Abstract
Antibiotics continue to be the standard-of-care for bacterial vaginosis (BV), although recurrence rates are high. Vaginal probiotics may improve durability of BV treatment, although few probiotics for vaginal health contain Lactobacillus spp. that commonly colonize the lower female genital tract. Characteristics of vaginal Lactobacillus strains from South African women were evaluated for their probiotic potential in vitro compared to strains from commercial vaginal products, including growth at varying pHs, ability to lower pH, produce D-/L-lactate and H2O2, influence growth of BV-associated Gardnerella vaginalis and Prevotella bivia, adherence to cervical cells and susceptibility to antibiotics. Fifty-seven Lactobacillus strains were purified from cervico-vaginal fluid, including L. crispatus, L. jensenii, L. gasseri, L. mucosae, and L. vaginalis. L crispatus strains grew better at pHs below 4.5 and lowered pH more effectively than other strains. Production of D-/L-lactate and H2O2 varied between Lactobacillus species and strains. Lactobacillus strains generally inhibited P. bivia more uniformly than G. vaginalis isolates. All vaginal Lactobacillus isolates were resistant to metronidazole while susceptibility to clindamycin varied. Furthermore, vaginal Lactobacillus strains tended to be broadly susceptible to penicillin, amoxicillin, rifampicin and rifabutin. Whole-genome-sequencing of five of the best-performing vaginal Lactobacillus strains confirmed their likely safety, due to antimicrobial resistance elements being largely absent, while putative intact prophages were present in the genomes of two of the five strains. Overall, vaginal Lactobacillus strains largely performed better in these in vitro assays than probiotic strains currently used in probiotics for vaginal health. Including the best-performing vaginal Lactobacillus isolates in a region-specific probiotic for vaginal health may result in improved BV treatment options.
Author summary
Lactobacillus species in the lower reproductive tract of healthy women lower vaginal pH and protect against sexually transmitted infections. However, women commonly suffer from bacterial vaginosis, a disruption in the optimal Lactobacillus-dominated genital microbiota to a more diverse one marked by higher pH, vaginal discharge and inflammation. Bacterial vaginosis is associated with adverse pregnancy outcomes and increased risk of sexually transmitted infections, including HIV. While antibiotics are the standard-of-care for bacterial vaginosis, most cases recur within six-months. Probiotics that include lactobacilli have been explored to improve treatment, although the majority of products do not contain species commonly found in the vagina. Here, we characterised a large panel of vaginal Lactobacillus strains from young African women (including growth, adhesion to host cells, ability to produce antimicrobial products, and pathogen inhibition), compared to isolates from commercial probiotic products for vaginal health. We also sequenced the genomes of top-performing isolates. Several vaginal Lactobacillus strains exhibited better probiotic profiles than commercial strains, suggesting that they would be beneficial in the development of probiotic treatment of bacterial vaginosis. A wider range of well-characterized Lactobacillus-containing probiotics may improve bacterial vaginosis treatment outcomes, which would hopefully lower the burden of adverse pregnancy outcomes and sexually transmitted infections.
Introduction
Maintenance of vaginal health is important in protecting women from adverse urogenital and reproductive health outcomes [1]. Optimally, the lower female genital tract (FGT) has a pH <4.5 [2,3] and a Lactobacillus-dominated microbiota [4,5]. However, the lower FGT microbiota frequently shifts to a non-optimal state that is characterised by a depletion of Lactobacillus spp. and high relative abundance of a diverse array of anaerobic bacteria, coinciding with elevation in vaginal pH ≥4.5—referred to as bacterial vaginosis (BV) [1]. BV can lead to severe reproductive complications [6–8], such as an increase in the risk for acquiring and transmitting sexually-transmitted infections (STIs) including human immunodeficiency virus (HIV) [9–11]. The current clinical standard of care (SOC) for BV is either oral or vaginal metronidazole or clindamycin [12]. However, antibiotic treatment of BV only results in a short-term cure as the recurrence rates are high, with ~50% of women recurring within six months [13,14]. As a result of this, several clinical studies evaluated Lactobacillus-containing probiotics as an adjunct to BV treatment and have shown largely beneficial although heterogeneous outcomes [14–17]. It is therefore crucial to develop more effective probiotic treatment strategies for BV. Few probiotics are explicitly marketed for vaginal health internationally and in South Africa [18], and only few of these contain Lactobacillus spp. commonly found in FGTs of women with optimal microbiota. Bacterial strains should fulfil specific biological criteria if their intended purpose is to be developed into a probiotic to improve FGT health–collectively referred to as the preferred product profile (PPP). In this study, we evaluated a range of PPP characteristics that should be considered in the development of vaginal probiotics. These included: (1) originating from the FGT, as vaginal Lactobacillus spp. are highly adapted for this specialized niche [19]; (2) ability to adhere well to FGT cells, as adherent isolates are more likely to remain locally [20–22]; (3) inhibiting the growth of BV-associated species, including G. vaginalis and P. bivia, [23–25] (4) tolerating low pH associated with vaginal health [26–28]; (5) lowering in vitro culture pH conditions, thereby inhibiting viral and bacterial pathogens [2,13,29,30]; (6) producing L- and D-lactate, which are involved in viral and bacterial pathogen inhibition [30–32]; and (7) tolerating antibiotics used to treat BV if administered in conjunction SOC [33–35]. All these PPP characteristics call for the use of bacterial strains adapted to the particular conditions of the FGT, which will mostly be met in Lactobacillus spp. originating from the FGT of generally healthy women. Thus, this study aimed to isolate and evaluate key characteristics of vaginal Lactobacillus strains from South African women to select the top performing strains for the development of a probiotic for vaginal health. Furthermore, the characteristics of vaginal strains were compared to those of commercially available probiotic strains currently used in probiotic formulations for vaginal health in South Africa and internationally.
Results
A total of 57 Lactobacillus strains were isolated from the FGT of 26 young (median age 18 years; IQR 17–19 years) South African women, including 10 L. crispatus, 9 L. gasseri, 18 L. jensenii, 8 L. vaginalis, and 12 L. mucosae strains (Table 1). Lactobacillus isolates of the same species, derived from the same woman, were only included if they showed different in vitro characteristics to avoid selection of identical strains. The characteristics of these vaginal Lactobacillus strains were compared to four Lactobacillus ATCC reference strains (L. crispatus 33197, L. gasseri 9857, L. jensenii 25258, and L. vaginalis 49540) and 10 Lactobacillus strains isolated from commercially available probiotics, including L. reuteri (n = 1), L. rhamnosus (n = 6) and L. acidophilus (n = 3) (Table 1).
Table 1. Details of vaginal, probiotic and ATCC reference Lactobacillus strains.
| Vaginal species | n | ID | Age@ | Cluster& | BV* | STI# | Isolate ID |
|---|---|---|---|---|---|---|---|
| L. crispatus | 10 | 70 80 94 95 96 100 73 |
19 18 18 17 17 18 16 |
- C2 None - - C2 - |
- - - - - - + |
- - - - - - - |
70.1PA, 70.6PA 80.3a 94.77PA 95.34PA 96.9PA, 96.9PB, 96.27PA 100.16a 73.55a |
| L. gasseri | 9 | 94 100 107 114 117 |
18 18 19 17 17 |
- C2 C3 C2 - |
- - - Int. + |
- - + + - |
94.98PB 107.10PB, 107.7PA 100.5PA, 100.46PA 114.1PA, 114.30PA, 114.12PB 117.73PA |
| L. jensenii | 18 | 88 94 95 96 72 93 84 73 89 92 |
20 18 17 17 16 17 18 16 18 18 |
- - - - C3 None C3 - - - |
- - - - - - Int + + + |
- - - - + + - - - - |
88.10PA, 88.33PA 94.70PA 95.1PA, 95.22PA, 95.31PA, 95.37PA 96.8PA, 96.45PA, 96.45PB 72.14PA, 72.22PA 93.18PA 73.2PA 84.35PA 89.50PA 92.1PA, 92.27PA |
| L. vaginalis | 8 | 80 88 91 100 73 81 79 |
18 20 18 18 16 18 18 |
C2 - C1 C2 - - C1 |
- - - - + + + |
- - - - - - + |
80.3b, 80.23b 88.5b 91.8a 100.13PA 73.27PA 81.17A 79.24PA |
| L. mucosae | 12 | 80 99 85 102 87 98 86 90 |
18 20 16 17 19 21 22 18 |
C2 C1 - C3 C1 C3 - C1 |
- - Int Int + + + + |
- - - - - - + + |
80.23a 99.1PA 85.1PA, 85.30PA 102.33PA 87.5PA, 87.21PA 98.46PA, 98.52PA 86.4PA, 86.30PA 90.13PA |
|
ATCC strains L. crispatus L. gasseri L. jensenii L. vaginalis |
4 |
33197 9857 25258 49540 |
|||||
|
Probiotic strains L. reuteri L. rhamnosus L. rhamnosus L. rhamnosus L. rhamnosus L. rhamnosus L. rhamnosus L. acidophilus L. acidophilus L. acidophilus |
10 Oral capsule Oral capsule Vaginal tablet Vaginal pessary Vaginal pessary Oral capsule Vaginal spray Oral capsule Vaginal spray Vaginal tablet |
RFZ1006 (Reuterina) RFZ1006 (Reuterina) 0154 (Provacare) 0200 (Gynophilus) 7447212 (Muvagyn) C21134 (Vagiforte) S21134 (Vagiforte) C21134 (Vagiforte) S21134 (Vagiforte) T20868 (Vagiforte) |
|||||
@Age in years
&C1- diverse microbiota, C2-L. crispatus-dominant, C3-L. iners-dominant; None-no cluster, -data n/a*by Nugent Scoring, with scores 0–3 being negative, 4–6 intermediate and 7–10 positive.
#including C. trachomatis, N. gonorrhoeae, T. vaginalis, M. genitalium, HSV-2, H. ducreyi, T. pallidum and L. venerum, ID-participant identification, CST-community-state-type
The majority of the South African women included in this study were BV and STI negative (Table 1), however, isolates from some women who were BV (n = 10) and/or STI (n = 6)-positive were included as well, to evaluate whether STI or BV status affects probiotic characteristics of vaginal Lactobacillus isolates. The vaginal microbiota of the participants belonged primarily to community cluster 1 (C1, diverse) and 3 (C3, L. iners-dominant), while only few belonged to cluster 2 (C2, L. crispatus-dominant) [36].
Growth kinetics of vaginal and probiotic Lactobacillus isolates at differing pHs
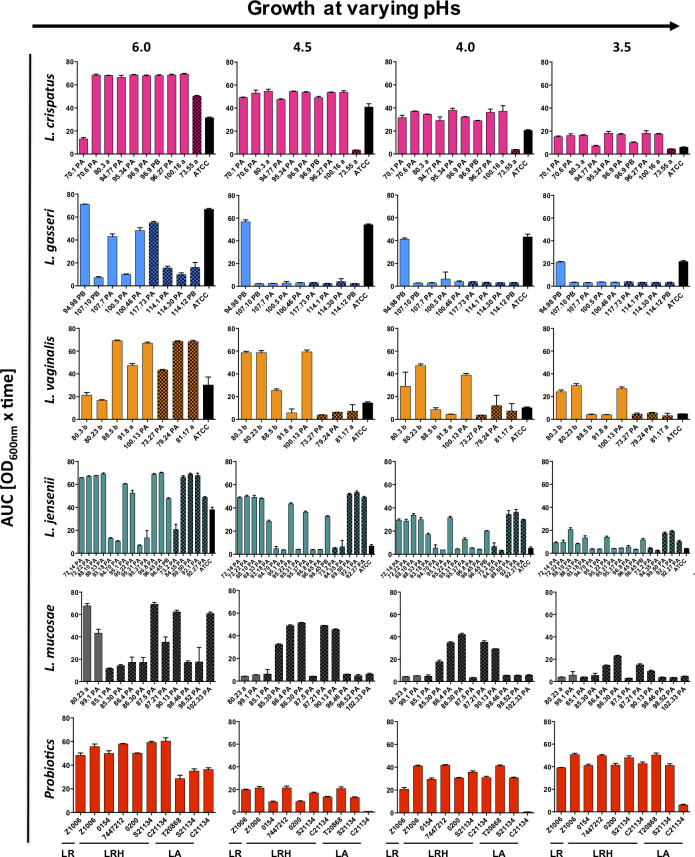
The in vitro growth kinetics of Lactobacillus strains under anaerobic conditions at pH 6.0 were evaluated, a characteristic that is important for commercial scale-up. The primary vaginal Lactobacillus isolates grew variably, between and within species (Fig 1). L. crispatus and L. jensenii strains grew the best, as quantified by calculation of the area under the curve (AUC), followed by L. vaginalis, L. mucosae and lastly L. gasseri strains.
Fig 1. Growth of vaginal and probiotic Lactobacillus isolates at varying culture pHs.
The growth of vaginal L. crispatus (pink), L. gasseri (blue), L. vaginalis (orange), L. jensenii (green) and L. mucosae (grey,) and commercially available probiotic strains (red, LR–L. reuteri, LRH = L. rhamnosus, LA = L. acidophilus) at varying pHs was measured over 48 hours. The AUC was calculated, and each bar represents the mean and standard deviation (SD) for each isolate. Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
The in vitro ability of isolates to grow at lower pHs was considered as important, since these conditions are optimal for the maintance of a healthy vagina [37,38]. All L. crispatus strains showed reduced growth at pHs 4.5, 4.0, and 3.5 compared to pH 6.0, although they appeared to tolerate low pHs better than other Lactobacillus spp. (Fig 1). In contrast, L. jensenii, L. vaginalis, L. gasseri, and L. mucosae strains appeared to be less acid tolerant, with the number of strains able to grow decreasing with lower pHs. Notably, there were no differences in growth at any pH between vaginal Lactobacillus strains isolated from women without BV or STIs versus those originating from women with BV and/or STIs (Fig 1). The majority of vaginal L. crispatus, L. vaginalis and L. jensenii strains grew better than their respective ATCC strain at all pHs tested. In comparison, the ten probiotic strains (including L. reuteri, L. rhamonosus and L. acidophilus) showed less growth than L. crispatus strains at pH 6.0, and the majority of probiotic strains showed some tolerance to pHs 3.5–4.5 (Fig 1).
Ability to lower culture pH
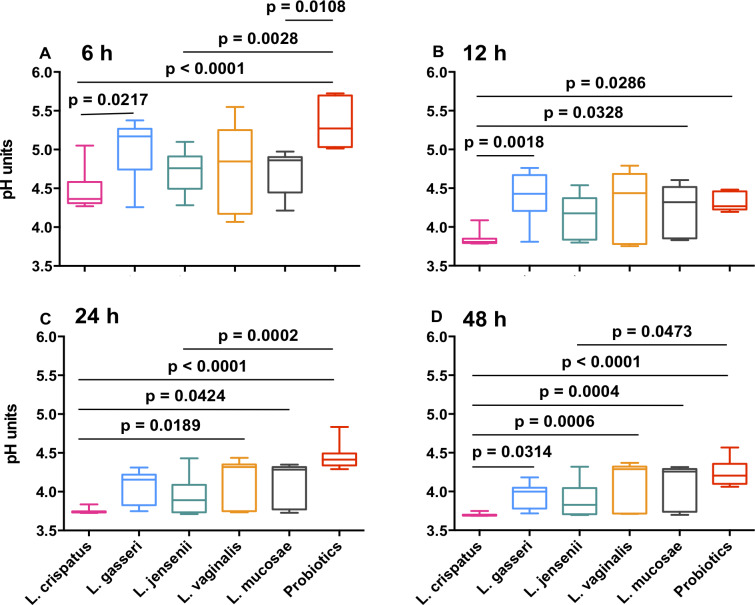
All vaginal Lactobacillus strains tended to have similar kinetics in their ability to lower pH under anaerobic conditions, with the lowest pH reached being pH 3.7, while none of the probiotic isolates lowered the pH <4.2 (S1 Fig). Overall, L. crispatus lowered the pH significantly more than L. gasseri, L. mucosae, L. vaginalis and any of the probiotic strains at 48 hours, after adjusting for multiple comparisons (Fig 2). Growth of individual isolates at pH 6.0 did not significantly predict their ability to lower culture pH at 48 hours (Spearman rho = -0.17, p = 0.165), indicating that differences in growth between strains did not account for differences in their ability to lower pH.
Fig 2. Ability of vaginal and probiotic Lactobacillus spp. to lower culture pH.
MRS culture pH was measured over 48 hours for vaginal L. crispatus (pink), L. gasseri (blue), L. jensenii (green), L. vaginalis (orange), L. mucosae (grey) and the probiotic isolates (red; including L. reuteri, L. rhamnosus and L. acidophilus strains). Non-parametric multiple comparisons were done using the Kruskal Wallis test. P<0.05, after adjusting for multiple comparisons, are shown.
L- and D-lactate production
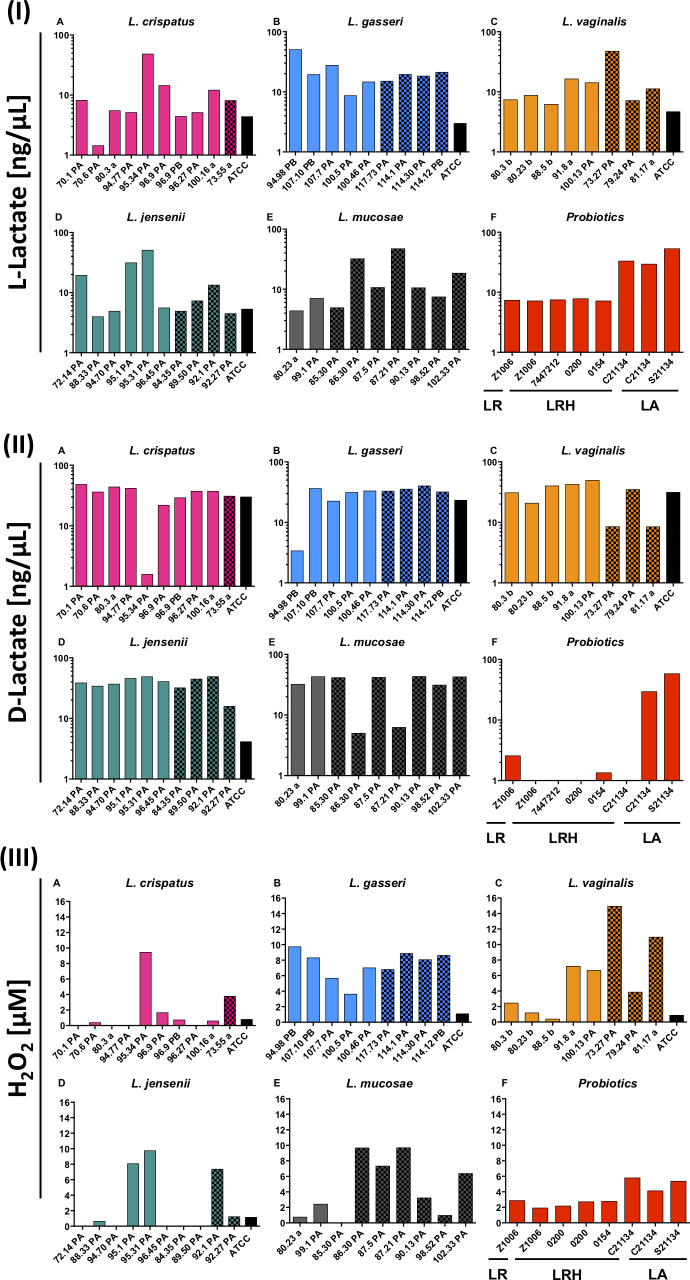
L- and D-lactate are considered important metabolic by-products of Lactobacillus strain metabolism that have anti-viral and anti-bacterial activity, respectively [30–32]. Therefore, the ability of vaginal Lactobacillus strains (46/57, due to assay limitations) to produce L- and D-lactate were evaluated under anaerobic conditions (Fig 3A and 3B). Vaginal Lactobacillus spp. tended to produce lower amounts of L- than D-lactate (median 10.6 ng/μL [IQR 5.6–19.4] vs. 35.8 ng/μL [30.4–41.9], p<0.0001), although all appeared to produce both isomers. Some vaginal strains produced high levels of L- but almost no D-lactate (such as L. crispatus 95.34PA), while others produced large amounts of D- but little L-lactate (such as L. jensenii 96.45PA). Again, STI and BV status of the donor did not influence L- and D-lactate levels measured (Fig 3A and 3B). Further, the majority of vaginal Lactobacillus isolates produced more of both isomers than their respective ATCC reference strains. With the exception of two probiotic L. acidophilus isolates (which produced similar levels of both isomers), all probiotic strains produced higher levels of L- than D-lactate, although concentrations of both were significantly lower than those of the vaginal strains (p<0.0001).
Fig 3. Ability of vaginal and probiotic Lactobacillus strains to produce (I) L-lactate, (II) D- lactate, and (III) H2O2 under anaerobic conditions.
Concentrations of H2O2, L- and D-lactate produced by L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), L. mucosae (E) and the commercial probiotic (F; including L. reuteri [LR], L. rhamnosus [LRH] and L. acidophilus [LA]) strains after 24 hours anaerobic incubation were measured. Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
The ability of strains to produce L- and D-lactate did not correlate (Spearman rho = 0.00, p = 0.9592), neither did production of D-lactate (Spearman rho = 0.05, p = 0.7250) or L-lactate (Spearman rho = -0.23, p = 0.0838) with growth. There was also no correlation between ability of isolates to lower culture pH and their ability to produce L- or D-lactate (Spearman rho = 0.15, p = 0.2604 for L-lactate; rho = -0.02, p = 0.8960 for D-lactate), indicating that lactate was not the only cause of lowering culture pH.
H2O2 production
Earlier studies suggested that H2O2 was the most influential Lactobacillus spp.-produced metabolite to inhibit pathogens in the lower FGT, although these studies were conducted aerobically [39,40]. To mimic in vivo conditions in the lower FGT, all experiments in this study were performed under anaerobic conditions. All of the vaginal L. gasseri and L. vaginalis strains produced small quantities of H2O2 (ranging from 0.5–15 μM), while some of the L. jensenii, L. crispatus, and L. mucosae strains did not produce any measurable H2O2 at all (Fig 3C). Of all previously evaluated in vitro characteristics, H2O2 only correlated with L-lactate levels positively (Spearman rho = 0.61, p<0.0001), but not with D-lactate (Spearman rho = -0.20, p = 0.1504).
Ability to inhibit P. bivia and G. vaginalis strains
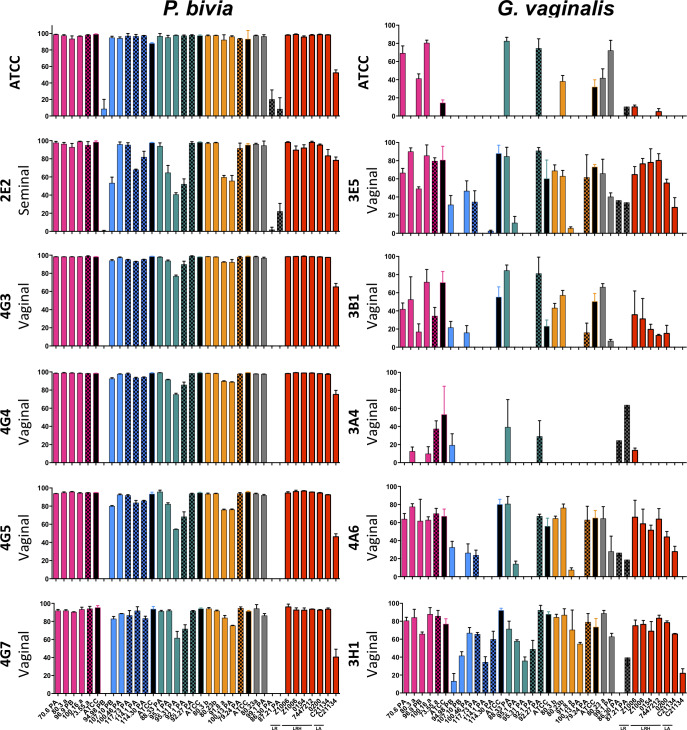
The ability of abiotic culture supernatants from a subset of vaginal Lactobacillus strains (25/57; selected based on performance in previous assays) to inhibit the growth of a panel of P. bivia and G. vaginalis strains was determined under anaerobic conditions (Fig 4). Most of the vaginal Lactobacillus isolates (besides L. gasseri 94.98PB, L. mucosae 86.30PA and 87.21PA) showed some degree of inhibition towards all clinical P. bivia strains, although the extent of inhibition differed by Lactobacillus and P. bivia strain (Fig 4). L. crispatus tended to inhibit P. bivia growth to a greater extent than other species, inhibiting all P. bivia strains > 90%. Vaginal Lactobacillus strains inhibited P. bivia similarly to their respective ATCC and the probiotic Lactobacillus strains (Fig 4).
Fig 4. Ability of Lactobacillus-conditioned media to inhibit the growth of P. bivia and G. vaginalis.
Conditioned media from L. crispatus (pink), L. gasseri (blue), L. jensenii (green), L. vaginalis (orange), L. mucosae (grey) and probiotic (red, LR–L. reuteri, LRH = L. rhamnosus, LA = L. acidophilus) cultures were co-incubated with reference ATCC and genital P. bivia and G. vaginalis strains for 48 hours and the AUC was calculated for each strain. The percentage of inhibition was determined by comparing the growth of P. bivia and G. vaginalis strains in the presence of non-conditioned control media to Lactobacillus-conditioned media. Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
In contrast, the inhibition of G. vaginalis strains was generally more variable and significantly lower (Fig 4). Only three vaginal Lactobacillus (L. crispatus 100.16A, L. jensenii 88.33PA and 92.27PA) and the L. crispatus ATCC strain inhibited the growth of the full panel of G. vaginalis strains. Inhibition appeared to be highly G. vaginalis strain-specific, as almost all Lactobacillus strains inhibited the vaginal G. vaginalis isolate 3H1 (Fig 4; bottom row), while only 9/25 inhibited the G. vaginalis ATCC strain (Fig 4; top row). Overall, L. crispatus strains tended to exhibit the strongest inhibitory activity against G. vaginalis, although this was not statistically significant. The ATCC reference Lactobacillus strains generally showed broader and better inhibitory activity against G. vaginalis than vaginal Lactobacillus isolates (Fig 4). Notably, the abilities of vaginal Lactobacillus strains to inhibit the pathogens tested were highly correlated (G. vaginalis and P. bivia: Spearman rho = 0.814, p<0.0001), indicating that individual Lactobacillus strains have a similar capacity to inhibit various BV-associated bacteria. Similarly to the previously assessed characteristics, BV/STI status of the donor did not influence inhibitory abilities.
Antibiotic susceptibility
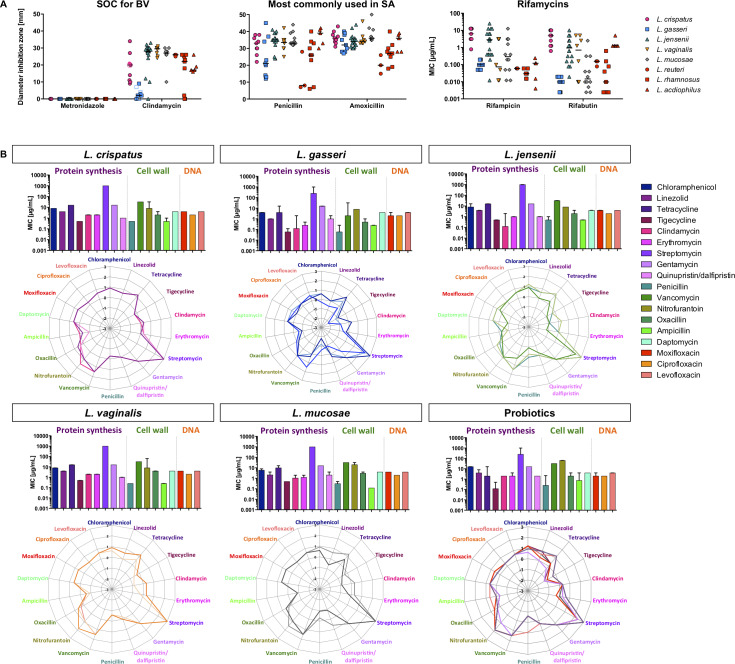
Metronidazole and clindamycin are SOC for BV treatment and penicillin and amoxicillin are commonly used antibiotics. All vaginal Lactobacillus isolates (n = 57) were resistant to metronidazole, while the susceptibility to clindamycin varied within and between Lactobacillus spp. (Fig 5A). L. vaginalis and L. jensenii isolates were the most susceptible to clindamycin, while L. gasseri strains were the least. The inhibition zones of the probiotic strains were smaller than those of most clinical strains (with the exception of L. gasseri), indicating that these might be less susceptible to clindamycin than the majority of vaginal Lactobacillus strains.
Fig 5. Antibiotic susceptibility of vaginal and probiotic Lactobacillus isolates.
(A) The susceptibility of L. crispatus (pink), L. gasseri (blue), L. jensenii (green), L. vaginalis (orange), and L. mucosae (grey) and probiotic (red) isolates to metronidazole (5 μg), clindamycin (2 μg), penicillin (2 μg) and amoxicillin (10 μg) was determined using disc diffusion assays. The susceptibility to rifampicin and rifabutin was determined using broth dilution assays. (B) For a subset of 20 Lactobacillus isolates, the MIC to antibiotics interfering with the protein synthesis (purple), cell wall and membrane (green) and DNA synthesis (red) was determined using Sensititre plates. Bar graphs show the median (IQR) for each species, while each line in the radar plot represent the susceptibility (log-transformed MICs) of individual isolates.
All vaginal Lactobacillus isolates showed similar susceptibility to penicillin, with the exception of L. gasseri isolates, which tended to be less susceptible (Fig 5A). Probiotic L. acidophilus strains were more susceptible to penicillin than most vaginal strains, while L. rhamnosus isolates tended to be similarly susceptible, and L. reuteri less. The inhibition zones for amoxicillin were highly comparable to those measured for penicillin, as was the intra-species variability (Fig 5A). L. crispatus isolates were significantly more susceptible to amoxicillin than L. rhamnosus (adj. p = 0.0497), and L. mucosae than L. reuteri (adj. p = 0.0128) and L. rhamnosus (adj. p = 0.0033), indicating that L. reuteri and L. rhamnosus but not L. acidophilus strains may be less susceptible to amoxicillin than the vaginal isolates.
The rifamycins rifampicin and rifabutin are commonly used to treat tuberculosis (TB) and form part of the six-month antibiotic regimen administered to ~500,000 people in South Africa who develop TB annually [41], and have previously been explored as a treatment option for BV [42,43]. All Lactobacillus isolates were highly susceptible to rifampicin (<12.5 μg/ml) and rifabutin (<10.0 μg/mL) and susceptibilities of the probiotic isolates were similar to the vaginal strains (Fig 5A).
Broader antibiotic resistance profiling to inhibitors of protein, cell wall/membrane and DNA synthesis was conducted on a subset of 14 vaginal and six probiotic Lactobacillus isolates (Fig 5B). Vaginal L. gasseri strains appeared to be susceptible to a wider range of antibiotics than other Lactobacillus spp. L. crispatus strains showed exactly the same antibiotic susceptibility, while the intra-species variability was higher for other vaginal Lactobacillus spp. There were no clear differences between vaginal and probiotic Lactobacillus spp. in antibiotic susceptibility profiles. Based on the clinical MIC breakpoints defined by the EUCAST (2019), all vaginal isolates would be considered sensitive to ampicillin (MIC ≤4 μg/mL), and the majority to chloramphenicol (MIC ≤8μg/mL). For penicillin, (sensitivity MIC≤0.25 μg/mL, resistance MIC >0.5μg/mL), all clinical L. gasseri, L. mucosae and L. vaginalis strains were sensitive, all L. crispatus strains and L. jensenii 88.33PA strains had an intermediate sensitivity, and L. jensenii 92.27PA was resistant. L. crispatus 100.16A, L. gasseri 117.73PA and 100.46PA, L. jensenii 92.17PA and 95.31PA, L. vaginalis 79.24PA and L. mucosae 102.33PA were susceptible to clindamycin (MIC ≤1 μg/mL), while all other isolates had MICs >1μg/mL. Since the breakpoint for clindamycin has been defined as >4μg/mL, accoding to the EUCAST 2019 guidelines, it was not possible to conclude with the current data whether these isolates are resistant.
Adhesion to ectocervical cells and inflammatory responses
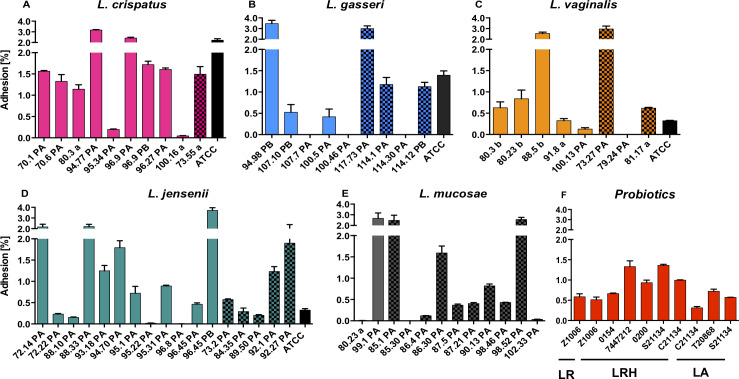
The selected Lactobacillus strains should strongly adhere to host cells [21,22] but not influence cell viability or cause inflammatory changes [44,45]. All vaginal L. crispatus (10/10), and the majority of L. vaginalis (7/8), L. jensenii (16/17) and L. mucosae (10/12) and two-thirds of the L. gasseri (6/9) strains adhered to Ca Ski cells to varying extents, independently of the BV/STI status of the donor (Fig 6). Overall, vaginal L. crispatus strains tended to adhere the strongest followed by L. mucosae, L. jensenii, L. vaginalis, and L. gasseri, and several vaginal isolates (2/10 L. crispatus, 2/9 L. gasseri, 6/8 L. vaginalis, and 11/18 L. jensenii) adhered better than their respective ATCC reference strain (Fig 6).
Fig 6. Adhesion of vaginal and probiotic Lactobacillus isolates to ectocervical Ca Ski cells.
Lactobacillus isolates were co-cultured with Ca Ski cells for 3 hours and the percentage of adhered bacteria was calculated for L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), L. mucosae (E) and the commercially available probiotic isolates (F, LR = L. reuteri, LRH = L. rhamnosus, LA = L. acidophilus). Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
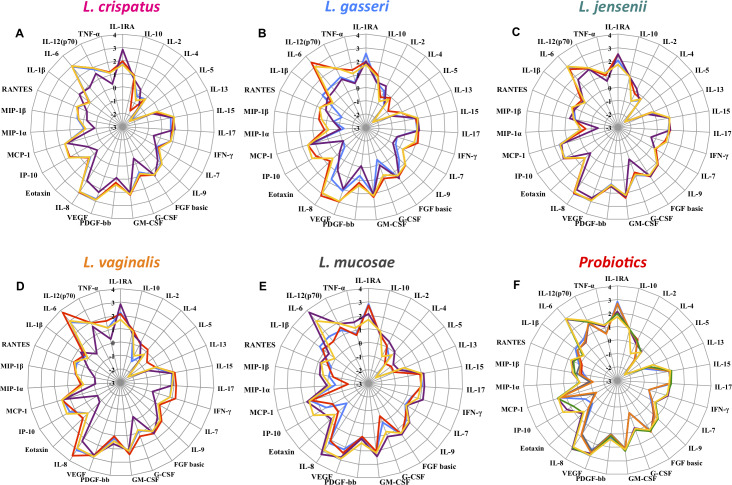
For a subset of vaginal Lactobacillus isolates that included three strains per vaginal Lactobacillus spp. and six probiotic strains, the effect on cervical epithelial Ca Ski cell viability and cytokine expression was determined (Fig 7). None of the vaginal strains decreased the viability of Ca Ski cells below 80%, as measured by trypan blue straining. After grouping the cytokines into their biological categories, all bacterial isolates up-regulated regulatory cytokines (including IL-1RA and IL-10) 1.5-fold (L. crispatus 100.16 a) to 10-fold (L. reuteri Z1006) compared to the control. In contrast, only five Lactobacillus strains (L. gasseri 100.46 PA, L. mucosae 80.23 a, L. vaginalis 79.24 PA, L. rhamnsus P0154 and C21134) induced adaptive cytokines (including IL-2/4/5/13/15/17 and IFN- γ) ~ 2-fold more than the control, while the remaining 16 strains downregulated adaptive cytokines up to 1.6-fold. Only for L. gasseri 100.46 PA slightly higher levels of growth factors and haematopoietic cytokines (including IL-7/9, FGF-basic, G-CSF, GM-CSF, PDGF-BB, and VEGF) were measured compared to the control, and the remaining strains downregulated this group of cytokines up to 4-fold. For about half of the strains (10/21) up to 4.2-fold higher levels of chemokines (including IL-8, Eotaxin, IP-10, MCP-1, MIP-1α/β and RANTES) were measured than for the control, while for the other half up to 24-fold lower levels were found. Four strains (L. gasseri 100.46 PA, L. mucosae 80.23 a, L. vaginalis 79.24 PA, L. rhamnosus C21134) induced higher inflammatory responses (1.02-, 2.44-, 5.84-, 5.88-fold, respectively, including IL-1β/6/12(p70) and TNF-α) than the control, while the remaining 17 Lactobacillus strains downregulated inflammatory responses up to 47-fold. In summary, the majority of bacterial strains induced cytokine responses comparable to the control and did not induce inflammatory responses. However, for L. gasseri 100.46 PA, L. mucosae 80.23 and L. vaginalis 79.24 PA higher levels of cytokines were measured in all cytokine categories, indicating that the inflammatory potential of these Lactobacillus strains requires further investigation.
Fig 7. Vaginal Ca Ski cell cytokine responses following co-culture with vaginal and probiotic Lactobacillus isolates.
The concentrations of 27 cytokines were measured in culture supernatants by Luminex. Abiotic cell culture supernatant was used as control (shown in yellow). The log-transformed concentrations of all measured cytokines for each three (shown in purple, blue and red) selected L. crispatus (A), L. gasseri (B), L. jensenii (C), L. vaginalis (D) and L. mucosae (E) and six probiotic (F) isolates are shown in radar plots. While all data points outside of the yellow radar (control) show an upregulation of cytokines, those inside the yellow radar indicate a downregulation of a certain cytokine.
Assessment of overall PPP performance
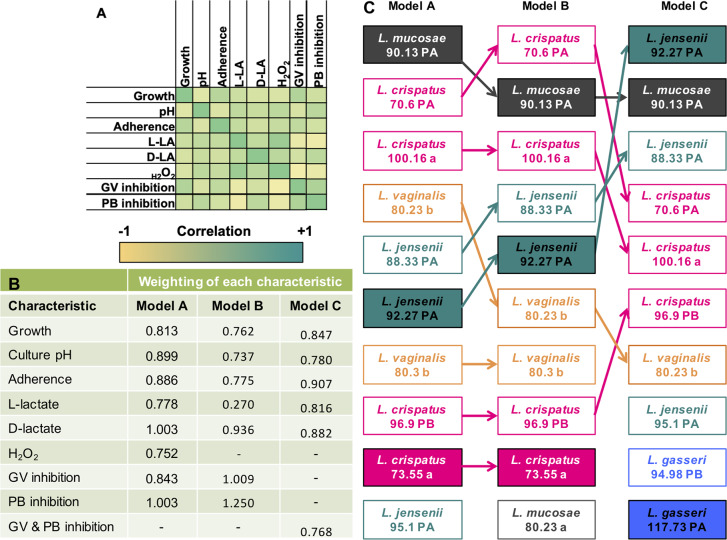
To identify the most promising vaginal Lactobacillus strains for the development of a probiotic for vaginal health, a weighted scoring system was devised based on PPP characteristics, including: their ability to grow well in vitro, lower pH, adhere to cervical epithelial cells, produce L- and D-lactate and H2O2, and inhibit growth of BV-associated species (G. vaginalis and P. bivia). Cytokine and antibiotic susceptibility profiles were not considered, as data were available only for a subset of vaginal strains. For this ranking, a total of 36/57 Lactobacillus strains (for which complete characteristic evaluations were available) were included. To allow objective comparisons, the performance of vaginal isolates was compared with their respective ATCC reference and the probiotic isolates. Because some of the characteristics included in the ranking correlated, a weighted scoring system was developed to account for co-linearity between characteristics (Fig 8A). Including all characteristics in the scoring matrix (model A; Fig 8B), L. mucosae 90.13PA, L. crispatus 70.6PA and 100.16A, L. vaginalis 80.23B, and L. jensenii 88.33PA and 92.27PA were the six highest scoring Lactobacillus strains, with similar points as the ATCC L. crispatus and ATCC L. gasseri strains, while all commercially available probiotics ranked lower. Further step-wise sensitivity analyses were performed to test the robustness of the selection. Model B: Because the concentrations of H2O2 produced by Lactobacillus were all below the level found to be microbicidal [30], a ranking model was considered in which H2O2 was excluded, which did not change the ranking of the top six strains (Fig 8C). Model C: In addition to excluding H2O2 production, the inhibitory effect on G. vaginalis and P. bivia were grouped to avoid skewing towards strains that performed generally well in the inhibition assays (Fig 8C), which resulted in two L. gasseri strains being included in the top-10 strains.
Fig 8. Selection of top-performing of Lactobacillus strains.
Correlation matrix of measured characteristics (A); and weighting of each characteristic in four different ranking models (A-C) that were considered (B). Model A included all characteristics. Model B excluded H2O2 production (as this may not be biologically relevant under anaerobic conditions) and Model C excluded H2O2 production in addition to grouping G. vaginalis and P. bivia inhibition into one category (since pathogen inhibition correlated strongly). Dark green indicates a strong positive correlation, while dark yellow indicates a strong negative correlation. (C) Relative ranking of vaginal Lactobacillus isolates according to the three different models that were considered. Species are color-coded (L. crispatus = pink, L. gasseri = blue, L. jensenii = green, L. vaginalis = orange, and L. mucosae = grey). Filled boxes indicate which strains were obtained from BV and/or STI-positive women.
The sensitivity analysis suggested that the scoring system was quite robust and showed that isolates from both STI/BV-negative and women with STIs or BV were among the top-performing strains, indicating that even Lactobacillus strains that originate from women with BV or STIs can have promising probiotic characteristics. Although all commercially available probiotic strains were included in the scoring system, none scored in the top ten in any of the models considered.
WGS analysis of selected Lactobacillus strains
Prior to selecting vaginal Lactobacillus strains for the development of a probiotic, we selected well-performing strains from a range of Lactobacillus spp. for whole genome sequencing and genome analysis to assess the presence of antimicrobial resistance determinants, mobile elements and prophages, including L. mucosae 90.13PA (ranked consistently in the top 2, from BV/STI-positive women), L. crispatus 70.6PA (highest ranking L. cripsatus strain from BV/STI-negative women) and L. crispatus 73.55a (highest ranking L. cripsatus strain from BV-positive women), L. gasseri 94.98PB (highest ranking L. gasseri strain, from BV/STI-negative women), and L. jensenii 95.1PA (from BV/STI-negative women).
The whole genomes of five selected strains were sequenced to a median depth of 117X (range 102-142X). Full assembly statistics are shown in S1 Table. Screening for the presence of well characterised antimicrobial resistance determinants revealed no matches to the Resfinder, CARD and AMRFinder databases for any of the assemblies. However, analysis of the RAST annotated assemblies revealed that all five strains harboured open reading frames (ORFs) with homology to the ldb0660 and copY-like genes in Lactobacillus delbrueckii subsp. bulgaricus that respectively encode a copper-translocating P-type ATPase and a negative transcriptional regulator, both of which play a role in copper homeostasis. In addition, three of the five strains (L. crispatus 70.6 PA, L. crispatus 73.55 a and L. mucosae 90.13 PA) harboured genes encoding putative TetM-type ribosomal protection proteins. Two strains (L. gasseri 94.98 PB and L. jensenii 95.1 PA) possessed potential beta-lactamase class A proteins, while L. crispatus 73.55 a harboured an ORF encoding a putative streptothricin acetyltransferase. The latter was flanked by genes encoding predicted mobile element-associated transposases. Finally, two classes of potential multidrug transporters were identified. All strains except L. gasseri 94.98 PB harboured genes encoding putative multidrug and toxin extrusion (MATE) family efflux pumps with homology to the MepA protein in Staphylococcus aureus and a further three strains (L. crispatus 70.6 PA, L. crispatus 73.55 a and L. jensenii 95.1 PA) harboured genes encoding putative major facilitator superfamily (MFS) class efflux pumps with homology to the Lde efflux pump in Listeria monocytogenes.
Putative intact prophages were present in the genomes of two of the five strains. L. crispatus 70.6 PA harboured a single predicted 43.9Kbp prophage, while L. mucosae 90.13 PA harboured three predicted prophages of 16.9Kbp, 37.5Kbp and 40.1Kbp, respectively. Complete CRISPR-Cas loci (i.e. one or more signature cas genes located alongside a CRISPR array) were identified in the genomes of three of the strains. L. crispatus 73.55 a harboured a type I-E locus with spacers showing matches to a lactococcal phage Tuc2009. L. mucosae 90.13 PA harboured two distinct loci encoding a type I-C and type III-A system, respectively. Spacers present in the type I-C locus showed homology to phage-like regions on the L. mucosae LM1 plasmid and the Streptococcus thermophilus phage Sfi19, while those in the type III-A locus showed homology to several plasmids (L. mucosae LM1, C. botulinum pCBH, and an unnamed plasmid in Lactobacillus fermentum DR9) as well as the Streptococcus phage Javan623. L. jensenii 95.1 PA harboured a single type II-A locus with spacers showing homology to several phages (an enterococcal phage, EFRM31 and the Lactobacillus phages Lf1 and Lj928).
Discussion
A limited variety of probiotics for vaginal health are currently available internationally and in South Africa, and few of these contain FGT commensals [18]. Thus, there is an urgent need for the development of additional well-designed probiotics for vaginal health. In this study, we evaluated key probiotic characteristics of a large panel of vaginal Lactobacillus strains, including L. crispatus, L. gasseri, L. jensenii, L. vaginalis and L. mucosae, from South African women. L. iners was not included, since its role in FGT health and disease remains unclear [46]. Overall, the majority of vaginal Lactobacillus strains performed better according to the PPP criteria considered than the included currently available commercial probiotic strains.
All Lactobacillus strains grew optimally at pH 6.0, similar to what has been described previously [28], despite existing at pHs <4.5 in the FGT [36,47] and actively lowering culture pH. All vaginal L. crispatus strains lowered the pH in vitro to 3.7, while only some strains of other vaginal Lactobacillus spp. achieved pHs <4.0. In vivo, women with an L. crispatus-dominated vaginal microbiota have been shown to have a lower FGT pH than women with a L. gasseri- or L. jensenii-dominated microbiota [4], suggesting good alignment between these in vitro characteristics and in vivo phenotypes.
The ability to produce lactate varied between Lactobacillus spp. and strains, although vaginal L. jensenii strains produced the lowest L-lactate (close to detection limit) concentrations, which supports previous studies showing that L. jensenii strains commonly only produce D-lactic acid [48,49]. Hutt et al. (2016) also found that L. gasseri produced significantly more lactate than L. jensenii and L. crispatus, which agrees with our findings. No correlation between lactate production and culture pH was found, but it has been suggested that other acids, such as acetic acid, contribute to lowering pH [50]. Under anaerobic conditions, vaginal Lactobacillus strains produced <20μM H2O2. Considering that the microbicidal activity of H2O2 has only been demonstrated at concentrations ≥1000 mM [30], H2O2 production by vaginal Lactobacillus spp. seems to be biologically irrelevant [51].
Vaginal and probiotic Lactobacillus strains inhibited G. vaginalis growth in a highly strain-specific manner. This indicates that G. vaginalis genotype may influence susceptibility to Lactobacillus-mediated inhibition. Previous studies have shown that metronidazole-resistant G. vaginalis strains were less susceptible to inhibition by Lactobacillus spp. [23,52], and biofilm-producing G. vaginalis strains were more tolerant to lactic acid than non-biofilm producing strains [53]. As such, G. vaginalis strain 3H1, which was inhibited broadly and strongly, belongs to genotype B, is not resistant to metronidazole and a low biofilm producer, which might explain the high susceptibility to Lactobacillus-mediated inhibition. In contrast, G. vaginalis 3B1 that showed low levels of inhibition belongs to genotype C and is highly resistant to metronidazole, which might explain why it was less susceptible to inhibition by Lactobacillus spp.
Antibiotic susceptibility has an impact on whether the probiotic could be administered concurrently with SOC [54]. All vaginal Lactobacillus strains were resistant to metronidazole, as expected, and indeed, concentrations between 128 and 256 μg/ml of metronidazole have been shown to stimulate the growth of vaginal Lactobacillus spp. in vitro [55]. Few Lactobacillus strains were resistant to clindamycin, as described by others [56–58], indicating that these strains could not be administered concurrently with SOC clindamycin treatment. Of note, all Lactobacillus strains were highly sensitive to rifamycins. Considering that these are administered to ~500,000 people annually in South Africa for six months to treat TB, effects of long-term administration of rifamycins on the FGT microbiota should be evaluated.
It is also important that probiotic candidates do not cause pathology to the host, such as an increase in genital inflammation [44], especially in a setting with extremely high STI and HIV rates like in South Africa. Promisingly, we found that all vaginal Lactobacillus strains up-regulated the regulatory cytokine IL-1RA, which inhibits binding of pro-inflammatory cytokines like IL-1α and IL-1β to their cognate receptors [59]. Some of the Lactobacillus strains even increased IL-8 production, which is important to consider, as IL-8 has been found to decrease HIV-1 transcription in both lymphocytes and ectocervical tissue explants [60]. Only four strains induced the inflammatory cytokines TNF-α, IL-12(p70), IL-6 and IL-1β, while the remaining Lactobacillus strains downregulated these, which is again reassuring as genital inflammation has been linked to HIV and STI risk [61]. Other cytokines that have been related to the risk of HIV acquisition in South African women include MIP-1α, MIP-1β and IP-10 [62], and it is encouraging that all besides three Lactobacillus strains downregulated these. Together, these findings indicate that administration of most vaginal Lactobacillus strains evaluated here is likely to be considered safe in a population with high STI and HIV prevalence but this needs confirmation in preclinical animal models and small interventional trial in healthy humans.
Using a weighted PPP scoring system, the identified top-performing strains included only vaginal Lactobacillus strains, obtained both from BV/STI negative South African women as well as some from donors who had BV and/or STIs, while none of the commercially available strains ranked as well, suggesting that there is potential to improve currently available probiotic formulations for vaginal health. Surprisingly, we did not find that strains from BV and STI-negative women consistently performed better than those from BV/STI-positive women, indicating that the probiotic potential of vaginal Lactobacillus strains is not necessarily dependent on the vaginal health of the donor.
The WGS of five of the best-performing vaginal Lactobacillus strains further confirmed the likelihood of their safety, due to antimicrobial resistance elements being largely absent. L. crispatus 73.55a harboured a streptothricin acetyltransferase sequence flanked by mobile elements, which is concerning as this could be one potential mechanism leading to the transfer of streptothricin resistance [63]. Prophage sequences were also identified, in agreement with other studies that observed functional and non-functional integrated bacteriophages in vaginal Lactobacillus spp. isolates [64–66]. While the relevance of potentially functional prophages for vaginal health is still unclear, it may be necessary to ensure that these prophage sequences are non-functional prior to inclusion of the strains into a commercial probiotic.
Several limitations of our study need to be acknowledged. No information on the strains that were included in the commercial probiotics were made available by all manufacturers. Not all characteristics were determined for all 57 strains, but we selected strains for further characterisation based on evidence of promising PPP characteristics. We also did not evaluate the mechanisms of pathogen inhibition.
In summary, this study evaluated probiotic characteristics of numerous vaginal Lactobacillus strains from South African women. The probiotic potential of these isolates was found to be strain-specific, and vaginal Lactobacillus strains largely performed better than probiotic strains currently used in probiotics for vaginal health, available internationally and in South Africa. Some of the best-performing, sequenced vaginal Lactobacillus isolates are now being considered for the development of a probiotic for vaginal health. This probiotic will be vaginally administered because vaginal administration affects vaginal health more quickly than does oral administration and enhances the viability of the administered microorganism [67]. It remains to be decided whether this probiotic should be region-specific, and only available to Southern African women due to the origin of its Lactobacillus strains, or worldwide. Once its effect on BV cure and recurrence has been tested in South Africa and found to be effective, the probiotic should also be tested in non-African women to determine whether these Lactobacillus strains show a similarly beneficial effect in other populations.
Materials and methods
Cohort from which vaginal Lactobacillus strains were isolated
Vaginal Lactobacillus isolates were derived from women (16–22 year old) enrolled in the Women’s Initiative in Sexual Health (WISH) study [68], approved by the Human Research Ethics Committees of the University of Cape Town (UCT HREC #267/2013). Women who were ≥18 years old provided written informed consent and those <18 years provided written assent, and consent was obtained from their parent(s) or legal guardian(s). Eligibility criteria included being HIV negative, generally healthy, not pregnant or menstruating at the time of sampling. Participants were tested for BV (Nugent scoring) and STIs (including C. trachomatis, N. gonorrhoeae, T. vaginalis, Mycoplasma genitalium, HSV-2, Hemophilus ducreyi, Treponema pallidum and Lymphogranuloma venerum). The vaginal microbiota was characterised by 16s rRNA gene sequencing [36]. Cervicovaginal secretions were collected using menstrual cups, diluted 1:4 in PBS and stored with glycerol (20%v/v) at -80°C for isolation of Lactobacillus strains.
Probiotics from which commercial Lactobacillus strains were isolated
Commercially available probiotics included Reuterina Femme (oral capsules, Ascendis Pharma, South Africa), Provacare Probiotic Vaginal Care (vaginal capsules, Provacare, Canada), Vagiforte Plus (oral capsules and vaginal tablet, Bioflora CC, South Africa), Vagiforte Plus Combo Pack (oral capsules and vaginal spray, Bioflora CC, South Africa), Gynophilus (vaginal pessary, biose, France) and Muvagyn (vaginal pessary, Hälsa Pharma GmbH, Germany).
Isolation of Lactobacillus strains
Cervicovaginal secretions or commercial probiotics dissolved in De-Man-Rogosa-Sharpe (MRS) were streaked onto MRS agar plates and incubated anaerobically (37°C, 48 hours). Single colonies with distinct morphologies were picked and re-streaked until homogenous. Pure colonies were inoculated into MRS broth, grown anaerobically (37°C, 48 hours), and stocks stored with glycerol (20% v/v) at –80°C. Matrix Assisted Laser Desorption/Ionization time-of-flight (MALDI-TOF, MALDI Biotyper, Bruker Daltonik, USA) was used to identify Lactobacillus spp. To determine bacterial concentrations at a standardized optical density (OD), overnight cultures were adjusted to OD600nm 0.1±0.01, serial dilutions plated onto MRS agar plates that were incubated anaerobically (37°C, 48 hours), colonies were counted, and CFU/mL calculated (S2 Fig).
Confirmation of Lactobacillus species identities by 16S rRNA gene sequencing
To confirm MALDI-TOF species identification, single colonies were incubated (37°C for 20 minutes, followed by 90°C for 15 minutes) in Tris-EDTA buffer (pH 7.4) with 20ng/mL proteinase K (BioLabs, USA). PCR reactions contained 8% (v/v) template, 0.5uM of F27 and R5 primers [69], 0.2mM PCR nucleotide mix (Promega, USA), 1x GoTaq Flexi Buffer (Promega, USA), 1.25 units GoTaq G2 Flexi DNA polymerase (Promega, USA), 2.5mM MgCl2 (Promega, USA) and nuclease-free H2O. PCR conditions were 96°C for 5 minutes; 30 cycles at 94°C for 30 seconds, 55°C for 30 seconds and 72°C for 1.5 minutes, and a final elongation at 72°C for 7 minutes. Products were Sanger sequenced at Macrogen Europe. 16S rRNA sequences were aligned to National Centre for Biotechnology Information (NCBI) database sequences with the Basic Local Alignment Search Tool (BLAST) algorithm, selecting the highest percentage identity match over 99% as the species identity.
Ability of Lactobacillus isolates to grow at low pH conditions
Cultures standardized to OD600nm 0.1±0.01 in MRS broth adjusted to pH 3.5, 4.0, 4.5 or 6.0 using HCl were incubated in triplicate under anaerobic conditions in 96-well plates at 37°C for 48 hours. OD600nm was measured at 0, 3, 6, 12, 18, 24, 36 and 48 hours and area under the curve (AUC) was calculated for each isolate. Experiments were repeated three times.
Ability of Lactobacillus isolates to lower culture pH
Cultures adjusted to OD600nm 0.1±0.01 in MRS broth were grown anaerobically at 37°C, and culture supernatant pH measured at 3, 6, 12, 24 and 48 hours using a calibrated pH meter. Experiments were performed in duplicate.
Measurement of anaerobic lactate and H2O2 production
Cultures adjusted to OD600nm 0.1±0.01 were incubated anaerobically for 24 hours at 37°C. Supernatants were filtered (0.2 μM) and concentrations of D- and L-lactate measured using colorimetric assays (Sigma-Aldrich, USA). H2O2 concentrations were measured using a fluorometric kit (Sigma-Aldrich, USA), according to the manufacturer’s instructions. Experiments were performed in duplicate.
Antimicrobial activity
G. vaginalis (ATCC 14018 and five vaginal isolates) and P. bivia (ATCC 29303 and five vaginal isolates) were grown in brain heart infusion (BHI) supplemented with 5% horse blood, standardised to OD600nm 0.4±0.01, and 100 μL was added in triplicate to 96-well plates. Abiotic Lactobacillus-conditioned MRS (100 μL; same as used for lactate measurement) was added and incubated anaerobically at 37°C for 48 hours. OD600nm was measured at 0, 3, 6, 12, 18, 24, 36 and 48 hours. As control, growth of G. vaginalis and P. bivia strains in the presence of plain MRS, at the same ratio as abiotic culture supernatants, was measured. AUC for each growth curve was calculated and percentage of growth inhibition in the presence of Lactobacillus-conditioned MRS compared to the control calculated. Experiments were repeated three times.
Adhesion to human ectocervical epithelial cells
Ca Ski cells (ATCC CRL-1550) were grown to 80% confluence in 10% fetal calf serum (FCS) Dulbecco’s Modified Eagle Medium (DMEM) in 24-well cell culture plates (37°C, 5%CO2). Lactobacillus isolates (4.2x106 CFUs) were added in triplicate and co-cultured for 3 hours at 37°C (5%CO2), after which unbound bacteria were gently removed by washing the cells three times with PBS. Cells and bound bacteria were detached using 0.1% Triton X-100, serially diluted, plated onto MRS agar plates, and incubated anaerobically at 37°C for 48 hours. The percentage of adhered bacteria was calculated as the number of bound cells compared to total number of bacterial cells initially added. Experiments were repeated three times.
Impact on cervical cell viability and cytokine responses
Ca Ski cells were grown in 10% FCS DMEM to 80% confluence at 37°C in 5% CO2. Lactobacillus strains (4.2x106 CFUs) were added in duplicate and incubated for 24 hours (37°C, 5% CO2). Ca Ski cells incubated without Lactobacillus strains were included as control. Supernatants were harvested and filtered (0.22 μm, Corning Costar Spin-X, Sigma-Aldrich, USA) to exclude bacterial cells. A Bio-Plex Pro Human Cytokine 27-Plex Luminex kit (Lot 64064139, Bio-Rad, USA) was used to measure concentrations of cytokines, chemokines and growth factors in the culture supernatant, according to the manufacturer’s instructions. Data were acquired using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories Inc, USA), and a 5PL regression line was used to determine concentrations from standard curves. All values below the detection limit were recorded as half of the lowest measured concentration for each cytokine. Viability of Ca Ski cells after co-culture was evaluated using trypan blue staining (0.4%, Sigma-Aldrich, USA).
Antibiotic susceptibility testing
Double-layer disc diffusion was used to determine the susceptibility to metronidazole (5μg), clindamycin (2μg), penicillin (2μg) and amoxicillin (10μg; Davies Diagnostics, South Africa), as described previously [58]. These experiments were performed in duplicate. For rifampicin and rifabutin (Sigma-Aldrich, USA), minimal inhibitory concentrations (MICs) were determined using two-fold serial dilutions according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2019 guidelines, with concentrations ranging from 5–0.00488μg/mL for rifabutin and 25–0.024μg/mL for rifampicin. MICs below the lowest or above the highest concentration tested were assigned a MIC half of the lowest or twice the highest concentration tested, respectively. Experiments were performed in duplicate. For a subset of Lactobacillus strains (n = 20), broader antibiotic susceptibility profiles were determined using Sensititre GPALL1F plates (including ampicillin, cefoxitin, chloramphenicol, ciprofloxacin, clindamycin, daptomycin, erythromycin, gentamicin, levofloxacin, linezolid, moxifloxacin, nitrofurantoin, oxacillin, penicillin, quinupristin/dalfopristin, rifampin, streptomycin, tetracycline, tigecycline, trimethoprim/sulamethoxazole and vancomycin; Thermo Fisher Scientific Inc., USA), according to the manufacturer’s instructions.
Whole genome sequencing
Genomic DNA was extracted from Lactobacillus isolates using the Quick-DNA Miniprep Kit (Zymo Research, USA) following the manufacturer’s instructions after a pre-lysis step which consisted of resuspension of the cell pellet in 200 μl PrimeStore Molecular Transport Medium (Longhorn Vaccines-Diagnostics, USA) and storage at -80°C for 24 hours. Libraries for sequencing were prepared using the Nextera DNA Flex kit (Illumina, USA), according to the manufacturer’s instructions, and sequenced using the Illumina MiSeq Reagent Micro Kit v2 on an Illumina MiSeq instrument in a paired-end, dual indexed 2 x 151 cycle sequencing run. Draft de novo assemblies were prepared using a customised assembly pipeline. Briefly, raw reads were trimmed using Trimmomatic v0.3.9 [70] using a sliding window approach (Phred quality cut-off of 15 averaged across 4 bases), a leading/trailing quality cut-off of 3 and the removal of reads below 30bp. Trimmed reads were assembled using SPAdes v3.13.0 [71] using the ‘—careful’ flag and omitting the initial read error correction step. To improve the draft assemblies, gap closure and contig extension were performed using GMcloser v1.6.2 [72]. The quality of the final assemblies for each strain was assessed using Quast v5.0.2 [73]. Rapid Annotation of microbial genomes using Subsystems Technology (RAST) was used to annotate the de novo assemblies [74].
Identification of putative antimicrobial resistance (AMR) determinants, prophages and mobile elements
Annotated draft assemblies were screened for the presence of putative antimicrobial resistance determinants using Resfinder v3.2. [75], CARD [76] and AMRFinder [77]. Putative prophages were identified using the online PHASTER server [78] [https://phaster.ca/]. CRISPR-Cas systems were identified using the CRISPRCasFinder online tool [79] [https://crisprcas.i2bc.paris-saclay.fr/] and spacer targets were identified using CRISPRTarget [80].
Statistical analyses
GraphPad Prism6 (GraphPad Software, USA), STATA version 11.0 (StataCorp, USA) and RStudio were used to generate graphs and analyse data. The Mann-Whitney U test was used for non-parametric variables, and Spearman Rank test was used for non-parametric correlations. The Kruskal-Wallis one-way analysis of variance was used to compare variables with three or more groups. A false-discovery rate step-down procedure [81] was used to adjust p-values for multiple comparisons, and adjusted p<0.05 were considered significant.
To rank Lactobacillus strains according to PPP criteria, a scoring system was developed. For each characteristic, Lactobacillus isolates were ranked into quartiles relative to the whole group: Scored “0” for characteristics <25th percentile (relative to all isolates); “1” for characteristics between 25–50th percentile; “2” for characteristics between 50–75th percentile, and “3” for characteristics between >75th percentile. Because some characteristics may correlate (e.g. ability to lower pH and H2O2 production), all scores were weighted to account for co-linearity: Weight = 1-(sum of column Spearman Rho÷sum of column score).
Supporting information
pH of vaginal L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), L. mucosae (E) and the probiotic (F) cultures were measured over 48 hours. Probiotics include L. reuteri, L. rhamnsus and L. acidophilus strains. As control, the pH of abiotic MRS was measured (shown in light grey). ATCC reference strains are shown as black lines. Physiological vaginal pH of women with optimal microbiota is shown as grey shading.
(TIFF)
Comparison of vaginal Lactobacillus spp. concentrations (measured in CFUs) at a standardized OD600nm 0.1 (±0.01): L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), and L. mucosae (E). Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
(TIFF)
The table shows the assembly statistics for the sequenced vaginal Lactobacillus strains. The sequences have been deposited in the European Nucleotide Archive (ENA) under accession number PRJEB37955.
(DOCX)
Data Availability
All sequencing data are available from the European Nucleotide Archive database at EMBL-EBI (accession number PRJEB37955).
Funding Statement
JSP is supported by EDCTP Strategic Primer SP.2011.41304.038; Poliomyelitis Research Foundation Award 15/23; SA National Research Foundation (NRF) South Africa / France Science and Technology Research collaboration Protea grant FSTR180523333842. AUH is supported by University of Cape Town Post-graduate publication incentive funding; Poliomyelitis Research Foundation Award; SA National Research Foundation (NRF). NW is supported by South African Medical Research Council Division of Research Capacity Development under National Medical Students Research Training Programme. The authors confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res Hum Retroviruses. 2019;35: 219–228. 10.1089/AID.2018.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67: 5170–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185: 375–379. 10.1067/mob.2001.115867 [DOI] [PubMed] [Google Scholar]
- 4.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108: 4680–4687. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4: 132ra52 10.1126/scitranslmed.3003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark P, Kurtzer T, Duff P. Role of Bacterial Vaginosis in Peripartum Infections. Infect Dis Obstet Gynecol. 1994;2: 179–183. 10.1155/S106474499400061X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson DB, Hanlon A, Nachamkin I, Haggerty C, Mastrogiannis DS, Liu C, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol. 2014;28: 88–96. 10.1111/ppe.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggerty CL, Totten PA, Tang G, Astete SG, Ferris MJ, Norori J, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect. 2016; sextrans-2015-052285. 10.1136/sextrans-2015-052285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van de Wijgert JHHM, Morrison CS, Cornelisse PGA, Munjoma M, Moncada J, Awio P, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48: 203–210. 10.1097/QAI.0b013e3181743936 [DOI] [PubMed] [Google Scholar]
- 10.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: A prospective cohort analysis among african couples. PLoS Med. 2012;9: 18 10.1371/journal.pmed.1001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto S, Soumillon M, et al. Inflammatory Responses in the Female Genital Tract. Immunity. 2016;42: 965–976. 10.1016/j.immuni.2015.04.019.Cervicovaginal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines. In: Sexually Transmitted Diseases Treatment Guidelines. 2015.
- 13.Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: A review. Clin Ther. 2008;30: 453–468. 10.1016/j.clinthera.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 14.Vicariotto F, Mogna L, Del Piano M. Effectiveness of the Two Microorganisms Lactobacillus Formulated in Slow-release Vaginal Tablets, in Women Affected by Bacterial Vaginosis. J Clin Gastroenterol. 2014;48: S106–S112. 10.1097/MCG.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 15.Marcone V, Rocca G, Lichtner M, Calzolari E. Long-term vaginal administration of Lactobacillus rhamnosus as a complementary approach to management of bacterial vaginosis. Int J Gynecol Obstet. 2010;110: 223–226. 10.1016/j.ijgo.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 16.Martinez RCR, Franceschini A, Patta MC, Quintana SM, Gomes BC, De Martinis ECP, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55: 133–138. 10.1139/w08-102 [DOI] [PubMed] [Google Scholar]
- 17.Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: Randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35: 131–134. 10.1016/S0928-8244(02)00465-0 [DOI] [PubMed] [Google Scholar]
- 18.Happel A-U, Jaumdally SZ, Pidwell T, Cornelius T, Jaspan HB, Froissart R, et al. Probiotics for vaginal health in South Africa: what is on retailers ‘ shelves? BMC Womens Health. 2017;17 10.1186/s12905-017-0362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes-soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative Functional Genomics of Lactobacillus spp. Reveals Possible Mechanisms for Specialization of Vaginal Lactobacilli to Their Environment. J Bacteriol. 2014;196: 1458–1470. 10.1128/JB.01439-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomás J, Zonenschain D, Morelli L, Nader-Macías ME. Characterisation of potentially probiotic vaginal lactobacilli isolated from Argentinean women Characterisation of potentially probiotic vaginal lactobacilli isolated from Argentinean women. Br J Biomed Sci. 2005;62: 170–174. 10.1080/09674845.2005.11732706 [DOI] [PubMed] [Google Scholar]
- 21.Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. Fem. 2006;48: 424–432. 10.1111/j.1574-695X.2006.00162.x [DOI] [PubMed] [Google Scholar]
- 22.Coudeyras S, Jugie G, Vermerie M, Forestier C. Adhesion of Human Probiotic Lactobacillus rhamnosus to Cervical and Vaginal Cells and Interaction with Vaginosis-Associated Pathogens. Infect Dis Obstet Gynecol. 2008. 10.1155/2008/549640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira GS, Carvalho FP, Arantes RME, Nunes AC, Moreira JLS, Mendonc M, et al. Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol. 2012;61: 1074–1081. 10.1099/jmm.0.041962-0 [DOI] [PubMed] [Google Scholar]
- 24.Castro J, Alves P, Sousa C, Cereija T, França Â, Jefferson KK, et al. Using an in-vitro biofilm model to assess the virulence potential of Bacterial Vaginosis or non- Bacterial Vaginosis Gardnerella vaginalis isolates. Sci Rep. 2015;5 10.1038/srep11640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreeva P, Shterev A, Danova S. Antimicrobial activity of vaginal lactobacilli against Gardnerella vaginalis and pathogens. Int J Adv Res Biol Sci. 2016;3: 200–207. [Google Scholar]
- 26.Huang Y, Adams MC. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol. 2004;91: 253–260. 10.1016/j.ijfoodmicro.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Silva J, Carvalho AS, Ferreira R, Vitorino R, Amado F, Domingues P, et al. Effect of the pH of growth on the survival of Lactobacillus delbrueckii subsp. bulgaricus to stress conditions during spray-drying. J Appl Microbiol. 2005;98: 775–782. 10.1111/j.1365-2672.2004.02516.x [DOI] [PubMed] [Google Scholar]
- 28.Hossein Nezhad M, Stenzel D, Britz M. Effect of growth at low pH on the cell surface properties of a typical strain of Lactobacillus casei group. Iran J Microbiol. 2010;2: 147–154. [PMC free article] [PubMed] [Google Scholar]
- 29.Nardini P, Alberto R, Palomino Ñ, Parolin C, Laghi L, Foschi C, et al. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci Rep. 2016;6 10.1038/srep29024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Hanlon DEO, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11 10.1186/1471-2334-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68: 2015–2025. 10.1093/jac/dkt156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valore E V, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obs Gynecol. 2002;187: 561–568. 10.1067/mob.2002.125280 [DOI] [PubMed] [Google Scholar]
- 33.Van Reenen CA, Dicks LMT. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Arch Microbiol. 2011;193: 157–168. 10.1007/s00203-010-0668-3 [DOI] [PubMed] [Google Scholar]
- 34.Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. PNAS. 2012;109: 1269–1274. 10.1073/pnas.1113246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gueimonde M, Sánchez B, Reyes-Gavilán CGDL, Margolles A. Antibiotic resistance in probiotic bacteria. Front Microbiol. 2013;4 10.3389/fmicb.2013.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ, et al. Microbial composition predicts genital inflammation and persistent bacterial vaginosis in adolescent South African women. Infect Immun. 2017;86: e00410–17. 10.1128/IAI.00410-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2: 543–546. 10.1016/s1286-4579(00)00313-0 [DOI] [PubMed] [Google Scholar]
- 38.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16: 1809–1813. 10.1093/humrep/16.9.1809 [DOI] [PubMed] [Google Scholar]
- 39.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2 O2-producing lac- tobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16: S273–281. 10.1093/clinids/16.supplement_4.s273 [DOI] [PubMed] [Google Scholar]
- 40.Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, et al. Hydrogen peroxide-produc- ing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174: 1058–63. 10.1093/infdis/174.5.1058 [DOI] [PubMed] [Google Scholar]
- 41.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society / Centers for Disease Control and Prevention / Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016;63: 853–867. 10.1093/cid/ciw566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn Microbiol Infect Dis. 1993;16: 111–118. 10.1016/0732-8893(93)90004-q [DOI] [PubMed] [Google Scholar]
- 43.Kharsany AB, Hoosen AA, Van den Ende J. Antimicrobial susceptibilities of Gardnerella vaginalis. Antimicrob Agents Chemother. 1993;37: 2733 LP– 2735. 10.1128/aac.37.12.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. Novel Vaginal Microflora Colonization Model Providing New Insight into Microbicide Mechanism of Action. MBio. 2011;2: 1–10. 10.1128/mBio.00168-11.Editor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomalka J, Ghneim K, Bhattacharyya S, Aid M, Barouch DH, Sekaly RP, et al. The sooner the better: innate immunity as a path toward the HIV cure. Curr Opin Virol. 2016;19: 85–91. 10.1016/j.coviro.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 46.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25: 182–191. 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 47.Anahtar MN, Byrne EH, Fichorova RN, Kwon DS, Anahtar MN, Byrne EH, et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Article Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity. 2015;42: 965–976. 10.1016/j.immuni.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of Vaginal Bacteria and D—and L -Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. MBio. 2013;4: e00460–13. 10.1128/mBio.00460-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutt P, Lapp E, Stsepetiva J, Smidt I, Taelma H, Borovkova N, et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb Ecol Health Dis. 2016;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168: 782–792. 10.1016/j.resmic.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 51.Tachedjian G, Hanlon DEO, Ravel J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLean NW, McGroarty JA. Growth Inhibition of Metronidazole-Susceptible and Metronidazole-Resistant Strains of Gardnerella vaginalis by Lactobacilli In Vitro. Appl Environ Microbiol. 1996;62: 1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson JL, Girerd PH, Karjane NW, Jefferson KK. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol. 2007;170: e1–e7. 10.1016/j.ajog.2007.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neut C, Mahieux S, Dubreuil LJ. Antibiotic susceptibility of probiotic strains: Is it reasonable to combine probiotics with antibiotics? Med Mal Infect. 2017;47: 477–483. 10.1016/j.medmal.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 55.Simoes J a, Aroutcheva a a, Shott S, Faro S. Effect of metronidazole on the growth of vaginal lactobacilli in vitro. Infect Dis Obstet Gynecol. 2001;9: 41–45. 10.1155/S1064744901000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charteris WP, Kelly HPM, Morelli L, Collins JK. Antibiotic Susceptibility of Potentially Probiotic Lactobacillus Species. J Food Prot. 1998;6: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 57.Delgado S, Flórez AB, Mayo B. Antibiotic Susceptibility of Lactobacillus and Bifidobacterium Species from the Human Gastrointestinal Tract. Curr Microbiol. 2005;50: 202–207. 10.1007/s00284-004-4431-3 [DOI] [PubMed] [Google Scholar]
- 58.Ocaña VS, Silva C, Nader-Macías ME. Antibiotic Susceptibility of Potentially Probiotic Vaginal Lactobacilli. Infect Dis Obstet Gynecol. 2006. 10.1155/IDOG/2006/18182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10: 89 Available: 10.1038/nri2691 [DOI] [PubMed] [Google Scholar]
- 60.Rollenhagen C, Asin SN. IL-8 Decreases HIV-1 Transcription in Peripheral Blood Lymphocytes and Ectocervical Tissue Explants. JAIDS J Acquir Immune Defic Syndr. 2010;54 Available: https://journals.lww.com/jaids/Fulltext/2010/08150/IL_8_Decreases_HIV_1_Transcription_in_Peripheral.3.aspx [DOI] [PubMed] [Google Scholar]
- 61.Mlisana K, Naicker N, Werner L, Roberts L, Van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206: 6–14. 10.1093/infdis/jis298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masson L, Passmore JAS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin Infect Dis. 2015;61: 260–269. 10.1093/cid/civ298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Hoek AHAM, Mevius D, Guerra B, Mullany P, Roberts AP, Aarts HJM. Acquired antibiotic resistance genes: an overview. Front Microbiol. 2011;2: 203 10.3389/fmicb.2011.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlova SI, Tao L. Induction of vaginal Lactobacillus phages by the cigarette smoke chemical benzo[a]pyrene diol epoxide. Mutat Res. 2000;466: 57–62. 10.1016/s1383-5718(00)00003-6 [DOI] [PubMed] [Google Scholar]
- 65.Kilic AO, Pavlova SI, Alpay S, Kilic SS, Tao L. Comparative Study of Vaginal Lactobacillus Phages Isolated from Women in the United States and Turkey: Prevalence, Morphology, Host Range, and DNA Homology. Clin Diagn Lab Immunol. 2001;8: 31–39. 10.1128/CDLI.8.1.31-39.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martín R, Soberón N, Escobedo S, Suárez JE. Bacteriophage induction versus vaginal homeostasis: role of H2O2 in the selection of Lactobacillus defective prophages. Int Microbiol. 2009;12: 131–136. 10.2436/20.1501.01.90 [DOI] [PubMed] [Google Scholar]
- 67.Dausset C, Patrier S, Gajer P, Thoral C, Lenglet Y, Cardot J, et al. Comparative phase I randomized open-label pilot clinical trial versus slow release muco-adhesive tablets. Eur J Clin Microbiol Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnabas SL, Dabee S, Passmore JS, Jaspan HB, Lewis DA, Jaumdally SZ, et al. Converging epidemics of sexually transmitted infections and bacterial vaginosis in southern African female adolescents at risk of HIV. Int J STD AIDS. 2017;0: 1–9. 10.1177/0956462417740487 [DOI] [PubMed] [Google Scholar]
- 69.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173: 697–703. 10.1128/jb.173.2.697-703.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014/04/01. 2014;30: 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012/04/16. 2012;19: 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kosugi S, Hirakawa H, Tabata S. GMcloser: closing gaps in assemblies accurately with a likelihood-based selection of contig or long-read alignments. Bioinformatics. 2015;31: 3733–3741. 10.1093/bioinformatics/btv465 [DOI] [PubMed] [Google Scholar]
- 73.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29: 1072–1075. 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2013/11/29. 2014;42: D206–D214. 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012/07/10. 2012;67: 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2019;48: D517–D525. 10.1093/nar/gkz935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob Agents Chemother. 2019;63: e00483–19. 10.1128/AAC.00483-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44: 16–21. 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Couvin D, Bernheim A, Toffano-Nioche C, Touchon M, Michalik J, Néron B, et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018;46: W246–W251. 10.1093/nar/gky425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biswas A, Gagnon JN, Brouns SJJ, Fineran PC, Brown CM. CRISPRTarget: bioinformatic prediction and analysis of crRNA targets. RNA Biol. 2013/03/14. 2013;10: 817–827. 10.4161/rna.24046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Columb MO, Sagadai S. Multiple comparisons. Curr Anaesth Crit Care. 2006;17: 233–236.doi: 10.1016/j.cacc.2006.03.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
pH of vaginal L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), L. mucosae (E) and the probiotic (F) cultures were measured over 48 hours. Probiotics include L. reuteri, L. rhamnsus and L. acidophilus strains. As control, the pH of abiotic MRS was measured (shown in light grey). ATCC reference strains are shown as black lines. Physiological vaginal pH of women with optimal microbiota is shown as grey shading.
(TIFF)
Comparison of vaginal Lactobacillus spp. concentrations (measured in CFUs) at a standardized OD600nm 0.1 (±0.01): L. crispatus (A), L. gasseri (B), L. vaginalis (C), L. jensenii (D), and L. mucosae (E). Isolates from BV/STI-negative women are shown by plain bars, those from BV and/or STI-positive women are patterned and ATCC strains are shown by black bars.
(TIFF)
The table shows the assembly statistics for the sequenced vaginal Lactobacillus strains. The sequences have been deposited in the European Nucleotide Archive (ENA) under accession number PRJEB37955.
(DOCX)
Data Availability Statement
All sequencing data are available from the European Nucleotide Archive database at EMBL-EBI (accession number PRJEB37955).