Abstract
Sarcopenia, which is characterized by the loss of skeletal muscle, has been reported to contribute to development of physical disabilities, various illnesses, and increasing mortality. MicroRNAs (miRNAs) are small non-coding RNAs that inhibit translation of target messenger RNAs. Previous studies have shown that miRNAs play pivotal roles in the development of sarcopenia. Therefore, this systematic review focuses on miRNAs that regulate sarcopenia.
Keywords: sarcopenia, microRNA, myotube, myocyte, myoblast, systematic review
Introduction
Sarcopenia, defined by the loss of skeletal muscle loss, contributes to developing physical disabilities, various illnesses, and increasing mortality (1, 2). MicroRNAs (miRNAs) have attracted attention as potential biomarkers and targets for specific therapies. MiRNAs are small non-coding RNAs (21–25 bases) that are not translated into proteins but inhibit the function of their target messenger RNAs (mRNAs) by destabilizing them and inhibiting their translation (3, 4). Previous studies have shown that miRNAs play pivotal roles in the development of sarcopenia (1–82). Therefore, this systematic review focuses on miRNAs that regulate sarcopenia.
Mechanism of the Development of Sarcopenia
Several factors, including chronic inflammation, increased reactive oxidative species, increased fibrosis of muscle, and increased loss of motor neurons, have been reported to contribute to development of sarcopenia by progressing muscle atrophy that results in lower muscle mass (26, 46). These factors have been reported to be tightly controlled by many signaling pathways and effector proteins, including some crosstalk with the protein synthesis pathway (32). Among these signaling pathways, transforming growth factor-β1 (TGF-β1) is considered as the main signaling molecule in the development of sarcopenia (47). TGF-β1 activates many downstream profibrotic signaling molecules, including mothers against decapentaplegic (Smad), extracellular signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and p38, which contribute to increasing transdifferentiation of myoblasts into myofibroblasts, resulting in development of muscle atrophy and fibrosis (47). Chronic inflammation has also been considered to contribute to the development of sarcopenia through the production of numerous proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β, which promote muscle catabolism (64).
Search Method
We searched for basic and clinical studies published in English in the PubMed database from 2007 to 2019. The literature search was conducted between August 3 and 13, 2019. The following medical subject headings were used: (“microrna AND sarcopenia” [Title/Abstract]), (“mirna AND sarcopenia” [Title/Abstract]), (“microrna AND frail” [Title/Abstract]), (“mirna AND frail” [Title/Abstract]), (“microrna AND frailty” [Title/Abstract]), and (“mirna AND frailty” [Title/Abstract]). The words “frailty” and “frail” were used for this review because they are involved in the condition of sarcopenia. Studies whose titles and abstracts did not meet selection criteria were excluded from this review. The remaining studies were carefully checked for eligibility for inclusion in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Figure 1; (83)]. The studies were included if (1) they reported the utility of miRNAs as potential biomarkers or targets for specific therapies of sarcopenia; and (2) they were published as full-text journal articles in English. Exclusion criteria were as follows: (1) they did not discuss specific miRNAs in sarcopenia; and (2) they included no description of sample settings. We could not perform a meta-analysis because the number of studies reporting miRNAs for sarcopenia was small, so statistical power would have been low.
Figure 1.
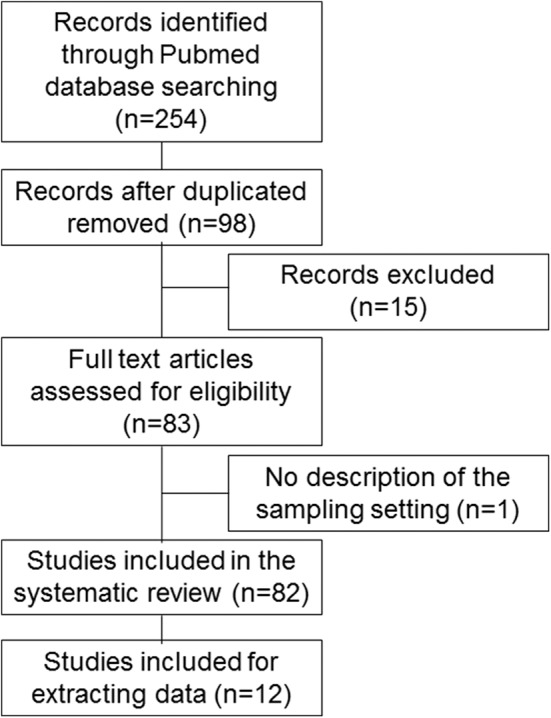
Flow diagram of this study.
Results
Search Results
A flow diagram of this study is shown in Figure 1 and Supplemental Table 1. Computer and manual searches identified 254 potentially suitable publications. After the removal of duplicates, the titles and abstracts of 98 remaining papers were screened. Of these, 15 publications were excluded because they did not describe specific miRNAs in sarcopenia, and one publication was excluded because it lacked a description of the sample setting; 82 studies were included in the final systematic review (1–82), and 12 studies were included for extracting data (1, 5, 6, 16, 28, 38, 40, 42, 60, 64, 71, 82).
MicroRNAs in Sarcopenia
Many studies investigated expression changes of miRNAs in muscles and/or blood (serum or plasma) of patients with sarcopenia (Table 1) and/or animal models of sarcopenia [Table 2; (1, 5, 16, 28, 38, 40, 42, 60, 64, 71, 82)]. Several studies investigated the effects of modulating miRNA expression on phenotypic changes using cultured muscle cells in vitro and rodent models of sarcopenia in vivo [Table 3; (1, 6, 42, 60, 71)].
Table 1.
Associations between miRNA expression levels and sarcopenia in patients.
| MiRNA | Samples | Expression change | Details | Target mRNA | References |
|---|---|---|---|---|---|
| MiRNA-10a-3p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-19a | Muscle | ↑ | No mention | PRKAA1 and PFKFB3 | (82) |
| MiRNA-21 | Serum | ↑ | The expression level was associated with self-reported exhaustion, time to walk 15 feet, and muscle weight loss | TGF-βR2 | (64) |
| MiRNA-34a | Muscle | ↑ | No mention | VEGFA | (82) |
| MiRNA-92a-3p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-185-3p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-194-3p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-203a-3p | Serum | ↑ | The expression level was associated with the psoas muscle mass index and intramuscular adipose tissue content | No mention | (1) |
| MiRNA-326 | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-424-5p | Muscle | ↑ | The expression level was associated with the result of 3-m gait speed and 6-m timed up and go test | Pol I R1A and UBTF | (16) |
| MiRNA-532-5p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-576-5p | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
| MiRNA-760 | Plasma | ↑ | The expression level was associated with muscle weight loss, grip strength, self-reported exhaustion, time to walk 15 feet, and kilocalories expended per week | No mention | (40) |
miRNA, microRNA; mRNA, messenger RNA; PFKFB3, 6-phosphofructo-2-kinase/fructose-2-,6-bisphosphatase 3; Pol I R1A, polymerase I receptor 1A; PRKAA1, protein kinase AMP-activated catalytic subunit alpha 1; Smad, mothers against decapentaplegic; TGF-βR2, transforming growth factor-β receptor 2; UBTF, upstream binding transcription factor; VEGFA, vascular endothelial growth factor A; ↑ means miRNA's upregulation in each samples.
Table 2.
Associations between miRNA expression levels and sarcopenia in rodent models.
| MiRNA | Sample | Expression change | Details | Target mRNA | References |
|---|---|---|---|---|---|
| MiRNA-1-3p | Muscle | ↑ | The expression level was associated with the soleus muscle weight/tibia length index, time to peak twitch tension, maximum tetanic contraction and half relaxation time of twitch, maximum tetanic relaxation rate, and fatigue index | TRIM63 and FBXO32 | (28) |
| MiRNA-29 | Muscle | ↑ | The expression level was associated with extensor digitorum longus and soleus muscle weight losses | B-myb, IGF-1, and p85 | (38) |
| MiRNA-29a-3p | Muscle | ↑ | The expression level was associated with the soleus muscle weight/tibia length index, time to peak twitch tension, maximum tetanic contraction and half relaxation time of twitch, maximum tetanic relaxation rate, and fatigue index | TRIM63 and FBXO32 | (28) |
| MiRNA-29b-3p | Muscle | ↓ | The expression level was associated with the soleus muscle weight/tibia length index, time to peak twitch tension, maximum tetanic contraction and half relaxation time of twitch, maximum tetanic relaxation rate, and fatigue index | TRIM63 and FBXO32 | (28) |
| MiRNA-98-5p | Muscle | ↑ | The expression level was associated with the size of muscle fibers | NGF | (5) |
| MiRNA-133a-3p | Muscle | ↑ | The expression level was associated with the soleus muscle weight/tibia length index, time to peak twitch tension, maximum tetanic contraction and half relaxation time of twitch, maximum tetanic relaxation rate, and fatigue index | TRIM63 and FBXO32 | (28) |
| MiRNA-133b-3p | Muscle | ↑ | The expression level was associated with the soleus muscle weight/tibia length index, time to peak twitch tension, maximum tetanic contraction and half relaxation time of twitch, maximum tetanic relaxation rate, and fatigue index | TRIM63 and FBXO32 | (28) |
| MiRNA-181a | Muscle | ↓ | The expression level was associated with the size of myotubes | Sirt1 | (71) |
| MiRNA-434-3p | Muscle | ↓ | No mention | Elf5A1 | (60) |
| MiRNA-455-3p | Muscle | ↓ | The expression level was associated with the size of myotubes | PITX1 and RXRB | (42) |
miRNA, microRNA; mRNA, messenger RNA; B-myb, myeloblastosis-related protein B; Elf5A1, eukaryotic translation initiation factor 5A1; Fbxo32, F-box protein 32; IGF-1, insulin-like growth factor-1; NGF, nerve growth factor; PITX1, paired-like homeodomain transcription factor 1; RXRB, retinoid X receptor-β; Sirt1, sirtuin 1; Trim63, tripartite motif containing 63 protein; ↓ means miRNA's downregulation in each samples; ↑ means miRNA's upregulation in each samples.
Table 3.
Effects of modulation of miRNA expression on frailty and sarcopenia in cells in vitro and rodent frailty and sarcopenia models in vivo.
| MiRNA | Tx | Rodent model and/or cells | Effects | Target mRNA | References |
|---|---|---|---|---|---|
| MiRNA-181a | OE | Mouse cultured myotubes of differentiated myoblasts (C2C12 cells) | Inhibition of Sirt1 induced a decrease in the myotube diameter | Sirt1 | (71) |
| MiRNA-203a-3p | OE | Human skeletal muscle cells | Inhibition of BIRC5 induced a decrease in the number of skeletal muscle cells | BIRC5 | (1) |
| MiRNA-434-3p | OE | Mice myocytes | Inhibition of Elf5A1 protected myocytes from apoptosis | EIF5A1 | (60) |
| MiRNA-455-3p | OE | Mouse cultured myotubes of differentiated myoblasts (C2C12 cells) | Inhibition of PITX1 and RXRB induced larger diameters of C2C12 myotubes | PITX1 and RXRB | (42) |
| MiRNA-672-5p | OE | Ovariectomy-induced sarcopenia mouse gastrocnemius muscle | Inhibition of Atrogin-1 and MuRF1 induced a decrease in lean muscle mass and Feret's diameter of muscle fibers and increase in the serum creatinine kinase level | Atrogin-1 and MuRF1 | (6) |
miRNA, microRNA; mRNA, messenger RNA BIRC, baculoviral inhibitors of apoptosis repeat containing; Elf5A1, eukaryotic translation initiation factor 5A1; MuRF1, muscle ring-finger protein-1; OE, overexpression; PITX1, paired-like homeodomain transcription factor 1; RXRB, retinoid X receptor-β; Sirt1, sirtuin 1; Tx, treatment.
Changes of the Expression Levels of microRNAs in Sarcopenia
Expression of 13 miRNAs (miRNA-10a-3p,−19a,−21, 34a,−92a-3p, 185-3p, 194-3p,−203a-3p,−326,−424-5p,−532-5p,−576-5p, and−760) was found to be changed in the muscles and/or blood (serum or plasma) of patients with sarcopenia [Table 1; (1, 16, 40, 64, 82)]. Among them, expression of three miRNAs in muscle (miRNA-19a,−34a, and−424-5p,) (16, 82), eight miRNAs in plasma (miRNA-10a-3p,−92a-3p,−185-3p,−194-3p,−326,−532-5p,−576-5p, and−760) (40), and two miRNAs in serum (miRNA-21 and−203a-3p) (1, 64) was changed and associated with physical functions including shrinking, weakness, poor endurance and energy, slowness, and low physical activity levels and expression of many signaling molecules, such as protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), 6-phosphofructo-2-kinase/fructose-2-,6-biphosphatase 3 (PFKFB3), transforming growth factor-β receptor 2 (TGF-βR2), vascular endothelial growth factor A (VEGFA), polymerase I receptor 1A (Pol I R1A), and upstream binding transcription factor (UBTF), which were shown to contribute to sarcopenia development [Table 1; (16, 64, 82)].
Expression of 10 miRNAs (miRNA-1-3p,−29,−29a-3p,−29b-3p,−98-5p,−133a-3p,−133b-3p,−181a,−434-3p, and−455-3p) was changed in muscles of rodent models of sarcopenia [Table 2; (5, 28, 38, 42, 60, 71)]. These miRNAs were associated with the expression levels of many signaling molecules, including tripartite motif containing 63 protein (TRIM63), F-box protein 32 (FBXO32), myeloblastosis-related protein B (B-myb), insulin-like growth factor-1 (IGF-1), p85, nerve growth factor (NGF), sirtuin 1 (Sirt1), eukaryotic translation initiation factor 5A1 (Elf5A1), paired-like homeodomain transcription factor 1 (PITX1), and retinoid X receptor-β (RXRB), which were shown to contribute to sarcopenia development [Table 2; (5, 28, 38, 42, 60, 71)].
Effects of microRNA Modulation on Sarcopenia
Several studies have reported that modulation of miRNAs has significant effects on sarcopenia in cultured myocytes in vitro (Table 3, Figures 2A,B). The specific miRNAs that have been reported to affect sarcopenia are described below.
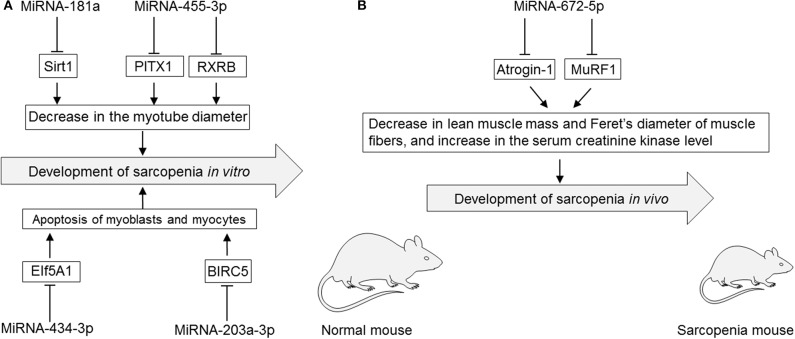
Figure 2.
(A) Roles of miRNAs in cultured myotubes, myoblasts, and myocytes in vitro. (B) Roles of miRNAs in rodent sarcopenia models in vivo. EIf5A1, eukaryotic translation initiation factor 5A1; miRNA, microRNA; PITX1, paired-like homeodomain transcription factor 1; RXRB, retinoid X receptor-β; Sirt1, sirtuin 1; MuRF1, muscle ring-finger protein-1.
MiRNA-181a
MiRNA-181a binds to the 3′-untranslated region of Sirt1 that is implicated in influencing aging, apoptosis, and inflammation (71). Overexpression of miRNA-181a using an miRNA-181a mimic by lipofection was shown to significantly decrease the myotube diameter, which was mediated by inhibiting its target Sirt1 in cultured myotubes of differentiated C2C12 cells, a subclone of mouse myoblasts. However, miRNA-181a knockdown using a miRNA-181a inhibitor led to an increase in the myotube diameter of cultured myotubes of differentiated C2C12 cells (71).
MiRNA-203a-3p
MiRNA-203a-3p binds to the 3′-untranslated region of baculoviral inhibitors of apoptosis repeat containing 5 (BIRC5), a member of the apoptosis inhibitor protein family that suppresses apoptosis via inhibition of the initiator caspase 9 and executers caspase 3 and 7 (84). MiRNA-203a-3p was upregulated in serum of colorectal cancer patients with sarcopenia as evaluated by a lower psoas muscle mass index compared with than in colorectal cancer patients without sarcopenia (1). Knockdown of miRNA-203a-3p using an miRNA-203-3p mimic by lipofection inhibited cell proliferation and induced apoptosis via increasing the expression level of the target BIRC5 in cultured human skeletal muscle cells (1).
MiRNA-434-3p
MiRNA-434-3p binds to the 3′-untranslated region of EIf5A1 that is involved in many cellular processes including cell division, apoptosis, and inflammation (60). Overexpression of miRNA-434-3p using an miRNA-434-3p mimic by lipofection inhibited the expression levels of EIf5A1, which prevented apoptosis of apoptosis-stimulated primary myocytes purified from hind limb muscles of C57BL/6J mice (60).
MiRNA-455-3p
Overexpression of miRNA-455-3p using an miRNA-455-3p mimic by lipofection inhibited the expression levels of PITX1 and RXRB, which are involved in muscle dystrophy and aging, resulting in a significant increase of the diameter of cultured myotubes differentiated from cultured mouse C2C12 myoblasts (42).
MiRNA-672-5p
Overexpression of miRNA-672-5p via tail vein injection of an miRNA-672-5p mimic in liposomes alleviated ovariectomy-induced sarcopenia in female BALB/c mice (6). Overexpression of miRNA-672-5p in ovariectomy-induced sarcopenia mice increased lean muscle mass but decreased the serum creatinine kinase level and increased Feret's diameter of muscle fibers with inhibited muscle atrogenes (Atrogin-1 and Murif1) that stimulate protein catabolism and negatively affect muscular health (6). Overexpression of miRNA-672-5p in ovariectomy-induced sarcopenia mice also increased osteoblastogenesis and mineralization, thereby reversing bone loss (6).
Discussion
Many miRNAs increase or decrease in muscles and blood of patients with sarcopenia and rodent models of sarcopenia. These expression changes are associated with the phenotypes of sarcopenia, such as lower physical functions and expression levels of many signaling molecules that mediate progression of sarcopenia. These lines of evidence suggest that miRNA levels in muscles and/or blood are potential biomarkers for sarcopenia. However, no study has reported the relationship between the expression changes of plasma/serum and muscle miRNAs in sarcopenia. It is necessary to investigate this relationship to clarify the mechanisms of miRNAs in each organ including muscles and their circulation form in blood for the development of sarcopenia as well as the utility of miRNAs in blood as biomarkers of sarcopenia. Additionally, several miRNAs have been demonstrated to affect sarcopenia in myocytes in vitro or rodent sarcopenia models in vivo. All studies reported the effects of miRNAs on sarcopenia in the setting of overexpression of these miRNAs as shown in Table 3. These results suggest that miRNAs are potential targets of gene therapy for sarcopenia. However, further studies are needed to investigate the mechanisms, target cells, and adverse effects of modulating these miRNAs. Additionally, so far, there is no clinical study that has directly investigated the functions of miRNAs in sarcopenia by modulation of their expression using a mimic and/or inhibitor. Future clinical studies will be necessary to confirm the effects of miRNAs on sarcopenia and their potential targets for gene therapy of sarcopenia. Our review has a number of limitations. First, we only searched for studies published in English. Second, we only used the PubMed database to identify publications. Third, meta-analysis could not be performed because the number of studies reporting miRNAs for sarcopenia was small, so statistical power would have been low. Therefore, further research is warranted to verify our conclusions.
Conclusion
Many miRNAs increase or decrease in muscles and blood of patients with sarcopenia and rodent models of sarcopenia. Additionally, several miRNAs have been demonstrated to affect sarcopenia in myocytes in vitro or rodent sarcopenia models in vivo. These results suggest that miRNAs are potential biomarkers and targets of gene therapy for sarcopenia. Further studies including clinical studies will be necessary to confirm the utility of miRNAs as biomarkers and targets for gene therapy of sarcopenia.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Funding. This review was supported by AMED under Grant No. JP19ek0210094h0003 and JSPS KAKENHI under Grant No. JP17K09708.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00180/full#supplementary-material
References
- 1.Okugawa Y, Toiyama Y, Hur K, Yamamoto A, Yin C, Ide S, et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J Cachexia Sarcopenia Muscle. (2019) 10:536–48. 10.1002/jcsm.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, et al. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. (2014) 28:4133–47. 10.1096/fj.14-254490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narasimhan A, Ghosh S, Stretch C, Greiner R, Bathe OF, Baracos V, et al. Small RNAome profiling from human skeletal muscle: novel miRNAs and their targets associated with cancer cachexia. J Cachexia Sarcopenia Muscle. (2017) 8:405–16. 10.1002/jcsm.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano-Arroquia A, McCormick R, Molloy AP, McArdle A, Goljanek-Whysall K. Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell. (2016) 15:361–9. 10.1111/acel.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aare S, Spendiff S, Vuda M, Elkrief D, Perez A, Wu Q, et al. Failed reinnervation in aging skeletal muscle. Skelet Muscle. (2016) 6:29. 10.1186/s13395-016-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad N, Kushwaha P, Karvande A, Tripathi AK, Kothari P, Adhikary S, et al. MicroRNA-672-5p identified during weaning reverses osteopenia and sarcopenia in ovariectomized mice. Mol Ther Nucleic Acids. (2019) 14:536–49. 10.1016/j.omtn.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altana V, Geretto M, Pulliero A. MicroRNAs and physical activity. Microrna. (2015) 4:74–85. 10.2174/2211536604666150813152450 [DOI] [PubMed] [Google Scholar]
- 8.Assar ME, Angulo J, Rodriguez-Manas L. Diabetes and ageing-induced vascular inflammation. J Physiol. (2016) 594:2125–46. 10.1113/JP270841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Band MM, Sumukadas D, Struthers AD, Avenell A, Donnan PT, Kemp PR, et al. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): study protocol for a randomised controlled trial. Trials. (2018) 19:6. 10.1186/s13063-017-2390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber L, Scicchitano BM, Musaro A. Molecular and cellular mechanisms of muscle aging and sarcopenia and effects of electrical stimulation in seniors. Eur J Transl Myol. (2015) 25:231–6. 10.4081/ejtm.2015.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berardi E, Annibali D, Cassano M, Crippa S, Sampaolesi M. Molecular and cell-based therapies for muscle degenerations: a road under construction. Front Physiol. (2014) 5:119. 10.3389/fphys.2014.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borras C, Serna E, Gambini J, Ingles M, Vina J. Centenarians maintain miRNA biogenesis pathway while it is impaired in octogenarians. Mech Ageing Dev. (2017) 168:54–7. 10.1016/j.mad.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Goljanek-Whysall K. microRNAs: modulators of the underlying pathophysiology of sarcopenia? Ageing Res Rev. (2015) 24(Pt B):263–73. 10.1016/j.arr.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 14.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. (2018) 47:214–77. 10.1016/j.arr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Bemben MG, Bemben DA. Bone and muscle specific circulating microRNAs in postmenopausal women based on osteoporosis and sarcopenia status. Bone. (2019) 120:271–8. 10.1016/j.bone.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Connolly M, Paul R, Farre-Garros R, Natanek SA, Bloch S, Lee J, et al. miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J Cachexia Sarcopenia Muscle. (2018) 9:400–16. 10.1002/jcsm.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cull AH, Rauh MJ. Success in bone marrow failure? Novel therapeutic directions based on the immune environment of myelodysplastic syndromes. J Leukoc Biol. (2017) 102:209–19. 10.1189/jlb.5RI0317-083R [DOI] [PubMed] [Google Scholar]
- 18.Dalmasso B, Hatse S, Brouwers B, Laenen A, Berben L, Kenis C, et al. Age-related microRNAs in older breast cancer patients: biomarker potential and evolution during adjuvant chemotherapy. BMC Cancer. (2018) 18:1014. 10.1186/s12885-018-4920-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dato S, Rose G, Crocco P, Monti D, Garagnani P, Franceschi C, et al. The genetics of human longevity: an intricacy of genes, environment, culture and microbiome. Mech Ageing Dev. (2017) 165(Pt B):147–55. 10.1016/j.mad.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Di Filippo ES, Mancinelli R, Pietrangelo T, La Rovere RM, Quattrocelli M, Sampaolesi M, et al. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem Biophys Res Commun. (2016) 473:462–70. 10.1016/j.bbrc.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 21.Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. (2008) 295:E1333–40. 10.1152/ajpendo.90562.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, et al. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics. (2011) 43:595–603. 10.1152/physiolgenomics.00148.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Souza RF, Zeng N, Markworth JF, Figueiredo VC, Hedges CP, Petersen AC, et al. Whey protein supplementation post resistance exercise in elderly men induces changes in muscle miRNA's compared to resistance exercise alone. Front Nutr. (2019) 6:91. 10.3389/fnut.2019.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebner N, Anker SD, von Haehling S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: highlights from the 11th cachexia conference. J Cachexia Sarcopenia Muscle. (2019) 10:218–25. 10.1002/jcsm.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erusalimsky JD, Grillari J, Grune T, Jansen-Duerr P, Lippi G, Sinclair AJ, et al. In search of 'omics'-based biomarkers to predict risk of frailty and its consequences in older individuals: the FRAILOMIC initiative. Gerontology. (2016) 62:182–90. 10.1159/000435853 [DOI] [PubMed] [Google Scholar]
- 26.Fan J, Kou X, Yang Y, Chen N. MicroRNA-regulated proinflammatory cytokines in sarcopenia. Mediat Inflamm. (2016) 2016:1438686. 10.1155/2016/1438686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fariyike B, Singleton Q, Hunter M, Hill WD, Isales CM, Hamrick MW, et al. Role of MicroRNA-141 in the aging musculoskeletal system: a current overview. Mech Ageing Dev. (2019) 178:9–15. 10.1016/j.mad.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlinger-Romero F, Yonamine CY, Junior DC, Esteves JV, Machado UF. Dysregulation between TRIM63/FBXO32 expression and soleus muscle wasting in diabetic rats: potential role of miR-1-3p,−29a/b-3p, and−133a/b-3p. Mol Cell Biochem. (2017) 427:187–99. 10.1007/s11010-016-2910-z [DOI] [PubMed] [Google Scholar]
- 29.Goljanek-Whysall K, Iwanejko LA, Vasilaki A, Pekovic-Vaughan V, McDonagh B. Ageing in relation to skeletal muscle dysfunction: redox homoeostasis to regulation of gene expression. Mamm Genome. (2016) 27:341–57. 10.1007/s00335-016-9643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham ZA, De Gasperi R, Bauman WA, Cardozo CP. Recombinant myostatin reduces highly expressed microRNAs in differentiating C2C12 cells. Biochem Biophys Rep. (2017) 9:273–80. 10.1016/j.bbrep.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. (2014) 23:99–108. 10.1016/j.jse.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. (2013) 43:12–21. 10.1007/s12020-012-9751-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases - complex signatures for multifactorial diseases? Mol Cell Endocrinol. (2016) 432:83–95. 10.1016/j.mce.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 34.Hamrick MW. Role of the cytokine-like hormone leptin in muscle-bone crosstalk with aging. J Bone Metab. (2017) 24:1–8. 10.11005/jbm.2017.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, et al. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. (2010) 400:379–83. 10.1016/j.bbrc.2010.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatse S, Brouwers B, Dalmasso B, Laenen A, Kenis C, Schoffski P, et al. Circulating MicroRNAs as easy-to-measure aging biomarkers in older breast cancer patients: correlation with chronological age but not with fitness/frailty status. PLoS ONE. (2014) 9:e110644. 10.1371/journal.pone.0110644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Liao K, Yang L, Pendyala G, Kook Y, Fox HS, et al. Tat-mediated induction of miRs-34a &−138 promotes astrocytic activation via downregulation of SIRT1: implications for aging in HAND. J Neuroimmune Pharmacol. (2017) 12:420–32. 10.1007/s11481-017-9730-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Z, Klein JD, Mitch WE, Zhang L, Martinez I, Wang XH. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging. (2014) 6:160–75. 10.18632/aging.100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibanez-Ventoso C, Driscoll M. MicroRNAs in C. elegans aging: molecular insurance for robustness? Curr Genomics. (2009) 10:144–53. 10.2174/138920209788185243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ipson BR, Fletcher MB, Espinoza SE, Fisher AL. Identifying exosome-derived MicroRNAs as candidate biomarkers of frailty. J Frailty Aging. (2018) 7:100–3. 10.14283/jfa.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung HJ, Lee KP, Kwon KS, Suh Y. MicroRNAs in skeletal muscle aging: current issues and perspectives. J Gerontol A Biol Sci Med Sci. (2019) 74:1008–14. 10.1093/gerona/gly207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung HJ, Lee KP, Milholland B, Shin YJ, Kang JS, Kwon KS, et al. Comprehensive miRNA profiling of skeletal muscle and serum in induced and normal mouse muscle atrophy during aging. J Gerontol A Biol Sci Med Sci. (2017) 72:1483–91. 10.1093/gerona/glx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JY, Park YK, Lee KP, Lee SM, Kang TW, Kim HJ, et al. Genome-wide profiling of the microRNA-mRNA regulatory network in skeletal muscle with aging. Aging. (2014) 6:524–44. 10.18632/aging.100677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunadian V, Neely RD, Sinclair H, Batty JA, Veerasamy M, Ford GA, et al. Study to improve cardiovascular outcomes in high-risk older patieNts (ICON1) with acute coronary syndrome: study design and protocol of a prospective observational study. BMJ Open. (2016) 6:e012091. 10.1136/bmjopen-2016-012091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis A, Lee JY, Donaldson AV, Natanek SA, Vaidyanathan S, Man WD, et al. Increased expression of H19/miR-675 is associated with a low fat-free mass index in patients with COPD. J Cachexia Sarcopenia Muscle. (2016) 7:330–44. 10.1002/jcsm.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma JF, Hall DT, Gallouzi IE. The impact of mRNA turnover and translation on age-related muscle loss. Ageing Res Rev. (2012) 11:432–41. 10.1016/j.arr.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 47.MacDonald EM, Cohn RD. TGFbeta signaling: its role in fibrosis formation and myopathies. Curr Opin Rheumatol. (2012) 24:628–34. 10.1097/BOR.0b013e328358df34 [DOI] [PubMed] [Google Scholar]
- 48.Margolis LM, Dawson-Hughes B, Rivas DA, Ezzyat Y, Fielding RA, Ceglia L. Effects of potassium bicarbonate supplements on circulating microRNA expression. J Endocr Soc. (2017) 1:1015–26. 10.1210/js.2017-00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolis LM, Lessard SJ, Ezzyat Y, Fielding RA, Rivas DA. Circulating MicroRNA are predictive of aging and acute adaptive response to resistance exercise in men. J Gerontol A Biol Sci Med Sci. (2017) 72:1319–26. 10.1093/gerona/glw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margolis LM, Rivas DA. Potential role of MicroRNA in the anabolic capacity of skeletal muscle with aging. Exerc Sport Sci Rev. (2018) 46:86–91. 10.1249/JES.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margolis LM, Rivas DA, Berrone M, Ezzyat Y, Young AJ, McClung JP, et al. Prolonged calorie restriction downregulates skeletal muscle mTORC1 signaling independent of dietary protein intake and associated microRNA expression. Front Physiol. (2016) 7:445. 10.3389/fphys.2016.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGregor RA, Poppitt SD, Cameron-Smith D. Role of microRNAs in the age-related changes in skeletal muscle and diet or exercise interventions to promote healthy aging in humans. Ageing Res Rev. (2014) 17:25–33. 10.1016/j.arr.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 53.Meng XM, Tang PM, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front Physiol. (2015) 6:82 10.3389/fphys.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michalska-Kasiczak M, Bielecka-Dabrowa A, von Haehling S, Anker SD, Rysz J, Banach M. Biomarkers, myocardial fibrosis and co-morbidities in heart failure with preserved ejection fraction: an overview. Arch Med Sci. (2018) 14:890–909. 10.5114/aoms.2018.76279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikovic J, Sadler K, Butchart L, Voisin S, Gerlinger-Romero F, Della Gatta P, et al. MicroRNA and long non-coding RNA regulation in skeletal muscle from growth to old age shows striking dysregulation of the callipyge locus. Front Genet. (2018) 9:548. 10.3389/fgene.2018.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell CJ, D'Souza RF, Schierding W, Zeng N, Ramzan F, O'Sullivan JM, et al. Identification of human skeletal muscle miRNA related to strength by high-throughput sequencing. Physiol Genomics. (2018) 50:416–24. 10.1152/physiolgenomics.00112.2017 [DOI] [PubMed] [Google Scholar]
- 57.Montano M, Long K. RNA surveillance-an emerging role for RNA regulatory networks in aging. Ageing Res Rev. (2011) 10:216–24. 10.1016/j.arr.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie M, Deng ZL, Liu J, Wang DZ. Noncoding RNAs, emerging regulators of skeletal muscle development and diseases. Biomed Res Int. (2015) 2015:676575. 10.1155/2015/676575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okugawa Y, Yao L, Toiyama Y, Yamamoto A, Shigemori T, Yin C, et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol Rep. (2018) 39:1555–64. 10.3892/or.2018.6270 [DOI] [PubMed] [Google Scholar]
- 60.Pardo PS, Hajira A, Boriek AM, Mohamed JS. MicroRNA-434-3p regulates age-related apoptosis through eIF5A1 in the skeletal muscle. Aging. (2017) 9:1012–29. 10.18632/aging.101207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SS, Kwon ES, Kwon KS. Molecular mechanisms and therapeutic interventions in sarcopenia. Osteoporos Sarcopenia. (2017) 3:117–22. 10.1016/j.afos.2017.08.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Portal-Nunez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. (2016) 108:1–10. 10.1016/j.bcp.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 63.Proctor CJ, Goljanek-Whysall K. Using computer simulation models to investigate the most promising microRNAs to improve muscle regeneration during ageing. Sci Rep. (2017) 7:12314. 10.1038/s41598-017-12538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rusanova I, Diaz-Casado ME, Fernandez-Ortiz M, Aranda-Martinez P, Guerra-Librero A, Garcia-Garcia FJ, et al. Analysis of plasma MicroRNAs as predictors and biomarkers of aging and frailty in humans. Oxid Med Cell Longev. (2018) 2018:7671850. 10.1155/2018/7671850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rusanova I, Fernandez-Martinez J, Fernandez-Ortiz M, Aranda-Martinez P, Escames G, Garcia-Garcia FJ, et al. Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp Gerontol. (2019) 124:110637. 10.1016/j.exger.2019.110637 [DOI] [PubMed] [Google Scholar]
- 66.Sannicandro AJ, Soriano-Arroquia A, Goljanek-Whysall K. micro(RNA)-managing muscle wasting. J Appl Physiol. (1985) 127:619–32. 10.1152/japplphysiol.00961.2018 [DOI] [PubMed] [Google Scholar]
- 67.Sarkozy M, Kovacs ZZA, Kovacs MG, Gaspar R, Szucs G, Dux L. Mechanisms and modulation of oxidative/nitrative stress in type 4 cardio-renal syndrome and renal sarcopenia. Front Physiol. (2018) 9:1648. 10.3389/fphys.2018.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seldeen KL, Pang M, Leiker MM, Bard JE, Rodriguez-Gonzalez M, Hernandez M, et al. Chronic vitamin D insufficiency impairs physical performance in C57BL/6J mice. Aging. (2018) 10:1338–55. 10.18632/aging.101471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shorter E, Sannicandro AJ, Poulet B, Goljanek-Whysall K. Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Curr Rheumatol Rep. (2019) 21:40. 10.1007/s11926-019-0839-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siracusa J, Koulmann N, Banzet S. Circulating myomiRs: a new class of biomarkers to monitor skeletal muscle in physiology and medicine. J Cachexia Sarcopenia Muscle. (2018) 9:20–7. 10.1002/jcsm.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soriano-Arroquia A, House L, Tregilgas L, Canty-Laird E, Goljanek-Whysall K. The functional consequences of age-related changes in microRNA expression in skeletal muscle. Biogerontology. (2016) 17:641–54. 10.1007/s10522-016-9638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki T, Springer J. MicroRNAs in muscle wasting. J Cachexia Sarcopenia Muscle. (2018) 9:1209–12. 10.1002/jcsm.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swan M. Meeting report: American aging association 40th Annual Meeting, Raleigh, North Carolina, June 3-6, 2011. Rejuvenation Res. (2011) 14:449–55. 10.1089/rej.2011.1216 [DOI] [PubMed] [Google Scholar]
- 74.Tan LJ, Liu SL, Lei SF, Papasian CJ, Deng HW. Molecular genetic studies of gene identification for sarcopenia. Hum Genet. (2012) 131:1–31. 10.1007/s00439-011-1040-7 [DOI] [PubMed] [Google Scholar]
- 75.Trovato E, Di Felice V, Barone R. Extracellular vesicles: delivery vehicles of myokines. Front Physiol. (2019) 10:522. 10.3389/fphys.2019.00522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang E. MicroRNA, the putative molecular control for mid-life decline. Ageing Res Rev. (2007) 6:1–11. 10.1016/j.arr.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 77.Weigl LG. Lost in translation: regulation of skeletal muscle protein synthesis. Curr Opin Pharmacol. (2012) 12:377–82. 10.1016/j.coph.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 78.Weilner S, Grillari-Voglauer R, Redl H, Grillari J, Nau T. The role of microRNAs in cellular senescence and age-related conditions of cartilage and bone. Acta Orthop. (2015) 86:92–9. 10.3109/17453674.2014.957079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yanai K, Ishii H, Aomatsu A, Ishibashi K, Morishita Y. MicroRNAs in peritoneal fibrosis: a systematic review. Discov Med. (2018) 26:271–80. [PubMed] [Google Scholar]
- 80.Zeng N, D'Souza RF, Mitchell CJ, Cameron-Smith D. Sestrins are differentially expressed with age in the skeletal muscle of men: a cross-sectional analysis. Exp Gerontol. (2018) 110:23–34. 10.1016/j.exger.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 81.Zeng P, Han W, Li C, Li H, Zhu D, Zhang Y, et al. miR-378 attenuates muscle regeneration by delaying satellite cell activation and differentiation in mice. Acta Biochim Biophys Sin. (2016) 48:833–9. 10.1093/abbs/gmw077 [DOI] [PubMed] [Google Scholar]
- 82.Zheng Y, Kong J, Li Q, Wang Y, Li J. Role of miRNAs in skeletal muscle aging. Clin Interv Aging. (2018) 13:2407–19. 10.2147/CIA.S169202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 84.Kotipatruni RR, Nalla AK, Asuthkar S, Gondi CS, Dinh DH, Rao JS. Apoptosis induced by knockdown of uPAR and MMP-9 is mediated by inactivation of EGFR/STAT3 signaling in medulloblastoma. PLoS ONE. (2012) 7:e44798. 10.1371/journal.pone.0044798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.