | Fission upregulation | LSC self-renewal | [131] |
| In LSCs | |||
| Prostate cancer | Mff repression resulting from BRD4 knockdown | CSC exhaustion | [133] |
| Prostate cancer | Increased fission | Tumorigenesis | [132] |
| Gastrointestinal stromal tumor | Inhibition of mitochondrial fission mediated by knockdown of Nestin | Cell proliferation and invasion | [134] |
| Colorectal cancer | Drp1 phosphorylation | Chemoresistance | [135] |
| Colorectal cancer | Suppression of Drp1 phosphorylation | Inhibition of carcinogenesis | [136] |
Hepatocellular cancer
Mitochondrial fission has been reported to promote cell migration, autophagy, tumor-associated macrophage infiltration and HCC progression [62,107]. The Drp1/Mfn1 expression ratio was found to be increased in HCC tissues and associated with poor prognosis. Enhanced mitochondrial fission mediated by elevated ROS production was found to promote the survival of HCC cells in vitro and in vivo [108]. Depletion of Mfn1 induced epithelial-to-mesenchymal transition of HCC cells [79]. However, another study showed that mitochondrial fusion also supported liver tumor cell growth [81]. Moreover, metabolic reprogramming via mitochondrial elongation was found to be essential for hepatocellular cancer cell survival and adaptation to energy stress [80].
Ovarian cancer
Ovarian cancer is the major cause of death among gynecologic cancers [109]. Previous studies found that ovarian cancer cells exhibit increased Drp1 expression. Drp1 was shown to be coexpressed with cell cycle genes and to support the proliferation of ovarian cancer cells [110]. Similarly, a study reported that under hypoxic conditions, mitochondrial fission caused cisplatin resistance in ovarian cancer cells [111]. In addition, salt-inducible kinase 2 was shown to enhance the Warburg effect in ovarian cancer cells through drp1-mediated fission [64].
Breast cancer
MYC was found to indirectly inhibit YAP/TAZ coactivators in breast cancer cells, thus suppressing cancer development. PLD6, an OMM-localized phospholipase, was identified as the mediator of MYC activity via enhancement of mitochondrial fusion [112]. In contrast, mitochondrial fission facilitates the survival, apoptosis and drug resistance of breast cancer cells [84,113,114]. The autophagy inhibitor liensinine was found to markedly increase apoptosis in breast cancer cells in combination with classical chemotherapeutic drugs by triggering DNM1L-mediated mitochondrial fission [115]. Moreover, novel Drp1 inhibitors, Drpitor1 and Drpitor1a, were identified to have antineoplastic potency in breast cancer cells [116].
Pancreatic cancer
The Ras oncogene is frequently mutated in pancreatic ductal adenocarcinomas, and its mutation is an early event in pancreatic tumorigenesis [117]. Expression of oncogenic Ras or MAPK pathway activation leads to increased mitochondrial fragmentation, but blocking mitochondrial fission through knockdown of Drp1 inhibits tumor growth via Erk2-mediated phosphorylation of Drp1 on Ser616 [23]. In addition, FAM49B was found to be a tumor suppressor in pancreatic ductal adenocarcinomas. FAM49B knockdown induced Drp1 phosphorylation and favored fission in cancer cells [118]. However, mitochondrial fission induced by knockdown of myoferlin was reported to inhibit cancer cell proliferation and ATP production [119].
Lung neoplasms
In non-small cell lung cancer (NSCLC), SIRT4 inhibited cancer progression by decreasing mitochondrial fission [120]. Downregulation of the oncoprotein AIM2 enhanced mitochondrial fusion [121]. Inhibition of PIM caused mitochondrial fragmentation and sensitized NSCLC cells to chemotherapy [122]. Another study showed that a fraction of endogenous MDM2 was actively imported into mitochondria and affected mitochondrial dynamics independent of p53 in lung neoplasms. MDM2 depletion resulted in enhanced phosphorylation of DRP1 on Ser637, leading to increased mitochondrial fission. Increased mitochondrial MDM2 levels strengthened the migratory and invasive properties of cancer cells [123].
Brain tumor
The identification of brain tumor initiating cells (BTICs) provided insights into human brain tumor pathogenesis. Drp1 showed activating phosphorylation in BTICs and inhibitory phosphorylation in bulk tumor cells. Suppression of Drp1 led to BTIC apoptosis and suppressed tumor growth. Drp1 activity regulated the downstream metabolic stress sensor AMP-activated protein kinase (AMPK). Furthermore, Drp1 activation was found to be related to poor prognosis in glioblastoma, implying that mitochondrial dynamics is a novel therapeutic target for brain tumors [124]. In glioma, NF-κB-inducing kinase was found to promote mitochondrial fission and cell invasion. Drp1 was essential for NF-κB-inducing kinase-dependent cell invasion [63].
Cervical cancer
MFN2 and Rab, as well as Ras Interactor 1 (RIN1), were identified as new Smad2 binding partners required for mitochondrial fusion in HeLa cells. Inactive cytoplasmic Smad2 rapidly promoted mitochondrial fusion by recruiting RIN1 into a complex with MFN2. These results implied functional connections between Smad proteins and mitochondrial dysfunction [125].
Melanoma
Nutrient-sensing mechanistic/mammalian target of rapamycin complex 1 (mTORC1), which is frequently activated in cancer, controls cell growth and metabolism [126]. mTOR stimulates translation of mitochondrial fission process 1 (MTFP1), which is coupled to pro-fission phosphorylation and mitochondrial recruitment of DRP1 in melanoma cells. Potent active site mTOR inhibitors induce mitochondrial hyperfusion due to diminished translation of MTFP1. Additionally, MTFP1 was identified as a critical effector of mTORC1 to govern cell fate decisions [127].
Immune cells in cancer
Mitochondria are important in innate immune responses to cellular damage, stress and infection [128]. Mitochondrial dynamics might affect Toll-like receptor agonist-mediated inflammatory responses and immune cell polarization. One study reported that Toll-like receptor-regulated switching to mitochondrial fission in tumor-associated macrophages via ablation of the OMM protein FAM73b resulted in T cell activation and enhancement of antitumor immunity. In addition, mitochondrial morphology was found to alter Parkin expression and the activity of its downstream CHIP-IRF1 axis, revealing new potential targets for cancer immunotherapy [67]. In another study, T cells with decreased surface expression of the NADase CD38 exhibited intrinsically higher NAD+ levels, increased oxidative phosphorylation, and shifted mitochondrial dynamics that greatly enhanced tumor control [129].
Leukemia
Mesenchymal stem cells (MSCs) protect T cell acute lymphoblastic leukemia (T-ALL) cells against chemotherapeutic agents. Mitochondrial fragmentation was observed in T-ALL cells cocultured with MSCs, and Drp1 phosphorylation on Ser616 was the underlying mechanism [130]. Leukemia stem cells (LSCs) are thought to be the driving factor of acute myeloid leukemia (AML) genesis and relapse after chemotherapy. Recently, depletion of Fis1 was demonstrated to attenuate mitophagy, resulting in cell cycle arrest and profound weakening of LSC self-renewal potential. Furthermore, inhibition of AMPK signaling rescued the biological effect of Fis1 loss [131].
Prostate cancer
Speckle-type POZ protein (SPOP) mutations contribute to prostate carcinogenesis. Experimental results demonstrated that expression of SPOP mutants augmented mitochondrial fission [132]. Furthermore, Drp1 expression promoted prostate cancer cell survival under metabolic stress conditions [78]. Recently, mitochondrial plasticity was found to be a new anticancer target in CSCs of human prostate cancer. BRD4 is one of extra-terminal domain BET proteins which bind to acetylated histones and transcription factors. Genetic knockdown of BRD4 blocked mitochondrial fission by repressing Mff and depleted CSCs. Ectopic expression of MFF rescued the exhaustion of CSCs [133].
Gastrointestinal cancers
Nestin was found to be upregulated in invasive gastrointestinal stromal tumor specimens. Knockdown of nestin inhibited the recruitment of Drp1 to mitochondria, thus changing mitochondrial dynamics [134]. In colorectal cancer, the release of high-mobility group box 1 protein promoted Drp1 phosphorylation, leading to chemoresistance [135]. Besides, Paris Saponin II exhibited antitumor capacity in colorectal cancer by modulating Drp1-mediated mitochondrial fission [136].
Conclusion
Great strides in the study of mitochondrial dynamics have been achieved in the past few years. Mitochondrial dynamics is particularly vital for the normal functions of mammalian cells. Indeed, as reviewed here, many kinds of human cancers are inextricably connected with dysregulated mitochondrial dynamics. In most cases, mitochondrial fission facilitates the proliferation, metastasis and drug resistance of cancer cells, causing cancer development. Inhibitors of mitochondrial fission effectors, such as Drpitor1 and Drpitor1a, have shown anticancer efficacy. However, in some cancers, mitochondrial fusion has been found to promote malignant phenotypes of cancer cells. Though current research achievements on the molecular mechanisms of mitochondrial dynamics have provided abundant therapeutic targets for cancer, means to achieve maximum benefit by manipulating mitochondrial dynamics in specific contexts need more investigation.
Furthermore, mitochondrial dynamics affects the proliferation of bulk cancer cells as well as the survival and stemness maintenance of CSCs, which are responsible for tumor recurrence and other malignant traits. Blocking mitochondrial fission weakens the self-renewal capacity of CSCs and leads to CSC exhaustion providing a new anticancer target. In addition, the function of immune cells such as T cells in the cancer microenvironment depends on tuning of mitochondrial fusion and fission, suggesting the feasibility of augmenting antitumor immunity by targeting mitochondrial dynamics.
Developing inhibitors against mitochondrial fusion and fission proteins is a promising new strategy to overcome resistance to chemotherapeutic drugs and cancer metastasis. However, few clinical trials of inhibitors targeting mitochondrial dynamics have been conducted to date, and much remains to be done before approaches targeting mitochondrial dynamics can be translated from the bench to the bedside.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81902804), a General Financial Grant from the China Postdoctoral Science Foundation (2017M621494) and The Science and Technology Commission of Shanghai (17DZ2260100, 19JC1410200).
Disclosure of conflict of interest
None.
Abbreviations
- MFN
Mitofusin
- OMM
Outer mitochondrial membrane
- OPA1
Optic atrophy protein 1
- IMM
Inner mitochondrial membrane
- PA
Phosphatidic acid
- DRP1
Dynamin-related protein 1
- ER
Endoplasmic reticulum
- SNPH
Syntaphilin
- SUMO
Small ubiquitin-like modifier
- CL
Cardiolipin
- PE
Phosphatidylethanolamine
- PS
Phosphatidylserine
- PISD
Phosphatidylserine decarboxylase
- DAG
Diacylglycerol
- ROS
Reactive oxygen species
- PINK1
Phosphatase and tensin homologue (PTEN)-induced putative kinase 1
- STX17
Syntaxin 17
- MTDNA
Mitochondrial DNA
- MICOS
Mitochondrial contact site and cristae organizing system
- HCC
Hepatocellular carcinoma
- MOMP
Mitochondrial outer membrane permeabilization
- AURKA
Serine/threonine kinase Aurora A
- MT-ND6
NADH-dehydrogenase 6
- NSCLC
Non-small cell lung cancer
- BTICs
Brain tumor initiating cells
- AMPK
AMP-activated protein kinase
- CDK
Cyclin-dependent kinase
- RIN1
Ras Interactor 1
- mTORC1
Mammalian target of rapamycin complex 1
- MTFP1
Mitochondrial fission process 1
- MSCs
Mesenchymal stem cells
- T-ALL
T cell acute lymphoblastic leukemia
- LSCs
Leukemia stem cells
- AML
Acute myeloid leukemia
- CSCs
Cancer stem cells
- SPOP
Speckle-type POZ protein
References
- 1.Schon EA. A toolkit for the cell’s powerhouse. Nat Biotechnol. 2008;26:294–296. doi: 10.1038/nbt0308-294. [DOI] [PubMed] [Google Scholar]
- 2.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal. 2012;16:1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brini M. Ca(2+) signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium. 2003;34:399–405. doi: 10.1016/s0143-4160(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 4.Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 5.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 2017;26:39–48. doi: 10.1016/j.cmet.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 8.Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19:630–641. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 10.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerbes RM, van der Klei IJ, Veenhuis M, Pfanner N, van der Laan M, Bohnert M. Mitofilin complexes: conserved organizers of mitochondrial membrane architecture. Biol Chem. 2012;393:1247–1261. doi: 10.1515/hsz-2012-0239. [DOI] [PubMed] [Google Scholar]
- 14.Van Laar VS, Berman SB, Hastings TG. Mic60/mitofilin overexpression alters mitochondrial dynamics and attenuates vulnerability of dopaminergic cells to dopamine and rotenone. Neurobiol Dis. 2016;91:247–261. doi: 10.1016/j.nbd.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S, Chinthapalli B. A proteomic screen with Drosophila Opa1-like identifies Hsc70-5/Mortalin as a regulator of mitochondrial morphology and cellular homeostasis. Int J Biochem Cell Biol. 2014;54:36–48. doi: 10.1016/j.biocel.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 17.Kameoka S, Adachi Y, Okamoto K, Iijima M, Sesaki H. Phosphatidic acid and cardiolipin coordinate mitochondrial dynamics. Trends Cell Biol. 2018;28:67–76. doi: 10.1016/j.tcb.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell. 2015;26:4466–4477. doi: 10.1091/mbc.E15-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Svitkina TM. Ultrastructure and dynamics of the actin-myosin II cytoskeleton during mitochondrial fission. Nat Cell Biol. 2019;21:603–613. doi: 10.1038/s41556-019-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, Counter CM, Kashatus DF. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, Inatome R, Yanagi S. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 27.Yu R, Jin SB, Lendahl U, Nister M, Zhao J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 2019;38 doi: 10.15252/embj.201899748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber N, Guimaraes S, Schrader M, Suter U, Niemann A. Charcot-Marie-Tooth disease-associated mutants of GDAP1 dissociate its roles in peroxisomal and mitochondrial fission. EMBO Rep. 2013;14:545–552. doi: 10.1038/embor.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barneo-Munoz M, Juarez P, Civera-Tregon A, Yndriago L, Pla-Martin D, Zenker J, Cuevas-Martin C, Estela A, Sanchez-Arago M, Forteza-Vila J, Cuezva JM, Chrast R, Palau F. Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of charcot-marie-tooth neuropathy. PLoS Genet. 2015;11:e1005115. doi: 10.1371/journal.pgen.1005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parfitt DA, Michael GJ, Vermeulen EG, Prodromou NV, Webb TR, Gallo JM, Cheetham ME, Nicoll WS, Blatch GL, Chapple JP. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum Mol Genet. 2009;18:1556–1565. doi: 10.1093/hmg/ddp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard M, Lariviere R, Parfitt DA, Deane EC, Gaudet R, Nossova N, Blondeau F, Prenosil G, Vermeulen EG, Duchen MR, Richter A, Shoubridge EA, Gehring K, McKinney RA, Brais B, Chapple JP, McPherson PS. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) Proc Natl Acad Sci U S A. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng ZH. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo JH, Agarwal E, Bryant KG, Caino MC, Kim ET, Kossenkov AV, Tang HY, Languino LR, Gabrilovich DI, Cohen AR, Speicher DW, Altieri DC. Syntaphilin ubiquitination regulates mitochondrial dynamics and tumor cell movements. Cancer Res. 2018;78:4215–4228. doi: 10.1158/0008-5472.CAN-18-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 36.Ban T, Ishihara T, Kohno H, Saita S, Ichimura A, Maenaka K, Oka T, Mihara K, Ishihara N. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol. 2017;19:856–863. doi: 10.1038/ncb3560. [DOI] [PubMed] [Google Scholar]
- 37.Stepanyants N, Macdonald PJ, Francy CA, Mears JA, Qi X, Ramachandran R. Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol Biol Cell. 2015;26:3104–3116. doi: 10.1091/mbc.E15-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald PJ, Stepanyants N, Mehrotra N, Mears JA, Qi X, Sesaki H, Ramachandran R. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol Biol Cell. 2014;25:1905–1915. doi: 10.1091/mbc.E14-02-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura Y, Sesaki H, Endo T. Phospholipid transport via mitochondria. Traffic. 2014;15:933–945. doi: 10.1111/tra.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frohman MA. Role of mitochondrial lipids in guiding fission and fusion. J Mol Med (Berl) 2015;93:263–269. doi: 10.1007/s00109-014-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adachi Y, Itoh K, Yamada T, Cerveny KL, Suzuki TL, Macdonald P, Frohman MA, Ramachandran R, Iijima M, Sesaki H. Coincident phosphatidic acid interaction restrains Drp1 in mitochondrial division. Mol Cell. 2016;63:1034–1043. doi: 10.1016/j.molcel.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashatus DF. Restraining the divider: a Drp1-phospholipid interaction inhibits Drp1 activity and shifts the balance from mitochondrial fission to fusion. Mol Cell. 2016;63:913–915. doi: 10.1016/j.molcel.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 43.Horvath SE, Bottinger L, Vogtle FN, Wiedemann N, Meisinger C, Becker T, Daum G. Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J Biol Chem. 2012;287:36744–36755. doi: 10.1074/jbc.M112.398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keckesova Z, Donaher JL, De Cock J, Freinkman E, Lingrell S, Bachovchin DA, Bierie B, Tischler V, Noske A, Okondo MC, Reinhardt F, Thiru P, Golub TR, Vance JE, Weinberg RA. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature. 2017;543:681–686. doi: 10.1038/nature21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell. 2011;20:376–387. doi: 10.1016/j.devcel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009;20:2049–2059. doi: 10.1091/mbc.E07-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 50.Ausman J, Abbade J, Ermini L, Farrell A, Tagliaferro A, Post M, Caniggia I. Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis. 2018;9:298. doi: 10.1038/s41419-018-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanchet L, Buydens MC, Smeitink JA, Willems PH, Koopman WJ. Isolated mitochondrial complex I deficiency: explorative data analysis of patient cell parameters. Curr Pharm Des. 2011;17:4023–4033. doi: 10.2174/138161211798764870. [DOI] [PubMed] [Google Scholar]
- 53.Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, Diaz-Ramos A, Hernandez-Alvarez MI, Sebastian D, Mauvezin C, Palacin M, Zorzano A. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013;32:2348–2361. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, Hu XX, Sloan CL, Pereira RO, Lira VA, Spitzer KW, Sharp TL, Shoghi KI, Sparagna GC, Rog-Zielinska EA, Kohl P, Khalimonchuk O, Schaffer JE, Abel ED. Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ Res. 2018;122:58–73. doi: 10.1161/CIRCRESAHA.117.311307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni XX, Nie J, Xie QY, Yu RH, Su L, Liu ZF. Protective effects of hyperbaric oxygen therapy on brain injury by regulating the phosphorylation of Drp1 through ROS/PKC pathway in heatstroke rats. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00811-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cid-Castro C, Hernandez-Espinosa DR, Moran J. ROS as regulators of mitochondrial dynamics in neurons. Cell Mol Neurobiol. 2018;38:995–1007. doi: 10.1007/s10571-018-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 60.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X, Cao H, Zhan L, Yin C, Wang G, Liang P, Li J, Wang Z, Liu B, Huang Q, Xing J. Mitochondrial fission promotes cell migration by Ca(2+) /CaMKII/ERK/FAK pathway in hepatocellular carcinoma. Liver Int. 2018;38:1263–1272. doi: 10.1111/liv.13660. [DOI] [PubMed] [Google Scholar]
- 63.Jung JU, Ravi S, Lee DW, McFadden K, Kamradt ML, Toussaint LG, Sitcheran R. NIK/MAP3K14 regulates mitochondrial dynamics and trafficking to promote cell invasion. Curr Biol. 2016;26:3288–3302. doi: 10.1016/j.cub.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao T, Zhang X, Zhao J, Zhou F, Wang Y, Zhao Z, Xing J, Chen B, Li J, Liu S. SIK2 promotes reprogramming of glucose metabolism through PI3K/AKT/HIF-1alpha pathway and Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett. 2020;469:89–101. doi: 10.1016/j.canlet.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 65.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, Chen Q, Huang SC, O’Neill CM, Edelson BT, Pearce EJ, Sesaki H, Huber TB, Rambold AS, Pearce EL. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Z, Li Y, Wang F, Huang T, Fan K, Zhang Y, Zhong J, Cao Q, Chao T, Jia J, Yang S, Zhang L, Xiao Y, Zhou JY, Feng XH, Jin J. Mitochondrial dynamics controls anti-tumour innate immunity by regulating CHIP-IRF1 axis stability. Nat Commun. 2017;8:1805. doi: 10.1038/s41467-017-01919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 69.Burman JL, Pickles S, Wang C, Sekine S, Vargas JNS, Zhang Z, Youle AM, Nezich CL, Wu X, Hammer JA, Youle RJ. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol. 2017;216:3231–3247. doi: 10.1083/jcb.201612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y, Han Z, Chen L, Gao R, Liu L, Chen Q. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702. doi: 10.1080/15548627.2016.1151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pryde KR, Smith HL, Chau KY, Schapira AH. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J Cell Biol. 2016;213:163–171. doi: 10.1083/jcb.201509003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xian H, Yang Q, Xiao L, Shen HM, Liou YC. STX17 dynamically regulated by Fis1 induces mitophagy via hierarchical macroautophagic mechanism. Nat Commun. 2019;10:2059. doi: 10.1038/s41467-019-10096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 76.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Lee YG, Nam Y, Shin KJ, Yoon S, Park WS, Joung JY, Seo JK, Jang J, Lee S, Nam D, Caino MC, Suh PG, Chan Chae Y. Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer. Cancer Lett. 2020;471:72–87. doi: 10.1016/j.canlet.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z, Li TE, Chen M, Xu D, Zhu Y, Hu BY, Lin ZF, Pan JJ, Wang X, Wu C, Zheng Y, Lu L, Jia HL, Gao S, Dong QZ, Qin LX. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming. Br J Cancer. 2020;122:209–220. doi: 10.1038/s41416-019-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Huang Q, Long X, Guo X, Sun X, Jin X, Li Z, Ren T, Yuan P, Huang X, Zhang H, Xing J. Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene. 2017;36:4901–4912. doi: 10.1038/onc.2017.98. [DOI] [PubMed] [Google Scholar]
- 81.Li M, Wang L, Wang Y, Zhang S, Zhou G, Lieshout R, Ma B, Liu J, Qu C, Verstegen MMA, Sprengers D, Kwekkeboom J, van der Laan LJW, Cao W, Peppelenbosch MP, Pan Q. Mitochondrial fusion via OPA1 and MFN1 supports liver tumor cell metabolism and growth. Cells. 2020;9 doi: 10.3390/cells9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagdas S, Kashatus JA, Nascimento A, Hussain SS, Trainor RE, Pollock SR, Adair SJ, Michaels AD, Sesaki H, Stelow EB, Bauer TW, Kashatus DF. Drp1 promotes KRas-driven metabolic changes to drive pancreatic tumor growth. Cell Rep. 2019;28:1845–1859. e1845. doi: 10.1016/j.celrep.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao X, Lee K, Reid MA, Sanderson SM, Qiu C, Li S, Liu J, Locasale JW. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 2018;22:3507–3520. doi: 10.1016/j.celrep.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng CT, Kuo CY, Ouyang C, Li CF, Chung Y, Chan DC, Kung HJ, Ann DK. Metabolic stress-induced phosphorylation of KAP1 Ser473 blocks mitochondrial fusion in breast cancer cells. Cancer Res. 2016;76:5006–5018. doi: 10.1158/0008-5472.CAN-15-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rambold AS, Pearce EL. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 2018;39:6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, McBride HM. MAPL SUMOylation of Drp1 stabilizes an ER/Mitochondrial platform required for cell death. Mol Cell. 2015;59:941–955. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 89.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Xie Q, Zhou X, Yao J, Zhu X, Huang P, Zhang L, Wei J, Xie H, Zhou L, Zheng S. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015;358:47–58. doi: 10.1016/j.canlet.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 91.Pyakurel A, Savoia C, Hess D, Scorrano L. Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol Cell. 2015;58:244–254. doi: 10.1016/j.molcel.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 93.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 95.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, Huang AY, Petrosiute A. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353:399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horbay R, Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016;21:1327–1335. doi: 10.1007/s10495-016-1295-5. [DOI] [PubMed] [Google Scholar]
- 98.Lee S, Park YY, Kim SH, Nguyen OT, Yoo YS, Chan GK, Sun X, Cho H. Human mitochondrial Fis1 links to cell cycle regulators at G2/M transition. Cell Mol Life Sci. 2014;71:711–725. doi: 10.1007/s00018-013-1428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Brito OM, Scorrano L. Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid Redox Signal. 2008;10:621–633. doi: 10.1089/ars.2007.1934. [DOI] [PubMed] [Google Scholar]
- 100.O’Flanagan CH, Morais VA, Wurst W, De Strooper B, O’Neill C. The Parkinson’s gene PINK1 regulates cell cycle progression and promotes cancer-associated phenotypes. Oncogene. 2015;34:1363–1374. doi: 10.1038/onc.2014.81. [DOI] [PubMed] [Google Scholar]
- 101.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 103.Betts BC, Veerapathran A, Pidala J, Yang H, Horna P, Walton K, Cubitt CL, Gunawan S, Lawrence HR, Lawrence NJ, Sebti SM, Anasetti C. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aai8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gong X, Du J, Parsons SH, Merzoug FF, Webster Y, Iversen PW, Chio LC, Van Horn RD, Lin X, Blosser W, Han B, Jin S, Yao S, Bian H, Ficklin C, Fan L, Kapoor A, Antonysamy S, Mc Nulty AM, Froning K, Manglicmot D, Pustilnik A, Weichert K, Wasserman SR, Dowless M, Marugan C, Baquero C, Lallena MJ, Eastman SW, Hui YH, Dieter MZ, Doman T, Chu S, Qian HR, Ye XS, Barda DA, Plowman GD, Reinhard C, Campbell RM, Henry JR, Buchanan SG. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9:248–263. doi: 10.1158/2159-8290.CD-18-0469. [DOI] [PubMed] [Google Scholar]
- 105.Maia AR, Maiato H. Aurora Mitochondrialis drives fission during mitosis. Dev Cell. 2011;21:387–388. doi: 10.1016/j.devcel.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 106.Bertolin G, Bulteau AL, Alves-Guerra MC, Burel A, Lavault MT, Gavard O, Le Bras S, Gagne JP, Poirier GG, Le Borgne R, Prigent C, Tramier M. Aurora kinase A localises to mitochondria to control organelle dynamics and energy production. Elife. 2018:7. doi: 10.7554/eLife.38111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bao D, Zhao J, Zhou X, Yang Q, Chen Y, Zhu J, Yuan P, Yang J, Qin T, Wan S, Xing J. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene. 2019;38:5007–5020. doi: 10.1038/s41388-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo X, Zhang J, Ji L, Ren T, An J, Liu B, Nie Y, Xing J. Increased mitochondrial fission promotes autophagy and hepatocellular carcinoma cell survival through the ROS-modulated coordinated regulation of the NFKB and TP53 pathways. Autophagy. 2016;12:999–1014. doi: 10.1080/15548627.2016.1166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 110.Tanwar DK, Parker DJ, Gupta P, Spurlock B, Alvarez RD, Basu MK, Mitra K. Crosstalk between the mitochondrial fission protein, Drp1, and the cell cycle is identified across various cancer types and can impact survival of epithelial ovarian cancer patients. Oncotarget. 2016;7:60021–60037. doi: 10.18632/oncotarget.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han Y, Kim B, Cho U, Park IS, Kim SI, Dhanasekaran DN, Tsang BK, Song YS. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene. 2019;38:7089–7105. doi: 10.1038/s41388-019-0949-5. [DOI] [PubMed] [Google Scholar]
- 112.von Eyss B, Jaenicke LA, Kortlever RM, Royla N, Wiese KE, Letschert S, McDuffus LA, Sauer M, Rosenwald A, Evan GI, Kempa S, Eilers M. A MYC-driven change in mitochondrial dynamics limits YAP/TAZ function in mammary epithelial cells and breast cancer. Cancer Cell. 2015;28:743–757. doi: 10.1016/j.ccell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 113.Chen L, Zhang J, Lyu Z, Chen Y, Ji X, Cao H, Jin M, Zhu J, Yang J, Ling R, Xing J, Ren T, Lyu Y. Positive feedback loop between mitochondrial fission and Notch signaling promotes survivin-mediated survival of TNBC cells. Cell Death Dis. 2018;9:1050. doi: 10.1038/s41419-018-1083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomkova V, Sandoval-Acuna C, Torrealba N, Truksa J. Mitochondrial fragmentation, elevated mitochondrial superoxide and respiratory supercomplexes disassembly is connected with the tamoxifen-resistant phenotype of breast cancer cells. Free Radic Biol Med. 2019;143:510–521. doi: 10.1016/j.freeradbiomed.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 115.Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming QL, Huang C, Li P, Gao N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy. 2015;11:1259–1279. doi: 10.1080/15548627.2015.1056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu D, Dasgupta A, Chen KH, Neuber-Hess M, Patel J, Hurst TE, Mewburn JD, Lima PDA, Alizadeh E, Martin A, Wells M, Snieckus V, Archer SL. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020;34:1447–1464. doi: 10.1096/fj.201901467R. [DOI] [PubMed] [Google Scholar]
- 117.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 118.Chattaragada MS, Riganti C, Sassoe M, Principe M, Santamorena MM, Roux C, Curcio C, Evangelista A, Allavena P, Salvia R, Rusev B, Scarpa A, Cappello P, Novelli F. FAM49B, a novel regulator of mitochondrial function and integrity that suppresses tumor metastasis. Oncogene. 2018;37:697–709. doi: 10.1038/onc.2017.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rademaker G, Hennequiere V, Brohee L, Nokin MJ, Lovinfosse P, Durieux F, Gofflot S, Bellier J, Costanza B, Herfs M, Peiffer R, Bettendorff L, Deroanne C, Thiry M, Delvenne P, Hustinx R, Bellahcene A, Castronovo V, Peulen O. Myoferlin controls mitochondrial structure and activity in pancreatic ductal adenocarcinoma, and affects tumor aggressiveness. Oncogene. 2018;37:4398–4412. doi: 10.1038/s41388-018-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fu L, Dong Q, He J, Wang X, Xing J, Wang E, Qiu X, Li Q. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36:2724–2736. doi: 10.1038/onc.2016.425. [DOI] [PubMed] [Google Scholar]
- 121.Qi M, Dai D, Liu J, Li Z, Liang P, Wang Y, Cheng L, Zhan Y, An Z, Song Y, Yang Y, Yan X, Xiao H, Shao H. AIM2 promotes the development of non-small cell lung cancer by modulating mitochondrial dynamics. Oncogene. 2020;39:2707–2723. doi: 10.1038/s41388-020-1176-9. [DOI] [PubMed] [Google Scholar]
- 122.Chauhan SS, Toth RK, Jensen CC, Casillas AL, Kashatus DF, Warfel NA. PIM kinases alter mitochondrial dynamics and chemosensitivity in lung cancer. Oncogene. 2020;39:2597–2611. doi: 10.1038/s41388-020-1168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arena G, Cisse MY, Pyrdziak S, Chatre L, Riscal R, Fuentes M, Arnold JJ, Kastner M, Gayte L, Bertrand-Gaday C, Nay K, Angebault-Prouteau C, Murray K, Chabi B, Koechlin-Ramonatxo C, Orsetti B, Vincent C, Casas F, Marine JC, Etienne-Manneville S, Bernex F, Lombes A, Cameron CE, Dubouchaud H, Ricchetti M, Linares LK, Le Cam L. Mitochondrial MDM2 regulates respiratory complex I activity independently of p53. Mol Cell. 2018;69:594–609. e598. doi: 10.1016/j.molcel.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, Ferguson AN, Kashatus DF, Bao S, Rich JN. Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci. 2015;18:501–510. doi: 10.1038/nn.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar S, Pan CC, Shah N, Wheeler SE, Hoyt KR, Hempel N, Mythreye K, Lee NY. Activation of mitofusin2 by Smad2-RIN1 complex during mitochondrial fusion. Mol Cell. 2016;62:520–531. doi: 10.1016/j.molcel.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 127.Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, Pearl D, Siddiqui N, Strack S, McGuirk S, St-Pierre J, Larsson O, Topisirovic I, Vali H, McBride HM, Bergeron JJ, Sonenberg N. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell. 2017;67:922–935. e925. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 128.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, Fu J, Zhang J, Nguyen H, Kang I, Toth K, Al-Homrani M, Husain M, Beeson G, Ball L, Helke K, Husain S, Garrett-Mayer E, Hardiman G, Mehrotra M, Nishimura MI, Beeson CC, Bupp MG, Wu J, Ogretmen B, Paulos CM, Rathmell J, Yu XZ, Mehrotra S. CD38-NAD(+)axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 2018;27:85–100. e108. doi: 10.1016/j.cmet.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai J, Wang J, Huang Y, Wu H, Xia T, Xiao J, Chen X, Li H, Qiu Y, Wang Y, Wang T, Xia H, Zhang Q, Xiang AP. ERK/Drp1-dependent mitochondrial fission is involved in the MSC-induced drug resistance of T-cell acute lymphoblastic leukemia cells. Cell Death Dis. 2016;7:e2459. doi: 10.1038/cddis.2016.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, Lai S, Rich JN, Inguva A, Shannon KM, Kim H, Tan AC, Myers JR, Ashton JM, Neff T, Pollyea DA, Smith CA, Jordan CT. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell. 2018;23:86–100. e106. doi: 10.1016/j.stem.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin X, Wang J, Gao K, Zhang P, Yao L, Tang Y, Tang L, Ma J, Xiao J, Zhang E, Zhu J, Zhang B, Zhao SM, Li Y, Ren S, Huang H, Yu L, Wang C. Dysregulation of INF2-mediated mitochondrial fission in SPOP-mutated prostate cancer. PLoS Genet. 2017;13:e1006748. doi: 10.1371/journal.pgen.1006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Civenni G, Bosotti R, Timpanaro A, Vazquez R, Merulla J, Pandit S, Rossi S, Albino D, Allegrini S, Mitra A, Mapelli SN, Vierling L, Giurdanella M, Marchetti M, Paganoni A, Rinaldi A, Losa M, Mira-Cato E, D’Antuono R, Morone D, Rezai K, D’Ambrosio G, Ouafik L, Mackenzie S, Riveiro ME, Cvitkovic E, Carbone GM, Catapano CV. Epigenetic control of mitochondrial fission enables self-renewal of stem-like tumor cells in human prostate cancer. Cell Metab. 2019;30:303–318. e6. doi: 10.1016/j.cmet.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Cai J, Huang Y, Ke Q, Wu B, Wang S, Han X, Wang T, Wang Y, Li W, Lao C, Song W, Xiang AP. Nestin regulates proliferation and invasion of gastrointestinal stromal tumor cells by altering mitochondrial dynamics. Oncogene. 2016;35:3139–3150. doi: 10.1038/onc.2015.370. [DOI] [PubMed] [Google Scholar]
- 135.Huang CY, Chiang SF, Chen WT, Ke TW, Chen TW, You YS, Lin CY, Chao KSC, Huang CY. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018;9:1004. doi: 10.1038/s41419-018-1019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen M, Ye K, Zhang B, Xin Q, Li P, Kong AN, Wen X, Yang J. Paris Saponin II inhibits colorectal carcinogenesis by regulating mitochondrial fission and NF-kappaB pathway. Pharmacol Res. 2019;139:273–285. doi: 10.1016/j.phrs.2018.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]