Abstract
Paracrine signaling between tumor and surrounding stromal cells is critical for the maintenance of tumor microenvironment during ovarian cancer progression. Small extracellular vesicles (sEVs; exosomes in particular) are nano-sized vesicles secreted actively by many cells including tumor cells and are found to have fundamental roles in intercellular communication through shuttling functional RNAs. Although microRNAs (also called miRNAs or miRs), small non-coding RNAs regulating gene expression, are selectively accumulated in tumor sEVs and can mediate intercellular communication, the exact biological mechanisms underlying the functions of exosomal miRNAs in ovarian tumor angiogenesis remain unclear. In this study, sEVs were isolated from conditioned medium of the human ovarian carcinoma cell line SKOV-3 using ExoQuick Exosome Precipitation Solution, and characterized by scanning electron microscopy, dynamic light scattering, and immunoblotting. To elucidate the possible paracrine effects on ovarian tumor cell-derived sEVs (TD-sEVs), we investigated the angiogenesis-related signaling events triggered by TD-sEVs in endothelial cells. Due to the possible role in ovarian tumor pathogenesis, we focused on miR-141-3p which was detected to be enriched in TD-sEVs compared with their corresponding donor cells. We identified that sEV transfer of miR-141-3p considerably reduced the expression levels of cytokine-inducible suppressors of cytokine signaling (SOCS)-5 leading to up-regulated JAK-STAT3 pathway in endothelial cells. We also observed that sEV-shuttled miR-141-3p may up-regulate the expression of VEGFR-2 in endothelial cells which leads to promoting endothelial cell migration and angiogenesis. The putative role of miR-141-3p shuttled by TD-sEVs in regulating VEGFR-2 expression was demonstrated by the ability of anti-miR-141-3p to rescue the promoting effects of TD-sEVs on the expression of VEGFR-2 in endothelial cells. Our results also revealed that TD-sEVs trigger the intracellular reactive oxygen species (ROS)-dependent activation of NF-κB signaling in endothelial cells. Taken together, our findings propose a novel model in which sEV transfer of epithelial ovarian cancer-secreted miR-141-3p plays as a significant mediator of intercellular communication, promoting endothelial cell angiogenesis.
Electronic supplementary material
The online version of this article (10.1007/s12079-020-00548-5) contains supplementary material, which is available to authorized users.
Keywords: Tumor angiogenesis, Small extracellular vesicles, Ovarian cancer-secreted miR-141-3p, JAK-STAT3 pathway
Introduction
Angiogenesis, the recruitment of blood vessels, has a hand in the growth and progression of ovarian carcinomas through ascites formation and metastatic spread (Bamias et al. 2012; Monk et al. 2016). The regulation of angiogenesis through paracrine signaling interactions between epithelial tumor cells and surrounding tumor stromal cells may be important in tumor growth as well as metastasis (Watnick 2012). Vascular endothelial growth factor (VEGF) signaling is the key mediator of angiogenesis and identifying its role and other signaling pathways related to the regulation of tumor angiogenesis would be of value against ovarian cancer (Weis and Cheresh 2011).
It has been postulated that the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway are substantial for tumorigenesis (Thomas et al. 2015). Indeed, the STAT proteins were elucidated as a group of latent cytoplasmic transcription factors that are activated in reaction to cytokine signaling (Mitchell and John 2005). The signaling by STATs is negatively governed by cytokine-inducible suppressors of cytokine signaling (SOCS) proteins as part of controlling negative feedback (Schindler et al. 2007; Shuai and Liu 2003). STAT3 is able to up-regulate VEGF expression in tumor cells, which in turn leads to the activation of VEGFR-2 in endothelial cells. Besides, VEGFR-2, which is mainly expressed in endothelial cells, is identified as one of the direct transcriptional targets of STAT3. As a positive correlation, the up-regulation of VEGFR-2 causes STAT3 activation in endothelial cells, which leads to VEGFR2 up-regulation and further activation. This STAT3-orchestrated interaction between endothelial and tumor cells provokes VEGFR-induced signaling and results in a number of angiogenesis-involved cellular activities in endothelial cells (Lee et al. 2015; Thomas et al. 2015). Nonetheless the JAK-STAT pathway is important in tumorigenesis and its activation may continue to have a role for cancer progression, the significance of the pathway in tumor stromal endothelial cells, particularly in terms of angiogenesis, still remains to be fully elucidated (Chen and Han 2008).
Small extracellular vesicles (sEVs; exosomes in particular) are diminutive (< 150 nm) membrane-bound extracellular vesicles proactively secreted by means of most cells (Thery et al. 2018) and can function in recipient cells either in their microenvironment or distant tissues (Colombo et al. 2014; Motavaf et al. 2016). Owing to harboring numerous biological macromolecules including proteins and RNAs as cargo, exosomes are identified to contribute to intercellular communication (Fu et al. 2016; Lee et al. 2012; Valadi et al. 2007). A growing body of evidence propose that tumor-secreted exosomes are able to induce phenotypic and functional alternations in the primary tumor microenvironment or metastatic niches (Ciardiello et al. 2016; Costa-Silva et al. 2015; Roma-Rodrigues et al. 2014). However, the biological functions of tumor-secreted exosomes in tumor stromal cells have not yet been fully elucidated.
Various studies have been elucidated that tumor cells release sEVs (or exosomes) involving microRNAs, also called miRNAs or miRs), a group of small non-coding RNAs regulating gene expression networks through post-transcriptional modification (Melo et al. 2014). The fact is that the uncontrolled expression of such molecules is imputed to the development and progression of human cancers, indicating that these molecules may operate as either oncogene or tumor suppressor (Babashah 2014; Babashah et al. 2012; Babashah and Soleimani 2011). miR-141, as one of the important members of miR-200 family, was demonstrated to be prominently overexpressed in ovarian carcinomas (Iorio et al. 2007; Koutsaki et al. 2017) raising the prospect that modulating the expression or activity of this miRNA may broaden the horizons toward using this miRNA as a therapeutic option in a clinical setting. Furthermore, since miR-141 was found to be involved in ovarian tumor pathogenesis (Choi and Ng 2017; Mateescu et al. 2011) and selectively accumulated in circulating tumor exosomes (Pan et al. 2018; Taylor and Gercel-Taylor 2008), this exosomal miRNA could be used as a biomarker to elucidate the ovarian cancer’s progression.
In this study, we objected to shed light on the possible paracrine consequences of ovarian tumor cell-derived sEVs (TD-sEVs) on endothelial cell angiogenesis. We found that miR-141-3p was enriched in both TD-sEVs and their corresponding donor cells and also observed that the transfer of sEVs-shuttled miR-141-3p promotes the migration of endothelial cells and their angiogenesis, partially, through up-regulating JAK-STAT3 pathway. We also illuminated that TD-sEVs-induced NF-κB activation may be adjusted by the intracellular reactive oxygen species (ROS) production which is happened in endothelial cells, showing a possible mechanism clarifying the paracrine effects of TD-sEVs on vascular reactions inside the microenvironment of ovarian tumor cells.
Materials and methods
Cell culture
The human ovarian carcinoma cell line SKOV-3 and human umbilical vein endothelial cells (HUVECs) were obtained from the Pasteur Institute of Iran and were cultured in Dulbecco’s modified Eagle’s medium (DMEM). The normal immortalized human ovarian surface epithelial cell line T1074 was maintained in Roswell Park Memorial Institute 1640 (RPMI 1640) medium. All media were supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 50 units/ml of penicillin G, and 50 μg/ml of streptomycin (all from Gibco BRL, Rockville, MD, USA) at 37 °C in a humidified atmosphere with 5% CO2.
Extracellular vesicle isolation and purification
The cells were cultured in T75 flasks with 5 × 104 cells/cm2 and propagated in the cell culture medium complemented with 10% exosome-depleted serum (EXO-FBS; System Biosciences) for 48 h. When the cells reached 80–90% confluency, the culture supernatants containing sEVs were harvested and subjected to differential centrifugation. Of note, the tumor cells were passaged 4–6 times and normal cells were passaged 2 times before using. Briefly, around 700 ml of the pooled conditioned media was centrifuged at 300×g for 10 min at 4 °C and then at 10,000×g for 30 min at 4 °C to eliminate residual cells and cellular debris, respectively. sEVs were pelleted by ExoQuick™ Exosome Precipitation Solution kit (System Biosciences) according to manufacturer’s instructions and re-suspended in PBS.
Dynamic light scattering measurements
To determine the size of isolated sEVs, we performed the dynamic light scattering (DLS) by applying a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) based on the given protocol.
Scanning electron microscopy
An aliquot of the sEV suspension was loaded on to a carbon-coated grid and fixed in 2.5% glutaraldehyde. The samples were undergone to a critical point of dehydration with the increasing grades of ethanol and then vacuum-dried on a glass substrate and sputter-coated with gold. To show the size and morphology of sEVs, scanning electron microscopy (SEM) was utilized.
Extracellular vesicle labeling assay
The PKH26 red fluorescent labeling kit (Sigma-Aldrich, USA) was applied to label the isolated TD-sEVs. The labeled TD-sEVs were incubated with endothelial cells for 12 h. For nuclear staining, the cells were fixed and stained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, USA). To monitor the fluorescence uptake, we utilized an inverted fluorescence microscope (Olympus CKX41).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the cells or sEVs with TRIzol® (Invitrogen, USA) and then a total of 1 μg of RNA was reverse-transcribed into complementary DNA (cDNA) using PrimeScript 1st Strand cDNA Synthesis kit (TAKARA Bio Inc., Otsu, Japan). The expression levels were determined by RT-qPCR using SYBR® Premix Taq™ II (TAKARA, Japan). In the way, the GAPDH gene was exploited as a suitable reference gene to normalize the number of transcripts in samples. The relative expression of each gene was indicated with mean Ct values using the 2-ΔΔCt method (Livak and Schmittgen 2001) (Hayat Nosaeid et al. 2009). To quantify miR-141, poly-(A)-tailing and cDNA synthesis were performed by reverse transcription of 1 μg total RNA using MiR-Amp Kit (ParsGenome, Iran), and then the expression levels of mature miRNA were determined using miR-141-specific primers as described previously (Bitaraf et al. 2019). The expression was normalized with U6 small nuclear RNA (snRNA) and RNU44 (Han et al. 2014).
Transfer of miRNA
To measure sEV transfer of miR-141-3p to endothelial cells, approximately 3 × 104 cells/well were co-incubated at different time points with 100 μg/ml TD-sEVs and transcription inhibitor α-amanitin (Sigma, 50 μg/ml) or only just with α-amanitin. Total RNA from HUVECs was isolated at time 0 and after 12 and 48 h of stimulation with TD-sEVs and/or α-amanitin. As an indirect measurement of sEV transfer of miRNA, the difference in Ct values between α-amanitin stimulated cells in the presence or absence of TD-sEVs at each experimental time point were calculated (Collino et al. 2010).
Downregulation of miR-141-3p using a miRNA inhibitor
HUVECs incubated with either TD-sEVs or vehicle control (PBS) were transfected with miRCURY LNA™ microRNA inhibitor for hsa-miR-141-3p or its negative control (Exiqon) at a final concentration of 100 nM using lipofectamine RNAiMAX (Invitrogen, USA) according to the manufacturer’s recommendation.
NF-κB activation assay
To measure NF-κB activity, the nuclear and cytosolic fractions were separated using a commercially available NF-κB Activation Assay Kit (FIVEphoton Biochemicals, San Diego, CA, USA) as stated by the manufacturer’s instructions. As a result, protein concentrations in the lysates were measured by Bradford assay, and the NF-κB p65 protein level in nuclear and cytoplasmic preparations was indicated by immunoblotting.
Immunoblotting
Cells or sEVs were lysed immediately with RIPA buffer which a cocktail of protease inhibitors (Roche) was added to Proteins were segregated on 10–12% SDS-polyacrylamide gels (SDS-PAGE) and transmitted to a polyvinylidene difluoride (PVDF) membrane. To block the membranes, 5% bovine serum albumin (Merck) in TBST (10 mM Tris-buffered saline with 0.05% Tween 20) was used. The blots were reprobed and incubated with the specific primary antibodies diluted in TBST (1:1000). After rinsing, horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated with blots and then subjected to chemiluminescence (ECL, Amersham, Buckinghamshire, UK). To do immunoblotting, various antibodies were employed suchlike rabbit monoclonal anti-CD9 (ab92726, Abcam), mouse monoclonal anti-CD81 (sc-166,029, Santa Cruz Biotechnology), and Rabbit polyclonal anti-p65 (NFKB-2, FIVEphoton Biochemicals). β-actin was used as a loading control.
Determination of intracellular reactive oxygen species production
The intracellularly reactive oxygen species (ROS) production was discerned by appending the 2′, 7′-dichlorofluorescein diacetate (DCFDA) (ab113851, Abcam) to the cell suspension. Briefly, the cells were washed and then incubated in the dark with 5 μM DCFDA for 45 min. The cells were washed and the fluorescent intensity was measured by flow cytometry (BD FACS Canto II, BD Bioscience).
Cell proliferation assay
The viable endothelial cells, incubated with either different concentrations of TD-sEVs (25, 50 and 100 μg/ml), 100 μg/ml normal, non-tumorigenic ovarian epithelial cell-derived sEVs (NT-sEVs) or vehicle control (PBS), were measured in triplicate by trypan blue exclusion 24 and 48 h after incubation.
Scratch wound healing assay
A confluent monolayer of serum-starved endothelial cells was subjected to a single vertical scratch. To display and quantify the wound healing at three different time points, the cells were treated with 100 μg/ml TD-sEVs, 100 μg/ml NT-sEVs or vehicle control (PBS). Consequentially, to analyze the wound closure, the online version of Wimasis WimScratch software was utilized.
In vitro capillary tube formation assay
To access the dynamics of vascular endothelial cell behavior, the in vitro formation of capillary-like tubes mediated by endothelial cells was monitored. In this assay, the cells, HUVECs, were kept in serum-starved condition in DMEM for 24 h and then 5 × 104 cells were transferred into 24-well plates pre-coated with Matrigel (Invitrogen, USA). For the next step, the incubation of the cells was executed with TD-sEVs (100 μg/ml) or vehicle control (PBS) for 12 h. We quantified the number of total branch points and tube length in 3 randomly selected fields.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) for at least three experiments. Differences between groups were assessed by one-way ANOVA with Graph-Pad Prism software v.7.0. For all statistical tests, p value <0.05 was considered statistically significant.
Results
Isolation, characterization and cellular uptake of purified TD-sEVs
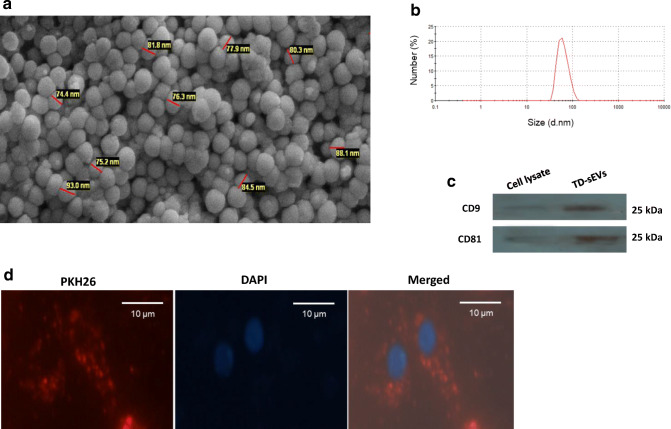
To uncover the paracrine effects of TD-sEVs on endothelial cell angiogenesis, sEVs were isolated from ovarian carcinoma cells. SEM analysis indicated that the purified TD-sEVs had a spherical shape with an approximate diameter ranging from about 50 to 150 nm (Fig. 1a). Also, the particle size distribution of sEVs by DLS revealed a mere peak at about 70 nm (Fig. 1b). The surface charge of sEV preparation, reflected by its zeta potential measurement, showed that the isolated sEVs were negatively charged (the range of −6.85 to −11.4 mV) and within the range reported previously (Deregibus et al. 2016) (Wang et al. 2015). In addition, immunoblotting demonstrated that the exosome-specific tetraspanin markers CD9 and CD81 were enriched in the TD-sEVs’ pool (Fig. 1c). Moreover, in order to examine whether TD-sEVs could be taken up by endothelial cells, PKH26-labeled sEVs were incubated with sub-confluent HUVECs for 12 h. Fluorescence microscopy imaging showed that TD-sEVs can be incorporated and concentrated into the endothelial cells’ cytoplasm (Fig. 1d).
Fig. 1.
Characterization and cellular internalization of isolated TD-sEVs. a A scanning electron micrograph of sEVs derived from ovarian tumor cells. Particles were approximately spherical and membrane encapsulated with the size range of 50–150 nm. b Representative dynamic light scattering (DLS) number distribution measurement of isolated TD-sEVs revealed a single peak (~70 nm diameter). c Detection of the exosome-specific markers CD9 and CD81 in purified sEVs obtained from tumor cells as demonstrated by immunoblotting. d Fluorescence microscopy analysis showed uptake of PKH26-labeled TD-sEVs by endothelial cells. Fluorescence micrograph reveals that TD-sEVs were incorporated and localized in the cytoplasm of HUVECs. The nuclear staining was performed by DAPI. The bar represents 20 μm
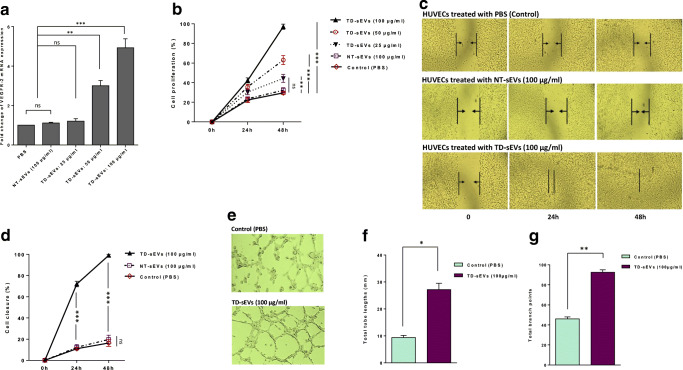
TD-sEVs promote endothelial cell migration and angiogenesis
Numerous studies have been identified that VEGF signaling is a consequential positive regulator of physiological as well as pathophysiological neovascularization (Goel and Mercurio 2013; Weis and Cheresh 2011). It was also demonstrated that VEGFR-2 is a vital endothelial cell-specific signal transducer receptor involved in VEGF-stimulated angiogenesis (Abhinand et al. 2016; Hicklin and Ellis 2005). To reveal the putative paracrine effects of TD-sEVs on endothelial cell angiogenesis, HUVECs were stimulated with different concentrations of sEVs derived from ovarian carcinoma cells, SKOV-3. RT-qPCR results showed that TD-sEVs caused an increase in the transcript levels of VEGFR-2 in HUVECs in a dose-dependent manner. As a control, there was no significant difference at the expression levels between NT-sEVs-treated and control PBS-treated cells (Fig. 2a).
Fig. 2.
TD-sEVs up-regulate VEGFR-2 expression and promote in vitro endothelial cell migration and angiogenesis. a Up-regulating of VEGFR-2 expression in HUVECs incubated with various concentrations of TD-sEVs (25, 50 and 100 μg/ml), 100 μg/ml NT-sEVs, or vehicle control (PBS) after 48 h. Columns represent means of three different experiments; bars represent SD. ns: non-significant (P > 0.05), **P < 0.01, ***P < 0.001. b Promoting effects of different concentrations of TD-sEVs on the proliferation rate of HUVECs at time points 24 and 48 h. No significant difference in the cell proliferation rate was observed between NT-sEVs-treated and control PBS-treated cells at various time points. Points represent means of three experiments; bars represent SD. c Representative micrographs of monolayer wounding assay in HUVECs treated with 100 μg/ml TD-sEVs, 100 μg/ml NT-sEVs, or vehicle control (PBS) at the time of scratch wounding (0 h), 24 and 48 h thereafter. d Quantitative analysis of wound closure at different time points indicated the promoting effects of TD-sEVs on the migration rates of HUVECs compared to those of cells treated with NT-sEVs or vehicle control (PBS). e Representative micrographs showing the effects of TD-sEVs on the capability of endothelial cells to form the capillary-like tube structures in vitro after 24 h. Quantitative analysis of the tube lengths (f) and branch points (g) of HUVECs following treatment with 100 μg/ml TD-sEVs or vehicle control (PBS) were calculated in three randomly selected fields. ns: non-significant, *P < 0.05, **P < 0.01, ***P < 0.001
Furthermore, various investigations have shown that VEGF signaling functions as a vigorous inducer of endothelial cell proliferation, migration, differentiation, and cell survival in addition to vessel permeability and dilation (Cebe-Suarez et al. 2006; Wang et al. 2008). Since TD-sEVs led to the up-regulation of VEGFR-2, we sought to assess whether TD-sEVs can exert promoting effects on the proliferation as well as migration rates of endothelial cells. To decipher, HUVECs were treated with either TD-sEVs or vehicle control (PBS) after which the cell proliferation in addition to the migration rates was evaluated. We identified that the proliferation of HUVECs stimulated with various concentrations of TD-sEVs significantly increased in a dose- and time-dependent manner. Importantly, we observed that NT-sEVs had no effects on the proliferation rate of HUVECs in comparison with cells treated with vehicle control PBS (Fig. 2b). Furthermore, to identify the effect of TD-sEVs on the migration of endothelial cells, a wound-healing assay was performed. As shown in Fig. 2c, TD-sEVs dramatically promoted the migration of HUVECs into the scratched areas of monolayers in a time-dependent manner. We also observed that the migration rate of endothelial cells incubated with NT-sEVs (100 μg/ml) did not show any significant difference between TD-sEVs-treated and control PBS-treated cells at various time points. Analysis of wound closure showed that HUVECs which were stimulated with 100 μg/ml TD-sEVs migrated significantly faster in comparison with the cells incubated with NT-sEVs (100 μg/ml) or PBS as vehicle control (Fig. 2d).
Additionally, to determine whether TD-sEVs affect the in vitro tubulogenesis of HUVECs, a capillary tube formation assay was conducted. Besides, we monitored the vascular behavior of endothelial cells. Compared with the control group, HUVECs stimulated with 100 μg/ml TD-sEVs displayed an increased ability to form vessel-like structures (Fig. 2e). As shown in Fig. 2f, g, TD-sEVs significantly increased the tube length and branch point formation by HUVECs in comparison with HUVECs formed by PBS-treatment. From the results, it is fair to suggest that sEVs derived from ovarian tumor cells may promote endothelial cell migration and angiogenesis through the up-regulation of VEGF signaling.
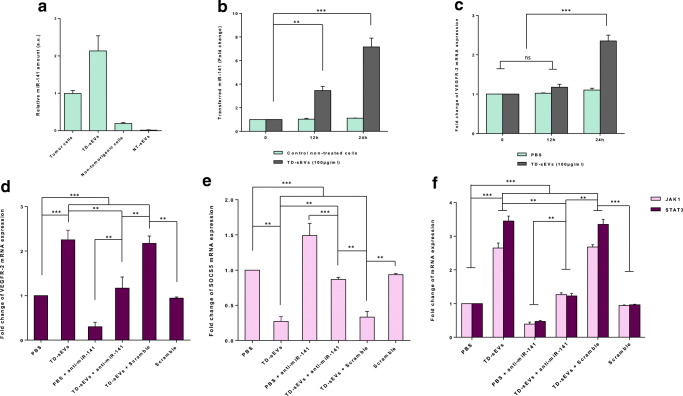
Tumor-secreted miR-141-3p regulates the JAK-STAT3 pathway through modulating suppressor of cytokine signaling 5 in endothelial cells
To describe the molecular mechanisms by which TD-sEVs promote endothelial cell angiogenesis, we conjectured that miRNAs shuttled by TD-sEVs might be account for pro-angiogenic effects of TD-sEVs on endothelial cells. In this regard, as miR-141 is considered as a critical miRNA involved in ovarian tumor pathogenesis (Iorio et al. 2007; Koutsaki et al. 2017; Mateescu et al. 2011) and also an enriched miRNA in ovarian tumor exosomes (Pan et al. 2018; Taylor and Gercel-Taylor 2008), we calculated and elucidated of this miRNA level in sEVs and corresponding donor cells. As shown in Fig. 3a, we highlighted that miR-141-3p was detectable in both TD-sEVs and corresponding donor cells. Importantly, no considerable levels of miR-141-3p were detected in sEVs derived from normal epithelial cells.
Fig. 3.
Extracellular vesicle transfer of miR-141-3p up-regulates the JAK-STAT pathway through modulating SOCS5 in endothelial cells. a The relative miR-141 levels determined by RT-qPCR in TD-sEVs and NT-sEVs as well as their corresponding donor cells. (b, c) sEV transfer of miR-141 led to a significant and time-dependent increase in VEGFR-2 mRNA expression. b Transfer of miR-141 was determined by RT-qPCR in HUVECs incubated with both TD-sEVs and α-amanitin compared to α-amanitin only treated cells (negative control) at time points 0, 12 and 24 h. c Mean normalized ratios for VEGFR-2 transcript levels measured by RT-qPCR in HUVECs treated with 100 μg/ml TD-sEVs (compared to PBS-treated control cells) at different time points. d sEV transfer of miR-141-3p caused the up-regulation of VEGFR-2 expression in endothelial cells. RT-qPCR data showed that the VEGFR-2 transcript levels in HUVECs treated with anti-miR-141-3p along with either TD-sEVs or vehicle control (PBS) were significantly lower than those of cells only treated with TD-sEVs. A significant reduction in VEGFR-2 expression in anti-miR-141-3p-transfected HUVECs was also found compared with a scramble-transfected negative control. e-f TD-sEVs up-regulates the JAK-STAT pathway via down-regulating SOCS5 in HUVECs. e Mean normalized ratios for SOCS5 transcript levels measured by RT-qPCR showed that TD-sEV-shuttled miR-141-3p caused down-regulation of SOCS5 expression in HUVECs. Importantly, the inhibitory effect of TD-sEVs on SOCS5 expression level was partially rescued when TD-sEVs-treated endothelial cells transfected along with anti-miR-141. f Mean normalized ratios for JAK1 and STAT3 transcript levels measured by RT-qPCR in TD-sEVs-treated HUVECs transfected with either anti-miR-141-3p or scramble negative control. Results proposed that JAK1 and STAT3 transcript expression levels were significantly increased in TD-sEVs-stimulated HUVECs, while in the presence of anti-miR-141-3p, the promoting effect of TD-sEVs on the JAK1 and STAT3 expression levels was partially diminished. Columns represent means of three different experiments; bars represent SD. ns: non-significant, **P < 0.01, ***P < 0.001
Given sEVs/exosomes represent a protective and enriched source of shuttle miRNAs, we sought to examine sEV transfer of epithelial ovarian cancer-secreted miR-141-3p into endothelial cells. For this purpose, HUVECs were co-stimulated with 100 μg/ml TD-sEVs by adding α-amanitin to repress transcriptional activation resulted from sEVs (Collino et al. 2010) (see Supplementary Fig. 1). As shown in Fig. 3b, results from RT-qPCR revealed that the levels of miR-141-3p were gradually increased in HUVECs stimulated with both TD-sEVs and α-amanitin compared to the negative control cells (α-amanitin alone).
To highlight the functional significance of epithelial ovarian cancer-secreted miR-141-3p, the expression level of VEGFR-2 in sEV-treated HUVECs was also evaluated at various time points. Interestingly, our results cast a new light on a significant, time-dependent increase of VEGFR-2 mRNA expression level, suggesting that TD-sEVs may directly or indirectly enhance VEGF signaling in endothelial cells (Fig. 3c). For further confirmation that miR-141-3p shuttled by TD-sEVs promotes VEGFR-2 up-regulation, HUVECs were transfected with anti-miR-141-3p (100 nM), as an antagomir to miR-141-3p, and stimulated with TD-sEVs (100 μg/ml) for 24 h. Intriguingly, in the presence of anti-miR-141-3p in HUVECs, the promoting effect of TD-sEVs on the VEGFR-2 mRNA expression level was partially rescued (Fig. 3d). Importantly, we also found that sEV transfer of miR-141-3p induced a significant increase in the expression of VEGF in endothelial cells (Supplementary Fig. 2). This finding supports the notion that the sEV transfer of tumor-secreted miR-141-3p may cause the up-regulation of VEGF signaling in endothelial cells.
Due to this fact that SOCS5 has been previously suggested as an undeviating target of miR-141-3p (Selth et al. 2012), the expression level of SOCS5 was evaluated in sEV-treated HUVECs. RT-qPCR results showed that the sEV transfer of miR-141-3p (Fig. 3b) was inversely associated with the SOCS5 mRNA expression level in a time-dependent manner in HUVECs incubated with 100 μg/ml TD-sEVs (Fig. 3e). As expected, transfection of anti-miR-141-3p into HUVECs significantly diminished the inhibitory effects of TD-sEVs on the SCOCS5 mRNA expression level (Fig. 3e). Additionally, because SOCS5 plays as a negative regulator of JAK-STAT3 signaling and STAT3 induces VEGF and VEGFR2 expression through binding to the corresponding promoters (Lee et al. 2015; Thomas et al. 2015), we concluded that tumor-secreted miR-141-3p may execute its influences on endothelial cell angiogenesis through altering the JAK-STAT3 pathway. Consistently, we identified that the transcript expression levels of JAK1 and STAT3 were remarkably increased in HUVECs following treatment with 100 μg/ml TD-sEVs, while in the presence of anti-miR-141-3p, the promoting effect of TD-sEVs on the JAK1 and STAT3 expression levels was partially rescued (Fig. 3f). Broadly translated our findings indicate that sEV transfer of tumor-secreted miR-141-3p resulted in reduced SOCS5 expression and may consequently up-regulate the VEGFR-2 expression in endothelial cells through promoting the JAK-STAT3 pathway.
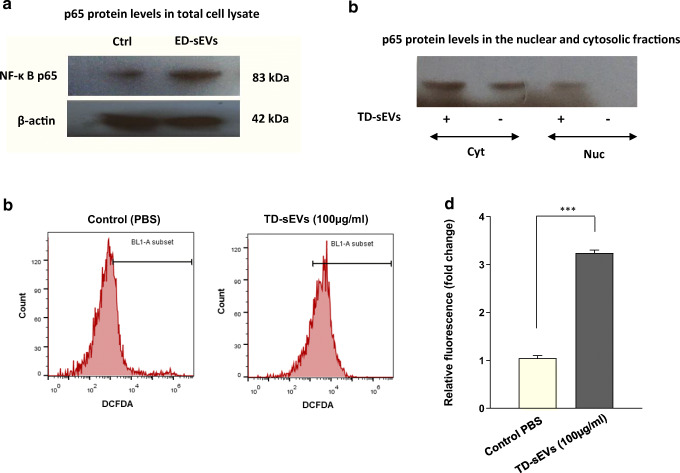
Increased nuclear translocation of NF-κB p65 in TD-sEV-treated endothelial cells is associated with elevated production of intracellular ROS
To our knowledge, the activation of NF-κB is a vital signaling factor for several vascular events in surrounding non-tumorigenic cells (Tabruyn and Griffioen 2008; Tabruyn et al. 2009), we sought to examine whether the transcription factor NF-κB is engaged in the paracrine effects of TD-sEVs on endothelial cell angiogenesis. To this end, HUVECs were incubated with 100 μg/ml TD-sEVs and the expression of endogenous NF-κB signaling subunit p65 in the whole-cell lysate was measured and compared to those cells which were treated with vehicle control (PBS). Substantiating our findings gained using HUVECs, sEV incubation caused a significant increase of NF-κB p65 protein level after 48 h (Fig. 4a). Considering the elevated protein levels of p65 in the total cell lysate of sEV-incubated HUVECs, moreover, we assessed the localization of p65 protein via immunoblotting. Results provide a basis for translocation of p65 into the nucleus of HUVECs following incubation with 100 μg/ml TD-sEVs after 48 h (Fig. 4b).
Fig. 4.
TD-sEVs-induced NF-κB activation may be mediated by ROS production in endothelial cells. a Immunoblotting analysis of NF-κB p65 protein levels in the total cell lysate of HUVECs, 48 h after treatment with 100 μg/ml TD-sEVs compared to control. β-actin was used as an endogenous loading control. b Activation of NF-κ B following sEV treatment revealed by elevated nuclear p65 protein levels (Nuc). Cyt: cytoplasmic fraction. c, d Promoting effect of TD-sEVs on the relative levels of intracellular ROS production in endothelial cells. c Representative histograms of DCFDA fluorescence intensity in HUVECs stimulated with 100 μg/ml TD-sEVs after 48 h. d Comparison of DCFDA fluorescent intensity between HUVECs treated with TD-sEVs (100 μg/ml) and control PBS-treated cells after 48 h. Columns represent means of three different experiments; bars represent SD. ***P < 0.001. ROS, reactive oxygen species; DCFDA, 2′, 7′-dichlorofluorescein diacetate
To shed light on the mechanism of NF-κB activation, we investigated intracellular ROS production in sEV-treated HUVECs. As depicted in Fig. 4c, d, the ROS generation in HUVECs stimulated 48 h with 100 μg/ml TD-sEVs was significantly higher than control PBS-treated cells. These findings propose that NF-κB activation upon sEV incubation may be intervened by ROS production in endothelial cells.
Discussion
The tumor microenvironment takes a center stage in ovarian cancer development and metastasis that are associated with disease progression and survival. This raises the intriguing possibility that modulating tumor microenvironment could provide new therapeutic opportunities for treating ovarian cancer, regardless of the genetic changes within tumor cells (Curtis et al. 2018). The tumor microenvironment involves complex biological signaling pathways with the contribution of surrounding tumor stromal cells that facilitate tumor development and progression. Paracrine signaling interactions between tumor cells and stromal endothelial cells are a key aspect of tumor progression, particularly in terms of angiogenesis (Watnick 2012).
Accumulating shreds of evidence have revealed that the cell-to-cell communication between epithelial tumor cells and surrounding stromal cells could be promoted through the horizontal transfer of functional RNAs through sEVs/exosomes (Bell and Taylor 2017; Liang et al. 2016; Pakravan et al. 2017; Wan et al. 2018). Similarly, recent investigations have demonstrated that tumor cells have an exacerbated secretion of sEVs/exosomes containing certain miRNAs that can modulate their hostile microenvironment to promote tumor angiogenesis (Lin et al. 2018; Taverna et al. 2014; van Balkom et al. 2013). Although some exciting insights have been obtained, the exact biological mechanisms underlying the functions of this kind of extracellular noncoding RNAs are incompletely characterized.
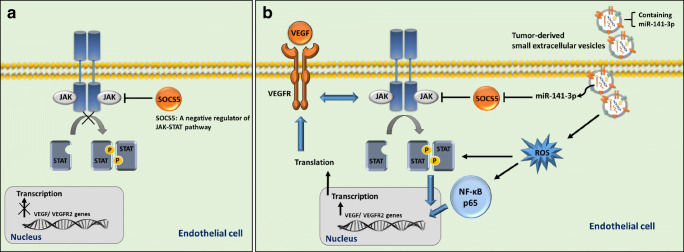
To our knowledge, the present investigation is the first study showing that the sEV-shuttled miR-141-3p from ovarian cancer cells may provoke in vitro angiogenesis through activating multiple angiogenic-related signaling pathways. An inverse correlation between SOCS5 and miR-141-3p has been previously described and SOCS5 was suggested as a bona fide target of this miRNA (Selth et al. 2012). On the other hand, SOCS5 is considered as a negative regulator of the JAK-STAT3 pathway and STAT3 participates in regulating angiogenesis, in large part, through inducing VEGF signaling (Chen and Han 2008) (Thomas et al. 2015). This result ties well with previous studies wherein we concluded that sEV transfer of tumor-secreted miR-141-3p may up-regulate the JAK-STAT pathway and VEGFR-2 signaling in part by targeting SCOC5 in endothelial cells (Fig. 5).
Fig. 5.
A proposed model clarifying the paracrine effects of TD-sEVs on endothelial cells. The model illustrates how sEVs derived from ovarian tumor cells deal with endothelial cells through modulating multiple angiogenic-related signaling pathways. a In a physiological state, SOCS5 functions as a negative regulator of the JAK-STAT pathway, which in turn modulates downstream signalings in endothelial cells. b Transfer of miR-141 by TD-sEVs may up-regulate the JAK-STAT pathway and VEGFR-2 signaling in part by targeting SCOC5 in HUVECs. Additionally, the induced NF-κB activation triggered by TD-sEVs which may be mediated by the intracellular ROS production is critical events in endothelial cell angiogenesis. This model may be an explanation for the vascular response within the microenvironment of ovarian cancer
As signaling via the VEGF pathway is crucial for angiogenic responses, we initially investigated the effects of TD-sEVs on VEGFR-2 signaling in endothelial cells and found a positive dose-response relationship between TD-sEVs and VEGFR-2 expression levels (Fig. 2a). We also observed a significant promoting effect of TD-sEVs on the proliferation ratio of endothelial cells in a dose-dependent manner (Fig. 2b) that highlights the role of VEGFR-2 signaling as an important regulator of endothelial cell proliferation. Interestingly, we also found that TD-sEVs increased the migratory capability of endothelial cells and significantly induced the ability of endothelial cells to make vessel-like structures (Fig. 2c-g). These findings indicate that the involvement of TD-sEVs to tumor angiogenesis may be in part imputed to VEGF signaling alternation and affirm to the putative beneficial effects of therapies targeting VEGF signaling for ovarian cancer.
The significance of VEGF and its endothelial cell receptors in promoting angiogenesis is firmly-established (Abhinand et al. 2016; Cebe-Suarez et al. 2006; Hicklin and Ellis 2005); however, other angiogenesis-related signaling events activated by paracrine effects of tumor cells resulting in endothelial cell angiogenesis still remain to be further clarified. The contribution of the JAK-STAT signaling in the construction of a unique tumor microenvironment and its miRNA-mediated regulation in endothelial cells and tumor angiogenesis has been previously suggested (Bartoli et al. 2003; Dong et al. 2010; Zhu et al. 2018). Doebele et al. (2010) demonstrated that members of the miR-17-92 cluster may function to inhibit angiogenesis in endothelial cells. They found JAK1 as a direct target of miR-17 and provided a shred of evidence for the contribution of this pathway in angiogenesis. It was also revealed that miR-106b, a miRNA belonging to the miR-106b-25 cluster, exerts an antiangiogenic function in endothelial cells through suppression of STAT3 (Maimaiti et al. 2016). The significance of a tumor-secreted miRNA in intercellular communication was suggested by another study regarding that the angiogenesis of endothelial cells can be triggered by tumor-secreted miR-9 which exerts its functions through stimulating the JAK-STAT pathway (Zhuang et al. 2012).
Consistent with the previous studies, we found a link between the up-regulation of the JAK-STAT3 pathway and endothelial cell angiogenesis in HUVECs stimulated with TD-sEVs. To decipher the molecular mechanisms for the paracrine effects of TD-sEVs on endothelial cell angiogenesis, we assessed and confirmed the expression levels of an sEV-shuttled miRNA, miR-141-3p that its possible role in ovarian cancer pathogenesis has been previously proposed (Choi and Ng 2017; Mateescu et al. 2011). Importantly, we demonstrated that TD-sEVs promote the expression of VEGFR-2 signaling in a miR-141-dependent manner (Fig. 3b-d) and involve in the JAK-STAT3 pathway (Fig. 3f) that can explain the vascular behavior of endothelial cells in ovarian cancer. We also found the transfer of sEV-shuttled miR-141-3p was consistently reduced SOCS5 expression in endothelial cells (Fig. 3b, e), resulted in up-regulation of the JAK-STAT3 signaling. This raises the intriguing possibility that miR-141-3p functions as a potent oncogenic miRNA by coincidently promoting multitudinous angiogenic-related signaling pathways.
The transcription factor NF-κB has been widely implicated in cancer development and progression (Patel et al. 2018). For instance, there are several reports demonstrate that the physiological interaction between NF-κB and STAT3 could lead to the nuclear retention of NF-κB (Hagihara et al. 2005; Lee et al. 2009). Consistently, we confirmed that the induced NF-κB activation triggered by TD-sEVs may be mediated by the intracellular ROS production in endothelial cells (Fig. 4). Of note, ROS are principally required to promote physiological angiogenesis; however, during pathological (tumor) conditions, continuous recruitment of tumor stromal cells results in uncontrolled, unleashed ROS production inducing pathological angiogenesis mainly on the VEGF signaling (Schreml et al. 2010). Importantly, there are also several studies revealing that VEGF promotes endothelial cell proliferation and migration by increasing intracellular ROS production (Wang et al. 2011) (Kim and Byzova 2014). The underlying molecular mechanisms of ROS production in tumor stromal endothelial cells appear to be partly related to the content of sEVs secreted by tumor cells (Bodega et al. 2019). As a possible mechanism, tumor cells through secreting sEVs containing a piece of pro-oxidant machinery can stimulate the production of cytokines and reactive nitrogen/oxygen intermediates by their target cells (Baj-Krzyworzeka et al. 2007; Popena et al. 2018). Consistently, our results showed that TD-sEVs possess an angiogenic activity in endothelial cells in a ROS-dependent mechanism. This activity is specific to the sEVs derived from ovarian carcinoma cells, SKOV-3, because such effects were not observed in the control group. Under certain observations, we speculated that ROS production and NF-κB activation promoted by TD-sEVs are critical points in endothelial cell angiogenesis and associated with up-regulation the JAK-STAT3 pathway and VEGFR-2 signaling.
In a nutshell, our data show that the sEV transfer of miRNAs from tumor cells may change the vascular responses within the microenvironment of ovarian tumor cells. Given that the proangiogenic activity of the TD-sEV-shuttled miR-141-3p depends on modulating the SOCS5-mediated JAK-STAT3 pathway and VEGFR-2 signaling, the data presented here identify a set of potential molecular targets for therapeutic intervention in tumor stromal endothelial cells. Obviously, further characterization of TD-sEV contents and a deeper understanding of the intercellular miRNA communication may result in identifying novel mechanisms of tumor angiogenesis and also could offer therapeutic opportunities for cancer treatment.
Electronic supplementary material
(PPTX 69 kb)
Acknowledgements
We would like to thank Dr. Majid Mossahebi-Mohammadi, Dr. Katayoon Pakravan, and Ms. Fahimeh-Sadet Norouzi for their technical assistance and advice. This work was supported financially by Tarbiat Modares University and Iran National Science Foundation (INSF) (Grant No. 96008647).
Abbreviations
- VEGF
Vascular endothelial growth factor
- VEGFR-2
Vascular endothelial growth factor receptor-2
- JAK/STAT
The Janus kinase/signal transducer and activator of transcription
- miRNAs, miRs
MicroRNAs
- sEVs
Small extracellular vesicles
- TD-sEVs
Ovarian tumor cell-derived small extracellular vesicles
- NT-sEVs
Non-tumorigenic ovarian epithelial cell-derived small extracellular vesicles
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- HUVECs
Human umbilical vein endothelial cells
- DLS
Dynamic light scattering
- U6snRNA
U6 small nuclear RNA
- RNU44
U44 Smal nucleolar RNA
- DCFDA
2′, 7′-dichlorofluorescein diacetate
- SEM
Scanning electron microscopy
- ROS
Reactive oxygen species
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10:347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babashah S. MicroRNAs: key regulators of oncogenesis. Switzerland: Springer International Publishing; 2014. [Google Scholar]
- Babashah S, Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. 2011;47:1127–1137. doi: 10.1016/j.ejca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Babashah S, Sadeghizadeh M, Tavirani MR, Farivar S, Soleimani M. Aberrant microRNA expression and its implications in the pathogenesis of leukemias. Cell Oncol (Dordr) 2012;35:317–334. doi: 10.1007/s13402-012-0095-3. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Zembala M. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett. 2007;113:76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bamias A, Pignata S, Pujade-Lauraine E. Angiogenesis: a promising therapeutic target for ovarian cancer. Crit Rev Oncol Hematol. 2012;84:314–326. doi: 10.1016/j.critrevonc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MB, Caldwell RB. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–1564. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- Bell E, Taylor MA. Functional roles for exosomal micrornas in the tumour microenvironment. Comput Struct Biotechnol J. 2017;15:8–13. doi: 10.1016/j.csbj.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitaraf A, Babashah S, Garshasbi M (2019) Aberrant expression of a five-microRNA signature in breast carcinoma as a promising biomarker for diagnosis. J Clin Lab Anal:e23063. 10.1002/jcla.23063 [DOI] [PMC free article] [PubMed]
- Bodega G, Alique M, Puebla L, Carracedo J, Ramirez RM. Microvesicles: ROS scavengers and ROS producers. J Extracell Vesicles. 2019;8:1626654. doi: 10.1080/20013078.2019.1626654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- Choi PW, Ng SW (2017) The functions of MicroRNA-200 family in ovarian Cancer: beyond epithelial-mesenchymal transition. Int J Mol Sci 18. 10.3390/ijms18061207 [DOI] [PMC free article] [PubMed]
- Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR, di Vizio D. Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci. 2016;17:175. doi: 10.3390/ijms17020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M, Mukherjee A, Lengyel E. The tumor microenvironment takes center stage in ovarian cancer metastasis. Trends Cancer. 2018;4:517–519. doi: 10.1016/j.trecan.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebele C, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- Dong Y, et al. Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis. 2010;31:2097–2104. doi: 10.1093/carcin/bgq167. [DOI] [PubMed] [Google Scholar]
- Fu H, Yang H, Zhang X, Xu W. The emerging roles of exosomes in tumor-stroma interaction. J Cancer Res Clin Oncol. 2016;142:1897–1907. doi: 10.1007/s00432-016-2145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Nishikawa T, Sugamata Y, Song J, Isobe T, Taga T, Yoshizaki K. Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid A gene expression. Genes Cells. 2005;10:1051–1063. doi: 10.1111/j.1365-2443.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- Han HS, Jo YN, Lee JY, Choi SY, Jeong Y, Yun J, Lee OJ. Identification of suitable reference genes for the relative quantification of microRNAs in pleural effusion. Oncol Lett. 2014;8:1889–1895. doi: 10.3892/ol.2014.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat Nosaeid M, et al. Validation and comparison of two quantitative real-time PCR assays for direct detection of DMD/BMD carriers. Clin Biochem. 2009;42:1291–1299. doi: 10.1016/j.clinbiochem.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Iorio MV, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsaki M, Libra M, Spandidos DA, Zaravinos A. The miR-200 family in ovarian cancer. Oncotarget. 2017;8:66629–66640. doi: 10.18632/oncotarget.18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- Lee HT, et al. Stat3 orchestrates interaction between endothelial and tumor cells and inhibition of Stat3 suppresses brain metastasis of breast cancer cells. Oncotarget. 2015;6:10016–10029. doi: 10.18632/oncotarget.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, Zhuang SM. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maimaiti A, Maimaiti A, Yang Y, Ma Y. MiR-106b exhibits an anti-angiogenic function by inhibiting STAT3 expression in endothelial cells. Lipids Health Dis. 2016;15:51. doi: 10.1186/s12944-016-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateescu B, et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TJ, John S. Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology. 2005;114:301–312. doi: 10.1111/j.1365-2567.2005.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BJ, Minion LE, Coleman RL. Anti-angiogenic agents in ovarian cancer: past, present, and future. Ann Oncol. 2016;27(Suppl 1):i33–i39. doi: 10.1093/annonc/mdw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motavaf M, Pakravan K, Babashah S, Malekvandfard F, Masoumi M, Sadeghizadeh M. Therapeutic application of mesenchymal stem cell-derived exosomes: A promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand) 2016;62:74–79. doi: 10.14715/cmb/2016.62.14.13. [DOI] [PubMed] [Google Scholar]
- Pakravan K, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- Pan C, Stevic I, Muller V, Ni Q, Oliveira-Ferrer L, Pantel K, Schwarzenbach H. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12:1935–1948. doi: 10.1002/1878-0261.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Horgan PG, McMillan DC, Edwards J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Popena I, et al. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17. doi: 10.1186/s12964-018-0229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int. 2014;2014:179486. doi: 10.1155/2014/179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- Selth LA, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int J Cancer. 2012;131:652–661. doi: 10.1002/ijc.26405. [DOI] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis. 2008;11:101–106. doi: 10.1007/s10456-008-9094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabruyn SP, et al. NF-kappaB activation in endothelial cells is critical for the activity of angiostatic agents. Mol Cancer Ther. 2009;8:2645–2654. doi: 10.1158/1535-7163.MCT-09-0383. [DOI] [PubMed] [Google Scholar]
- Taverna S, Amodeo V, Saieva L, Russo A, Giallombardo M, De Leo G, Alessandro R. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Mol Cancer. 2014;13:169. doi: 10.1186/1476-4598-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Thery C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van Balkom BW, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, Liu L. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8:1661–1673. [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Phys Cell Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick RS. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006676. doi: 10.1101/cshperspect.a006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Zhu M, et al. YAP via interacting with STAT3 regulates VEGF-induced angiogenesis in human retinal microvascular endothelial cells. Exp Cell Res. 2018;373:155–163. doi: 10.1016/j.yexcr.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Zhuang G, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 69 kb)