Abstract
Interleukin-18 (IL-18) is a member of the IL-1 family of cytokines and was initially described as an IFN-γ-inducing factor derived from anti-CD3-stimulated T-helper (Th)1 cells. IL-18 plays a significant role in the activation of hematopoietic cell types mediating both Th1 and Th2 responses and is the primary inducer of interferon-γ in these cells. The biological activity of IL-18 is mediated through its binding to the IL-18 receptor complex and activation of nuclear factor-κB (NF-κB), culminating in the production and release of several cytokines, chemokines, and cellular adhesion molecules. In certain cell types, IL-18 also activates mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase/ AKT serine/threonine kinase (PI3K/AKT) signaling modules leading to the production and release of proinflammatory cytokines. IL-18-mediated signaling acts as one of the vital components of the immunomodulatory cytokine networks involved in host defense, inflammation, and tissue regeneration. Albeit its biomedical importance, a comprehensive resource of IL-18 mediated signaling pathway is currently lacking. In this study, we report on the development of an integrated pathway map of IL-18/IL-18R signaling. The pathway map was developed through literature mining from published literature based on manual curation guidelines adapted from NetPath and includes information on 16 protein-protein interaction events, 38 enzyme-catalysis events, 12 protein translocation events, 26 activations/inhibition events, transcriptional regulators, 230 gene regulation events and 84 induced protein expression events. The IL-18 signaling pathway can be freely accessed through the WikiPathways database (https://www.wikipathways.org/index.php/Pathway:WP4754).
Electronic supplementary material
The online version of this article (10.1007/s12079-019-00544-4) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Proinflammatory cytokine, Post-translational modifications, Protein-protein interactions, Signaling pathways
Introduction
Interleukin-18 (IL-18), a member of the IL-1 family of cytokine, was initially termed as IFN-gamma inducing factor (Nakamura et al. 1989) and was later defined as a proinflammatory cytokine with the ability to induce IFNγ (Dinarello 2001). The gene encoding IL18 is located on chromosomes 9 and 11 in mice and humans, respectively. It contains 7 exons with two distinct promoters on exon 1 and 2, including an interferon consensus sequence binding protein and a PU.1 binding sites (Nakanishi et al. 2001b). IL18 encodes a 193 amino acid precursor, first synthesized as an inactive 24-kDa protein without a signal peptide, and is predominantly localized in the cytoplasm (Arend et al. 2008; Carta et al. 2013; Dinarello 2018). The IL-18 precursor was primarily found to be expressed at high levels in Kupffer cells (Matsui et al. 1997; Tsutsui et al. 1997). Subsequent reports demonstrated that similar to other members of the IL-1 family such as IL-1α and IL-33 but not IL-1β, IL-18 is constitutively expressed in most cell types including human peripheral blood mononuclear cells (PBMCs), macrophages, dendritic cells (DCs) (Chen et al. 2013), osteoblasts, epithelial cells, chondrocytes, and epidermal keratinocytes (Gerdes et al. 2002; Sanders and Mishra 2016) as well as in mouse peritoneal macrophages and spleen, thereby suggesting its vital pathophysiological role in health and disease. Additionally, the expression of a membrane-bound form of IL-18 in a subset of monocytes differentiated in vitro to macrophages by M-CSF has been reported (Bellora et al. 2012).
IL-18 similar to IL1-β, is synthesized as an inactive precursor form and cleaved to its active form (18KDa) by caspase-1 in response to inflammasome activation mediated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) recognition (Fabbi et al. 2015; Jacobs and Damania 2012; Zitvogel et al. 2012). The active form of IL-18 is released primarily from macrophages and dendritic cells. Additionally, caspase-1 independent mechanisms of IL-18 processing have been reported. Importantly, caspase-8 mediated maturation and release of IL-18 in myeloid cells have been demonstrated as Fas-dependent but independent of RIP3 or inflammasomes (Bossaller et al. 2012; Tsutsui et al. 1999). Furthermore, proIL-18, similar to IL-1α and IL-33, is also released from dying cells and is likely processed extracellularly by neutrophil proteases such as neutrophil-derived proteinase 3 (Sugawara et al. 2001), Granzyme B produced mainly by NK cells and cytotoxic T lymphocytes (Omoto et al. 2010), or by mast cell chymase (Omoto et al. 2006).
Signaling by IL-18 is mediated through IL-18 receptor, a heterodimeric complex consisting of the ligand-binding chain termed as IL-18Rα, and the co-receptor chain or the signal-transducing chain termed as IL-18Rβ (Boraschi and Tagliabue 2013; Kim et al. 2001) belonging to the IL-1 receptor family (Lee et al. 2004). The alpha chain (IL-18Rα) was initially reported as an orphan receptor termed as the IL-1R-related protein (IL-1Rrp), which was later identified and demonstrated to have increased binding capacity to IL-18, leading to the activation of NF-κB-driven luciferase reporter gene (Torigoe et al. 1997). The co-receptor or the beta chain (IL-18Rβ) has a low binding affinity to IL-18 alone but demonstrates a higher affinity when IL-18 is coupled to IL-18Rα chain (Born et al. 1998) resulting in the initiation of downstream signaling and induction of inflammatory mediators (Bossaller et al. 2012). X-ray crystallography studies reveal similarities in the 3D-structure of IL-18 and its co-receptor with the IL-1β receptor complex (Tsutsumi et al. 2014). IL-18R is mainly expressed in hematopoietic cells such as CD4 + NKT cells, mast cells, basophils, T-cells with the highest expression observed in NK cells driving its differentiation and activation (Nakamura et al. 2000). The expression of IL18R on Th1 cells and B cells is mainly driven by IL-12 (Yoshimoto et al. 1998) and in human NK and T cells by IL-12 and IFNα (Sareneva et al. 2000). Recent studies also indicate the expression of IL18R in non-immune cells, such as nerve cells and epithelial cells (Nakanishi 2018).
On binding to its receptor complex, IL-18 activates multiple signaling pathways by first recruiting MyD88, probably mediated by TRAM (Ohnishi et al. 2012), followed by IRAK4 (Suzuki et al. 2003) and IRAK1/2 forming a molecular assembly called Myddosome (Tsutsumi et al. 2014). This complex recruits TRAF6 eventually activating NF-κB and mitogen-activated protein kinase (MAPK) pathways (Adachi et al. 1998; Cao et al. 1996). IL-18-mediated signaling is responsible for the induction of various inflammatory factors involved in both innate and adaptive immune responses. In an IL-12 or IL-15 dependent setting, IL-18 induces Th1-mediated immune responses by activating NK cells and Th1 cells, essential for host defense against intracellular pathogen infection through IFNγ production (Chaix et al. 2008). Additionally, IL-18 also induces the production of TNF and FasL involved in growth, survival, and apoptosis (Tsutsui et al. 1999; Zhang et al. 2011). However, independent of synergistic signals, IL-18 potentially induces IL-4 and IL-13 production in T cells, NK cells, mast cells, and basophils driving a Th2 response (Nakanishi et al. 2001a; Yoshimoto et al. 1999). Other mediators induced by IL-18 include inducible nitric oxide; cyclooxygenase (Cox-2); proinflammatory cytokines IL-1β, IL-6; chemokines IL-8, MCP-1, and MIP-1α; intracellular adhesion molecule ICAM-1; growth factor GM-CSF (Dinarello 1999; Kohka et al. 1998; Nakanishi et al. 2001b; Olee et al. 1999). IL-18 also induces the production of cytokines such as IL-12, IL-2 (Okamura et al. 1998; Takeuchi et al. 1997) which was observed in experimental murine dengue models, where the synergetic effect of IL-12 and IL-18 are considered as critical mediators in dengue host defense (Fagundes et al. 2011). Given the pleiotropic functions of IL-18, regulation of IL-18 activity is vital to prevent an aberrant immune response. The cell activation mediated by IL-18 is mainly regulated by IL-18 binding protein (IL-18BP) (Dinarello et al. 2013) which binds to IL-18 at a higher affinity than IL-18Rα (Novick et al. 1999) and thereby suppresses its Th-1 response in physiological conditions (Kim et al. 2000; McInnes et al. 2000). Recent evidence suggests IL-37, an anti-inflammatory cytokine belonging to the IL1 family, acts as a negative regulator by binding to IL-18Rα with low affinity, resulting in the loss of recruitment of IL-18Rβ and downstream signaling (Nold-Petry et al. 2015).
The role of IL-18 in host defense mechanisms against intracellular pathogens has been well documented. Increasing evidence also suggests its vital role in the maintenance of metabolic homeostasis (Lindegaard et al. 2013; Netea et al. 2006; Zorrilla et al. 2007) and protection against the development of metabolic syndrome (De Nardo and Latz 2011; Murphy et al. 2016). Elevated signaling by IL-18 has been implicated in a number of pathological conditions such as several acute and chronic inflammatory diseases, including COPD, asthma, atopic dermatitis, rheumatoid arthritis, lupus erythematosus, atherosclerosis, graft-versus-host disease, renal diseases and hepatitis (Briend et al. 2017; Lee et al. 2015; Liew et al. 2003; Sharma et al. 2009; Xu et al. 2017). Interestingly, the role of IL-18 in ameliorating cancer metastasis has been increasingly recognized (Dupaul-Chicoine et al. 2015; Smyth et al. 2004).
Despite the importance of IL-18-mediated signaling, detailed documentation of IL-18 mediated signaling events is currently lacking. Though the signaling pathway map of IL-18 is available in KEGG and Reactome pathway database, it currently provides only a generic view of the signaling modules regulated by IL-18. In the current study, we developed a resource of signaling events mediated by IL-18/IL-18R in similar lines to earlier reports on comprehensive signaling maps (Bhat et al. 2019; Pinto et al. 2018; Subbannayya et al. 2014). Our resource contains the complete cascade of IL-18 signaling, including 319 molecules and 402 reactions stimulated by IL-18 through manual annotation of the data mined from the publicly available literature. The signaling pathway map is made available through WikiPathways (https://www.wikipathways.org/index.php/Pathway:WP4754).
Methodology
Literature survey and catalog of signaling events
An extensive literature search was carried out in PubMed using key terms “IL-18” OR “IL18” OR “Interleukin-18” OR “IL-18 induced” AND “Signaling” OR “Pathway” NOT ‘Review.’ The downstream signaling cascade under the influence of IL-18 was screened, and only those molecular events regulated/influenced by IL-18 were considered for further documentation as per NetPath criteria (Kandasamy et al. 2010). From the screened research articles, information on IL-18 induced protein-protein interactions (PPIs), post-translational modifications (PTMs), gene regulation (upregulated or downregulated in response to IL-18 stimulation), protein activation/inhibition and protein translocation events were systematically annotated based on NetPath criteria. The information manually annotated includes the experimental conditions- in vivo or in vitro, cell line model, protein site/domain involved in PPI, transcription regulators (if present). Additional information about post-translational modifications, including the residue and site of modification, has been included. The curated data were subjected to quality control by both internal and external review process.
Development of signaling pathway map
The signaling pathway map of IL-18-mediated signaling events has been developed using PathVisio software (van Iersel et al. 2008). The final IL-18 mediated signaling pathway was visualized based on NetPath criteria and implemented in WikiPathways (https://www.wikipathways.org/index.php/WikiPathways). The reactions activated/induced by IL-18 are arranged in a topological order starting from the ligand-receptor interaction to signaling modulators, transcription factors, and transcriptionally regulated genes. Pathway modules such as MAPK signaling, PI3K/AKT signaling, which are regulated by IL-18, have been depicted in the pathway map.
Results
The development of the IL-18/IL-18R signaling pathway map involved screening of a total of 1748 research articles from PubMed until July 2019 using keywords as described in the methods section. Of these, 292 research articles had information related to IL-18 induced signaling as compared with an unstimulated condition in humans or mammals. From the manually screened articles, a total of 406 IL-18 mediated signaling events arbitrated by 324 molecules were curated (Supplementary Data S1-S7). These events included 16 protein-protein interaction events, 38 enzyme-catalysis events, 12 protein translocation, 26 activations/inhibition 230 gene regulation, and 84 induced protein expression events were manually annotated based on modified NetPath criteria (Kandasamy et al. 2010). The PPIs included both ‘binary’ and ‘complex’ (multimeric) associations. In the case of enzyme-substrate reactions, depending on the information available on the upstream enzyme, the reactions were classified as “direct” or “induced” reactions. Additionally, the site and residue of PTMs were documented depending on the available literature evidence. In all, information was available for 38 PTM events. All the events that have been annotated include a link to the corresponding references.
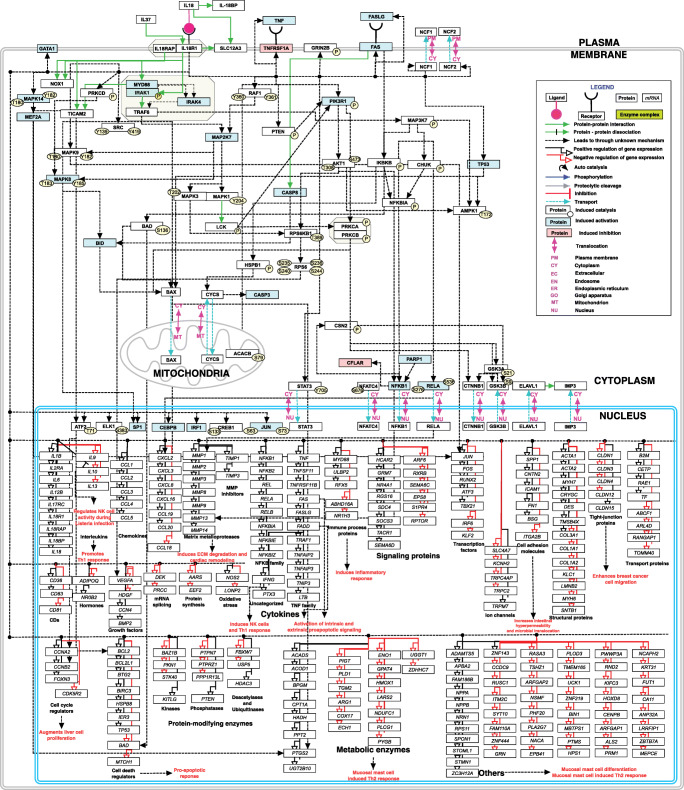
The pathway map was drawn in PathVisio. All curated events were included in the map. Pathway topology was determined based on known signaling modules, by comparison with existing pathway resources, and comparing with IL-1 signaling (Fig. 1).
Fig. 1.
Schematic representation of IL-18 signaling pathway. Schematic representation of IL-18 induced signaling reactions. The signaling pathway map represents molecules involved in ligand-receptor interactions and IL-18 induced downstream molecular events including molecular association, catalysis, translocation, and gene regulation events. Information regarding the post-translational modification site and the residue is also shown in the pathway.
Summary of IL-18 mediated signal transduction
IL-18 is an important inflammatory cytokine that initiates signaling by binding to its receptor-binding protein IL-18 alpha chain (IL-18Rα) and co-receptor (IL-18Rβ), forming a ternary complex (Dinarello et al. 2013). The central role of IL-18Rα was demonstrated by Hoshino K et al. using IL-18Rα- deficient mice, where they observed a lack of Th1 response upon IL-18 stimulation. Other studies also reported inhibition of c-Jun N-terminal kinase and NF-κB activation in Th1 cells from IL-18Rα−/− mice (Hoshino et al. 1999; Yoshimoto et al. 1998). Following the complex formation, the classical Myddosome complex (MYD88/ IRAK/TRAF6) is recruited (Kojima et al. 1998), and the recruitment of MYD88 likely involves TRAM, a sorting adaptor known for its role in TLR signaling (Ohnishi et al. 2012). It is presumed that similar to IL-1/IL1R signaling, IL-18 mediated TRAF6 ubiquitination of IκBα kinase (CHUK) results in its degradation and concomitant activation and release of NFκB that translocates to the nucleus to induce transcription of inflammatory genes, including proinflammatory cytokines, chemokines, and adhesion molecules (Chandrasekar et al. 2004, 2006b, 2008; Doffinger et al. 2001; Finotto et al. 2004; Lee et al. 2004; Leyfer et al. 2004; Reddy et al. 2010, 2011; Zabalgoitia et al. 2008). IL-18 also activates the MAPK cascade to induce STAT3 activation, IFNγ production and resultant cytotoxic activity in NK cells (Kalina et al. 2000). Additionally, IL-18 increases the expression of nitric oxide synthase (iNOS) in leukocytes via IL-18R/ p38-MAPK phosphorylation (Jablonska et al. 2008).
IL-18 also activates phosphatidylinositol-kinase (PI-3K)/AKT/ mammalian target of rapamycin (mTOR) pathway to regulate pathogenic Th17 cell differentiation and expression of Bcl-xL and Bcl2 (Deason et al. 2018; El-Darawish et al. 2018). Additionally, Akt phosphorylation is also induced in several hematopoietic and epithelial cells such as neutrophils, basophils, macrophages, HCF, HCMEC, VSMC, SMCs, and mouse aortic smooth muscle cells (ASMC) (Chandrasekar et al. 2005, 2006b, 2008; Finotto et al. 2004; Kroeger et al. 2009; Morel et al. 2002; Reddy et al. 2008, 2010, 2011; Venkatesan et al. 2009; Yoo et al. 2005). In rheumatoid arthritis synovial tissue fibroblasts, IL-18 induced the cell surface expression of VCAM1 through two distinct signaling mechanisms involving direct activation of Src by IL-18 which in turn activates transcription factor AP-1 through Ras-Raf1 induced ERK1/2 activation. Independent of Src, IL-18 also activated the PI3K-AKT module to induce VCAM1 (Morel et al. 2002). Crosstalk between GRP78, phospho-Akt, NF-κB, and XIAP is necessary for the aggressive growth of cancer via CASP3 in normal human neonatal foreskin epidermal keratinocytes (Hosotani et al. 2008). IL-18 also induces JNK/Sp1 signaling and MMP-9 expression in part via EMMPRIN/ BSG and through MAPK mediated AP-1 and NFkB activation (Chandrasekar et al. 2006a; Reddy et al. 2010).
In addition to the activation and inhibition of signaling events in different cell types, the downstream signaling proteins that are induced by IL-18 also varies with cell types. IL-18 induces the activation and nuclear translocation of cytosolic NFκB1 proteins in normal human epidermal melanocytes (NHEM), cortical neurons, myelomonocytic cells, cardiac microvascular endothelial cells (EC) which results in the production and release of MIP1-alpha and -beta, MIP2-alpha and -beta, and IL-8 (Leyfer et al. 2004; Zabalgoitia et al. 2008; Zhou et al. 2013). In chondrocytes, embryonic stem (ES) cells, human cardiac microvascular endothelial cells, and human alveolar basal epithelial cells, IL-18 induces the expression of IL-6 and IL-8. In murine peritoneal macrophages, IL-18 produces TNFα, IL-6, IL-1α, and IL-1β, which leads to joint inflammation resulting in cartilage destruction (Dai et al. 2004; Hosotani et al. 2008; Volin and Koch 2011). IL-18 stimulation also induces IL23A, IL17A, and MCP-1 expression in macrophage and microglia (Chandrasekar et al. 2004; Cheung et al. 2005; Olee et al. 1999).
Regulation of IL-18 expression and activity occurs at several levels. Several repressors at the genomic and post-transcriptional levels have been identified, including B cell lymphoma 6 protein (Bcl6) that has been demonstrated as one of the critical repressors of the IL18 gene (Takeda et al. 2003). miRNAs such as miR-346 in synovial cells (Alsaleh et al. 2009), miR-197 in hepatitis B and chronic liver failure (Chen et al. 2013), miR-134 in adult-onset Still’s disease (Liao et al. 2017) have shown to lower IL-18 expression and secretion. The biological activity of soluble IL-18 is mainly mediated by IL-18 binding protein (IL-18BP) (Dinarello et al. 2013) which binds to IL-18 at a higher affinity than IL-18Rα (Novick et al. 1999) and thereby suppressing its Th-1 response in physiological conditions (Kim et al. 2000; McInnes et al. 2000). A feedback response by IL-18BP to elevated IL-18 helps regulate untoward IFN-γ signaling, thus reducing the damage resulting from excessive “free” IL-18. IL-18BP also acts as a carrier protein circulating with IL-18 and is required for the normal NK cell function (Harms et al. 2017). The severity of the disease is associated with an imbalance of both IL-18 and IL-18BP, with elevated levels of free IL-18 associated with the risk of development of disorders such as autoimmune disorders, schizophrenia, sepsis, among others (Michels et al. 2015). Several studies have demonstrated considerable changes in pathology when administered with IL-18BP in experimental murine models of arthritis, colitis, endotoxic shock, ischemia-reperfusion injury and type 1 diabetes (Banda et al. 2003; Colafrancesco et al. 2012; Faggioni et al. 2001; He et al. 2008). A recent study also demonstrated the association of IL-18BP deficiency with the development of exacerbated colitis and arrested maturation of goblet cells (Nowarski et al. 2015). Considering the vital role of IL18BP in IL18 mediated signaling, further studies are required for the better understanding of the mechanism of action of IL-18BP in the IL-18 signaling pathway. The signaling pathway map (Fig. 1) provides a complete picture of the signaling events mediated by IL-18, and we hope that this will enable researchers to obtain insights into the signaling cross-talk across the various signaling modules regulated by IL-18.
IL-18 signaling in disease
IL-18 and its receptor (IL-18R) are closely involved in regulating both adaptive and innate immune responses. With increasing evidence suggesting a crucial role of inflammasomes in regulating IL-1β and IL-18 secretion, several genetic disorders associated with the components of inflammasomes may likely result in their increased levels in circulation, and thereby resulting in autoinflammatory syndromes such as cryopyrin-associated periodic syndrome (CAPS) (Hoffman et al. 2001), macrophage-activating syndrome (MAS) (Canna et al. 2014; Girard-Guyonvarc’h et al. 2018; Yasin et al. 2019), adult-onset Still’s disease (AOSD) (Girard et al. 2016) and several other autoinflammatory and autoimmune diseases including inflammatory bowel disease, schizophrenia and sarcoidosis (McInnes et al. 2000; Zhang et al. 2016). Additionally, genetic polymorphisms in IL18 or IL18R also contribute to allergic reactions, metabolic and autoimmune disorders. Several studies have demonstrated the presence of IL-18 and its receptor subunit in the neurons confirming its ability to cross the blood-brain barrier (Alboni et al. 2010). Studies have also tried to demonstrate its role in neuro-physiological and neuro-pathological diseases (Andoh et al. 2008; Culhane et al. 1998; Wang et al. 2006; Wheeler et al. 2000). The level of IL-18 and IL-18R subunits can be induced and regulated in the central nervous system (CNS) as demonstrated in hippocampus of mouse by increased level of IL-18 and IL-18Rα during kainic acid (KA)-induced excitotoxicity and marked increase of IL-18 in mouse microglia during hypoxic-ischemic brain injury (Hedtjarn et al. 2002; Jeon et al. 2008; Miyoshi et al. 2008). Studies have reported increased level of IL-18 in autoimmune neurodegenerative diseases such as multiple sclerosis (MS), Alzheimer’s disease (AD) and autoimmune encephalomyelitis (EAE) confirming its pivotal role in the pathogenesis of the disease (Huang et al. 2004; Jander and Stoll 1998; Motta et al. 2007; Nicoletti et al. 2001; Ojala et al. 2009; Wildbaum et al. 1998). It has also been reported in mediating angiogenesis and vascular remodeling in inflammageing (Fahey and Doyle 2019; Rodriguez-Menocal et al. 2014). Induced IL-18 and IL-18Rα play a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation (Kang et al. 2007). IL-18 has been associated with inflammatory disorders and numerous reactions in human body such as atherosclerosis, atopic eczema, COPD, maintenance of homeostasis, development of autoimmune disease along with the significant role in the prevention of infectious disease such as tuberculosis(Akdis et al. 2016; Blankenberg et al. 2002; Briend et al. 2017; Lee et al. 2015; Terada et al. 2006; Wawrocki et al. 2016; Wawrocki et al. 2019). Hence, the neutralization of IL-18 antibody can be utilized in the preclinical models of inflammation, metastasis, and tissue injury (Lauw et al. 2002; Nakajima and Owen 2012; Yu et al. 2002). The inhibition of IL-18 in autoimmune diseases such as Crohn’s disease and psoriasis could act as an attractive therapeutic regimen as IFNγ production decreases with the reduced IL-18 (Dinarello et al. 2013; Joosten et al. 2000).
Conclusions
IL-18, a member of the IL-1 family, is reportedly involved in the activation of hematopoietic cell types and plays a crucial role in the regulation of autoimmune diseases and cancers. The importance IL-18 mediated signaling pathway and the absence of signaling resources led us to drive manual curation efforts from published literature and catalog signaling events upon IL-18 stimulation. We anticipate that the detailed pathway map of IL-18 and the compendium of IL-18 signaling events emanating from this study will serve as a useful resource for researchers. Besides expanding understanding of IL-18 signaling in both normal physiology and disease, the availability of this pathway resource on the WikiPathways resource will enable researchers to use this signaling pathway map to carry out pathway analysis of high-throughput omics data obtained from various platforms.
Electronic supplementary material
S1. Statistics of IL-18 signaling pathway (XLSX 10 kb)
S2. IL-18 induced protein-protein interactions (XLSX 13 kb)
S3. IL-18 induced activation/inhibition of proteins (XLSX 13 kb)
S4. IL-18 induced post-translational modifications of proteins mediated by enzyme catalysis (XLSX 17 kb)
S5. IL-18 induced protein translocation (XLSX 11 kb)
S6. IL-18 induced gene regulation (XLSX 28 kb)
S7. IL-18 induced regulation of protein expression (XLSX 17 kb)
Acknowledgments
We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka, for the support to Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). RDAB is a recipient of the Senior Research Fellowship from the Indian Council of Medical Research (ICMR), Government of India. SMP is a recipient of the INSPIRE Faculty Award from the Department of Science and Technology (DST), Government of India.
Abbreviations
- IL-18
Interleukin-18
- IFNγ
Interferon gamma
- IL-1β
Interleukin-1beta
- M-CSF
Macrophage colony-stimulating factor
- NF-κB
Nuclear factor-κB
- PPI
Protein-protein interaction
- IL-18BP
Interleukin-18 binding protein
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D.A.B Rex, Email: rexprem@yenepoya.edu.in.
Nupur Agarwal, Email: nupur@yenepoya.edu.in.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in.
Richard K. Kandasamy, Email: richard.k.kandasamy@ntnu.no
Yashwanth Subbannayya, Email: yashwanth.subbannayya@gmail.com, Email: yashwanth.subbannayya@ntnu.no.
Sneha M. Pinto, Email: sneha@yenepoya.edu.in, Email: sneha.mp@gmail.com
References
- Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Akdis M, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Alboni S, Cervia D, Sugama S, Conti B (2010) Interleukin 18 in the CNS. J Neuroinflammation 7:9. 10.1186/1742-2094-7-94-2094-7-9 [DOI] [PMC free article] [PubMed]
- Alsaleh G, et al. Bruton’s tyrosine kinase is involved in miR-346-related regulation of IL-18 release by lipopolysaccharide-activated rheumatoid fibroblast-like synoviocytes. J Immunol. 2009;182:5088–5097. doi: 10.4049/jimmunol.0801613. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kishi H, Motoki K, Nakanishi K, Kuraishi Y, Muraguchi A. Protective effect of IL-18 on kainate- and IL-1 beta-induced cerebellar ataxia in mice. J Immunol. 2008;180:2322–2328. doi: 10.4049/jimmunol.180.4.2322. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Banda NK, et al. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003;170:2100–2105. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- Bellora F, et al. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol. 2012;42:1618–1626. doi: 10.1002/eji.201142173. [DOI] [PubMed] [Google Scholar]
- Bhat SA, et al. A network map of netrin receptor UNC5B-mediated signaling. J Cell Commun Signal. 2019;13:121–127. doi: 10.1007/s12079-018-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, Rupprecht HJ. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.CIR.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25:394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- Bossaller L, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend E, et al. IL-18 associated with lung lymphoid aggregates drives IFNgamma production in severe COPD. Respir Res. 2017;18:159. doi: 10.1186/s12931-017-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- Carta S, Lavieri R, Rubartelli A. Different members of the IL-1 family come out in different ways: DAMPs vs. cytokines? Front Immunol. 2013;4:123. doi: 10.3389/fimmu.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix J, et al. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem. 2008;283:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, et al. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281:15099–15109. doi: 10.1074/jbc.M600200200. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, et al. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem. 2005;280:26263–26277. doi: 10.1074/jbc.M502586200. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Valente AJ, Freeman GL, Mahimainathan L, Mummidi S. Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-kappaB-dependent PTEN activation. Biochem Biophys Res Commun. 2006;339:956–963. doi: 10.1016/j.bbrc.2005.11.100. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, Mummidi S. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem. 2004;279:20221–20233. doi: 10.1074/jbc.M313980200. [DOI] [PubMed] [Google Scholar]
- Chen L, Li C, Peng Z, Zhao J, Gong G, Tan D. miR-197 expression in peripheral blood mononuclear cells from hepatitis B virus-infected patients. Gut Liver. 2013;7:335–342. doi: 10.5009/gnl.2013.7.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H, Chen NJ, Cao Z, Ono N, Ohashi PS, Yeh WC. Accessory protein-like is essential for IL-18-mediated signaling. J Immunol. 2005;174:5351–5357. doi: 10.4049/jimmunol.174.9.5351. [DOI] [PubMed] [Google Scholar]
- Colafrancesco S, Priori R, Alessandri C, Perricone C, Pendolino M, Picarelli G, Valesini G. IL-18 serum level in adult onset Still’s disease: a marker of disease activity. Int J Inflam. 2012;2012:156890. doi: 10.1155/2012/156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane AC, Hall MD, Rothwell NJ, Luheshi GN. Cloning of rat brain interleukin-18 cDNA. Mol Psychiatry. 1998;3:362–366. doi: 10.1038/sj.mp.4000389. [DOI] [PubMed] [Google Scholar]
- Dai SM, Matsuno H, Nakamura H, Nishioka K, Yudoh K. Interleukin-18 enhances monocyte tumor necrosis factor alpha and interleukin-1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum. 2004;50:432–443. doi: 10.1002/art.20064. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deason K, et al. BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation. J Exp Med. 2018;215:2413–2428. doi: 10.1084/jem.20171810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2001) Novel targets for interleukin 18 binding protein. Ann Rheum Dis 60 (Suppl 3): iii18-24. 10.1136/ard.60.90003.iii18 [DOI] [PMC free article] [PubMed]
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doffinger R, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, et al. The Nlrp3 Inflammasome suppresses colorectal Cancer metastatic growth in the liver by promoting natural killer cell Tumoricidal activity. Immunity. 2015;43:751–763. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- El-Darawish Y, et al. Frontline science: IL-18 primes murine NK cells for proliferation by promoting protein synthesis, survival, and autophagy. J Leukoc Biol. 2018;104:253–264. doi: 10.1002/JLB.1HI1017-396RR. [DOI] [PubMed] [Google Scholar]
- Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97:665–675. doi: 10.1189/jlb.5RU0714-360RR. [DOI] [PubMed] [Google Scholar]
- Faggioni R, et al. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- Fagundes CT, et al. IFN-gamma production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 2011;5:e1449. doi: 10.1371/journal.pntd.0001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey E, Doyle SL. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol. 2019;10:1426. doi: 10.3389/fimmu.2019.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finotto S, et al. Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut. 2004;53:392–400. doi: 10.1136/gut.2003.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Guyonvarc'h C, Palomo J, Martin P, Rodriguez E, Troccaz S, Palmer G, Gabay C. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood. 2018;131:1430–1441. doi: 10.1182/blood-2017-06-789552. [DOI] [PubMed] [Google Scholar]
- Girard C, et al. Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology (Oxford) 2016;55:2237–2247. doi: 10.1093/rheumatology/kew300. [DOI] [PubMed] [Google Scholar]
- Harms RZ, Creer AJ, Lorenzo-Arteaga KM, Ostlund KR, Sarvetnick NE. Interleukin (IL)-18 binding protein deficiency disrupts natural killer cell maturation and diminishes circulating IL-18. Front Immunol. 2017;8:1020. doi: 10.3389/fimmu.2017.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, et al. Interleukin-18 binding protein transgenic mice are protected against ischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1414–F1421. doi: 10.1152/ajprenal.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041–5044. [PubMed] [Google Scholar]
- Hosotani Y, et al. Interleukin-18 prevents apoptosis via PI3K/Akt pathway in normal human keratinocytes. J Dermatol. 2008;35:514–524. doi: 10.1111/j.1346-8138.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- Huang WX, Huang P, Hillert J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult Scler. 2004;10:482–487. doi: 10.1191/1352458504ms1071oa. [DOI] [PubMed] [Google Scholar]
- Jablonska E, Ratajczak W, Jablonski J. Role of the p38 MAPK pathway in induction of iNOS expression in human leukocytes. Folia Biol. 2008;56:83–89. doi: 10.3409/fb56_1-2.83-89. [DOI] [PubMed] [Google Scholar]
- Jacobs SR, Damania B. NLRs, inflammasomes, and viral infection. J Leukoc Biol. 2012;92:469–477. doi: 10.1189/jlb.0312132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Stoll G. Differential induction of interleukin-12, interleukin-18, and interleukin-1beta converting enzyme mRNA in experimental autoimmune encephalomyelitis of the Lewis rat. J Neuroimmunol. 1998;91:93–99. doi: 10.1016/S0165-5728(98)00162-3. [DOI] [PubMed] [Google Scholar]
- Jeon GS, Park SK, Park SW, Kim DW, Chung CK, Cho SS. Glial expression of interleukin-18 and its receptor after excitotoxic damage in the mouse hippocampus. Neurochem Res. 2008;33:179–184. doi: 10.1007/s11064-007-9434-6. [DOI] [PubMed] [Google Scholar]
- Joosten LA, et al. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000;165:6553–6558. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- Kalina U, et al. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol. 2000;165:1307–1313. doi: 10.4049/jimmunol.165.3.1307. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178:1948–1959. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, et al. Functional reconstitution and regulation of IL-18 activity by the IL-18R beta chain. J Immunol. 2001;166:148–154. doi: 10.4049/jimmunol.166.1.148. [DOI] [PubMed] [Google Scholar]
- Kohka H, et al. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J Leukoc Biol. 1998;64:519–527. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- Kojima H, et al. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophys Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauw FN, Branger J, Florquin S, Speelman P, van Deventer SJ, Akira S, van der Poll T. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol. 2002;168:372–378. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho DH, Park HJ. IL-18 and cutaneous inflammatory diseases. Int J Mol Sci. 2015;16:29357–29369. doi: 10.3390/ijms161226172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci USA. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer D, et al. Cis-element clustering correlates with dose-dependent pro- and antisignaling effects of IL18. Genes Immun. 2004;5:354–362. doi: 10.1038/sj.gene.6364099. [DOI] [PubMed] [Google Scholar]
- Liao TL, et al. Upregulation of circulating microRNA-134 in adult-onset Still’s disease and its use as potential biomarker. Sci Rep. 2017;7:4214. doi: 10.1038/s41598-017-04086-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Wei XQ, McInnes IB. Role of interleukin 18 in rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl 2):ii48–ii50. doi: 10.1136/ard.62.suppl_2.ii48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard B, et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62:3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, et al. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- McInnes IB, Gracie JA, Leung BP, Wei XQ, Liew FY. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–315. doi: 10.1016/S0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- Michels M, et al. Normal free interleukin-18 (IL-18) plasma levels in dengue virus infection and the need to measure both total IL-18 and IL-18 binding protein levels. Clin Vaccine Immunol. 2015;22:650–655. doi: 10.1128/CVI.00147-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–12787. doi: 10.1523/JNEUROSCI.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel JC, Park CC, Zhu K, Kumar P, Ruth JH, Koch AE. Signal transduction pathways involved in rheumatoid arthritis synovial fibroblast interleukin-18-induced vascular cell adhesion molecule-1 expression. J Biol Chem. 2002;277:34679–34691. doi: 10.1074/jbc.M206337200. [DOI] [PubMed] [Google Scholar]
- Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer’s disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 2016;23:155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Owen CA. Interleukin-18: the master regulator driving destructive and remodeling processes in the lungs of patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2012;185:1137–1139. doi: 10.1164/rccm.201204-0590ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/IAI.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Otani T, Okura R, Ijiri Y, Motoda R, Kurimoto M, Orita K. Expression and responsiveness of human interleukin-18 receptor (IL-18R) on hematopoietic cell lines. Leukemia. 2000;14:1052–1059. doi: 10.1038/sj.leu.2401789. [DOI] [PubMed] [Google Scholar]
- Nakanishi K. Unique action of Interleukin-18 on T cells and other immune cells. Front Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Netea MG, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, et al. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology. 2001;57:342–344. doi: 10.1212/WNL.57.2.342. [DOI] [PubMed] [Google Scholar]
- Nold-Petry CA, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/S1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Nowarski R, et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163:1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, et al. TRAM is involved in IL-18 signaling and functions as a sorting adaptor for MyD88. PloS One. 2012;7:e38423. doi: 10.1371/journal.pone.0038423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttila T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/S0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162:1096–1100. [PubMed] [Google Scholar]
- Omoto Y, et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177:8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- Omoto Y et al. (2010) Granzyme B is a novel interleukin-18 converting enzyme J Dermatol Sci 59:129–135. 10.1016/j.jdermsci.2010.05.004 [DOI] [PubMed]
- Pinto SM, et al. A network map of IL-33 signaling pathway. J Cell Commun Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VS, et al. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J Cell Physiol. 2008;215:697–707. doi: 10.1002/jcp.21348. [DOI] [PubMed] [Google Scholar]
- Reddy VS, et al. Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2010;299:H1242–H1254. doi: 10.1152/ajpheart.00451.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011;226:3303–3315. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Menocal L, et al. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am J Physiol Heart Circ Physiol. 2014;306:H641–H653. doi: 10.1152/ajpheart.00641.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders NL, Mishra A. Role of interleukin-18 in the pathophysiology of allergic diseases. Cytokine Growth Factor Rev. 2016;32:31–39. doi: 10.1016/j.cytogfr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165:1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- Sharma A, Chakraborti A, Das A, Dhiman RK, Chawla Y. Elevation of interleukin-18 in chronic hepatitis C: implications for hepatitis C virus pathogenesis. Immunology. 2009;128:e514–e522. doi: 10.1111/j.1365-2567.2008.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya Y, Anuja K, Advani J, Ojha UK, Nanjappa V, George B, Sonawane A, Kumar RV, Ramaswamy G, Pandey A, Somani BL, Raju R. A network map of the gastrin signaling pathway. J Cell Commun Signal. 2014;8:165–170. doi: 10.1007/s12079-014-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- Suzuki N, et al. IL-1 receptor-associated kinase 4 is essential for IL-18-mediated NK and Th1 cell responses. J Immunol. 2003;170:4031–4035. doi: 10.4049/jimmunol.170.8.4031. [DOI] [PubMed] [Google Scholar]
- Takeda N, et al. Bcl6 is a transcriptional repressor for the IL-18 gene. J Immunol. 2003;171:426–431. doi: 10.4049/jimmunol.171.1.426. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ikeda M, Kurimoto M. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- Terada M, et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci USA. 2006;103:8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity. 1999;11:359–367. doi: 10.1016/s1074-7613(00)80111-9. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, et al. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–3967. [PubMed] [Google Scholar]
- Tsutsumi N, et al. The structural basis for receptor recognition of human interleukin-18. Nat Commun. 2014;5:5340. doi: 10.1038/ncomms6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C (2008) Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics 9:–399. 10.1186/1471-2105-9-399, Presenting and exploring biological pathways with PathVisio 130–2105–9-399 [pii] [DOI] [PMC free article] [PubMed]
- Venkatesan B, Valente AJ, Reddy VS, Siwik DA, Chandrasekar B. Resveratrol blocks interleukin-18-EMMPRIN cross-regulation and smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2009;297:H874–H886. doi: 10.1152/ajpheart.00311.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volin MV, Koch AE. Interleukin-18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res. 2011;31:745–751. doi: 10.1089/jir.2011.0050. [DOI] [PubMed] [Google Scholar]
- Wang N, Sugama S, Conti B, Teramoto A, Shibasaki T. Interleukin-18 mRNA expression in the rat pituitary gland. J Neuroimmunol. 2006;173:117–125. doi: 10.1016/j.jneuroim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wawrocki S, Druszczynska M, Kowalewicz-Kulbat M, Rudnicka W. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim Pol. 2016;63:59–63. doi: 10.18388/abp.2015_1153. [DOI] [PubMed] [Google Scholar]
- Wawrocki S, Kielnierowski G, Rudnicka W, Druszczynska M (2019) Lack of significant effect of interleukin-18 gene variants on tuberculosis susceptibility in the Polish population. Acta Biochim Pol. 10.18388/abp.2019_2797 [DOI] [PubMed]
- Wheeler RD, Culhane AC, Hall MD, Pickering-Brown S, Rothwell NJ, Luheshi GN. Detection of the interleukin 18 family in rat brain by RT-PCR. Mol Brain Res. 2000;77:290–293. doi: 10.1016/S0169-328X(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Youssef S, Grabie N, Karin N. Neutralizing antibodies to IFN-gamma-inducing factor prevent experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6368–6374. [PubMed] [Google Scholar]
- Xu MH, Yuan FL, Wang SJ, Xu HY, Li CW, Tong X. Association of interleukin-18 and asthma. Inflammation. 2017;40:324–327. doi: 10.1007/s10753-016-0467-3. [DOI] [PubMed] [Google Scholar]
- Yasin S et al (2019) IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology (Oxford). 10.1093/rheumatology/kez282 [DOI] [PMC free article] [PubMed]
- Yoo JK, Kwon H, Khil LY, Zhang L, Jun HS, Yoon JW. IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J Immunol. 2005;175:8280–8286. doi: 10.4049/jimmunol.175.12.8280. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- Yoshimoto T, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Chen Z, Mix E, Zhu SW, Winblad B, Ljunggren HG, Zhu J. Neutralizing antibodies to IL-18 ameliorate experimental autoimmune neuritis by counter-regulation of autoreactive Th1 responses to peripheral myelin antigen. J Neuropathol Exp Neurol. 2002;61:614–622. doi: 10.1093/jnen/61.7.614. [DOI] [PubMed] [Google Scholar]
- Zabalgoitia M, et al. Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic Biol Med. 2008;44:284–298. doi: 10.1016/j.freeradbiomed.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hile KL, Asanuma H, Vanderbrink B, Franke EI, Campbell MT, Meldrum KK. IL-18 mediates proapoptotic signaling in renal tubular cells through a Fas ligand-dependent mechanism. Am J Physiol Renal Physiol. 2011;301:F171–F178. doi: 10.1152/ajprenal.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, et al. Serum IL-18 level, clinical symptoms and IL-18-607A/C polymorphism among chronic patients with schizophrenia in a Chinese Han population. Psychoneuroendocrinology. 2016;68:140–147. doi: 10.1016/j.psyneuen.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shang J, Song J, Ping F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-kappaB pathway. Int J Biochem Cell Biol. 2013;45:308–316. doi: 10.1016/j.biocel.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–351. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Statistics of IL-18 signaling pathway (XLSX 10 kb)
S2. IL-18 induced protein-protein interactions (XLSX 13 kb)
S3. IL-18 induced activation/inhibition of proteins (XLSX 13 kb)
S4. IL-18 induced post-translational modifications of proteins mediated by enzyme catalysis (XLSX 17 kb)
S5. IL-18 induced protein translocation (XLSX 11 kb)
S6. IL-18 induced gene regulation (XLSX 28 kb)
S7. IL-18 induced regulation of protein expression (XLSX 17 kb)