Introduction
Mammalian erythropoiesis occurs in three stages; the primitive, the fetal definitive and the adult definitive stages.1 After the primitive stage, which takes place in the yolk sac, definitive erythropoiesis moves to the fetal liver and the spleen but is finally restricted to the bone marrow, as adult definitive red blood cells, for the rest of the life.2 After birth, the location of erythropoiesis gradually switches to spongy flat bones, such as ilium, sternum, ribs, and cranium, the sites which adults rely on mostly for steady-state erythropoiesis.3 Erythrocytes are constantly produced under a highly orchestrated process regulated by multiple factors in adult bone marrow niches and local tissue microenvironments that control hematopoietic stem cell maintenance/survival.4 Nonetheless, stresses such as anemia, chronic infection, pregnancy, cancer, hematologic disorders, and stromal abnormalities disrupt this balance in the bone marrow, causing erythropoiesis to occur outside of the bone marrow (e.g. in spleen and liver).5,6 It is worth noting that stress erythropoiesis may be a better reflection of this phenomenon than extramedullary erythropoiesis (EE) in some cases. However, as will be discussed in this perspective, there is a rich body of evidence demonstrating the occurrence of EE under different physiological and pathological conditions.
EE implies the generation of erythrocytes outside of medullary spaces of the bone marrow.7 Under these circumstances, EE is considered to be the main cause of the abundance of erythroid precursors in the periphery. This may occur as a result of passive incontinence of hematopoietic cells from the sites of EE, where tissue structure/control of cell egress is less efficient than that of the bone marrow.8 However, the clinical implication of the expansion of erythroid precursors outside of the bone marrow has not been well defined. This perspective aims to provide the reader with an overview of the current understanding of the immunological consequences of EE.
Erythroid precursors are the newborn’s first-time enemies but lifelong friends
Newborns are highly susceptible to fatal infections. This susceptibility has generally been ascribed to the immaturity of the neonatal immune system. Nevertheless, this old dogma has been challenged with the discovery of the physiological abundance of immunosuppressive erythroid precursors during this developmental stage of life. It has been reported that the spleen of neonatal mice is impressively enriched with erythroid precursors co-expressing the transferrin receptor CD71 and the erythroid lineage marker TER119. The levels of these cells were maximal between days 6-9 but gradually declined to the adult level by day 21 in experiments performed in Cincinnati, USA9 and by day 28 in experiments performed in Edmonton, Canada.10 This difference might be related to geographical factors, such as altitude, or differences in the animals’ microbiome. Likewise, human cord blood and placenta accommodate an equally enriched proportion of erythroid precursor cells co-expressing CD71 and the erythroid lineage marker CD235a (glycophorin A). However, these cells are sparse in the peripheral blood of healthy adults.9 Since their discovery, we have defined these cells as “CD71+ erythroid cells (CEC)”.11 CEC are mainly erythroid precursors expressing high levels of CD71, including reticulocytes but excluding mature red blood cells. Neonatal CEC express arginase-2, whose activity is essential for the cells’ immunosuppressive properties.9 The presence of CEC was found to be associated with increased neonatal susceptibility to infection.9 However, CEC-mediated susceptibility to infection was counterbalanced by these cells’ protective role against aberrant immune cell activation in the intestine, allowing swift colonization by microbial communities after parturition.9 This was shown by increased immune cell activation and production of pro-inflammatory cytokines [interleukin (IL)-6 and tumor necrosis factor (TNF)-α] by antigen-presenting cells in the intestine when CEC were partially depleted in wildtype compared to germ-free mice.9 In addition, following studies in a murine model of whooping cough, it was reported that CEC impaired innate immune responses against Bordetella pertussis infection.10 Specifically, depletion of CEC unleashed an innate immune response characterized by enhanced production of protective cytokines [interferon (IFN)-γ, TNF-α, and IL-12] and resulted in the recruitment of natural killer cells and antigen-presenting cells in the lungs of neonatal mice, which restored resistance to B. pertussis infection. In contrast, neonatal CEC adoptively transferred into adult recipients by intravenous injection impaired the adults’ innate immune response against B. pertussis infection.10 Moreover, the enzymatic activity of arginase-2 secreted by CEC inhibited phagocytosis of B. pertussis in vitro.10 These observations challenged the notion of neonatal susceptibility to infection being due to intrinsic defects of immune cells, and instead highlighted active immune suppression mediated by the abundance of CEC in the newborn. These findings provided additional support to the novel concept in neonatal immunology that immuno-suppression is essential to dampen costly robust immune responses in newborns. Further studies demonstrated that CEC hindered adaptive cellular and humoral immune responses to infection with B. pertussis and vaccination against this pathogen in neonatal mice. Depletion of CEC before vaccination resulted in a substantial increase in the induction of antigen-specific protective cytokines (IFN-γ and IL-17) and antibodies (IgA and IgG) against B. pertussis.12 Similarly, the ablation of CEC before a primary infection resulted in more robust, protective immunity following re-infection with B. pertussis in neonatal mice.12 These observations suggest that the accumulation of CEC in the periphery could have detrimental effects on both the innate and adaptive immune responses to pathogens. Furthermore, CEC from human cord blood and placenta have immunosuppressive effects following stimulation with different bacterial ligands or anti-CD3/CD28 in vitro.9,12,13 In a complementary study, pre-term labor-derived human cord blood CEC were shown to participate in the suppression of CD4+ and CD8+ T-cell proliferation and modulate cytokine production by antigen-presenting cells in the presence of heat-killed Listeria monocytogenes.14 These observations raised the possibility that CEC might have immunomodulatory rather than immunosuppressive properties, leading to enhanced pro-inflammatory cytokine production under specific circumstances (e.g., CEC from pre-term versus full-term cord blood). Although CEC impair both innate and adaptive immune responses against pathogens in the neonate, their crucial role in the host’s adaptation to microbial communities has lifelong benefits and deserves appreciation (Figure 1).
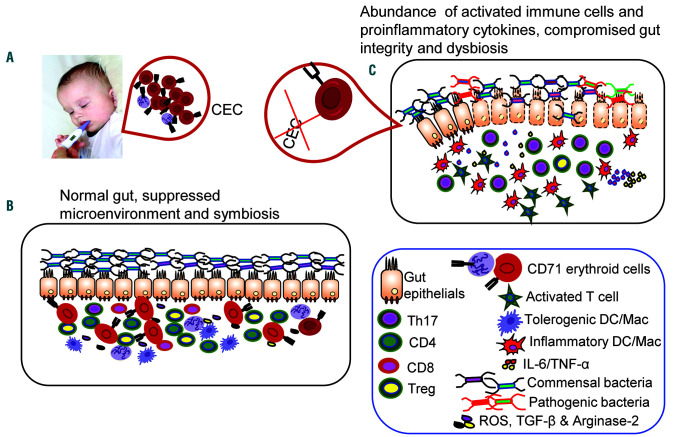
Figure 1.
CD71+ erythroid cells play an important role in gut homeostasis. (A) Physiological abundance of CD71+ erythroid cells (CEC) is associated with increased neonatal susceptibility to infections. (B) Model illustrating the putative role of CEC in providing an immunosuppressive environment upon release of regulatory mediators such as reactive oxygen species, transforming growth factor-β, arginase-2 as well as the induction of regulatory T cells (Treg), which might contribute to maintaining symbiosis with the microbiome and intestinal integrity. (C) An absence or reduction of CEC results in a pro-inflammatory state associated with raised levels of tumor necrosis factor-α and interleukin-6, and hyper-immune activation, but lower numbers of Treg, which results in compromised intestinal integrity and dysbiosis. DC: dendritic cell; Mac; macrophage; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; ROS: reactive oxygen species; TGF-β: transforming growth factor-β.
Future research should be directed at understanding the cross-talk between CEC and microbial communities to determine any therapeutic benefit in human newborns. Such novel studies will establish the scientific framework for more in-depth translational studies in the future.
Immunological benefits of extramedullary erythropoiesis in pregnancy and gut homeostasis
A good pregnancy outcome requires selective silencing of maternal immune effector cells against the father’s fetal alloantigens.15,16 The fetus is antigenically similar to a semi-allogeneic transplant, with the risk of immunological rejection. As such, the mother’s immune response during gestation requires tolerance to alloantigens, preventing potentially damaging immune responses that may result in abortion or preterm delivery.17 The maintenance of pregnancy does, therefore, represent a major challenge for the maternal immune system, since it has to tolerate a semi-allogeneic fetus and at the same time protect both the mother and the fetus against potential pathogens. Several mechanisms have been reported to be involved in blocking the immunological rejection of the fetus,18,19 including those modified by CEC. During pregnancy, especially after mid-gestation, the total red blood cell count increases to meet the increased demand for blood supply by the mother and the fetus. The normal range of erythropoietin concentration in pregnant women is varied, although erythropoietin concentration rises as the demand for blood supply increases.20 This high level of erythropoietin, alongside other factors, such as 27-hydroxycholesterol, the cholesterol metabolite, which induces hematopoietic stem cell mobilization through the estrogen receptor α, is required for EE formation during gestation.21 In concert with estradiol, 27-hydroxycholes-terol promotes EE by regulating estrogen receptor α function in hematopoietic stem cell mobilization. In agreement with this concept, Delyea et al. found physiological expansion of CEC in the spleen and peripheral blood of an allogeneic mouse model of pregnancy.22 Although a moderate expansion of CEC was observed in syngeneic pregnancy, it was significantly lower than that in the allogeneic mice.22 This suggests a potential role for CEC in response to alloantigens. In support of these hypotheses, an abundance of CEC was found at the feto-maternal interface during pregnancy.22 Maternal CEC, like the neonatal CEC, expressed arginase-2 and activity of this enzyme was required for the cells’ inhibitory effect against the aggressive allogeneic response at the feto-maternal interface. These CEC from the spleen and placental tissues of pregnant mice, unlike neonatal CEC, expressed substantial levels of programmed death-1/2 ligands (PDL-1/PDL-2) and subsequently suppressed PD-1-expressing T cells at the feto-maternal interface.22 Furthermore, the ablation of CEC in allogeneic mice skewed the immune response toward a Th1 response characterized by upregulation of inflammatory cytokines and chemokines (e.g. TNF-α and IFN-γ, IL-6 and CXCL-1) resulting in fetal resorption.22 Similarly, expansion of CEC was observed in the peripheral blood of pregnant women in the second and third trimesters of pregnancy.13 CEC from either peripheral blood or cord blood/placental tissues of healthy mothers exhibited immunosuppressive properties. However, the frequency of CEC was lower in mothers with inflammatory bowel disease (IBD) and the cells were functionally impaired when examined in vitro.13 IBD is associated with intestinal dysbiosis and dysfunctional interaction between the microbiota and the gut mucosal immune system, which results in a dysregulated immune response against commensal microbial antigens.23 The reduced frequency and/or impaired functionality of CEC during pregnancy may, therefore, predispose patients with IBD to a more pro-inflammatory milieu in their gastrointestinal tract, characterized by lower numbers of regulatory T cells (Treg), higher concentrations of IL-6 and TNF-α, and dysbiosis.13 In line with this, in the absence of CEC, upregulation of IL-6 and TNF-α production by residential antigen-presenting cells in the gut was observed in a mouse model of pregnancy.13,22 Immune activation following upregulation of TNF-α production may result in excessive tissue damage and disruption of tight junctions in IBD mothers.24 In agreement with this, an increased permeability of the intestinal epithelial barrier was noted in pregnant mice when CEC were depleted.13 Compromised intestinal barrier integrity may result in translocation of bacteria and their products, triggering a vicious cycle of inflammatory response.
An inflammatory milieu may influence the diversity and frequency of microbial communities in the gut. As such, the ablation of CEC was associated with dysbiosis during pregnancy, suggesting a crucial role for these cells in maintaining homeostasis and symbiosis.13 The initial establishment of the neonatal microbiome is mainly determined by maternal-newborn exchanges of the microbiota. During normal vaginal delivery, the newborn is exposed to an army of new allies, which colonize the urogenital tract of the mother.25 Delivery via Cesarean section deprives the newborn of these microbial communities.26 Thus, vaginal delivery and subsequent exposure to maternal microbiota via nursing are evolutionarily important to the development of the newborn’s immune system. Interestingly, not only was the frequency of CEC observed in the cord blood and placenta of a twin delivered by Cesarean section lower than that of the vaginally delivered twin, but the CEC of the two twins also had a different gene profile.27 It can, therefore, be speculated that lower levels of CEC in IBD patients may result in intestinal dysbiosis and poor pregnancy outcomes.28,29 In support of this hypothesis, lower CEC frequency was associated with preterm deliveries and emergency Cesarean sections.30 Therefore, during gestation EE is not only required as a physiological response to the demand for a greater blood supply but it also plays a crucial role in feto-maternal tolerance and symbiosis. However, whether the source of abundant CEC in pregnant women, similar to mice, is splenic EE or due to passive incontinence from the bone marrow is unknown.
How erythroid precursors modulate immune responses in cancer
Anemia has been described as a primary consequence of tumor development in some oncological patients and animal models of cancer.31 The pathogenesis of cancer-related anemia is complicated and can be multifactorial. There are several reports on EE development in malignant solid tumors such as breast and lung cancers.32,33 Although the principal explanation for the formation of EE in solid tumors is unknown, it appears that erythropoiesis-stimulating agents may play a pivotal role in the emergence of EE niches in cancer patients.34 A recent study highlighted the expansion of CEC (named Ter-cells in that study) in the spleen of an animal model of hepatocellular carcinoma.35 Han et al. reported that tumor-derived transforming growth factor (TGF)-β activates the Smad3 downstream signaling pathway, which induces CEC from erythroid progenitor cells in the spleen. These CEC, by releasing artemin, a member of the glial cell line-derived neurotrophic factor family, directly promote the development and metastasis of hepatocellular carcinoma via an interaction of artemin with its receptor GFRα3 on tumor cells.35 Although Han et al. claimed that these erythroid precursors lacked immunosuppressive properties, a more recent study demonstrated immunosuppressive effects of CEC in advanced cancer.36 The latter study reported an association between the expansion of immunosuppressive CEC and impaired Epstein-Barr virus-specific CD8+ T-cell proliferation in patients with advanced cancer who were anemic.36 This research group also described strongly impaired CD8+ and CD4+ T-cell proliferation from melanoma-bearing C57BL/6 mice by splenic CEC when co-cultured in vitro,36 and that CD45+-expressing CEC showed more robust production of reactive oxygen species (ROS) compared to CD45− CEC.36 Accordingly, CD45+ CEC exhibited more potent suppression of virus-specific CD8+ T-cell proliferation compared to their CD45− counterparts in a mouse model of chronic lymphocytic choriomeningitis virus infection.36
In addition to ROS production, we believe that CEC may utilize other soluble regulatory mediators (e.g. arginase-2, TGF-β, and galectins) or, via cell-cell interactions, modulate the functionality of immune cells in different conditions including cancer (Figure 2). As proof-of-concept, Shahbaz et al. found that a subpopulation of neonatal CEC, which express the inhibitory molecule V-domain Ig suppressor of T-cell activation (VISTA), promotes the development of Treg through TGF-β37 as VISTA+ CEC were the major source of TGF-β production compared to CD71+ VISTA− erythroid cells.37 Subsequently, CD71+ VISTA+ cells, via TGF-β, promoted the generation of Treg from naïve CD4+ T cells in vitro.37 It is, therefore, possible that expanded CEC in chronic conditions such as cancer can also promote the development of Treg and indirectly suppress T-cell effector functions.
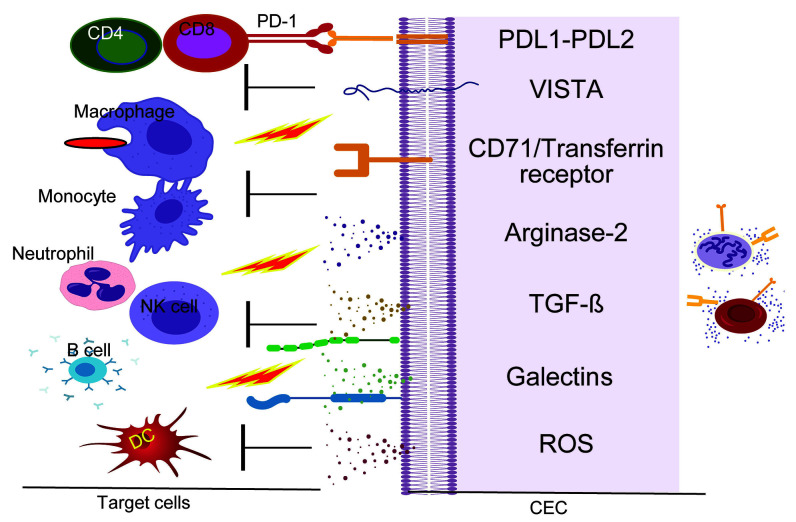
Figure 2.
Putative immunoregulatory properties of CD71+ erythroid cells. The proposed model hypothesizes that CD71+ erythroid cells via cell-cell interactions (VISTA:?, PD-1:PDL1/PDL-2 and Galectins) or via soluble factors (e.g. TGF-β, Arginase-2, ROS and Galectins) suppress or modulate the functionality of different immune cells. PD-1: programmed cell death protein 1; PDL: programmed death ligand; VISTA: V-domain Ig suppressor of T-cell activation; TGF-β: transforming growth factor-β. ROS: reactive oxygen species; DC: dendritic cells; CEC: CD71+ erythroid cells.
Human immunodeficiency virus binds to erythroid precursors via CD235a
Although an interaction of human immunodeficiency virus (HIV) with red blood cells via the Duffy antigen receptor for chemokines and complement receptor-1 has been documented,38,39 the role of CEC in HIV pathogenicity has not been investigated until now. Expansion of CEC in the blood of HIV-infected and anemic individuals was recently reported.40 It was demonstrated that these cells, via ROS, exacerbate HIV replication/infection in CD4+ T cells and even make CD4+ T cells more permissive to HIV infection. Besides, by binding to CD235a on the surface of CEC, HIV can travel to different parts of the body.40 In support of this, a positive correlation between plasma viral load and the frequency of these cells was found in HIV patients. More importantly, it was observed that infective HIV particles reside inside CEC but not inside mature red blood cells. Therefore, by harboring HIV, CEC can play an important role in the pathogenesis of HIV-related disease.
Conclusion
In general, EE is not considered a physiological event in adults but a compensatory mechanism occurring secondary to inadequate medullary function in adults.41 EE is, therefore, identified as secondary or accessory to another factor that directly affects the bone marrow or to a systemic event that subsequently impacts the bone marrow. For instance, EE can be related to stromal abnormalities in the bone marrow such as osteopetrosis, and marrow fibrosis in which narrow storage becomes limited. Moreover, EE can be prompted by hematologic disorders, chronic infections and cancer.41,42 Overall, in terms of clinical relevance, EE should be considered a risk factor for an underlying condition in adults. However, EE may be considered a normal physiological process during pregnancy and in developing newborns.
Regardless of the underlying mechanism, EE results in the development of erythroblastic islands in other organs/tissues, in particular the spleen and liver. EE results in an abundance of erythroid precursors or CEC in the periphery, which can be costly for the host. As we discussed above, erythroid precursors have immunosuppressive or immunomodulatory properties and their expansion can, therefore, have an impact on the effector functions of various different immune cells. CEC compromise neonatal innate immune responses against prenatal pathogens9,10 and also impair adaptive immunity in newborns.12 We and others have demonstrated that CEC, via soluble mediators such as arginase-2, TGF-β, and ROS or through cell-cell interactions (e.g. PD-1:PDL-1, VISTA:?), suppress/modulate different immune cells in vitro and in vivo.11,14,36,43 These observations highlight the diverse immunosuppressive and/or immunomodulatory properties of CEC in different scenarios. The immunological consequences of EE in different pathological conditions such as autoimmune diseases, hematologic disorders, chronic infections, malnutrition, anemia, parasitic infections, and cancer should, therefore, be taken into consideration. Moreover, understanding the mechanisms controlling the extramedullary microenvironment might lead to a better comprehension of the rules balancing immunity and tolerance induction contributed by CEC, particularly during pregnancy and in newborns. In conclusion, more in-depth investigations are required to better appreciate the diverse immunological properties of these forgotten cells in different circumstances such as thalassemia, malaria and other hematologic disorders.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR), a CIHR New Investigator Salary Award (360929) and a CIHR Foundation Scheme Grant (353953) (all to SE).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/6/1478
References
- 1.Palis J. Ontogeny of erythropoiesis. Curr Opin Hematol. 2008;15(3):155–161. [DOI] [PubMed] [Google Scholar]
- 2.Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119(21):4828–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3(4): a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto K, Miwa Y, Abe-Suzuki S, et al. Extramedullary hematopoiesis: elucidating the function of the hematopoietic stem cell niche (review). Mol Med Rep. 2016;13(1): 587–591. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med. 2010;1:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan N, Lavu S, Hanson CA, Tefferi A. Extramedullary hematopoiesis in the absence of myeloproliferative neoplasm: Mayo Clinic case series of 309 patients. Blood Cancer J. 2018;8(12):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49(3):508–523. [DOI] [PubMed] [Google Scholar]
- 9.Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504(7478): 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid suppressor cells compromise neonatal immune response against Bordetella pertussis. J Immunol. 2017;199(6):2081–2095. [DOI] [PubMed] [Google Scholar]
- 11.Elahi S. Neglected cells: immunomodulatory roles of CD71(+) erythroid cells. Trends Immunol. 2019;40(3):181–185. [DOI] [PubMed] [Google Scholar]
- 12.Namdar A, Koleva P, Shahbaz S, Strom S, Gerdts V, Elahi S. CD71+ erythroid suppressor cells impair adaptive immunity against Bordetella pertussis. Sci Rep. 2017;7(1):7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunsmore G, Koleva P, Ghobakhloo N, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis. 2019;40(3):181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller D, Romero R, Unkel R, et al. CD71+ erythroid cells from neonates born to women with preterm labor regulate cytokine and cellular responses. J Leukoc Biol. 2018;103(4):761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107 (20):9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. [DOI] [PubMed] [Google Scholar]
- 18.Rowe JH, Ertelt JM, Xin LJ, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L, Ertelt JM, Rowe JH, et al. Cutting edge: committed Th1 CD4+ T cell differentiation blocks pregnancy-induced Foxp3 expression with antigen-specific fetal loss. J Immunol. 2014;192(7):2970–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotes PM, Canning CE, Lind T. Changes in serum immunoreactive erythropoietin during the menstrual cycle and normal pregnancy. Br J Obstet Gynaecol. 1983;90(4):304–311. [DOI] [PubMed] [Google Scholar]
- 21.Oguro H, McDonald JG, Zhao Z, Umetani M, Shaul PW, Morrison SJ. 27-Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. J Clin Invest. 2017;127(9):3392–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delyea C, Bozorgmehr N, Koleva P, et al. CD71(+) erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J Immunol. 2018; 200(12):4044–4058. [DOI] [PubMed] [Google Scholar]
- 23.Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest. 2014;124(10):4190–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabinger T, Bode KJ, Demgenski J, et al. Inhibitor of apoptosis protein-1 regulates tumor necrosis factor-mediated destruction of intestinal epithelial cells. Gastroenterology. 2017;152(4):867–879. [DOI] [PubMed] [Google Scholar]
- 25.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunsmore G, Koleva P, Sutton RT, Ambrosio L, Huang V, Elahi S. Mode of delivery by an ulcerative colitis mother in a case of twins: immunological differences in cord blood and placenta. World J Gastroenterol. 2018;24(42):4787–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56(6):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephansson O, Larsson H, Pedersen L, et al. Congenital abnormalities and other birth outcomes in children born to women with ulcerative colitis in Denmark and Sweden. Inflamm Bowel Dis. 2011;17(3):795–801. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Lopez N, Romero R, Xu Y, et al. Umbilical cord CD71+ erythroid cells are reduced in neonates born to women in spontaneous preterm labor. Am J Reprod Immunol. 2016;76(4):280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim A, Rivera S, Shprung D, et al. Mouse models of anemia of cancer. PloS One. 2014;9(3):e93283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu FI, Filippa DA, Castro-Malaspina H, Downey RJ. Extramedullary hematopoiesis mimicking metastatic lung carcinoma. Ann Thorac Surg. 1998;66(4):1411–1413. [DOI] [PubMed] [Google Scholar]
- 34.Redmond J, 3rd, Kantor RS, Auerbach HE, Spiritos MD, Moore JT. Extramedullary hematopoiesis during therapy with granulocyte colony-stimulating factor. Arch Pathol Lab Med. 1994;118(10):1014–1015. [PubMed] [Google Scholar]
- 35.Han Y, Liu Q, Hou J, et al. Tumor-induced generation of splenic erythroblast-like Ter-cells promotes tumor progression. Cell. 2018;173(3):634–648. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, He R, Long H, et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat Med. 2018;24(10):1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahbaz S, Bozorgmehr N, Koleva P, et al. CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-beta. PLoS Biol. 2018;16(12):e2006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He W, Neil S, Kulkarni H, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe. 2008;4(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montefiori DC, Graham BS, Zhou JY, Zhou JT, Ahearn JM. Binding of human immunodeficiency virus type 1 to the C3b/C4b receptor CR1 (CD35) and red blood cells in the presence of envelope-specific antibodies and complement. National Institutes of Health AIDS Vaccine Clinical Trials Networks. J Infect Dis. 1994;170(2):429–432. [DOI] [PubMed] [Google Scholar]
- 40.Namdar A, Dunsmore G, Shahbaz S, et al. CD71(+) erythroid cells exacerbate HIV-1 susceptibility, mediate trans-infection, and harbor infective viral particles. mBio. 2019;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohawon D, Lau KK, Lau T, Bowden DK. Extramedullary haematopoiesis: a pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol. 2012;56(5):538–544. [DOI] [PubMed] [Google Scholar]
- 42.Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48(6):1426–1437. [DOI] [PubMed] [Google Scholar]
- 43.Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol. 2014;5:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.