Abstract
Adverse life events can lead to stable changes in brain structure and function and are considered primary sources of risk for post-traumatic stress disorder, depression, and other neuropsychiatric disorders. However, most individuals do not develop these conditions following exposure to traumatic experiences, and research efforts have identified a number of experiential factors associated with an individual’s ability to withstand, adapt to, and facilitate recovery from adversity. While multiple animal models of stress resilience exist, so that the detailed biological mechanisms can be explored, studies have been disproportionately conducted in male subjects even though the prevalence and presentation of stress-linked disorders differ between sexes. This review focuses on 1) the mechanisms by which experiential factors (behavioral control over a stressor, exercise) reduce the impact of adverse events as studied in males; 2) whether other manipulations (ketamine) that buffer against stress-induced sequelae engage the same circuit features; and 3) whether these processes operate similarly in females. We argue that investigation of experiential factors that produce resistance/resilience rather than vulnerability to adversity will generate a unique set of biological mechanisms that potentially underlie sex differences in mood disorders.
Keywords: stress, coping, exercise, ketamine, medial prefrontal cortex
Graphical Abstract
Women are at a higher risk for certain stress-linked psychiatric disorders and accounting for sex as a biological variable in preclinical research is fundamental for developing effective therapies for both men and women. We review the neural and behavioral sequelae of stress-buffering factors in each of the sexes.
Introduction
Exposure to adverse events is a risk factor for a variety of negative health outcomes including the development of psychiatric disorders such as depression, generalized anxiety, and posttraumatic stress disorder. Although polygenic factors certainly play a role, many resilience studies have focused on factors that change how an individual experiences trauma (e.g., coping strategies, pharmaceuticals, physical health, safety learning). These factors are associated with positive mental health outcomes and are modifiable through experience, which can be leveraged by interventions designed to inculcate resilience to adversity. Unfortunately, treatments for stress-related disorders are not always effective and this is may be due to different responses to resilience factors including differences that arise from sex (Breslau, 2009). Indeed, many psychiatric disorders that have stress as a contributing factor are sex-biased in their prevalence and/or presentation. Women are over twice as likely to develop mood and anxiety disorders whereas men are nearly three times as likely to develop a substance use disorder (Steel et al., 2014). Due to the limited use of females in preclinical research (Beery & Zucker, 2011), it is not understood how the neural circuits underlying resilience factors compare between the sexes and whether they produce similar or different behavioral outcomes. The purpose of this review is to provide a current summary of the neural mechanisms and behavioral phenomena induced by stress-protective factors in each of the sexes and to identify future research avenues.
Psychological variables such as a perceived ability to cope with or control a stressor can enhance long term psychosocial health and wellbeing (Kohn et al.,1994; Yi et al., 2005; Dijkstra et al., 2016). Similarly, physical health through aerobic exercise pre or post trauma can reduce depression ratings and relapse to the same degree as many current pharmacotherapies (Blumenthal et al., 2012). Whether these protective factors lead to a resilient phenotype that differs between men and women is not fully understood. There is evidence that men and women cope with adversity differently, which may be due to the fact that certain resilience factors are more effective for one sex than the other (Hamilton & Fagot, 1998; Tamres et al., 2002). Current research efforts are directed at identifying the shared and sex-specific features, and corresponding neural processes, of experiential resilience factors in order to maximize the applicability of preventative and durable treatment strategies for stress-linked disorders.
Animal models of stress have allowed us to study possible neural mechanisms driving the behavioral sequelae of stress exposure. One approach to studying stress vulnerability/resistance is to examine natural variation in the stress response. For example, an experimental manipulation (e.g. social defeat) will cause a measurable outcome (e.g. social interaction) that follows some distribution pattern. A criterion set by the experimenter can then be used to separate these groups (stress susceptible vs. stress resilient) in order to identify the neural circuit, endocrine, and molecular mediators that underlie the difference in response.
For some outcome measures, like freezing in fear conditioning/extinction tasks, male and female rodent models have been reported to produce a similar distribution pattern, termed sex convergence (Iwasaki-Sekino et al., 2009). However, others report sex divergence in which freezing is greater in males than in females (Kudielka et al., 2005; Amat et al., 2006; Baratta et al., 2008). Indeed, sex divergence is most commonly seen when the behavioral outcome measure involves ambulation, such as in the sidman avoidance, open field, and emergence (sheltered to exposed) tasks (Archer, 1975) in which female rodents are more active in avoiding threat to a greater degree than males. Higher ambulatory activity in females is often interpreted as a blunted fear response, despite the possibility that this is a female specific fear behavior (Gruene et al., 2015). Divergence in the opposite direction has also been reported. In response to chronic variable stress, females show stress induced sequelae in a vast array of behavioral tests in which males do not (Hodes et al., 2015). In addition, females exhibit a greater physiological response to footshock and handling. Given that male and female responses to stress in rodents do not necessarily parallel the sex differences in susceptibility to stress related disorders, future studies would benefit from a better understanding of sex specific behavioral responses to stress and the experiential factors that influence these responses.
An alternative approach to studying the neural mechanisms underlying resistance/resilience to stress is to manipulate experiential factors that are associated with resilience in humans. Manipulating the function of neural elements (gene to circuit) during an event known to produce resilience has helped to identify resilience mechanisms, with the majority of work conducted in males. We are only beginning to understand how stress resilience/resistance models work in females and how the underlying elements compare to males. Many experiential factors either occurring before, during, or after stress have been identified as stress protective. Here, we will focus on three that have been reported to be protective or predict protection from stress outcomes in humans: the use of coping strategies, ketamine and physical exercise. In preclinical models these factors are protective, provide enduring protection to a diverse set of stressors and engage distinct neural mechanisms.
Coping with stress
In humans, many of the factors associated with the ability to withstand (resistance) or facilitate recovery (resilience) from the impact of an adverse life event revolve around coping factors. Strategies aimed at eliminating or blunting the cause of the stressor (problem-focused coping) or reducing the emotional impact of the stressor (emotion-focused coping) are viewed as key processes in exerting actual or perceived control over negative circumstances in an individual’s life (Billings & Moos, 1981; Folkman et al., 1984). Behavioral control over some aspect of the stressor (Pearlin & Carmi, 1978; Baker & Berenbaum, 2007; Diehl & Hay, 2010) represents one of the few aspects of coping that can be experimentally manipulated in animals in order to better understand how resilience is mediated at a neural level.
Behavioral control has been studied in a variety of species, but the rat has been most frequent. Here, rats that receive physically identical stressors are compared, with one group having behavioral control over the termination of the stressor, and another group having no control. In order to isolate the variable of control, each subject is placed into small boxes with a wheel mounted on the front wall. The rat’s tail extends from the back of the box and is affixed to electrodes. For one group (ES; escapable stress), subjects receive a series of tailshocks each of which is terminated when the rat performs a given instrumental escape response (turning the wheel). Thus, ES subjects can exert control over one aspect of the stressor - the duration of each of the shocks. Each member of a second group (IS; inescapable stress) is paired with one from the escape group and simply receives tailshocks of the same duration as determined by its ES partner. For the IS subject, turning the wheel has no consequence. A third subject receives no tailshock, and serves as a no stress control group. Tailshock is used in rat studies to ensure that subjects with and without control receive physically identical shocks (number, intensity, duration, temporal pattern, etc.), a necessary requirement for isolating the impact of controllability. It may be the case that behavioral control would mitigate the outcome of stressors other than tailshock (e.g. social defeat, restraint), but since controllability can’t be readily manipulated in these paradigms its potential mitigating role cannot be determined.
Numerous behavioral changes are produced in subjects exposed to IS but do not occur if the subject is allowed to exert behavioral control over the stressor (ES). Behavioral changes (often termed “learned helplessness effects”) that follow IS, but not ES, include impaired shuttle box escape behavior, increased social avoidance, neophobia, potentiated morphine conditioned place preference, reduced aggression, resistance to fear extinction, and exaggerated shock-elicited freezing when tested in a novel environment 24 hr after stress treatment (Weiss, 1968; Maier & Watkins, 1998; Maier & Watkins 2005; Baratta et al., 2007; Rozeske et al., 2011). It is important to note that behavioral control doesn’t block or reduce all of the neurochemical or behavioral outcomes of IS. For instance, tail shock leads to robust changes in autonomic (Thompson et al., 2013), endocrine (Maier et al., 1986; Helmreich et al., 1999; Helmreich et al., 2012), brain immune (Frank et al., 2007), neural (McDevitt et al., 2009) and behavioral (Woodmansee et al., 1993) endpoints that are insensitive to control. Thus, it’s not the case that uncontrollable stress is simply more “aversive” or “potent” than controllable stress.
Additionally, the stress-buffering effects of ES extend beyond the stressor being controlled. That is, an initial experience with behavioral control buffers against the neural and behavioral sequelae of future inescapable stressors occurring much later (enduring) and in a different apparatus/environment from the original control experience (transsituational; Williams & Maier, 1977; Amat et al., 2006; Baratta et al., 2008; Amat et al., 2010). For example, Amat et al. (2006) showed that a previous experience of ES blocked the typical neurochemical and behavioral consequences (shuttle box escape interference and exaggerated shock-elicited freezing) produced by IS one week later, a process termed “behavioral immunization”. Prior exposure to IS had no effect on later IS, suggesting that the prior experience of control over shock, rather than shock per se, is the active ingredient in the development of resilience. It is doubtful that ES buffers against all future adversity, yet the boundary conditions of its protection remain largely unexplored. Of note, the proactive effects of ES extend to future uncontrollable stressors that don’t involve shock, namely social defeat. Similar to IS, acute social defeat leads to interference with shuttle box escape and increases social avoidance. ES occurring 7 days prior to social defeat blocked these social defeat-induced behavioral outcomes (Amat et al., 2010).
The initial work investigating the neural mediation of controllability effects focused on the mechanisms that produced the behavioral sequelae following uncontrollable stress. This has been reviewed in detail elsewhere (Maier & Watkins, 1998) and it involves a neural circuit that involves intense activation of serotonergic (5-HT) neurons in the dorsal raphe nucleus (DRN) which is critical for the production of IS-induced behavioral effects. The intense activation of DRN 5-HT neurons by uncontrollable stressors leads to a persistent sensitization of these neurons such that later testing procedures (footshock in the shuttlebox, presence of a juvenile, etc.) produce an exaggerated release of 5-HT into DRN projection regions that are the proximal mediators of the behaviors (Amat et al., 1998; Grahn et al., 1999; Bland et al., 2003; Strong et al., 2011). Activation of the DRN is both necessary and sufficient for producing many of the behaviors that follow IS.
Not surprisingly, behavioral control over shock does not lead to potent activation of DRN 5-HT and its exaggerated release in target regions of the DRN (Amat et al., 1998; Maswood et al., 1998; Strong et al., 2011). Rather, the experience of control engages the prelimbic (PL) region of the mPFC and provides top-down inhibition over DRN 5-HT activity (Amat et al., 2005), thereby preventing the shock-induced behavioral changes. This inhibition occurs because glutamatergic output neurons from the PL preferentially synapse onto GABAergic interneurons within the DRN (Jankowski & Sesack, 2004) which provide monosynaptic inhibition over 5-HT activity (Challis et al., 2014). Electrical stimulation of the mPFC (Hajos et al., 1998; Varga et al., 2001) and pathway-specific photostimulation of DRN-projecting mPFC neurons (Challis et al., 2014) both lead to DRN 5-HT inhibition through a GABAergic mechanism. In contrast, IS does not engage the PL projection to the DRN and pharmacological inhibition of the PL has no impact on the neurochemical and behavioral outcomes of IS (Amat et al., 2005; Baratta et al., 2009). These findings underscore the view that resilience mechanisms are not simply the absence of changes that drive vulnerability; rather, it engages a distinct set of neural mechanisms.
Engagement of the PL by behavioral control is also required for its enduring stress-buffering effects against future adverse events, a protection that lasts up to two months (Amat et al., 2006; Kubala et al., 2012). Efforts directed at understanding how an initial experience of coping with stress produces resistance to uncontrollable stressors experienced at a much later point in time have focused on N-methyl-D-aspartate receptor (NMDAR)-dependent processes. Activation of the extracellular signal-regulated kinase (ERK) cascade, an essential component of NMDAR signaling, is involved in the encoding and consolidation of an experience that leads to long-term behavioral change (Malenka et al., 1993; Thomas & Huganir, 2004; Morris, 2013). Interestingly, controllable stress increases phosphorylated ERK1 and ERK2 within the PL and blockade of NMDAR and ERK-dependent signaling in the PL during the experience of control eliminates behavioral immunization (Christianson et al., 2014). Although synaptic plasticity in the PL has never been directly assessed, behavioral control induces PL layer 5 pyramidal cell excitability by increasing intrinsic membrane excitability (Varela et al., 2012).
The reviewed work above clearly demonstrates that the PL regulates the DRN 5-HT response to shock in the presence of control, but is the mPFC also involved in the acquisition/detection of the instrumental wheel-turn controlling response? In the appetitive domain, there are two distinct instrumental learning systems with largely nonoverlapping circuitries mediating the acquisition and performance of instrumental action. One system termed “action-outcome” is mediated by a corticostriatal circuit involving the dorsal medial striatum (DMS) and the PL. The action-outcome system records the association between the action (wheel-turn) and the outcome (stress termination) and is sensitive to contingency (e.g. the probability of the outcome given the response integrated with the probability of the outcome given no response). The other system termed “habit” is mediated by the dorsolateral striatum (DLS) and sensorimotor cortex and records stimulus response associations in a contingency-independent manor (Yin et al., 2005; O’Hare et al., 2016). Recent data demonstrate that both the acute and long-term protective effects of behavioral control require the PL and the DMS (action-outcome circuit), but not the DLS (habit circuit; Amat et al., 2014).
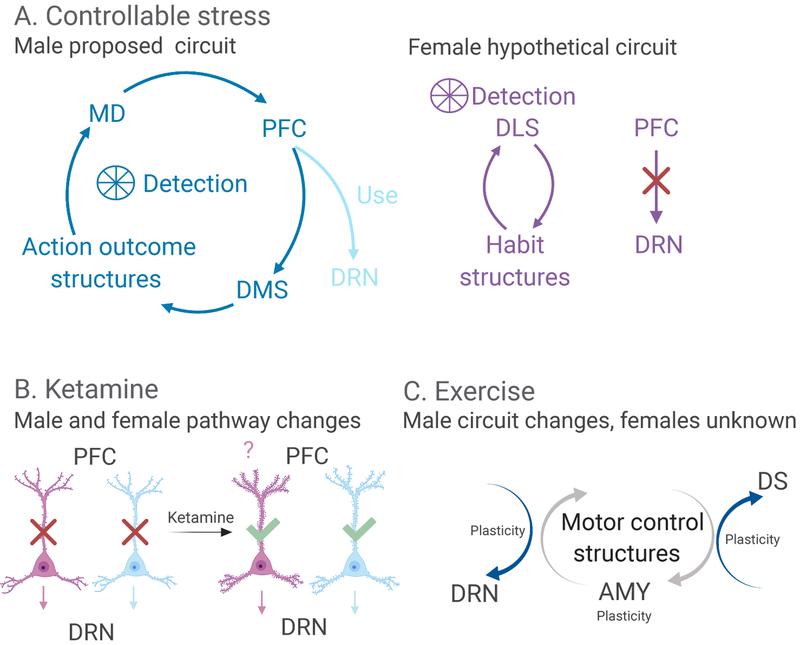
These aforementioned data suggest that the PL is important for both the detection of the instrumental controlling response and regulation of the DRN, yet whether these processes occur through the same or different PL ensembles is unclear. The existing data are sparse, but it appears that the PL pyramidal neurons that project to the DMS and the PL neurons that project to the DMS are intermixed within layer 5 and are largely non-overlapping (Baratta & Maier, 2017). Thus, it appears that embedded within the PL are two separable ensembles associated with two distinct features of behavioral control: its ‘detection’ the subsequent ‘use’ of that information to modulate downstream structures (Figure 1).
Figure 1.
Simplified illustration of the circuits engaged by controllable stress, ketamine and exercise in males and females. (A) Proposed overlapping circuits involved in the two separable features of controllable stress in males (blue) and females (pink). ‘Detection’ of the instrumental wheel-turn response through the corticostriatal action-outcome system (light blue). The ‘use’ pathway (dark blue) showing prelimbic (PL) top-down inhibition over dorsal raphe nucleus (DRN). In females, ‘detection’ of the wheel-turn response may be acquired by the instrumental habit learning system (light pink), which does not lead to PL-DRN engagement (dark pink). (B) Schematic of ketamine’s direct engagement of the PL-DRN pathway in males and females. In both males and females, evidence for ketamine-induced spine formation specific to the PL-DRN pathway is unknown. (C) Motor structures engaged by exercise-induce plasticity in stress-responsive sites. The circuitry underlying the stress protective effects of exercise in females is unknown. Amy, amygdala; DS, dorsal striatum.
Recent mandates introduced by the National Institutes of Health and the Canadian Institutes of Health Research require investigators to examine sex effects in preclincal research in order to address health disparities (Clayton & Collins, 2014). The work described above was conducted entirely in male rodents, and prior to implementation of these policies, it was largely unknown whether behavioral control operates in females as it does in males, and if so, whether the same circuit mechanisms are involved. Few studies have manipulated controllability in females, and interestingly, control was without an effect (Dalla et al., 2008). There are a number of published female studies that utilize the term “learned helplessness”, however the term is often used in the literature in a way that differs from its definition (Maier & Seligman, 1976). Learned helplessness refers to the behavioral and neurochemical outcomes of the stressor that depend on the uncontrollability of the stressor. That is, to qualify as a learned helplessness effect a behavioral change (e.g., poor escape behavior) must follow exposure to IS, but not physically identical ES. This is what distinguishes a helplessness effect from a generic stress effect, and without this distinction, all effects of a stressor would be labeled as a learned helplessness effect. Indeed, it has been shown that not all outcomes that follow an uncontrollable stressor are learned helplessness effects. For example, IS produces reduced daily activity in running wheels, but ES produces the same reduction (Woodmansee et al., 1993) and so this reduction in activity is not produced by learned helplessness but is instead a simple stress effect. Prior female learned helplessness studies have not manipulated the controllability of the stressor (e.g., Jenkins et al., 2001), and when they have, there has often been no difference between the uncontrollable stress group and no stress controls. In either case, the impact of behavioral control cannot be determined.
Baratta et al. (2018) investigated the impact of controllability in female rats using the identical conditions that produce the differential behavioral consequences observed in male rats. In sharp contrast to what has previously been observed in males, the beneficial effects of behavioral control were absent in females, that is, both female ES and IS groups show identical levels of stress-induced sequelae when compared to no stress controls. ES and IS females exhibited greater c-Fos activation in DRN 5-HT expressing neurons, fail to escape in a shuttle box, and reduced juvenile social exploration (JSE; Baratta et al., 2018). That is, the controllability of the stressor does not determine resilience in females. Furthermore, c-Fos expression is absent in DRN-projecting PL neurons. Pharmacological activation of the PL reverses this phenotype, effectively blocking the effects of tail shock in uncontrollable and controllable stress females. These data suggest that mPFC mediated regulation of DRN 5-HT is not engaged by control in female subjects. It is currently unknown whether the lack of protection is due to sex differences in the detection of behavioral control or the use of control information to initiate regulation of the DRN.
The failure of control to provide protection in females is striking given that females rapidly acquire the wheel-turn controlling response at a rate comparable to males. As mentioned above, there are two separable systems involved in the encoding of instrumental responses and one untested possibility is that females acquire the escape response with the PL-independent habit system rather than the corticostriatal action-outcome system. Elevated catecholamine levels in the mPFC, such as those that may occur during stress exposure, can disrupt prefrontal top-down function (Arnsten, 2009) as well as strengthen habit circuit systems (Fournier et al., 2017). Stress-evoked release of catecholamines have been reported to be greater in females (Mitsushima et al., 2006; Staiti et al., 2011) across several brain regions. Moreover, stress-induced corticosterone levels, which have been reported to be higher in females (Heck & Handa, 2018), can shift the acquisition of instrumental learning to the habit system (Schwabe & Wolf, 2011). Thus, one possibility is that stress-evoked catecholamine release in the mPFC biases females to acquire the wheel-turn controlling response with the habit learning system, evading engagement of the PL and top-down regulation of the DRN during tail shock.
The absence of a modulating effect of control in female ES subjects also extends to behavioral immunization. Baratta et al. (2019) investigated immunization effects by giving male and female rats ES, IS, and no stress home cage (HC) one week prior to an uncontrollable stressor. As is typical, ES males were resistant to the behavioral outcomes of the later experienced uncontrollable stressor, whereas ES females were not. In addition, structural plasticity in the PL-to-DRN pathway following ES and IS differed between the sexes. In males, IS lead to non-pathway-specific alterations in spine enlargement, while ES elicited spine changes only in DRN-projecting PL neurons. Thus, structural plasticity in the PL-to-DRN pathway following ES is consistent with prior work implicating the involvement of this pathway in the long-term protective effects of controllable stress (Amat et al., 2006; Varela et al., 2012; Christianson et al., 2014). In females, the pattern of spine changes differed from males. ES-elicited broad, non-specific spine changes in the mPFC while IS led to only minor alterations. Whether these sex-specific patterns of structural plasticity are related to the differential behavioral outcomes remains to be determined.
Stressor controllability produces sex-divergent behaviors in rodents; males given a controlling instrumental response over stress termination are resistant to the behavioral outcomes of that stressor, whereas in corresponding females the controlling response is without benefit. Future work dissecting the neural circuit components that respond to coping with stress, and how they differ between the sexes, may serve as an important model for understanding sex differences in susceptibility to stress-related disorders.
Stress-buffering and restorative effects of subanesthetic ketamine
Depression is estimated to be among the most burdensome disorders worldwide affecting more than 300 million people (Friedrich, 2017). Current pharmacotherapies for moderate and severe depressive disorders are only partially effective, at best, and treatment-resistant individuals require alternative therapeutic approaches with novel mechanisms of action (Wiedemann, 2011). Over the last two decades, clinical studies have found that acute treatment with a sub-anesthetic dose of the NMDAR antagonist, ketamine, produces rapid (i.e. within hours) and sustained (up to two weeks) antidepressant effects in individuals with treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006; Price et al., 2009; Zarate et al., 2012; Murrough et al., 2013; Lai et al., 2014). The antidepressant effects of ketamine are observed similarly in both men and women (Carrier et al., 2013; Saland et al., 2017; Freeman et al., 2019), though a few report the effects last longer in men (Coyle et al., 2015; Rybakowski et al., 2017). The mechanisms underlying the antidepressant effects of ketamine appear to go beyond NMDA antagonism (Newport et al., 2015) and preclinical studies suggest they are also sex-differentiated.
Although ketamine is the prototype for a new generation of antidepressants for treatment-resistant depressive patients, ketamine has properties that limit its utility as a therapeutic. Ketamine has a potential for abuse and can be accompanied by dissociative and psychotomimetic side effects. This has led to efforts directed at testing and developing structural analogs of ketamine with a safer profile (Lener et al., 2017) and to understand the mechanism(s) by which ketamine is effective. In preclinical studies, which have mostly been conducted in males, low dose ketamine in male rats and mice facilitates fear extinction (Girgenti et al., 2017) and blunts the behavioral outcomes of stressor exposure (e.g. chronic corticosterone administration, inescapable tail shock, chronic mild stress) when administered prior to (Maeng et al., 2008; Dolzani et al., 2018) or after stress treatment (Li et al., 2011). The effects of ketamine are sufficiently diverse, buffering against and reversing stress-induced changes in forced swim, tail suspension, juvenile social investigation, shuttle box escape, and elevated plus maze (Armario et al., 1991; Maeng et al., 2008; Engin et al., 2009; Autry et al., 2011; Dolzani et al., 2018).
Low dose ketamine also provides stress-protection in female rodents, and the effective dose and time course of its stress-reducing effects appear to be sex divergent (Carrier & Kabbaj, 2013; Franceschelli et al., 2015; Dossat et al., 2018). In female rats, systemic administration of low dose (2.5 mg/kg) ketamine prior to testing (30 min) reduces time spent immobile in a forced swim test and decreases time to feed in a novelty suppressed feeding task. Males require at a higher dose (5 mg/kg) for similar effects (Carrier & Kabbaj, 2013). Further, ketamine delivered following 10 days of chronic mild stress can ameliorate stressed-induced behaviors for up to 24 hrs in females and 7 days for males (Franceschelli et al., 2016). Notably, some human studies have also reported that the effects of ketamine last longer in males (Coyle & Laws, 2015; Rybakowski et al., 2017). The stress-buffering effects of low dose ketamine are diminished in ovariectomized females and restored upon estrogen and progesterone supplementation (Carrier & Kabbaj, 2013). This suggests that gonadal hormones may play an important role in ketmaine’s mechanism of action in females.
Ketamine has been shown to modulate neurochemical activity in the prefrontal cortex, with some effects comparable between the sexes, in both clinical and preclinical studies. Ketamine administration in men and women with or without major depressive disorder produces an increase in glutamate transmission in the mPFC (Abdallah et al., 2018). Similarly, in male rodents, systemic administration of ketamine stimulates the release of glutamate resulting from acute, transient inhibition of GABAergic activity (Duman et al., 2019) and pharmacological inhibition of the mPFC prevents ketamine’s protective effects against stress (Amat et al., 2016). Interestingly, intra-mPFC administration of ketamine leads to dopamine release (Lorrain et al., 2003) and optogenetic inhibition of dopamine receptor 1-expressing mPFC neurons eliminates the protective effects of ketamine in both male and female mice (Hare et al., 2019). Collectively, these data implicate the induction of dopaminergic and glutamatergic signaling in the prefrontal cortex for ketamine’s antidepressant effects.
Behavioral sequelae induced by stress exposure are often associated with changes in structural plasticity, such as increased elimination or decreased formation of dendritic spines, across numerous brain regions (Moda-Sava et al., 2019). In both male and female naive rodents, low-dose ketamine can enhance synaptic plasticity and spine formation (Ruddy et al., 2015; Duman et al., 2016; Phoumthipphavong et al., 2016; Pryazhnikov et al., 2018). At least in males, spine formation and stable maintenance is critical for ketamine’s stress-reducing effects. Using a novel photosensitive tool, activated synapse-targeting photoactivatable Rac1 (AS-PaRac1), Moda-Sava et al. (2019) demonstrated that spine development by ketamine is necessary for its long-lasting buffering effects on stress outcome. Synaptic remodeling appears to be a critical feature of ketamine’s ability to reverse the impact of stress. The mammalian target of rapamycin (mTOR) pathway, an important player in spine formation and maturation, is necessary for ketamine-induced increases in spine formation in the mPFC and it’s ability to blunt stress effects on forced swim, inescapable stress, and the novelty suppressed feeding test (Li et al., 2010). Interestingly, brain-derived neurotrophic factor (BDNF), but not mTOR, signaling is necessary for the acute antidepressant effects (Autry et al., 2011). These data suggest mTOR signaling and spine formation is most likely important for sustaining the protective effects of ketamine whereas other mechanisms may underlie the rapid protective effects.
The impact of ketamine on prefrontal structural plasticity may be sex-divergent (Table 1), particularly in the mPFC (Sarkar & Kabbaj, 2016). In males, ketamine increases prefrontal spine density and synaptic protein expression, which is not observed in females (Sarkar & Kabbaj, 2016). An increase in synaptic plasticity related proteins following ketamine administration is observed in males and only in females that are in diestrus 1 and proestrus (Dossat et al., 2018). Future work regarding the effects of ketamine on long-term changes in neuronal architecture and signaling would benefit from including both sexes and closely examining the effect of estrus cycle phase.
Table 1. Female studies relevant to behavioral control, exercise, and ketamine.
Non-exhaustive list of female rodent studies relevant to the impact of behavioral control, ketamine, and exercise on adverse events. Note that references for learned helplessness studies in which the endpoint measure was unaffected by uncontrollable stress and/or did not include a comparison controllable stress group, are not listed.
| Author, Year | Resilience Factor | Sex | Main Phenotypes |
|---|---|---|---|
| Baratta et al. (2018) | Behavioral control | F | Behavioral control in female rats does not mitigate stress-induced behaviors - social avoidance, enhanced freezing, impaired escape. Prefrontal regulation of dorsal raphe serotonin is absent. |
| Baratta et al. (2019) | Behavioral control | F | M | Unlike males, behavioral control in female rats does not buffer against outcomes of future adverse events. Behavioral control in males, but not females, induces pathway-specific spine changes in dorsal raphe-projecting prefrontal neurons. |
| Carrier and Kabbaj (2013) | Ketamine | F | M | Females respond to a lower dose of ketamine in the forced swim test than male rats, an effect that is eliminated following ovariectomy and restored following estrogen and progesterone supplement. |
| Dolzani et al. (2018) | Ketamine | F | In female rats, ketamine produces persistent changes in prefrontal-to-dorsal raphe circuit activity such that the serotonergic response to and behavioral outcome of future stressors are blunted. |
| Dossat et al. (2018) | Ketamine | F | M | Proestrus female mice exhibit behavioral sensitivity to doses of ketamine that are ineffective in males and diestrus females. |
| Franceschelli et al. (2015) | Ketamine | F | M | Female mice displayed differential sensitivity to the rapid (30 min) and sustained (24 hr) effects of low dose ketamine in the forced swim test compared to males. |
| Goodwill et al. (2019) | Ketamine | F | M | Female, but not male, mice exposed to early life stress exhibit decreased grooming and elevated resting behavior in adulthood. Both phenotypes are reversed following acute ketamine administration. |
| Hare et al. (2019) | Ketamine | F | M | The rapid-acting effects of ketamine on novelty suppressed feeding and forced swim immobility require activity in Drd1-expressing pyramidal cells in the mouse medial prefrontal cortex. |
| Newman et al. (2019) | Ketamine | F | Ketamine reverses chronic defeat-induced social avoidance in female mice 24 hr after administration. |
| Phoumthipphavong et al. (2016) | Ketamine | F | M | A single subanesthetic dose of ketamine enhances dendritic spine formation that persists for up to two weeks in the medial frontal cortex of female and male mice. |
| Thelen et al. (2019) | Ketamine | F | M | A single subanesthetic dose of ketamine induces synaptic plasticity-related markers and sustained dendritic spine formation in the medial prefrontal cortex of male, but not female, mice. |
| Sarkar and Kabbaj (2016) | Ketamine | F | M | Chronic social isolation reduces medial prefrontal cortex spine density in both female and male rats. Ketamine rapidly reverses this effect in males but not females. |
| Bouchet et al. (2017) | Exercise | F | M | Phase of estrous cycle, but not exercise condition, blocks cued fear renewal in rats. Proestrus and estrus, but not metestrus and diestrus, during extinction training protects against later fear renewal. |
| Dishman et al. (1997) | Exercise | F | Chronic wheel running in female rats enhances shuttle box performance and reduces amygdala serotonin content following uncontrollable stress. |
| Jones et al. (2016) | Exercise | F | Brief exposure to wheel running attenuates restraint-induced corticosterone in ovariectomized female rats only if estrogen is replaced. |
| Kannangara et al. (2011) | Exercise | F | Wheel running in aged (17–18 months old) female mice reduces corticosterone levels in socially housed subjects and promotes hippocampal cell proliferation independent of housing condition. |
| Lalanza et al., (2015) | Exercise | F | M | Long-term treadmill exercise enhances shuttle box avoidance/escape performance in female and male rats. In females, prior exercise did not modify the endocrine response to shuttle box testing. |
| Tanner et al. (2019) | Exercise | F | Six weeks of wheel running in female rats buffers against behavioral sequelae of uncontrollable stress. |
| Robinson et al. (2019) | Exercise | F | Following stress treatment, four weeks of wheel running reduces impact of prior stress on open field, elevated plus maze, and cued fear conditioning in female rats. |
Nonetheless, activation of the PL-to-DRN pathway appears to be important for the long-term protective effects of ketamine in both males and females (Figure 1). Using an activity-dependent marking system (robust activity marking system, RAM), Dolzani et al. (2018), demonstrated that ketamine administered induces activity in PL ensembles that are later reactivated during exposure to uncontrollable stress. In addition, inhibition of the PL-DRN pathway using a Cre-inducible inhibitory designer receptor activated by designer drugs (DREADDs) elminitates the protective effects of ketamine (Dolzani et al., 2018). These results suggest that ketamine alters the PL-to-DRN pathway in such a way that future uncontrollable stressors that normally don’t activate the PL-to-DRN pathway, now do so, leading to a blunting of the DRN 5-HT and preventing stress-induced behavioral sequelae. Whether this process involves stable spine changes, and whether it differs between the sexes, is unknown (Figure 1).
Exercise-induced stress resistance
Increasing physical activity, either before or after stressor exposure, is another way to reduce stress outcomes. Recent longitudinal studies following large numbers of subjects, for example, find strong associations between exercise and mental health (Chekroud et al., 2018; Harvey et al., 2018). Both of these studies indicate that outcomes are not influenced by exercise intensity, age or sex, and both report similar optimal durations of exercise of an hour or less. In fact, Harvey et al. (2018) report that only 60 min of exercise per week is associated with lower incidence of new-onset depression. In addition to these protective effects of exercise, exercise can reduce symptoms of existing stress-related disorders. Exercise, either alone or as an adjunct to conventional treatment strategies, can reduce symptoms of depression and anxiety in both males and females (King et al., 1991; Bartholomew & Linder 1998; Broocks et al., 1998; Blumenthal et al., 1999; Goodwin, 2003; De Moor et al., 2006; Blumenthal et al., 2007; Ströhle et al., 2007; Merom et al., 2008; Smits et al., 2008; Asmundson et al., 2013; Wegner et al., 2014). Exercise is also emerging as an effective strategy to overcome drug addiction in both males and females (Zhou et al., 2016; Lynch et al., 2017). Some important themes emerging from these clinical studies are that maintaining regular exercise seems to be more effective than exercising at a high intensity, and exercise benefits both sexes.
Rodent models provide a useful tool to investigate features of, and mechanisms underlying, exercise-induced stress resilience (Greenwood, 2019b). Rats allowed voluntary access to running wheels demonstrate resilience against a variety of stressors, such as morphine withdrawal (Miladi-Gorji et al., 2012), maternal separation (Masrour et al., 2018), forced swimming (Duman et al., 2008), olfactory bulbectomy (Van Hoomissen et al., 2011), chronic mild stress (Solberg et al., 1999), and inescapable stress (Dishman et al., 1997c; Greenwood et al., 2003; Greenwood et al., 2007a). It is this latter stressor, inescapable stress, with which exercise-induced stress resilience has been most well characterized (Greenwood & Fleshner, 2008; Greenwood & Fleshner, 2011). Male rats allowed voluntary access to running wheels for six weeks prior to exposure to inescapable tail shock are protected from the shuttle box escape deficit (Greenwood et al., 2003), exaggerated freezing (Greenwood et al., 2003), social avoidance (Greenwood et al., 2012a), circadian disruption (Thompson et al., 2016), amnesia (Fleshner et al., 2014), and potentiation of the rewarding effects of morphine (Rozeske et al., 2011), typically produced by exposure to inescapable stress in sedentary rats. Although forced treadmill training generally fails to produce stress resilience, stress resilience can be produced by forced wheel running of a pattern similar to voluntary running (Greenwood et al., 2013). In humans, the intensity of exercise is not a key factor in maintaining mental health. Similarly in rats, the protective effects of wheel running against inescapable stress are seldom related to running distance (Greenwood et al., 2003; Greenwood et al., 2013). Rather, it is the duration of wheel access that appears to be a critical factor. In male rats, six weeks of wheel running enables stress resilience, but three weeks is insufficient (Greenwood et al., 2005a; Greenwood et al., 2005b). Stress resilience produced by six weeks of wheel running is not permanent, but does persist about 15 days following forced cessation of exercise (Greenwood et al., 2012a).
Although stress resilience produced by exercise has been well characterized, the above studies focus on the effects of exercise in male rodents. Much less is known about exercise-induced stress resilience in females. Wheel running can decrease vulnerability to substance use disorders in both sexes (Zlebnik & Carroll, 2015; Lynch et al., 2017), with exercise appearing to benefit females more than males (Lynch et al., 2017). Similarly, four weeks of wheel running has been reported to reverse anxiety-like behavior following exposure to a complex stressor in female rats (Robinson et al., 2019), but another study reported that wheel running reverses depression-like behavior produced by perinatal ethanol exposure in males only (Brocardo et al., 2012). These studies provide evidence that the stress-protective effects of exercise may be sex divergent.
One component of stress resilience is protection from fear relapse following fear extinction. In male rats, an acute bout of wheel running during (Mika et al., 2015) or immediately following (Siette et al., 2014; Tanner et al., 2018) fear extinction can enhance fear extinction retrieval and reduce relapse. This beneficial effect of exercise appears to be sex dependent. An acute bout of wheel running immediately after auditory fear extinction fails to enhance extinction retrieval or reduce relapse in female rats (Bouchet et al., 2017). However, many of the rats in this experiment were in the proestrus phase of the estrous cycle during fear extinction training. Estrogen levels are high during proestrus, and estrogen is known to support strong fear extinction memory relative to females in estrous phases with low levels of estrogen, such as metestrus (Velasco et al., 2019). In fact, females in the Bouchet et al. (2017) study exposed to fear extinction training during estrous phases with high estrogen were protected from fear renewal, one form of relapse. It is possible that exercise could not further enhance fear extinction in these females already benefiting from high levels of estrogen. Similarly, Jones et al. (2016) found that 2 days of wheel running was able to attenuate stress-induced corticosterone in ovariectomized female rats only if estrogen was replaced. Running actually potentiated corticosterone increases following stress in ovariectomized females in the absence of estrogen replacement (Jones et al., 2016). Together, these data indicate that the effects of exercise in females could depend on cycling ovarian hormones.
Clearly, more work needs to be done characterizing potential sex differences in exercise-induced stress resilience in animal models. Based on the favorable results of clinical exercise studies that include female subjects, we expect that exercise can enable stress resilience in female rodents as it can in males. Because exercise-induced stress resilience has been well characterized in males using the inescapable stress model, we set out to determine if wheel running could prevent the behavioral consequences of inescapable stress in females. We found that adult, female Sprague Dawley rats allowed access to running wheels for six weeks are indeed protected from both the exaggerated fear and reduction in JSE produced by IS (Tanner et al., 2019). Although males were not included in this experiment, the magnitude of the protective effect produced by wheel running in females is similar to that of males. Exposure to inescapable stress produces a large (between 170 – 250% above control levels) increase in shock-elicited freezing in both males and females (Greenwood et al., 2013; Baratta et al., 2018; Tanner et al., 2019). In contrast, levels of shock-elicited freezing in male and female runners exposed to inescapable stress have been reported to be 97.3 +/− 19.7 % (Greenwood et al., 2013) and 94.2 +/− 15.7 % (Tanner et al., 2019) of the freezing levels observed in non-stressed male and female runners, respectively (t-test between males and females: p > 0.05). Similarly, exposure to inescapable stress reduces exploration during a 3 min JSE test to approximately 75% of the levels displayed by non-stressed male and female rats (Baratta et al., 2018; Greenwood et al., 2012a; Tanner et al., 2019), but exercise maintains social exploration after stress to 113.7 +/− 5.6 % (Greenwood et al., 2012a) and 92.3 +/− 8.9 % (Tanner et al., 2019) of non-stressed male and female runners, respectively (t-test between males and females: p > 0.05).
As in prior work in males, the distance run in females did not influence the strength of the stress-protective effect of exercise in females, despite the fact that female rats run about twice the nighty distance as males (Tanner et al., 2019). Since the duration of wheel running is an important factor in determining exercise-induced stress resilience in males, it is critical to determine if similar durations of wheel running are required to enable stress resilience in females. In male rats, six weeks of wheel running prevents and reverses the behavioral consequences of inescapable stress, while three weeks of running is unable to produce these stress resilience effects (Greenwood et al., 2005a; Greenwood et al., 2007a). It is unknown if three weeks of wheel running is sufficient to protect females from the negative outcomes of inescapable stress. Therefore, we sought to determine the effect of three weeks of prior voluntary wheel running on the behavioral outcomes of inescapable stress in females. Adult, female Sprague Dawley rats were randomly assigned to either voluntary wheel running (“run”) or sedentary conditions. Run rats were given access to unlocked running wheels for three weeks, while the wheels in cages of sedentary rats remained locked. Following three weeks of running or sedentary conditions, rats were either left undisturbed in their home cages (HC) or exposed to IS as in our prior work (Tanner et al., 2019). Twenty-four hours after stress, all rats (n = 8/group) were tested for JSE and shock-elicited freezing, as previously described (Tanner et al., 2019). Run rats not exposed to stress were not included in the study, because we already know that wheel running does not alter social exploration or freezing behavior in non-stressed females (Tanner et al., 2019). All experimental procedures were approved by the University of Colorado Denver Institutional Animal Care and Use Committee and raw data are available upon request.
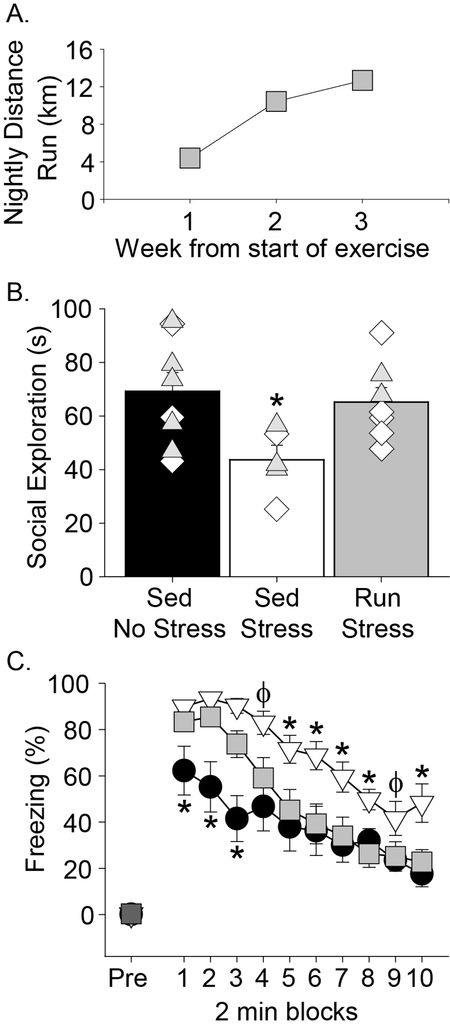
Results of the experiment are shown in Figure 2. Sedentary (179.6 ± 3.4g) and run (178.5 ± 3.2g) rats weighed similar amounts prior to the start of wheel running, but run rats gained less weight than sedentary rats over the three weeks of exercise (exercise by time interaction: F(3, 42) = 8.5; p = 0.0002; data not shown). The average daily running distance increased over the 3 weeks (F(7, 20) = 17; p < 0.0001; Figure 2A). A technical malfunction resulted in the loss of four video recordings of social exploration, thus reducing group sizes used for social exploration. IS reduced social exploration in sedentary females (n = 5), whereas the levels of social exploration in IS female runners (n = 7) were indistinguishable from those of HC females (n = 8; F(2, 17) = 4; p < 0.05; Figure 2B). Similar results were observed for shock-elicited freezing (Figure 2C). Freezing prior to the two foot shocks was minimal and did not differ between groups (Figure 2C, “pre”). Freezing behavior increased in all groups following the two foot shocks, and then subsequently decreased over the 20-minute scoring session (main effect of time: F(9, 189) = 54.3; p < 0.0001). Repeated measures ANOVA revealed an interaction between group and time (F(18, 189) = 2.7; p < 0.001), such that sedentary IS rats displayed impaired within-session extinction compared to rats in other groups (Figure 2C). As in our prior work (Tanner et al., 2019), neither distance run nor phase of estrous cycle during stress or testing (shown for JSE in Figure 2B) impacted the behavioral outcomes, although the experiment was not adequately powered to determine whether the effects of exercise depend on estrous phase. These data indicate that a shorter duration of voluntary exercise can enable stress resilience in females than in males. Thus, exercise seems to be a highly effective stress resilience factor in females. These results justify more intensive investigations into sex-dependent mechanisms of exercise-induced stress resilience.
Figure 2.
Three weeks of wheel running enables stress resilience in females. (A) Distance run over the duration of the 3 week experiment. (B) Time spent interacting with juvenile conspecific during a 3 minute juvenile social exploration test. Diamonds represent individual rats in metestrous or diestrus at the time of behavioral testing. Triangles represent individual rats in proestrus or estrus at the time of behavioral testing. (C) Levels of freezing prior to (pre) or following 2 foot shocks. Data represent group means ± SEM. Sed, sedentary; Run, voluntary running; HC, Home cage controls; IS, inescapable stress. * p < 0.05 relative to all other groups; ϕ p < 0.05 relative to Sed HC only.
Exercise impacts a variety of nervous system factors and processes that could contribute to stress resilience (Morgan et al., 2015). Based on the observation that exercise elicits stress resilience in females faster than in males, it is possible that males and females differentially engage stress resilience mechanisms. Supporting the idea that some of the effects of exercise may be bigger in females than in males are emerging data suggesting the cognitive benefits of exercise are also more powerful in females (Barha et al., 2017a; Barha et al., 2017b; Barha & Liu-Ambrose, 2018). However, the majority of mechanistic studies have so far focused on males.
In males, exercise impacts monoaminergic systems (Dishman, 1997a; Dishman, 1997b; Greenwood & Fleshner, 2011; Nicastro & Greenwood, 2016; Greenwood, 2019a), as well as growth factors and other mediators of neural plasticity including BDNF, galanin and mTOR (Cotman et al., 2007; Sciolino & Holmes, 2012; Lloyd et al., 2017; El-Sayes et al., 2019). Monoaminergic systems appear to be sensitive to exercise in females (Dishman, 1997b), but much remains unknown. Exercise increases BDNF (Neeper et al., 1995) and neurogenesis (van Praag et al., 1999; Triviño-Paredes et al., 2016; Kim et al., 2019) in both male and female rodents; both of which have been implicated in stress resilience (Duman, 2009; Levone et al., 2015). In female rats, the increase in BNDF produced by exercise is dependent on estrogen (Berchtold et al., 2001). Exercise also attenuates the hypothalamic-pituitary-adrenal response to acute and chronic stressors in males (Droste et al., 2003; Campeau et al., 2010; Sasse et al., 2013); an effect that seems to vary with estrous phase in females (Jones et al., 2016).
There are multiple mechanisms by which exercise could prevent specifically the behavioral consequences of inescapable stress (Christianson & Greenwood, 2014; Nicastro & Greenwood, 2016). Neither attenuation of the hypothalamic-pituitary-adrenal axis response to inescapable stress, nor hippocampal BDNF (Greenwood et al., 2007b) appear to be necessary for the ability of wheel running to prevent inescapable stress-induced behaviors. Because of the critical role of DRN 5-HT neurons in mediating the behavioral effects of inescapable stress, much research has focused on the effects of exercise on central serotonergic systems (Greenwood & Fleshner, 2011). In males, six weeks of wheel running attenuates activity of DRN 5-HT neurons as measured with c-Fos and increases mRNA coding for the 5-HT1A inhibitory autoreceptor (Greenwood et al., 2003; Greenwood et al., 2005b; Loughridge et al., 2013), which could enhance 5-HT1A-mediated autoinhibition of DRN 5-HT neurons during stress. Consistent with the c-Fos data, six weeks of wheel running also prevents the IS-induced potentiation of 5-HT efflux in the dorsal striatum (Clark et al., 2015), where exaggerated 5-HT release contributes to the IS-induced shuttle box escape deficit (Strong et al., 2011). 5-HT adaptations to exercise are not limited to DRN 5-HT neurons. Six weeks of wheel running also reduces the expression of 5-HT2C receptors in the dorsal striatum and amygdala (Greenwood et al., 2012b). See Figure 1 for a summary of the circuit on which exercise-induced plasticity is proposed to produce stress resilience in males. If these adaptations are also important for enabling stress resilience in females, then we would expect these exercise adaptations to occur more rapidly in the brains of females. Interestingly, and in stark contrast to ketamine and escapable stress which rely on prefrontal mechanisms, exercise appears to constrain the DRN 5-HT response to stress through mechanisms other than prefrontal cortex inhibition (Greenwood et al., 2013; Christianson & Greenwood, 2014). These findings suggest that the circuits contributing to stress resilience differ between exercise and other resilience factors, namely ketamine and behavioral control.
Conclusions
The ability of experiential factors such as coping with stress and exercise to protect against stress-induced outcomes differs between the sexes. Recent work from stressor controllability studies shows that unlike males, the acute and long-term benefits afforded by behavioral control is absent in females. These findings suggest that the neural processing of control differs in females and the lack of engagement and structural plasticity within the PL-to-DRN pathway represents a potential mechanism. Since males and females exhibit different spine changes in this pathway following behavioral control, future work should employ novel circuit-targeting strategies for labeling and manipulating spines formed in response to an experience of control. The emergence of optical tools like AS-PaRAC1, which has been used to establish a causal link between ketamine-induced spine changes and stress protection (Moda-Sava et al., 2019), will drive future mechanistic studies that explore the causal relationship between experiential factors and the stable circuit changes that produce their protective effects, and whether or not these changes are shared between factors. Identifying common circuit endophenotypes between resilience factors enhances the possibility of translating basic research findings into clinical interventions.
Women are more susceptible to stress-related disorders and factors that change how an individual experiences stress either before, during, or after the event can be utilized to develop strategies to help reduce the incidence of these disorders. Recent preclinical findings from ketamine have revealed that experiential resilience factors sexually converge at the level of behavioral outcome, yet diverge at a neural circuit level. Not all paths to resilience are concordant between the sexes nor do all resilience factors operate equally at a circuit level. Identifying these mechanistic differences represents an important step in developing sex-specific treatments for stress-linked disorders.
Acknowledgements
Supported by National Institutes of Health grants R15 MH114025 (BNG), R01 MH050479 (MVB), R21 MH106817 (MVB), and a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (MVB). Key features of the graphical abstract and figure one designed with Bio-render
Abbreviations
- 5-HT
serotonin
- AS-PaRac1
activated synapse-targeting photoactivatable Rac1
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- DREADD
designer receptors exclusively activated by designer drugs
- DRN
dorsal raphe nucleus
- ERK
extracellular signal-regulated kinase
- ES
escapable stress
- HC
home cage control
- IEG
immediate early gene
- IS
inescapable stress
- JSE
juvenile social exploration
- mPFC
medial prefrontal cortex
- mTOR
mammalian target of rapamycin
- NMDAR
N-methyl-D-aspartate receptor
- PL
prelimbic cortex
- PTSD
posttraumatic stress disorder
- RAM
robust activity marking system
Footnotes
Conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, de Graaf RA, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G & Mason GF (2018) The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology, 43, 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR & Maier SF (1998) Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res, 812, 113–120. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR & Maier SF (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci, 8, 365–371. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR & Maier SF (2006) Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: Role of the ventral medial prefrontal cortex. J. Neurosci, 26, 13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR & Maier SF (2010) Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience, 165, 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, Sperr K, Ciancio N, Watkins LR & Maier SF (2016) Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J. Neurosci, 36, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J (1975) Rodent sex differences in emotional and related behavior. Beh. Biol, 14, 451–479. [DOI] [PubMed] [Google Scholar]
- Armario A, Gil M, Marti J, Pol O & Balasch J (1991) Influence of various acute stressors on the activity of adult male rats in a holeboard and in the forced swim test. Pharmacol. Biochem. Behav, 39, 373–377. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci, 10, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Fetzner MG, DeBoer LB, Powers MB, Otto MW & Smits JA (2013) Let’s get physical: A contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depress. Anxiety, 30, 362–373. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P, Kavalali ET & Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature, 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JP & Berenbaum H (2007) Emotional approach and problem-focused coping: A comparison of potentially adaptive strategies. Cogn. Emot, 21, 95–118. [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR & Maier SF (2007) Controllable versus uncontrollable stressors bidirectionally modulate conditioned but not innate fear. Neuroscience, 146, 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR & Maier SF (2008) Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn. Mem, 15, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR & Maier SF (2009) Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur. J. Neurosci, 30, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV & Maier SF (2017) New tools for understanding coping and resilience. Neurosci. Lett, 6, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, Watkins LR & Maier SF (2018) Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur. J. Neurosci, 8, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Gruene TM, Dolzani SD, Chun LE, Maier SF & Shansky RM (2019) Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct. Funct, 224, 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Davis JC, Falck RS, Nagamatsu LS & Liu-Ambrose T (2017a) Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol, 46, 71–85. [DOI] [PubMed] [Google Scholar]
- Barha CK, Falck RS, Davis JC, Nagamatsu LS & Liu-Ambrose T (2017b) Sex differences in aerobic exercise efficacy to improve cognition: A systematic review and meta-analysis of studies in older rodents. Front. Neuroendocrinol, 46, 86–105. [DOI] [PubMed] [Google Scholar]
- Barha CK & Liu-Ambrose T (2018) Exercise and the Aging Brain: Considerations for sex differences. Brain Plast, 4, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew JB & Linder DE (1998) State anxiety following resistance exercise: The role of gender and exercise intensity. J. Behav. Med, 2, 205–219. [DOI] [PubMed] [Google Scholar]
- Beery AK & Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev, 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA & Cotman CW (2001) Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur. J. Neurosci, 14, 1992–2002. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS & Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry, 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Billings AG & Moos RH (1981) The role of coping responses and social resources in attenuating the stress of life events. J. Behav. Med, 4, 139–157. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR & Maier SF (2003) Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology, 28, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM & Krishnan KR (1999) Effects of exercise training on older patients with major depression. Arch. Intern. Med, 159, 2349–2356. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A & Sherwood A (2007) Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med, 69, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Smith PJ & Hoffman BM (2012) Is exercise a viable treatment for depression? ACSMs Health Fit. J, 16, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN & Toedebusch RG (2017) Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev, 97, 1351–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet CA, Lloyd BA, Loetz EC, Farmer CE, Ostrovskyy M, Haddad N, Foright RM & Greenwood BN (2017) Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn. Mem, 24, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N (2009) The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse, 10, 198–210. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J & Christie BR (2012) Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology, 62, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Hillmer-Vogel U & Rüther E (1998) Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am. J. Psychiatry, 5, 603–609. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, Babb JA, Greenwood BN, Fleshner M & Day HE (2010) Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J. Neuroendocrinol, 22, 872–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N & Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology, 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Challis C, Beck SG & Berton O (2014) Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front. Behav. Neurosci, 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH & Chekroud AM (2018) Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry, 5, 739–746. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, Watkins LR & Maier SF (2014) Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front. Behav. Neurosci, 8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP & Greenwood BN (2014) Stress-protective neural circuits: Not all roads lead through the prefrontal cortex. Stress, 17, 1–12. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Amat J, McConnell SO, Ghasem PR, Greenwood BN, Maier SF & Fleshner M (2015) Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLoS One, 10:e0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA & Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature, 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC & Christie LA (2007) Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci, 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Coyle CM & Laws KR (2015) The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum. Psychopharmacol, 30, 152–163. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, & Shors TJ (2008) Females do not express learned helplessness like males do. Neuropsychopharmacology, 33, 1559–1569. [DOI] [PubMed] [Google Scholar]
- De Moor MH, Beem AL, Stubbe JH, Boomsma DI & De Geus EJ (2006) Regular exercise, anxiety, depression and personality: A population-based study. Prev. Med, 4, 273–279. [DOI] [PubMed] [Google Scholar]
- Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, Dutheil S, Dwyer JM, Taylor SR, Picciotto MR & Duman RS (2019) Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am. J. Psychiatry, 176, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M & Hay EL (2010) Risk and resilience factors in coping with daily stress in adulthood: The role of age, self-concept incoherence, and personal control. Dev. Psychol, 46, 1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra MT & Homan AC (2016) Engaging in rather than disengaging from stress: Effective coping and perceived control. Front. Psychol, 7:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK (1997a) Brain monoamines, exercise, and behavioral stress: Animal models. Med. Sci. Sports Exerc, 29, 63–74. [DOI] [PubMed] [Google Scholar]
- Dishman RK (1997b) The norepinephrine hypothesis, In Morgan WP (ed), Series in health psychology and behavioral medicine. Physical activity and mental health, Taylor & Francis, Philadelphia, pp. 199–212. [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH & Meyerhoff JL (1997c) Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res. Bull, 42, 399–406. [DOI] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sørensen AT, Watkins LR, Lin Y & Maier SF (2018) Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro., 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Wright KN, Strong CE & Kabbaj M (2018) Behavioral and biochemical sensitivity to low doses of ketamine: Influence of estrous cycle in C57BL/6 mice. Neuropharmacology, 130, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst AC & Reul JM (2003) Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology, 144, 3012–3023. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS & Duman RS (2008) Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res, 1199, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS (2009) Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin. Neurosci, 11, 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G & Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med, 22, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Sanacora G & Krystal JH (2019) Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron, 102, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayes J, Harasym D, Turco CV, Locke MB & Nelson AJ (2019) Exercise-induced neuroplasticity: A mechanistic model and prospects for promoting plasticity. Neuroscientist, 25, 65–85. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D & Dickson CT (2009) Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience, 161, 359–369. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Greenwood BN & Yirmiya R (2014) Neuronal-glial mechanisms of exercise-evoked stress robustness. Curr. Top. Behav. Neurosci, 18, 1–12. [DOI] [PubMed] [Google Scholar]
- Folkman S (1984) Personal control and stress and coping processes: A theoretical analysis. J. Pers. Soc. Psychol, 46, 839–852. [DOI] [PubMed] [Google Scholar]
- Fournier M, d’Arripe-Longueville F & Radel R (2017) Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology, 77, 275–283. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C & Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience, 290, 49–60. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR & Maier SF (2007) Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun, 21, 47–59. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew S, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH & Fava M (2019) Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J. Psychiatr. Res, 110, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ (2017) Depression is the leading cause of disability around the world. JAMA, 317:1517. [DOI] [PubMed] [Google Scholar]
- Girgenti MJ, Ghosal S, LoPresto D, Taylor JR & Duman RS (2017) Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol. Dis, 100, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T & Bath KG (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, 44, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD (2003) Association between physical activity and mental disorders among adults in the United States. Prev. Med, 6, 698–703. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR & Maier SF (1999) Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res, 826, 35–43. [DOI] [PubMed] [Google Scholar]
- Greenwood BN (2019a) The role of dopamine in overcoming aversion with exercise. Brain Res, 1713, 102–108. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF & Fleshner M (2003) Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. J Neurosci, 23, 2889–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF & Fleshner M (2005a) The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res, 1033, 164–178. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S & Fleshner M (2005b) Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol. Psychiatry, 57, 559–568. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA & Fleshner M (2007a) Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav. Neurosci, 121, 992–1000. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS & Fleshner M (2007b) Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience, 144, 1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN & Fleshner M (2008) Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med, 10, 81–98. [DOI] [PubMed] [Google Scholar]
- Greenwood BN & Fleshner M (2011) Exercise, stress resistance, and central serotonergic systems. Exerc. Sport Sci. Rev, 39, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP & Fleshner M (2012a) The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav. Brain Res, 233, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Loughridge AB, Day HE, Clark PJ, Mika A, Hellwinkel JE, Spence KG & Fleshner M (2012b) 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PloS One, 7:e46118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC & Fleshner M (2013) Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur. J. Neurosci, 37, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN & Fleshner M (2019b) Voluntary wheel running: A useful rodent model for investigating mechanisms of stress robustness and exercise motivation. Curr. Opin. Behav. Sci, 28, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD & Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. eLife., 4:e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Richards CD, Székely AD & Sharp T (1998) An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience, 87, 95–108. [DOI] [PubMed] [Google Scholar]
- Hamilton S & Fagot BI (1988) Chronic stress and coping styles: A comparison of male and female undergraduates. J. Pers. Soc. Psychol, 55, 819–823. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ & Duman RS (2019) Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun, 10:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A & Hotopf M (2018) Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am. J. Psychiatry, 175, 28–36. [DOI] [PubMed] [Google Scholar]
- Heck AL & Handa RJ (2018) Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology, 44, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H & Watson SJ. (1999) The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol, 11, 121–128. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Tylee D, Christianson JP, Kubala KH, Govindarajan ST, O’Neill WE, Becoats K, Watkins L & Maier SF (2012) Active behavioral coping alters the behavioral but not the endocrine response to stress. Psychoneuroendocrinology, 12, 1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Ménard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ & Russo SJ (2015) Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci, 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP & Sesack SR (2004) Prefrontal cortical projections to the rat dorsal raphe nucleus: Ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J. Comp. Neurol, 468, 518–529. [DOI] [PubMed] [Google Scholar]
- Jenkins JA, Williams P, Kramer GL, Davis LL & Petty F (2001) The influence of gender and the estrous cycle on learned helplessness in the rat. Biol. Psychol, 58, 147–158. [DOI] [PubMed] [Google Scholar]
- Jones AB, Gupton R & Curtis KS (2016) Estrogen and voluntary exercise interact to attenuate stress-induced corticosterone release but not anxiety-like behaviors in female rats. Behav. Brain Res, 311, 279–286. [DOI] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR & van Praag H (2011) Running reduces stress and enhances cell genesis in aged mice. Neurobiol. Aging, 32, 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DL, McLaren DG, Mathy RM & Nitschke JB (2012) Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front. Psychol, 3:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liu QF, Urnuhsaikhan E, Jeong HJ, Jeon MY & Jeon S (2019) Moderate-intensity exercise induces neurogenesis and improves cognition in old mice by upregulating hippocampal hippocalcin, otub1, and spectrin-α. Mol. Neurobiol, 56, 3069–3078. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Taylor CB, Kraemer HC, and DeBusk RF (1991) Group- vs home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA, 11, 1535–1542. [PubMed] [Google Scholar]
- Kohn PM, Hay BD & Legere JJ (1994) Hassles, coping styles, and negative wellbeing. Pers. Individ. Dif, 17, 169–179. [Google Scholar]
- Kubala KH, Christianson JP, Kaufman RD, Watkins LR & Maier SF (2012) Short- and long-term consequences of stressor controllability in adolescent rats. Behav. Brain Res, 234, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM & Kirschbaum C (2005) Sex differences in HPA axis responses to stress: A review. Biol. Psychol, 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S & Loo CK (2014) Pilot dose-response trial of i.v. ketamine in treatment-resistant depression. World J. Biol. Psychiatry, 15, 579–584. [DOI] [PubMed] [Google Scholar]
- Lalanza JF, Sanchez-Roige S, Cigarroa I, Gagliano H, Fuentes S, Armario A, Capdevila L & Escorihuela RM. (2015) Long-term moderate treadmill exercise promotes stress-coping strategies in male and female rats. Sci. Rep, 5:16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Kadriu B & Zarate CA Jr. (2017) Ketamine and beyond: Investigations into the potential of glutamatergic agents to treat depression. Drugs, 4, 381–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cryan JF & O’Leary OF (2014) Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress, 1, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G & Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X-Y, Aghajanian G & Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry, 69, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Fuchikami M, Dwyer JM, Lepack AE, Duman RS & Aghajanian GK (2013) GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology, 38, 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST & Greenwood BN (2017) Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav. Brain Res, 323, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ & Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience, 117, 697–706. [DOI] [PubMed] [Google Scholar]
- Loughridge AB, Greenwood BN, Day HE, McQueen MB & Fleshner M (2013) Microarray analyses reveal novel targets of exercise-induced stress resistance in the dorsal raphe nucleus. Front. Behav. Neurosci, 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Robinson AM, Abel J & Smith MA (2017) Exercise as a prevention for substance use disorder: A review of sex differences and neurobiological mechanisms. Curr. Addict. Rep, 4, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G & Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry, 63, 349–352. [DOI] [PubMed] [Google Scholar]
- Maier SF & Seligman MEP (1976) Learned helplessness: Theory and evidence. J. Exp. Psychol. Gen, 105, 3–46. [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM & Kalin NH (1986) Stressor controllability and the pituitary-adrenal system. Behav. Neurosci, 100, 669–674. [DOI] [PubMed] [Google Scholar]
- Maier SF & Watkins LR (1998) Stressor controllability, anxiety, and serotonin. Cognit. Ther. Res, 22, 595–613. [Google Scholar]
- Maier SF & Watkins LR (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev, 29, 829–841. [DOI] [PubMed] [Google Scholar]
- Malenka RC & Nicoll RA (1993) NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci, 16, 521–527. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR & Maier SF (1998) Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res., 783, 115–120. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Szot P, Baratta MV, Bland ST, White SS, Maier SF & Neumaier JF (2009) Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res, 1285, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merom D, Phongsavan P, Wagner R, Chey T, Marnane C, Steel Z, Silove D & Bauman A (2008) Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders - a pilot group randomized trial. J. Anxiety Disord, 6, 959–968. [DOI] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, Day HE, Campeau S, Fleshner M & Greenwood BN (2015) Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol. Learn. Mem, 125, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miladi-Gorji H, Rashidy-Pour A & Fathollahi Y (2012) Anxiety profile in morphine-dependent and withdrawn rats: Effect of voluntary exercise. Physiol. Behav, 105, 195–202. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T & Kimura F (2006) Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur. J. Neurosci, 24, 3245–3254. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Corrigan F & Baune BT (2015) Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry, 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM (2013) NMDA receptors and memory encoding. Neuropharmacology, 74, 32–40. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS & Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-Site randomized controlled trial. Am. J. Psychiatry, 170, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J & Cotman C (1995) Exercise and brain neurotrophins. Nature, 373, 109. [DOI] [PubMed] [Google Scholar]
- Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF & Miczek KA (2019) Fighting Females: Neural and behavioral consequences of social defeat stress in female mice. Biol. Psychiatry, 86, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB & APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry, 172, 950–966. [DOI] [PubMed] [Google Scholar]
- Nicastro TM & Greenwood BN (2016) Central monoaminergic systems are a site of convergence of signals conveying the experience of exercise to brain circuits involved in cognition and emotional behavior. Curr. Zool, 62, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare JK, Ade KK, Sukharnikova T, Van Hooser SD, Palmeri ML, Yin HH & Calakos N (2016) Pathway-specific striatal substrates for habitual behavior. Neuron, 89, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI & Schooler C (1978) The structure of coping. J. Health Soc. Behav, 19, 2–21. [PubMed] [Google Scholar]
- Phoumthipphavong V, Barthas F, Hassett S & Kwan AC (2016) Longitudinal effects of ketamine on dendritic Architecture in vivo in the mouse medial frontal cortex. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS & Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry, 66, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryazhnikov E, Mugantseva E, Casarotto P, Kolikova J, Fred SM, Toptunov D, Afzalov R, Hotulainen P, Voikar V, Terry-Lorenzo R, Engel S, Kirov S, Castren E & Khiroug L (2018) Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine. Sci. Rep, 8:6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Christ CC, Cahill MM, Aldrich SJ & Taylor-Yeremeeva E (2019) Voluntary exercise or systemic propranolol ameliorates stress-related maladaptive behaviors in female rats. Physiol. Behav, 198, 120–133. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Greenwood BN, Fleshner M, Watkins LR & Maier SF (2011) Voluntary wheel running produces resistance to inescapable stress-induced potentiation of morphine conditioned place preference. Behav. Brain Res, 219, 378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy RM, Chen Y, Milenkovic M & Ramsey AJ (2015) Differential effects of NMDA receptor antagonism on spine density. Synapse, 69, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Permoda-Osip A & Bartkowska-Sniatkowska A (2017) Ketamine augmentation rapidly improves depression scores in inpatients with treatment-resistant bipolar depression. Int. J. Psychiatry Clin. Pract, 21, 99–103. [DOI] [PubMed] [Google Scholar]
- Saland SK, Duclot F & Kabbaj M (2017) Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr. Opin. Behav. Sci, 14, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A & Kabbaj M (2016) Sex differences in effects of ketamine on behavior, spine density and synaptic proteins in socially isolated rats. Biol. Psychiatry, 80, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse SK, Nyhuis TJ, Masini CV, Day HE & Campeau S (2013) Central gene expression changes associated with enhanced neuroendocrine and autonomic response habituation to repeated noise stress after voluntary wheel running in rats. Front. Physiol, 4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L & Wolf OT (2011) Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav. Brain Res, 2, 321–328. [DOI] [PubMed] [Google Scholar]
- Sciolino NR & Holmes PV (2012) Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev, 36, 1965–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Reichelt AC & Westbrook RF (2014) A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learn. Mem, 21, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E & Otto MW (2008) Reducing anxiety sensitivity with exercise. Depress. Anxiety, 8, 689–699. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Horton TH & Turek FW (1999) Circadian rhythms and depression: Effect of exercise in an animal model. Am. J. Physiol, 276, R152–161. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J & Palenicek T (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro. Endocrinol. Lett, 34, 287–293. [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC & Mokler DJ (2011) A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology, 61, 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V & Silove D (2014) The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol, 43, 476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Höfler M, Pfister H, Müller AG, Hoyer J, Wittchen HU & Lieb R (2007) Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med, 11, 1657–1666. [DOI] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M & Greenwood BN (2011) 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience, 197, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamres LK, Janicki D & Helgeson VS (2002) Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Pers. Soc. Psychol. Rev, 6, 2–30. [Google Scholar]
- Tanner MK, Hake HS, Bouchet CA & Greenwood BN (2018) Running from fear: Exercise modulation of fear extinction. Neurobiol. Learn. Mem, 151, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner MK, Fallon IP, Baratta MV & Greenwood BN (2019) Voluntary exercise enables stress resistance in females. Behav. Brain Res, 369:111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM & Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci, 5, 173–183. [DOI] [PubMed] [Google Scholar]
- Thompson RS, Christianson JP, Maslanik TM, Maier SF, Greenwood BN & Fleshner M (2013) Effects of stressor controllability on diurnal physiological rhythms. Physiol. Behav, 112–113, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RS, Roller R, Greenwood BN & Fleshner M (2016) Wheel running improves REM sleep and attenuates stress-induced flattening of diurnal rhythms in F344 rats. Stress, 19, 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triviño-Paredes J, Patten AR, Gil-Mohapel J & Christie BR (2016) The effects of hormones and physical exercise on hippocampal structural plasticity. Front. Neuroendocrinol, 41, 23–43. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen J, Kunrath J, Dentlinger R, Lafrenz A, Krause M & Azar A (2011) Cognitive and locomotor/exploratory behavior after chronic exercise in the olfactory bulbectomy animal model of depression. Behav. Brain Res, 222, 106–116. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G & Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci, 2, 266–270. [DOI] [PubMed] [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF & Cooper DC (2012) Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J. Neurosci, 32, 12848–12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Székely AD, Csillag A, Sharp T & Hajós M (2001) Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience, 106, 783–792. [DOI] [PubMed] [Google Scholar]
- Velasco ER, Florido A, Milad MR & Andero R (2019) Sex differences in fear extinction. Neurosci. Biobehav. Rev, 103, 81–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O & Budde H (2014) Effects of exercise on anxiety and depression disorders: review of meta- analyses and neurobiological mechanisms. CNS Neurol. Disord. Drug Targets, 13, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Weiss JM (1968) Effects of coping responses on stress. J. Comp. Physiol. Psychol, 65, 251–260. [DOI] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE & Dolan RJ (2006) Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J. Neurosci, 26, 11501–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann K (2011) Biomarkers in development of psychotropic drugs. Dialogues Clin. Neurosci, 13, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL & Maier SF (1977) Transituational immunization and therapy of learned helplessness in the rat. J. Exp. Psychol.: Anim. Behav. Process, 3, 240–252. [Google Scholar]
- Woodmansee WW, Silbert LH & Maier SF (1993) Factors that modulate inescapable shock-induced reductions in daily activity in the rat. Pharmacol. Biochem. Behav, 45, 553–559. [DOI] [PubMed] [Google Scholar]
- Yi JP, Smith RE & Vitaliano PP (2005) Stress-resilience, illness, and coping: A person-focused investigation of young women athletes. J. Behav. Med, 28, 257–265. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ & Balleine BW (2005) Blockade of NMDA receptors in the dorsomedial striatum prevents action–outcome learning in instrumental conditioning. Eur. J. Neurosci, 22, 505–512. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS & Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry, 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V & Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol. Psychiatry, 71, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao M, Zhou C & Li R (2016) Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front. Neuroendocrinol, 40, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE & Carroll ME (2015) Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology, 232, 3507–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]