Abstract
Five new withanolides (1–5) along with five known ones (6–10) were isolated from the whole plants of Physalis minima Linn. The chemical structures of the new compounds were identified as (20S,22R) 15a-acetoxy-5β,6β-epoxy-4β,14a,28-trihydroxy-3β-methoxy-1-oxowitha-16,24-dienolide (1), (20S,22R) 15a-acetoxy-5β,6β-epoxy-3β,4β,14β,17β,20β-pentahydroxy-1-oxowitha-24-enolide (2), (20R,22R) 15α-acetoxy-4β,5α,6β,14α,20β-pentahydroxy-1-oxowitha-2,24-dienolide (3), (20R,22R) 15α-acetoxy-5α,6β,14α,20β-tetrahydroxy-1-oxowitha-2,24-dienolide (4), and (20S,22R) 5α,6β,14β-trihydroxy-1,15-dioxowitha-2,16,24-trienolide (5) on the basis of integration combining IR, UV, HR-ESI-MS, 1D-NMR, and 2D-NMR analyses. Biologically, compounds (1–10) were subjected to evaluate their anti-inflammatory activities via inhibiting nitric oxide production in lipopolysaccharide-stimulated murine RAW 264.7 cells in vitro. The activity screening indicated that all of the compounds showed a moderate inhibitory effect against nitric oxide production with IC50 values of 23.53–66.28 μM.
1. Introduction
As a part of the Solanaceae family, Physalis minima Linn. is a branched annual shrub and distributed throughout subtropical and tropical areas.1,2 At the same time, its whole plant has been used as a traditional Chinese folk medicine for the treatment of cough phlegm, cold fever, sore throat, asthma and so on.3 Previous research on P. minima Linn. lead to the isolation of bioactive withanolides and physalins, which showed heightened anti-inflammatory and cytotoxic activities.3,4
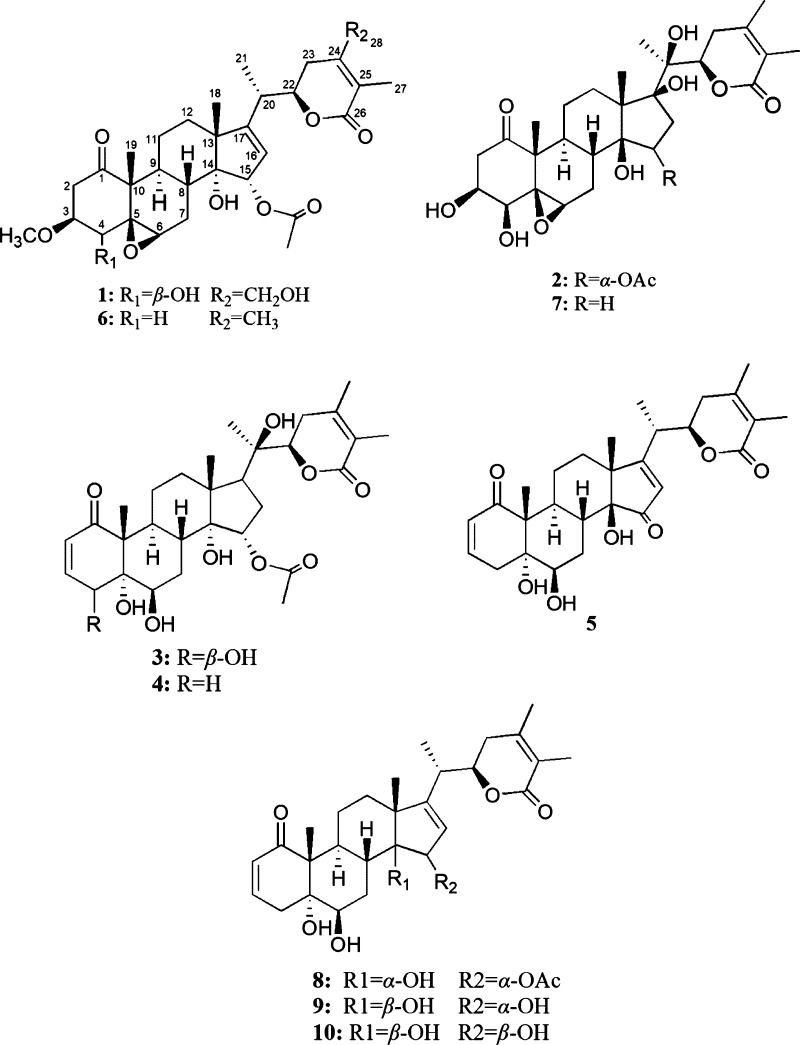
Withanolides are the main secondary metabolites of Solanaceae family. Their parent nuclear structure are highly oxygenated C28 ergostane-type steroids, of which their 23-hydroxy-26-oic or 22-hydroxy-26-oic analogue oxidized to generate a γ or δ-lactone rings.5−8 Recently, withanolides have gained increasing attentions through their diverse structures and broadly biological activities. Moreover, recent pharmacological researches have illustrated that withanolides show anti-inflammatory, antimicrobial, immunomodulatory, and cytotoxic activities.9,10 As part of ongoing research project on P. minima to discover the clinical candidate compounds with anti-inflammatory activities, the 85% EtOH extract of the whole plants of P. minima was separated over D101 marcroporouse resin, silica gel, medium pressure liquid chromatography (MPLC), Sephadex LH-20, and semipreparative high performance liquid chromatography (HPLC) to obtain 10 withanolides (Figure 1), including five new ones (1–5). In addition, their anti-inflammatory activities were evaluated against lipopolysaccharide (LPS)-activated RAW 264.7 cells. Herein, the isolation, structure elucidation, and anti-inflammatory activities of these withanolides were presented.
Figure 1.
Structures of withanolides from the whole plants of P. minima Linn.
2. Results and Discussion
Compound 1 was obtained as an amorphous powder. Its molecular formula was inferred as C31H42O10 from the HR-ESI-MS [M + FA – H]− ion peak at m/z: 619.2805 (calcd 619.2800), indicating eleven degrees of unsaturation. The IR spectrum of 1 indicated the absorptions of an α,β-unsaturated δ-lactone (1730 cm–1) and an olefinic function (1642 cm–1). The 13C NMR spectrum displayed the resonances of 31 carbons, owing to 6 methyls, 6 methylenes, 9 methines and 10 quaternary carbons. The 1H and 13C spectroscopic data (Tables 1 and 2) of 1 indicated four methyl at δH 1.07 (s, 3H), 1.25 (s, 3H), 1.19 (d, J = 7.2 Hz, 3H) and 1.88 (s, 3H), which were assigned to H-18, H-19, H-21, and H-27. An obviously doublet at δH 4.39 (H-22, 1H, dd, J = 12.0, 3.6 Hz) and along with carbon signals (δC 167.7, 120.8, and 152.8) suggested the existence of an α,β-unsaturated δ-lactone of withanolide skeleton. Comparison of its NMR data with those of physagulin N (6)2 suggested that compound 1 was 15a-acetoxy-5β,6β-epoxy-14a-hydoxy-3β-methoxy-1-oxowitha-16,24-dienolide type of withanolide. The obvious difference was the signals for ring A and ring E. The downfield signal of C-4 (+41.1 ppm) at δC 77.6 and HMBC correlation from H-4 at δH 3.68 (1H, d, J = 3.6 Hz) to C-6 at δC 58.6, C-10 at δC 52.1, and C-2 at δC 39.9 indicated that a hydroxyl group was attached to C-4 in 1. At the same time, the NOE correlation from δH H-4 at δH 3.68 (1H, d, J = 3.6 Hz) to H-6 at δH 3.28 (1H, br s) suggested that H-4 and H-6 were a-oriented, while the hydroxy group attached to C-4 was β-oriented. In ring E, downfield shift of C-28 (+39.1 ppm) at δC 60.2 showed that a hydroxyl group was located at C-28 in 1, and another hydroxy group was attached to C-28 (δC 60.2), as suggested by the key HMBC correlation from H-28 at δH 4.28, 4.37 (2H, m) to C-25 at δC 120.8 (Figure 2). This assignment was confirmed by the phenomenon that the C-24 was de-shielded from 149.2 to 152.8 ppm, while C-23 was shielded from 32.3 to 27.1 ppm by the β and γ effects of the 28-hydroxy group. The chemical shift of C-12 at δC 32.9 indicated the hydroxy group attached to C-14 was a-oriented. Because a 14a hydroxy group would shield C-12 from around 37.0 to around 32.0 ppm through a γ effect, while a 14β hydroxy group did not have such effect. The NOE correlation from H-20 at δH 2.57 (1H, m) to CH3-18 at δH 1.07 (3H, s) suggested that H-20 and H-18 were a-oriented, and thus a 20S configuration was indicated. The absolute configuration at C-22 was assigned as 22R from the positive Cotton effect at 250 nm from the n−π* transition of a typical α,β-unsaturated δ-lactone in the ECD spectrum.1 Thus, the structure of 1 was assigned as (20S,22R) 15a-acetoxy-5β,6β-epoxy-4β,14a,28-trihydoxy-3β-methoxy-1-oxowitha-16,24-dienolide, named physaliolide L.
Table 1. 13C NMR (150 MHz) Spectroscopic Data for Compounds 1–5 in CD3ODa.
| no. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 210.7 | 213.0 | 206.2 | 205.8 | 206.9 |
| 2 | 39.9 | 43.2 | 127.6 | 127.6 | 128.7 |
| 3 | 77.6 | 69.3 | 142.7 | 142.3 | 144.5 |
| 4 | 73.7 | 78.4 | 76.0 | 35.2 | 36.6 |
| 5 | 63.5 | 64.7 | 73.4 | 76.5 | 78.3 |
| 6 | 58.6 | 60.7 | 73.4 | 73.1 | 75.4 |
| 7 | 24.1 | 27.1 | 27.8 | 27.8 | 28.8 |
| 8 | 35.0 | 34.0 | 35.7 | 33.5 | 34.7 |
| 9 | 38.0 | 41.8 | 35.3 | 34.4 | 33.2 |
| 10 | 52.1 | 52.0 | 51.6 | 51.6 | 55.5 |
| 11 | 20.2 | 21.8 | 23.0 | 22.1 | 22.5 |
| 12 | 32.9 | 37.5 | 32.3 | 32.8 | 36.7 |
| 13 | 50.3 | 54.4 | 44.2 | 43.2 | 54.0 |
| 14 | 81.3 | 82.2 | 81.1 | 82.0 | 79.4 |
| 15 | 83.4 | 88.4 | 82.6 | 83.4 | 211.5 |
| 16 | 121.1 | 39.9 | 32.9 | 33.3 | 129.8 |
| 17 | 161.6 | 86.7 | 52.2 | 51.9 | 189.4 |
| 18 | 14.8 | 21.2 | 14.1 | 15.0 | 19.9 |
| 19 | 13.3 | 15.0 | 14.0 | 13.0 | 14.4 |
| 20 | 35.0 | 83.7 | 78.6 | 79.4 | 38.2 |
| 21 | 16.8 | 15.0 | 10.9 | 19.8 | 17.6 |
| 22 | 79.9 | 80.6 | 78.6 | 77.0 | 81.0 |
| 23 | 27.1 | 33.9 | 32.3 | 28.6 | 34.5 |
| 24 | 152.8 | 153.9 | 152.8 | 153.1 | 152.5 |
| 25 | 120.8 | 121.7 | 120.2 | 120.2 | 122.2 |
| 26 | 167.7 | 169.6 | 168.4 | 168.8 | 168.7 |
| 27 | 10.5 | 9.2 | 8.6 | 10.3 | 12.4 |
| 28 | 60.2 | 21.4 | 19.1 | 18.5 | 20.4 |
| CH3CO-1′ | 170.1 | 171.4 | 170.1 | 171.0 | |
| CH3CO-2′ | 19.9 | 20.5 | 19.9 | 19.1 | |
| OMe | 52.1 |
Chemical shifts are in ppm, and the assignments were based on HSQC, HMBC, and NOSEY spectra.
Table 2. 1H NMR (600 MHz) Spectroscopic Data for Compounds 1–5 in CD3ODa.
| no. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | α: 2.65 dd (16.2, 3.0) | α: 2.55 dd (15.6, 3.2) | 5.80 dd (10.2,3.6) | 5.79 dd (10.2, 3.6) | 5.76 dd (10.2, 3.6) |
| β: 2.89 dd (16.2, 7.8) | β: 2.91 dd (15.6, 7.2) | ||||
| 3 | 3.49 dt (7.8, 3.0) | 4.08 m | 6.66 ddd (10.2, 5.4, 3.6) | 6.67 ddd (10.2, 5.4, 3.6) | 6.66 ddd (10.2, 5.4, 3.6) |
| 4 | 3.66 d (3.0) | 3.21 d (3.2) | 3.60 d (3.6) | α: 2.07 dd (10.2, 5.4) | α: 2.05 dd (10.2, 5.4) |
| β: 3.26 dt (16.2, 3.6) | β: 3.24 dt (16.2, 3.6) | ||||
| 6 | 3.28 br s | 3.30 br s | 4.37 t (3.6) | 4.62 t (3.6) | 3.61 t (3.6) |
| 7 | α: 1.60 m | α: 2.49 m | α: 1.83 m | α: 1.97 m | α: 2.01 m |
| β: 2.59 m | β: 2.60 m | β: 2.09 m | β: 2.21 m | β: 2.05 m | |
| 8 | 2.59 m | 2.63 m | 1.95 m | 1.95 m | 2.27 m |
| 9 | 1.93 m | 2.57 m | 2.42 m | 2.42 m | 2.08 m |
| 11 | 1.35 m, 1.48 m | 1.48 m, 1.70 m | 2.16 m, 2.12 m | 2.16 m, 2.12 m | 1.62 m, 1.64 m |
| 12 | 1.78 m, 1.47 m | 1.51 m, 2.21 m | 1.82 m, 2.31 m | 1.82 m, 2.31m | 1.55 m, 1.82 m |
| 15 | 5.27 d (3.0) | 5.04 d (3.2) | 5.27 d (2.4) | 5.07 d (2.4) | |
| 16 | 5.71 d (3.0) | 3.67 m, 3.76 m | 2.44 m, 1.41 m | 2.22 m, 2.27 m | 6.23 s |
| 17 | 1.27 m | 1.42 m | |||
| 18 | 1.07 s | 1.21 s | 1.26 s | 1.09 s | 1.17 s |
| 19 | 1.25 s | 1.26 s | 1.25 s | 1.32 s | 1.35 s |
| 20 | 2.57 m | 2.84 m | |||
| 21 | 1.19 d (7.2) | 1.07 s | 1.88 s | 1.12 s | 1.27 d (7.2) |
| 22 | 4.39 dd (12.0, 3.6) | 5.08 dd (12.0, 3.6) | 5.11 dd (12.0, 3.6) | 4.75 dd (12.0, 3.6) | 4.47 dd (12.0, 3.6) |
| 23 | 2.59 m, 2.29 m | 1.86 m, 1.96 m | 2.58 m, 2.29 m | 2.58 m, 2.29 m | 2.41 m, 2.51 m |
| 27 | 1.88 s | 1.86 s | 1.06 s | 1.06 s | 1.84 s |
| 28 | 4.28 m, 4.37 m | 1.99 s | 1.99 s | 2.01 s | 1.99 s |
| CH3CO | 2.07 s | 2.10 s | 2.07 s | 2.07 s | |
| OMe | 3.21 s |
Chemical shifts are in ppm, and coupling constants (J) in Hz are given in parentheses. The assignments were based on HSQC, HMBC, and NOSEY spectra.
Figure 2.
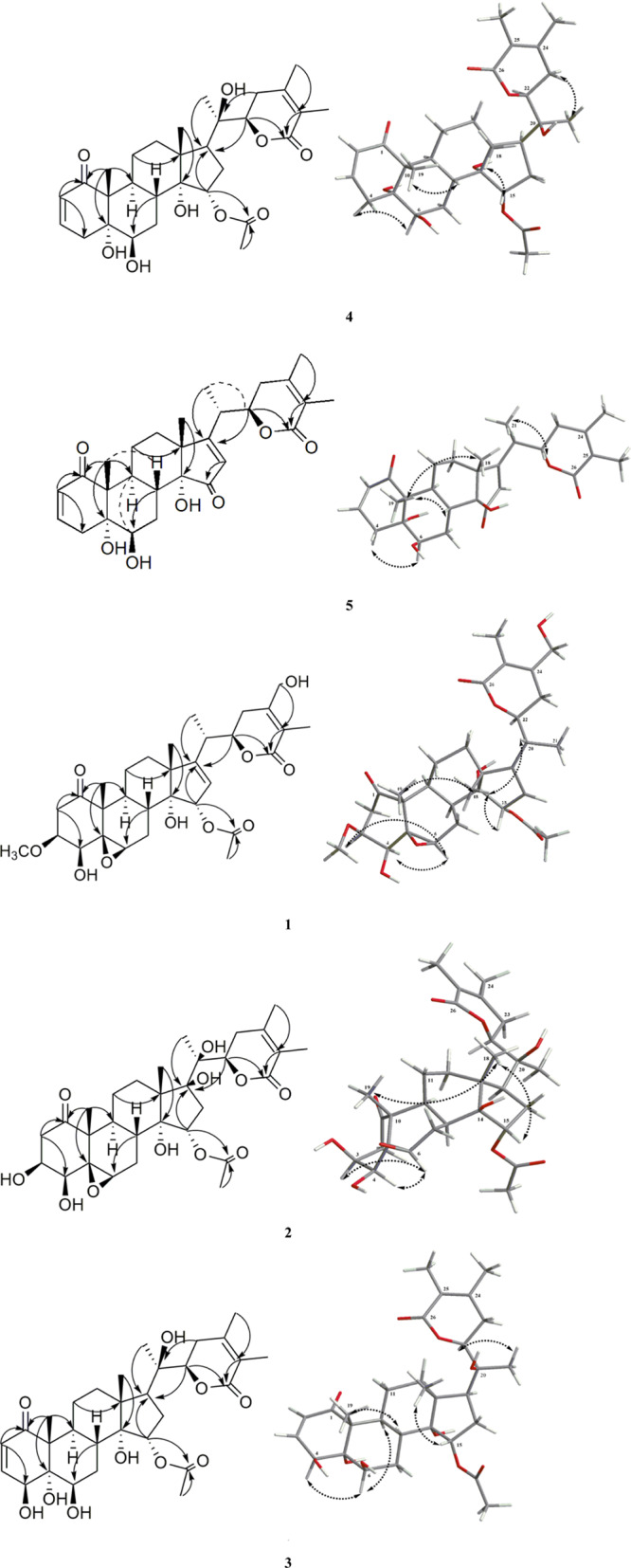
Key HMBC and NOE correlations for compounds 1–5.
Compound 2 was isolated as an amorphous powder. The molecular formula of compound 2 was deduced to be C30H42O11 by the HR-ESI-MS [M + FA – H]− ion peak at m/z: 623.2704 (calcd 623.2735). The IR spectrum showed the hydroxy group (3442 cm–1) and α,β-unsaturated δ-lactone (1735 cm–1) absorptions. The 1H and 13C NMR spectra of 2 were similar to those of phyperunolide E (7).1 The main difference is the signals of the ring D, where an acetoxyl moiety [(δC: 171.4, 20.5; δH: 2.10 (3H, s)] was present in 2. The HMBC correlation from δH 4.84 (H-15, m) to δC 171.4 (C-OAc-1′) suggested that 2 was 15-acetoxy-phyperunolide E. Furthermore, the NOESY correlation between H-15 (δH 4.84, m) and H-18 (δH 1.21, s) indicated that the 15-acetoxy group was a-oriented. Compared with 1, the chemical shift of C-12 at δC 37.5 indicated the orientation of 14-hydroxy group was β in 2 because a 14β hydroxy group did not have a γ effect. Thus, 2 had the same configurations (20S,22R) as 7via biosynthetic considerations.1 Therefore, the structure of 2 was deduced to be (20S,22R) 15a-acetoxy-5β,6β-epoxy-3β,4β,14β,17β,20β-pentahydroxy-1-oxowitha-24-enolide, named physaliolide M.
Compound 3 was purified as an amorphous power. The molecular formula of 3 was deduced to be C30H42O10 by the [M + FA – H]− ion peak at m/z: 607.2799 (calcd 607.2765) in HR-ESI-MS. 1H and 13C NMR and 2D NMR spectra showed that it is a withanolide-type compound.4 Though their 1H and 13C NMR spectra data were similar to those of physaliolide E (8),4 differences were observed in the signals of rings A and D. In ring D, upfield shift of C-16 (−91.3 ppm) at δC 32.9 and C-17 (−112.0 ppm) at δC 52.2 in 3 indicated that the Δ16(17) double bond was absent in 3, as indicated by 1H–1H COSY correlation observed between H-16 and H-17 in 3. This has been supported by the HMBC corrections between H-16 at δH [2.44 (1H, m), 1.41 (1H, m)] and C-14 at δC 82.6, and between H-17 at δH 1.27 (1H, m) and C-21 at δC 10.9. In ring A, an additional hydroxy group at the C-4 position could be indicated, as supported by the downfield shift of C-4 (+37.0 ppm) at δC 74.0, and the key HMBC correlations from H-4 at δH (3.60, d, J = 3.6 Hz) to C-2 at δC 127.6, C-6 at δC 73.4, and C-10 at δC 51.6. The relative configuration of the hydroxy group linked to C-4 was β-orientation due to the correlation between H-4 at δH (3.60, d, J = 3.6 Hz) and H-6 at δH 4.37 (H-6, t, J = 3.0 Hz) observed in the NOESY 2D NMR spectrum of 3. The chemical shift value of the C-19 methyl group was important for deducing the stereochemistry of the A/B ring junction in 5,6-dihydroxy and 4,5,6-trihydroxy withanolides. In the trans-isomer, the chemical shift of C-19 appears at around δC 15.0 ppm, while in a cis-junction, the C-19 resonates near δC 9.0 ppm. The chemical shift value of the C-19 methyl group (δC 14.0 ppm) indicated trans-fusion for rings A and B and showed the orientation of 5-hydroxy group was α in 3.1 At the same time, it was readily confirmed that 3 had the same relative configuration of 14-hydroxy group with 1 by comparing their chemical shift values of C-12. Thus, the structure of 3 was proposed as (20R,22R) 15α-acetoxy-4β,5α,6β,14α,20β-pentahydroxy-1-oxowitha-2,24-dienolide, named physaliolide N.
Compound 4 was shown to have the molecular formula C30H42O9 by the [M + FA – H]− ion peak at m/z: 591.2848 (calcd 591.2815) in HR-ESI-MS. The 1H and 13C NMR spectroscopic data of 4 were similar to those of 3, except for those of ring A. The 13C NMR spectrum of 4 indicated that the C-4 hydroxy group was absent, as indicated by the upfield shift of C-4 (−38.8 ppm) at δC 35.2. Also, the assignment of C-4 was confirmed by the HMBC correlation from H-4 at δH 2.07 (1H, d, J = 3.6 Hz) to C-2 at δC 127.6, C-6 at δC 73.1, and C-10 at δC 51.6. It was readily confirmed that 4 had the same relative configurations of 5-hydroxy group and 14-hydroxy group with 3 by comparing their chemical shift values of C-19 and C-12. The absolute configurations (20R,22R) were elucidated via biosynthetic considerations and compar. Thus, the structure of 4 was elucidated to be (20R,22R) 15α-acetoxy-5α,6β,14α,20β-tetrahydroxy-1-oxowitha-2,24-dienolide, named physaliolide O.
Compound 5 was isolated and purified as white amorphous powder, and the molecular formula was determined as [M + FA – H]− ion peak at m/z: 529.2462 (calcd 529.2438), referring to a molecular formula of C28H36O7. The IR spectrum showed three absorption bands at 3454, 1732, and 1706 cm–1, which were in accordance with a hydroxyl, α, β-unsaturated ketone, and α, β-unsaturated δ-lactone. The structure of 5 was similar to that of physangulatin A (9),1 as implied by their 1H and 13C spectroscopic data. The hydroxy group attached to C-15 in physangulatin A was changed to instead of a carbonyl group in 5, as confirmed by the HMBC correlations from H-8 at δH 2.27 (1H, m) and H-16 at δH 6.23 (1H, br s) to C-15 at δC 211.5 in 5. The structure of 5 could be (20S,22R) 5α,6β,14β-trihydroxy-1,15-dioxowitha-2,16,24-trienolide, named physaliolide P.
In addition to the new withanolides, five known withanolides, including physagulin N (6),2 phyperunolide E (7),12 withaminimin (8),1 physangulatin A (9),1 and physangulatin B (10)1 were identified from P. minima (Figure 1). The known compounds were characterized based on comparison of their NMR, and mass spectroscopic data with those in the literature values. Compounds 1–10 were examined for their anti-inflammatory ability to inhibit nitric oxide (NO) production in LPS-activated murine RAW 264.7 cells. Meanwhile, cell viability was also tested by the MTT method to explain whether the inhibition was owing to the cytotoxicity of the tested compounds. All tested compounds exhibited weak cytotoxicity against RAW 264.7 cells (IC50 > 80 μM and data do not show here). As the results shown in Table 3, compounds 1–10 all exhibited moderate anti-inflammatory activities via inhibiting nitric oxide (NO) production in LPS-stimulated murine RAW 264.7 cells.
Table 3. Inhibitory Effects of Compounds 1–10 on NO Production Induced by LPS in Macrophages (IC50 Values in μM)a.
| compounds | IC50 ± SD (μM) |
|---|---|
| 1 | 27.35 ± 1.53 |
| 2 | 23.53 ± 1.15 |
| 3 | 28.96 ± 1.78 |
| 4 | 30.25 ± 1.78 |
| 5 | 66.28 ± 1.59 |
| 6 | 25.72 ± 0.98 |
| 7 | 24.33 ± 1.32 |
| 8 | 31.15 ± 0.84 |
| 9 | 34.36 ± 1.12 |
| 10 | 38.72 ± 1.06 |
| l-NAME | 36.75 ± 1.32 |
l-NAME was used as the positive control.
3. Conclusions
In a word, five new withanolides (1–5), along with five known analogues (6–10), were isolated from the whole plants of P. minima L. These compounds were all evaluated for their anti-inflammatory activities via inhibiting nitric oxide (NO) production in LPS-stimulated murine RAW 264.7 cells in vitro. All of these compounds showed moderate inhibitory effect on nitric oxide production, and the inhibition of NO production activity was not related to their cytotoxicity. It is accordingly suggested that the withanolides with 5β,6β-epoxy group may have better anti-inflammatory activity.
4. Materials and Methods
4.1. General
The UV spectra were measured on a Shimadzu UV-2501 PC spectrophotometer. The specific optical rotations were determined by a PerkinElmer polarimeter model 241, and IR spectra were performed by using a PerkinElmer 983 G spectrometer (PerkinElmer, Inc., Waltham, MA, USA). The ECD spectra were recorded on a JASCO 810 spectropolarimeter. The NMR spectra were obtained by using a Bruker AVANCE III 600 spectrometer (Karlsruhe, Germany), and HRESI mass spectra were determined by using Micromass Q-TOF2 spectrometer (AB SCIEX, Canada). Semipreparative HPLC was performed by using Waters 2535 (Waters, Milford, MA, USA) with the YMC-Pack ODS-A column (5 μm, 10 × 250 mm, YMC, Kyoto, Japan), which contained a Waters 2489 UV detector. Its flow rate was 2.0 mL/min and the wavelength for detection was 223 nm. The MPLC system was carried out by using a Büchi Flash chromatography with a C-650 pump and a flash column (460 mm × 26 mm i.d., Büchi Corp., Flawil, Switzerland), while its flow rate was 15.0 mL/min. Sephadex LH-20 column chromatography was purchased from GE Corp. D101 macroporous resin was acquired from Xi’an Sunresin New Material Co. Ltd. Silica gel (200–300 mesh) and silica gel TLC plates were obtained from Qingdao Marine Chemical Factory.
4.2. Plant Materials
The whole dried plants of P. minima Linn. were collected in Guangzhou, Guangdong Province of China in November 2014 and identified by Prof. Xiaoran Li. The voucher specimen (no. 14-15-06-01) is deposited in the herbarium of College of Pharmaceutical Science, Soochow University.
4.3. Extraction and Isolation
The dried and powdered plant (20 kg) was extracted by reflux at 80 °C with 85% EtOH (80 L), and a dark green residue (0.45 kg) was obtained from evaporation of the extract under reduced pressure. The residue was suspended in purified water and then passed through a D101 macroporous resin column, and gradient elution was carried out by EtOH–H2O (0, 30, 60 and 80%) to obtain four fractions: Frs. A–D. Then, Fr. C was suspended in purified water and partitioned with dichloromethane (DCM), EtOAc, and then nBuOH. The DCM extraction (65 g) was passed through silica gel (200–300 mesh) column, and gradient elution was carried out by the CH2Cl2–MeOH solvent system (25:1, 15:1, 10:1, 5:1, and then 1:1) to obtain five subfractions (Frs. C-1–C-5). After that, Fr. C-2 (20 g) was resubjected to silica gel column following the former gradients to afford five fractions (Frs. C-2-1–C-2-5). Fr. C-2-1 (5 g) was subjected to MPLC (460 mm × 26 mm), with a gradient elution of MeOH–H2O (60:40, 80:20, and then 100:0) to afford three fractions (Frs. C-2-1a–C-2-1c). Fr. C-2-1a (1.2 g) was subjected to a Sephadex-LH 20 column and eluted with MeOH, then separated by semipreparative HPLC using ODS column with the MeOH–H2O (60:40) elution yielded compounds 1 (25 mg, tR = 26.4 min), 6 (13 mg, tR = 28.0 min), 2 (9 mg, tR = 35.2 min), 7 (8 mg, tR = 40.5 min). Similarly, Fr. C-2-1b (760 mg) was also purified by semipreparative HPLC, and eluted with MeOH–H2O (68:32) to yield compounds 3 (15 mg, tR = 32.2 min), 4 (15 mg, tR = 36.6 min), 5 (13 mg tR = 42.2 min), and 8 (6 mg, tR 48.5 = min). Fr. C-2-1c (350 mg) was purified by semipreparative HPLC with MeOH–H2O (60:40) to yield compounds 9 (13 mg, tR = 30.5 min) and 10 (12 mg, tR = 34.2 min).
4.3.1. Physaliolide L (1)
White amorphous powder; [α]D20 +16.7 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.25), 224 (4.18) nm; IR νmax (KBr): 3460, 2935, 1768, 1730, 1642, 1640, 1605, 1162 cm–1; ECD (MeOH) nm (Δε): 252 (+15.6), 300 (−9.2); 13C NMR (150 MHz) and 1H NMR (600 MHz) spectroscopic data, see Tables 1 and 2; HR-ESI-MS m/z: 619.2805 [M + FA – H]− (calcd for C32H43O12 619.2800).
4.3.2. Physaliolide M (2)
White amorphous powder; [α]D20 +12.8 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.02), 224 (4.03) nm; IR νmax (KBr): 3442, 2922, 1735, 1710, 1645, 1641, 1602, 1168 cm–1; 13C NMR (150 MHz) and 1H NMR (600 MHz) spectroscopic data, see Tables 1 and 2; HR-ESI-MS m/z: 623.2704 [M + FA – H]− (calcd for C31H43O13 623.2735).
4.3.3. Physaliolide N (3)
White amorphous powder; [α]D20 +12.4 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.07), 224 (4.27) nm; IR νmax (KBr): 3450, 2925, 1738, 1706, 1642, 1640, 1608, 1158 cm–1; 13C NMR (150 MHz) and 1H NMR (600 MHz) spectroscopic data, see Tables 1 and 2; HR-ESI-MS m/z: 607.2799 [M + FA – H]− (calcd for C31H43O12 607.2765).
4.3.4. Physaliolide O (4)
White amorphous powder; [α]D20 +18.6 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.15), 224 (4.38) nm; IR νmax (KBr): 3455, 2920, 1730, 1705, 1642, 1640, 1600, 1160 cm–1; 13C NMR (150 MHz) and 1H NMR (600 MHz) spectroscopic data, see Tables 1 and 2; HR-ESI-MS m/z: 591.2848 [M + FA – H]− (calcd for C31H43O11 591.2815).
4.3.5. Physaliolide P (5)
White amorphous powder; [α]D20 +15.3 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.54), 224 (4.67) nm; IR νmax (KBr): 3454, 2928, 1732, 1706, 1642, 1638, 1601, 1158 cm–1; 13C NMR (150 MHz) and 1H NMR (600 MHz) spectroscopic data, see Tables 1 and 2; HR-ESI-MS m/z: 529.2462 [M + FA – H]− (calcd for C29H37O9 529.2438).
4.4. Anti-inflammatory Assay
All compounds were assayed for their anti-inflammatory activities via inhibiting nitric oxide production in LPS-stimulated murine RAW 264.7 cells using the Griess method.11 The RAW 264.7 cells were cultivated into 96-well plates (2 × 105 cells/well) under 37 °C for 24 h and then activated by LPS (1 μg/mL) with or without experimental compounds at different concentrations from 6.25 to 100 μM and NG-nitro-l-arginine methyl ester (l-NAME) (the positive control). Medium was collected, and Griess reagent was used to measure the NO production level with absorbance at 540 nm using a microplate reader.
4.5. Cell Viability
Cell viability was tested by using the MTT method.4 to explain whether the production was due to the cytotoxicity. RAW 264.7 cells were cultivated into 96-well plates (2 × 104 cells/well) for 24 h. The cells were exposed to the text compounds with indicated concentrations for 24 h. Then MTT (0.5 mg/mL) was added into the wells, which were incubated for 4 h. Dimethyl sulfoxide (100 μL) was transferred into each well to dissolve the crystals and recorded the absorbance at wavelength of UV 570 nm by using a BioTek microplate reader. Experiment paralleled for three times, and the IC50 values of tested compounds 1–10 were calculated, as shown in Table 3.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00467.
13C NMR and 1H NMR spectroscopic data for compounds 6–10 (in CD3OD), ECD spectrum of 1, and HR-ESI-MS, 13C NMR and 1H NMR, HSQC, HMBC, COSY, and NOESY spectra of compounds 1–5 (in CD3OD) (PDF)
Author Contributions
Z.Z., Y.G., and Q.X., designed the study; J.W. performed the experiments with helps from T.Z., M.Y., H.Z., and H.J. The manuscript was prepared by J.W. and Z.Z. All authors discussed the results and their interpretation and commented on the manuscript at all stages.
The authors sincerely thank Syngenta for the studentship awarded to J.W. This work was financially supported by the Chinese National S&T Special Project on Major New Drug Innovation (2019ZX09735-002 and 2017ZX09301059), and the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-3-015).
The authors declare no competing financial interest.
Dedication
This paper is dedicated to Professor Youyou Tu, the 2015 Nobel Prize Laureate of Physiology or Medicine on the Occasion of Her 90th Birthday.
Supplementary Material
References
- Sun C.-P.; Qiu C.-Y.; Yuan T.; Nie X.-F.; Sun H.-X.; Zhang Q.; Li H.-X.; Ding L.-Q.; Zhao F.; Chen L.-X.; Qiu F. Antiproliferative and Anti-inflammatory Withanolides from Physalis angulata. J. Nat. Prod. 2016, 79, 1586–1597. 10.1021/acs.jnatprod.6b00094. [DOI] [PubMed] [Google Scholar]
- Abe F.; Nagafuji S.; Okawa M.; Kinjo J. Trypanocidal Constituents in Plants 6. Minor Withanolides from the Aerial Parts of Physalis angulata. Chem. Pharm. Bull. 2006, 54, 1226–1228. 10.1248/cpb.54.1226. [DOI] [PubMed] [Google Scholar]
- Guan Y.-Z.; Shan S.-M.; Zhang W.; Luo J.-G.; Kong L.-Y. Withanolides from Physalis minima and their inhibitory effects on nitric oxide production. Steroids 2014, 82, 38–43. 10.1016/j.steroids.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Wu J.; Li X.; Zhao J.; Wang R.; Xia Z.; Li X.; Liu Y.; Xu Q.; Khan I. A.; Yang S. Anti-inflammatory and cytotoxic withanolides from Physalis minima. Phytochemistry 2018, 155, 164–170. 10.1016/j.phytochem.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Gao C.; Li R.; Zhou M.; Yang Y.; Kong L.; Luo J. Cytotoxic withanolides from Physalis angulata. Nat. Prod. Res. 2018, 32, 676–681. 10.1080/14786419.2017.1338281. [DOI] [PubMed] [Google Scholar]
- Zhu X.-H.; Takagi M.; Ikeda T.; Midzuki K.; Nohara T. Withanolide-type steroids from Solanum cilistum. Phytochemistry 2001, 56, 741–745. 10.1016/s0031-9422(00)00487-8. [DOI] [PubMed] [Google Scholar]
- Sun C.-P.; Kutateladze A. G.; Zhao F.; Chen L.-X.; Qiu F. A novel withanolide with an unprecedented carbon skeleton from Physalis angulata. Org. Biomol. Chem. 2017, 15, 1110–1114. 10.1039/c6ob02656g. [DOI] [PubMed] [Google Scholar]
- Sun C.-P.; Zhao F.; Kang N.; Chen L. X.; Qiu F. Physalins V-IX, 16,24-cyclo-13,14-seco-withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057. 10.1038/s41598-017-03849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.-P.; Ma L.; Luo J.-Y.; He F.-Y.; Lou L.-G.; Hu L.-H. Cytotoxic Withanolides fromPhysalis angulata L. Chem. Biodiversity 2007, 4, 443–449. 10.1002/cbdv.200790036. [DOI] [PubMed] [Google Scholar]
- Gao C.-Y.; Ma T.; Luo J.; Kong L. Y. Three New Cytotoxic Withanolides from the Chinese Folk Medicine Physalis angulata. Nat. Prod. Commun. 2015, 10, 2059–2062. 10.1177/1934578x1501001211. [DOI] [PubMed] [Google Scholar]
- Kang N.; Yuan R.; Huang L.; Liu Z.; Huang D.; Huang L.; Gao H.; Liu Y.; Xu Q.-M.; Yang S. Atypical Nitrogen-Containing Flavonoid in the Fruits of Cumin (Cuminum cyminum L.) with Anti-inflammatory Activity. J. Agric. Food Chem. 2019, 67, 8339–8347. 10.1021/acs.jafc.9b02879. [DOI] [PubMed] [Google Scholar]
- Lan Y.-H.; Chang F.-R.; Pan M.-J.; Wu C.-C.; Wu S.-J.; Chen S.-L.; Wang S.-S.; Wu M.-J.; Wu Y.-C. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009, 116, 462–469. 10.1016/j.foodchem.2009.02.061. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.