Abstract
The extracellular matrix microenvironment of adipose tissue is of critical importance for the differentiation, remodeling and function of adipocytes. Fibrillin-1 is one of the main components of microfibrils and a key player in this process. Furin processing of profibrillin-1 results in mature fibrillin-1 and releases the C-terminal propeptide as a circulating hunger hormone, asprosin. Mutations in the fibrillin-1 gene lead to adipose tissue dysfunction and causes Marfan syndrome, marfanoid progeroid lipodystrophy syndrome, and neonatal progeroid syndrome. Increased TGF-β signaling, altered mechanical properties and impaired adipogenesis are potential causes of adipose tissue dysfunction, mediated through deficient microfibrils. Circulating asprosin on the other hand is secreted primarily by white adipose tissue under fasting conditions and in obesity. It increases hepatic glucose production and drives insulin secretion and appetite stimulation through inter-organ cross talk. This review discusses the metabolic consequences of fibrillin-1 and fibrillin-1-derived asprosin in pathological conditions. Understanding the dynamic role of fibrillin-1 in the adipose tissue milieu and of circulating asprosin in the body can provide novel mechanistic insights into how fibrillin-1 may contribute to metabolic syndrome. This could lead to new management regimens of patients with metabolic disease.
Keywords: Fibrillin-1, Asprosin, Extracellular matrix, Elastin, Marfan syndrome, Marfanoid progeroid lipodystrophy syndrome, Adipose tissue, Metabolic disorders
Introduction
Adipose tissue and metabolic disorders
Energy metabolism involves a balanced action of energy intake, storage and expenditure maintained by inter-organ crosstalk in the body. The central organ is the brain and the peripheral organs include stomach, intestine, liver, adipose tissue, pancreas, and skeletal muscle. Adipose tissue is a unique form of loose connective tissue critically important to regulate energy storage and expenditure (Fruhbeck 2008). It is classified into white, brown and beige adipose tissue. White adipose tissue is present throughout the body and functions primarily as storage of energy-rich lipids (Gesta et al. 2007). It was initially considered as a caloric reservoir of triglycerides until the discovery of the hormone leptin (Zhang et al. 1994). It is now well recognized that white adipose tissue secretes a variety of “adipokines” and represents a major endocrine organ. Important adipokines include leptin, adiponectin, resistin, and asprosin (Zhang et al. 1994; Minokoshi et al. 1999; Gesta et al. 2007; Denroche et al. 2012; Choe et al. 2016; Romere et al. 2016; D’Souza et al. 2017). Brown adipose tissue is mainly involved in thermogenesis (Smith and Horwitz 1969; Saely et al. 2012). Beige adipose tissue also plays a role in thermogenesis, but it requires induction (Wu et al. 2012). Like white adipocytes, brown and beige adipocytes also secrete hormones and growth factors termed “batokines” that regulate glucose homeostasis (Kajimura et al. 2015). Metabolic syndrome is a collection of conditions that increases the risk of developing type 2 diabetes mellitus and cardiovascular diseases (Kassi et al. 2011). For example, obesity and lipodystrophy, when associated with insulin resistance, hyperglycemia, hyperinsulinemia, and liver hepatosteatosis, lead to the risk of developing type 2 diabetes mellitus and cardiovascular diseases (Herranz et al. 2008; Unger and Scherer 2010; Virtue and Vidal-Puig 2010; Kassi et al. 2011; Bindlish et al. 2015; Czech 2017).
Adipose tissue components and remodeling
Adipose tissue consists of both cellular and non-cellular extracellular matrix (ECM) components. The cellular components include adipocytes, pre-adipocytes, mesenchymal stem cells, fibroblasts, pericytes, endothelial cells, macrophages, and T cells (Eto et al. 2009; Garcia-Rubio et al. 2018). Approximately 10% of all adipocytes are replenished each year (Spalding et al. 2008). The main ECM components in adipose tissues include fibronectin, fibrillin-1, elastin, collagen I, III, IV, V and VI, as well as laminin α2 and α4 (Mariman and Wang 2010; Vaicik et al. 2014; Martinez-Santibanez et al. 2015; Davis et al. 2016).
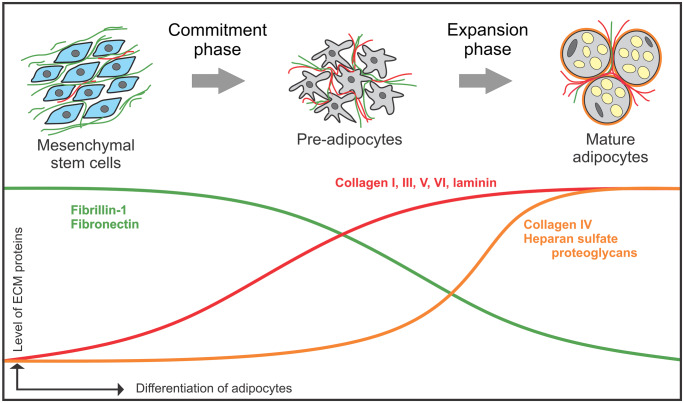
Although not well studied, some evidence shows the importance of ECM-adipocyte interactions in adipogenesis. During adipose tissue differentiation, pre-adipocytes transform into mature adipocytes. Fibronectin appears early at the pre-adipocyte stage of adipogenesis, but is degraded during the differentiation process (Kubo et al. 2000). Pref-1, an inhibitor of adipocyte differentiation directly interacts with fibronectin and downregulates the integrin signaling cascade to inhibit adipocyte differentiation (Wang et al. 2010). Emerging evidence suggests that fibrillin-1 follows a similar expression pattern than fibronectin during adipogenesis (Davis et al. 2016). Several interstitial ECM collagens (collagen I, III, V, VI), as well as the basement membrane protein laminin appear during mid differentiation and are present until later stages (Kubo et al. 2000). Other basement membrane components (collagen IV and heparan sulfate proteoglycan) are detectable around mature adipocytes (Pierleoni et al. 1998). These data suggest that adipocytes assemble basement membranes in later differentiation stages (Fig. 1).
Fig. 1.
Schematic stages of adipogenesis in relation to ECM protein expression. Key steps of adipogenesis are indicated. Relative ECM protein expression is shown as curves. Fibronectin and fibrillin-1 (green curve) is present in the early commitment phase and decreases during adipocyte differentiation, whereas collagen I, III, V, VI and laminin (red curve) appear during mid-differentiation and increase further until maturation. Basement membrane components, including collagen IV and heparan sulfate proteoglycans (orange curve) are mainly present when adipocytes are differentiated
The interstitial ECM of adipose tissue and the basement membranes undergo extensive remodeling to allow adipocytes to expand or shrink in size during weight gain or loss, respectively (Mariman and Wang 2010). This contributes to diet-induced insulin resistance in several metabolic tissues such as adipose tissue and skeletal muscles (Williams et al. 2015). mRNA expression levels of several basement membrane components (COL4A1, LAMC1, SPARC, HSPG2, NID1) were increased in visceral adipose tissue of obese compared to non-obese humans. Also, increased COL4A1 positively correlated with parameters of glucose metabolism (Reggio et al. 2016). Fibrosis in adipose tissue is commonly observed in obesity which is aggravated by increased inflammatory cytokines, among others (Huber et al. 2007; Halberg et al. 2009).
Marfan syndrome and related disorders with adipose tissue abnormalities
Fibrillin-1 is a large ECM protein ubiquitously present in many tissues throughout the body (Sakai et al. 1986). Mutations in the fibrillin-1 gene (FBN1) cause a wide spectrum of type I fibrillinopathies (Dietz et al. 1991; Collod-Beroud et al. 2003). While most of them are relatively rare disorders, Marfan syndrome (MFS) is more common with a prevalence of about 2–3 in 10,000 (Judge and Dietz 2005; Groth et al. 2015). Type I fibrillinopathies are characterized by clinical symptoms in the skeletal, cardiovascular, ocular, and adipose tissues (Pyeritz 2000; Summers et al. 2005; Robinson et al. 2006; Yetman and McCrindle 2010). More than 1,800 different mutations in FBN1 leading to MFS have been reported (Collod-Beroud et al. 2003). The underpinning pathogenetic mechanism leading to MFS can include a dominant negative mechanism, where mutated fibrillin-1 becomes incorporated into microfibrils and compromises their function, or by haploinsufficiency with reduced fibrillin-1 protein present in the ECM due to a variety of mechanisms including nonsense mediated mRNA decay, or secretion deficiency (Hubmacher and Reinhardt 2011). The effective physiological outcome in both cases is a reduced level of fully functional microfibrils in tissues and elevated levels of active TGF-β (Hubmacher and Reinhardt 2011). In addition to altering TGF-β levels, fibrillin-1 sequesters other bioactive molecules, such as bone morphogenetic protein (BMP)-2, -4, -5, -7 and − 10 (Sengle et al. 2011; Wohl et al. 2016), and osteoclastogenic cytokine receptor activator of nuclear factor κβ ligand (RANKL) (Tiedemann et al. 2013). However, these interactions have not been shown to be involved in MFS disease pathogenesis.
Many individuals affected with MFS have an asthenic body habitus. A subgroup of MFS patients classified as marfanoid progeroid lipodystrophy syndrome (MPLS) caused by mutations in the C-terminal domain of fibrillin-1 (Passarge et al. 2016), exhibit a progeroid appearance and/or a lipodystrophic phenotype (Goldblatt et al. 2011; Horn and Robinson 2011; Takenouchi et al. 2013; Garg and Xing 2014; Jacquinet et al. 2014; Lin et al. 2019). Neonatal progeroid syndrome (NPS) can also be caused by C-terminal fibrillin-1 mutations and is characterized by lipodystrophy from birth and premature aging (O’Neill et al. 2007; Romere et al. 2016). However, there is also a subgroup of MFS patients who fall under the obese category with a body mass index of > 30 kg/m2 (22% - similar to the common population) (Yetman and McCrindle 2010). This shows that MFS patients are not protected from obesity. Obesity in MFS patients increases the risk of pre-existing cardiovascular complications, such as aneurysm formation and aortic dissection (Orio et al. 2007; Bastien et al. 2012).
Fibrillin-1 and adipose tissue metabolism
Fibrillin-1 and microfibrils
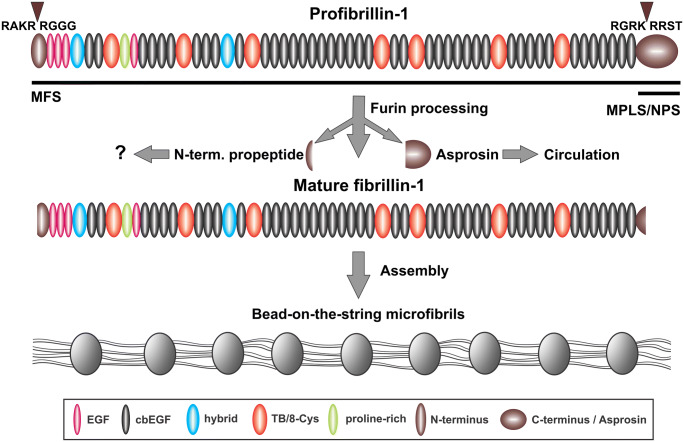
In humans and most other mammals, fibrillins are encoded by three active genes FBN1, FBN2 and FBN3, whereas in rodents the FBN3 gene is inactivated (Zhang et al. 1995; Corson et al. 2004). Fibrillin-1 is expressed throughout life, whereas fibrillin-2 and 3 is mainly present during fetal and embryonic development (Zhang et al. 1995; Corson et al. 2004; Sabatier et al. 2011). Due to its relevance to the review topic, we focus on fibrillin-1. It is a 350 kDa glycoprotein typically expressed in cells of mesenchymal origin (Summers et al. 2010; Davis et al. 2014). It is a multi-domain protein containing 47 epidermal growth factor-like EGF domains (Pereira et al. 1993), the majority of them (43) bind calcium (Corson et al. 1993) (Fig. 2). Other domains in fibrillin-1 include seven transforming growth factor beta (TGF-β) binding protein domains, two hybrid domains, and unique N- and C-terminal domains. Fibrillin-1 is synthesized as a precursor profibrillin which is processed by the endoprotease furin that cleaves in the N- and C-terminal prodomains giving rise to the mature ~ 320 kDa fibrillin-1 (Milewicz et al. 1995; Reinhardt et al. 1996b; Lönnqvist et al. 1998) (Fig. 2). Processed fibrillin-1 assembles together with other proteins, including microfibril-associated glycoproteins (MAGPs) into bead-on-a-string microfibrils via N-to-C-terminal interactions in a periodic fashion (Keene et al. 1991; Reinhardt et al. 1996a; Hubmacher et al. 2008). One of the main function of fibrillin-containing microfibrils is their essential contribution in providing a scaffold for elastic fiber formation in elastic tissues. In non-elastic tissues fibrillin-containing microfibrils can serve structural roles by intersecting firmly with basement membranes, such as in the eye or kidney (Kriz et al. 1990; Tiedemann et al. 2005). Another crucial function of fibrillin-1 is to regulate TGF-β bioavailability in the ECM through direct interaction with latent TGF-β binding protein (LTBP)-1 and − 4 and by stabilizing the large latent TGF-β complex (Isogai et al. 2003; Neptune et al. 2003; Chaudhry et al. 2007; Zilberberg et al. 2012). Microfibril-associated MAGP-1 binds the active form of TGF-β and bone morphogenetic protein-7, suggesting a critical role in microfibril-mediated growth factor signaling (Weinbaum et al. 2008; Broekelmann et al. 2020). Ablation of MAGP-1 results in increased overall adiposity and body fat and is associated with elevated TGF-β levels (Weinbaum et al. 2008; Craft et al. 2014).
Fig. 2.
Furin processing of profibrillin-1 leads to microfibril assembly and asprosin release. Profibrillin-1 is processed by furin cleavage in the N- and C- terminals domains (arrowheads), giving rise to mature fibrillin-1 and the N- and C-terminal propeptides. The C-terminal propeptide (asprosin) is released into the circulation, whereas it is not known whether the N-terminal propeptide fulfils further functions. Processed mature fibrillin-1 assembles to form bead-on-the-string microfibrils. Regions in the fibrillin-1 protein where mutations lead to MFS and MPLS/NPS are indicated by black horizontal bars
Regulation of TGF-β signaling by fibrillin-1 in adipose tissue metabolism
MFS and MPLS share characteristically reduced subcutaneous adipose tissue. Previous reports indicated that fibrillin-1 mutations in both patient groups upregulate TGF-β signaling (Andelfinger et al. 2016; Lin et al. 2019). There is also evidence that TGF-β is upregulated in obesity. 184 non-diabetic obese human subjects from a diverse background showed increased circulating TGF-β levels (Yadav et al. 2011). In addition, the levels of TGF-β positively correlated with the levels of adipose tissue mass and negatively correlated with the energy expenditure in overweight subjects (Yadav et al. 2011). Another study identified that the levels of TGF-β from white adipose tissue positively correlated with the body mass index from 13 female individuals after a bariatric surgery (Fain et al. 2005). Two mouse models of obesity, Leptinob/ob and high fat diet-induced obesity, showed increased TGF-β in circulating plasma and in white adipose tissue, as well as increased TGF-β signaling in white adipose tissue (Samad et al. 1997; Yadav et al. 2011). In addition, intraperitoneal injection of TGF-β increased the gene expression levels of adipogenic markers in white adipose tissue (Yadav et al. 2011). It is also important to note that obese women were identified with increased FBN1 gene expression levels in white adipose tissue (Davis et al. 2016). Therefore, abnormal adipose tissue metabolism strongly correlates with elevated TGF-β levels in both humans and mice.
To unravel if fibrillin-1 contributes to adipose tissue homeostasis through TGF-β signaling, Walji et al.. used three FBN1 mouse models, heterozygous Fbn1+/− mice, mice lacking the first hybrid domain in fibrillin-1 (Fbn1H1Δ/H1Δ), and mice with a MFS causing mutation (Fbn1C1039G/+) (Walji et al. 2016). Surprisingly, none of these mouse models showed a lipodystrophic phenotype, which contrasts with the presentation of human MFS caused by either dominant negative or haploinsufficient mechanisms of the mutated fibrillin-1. Moreover, these mice were slightly heavier than their wild-type littermates. Interestingly, at 24 weeks these mice showed an insulin resistance phenotype, possibly explained by slightly increased body weight and excess circulating TGF-β levels. TGF-β signaling was not different in white adipose tissue of all three mouse models, whereas it was increased in bones analyzed without the bone marrow compared to wild-type mice.
The contradiction between data obtained from human and mouse also becomes evident from a study addressing the role of microfibril-associated MAGP-1. Ablation of the gene for MAGP-1 leads to an obese phenotype in mice, which is augmented with the intake of a high-fat diet (Craft et al. 2014). This suggests that MAGP-1 is protective against obesity in mice. MAGP-1 deficiency did surprisingly not alter the backbone of microfibrils, but rather increased TGF-β signaling. These authors have also shown that the gene expression of MAGP-1 in white adipose tissue of obese human individuals was higher than in normal controls, not correlating with the mouse studies. However, MAGP-1 protein expression was not addressed in this study.
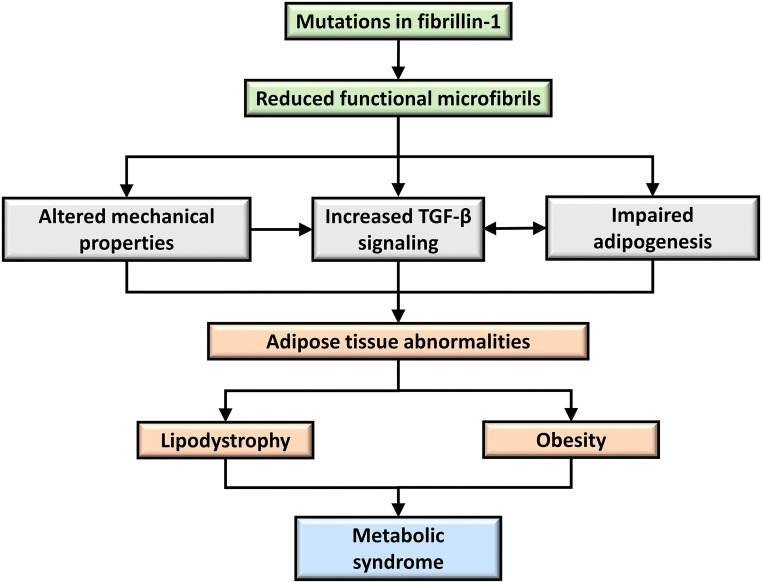
Overall, increased TGF-β signaling is associated with obesity in humans and mice. However, MFS patients identified with mutations in fibrillin-1 have reduced body fat in the presence of increased TGF-β signaling (Fig. 3). The tested fibrillin-1 relevant mouse models do not mimic the lipodystrophic phenotype observed in human MFS. Importantly, these mouse models either have a relatively mild MFS (Fbn1C1039G/+), or no MFS at all (Fbn1H1Δ/H1Δ; Fbn1+/−). It is possible, that a more severe MFS mouse model would better mimic the human conditions. In this regard, Fbn1mgR/mgR mice expressing ~ 20% of normal fibrillin-1 may be more useful to understand the mechanism of fibrillin-1 in adipose tissue function and homeostasis. In addition, the recently developed mouse (Fbn1NPS/+) and rabbit (Fbn1 het) models harboring C-terminal deletions of fibrillin-1 show severe lipodystrophic phenotypes and should thus be well suited for such studies (Duerrschmid et al. 2017; Chen et al. 2018).
Fig. 3.
The proposed role of fibrillin-1 in adipose tissue metabolism and disease. Mutations in fibrillin-1 results in reduced functional microfibrils (green boxes). Presumably, this leads to elevated TGF-β signaling, altered mechanical properties, and impaired adipogenesis in adipose tissue (gray boxes). Either individually or combinations of these factors cause adipose tissue abnormalities such as lipodystrophy in MFS, MPLS, and NPS, or obesity (light orange boxes). Both situations can lead to metabolic syndrome (blue box)
Potential role of fibrillin-1 in maintaining the mechanical properties of adipose tissue
Work over recent years clarified for the aortic disease pathogenesis in MFS and related disorders that TGF-β upregulation is not the primary disease driver. Instead, mutations in FBN1 and other genes coding for proteins of the contractile unit in smooth muscle cells lead to compromised mechanosensing and mechanoregulating responses, and TGF-β upregulation appears to be a secondary unproductive response (Milewicz et al. 2017a, b). It remains to be explored whether altered ECM mechanical properties acting on adipose cells can lead to similar cellular and functional responses (Fig. 3). It is clear for example that mechanical regulation mediated through the YAP/TAZ pathway plays a key role in mesenchymal stem cell differentiation into adipocytes (Oliver-De La Cruz et al. 2019).
Generally, in obesity or lipodystrophy, the alterations in the adipose tissue initiate fibrosis which is further enhanced by inflammatory mechanisms (Buechler et al. 2015). In fibrotic fat tissues of human and mice, several collagens are deposited in large amounts (Huber et al. 2007; Khan et al. 2009; Pasarica et al. 2009). In particular, collagen VI is upregulated in adipose tissue of obese individuals as well as in leptin receptor-deficient obese mice (Khan et al. 2009; Pasarica et al. 2009). Hypoxic conditions during the adipose tissue expansion in obese mice induce hypoxia inducible factor-1α (HIF-1α), a critical molecule that initiates adipose tissue fibrosis (Halberg et al. 2009). It has also been shown in mice that loss of membrane type matrix metalloproteinase-1 gives rise to enlarged collagen fibrils and leads to adipocyte dysfunction (Chun et al. 2006). Therefore, it is possible that the fibrotic ECM in fat tissue establishes a mechanical barrier for the transport of metabolic hormones, including glucose and insulin, which may lead to defective insulin signaling and insulin resistance (Williams et al. 2015) (Fig. 3). In addition, altered mechanical properties of the adipose tissue alters angiogenesis that leads to adipose tissue dysfunction (Corvera and Gealekman 2014).
Fibrillin-1 containing microfibrils are key elements for the formation of elastic fibers in elastic tissues where they act as scaffolds together with other proteins for deposition of the soluble tropoelastin precursor (Kozel and Mecham 2019). Elastin is present in the ECM of white adipose tissue where it provides structural elasticity and interacts with collagens (Khan et al. 2009; Alkhouli et al. 2013). During the development of adipose tissue fibrosis in obese mice and humans, the levels of elastin is downregulated in fibrotic areas compared to lean white adipose control tissue (Khan et al. 2009; Spencer et al. 2011; Martinez-Santibanez et al. 2015). This results in structural rigidity in the white adipose tissue (Halberg et al. 2009). It is expected that deficient fibrillin-1 protein (either in amount or function) in the ECM surrounding adipocytes would alter the formation and stability of elastic assemblies. While it is clear that the peri-adipocyte matrix contains elastin, it is not known whether the macromolecular assembly status represents fully functional elastic fibers that can confer micro-elasticity to the peri-adipocyte ECM. Further in-depth studies need to be performed to identify the role of fibrillin-1 in adipose tissue metabolism and how it affects the mechanical cues in the microenvironment.
Role of fibrillin-1 in adipogenesis
Adipogenesis is a multi-step differentiation process which involves two phases, an early commitment phase where mesenchymal stem cells differentiate into pre-adipocytes and a subsequent expansion phase which coincides with ECM remodeling to form mature adipocytes (Lilla et al. 2002) (Fig. 1). As mentioned above, fibrillin-1 is upregulated in the adipose tissue of obese women, correlating with a size increase of the adipocytes, but not with the cell number (Davis et al. 2016). This indicates that fibrillin-1 plays a role in adipocyte metabolism rather than proliferation, either by providing mechanical support to adipocytes or by signaling mechanisms induced by apipocyte-fibrillin-1 interactions. Correlating with these data, when wild-type mice were fed with a high-fat diet, the mRNA expression levels of fibrillin-1 increased in white adipose tissue (Moraes et al. 2003; López et al. 2004). Analyses of the whole-body fat mass and body mass index in different wild-type mouse strains (DBA/2J, CBA/J, C3H/HeJ and C57Bl/6J) correlated positively with levels of fibrillin-1 mRNA expression. Based on these limited data in combination with the lipodystrophic phenotype in MFS, NPS and MPLS (Passarge et al. 2016), the emerging role of fibrillin-1 involves positive regulation of adipose tissue development (Fig. 3).
In vitro data show that the fibrillin-1 protein and gene expression levels are high in undifferentiated adipose tissue-derived mesenchymal stem cells and decline as the cells differentiate into mature adipocytes (Davis et al. 2016) (Fig, 1). These results suggest that fibrillin-1 plays a role in the early phase of adipocyte differentiation, but they cannot explain the positive correlation in the human and mouse studies outlined above. In vitro adipocyte differentiation may not exactly mimic the process under physiological conditions in vivo.
To further dissect the role of fibrillin-1 in adipogenesis, detailed studies are required to identify the stage in which fibrillin-1 interacts with the mesenchymal stem cells, as well as its interacting partners and the receptors critical for adipocyte differentiation. Such studies would likely provide new and exciting insights in the molecular and functional mechanisms of adipose tissue in normal and pathological conditions.
Asprosin
Adipokines secreted by adipocytes play a major role in whole body energy metabolism (Trayhurn et al. 2006). Alterations in adipokine levels can contribute significantly to the development of metabolic disorders. Recent studies show the importance of a newly discovered fibrillin-1-derived hormone, asprosin, that plays a role in NPS and obesity. This part of the review discusses recent advancements in asprosin biology and function (Fig. 4). It also highlights some existing controversies, and summarizes approaches that will advance this research.
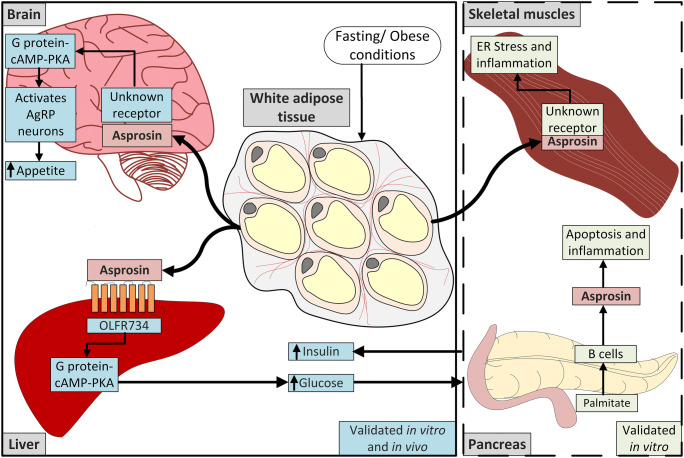
Fig. 4.
The role of asprosin in metabolic organs. Left solid box: During fasting or in obesity, increased circulating asprosin promotes hepatic glucose production and increases insulin secretion from the pancreas (Romere et al. 2016). Asprosin also activates the AgRP neurons in the brain and increases appetite (Duerrschmid et al. 2017). Both asprosin functions have been tested and validated in vivo and in vitro experiments. Right dashed box: Other in vitro studies have shown that increased asprosin levels lead to apoptosis and inflammation in pancreatic B cells (Lee et al. 2019), and to inflammation and ER stress in the skeletal muscle cells (Jung et al. 2019). However, further in vivo validation is required for these functional aspects
Asprosin, the C-terminal propeptide of fibrillin-1
It is now clear that cleavage of the C-terminal region of several extracellular matrix proteins produce circulating peptides with completely different functions than the ECM proteins the fragments originate from. One prominent example is the angiogenesis inhibitor endostatin proteolytically produced from collagen XVIII (Wenzel et al. 2006). Recently, a new member following this principal in biology was discovered, asprosin (Romere et al. 2016). Asprosin is the C-terminal prodomain of fibrillin-1 cleaved during or directly after secretion by the proprotein convertase furin, giving rise to a bioactive 140 amino acid cleavage product (Lönnqvist et al. 1998; Wallis et al. 2003; Romere et al. 2016) (Fig. 2). This propeptide migrates much higher than expected in SDS gels (~ 30–32 kDa), likely due to the presence of three N-linked glycans (Lönnqvist et al. 1998). Processing of the C-terminal propeptide is a prerequisite for the ~ 320 kDa mature fibrillin-1 to assemble into the typical bead-on-the-string microfibrils (Raghunath et al. 1999; Jensen et al. 2014) (Fig. 2). The propeptide blocks a critical N- to C-terminal self-interaction site in fibrillin-1 to prevent premature microfibril assembly during secretion (Hubmacher et al. 2008; Jensen et al. 2014).
Several mutations in the C-terminal fibrillin-1 propeptide giving rise to MFS inhibit processing of the propeptide (Milewicz et al. 1995; Lönnqvist et al. 1998). Other mutations in this region lead to NPS and MPLS (Graul-Neumann et al. 2010; Goldblatt et al. 2011; Horn and Robinson 2011; Takenouchi et al. 2013; Garg and Xing 2014; Jacquinet et al. 2014; Romere et al. 2016; Lin et al. 2019), indicating a role of the fibrillin-1 C-terminal prodomain in the regulation of adipose tissue. Interestingly, a proteomic study analyzing microfibrils from different tissues identified the C-terminal propeptide associated with assembled microfibrils, suggesting that it fulfills other functions in addition to controlling fibrillin-1 assembly (Cain et al. 2006). Since no alternative splicing mechanisms have been identified for fibrillin-1 that would produce exclusively the C-terminal propeptide, each molecule of the ~ 30–32 kDa C-terminal propeptide is produced through the synthesis of the full length ~ 350 kDa proform of fibrillin-1. This is an important aspect to realize for the discussion below about the function and turnover of asprosin. A recent discovery showed that under starving conditions, circulating asprosin levels increase significantly and promote hepatic glucose production (Romere et al. 2016). Asprosin is elevated in humans and mice affected with insulin resistance, obesity and diabetes mellitus (Romere et al. 2016; Alan et al. 2019; Wang et al. 2019a; Zhang et al. 2019b), but it is decreased in NPS patients characterized by a severe lipodystrophic phenotype (Romere et al. 2016). The principle functions of asprosin are summarized for different tissues in the following paragraphs and in Table 1.
Table 1.
Summary of the principle roles of asprosin in different metabolic tissues and organs
| Tissue | Principle Function of Asprosin | References |
|---|---|---|
| Adipose Tissue | Main source of asprosin production and secretion | Romere et al. 2016 |
| Liver | Primary target tissue; promotes hepatic glucose production |
Romere et al. 2016 Ko et al. 2019 |
| Receptor binding (OLFR734) | Li et al. 2019 | |
| Pancreas | Reduces insulin secretion; amplifies inflammatory response and apoptosis of pancreatic cells | Lee et al. 2019 |
| Brain | Increases appetite and food intake | Duerrschmid et al. 2017 |
| Skeletal Muscle | Augments endoplasmic reticulum stress and inflammation | Jung et al. 2019 |
Asprosin source and function in metabolic tissues
Adipose Tissue
White adipocytes are the primary source of asprosin (Romere et al. 2016) (Fig. 4). This was confirmed by (i) RNA sequencing and qPCR data, demonstrating highly expressed FBN1 mRNA levels in white adipose tissue in healthy humans and mice, respectively (Romere et al. 2016); (ii) ablation of adipose tissue in mice (Bscl2 knockout mice) led to a 2-fold reduction in circulating asprosin; (iii) in vitro, mature adipocytes produced and secreted asprosin, whereas pre-adipocytes did not. In vitro and in vivo, glucose suppresses the production of asprosin in white adipose tissue (Romere et al. 2016). For example, treating mature adipocytes with high glucose suppressed production of asprosin compared to glucose-free conditions. In addition, wild-type mice treated with streptozotocin to damage the functions of pancreatic cells have high blood glucose and lower asprosin levels than the non-treated mice.
Liver
Liver is the target organ of asprosin (Romere et al. 2016; Ko et al. 2019) (Fig. 4). A single dose of recombinant asprosin injected subcutaneously in mice significantly increased blood glucose levels by activating hepatic glucose production (Romere et al. 2016). Asprosin did not directly affect insulin production in the pancreas. This was confirmed by a hyperinsulinemic-euglycemia clamp study with infusion of insulin at high concentrations in wild-type mice, which elevated circulating asprosin levels and glucose production in the liver, whereas other metabolic organs were not affected. Consistent with these results, single photon emission computed tomography after injection of iodinated recombinant asprosin (125I-asprosin) in mice showed that the primary site of asprosin accumulation was the liver (Romere et al. 2016). Primary mouse hepatocytes exposed to recombinant asprosin increased glucose production in a dose-dependent manner, validating that asprosin directly acts on hepatocytes. It was further identified by in vitro and in vivo assays that asprosin acts via the G protein-cAMP-protein kinase A signaling axis (Romere et al. 2016) (Fig. 4). Aerobic exercise training reduced the concentrations of asprosin in rats with type 1 diabetes mellitus. This inhibited glucose production, TGF-β levels and protein kinase A in the liver (Ko et al. 2019). However, this study was mainly limited to liver and the consequence of aerobic training on the skeletal muscles was not addressed.
Pancreas
Glucose homeostasis in the body is maintained by insulin secretion from pancreatic β-cells. Insulin resistance leading to obesity is associated with dysfunction of pancreatic β-cells resulting in reduced insulin secretion (Cerf 2013). Several fat tissue-derived adipokines promote failure of β-cell function (Dunmore and Brown 2013). In obesity, uncontrolled production of hepatic glucose is the main cause of hyperglycemia and therefore asprosin-dependent hepatic glucose production could potentially lead to glucose toxicity which affects pancreatic function (Petersen and Shulman 2018). As expected, plasma asprosin levels are negatively correlated with first-phase insulin secretion (Wang et al. 2018). Together, the data suggest that increased plasma asprosin concentrations in obesity might contribute to β-cell malfunction and modify glucose homeostasis.
Another problem that arises from hyperglycemia is pancreatic inflammation that can lead to the development of type 2 diabetes mellitus (Cerf 2013) (Fig. 4). Treatment of mouse insulinoma pancreatic cells (MIN6) with palmitate increased expression and secretion of asprosin from the MIN6 cells, which further activated inflammatory markers and cytokines (NFκβ, TNFα, MCP1) (Lee et al. 2019). Addition of recombinant asprosin to MIN6 cells amplified the inflammatory response, whereas siRNA-mediated inhibition of fibrillin-1 mRNA (and consequently inhibition of asprosin) reversed these changes. Asprosin induces inflammation through the TLR4/JNK mediated pathway and promotes apoptosis in the MIN6 cells (Lee et al. 2019). However, a human cohort (143 subjects) with groups of normal glucose regulation, impaired glucose regulation, and newly diagnosed type 2 diabetes mellitus showed no correlation between plasma asprosin levels and the highly sensitive C-reactive protein, a systemic marker of inflammation in all three groups (Wang et al. 2018).
Brain
NPS patients and Fbn1NPS/+ mice (secrete less than 50% asprosin compared to wild-type, similar to human NPS) are characterized by reduced appetite and food intake (Duerrschmid et al. 2017). Subcutaneous injection of recombinant asprosin increased the appetite in Fbn1NPS/+ mice. The injected recombinant asprosin crossed the blood-brain barrier and activated orexigenic agouti-related peptide (AgRP) neurons in the brain through the cAMP pathway, leading to stimulation of appetite and increased food intake (Duerrschmid et al. 2017). Also, individuals with obesity show elevated circulating asprosin, causing increased appetite and food intake (Romere et al. 2016; Wang et al. 2018; Zhang et al. 2019a). Immunological sequestration of asprosin in obese mice (Leprdb/db) protected against obesity by reducing food intake and body weight (Duerrschmid et al. 2017). Overall, the data support the concept of asprosin acting centrally as an orexigenic hormone (Fig. 4).
Several aspects of the well-studied hunger hormone ghrelin are similar to those of asprosin. This includes stimulation of appetite by activating the AgRP neurons (Goto et al. 2006; Wang et al. 2014). Also, fasting rats demonstrated higher ghrelin levels that decreased after feeding (Nakazato et al. 2001). In addition, in humans and rats, administration of ghrelin prior to meals increased food intake (Wren et al. 2001a, b). However, unlike the Fbn1NPS/+ mice, ablation of ghrelin does not cause reduction in body weight (Tschop et al. 2001). Also, obese individuals are resistant to ghrelin and they show reduced amounts of circulating ghrelin in the blood. Therefore, the mechanism of asprosin is likely different than that of ghrelin.
Skeletal Muscle
While skeletal muscle is the principal organ for glucose uptake, very little is known about the role of asprosin in this tissue. Insulin resistance in skeletal muscle leads to pancreatic β-cells dysfunction in obese individuals (Czech 2017). Jung et al.., reported that asprosin treatment of differentiated mouse skeletal C2C12 cells disrupted insulin signaling through the protein kinase Cδ pathway, which increased endoplasmic reticulum stress (phosphorylated inositol-requiring enzyme 1, eukaryotic initiation factor 2, and CCAAT-enhancer-binding homologous protein expression), as well as inflammatory markers (interleukin-6, NFκβ) (Jung et al. 2019) (Fig. 4).
An olfactory receptor is a receptor for asprosin
The identification of the receptor for asprosin in liver represents the most recent advancement in the field. Li et al.. has identified the olfactory receptor OLFR734, a G-protein coupled receptor of the rhodopsin family, as receptor for asprosin in the liver (Li et al. 2019) (Fig. 4). Surface plasmon resonance spectroscopy confirmed strong binding of asprosin to OLFR734 with a KD of 18 nM. This is in the range of circulating levels of asprosin under fasting conditions (5–15 nM) (Romere et al. 2016). As expected, OLFR734 knockout mice did not respond to asprosin. Under both, basal fasting conditions and high fat diet-induced obese conditions, OLFR734 knockout mice displayed improved insulin sensitivity (Li et al. 2019). OLFR734 knockout mice are characterized by low levels of circulating glucagon, a critical regulator of the glucose homeostasis pathway. A comparison of the signaling axis of asprosin/OLFR734 with the glucagon/glucagon receptor axis in primary hepatocytes showed that intracellular cAMP levels and hepatic glucose production was 2-fold higher in the presence of glucagon compared to asprosin. However, exposure of these cells to asprosin together with glucagon increased hepatic glucose production synergistically. In addition, OLFR734 deficiency in mice abolished only the effect of asprosin but not that of glucagon. This suggests that the asprosin/OLFR734 pathway works independent of the glucagon/glucagon receptor pathway and both are important for hepatic glucose production (Li et al. 2019).
Clinical and biochemical analyses of asprosin in metabolic diseases
Clinical studies investigated the levels of plasma asprosin and its correlation with factors of obesity and diabetes mellitus. Circulating asprosin concentrations were high in patients with type 2 diabetes mellitus observed in four independent studies (Li et al. 2018; Wang et al. 2018; Zhang et al. 2019a, b). In addition, plasma asprosin levels also dynamically increased the blood glucose levels, fasting insulin concentrations, markers of obesity and lipid profiles, but negatively correlated with β-cell function in type 2 diabetes mellitus patients (Wang et al. 2018; Zhang et al. 2019b). The response of asprosin to glucose fluctuation was impaired in type 2 diabetes mellitus patients compared to the control group, which might be one of the reasons for the onset of type 2 diabetes mellitus (Zhang et al. 2019b). Saliva and plasma asprosin concentrations were also shown to be associated with indices of obese individuals (Ugur and Aydin 2019).
A study with 87 obese children showed decreased levels of plasma asprosin compared to the control group (Long et al. 2019). The circulating asprosin levels in this study were more reduced in boys compared to girls, demonstrating a sexual dimorphism. However, a recent publication with 119 Chinese children showed the opposite, increased asprosin levels, and the results positively correlated with insulin resistance (Wang et al. 2019b).
Altered asprosin levels were also observed in several other metabolic disorders, some examples are listed below. Plasma asprosin was demonstrated to be high in polycystic ovary syndrome, which predisposes to type 2 diabetes mellitus in female patients (Li et al. 2018; Alan et al. 2019). The concentrations of circulating asprosin was significantly increased in mothers with gestational diabetes, as well as with preeclampsia (Baykus et al. 2019). Acara et al.. have shown that circulating asprosin levels correlate with the severity of acute coronary syndrome with unstable angina pectoris (Acara et al. 2018). Reduced serum asprosin levels were associated with weight loss after bariatric surgery, suggesting that asprosin could serve as an important marker in this surgical approach (Wang et al. 2019a). Future clinical trials and research will be necessary to understand the relevance of these findings.
These results indicate the potential use of asprosin as a biomarker to monitor the development of metabolic diseases for example diabetes mellitus. The asprosin levels in these studies were determined by commercially available ELISA kits. However, the quality of these procedures is not always transparent. To ensure consistency and reliability of these tests, specificity and sensitivity for asprosin should be demonstrated and harmonized between the products.
Open Questions
A number of critical aspects requires further studies to fully understand the mechanisms fibrillin-1 and fibrillin-1-derived asprosin adopt in fat tissue metabolism and metabolic disease. One important point refers to the cyclic food intake-dependent generation of asprosin that is inherently coupled to the synthesis of the 350 kDa fibrillin-1. Fluctuating asprosin levels during the course of a day would either require new fibrillin-1 synthesis, or alternatively, release of stored asprosin from intra- or extracellular storage sites. Virtually nothing is known about the turnover of fibrillin-1 in the extracellular matrix. In elastic tissues the fibrillin-containing microfibrils are located on the surface of elastic fibers, which are characterized by minimal elastin synthesis and persistence during an entire human lifespan (Shapiro et al. 1991). In regenerating skin, the time frame for new microfibrils to form close to the basement membrane is days to months (Raghunath et al. 1996). We also know that microfibrils are heavily cross-linked by transglutaminase and disulfide-bond mediated mechanisms (Qian and Glanville 1997), which presumably slows down protein turnover within a microfibril. Overall, it is presently difficult to understand how fibrillin-1 would rapidly turn over to produce the required amounts of asprosin in a timely manner several times during a day. Therefore, the field urgently needs to understand the turnover of fibrillin-1 in all relevant tissues, as well as how asprosin is possibly stored inside or outside of cells to be readily available when needed.
The cellular source of fibrillin-1 in adipose tissue is presently not clear. Besides mesenchymal stem cells, pre-adipocytes, and differentiated adipocytes, other cells such as fibroblasts, pericytes, and endothelial cells could also synthesize fibrillin-1. It is also not known whether fibrillin-1 in the peri-adipocyte matrix is indeed present in the typical beaded microfibrils known from other tissues (Keene et al. 1991; Davis et al. 2002), or whether there is possibly another organizational form that better allows liberation of asprosin. For example, in skin, fibrillin-1 labeling was in some instances not only localized to microfibrils intersecting with the basement membrane, but also to the lamina densa of the basement membrane in the absence of beaded microfibrils (Dzamba et al. 2001). It is possible that fibrillin-1 in the adipose extracellular matrix is associated with the basement membrane that surrounds each adipocyte. High resolution electron microscopy and pulse chase studies would help shedding light on these important questions to fully understand the source of asprosin.
One important feature of the C-terminal fibrillin-1 propeptide is the presence of three predicted N-linked glycans. Comparing the recombinant propeptide/asprosin produced by bacterial systems to either eukaryotic recombinant asprosin or to human plasma derived asprosin strongly suggests that these sites are indeed occupied (Lönnqvist et al. 1998; Romere et al. 2016; von Herrath et al. 2019). The role of these highly concentrated glycan chains within a 36 amino acid residue sequence close to the furin processing site has not been explored in detail. It should be analyzed whether they contribute to the stability and clearance of circulating asprosin and/or whether they are critical for target receptor binding.
Another unanswered aspect ranks around the reproducibility of some data produced in this field. Recently, a group of researchers from Novo Nordisk published a case report describing extensive efforts to reproduce some of the functional aspects of asprosin with many recombinant asprosin constructs produced by bacteria as well as by mammalian cells (Li et al. 2019; von Herrath et al. 2019). Despite significant efforts, these authors could not reproduce some of the original data obtained in cell culture and in mouse models (Romere et al. 2016). One interpretation alluded to potentially impure and insufficiently validated purity of the asprosin used in the original publication (von Herrath et al. 2019). This is an important aspect to consider, especially for future approaches with recombinant asprosin produced by mammalian cells. We have for example shown that fibrillin-1 fragments produced in HEK293 cells and purified by Ni2+-chelating chromatography consistently contains small traces of bioactive TGF-β1 (Kaur and Reinhardt 2012). Another possibility of the above described lack of reproducibility could be the differences in the recombinant asprosin in terms of protein tags (His tag versus GST fusion protein) as well as details in the purification procedures.
In summary, a number of open questions need to be addressed to advance our understanding of these exciting new roles of fibrillin-1 and the fibrillin-1-derived asprosin in adipose tissue function and metabolic disorders.
Acknowledgements
This work was supported by the Canadian Institute of Health Research (MOP-137091 and PJT-162099 to DPR) and by the Fonds de Recherche du Québec - Santé (No. 277476 to MLM).
Abbreviations
- ECM
Extracellular matrix
- FBN1
Fibrillin-1 gene
- MFS
Marfan syndrome
- MPLS
Marfanoid progeroid lipodystrophy syndrome.
- NPS
Neonatal progeroid syndrome
- TGF-β
Transforming growth factor-β
- MAGP-1
Microfibril-associated glycoprotein 1
- cAMP
Cyclic adenosine monophosphate
- OLFR734
Olfactory receptor 734
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acara AC, Bolatkale M, Kiziloglu I, Ibisoglu E, Can C. A novel biochemical marker for predicting the severity of ACS with unstable angina pectoris: Asprosin. Am J Emerg Med. 2018;36:1504–1505. doi: 10.1016/j.ajem.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Alan M, Gurlek B, Yilmaz A, Aksit M, Aslanipour B, Gulhan I, Mehmet C, Taner CE. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:220–223. doi: 10.1080/09513590.2018.1512967. [DOI] [PubMed] [Google Scholar]
- Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K, Winlove CP. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab. 2013;305:E1427–E1435. doi: 10.1152/ajpendo.00111.2013. [DOI] [PubMed] [Google Scholar]
- Andelfinger G, Loeys B, Dietz H. A decade of discovery in the genetic understanding of thoracic aortic disease. Can J Cardiol. 2016;32:13–25. doi: 10.1016/j.cjca.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Bastien M, Dagenais F, Dumont E, Vadeboncoeur N, Dion B, Royer M, Gaudet-Savard T, Poirier P. Assessment of management of cardiovascular risk factors in patients with thoracic aortic disease. Blood Press Monit. 2012;17:235–242. doi: 10.1097/MBP.0b013e32835b9e74. [DOI] [PubMed] [Google Scholar]
- Baykus Y, Yavuzkir S, Ustebay S, Ugur K, Deniz R, Aydin S. Asprosin in umbilical cord of newborns and maternal blood of gestational diabetes, preeclampsia, severe preeclampsia, intrauterine growth retardation and macrosemic fetus. Peptides. 2019;120:170132. doi: 10.1016/j.peptides.2019.170132. [DOI] [PubMed] [Google Scholar]
- Bindlish S, Presswala LS, Schwartz F. Lipodystrophy: Syndrome of severe insulin resistance. Postgrad Med. 2015;127:511–516. doi: 10.1080/00325481.2015.1015927. [DOI] [PubMed] [Google Scholar]
- Broekelmann TJ, Bodmer NK, Mecham RP. Identification of the growth factor-binding sequence in the extracellular matrix protein MAGP-1. J Biol Chem. 2020;295:2687–2697. doi: 10.1074/jbc.RA119.010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler C, Krautbauer S, Eisinger K. Adipose tissue fibrosis. World J Diabetes. 2015;6:548–553. doi: 10.4239/wjd.v6.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGF-beta1. J Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yao B, Yang Q, Deng J, Song Y, Sui T, Zhou L, Yao H, Xu Y, Ouyang H, Pang D, Li Z, Lai L. Truncated C-terminus of fibrillin-1 induces Marfanoid-progeroid-lipodystrophy (MPL) syndrome in rabbit. Dis Model Mech. 2018;11:dmm031542. doi: 10.1242/dmm.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Collod-Beroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, Holman K, Kaitila I, Loeys B, Matyas G, Nuytinck L, Peltonen L, Rantamaki T, Robinson P, Steinmann B, Junien C, Beroud C, Boileau C. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat. 2003;22:199–208. doi: 10.1002/humu.10249. [DOI] [PubMed] [Google Scholar]
- Corson GM, Chalberg SC, Dietz HC, Charbonneau NL, Sakai LY. Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5’ end. Genomics. 1993;17:476–484. doi: 10.1006/geno.1993.1350. [DOI] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842:463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft CS, Pietka TA, Schappe T, Coleman T, Combs MD, Klein S, Abumrad NA, Mecham RP. The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-beta. Diabetes. 2014;63:1920–1932. doi: 10.2337/db13-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Roth RA, Heuser JE, Mecham RP. Ultrastructural properties of ciliary zonule microfibrils. J Struct Biol. 2002;139:65–75. doi: 10.1016/s1047-8477(02)00559-2. [DOI] [PubMed] [Google Scholar]
- Davis MR, Andersson R, Severin J, de Hoon M, Bertin N, Baillie JK, Kawaji H, Sandelin A, Forrest AR, Summers KM, Consortium F. Transcriptional profiling of the human fibrillin/LTBP gene family, key regulators of mesenchymal cell functions. Mol Genet Metab. 2014;112:73–83. doi: 10.1016/j.ymgme.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MR, Arner E, Duffy CR, De Sousa PA, Dahlman I, Arner P, Summers KM. Expression of FBN1 during adipogenesis: Relevance to the lipodystrophy phenotype in Marfan syndrome and related conditions. Mol Genet Metab. 2016;119:174–185. doi: 10.1016/j.ymgme.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig. 2012;3:115–129. doi: 10.1111/j.2040-1124.2012.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6:1052–1065. doi: 10.1016/j.molmet.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, Jia P, Zhao Z, Farias M, Wu Q, Milewicz DM, Sutton VR, Moore DD, Butte NF, Krashes MJ, Xu Y, Chopra AR. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23:1444–1453. doi: 10.1038/nm.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunmore SJ, Brown JE. The role of adipokines in beta-cell failure of type 2 diabetes. J Endocrinol. 2013;216:T37–T45. doi: 10.1530/JOE-12-0278. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Keene DR, Isogai Z, Charbonneau NL, Karaman-Jurukovska N, Simon M, Sakai LY. Assembly of epithelial cell fibrillins. J Invest Dermatol. 2001;117:1612–1620. doi: 10.1046/j.0022-202x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- Fain JN, Tichansky DS, Madan AK. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism. 2005;54:1546–1551. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- Garcia-Rubio J, Leon J, Redruello-Romero A, Pavon E, Cozar A, Tamayo F, Caba-Molina M, Salmeron J, Carazo A. Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci Rep. 2018;8:15203. doi: 10.1038/s41598-018-33488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Xing C. De novo heterozygous FBN1 mutations in the extreme C-terminal region cause progeroid fibrillinopathy. Am J Med Genet A. 2014;164A:1341–1345. doi: 10.1002/ajmg.a.36449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Goldblatt J, Hyatt J, Edwards C, Walpole I. Further evidence for a marfanoid syndrome with neonatal progeroid features and severe generalized lipodystrophy due to frameshift mutations near the 3’ end of the FBN1 gene. Am J Med Genet A. 2011;155A:717–720. doi: 10.1002/ajmg.a.33906. [DOI] [PubMed] [Google Scholar]
- Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, Nagasaki H, Oiso Y. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology. 2006;147:5102–5109. doi: 10.1210/en.2006-0104. [DOI] [PubMed] [Google Scholar]
- Graul-Neumann LM, Kienitz T, Robinson PN, Baasanjav S, Karow B, Gillessen-Kaesbach G, Fahsold R, Schmidt H, Hoffmann K, Passarge E. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3’ terminus of the FBN1-gene. Am J Med Genet A. 2010;152A:2749–2755. doi: 10.1002/ajmg.a.33690. [DOI] [PubMed] [Google Scholar]
- Groth KA, Hove H, Kyhl K, Folkestad L, Gaustadnes M, Vejlstrup N, Stochholm K, Ostergaard JR, Andersen NH, Gravholt CH. Prevalence, incidence, and age at diagnosis in Marfan syndrome. Orphanet J Rare Dis. 2015;10:153. doi: 10.1186/s13023-015-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz P, de Lucas R, Perez-Espana L, Mayor M. Lipodystrophy syndromes. Dermatol Clin. 2008;26:569–578 ix. doi: 10.1016/j.det.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D, Robinson PN. Progeroid facial features and lipodystrophy associated with a novel splice site mutation in the final intron of the FBN1 gene. Am J Med Genet. 2011;155A:721–724. doi: 10.1002/ajmg.a.33905. [DOI] [PubMed] [Google Scholar]
- Huber J, Loffler M, Bilban M, Reimers M, Kadl A, Todoric J, Zeyda M, Geyeregger R, Schreiner M, Weichhart T, Leitinger N, Waldhausl W, Stulnig TM. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes. 2007;31:1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, El-Hallous E, Nelea V, Kaartinen MT, Lee ER, Reinhardt DP. Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 C-terminus into bead-like structures enables self-assembly. Proc Natl Acad Sci USA. 2008;105:6548–6553. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D, Reinhardt DP (2011) Microfibrils and fibrillin. In: Biology of Extracellular Matrix (Mecham RP ed.). Springer, New York, pp 233–265
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Jacquinet A, Verloes A, Callewaert B, Coremans C, Coucke P, de Paepe A, Kornak U, Lebrun F, Lombet J, Pierard GE, Robinson PN, Symoens S, Van Maldergem L, Debray FG. Neonatal progeroid variant of Marfan syndrome with congenital lipodystrophy results from mutations at the 3’ end of FBN1 gene. Eur J Med Genet. 2014;57:230–234. doi: 10.1016/j.ejmg.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Aspinall G, Handford PA. C-terminal propeptide is required for fibrillin-1 secretion and blocks premature assembly through linkage to domains cbEGF41-43. Proc Natl Acad Sci USA. 2014;111:10155–10160. doi: 10.1073/pnas.1401697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Kim HC, Kim HU, Park T, Park J, Kim U, Kim MK, Jeong JH. Asprosin attenuates insulin signaling pathway through PKCdelta-activated ER stress and inflammation in skeletal muscle. J Cell Physiol. 2019;234:20888–20899. doi: 10.1002/jcp.28694. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Reinhardt DP. Immobilized metal affinity chromatography co-purifies TGF-beta1 with histidine-tagged recombinant extracellular proteins. PLoS One. 2012;7:e48629. doi: 10.1371/journal.pone.0048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene DR, Maddox BK, Kuo HJ, Sakai LY, Glanville RW. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991;39:441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JR, Seo DY, Kim TN, Park SH, Kwak HB, Ko KS, Rhee BD, Han J. Aerobic exercise training decreases hepatic asprosin in diabetic dats. J Clin Med. 2019;8:666. doi: 10.3390/jcm8050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel BA, Mecham RP. Elastic fiber ultrastructure and assembly. Matrix Biol. 2019;84:31–40. doi: 10.1016/j.matbio.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, Elger M, Lemley K, Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int Suppl. 1990;30:S2–S9. [PubMed] [Google Scholar]
- Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F (2000) Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. In Vitro Cell Develop Biol Anim 36:38–44. 10.1290/1071-2690(2000)036%3C0038:OOEMCD%3E2.0.CO;2 [DOI] [PubMed]
- Lee T, Yun S, Jeong JH, Jung TW. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol. 2019;486:96–104. doi: 10.1016/j.mce.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Li X, Liao M, Shen R, Zhang L, Hu H, Wu J, Wang X, Qu H, Guo S, Long M, Zheng H. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators Inflamm. 2018;2018:7375294. doi: 10.1155/2018/7375294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Shan H, Chen L, Long A, Zhang Y, Liu Y, Jia L, Wei F, Han J, Li T, Liu X, Deng H, Wang Y. OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab. 2019;30:319–328 e318. doi: 10.1016/j.cmet.2019.05.022. [DOI] [PubMed] [Google Scholar]
- Lilla J, Stickens D, Werb Z. Metalloproteases and adipogenesis: a weighty subject. Am J Pathol. 2002;160:1551–1554. doi: 10.1016/S0002-9440(10)61100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Liu Z, Liu G, Zhao S, Li C, Chen W, Coban Akdemir Z, Lin J, Song X, Wang S, Xu Q, Zhao Y, Wang L, Zhang Y, Yan Z, Liu S, Liu J, Chen Y, Zuo Y, Yang X, Sun T, Yang XZ, Niu Y, Li X, You W, Qiu B, Ding C, Liu P, Zhang S, Carvalho CMB, Posey JE, Qiu G, Deciphering Disorders Involving S, study Lupski CO, Wu JR, Zhang Z, Wu J N (2019) Genetic and molecular mechanism for distinct clinical phenotypes conveyed by allelic truncating mutations implicated in FBN1. Mol Genet Genomic Med:e1023. 10.1002/mgg3.1023 [DOI] [PMC free article] [PubMed]
- Long W, Xie X, Du C, Zhao Y, Zhang C, Zhan D, Li Z, Ning Q, Luo X. Decreased circulating levels of asprosin in obese children. Horm Res Paediatr. 2019;91:271–277. doi: 10.1159/000500523. [DOI] [PubMed] [Google Scholar]
- Lönnqvist L, Reinhardt DP, Sakai LY, Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998;7:2039–2044. doi: 10.1093/hmg/7.13.2039. [DOI] [PubMed] [Google Scholar]
- López IP, Milagro FI, Martí A, Moreno-Aliaga MJ, Martínez MJ, De Miguel C. Gene expression changes in rat white adipose tissue after a high-fat diet determined by differential display. Biochem Biophys Res Commun. 2004;318:234–239. doi: 10.1016/j.bbrc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Santibanez G, Singer K, Cho KW, DelProposto JL, Mergian T, Lumeng CN. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte. 2015;4:264–272. doi: 10.1080/21623945.2015.1027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T. A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest. 1995;95:2373–2378. doi: 10.1172/JCI117930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Prakash SK, Ramirez F. Therapeutics targeting drivers of thoracic aortic aneurysms and acute aortic dissections: insights from predisposing genes and mouse models. Annu Rev Med. 2017;68:51–67. doi: 10.1146/annurev-med-100415-022956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, Stull JT. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol. 2017;37:26–34. doi: 10.1161/ATVBAHA.116.303229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Bégeot M. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinol Metab Clin North Am. 2003;144:4773–4782. doi: 10.1210/en.2003-0456. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Oliver-De La Cruz J, Nardone G, Vrbsky J, Pompeiano A, Perestrelo AR, Capradossi F, Melajova K, Filipensky P, Forte G. Substrate mechanics controls adipogenesis through YAP phosphorylation by dictating cell spreading. Biomaterials. 2019;205:64–80. doi: 10.1016/j.biomaterials.2019.03.009. [DOI] [PubMed] [Google Scholar]
- O’Neill B, Simha V, Kotha V, Garg A. Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am J Med Genet. 2007;143A:1421–1430. doi: 10.1002/ajmg.a.31840. [DOI] [PubMed] [Google Scholar]
- Orio F, Jr, Palomba S, Cascella T, Savastano S, Lombardi G, Colao A. Cardiovascular complications of obesity in adolescents. J Endocrinol Invest. 2007;30:70–80. doi: 10.1007/BF03347399. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarge E, Robinson PN, Graul-Neumann LM. Marfanoid-progeroid-lipodystrophy syndrome: a newly recognized fibrillinopathy. Eur J Hum Genet. 2016;24:1244–1247. doi: 10.1038/ejhg.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, D’Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993;2:961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierleoni C, Verdenelli F, Castellucci M, Cinti S. Fibronectins and basal lamina molecules expression in human subcutaneous white adipose tissue. Eur J Histochem. 1998;42:183–188. [PubMed] [Google Scholar]
- Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481–510. doi: 10.1146/annurev.med.51.1.481. [DOI] [PubMed] [Google Scholar]
- Qian RQ, Glanville RW. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry. 1997;36:15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Bächi T, Meuli M, Altermatt S, Gobet R, Bruckner-Tuderman L, Steinmann B. Fibrillin and elastin expression in skin regenerating from cultured keratinocyte autografts: morphogenesis of microfibrils begins at the dermo-epidermal junction and precedes elastic fiber formation. J Invest Dermatol. 1996;106:1090–1095. doi: 10.1111/1523-1747.ep12339373. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschödrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112:1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Reggio S, Rouault C, Poitou C, Bichet JC, Prifti E, Bouillot JL, Rizkalla S, Lacasa D, Tordjman J, Clement K. Increased basement membrane components in adipose tissue during obesity: links With TGFbeta and metabolic phenotypes. J Clin Endocrinol Metab. 2016;101:2578–2587. doi: 10.1210/jc.2015-4304. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, Pöschl E, Bächinger HP, Gambee JE, Sakai LY. Fibrillin 1: Organization in microfibrils and structural properties. J Mol Biol. 1996;258:104–116. doi: 10.1006/jmbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Sasaki T, Dzamba BJ, Keene DR, Chu ML, Göhring W, Timpl R, Sakai LY. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J Biol Chem. 1996;271:19489–19496. doi: 10.1074/jbc.271.32.19489. [DOI] [PubMed] [Google Scholar]
- Robinson P, Arteaga-Solis E, Baldock C, Collod-Beroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, Kielty CM, Loeys B, Milewicz DM, Ney A, Ramirez F, Reinhardt DP, Tiedemann K, Whiteman P, Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, Sarkar P, Rendon DA, Gaber MW, LeMaire SA, Coselli JS, Milewicz DM, Sutton VR, Butte NF, Moore DD, Chopra AR. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165:566–579. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30:43–52. doi: 10.1016/j.matbio.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58:15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37–48. doi: 10.1007/BF03401666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev. 1969;49:330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KM, Nataatmadja M, Xu D, West MJ, McGill JJ, Whight C, Colley A, Ades LC. Histopathology and fibrillin-1 distribution in severe early onset Marfan syndrome. Am J Med Genet. 2005;139:2–8. doi: 10.1002/ajmg.a.30981. [DOI] [PubMed] [Google Scholar]
- Summers KM, Raza S, van Nimwegen E, Freeman TC, Hume DA. Co-expression of FBN1 with mesenchyme-specific genes in mouse cell lines: implications for phenotypic variability in Marfan syndrome. Eur J Hum Genet. 2010;18:1209–1215. doi: 10.1038/ejhg.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Hida M, Sakamoto Y, Torii C, Kosaki R, Takahashi T, Kosaki K. Severe congenital lipodystrophy and a progeroid appearance: Mutation in the penultimate exon of FBN1 causing a recognizable phenotype. Am J Med Genet. 2013;161A:3057–3062. doi: 10.1002/ajmg.a.36157. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Sasaki T, Gustafsson E, Göhring W, Bätge B, Notbohm H, Timpl R, Wedel T, Schlötzer-Schrehardt U, Reinhardt DP. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J Biol Chem. 2005;280:11404–11412. doi: 10.1074/jbc.M409882200. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Boraschi-Diaz I, Rajakumar I, Kaur J, Roughley P, Reinhardt DP, Komarova SV. Fibrillin-1 directly regulates osteoclast formation and function by a dual mechanism. J Cell Sci. 2013;126:4187–4194. doi: 10.1242/jcs.127571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines–energy regulation from the human perspective. J Nutr. 2006;136:1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol. 2019;2019:2521096. doi: 10.1155/2019/2521096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaicik MK, Thyboll Kortesmaa J, Moverare-Skrtic S, Kortesmaa J, Soininen R, Bergstrom G, Ohlsson C, Chong LY, Rozell B, Emont M, Cohen RN, Brey EM, Tryggvason K. Laminin alpha4 deficient mice exhibit decreased capacity for adipose tissue expansion and weight gain. PLoS One. 2014;9:e109854. doi: 10.1371/journal.pone.0109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome - an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- von Herrath M, Pagni PP, Grove K, Christoffersson G, Tang-Christensen M, Karlsen AE, Petersen JS. Case reports of pre-clinical replication studies in metabolism and diabetes. Cell Metab. 2019;29:795–802. doi: 10.1016/j.cmet.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Walji TA, Turecamo SE, DeMarsilis AJ, Sakai LY, Mecham RP, Craft CS. Characterization of metabolic health in mouse models of fibrillin-1 perturbation. Matrix Biol. 2016;55:63–76. doi: 10.1016/j.matbio.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis DD, Putnam EA, Cretoiu JS, Carmical SG, Cao SN, Thomas G, Milewicz DM. Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones. J Cell Biochem. 2003;90:641–652. doi: 10.1002/jcb.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao L, Smas C, Sul HS. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol. 2010;30:3480–3492. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qu H, Xiong X, Qiu Y, Liao Y, Chen Y, Zheng Y, Zheng H (2018) Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediat Inflamm 2018:9471583. 10.1155/2018/9471583 [DOI] [PMC free article] [PubMed]
- Wang CY, Lin TA, Liu KH, Liao CH, Liu YY, Wu VC, Wen MS, Yeh TS. Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes. 2019;43:1019–1025. doi: 10.1038/s41366-018-0248-1. [DOI] [PubMed] [Google Scholar]
- Wang M, Yin C, Wang L, Liu Y, Li H, Li M, Yi X, Xiao Y (2019b) Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab:1–8. 10.1159/000503808 [DOI] [PubMed]
- Weinbaum JS, Broekelmann TJ, Pierce RA, Werneck CC, Segade F, Craft CS, Knutsen RH, Mecham RP. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J Biol Chem. 2008;283:25533–25543. doi: 10.1074/jbc.M709962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel D, Schmidt A, Reimann K, Hescheler J, Pfitzer G, Bloch W, Fleischmann BK. Endostatin, the proteolytic fragment of collagen XVIII, induces vasorelaxation. Circ Res. 2006;98:1203–1211. doi: 10.1161/01.RES.0000219899.93384.ed. [DOI] [PubMed] [Google Scholar]
- Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26:357–366. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl AP, Troilo H, Collins RF, Baldock C, Sengle G. Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1. J Biol Chem. 2016;291:12732–112746. doi: 10.1074/jbc.M115.704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetman AT, McCrindle BW. The prevalence and clinical impact of obesity in adults with Marfan syndrome. Can J Cardiol. 2010;26:137–139. doi: 10.1016/s0828-282x(10)70370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen C, Zhou N, Fu Y, Cheng X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta. 2019;489:183–188. doi: 10.1016/j.cca.2017.10.034. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang H, Ma X, Wu H. Increased serum level and impaired response to glucose fluctuation of asprosin is associated with type 2 diabetes mellitus. J Diabetes Investig. 2019 doi: 10.1111/jdi.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB. Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol. 2012;227:3828–3836. doi: 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]