Summary
The COVID‐19 pandemic is causing a significant increase in the number of patients requiring relatively prolonged invasive mechanical ventilation and an associated surge in patients who need a tracheostomy to facilitate weaning from respiratory support. In parallel, there has been a global increase in guidance from professional bodies representing staff who care for patients with tracheostomies at different points in their acute hospital journey, rehabilitation and recovery. Of concern are the risks to healthcare staff of infection arising from tracheostomy insertion and caring for patients with a tracheostomy. Hospitals are also facing extraordinary demands on critical care services such that many patients who require a tracheostomy will be managed outside established intensive care or head and neck units and cared for by staff with little tracheostomy experience. These concerns led NHS England and NHS Improvement to expedite the National Patient Safety Improvement Programme’s ‘Safe Tracheostomy Care’ workstream as part of the NHS COVID‐19 response. Supporting this workstream, UK stakeholder organisations involved in tracheostomy care were invited to develop consensus guidance based on: expert opinion; the best available published literature; and existing multidisciplinary guidelines. Topics with direct relevance for frontline staff were identified. This consensus guidance includes: infectivity of patients with respect to tracheostomy indications and timing; aerosol‐generating procedures and risks to staff; insertion procedures; and management following tracheostomy.
Keywords: coronavirus, COVID‐19, personal protective equipment, tracheostomy
Key points.
Tracheostomy facilitates weaning from prolonged ventilation when a primary tracheal extubation has or is highly likely to fail. Laryngeal oedema may be an additional problem for patients with COVID‐19.
Tracheostomy insertion and subsequent care are aerosol‐generating procedures. Positive‐pressure ventilation increases the aerosolisation risk.
Delaying tracheostomy reduces risks for healthcare workers involved at insertion but exposes patients to the risks of prolonged tracheal intubation.
Infectivity is likely to be low around the time of tracheostomy and significantly lower than the time of tracheal intubation. The role of antigen and antibody testing to determine optimal timing for tracheostomy is unclear, but a negative SARS‐CoV‐2 antigen test is not necessary before undertaking tracheostomy.
A tracheostomy may provide a more controlled situation for weaning as compared with a high‐risk primary tracheal extubation, particularly if reintubation or the use of rescue therapies is a concern.
Modifications to tracheostomy insertion techniques are required to minimise aerosol generation. Optimal location for tracheostomy insertion should be discussed and agreed locally.
A pre‐procedural apnoea test can be used to assess physiological stability before embarking on a tracheostomy.
Open surgical procedures are preferred, but percutaneous procedures are not contraindicated. Planning, rehearsal and communication is critical.
Neuromuscular blockade should be monitored and maintained throughout the procedure.
Postprocedural care should be adapted to minimise airway procedures and aerosol‐generating procedures. This includes: reviewing humidification needs; specifying the frequency of suction; daily inner tube care; and discontinuing positive pressure ventilation, whenever possible, during disconnection from the ventilator circuit.
Safe cohort locations should be equipped appropriately and supported, with care led by experienced multidisciplinary teams.
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic is having a significant impact on the delivery of health and social care in the UK, with a significant surge in the numbers of patients requiring prolonged mechanical ventilation. Our experience mirrors that from China and elsewhere in Europe, and critically ill patients requiring invasive ventilation have up to 50% mortality, with survivors requiring prolonged support [1, 2, 3, 4, 5, 6, 7]. Prolonged trans‐laryngeal tracheal intubation and ventilation is associated with respiratory muscle weakness and delayed recovery, together with overt and occult laryngeal dysfunction. These factors led to tracheostomy being performed in 8‐13% of all critically ill patients before the COVID‐19 pandemic, usually at the bedside in the intensive care unit (ICU) by intensivists, typically using a percutaneous technique [8, 9]. In‐hospital mortality was around 20% for patients who required tracheostomy, though most commonly due to their underlying disease processes, and thereafter about 50% do not survive beyond 1 year [10, 11].
There has been an initial reluctance to perform tracheostomy in patients with COVID‐19 due to early reports of high mortality and concerns regarding transmission of infection to healthcare workers. Whereas SARS‐CoV‐2, the virus responsible for COVID‐19, has lower case fatality rates than coronaviruses which cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), it has significantly higher infectivity [12, 13]. The virus is transmitted directly through surface spread from respiratory droplets, with later mucosal contact and via direct respiratory droplet inhalation. Transmission to healthcare staff is also possible during aerosol‐generating procedures, which include tracheostomy insertion and subsequent management. There was an increased risk of contracting SARS among healthcare workers involved in: tracheal intubation (OR 6.6); tracheostomy (OR 4.2); non‐invasive ventilation (OR 3.1); and manual ventilation before intubation (OR 2.8) [14]. Reports of viral transmission have also emerged in the current pandemic [15, 16, 17].
Emerging data around infectivity, viral and antibody testing, and the volume of patients requiring tracheostomy have fuelled uncertainty and anxiety among the many multidisciplinary staff required to safely manage patients. Caring for patients outside established critical care or head and neck units or managing patients with new or existing tracheostomies in the community further compounds these problems, especially when bed‐side staff may have little tracheostomy experience.
The stakeholder organisations represented in this short‐life working party have responded to individual and collective requests to provide tracheostomy guidance for front‐line staff, modified for COVID‐19. Our aim was to rapidly synthesise multiple guidelines and to deliver consistent, authoritative, patient‐focused resources and guidance for staff during the pandemic. The guidance compliments the NHS England and NHS Improvement National Patient Safety Improvement Programme (NatPatSIP) ‘Safe Tracheostomy Care’.
Methods
The working party was convened rapidly by invitation to the known UK stakeholder organisations involved in tracheostomy care. Given the pressing need for cohesive guidance, formal endorsement was not sought from individual organisations, but much of the content was derived from updated or new COVID‐19 pandemic specific guidance published and approved by these stakeholders. One author (BAM) conducted a literature review using combinations of the search terms: coronavirus; COVID‐19; tracheostomy; and tracheotomy, searching PubMed, EMBASE, Medline and the Google from 1 January to 15 April 2020. These consensus recommendations are based on expert opinion, informed by best evidence and published guidance where possible.
COVID‐19 infectivity and the influence on tracheostomy timing
Whereas a detailed review of the characteristics of SARS‐CoV‐2 are beyond the scope of this document, an understanding of the natural history of the disease is relevant to the timing and conduct of tracheostomy insertion, subsequent management and the protection of staff. The median incubation period from exposure to symptoms is approximately 5 days [1, 18]. Viral RNA can be detected in upper respiratory tract secretions by polymerase chain reaction (PCR), peaking around the time of symptom onset, and declining over the following 3–4 days in mild illness [19]. The body’s immune response is to produce specific antiviral antibodies, which appears around 1 week after symptom onset, and these are detectable in 90% of those affected by day 12 [20]. The infectivity of a person relates to their ability to transmit live virus to another and can only be assessed by culture of detected virus. The quantity of virus required to infect another is currently unknown, but the highest risk is associated with respiratory secretions. The detection of viral RNA by PCR (often referred to as ‘viral shedding’) does not necessarily indicate infectivity, as non‐viable virus can be detected. The anatomical source and the performance of the PCR test also influences detection.
In critically ill patients (those more likely to require tracheostomy), viral RNA load has been shown to be significantly higher and decline more slowly [2, 20, 21]. The typical clinical course of severe COVID‐19 can be described based on published case series, corroborated following personal communication with colleagues in China and Europe (Fig. 1) [1, 2, 3, 4, 5, 6, 7]. Whereas geographical variation occurs, 6–12% of symptomatic cases with detectable virus require critical care admission and up to 70–80% of these patients require invasive ventilation. Of those requiring invasive ventilation, 40–60% will not survive [22]. Emerging UK data are skewed towards shorter intensive care lengths of stay, but around 10% of patients admitted to intensive care can be expected to require ventilation beyond 14 days [1, 2, 3, 4, 5, 6, 7]. By pooling data from two studies of 181 patients [20, 23], which included 32 (17.7%) ‘critical’ and 72 (40%) ‘severe’ cases (based on overlapping case series [2]), the viral profile can be characterised (Fig. 2). Whereas the true level of infectivity is unknown, it is likely to be low around the time that tracheostomy is considered, some 10–14 days after tracheal intubation and approximately 20–24 days after the onset of symptoms. This is especially the case in the presence of antiviral antibody or if a patient is showing signs of physiological improvement, associated with a reduction in inflammatory markers.
Figure 1.
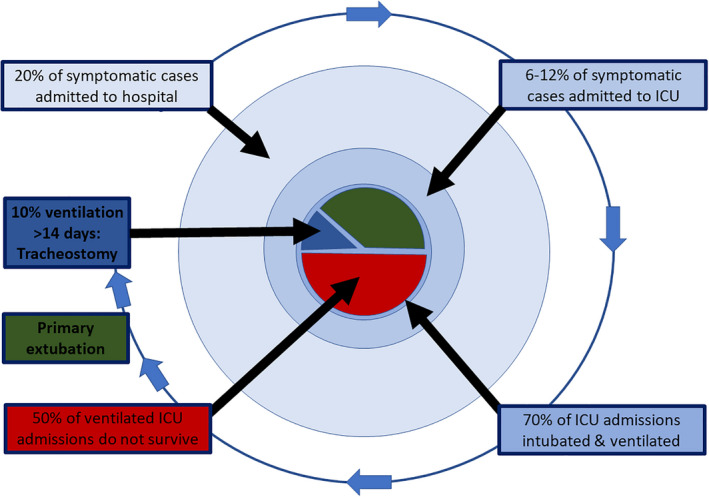
Schematic flow of symptomatic COVID‐19 patients admitted to UK hospitals.
Figure 2.
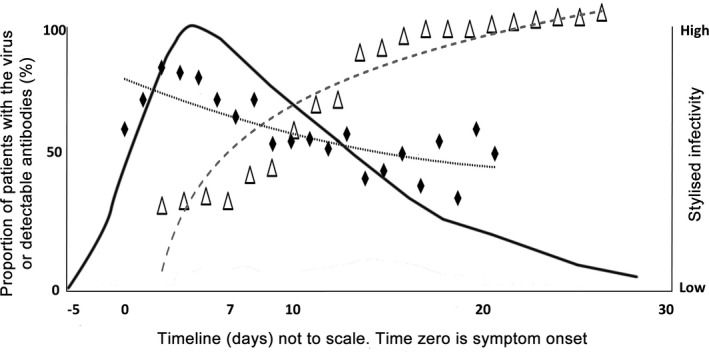
Stylised viral profile from pooled data from two studies of 181 patients [20, 23]. The curves show; the proportion of patients with detectable SARS COV‐2 RNA on polymerase chain reaction (diamonds); antiviral antibody (triangles); and inferred infectivity (high to low). Timeline (not to scale) highlights initial exposure followed by typical symptom onset, hospital admission, ICU admission, and the tracheostomy window. Tracheostomy is considered 10–14 days after ICU admission, or approximately 20–24 days after the onset of symptoms.
The COVID‐19 pandemic notwithstanding, optimal timing of tracheostomy in the critically ill is unclear [24, 25, 26, 27, 28]. Delaying tracheostomy for patients harbouring SARS‐CoV‐2 may reduce infection risks for staff, but these risks must be balanced against the complications of prolonged trans‐laryngeal tracheal intubation. Tracheostomy should only be considered in patients who are recovering from COVID‐19‐associated pneumonitis, recognising that this can be difficult to judge. Prone positioning of a patient with a tracheostomy is difficult and tracheostomy should only be considered when a patient has recovered sufficiently that prone positioning is unlikely to be required. Using current case series, evidence and expert opinion as a guide, tracheostomy should be delayed until at least day 10 of mechanical ventilation and only considered when patients are showing signs of clinical improvement. Currently, the potential role of specific tests does not replace clinical judgement regarding optimal timing.
Indications for tracheostomy in the COVID‐19 pandemic
The predominant indication for tracheostomy remains facilitating, and weaning from, prolonged mechanical ventilation. Actual or anticipated airway obstruction remains the primary surgical indication. Other indications include inadequate laryngeal reflexes and the need for invasive pulmonary hygiene in patients unable to clear respiratory secretions. However, decision‐making needs to be adapted for the COVID‐19 pandemic.
Delaying tracheostomy insertion exposes patients to the risks of: direct airway complications of prolonged trans‐laryngeal tracheal intubation; lung injury from prolonged mechanical ventilation; and prolonged critical care stay, such as excessive sedation, nosocomial infection and physiological deconditioning. Patient selection for attempted primary tracheal extubation should be based on established practice [29]. However, failed primary tracheal extubation associated with urgent rescue oxygenation and re‐intubation risks exposing patients to potential morbidity and mortality, and exposes clinical staff to potential infection. Therapies for failed extubation, such as high‐flow nasal oxygenation, continuous positive airway pressure or non‐invasive ventilation, generate potentially hazardous aerosols to varying degrees and may not be appropriate. They also impose a strain on ward‐level hospital oxygen delivery capacity [30]. A conservative approach to attempted primary tracheal extubation is recommended, restricted to those predicted to have a high chance of success. Elective tracheostomy offers a ‘closed’ system for controlled weaning of respiratory support which may be preferable to a high‐risk primary tracheal extubation strategy. Patients continuing to receive ventilatory support via a tracheostomy can be managed with reduced or no sedation, which may ease logistical work‐loads, reduce the requirement for specialist critical care nursing, and facilitate transfer to lower acuity care areas to maintain intensive care capacity.
Anecdotal reports are emerging of significant laryngeal oedema and laryngeal ulceration in patients with COVID‐19 [31]. Whereas post‐intubation laryngeal oedema is not uncommon in the critically ill, coronaviruses are known to cause laryngitis and it is plausible that SARS‐CoV‐2 has additional direct laryngeal effects in some people [32, 33]. A pre‐extubation tracheal tube cuff deflation ‘leak test’ should be considered as a screening surrogate of upper airway patency, with laryngoscopy indicated if there is doubt. Tracheostomy may be indicated if airway oedema does not improve over time, especially when the larynx appears significantly oedematous at laryngoscopy [34]. The role of steroids in treating airway oedema in the setting of COVID‐19 is uncertain.
Tracheostomy‐associated aerosol generation
Droplet transmission of SARS‐CoV‐2 occurs via large respiratory droplets, which are subject to gravity and typically travel less than 1 metre in air [35]. Airborne transmission occurs when smaller respiratory particles circulate in the air. Whereas COVID‐19 is currently not considered an airborne infection, aerosolisation of respiratory droplets can occur when procedures accelerate air/gas across a fluid surface and produce virus‐containing aerosols that risk transmission beyond 2 metres. The infective potential of aerosols depends on: where the fluid originates; the viral load present at the anatomical source; and the force with which aerosols are generated. Aerosol generation includes sneezing and coughing (although typically associated with larger droplets and so shorter spread) and airway management, including: tracheal intubation; tracheal extubation; tracheostomy insertion; tracheal suction; airway endoscopy; bronchoscopy; and mask ventilation [14]. To reduce the risk of transmission via aerosolisation, strategies to reduce coughing, reduce positive pressure delivered to an unsealed airway, and reduce exposure to respiratory droplets are recommended [30]. These recommendations can be reasonably extrapolated to include positive pressure applied to an uncuffed, fenestrated, or cuff‐deflated tracheostomy tube. Interventions that bring staff into proximity of respiratory, oral and aerodigestive tract secretions should also be considered aerosol‐generating, especially if patients are receiving positive‐pressure ventilation.
There are a variety of logistical and practical considerations that influence optimal location for tracheostomy insertion in the critically ill. The logistical challenges of transfer to an operating theatre are made more complex by the requisite infection control precautions during this pandemic. Tracheostomy can be performed in the ICU (often with sub‐optimal equipment, lighting, assistants and positioning on wide beds) or in the operating theatre (requiring transfer, transmission risks to multiple staff and associated logistics). In most tracheostomies reported during the SARS outbreak, open tracheostomy was performed at the bed‐side in intensive care negative‐pressure rooms [13]. The ideal negative‐pressure single‐occupancy rooms suitable for tracheostomy are not available in most UK facilities and therefore choice of operative location is a balance of patient and staff risks [13]. Portable high‐efficiency particulate air‐filtration (HEPA) systems may provide an acceptable alternative to a negative‐pressure room. Positive pressure/laminar flows are more likely to contaminate the immediate operative environment, but locations with rapid air turnover are probably more important than whether the flow is positive or negative [35]. Systems for donning and doffing of personal protective equipment (PPE) and provision of additional equipment from a ‘clean’ location should be planned and rehearsed. Critical care and surgical teams should review optimal locations for tracheostomy insertion during the pandemic, balancing the risks to patients and staff, and considering local facilities and expertise [13].
Tracheostomy insertion procedure for patients with COVID‐19
Modifications to tracheostomy insertion techniques have been proposed by both surgical and intensive care groups due to the high infectivity of SARS‐CoV‐2 and the aerosol generation potential. There are additional human factors and logistical challenges to working in unfamiliar circumstances, wearing PPE, and undertaking procedures which can invoke significant concern among healthcare providers. These are best mitigated against by open, sympathetic multidisciplinary discussion, detailed planning and thorough rehearsal.
Successful percutaneous, open surgical or hybrid approaches have been described in the current pandemic, all of which can be used in operating theatres or appropriate intensive care locations. The choice of technique is determined by local expertise and resources, with confidence and experience essential. Detailed guidance to minimise aerosol generation during tracheostomy in high‐consequence infectious diseases is well documented [13]. Before the current pandemic, percutaneous tracheostomy was the predominant technique in the critically ill, with surgical approaches usually reserved for patients with more complex anatomy. Percutaneous techniques involve airway manipulations that leave the tracheal tube cuff at the level of the larynx, risking leakage of exhaled gas during tidal ventilation via the upper airways. Packing of the hypopharynx and continuous suctioning from the mouth have been proposed as mitigation strategies [13, 36]. Bronchoscopic guidance of the tracheal puncture likely improves the safety of the procedure, but makes aerosol generation even more likely [37]. The choice of using bronchoscopy during percutaneous tracheostomy in a patient with COVID‐19 should reside with the operative team. If used, single‐use bronchoscopes with a sealed ventilator circuit are recommended [38]. Whereas ultrasound guidance of the puncture (in addition to pre‐procedural ultrasound of the neck to identify vasculature) and para‐tracheal tube bronchoscopy have been described to reduce aerosolisation [39], operators should only undertake procedures with which they are familiar and are competent to perform.
Surgical tracheostomies were favoured over percutaneous tracheostomies during the SARS outbreak, with a controlled open approach thought to have less aerosol potential than a percutaneous approach [13, 40, 41]. This conclusion is shared by some groups in the current pandemic, although it is noteworthy that percutaneous techniques have since advanced [36]. Strategies for the prevention of aerosol generation during surgical tracheostomy include: advancing the tracheal tube distal to the operative site before opening the trachea; hyperinflation of the tracheal tube cuff; pausing ventilation at key points; and covering the operative site with gauze swabs when ventilation recommences. Detailed descriptions of the surgical technique have been provided by ENT‐UK (www.entuk.org), the British Laryngological Society (www.laryngology.uk) and the British Association of Oral and Maxillofacial Surgeons (www.baoms.org.uk), among others [13, 36, 37, 42, 43, 44, 45]. Clamping of the tracheal tube before manipulation should not be necessary during apnoea and may damage the tube.
Pausing ventilation at key points during the procedure is a recognised strategy to minimise aerosolisation. This should be considered during initial manipulation of the tracheal tube below the tracheostomy site for surgical procedures, above it during percutaneous approaches, and when the trachea is opened and punctured. As apnoea can rapidly cause life‐threatening hypoxia in ventilator‐dependant critically ill patients, to demonstrate physiological suitability for tracheostomy, it is wise to perform an apnoea trial in the supine position in the ICU before committing to the procedure. Rapid desaturation predicts a similar response during tracheostomy. For spontaneously breathing patients, a reduction in pressure support may suffice. The ability to conduct or tolerate an apnoea trial should not replace multidisciplinary clinical judgement regarding the risks and benefits of a tracheostomy. The technique of ‘apnoeic tracheostomy’ should only be performed by experienced operators who have agreed a detailed plan of how critical desaturation will be managed.
Many critically ill tracheostomy candidates will be anticoagulated. There is no definitive evidence to guide the best technique to minimise complications in anticoagulated patients [46]. Regardless of technique, complete paralysis is essential and eliminates patient movement and coughing. For patients receiving infusions of neuromuscular blocking drugs, tachyphylaxis can occur and neuromuscular monitoring is recommended.[47, 48]
Protection of staff during the insertion procedure is crucial. In published case series of tracheostomies performed in Singapore, Hong Kong and Canada during the SARS outbreak, operators used PPE measures comprising a ‘fit‐tested’ respiratory mask (FFP3 or N95 standard) with additional fluid‐resistant face shields to protect the conjunctiva, and/or powered air‐purifying respirators (PAPRs) [13]. These measures appeared effective, as all members of the tracheostomy surgical teams remained healthy after performing 23 tracheostomies across institutions. Whichever technique is chosen, personnel should be kept to an absolute minimum with the most senior operator and anaesthetist involved. The team should prepare, rehearse, communicate effectively (including non‐verbal communication) and use Local Safety Standards for Invasive Procedures (LocSSIPs) to standardise the procedure. Use of dedicated surgical, anaesthetic and theatre teams are likely to improve performance.
In summary, there are advantages of both surgical and percutaneous techniques which must be considered alongside current established local practice. Whatever the technique, careful planning around location, timing, anticoagulation, and tube choice, alongside meticulous evaluation of final tube position will ensure timely and safe tracheostomy whereas minimising risks of later complications.
Management following tracheostomy
The care of tracheostomised patients with COVID‐19 follows established principles of high‐quality multi‐disciplinary tracheostomy care [49, 50]. However, due to the risk of viral transmission to healthcare workers, it must be modified. Whereas infectivity reduces with time, it is currently unclear when a patient with COVID‐19 ceases to be an infection risk to healthcare staff and others. This is particularly the case for patients recovering from critical illness. Until such time that a test, or combination of tests, can clarify this situation, tracheostomised patients recovering from SARS‐CoV‐2 infection should be seen as potentially hazardous to staff, although a tailored individual approach to evaluating infection risks may be possible with appropriate local expertise.
Scarcity of critical care beds means many patients, including those with tracheostomies, will receive ongoing care outside of ICUs, which poses additional challenges to patient safety and weaning. Healthcare professionals may manage patients with clinical needs that are outside their usual scope of practice, providing interventions they have never previously delivered, often in unfamiliar environments, and must have the supervised support of competent clinical professionals. The additional infection control measures and adaptations to usual practice may compromise best care to protect staff and preserve patient flow. These challenges will continue beyond hospital care and are relevant to patients with both new and existing tracheostomies managed in the community during the pandemic.
In response, NHS England and NHS Improvement has produced a toolkit from the National Patient Safety Improvement Programme to support healthcare staff (Table 1 and www.tracheostomy.org.uk). Chiefly, it is intended for healthcare workers in hospitals but much of its content is readily applicable to primary care or community settings. The material is adapted from existing resources and uses multimedia platforms to convey key principles of care where possible [49, 50, 51, 52, 53]. Wherever it is used, the toolkit’s objective is the same: to ensure that healthcare staff caring for patients with tracheostomies in these challenging circumstances are able to do so safely. Safe locations must be identified, staffed, equipped and supported to manage patients with differing clinical needs. Bedhead signs and tracheostomy passports convey essential information about the patient’s airway and should be used [49]. It is important to ensure that tracheostomy care is led or supervised by experienced, tracheostomy‐trained staff [49]. Novel options beyond standard hospital ward settings, such as large temporary hospitals, may be required.
Table 1.
The key elements of the National Tracheostomy Safety Project daily care bundle for adults. Minimum frequency ranges extended for COVID‐19 patients, but this must be reviewed daily. Adapted from NHS England and NHS Improvement NatPatSIP.
| Action | Minimum frequency | |
|---|---|---|
| Tube care |
Secure the tube (tapes or ties) Inner cannula (check and clean) Cuff pressure check Sub‐glottic secretions aspirated |
8 hourly – daily 8 hourly – daily 8 hourly – daily 4–12 hourly |
| Resuscitation |
Review red flags Know what to do |
Start of every shift |
| Airway | Suction to keep the airway clear | 4–8 hourly |
| Care of the stoma |
Keep skin clean, healthy and dry Change dressings Skin care |
Daily Daily Daily |
| Humidification |
Keep secretions loose Humidification ladder Respiratory physiotherapy |
8 hourly 8 hourly 8 hourly |
| Environment |
Bedhead sign Equipment |
Check at the start of every shift |
| Communication |
Non‐verbal communication aids Communication plan Discuss with speech and language therapist |
Per shift Per shift Per shift |
| Mouth care |
Oral secretion management Brush the teeth Saliva replacement and oral gel |
8 hourly 8 hourly 8 hourly |
| Swallowing and nutrition |
Discuss with speech and language therapists and nutrition teams Swallowing assessment Assess adequacy of nutrition |
Daily (if changes) Daily Daily |
Several modifications to traditional care should be considered during the pandemic. Key principles include: infection prevention and control; a focus on essential care and the avoidance of unnecessary interventions (especially those that generate aerosols); early recognition of deterioration; and timely responses to emergencies. Airway interventions should be planned when possible. The use of appropriate PPE by all staff remains a priority, even in emergencies, and systems to summon help from adjacent areas where staff may already be wearing PPE are recommended. Potential aerosolisation can be minimised during positive‐pressure ventilation by ensuring the breathing circuit remains ‘closed’ as much as possible. The use of a cuffed, non‐fenestrated tube with ‘in‐line’ (closed) suction will mitigate aerosol generation [54, 55]. The frequency of routine care should be reduced to the minimum required, but it is essential that this is reviewed daily. Cuffs should remain inflated initially at 20–30 cmH2O (or above ventilator peak inflation pressures) with pressures checked approximately every 12 h. Cuff pressure may need to be increased during recruitment manoeuvres. Minimal leak tests should be avoided, and tube changes should be avoided or delayed if possible [42, 55].
Humidification and removable inner cannulae are routinely used during ventilated and non‐ventilated tracheostomy care to prevent tube occlusion from respiratory secretions and reduce the need for suction [42, 43]. Commencing immediate postoperative care with a simple heat and moisture exchange (HME) filter (which can be changed every 7 days in a ‘dry’ circuit) combined with inner tube inspection and change every 24 h appears safe [56]. However, experience from other countries suggests that secretions may become thicker over time and active, water‐based humidification may become necessary. The role of the respiratory physiotherapist as part of the multidisciplinary team is critical in maintaining chest clearance. Mucolytic drugs may be a useful alternative or adjunct. Saline or hypertonic saline nebulisers can be of benefit, but the addition of humidification or saline significantly reduces HME filter efficiency [57]. The HME filters should be inspected daily and at any time when there is a deterioration in ventilation [30]. Whereas patients are considered infectious, ventilation should be paused following pre‐oxygenation before circuit breaks to change inner tubes or HME filters. Staff should be familiar with how to do this on the range of ventilators they are working with.
Weaning from mechanical ventilation is managed generally by using progressive reductions in support alongside laryngeal rehabilitation. As patients recover, their infectivity will reduce, although as discussed above, determining when patients become non‐infectious is currently not possible. Optimal care must balance the ever decreasing (but unknown) risk to staff with proactive weaning and rehabilitation. Cuff deflation, ventilator‐adjusted leak facilitated speech, one‐way speaking valves, above‐cuff vocalisation strategies and induced coughing risk aerosolisation, especially when positive‐pressure ventilation is ongoing [58]. A cautious approach to weaning with the cuff remaining inflated must be balanced against delaying recovery and restricting communication. Early involvement of speech and language therapists using creative methods of augmentative and alternative communication will reduce patient anxiety and facilitate communication and participation in care [59]. Key components indicating readiness to commence cuff deflation trials include: minimal pressure support; cough strength sufficient to expectorate sputum; safe swallowing of saliva; absence of reliance on tracheal suctioning for secretion management; and signs of a patent upper airway (good airflow in and out of the mouth with the cuff down and one‐way valve in place). Potentially infectious patients, who are clinically ready to commence cuff deflation trials, should be managed in dedicated COVID‐19 locations by experienced staff protected by appropriate PPE. Different ventilators generate variable peak flows which staff must appreciate before cuff deflation. Minimum or no positive pressure delivered during trials, used alongside facemasks or tracheostomy shields for patients, will reduce aerosolisation.[14]
Bed‐side assessment of laryngeal function or oedema should rely initially on clinical skills rather than endoscopy or laryngoscopy, if possible, and a multidisciplinary approach is recommended [60]. Laryngeal visualisation may be required if upper airway pathology is suspected, for example in the absence of vocalisation, an inability to manage oral secretions, or when swallowing is unsafe. If endoscopy is essential to guide care, it should be undertaken by experienced staff, recorded to minimise duplication, abbreviated to reduce exposure time, reviewed by the multidisciplinary team and undertaken with the minimal positive pressure possible, if cuff deflation is used as part of the assessment. Staff should wear appropriate PPE and the patient should wear a facemask. Decannulation should be considered as soon as is safely possible, managed by a multidisciplinary tracheostomy team.
Patients can be managed on designated tracheostomy wards when they no longer need ventilatory support and their medical and nursing needs become less intensive. Most patients will be suitable for decannulation during their hospital admission, but some will require ongoing care and rehabilitation before decannulation. Community locations should be staffed and equipped to deliver safe care, following the principles described in the NatPatSIP toolkit (Table 1). Clear plans for daily care, PPE requirements, review and decannulation should be communicated to the patient, their family and the community care teams when planning discharge. Multidisciplinary follow‐up clinics are recommended [61].
Personal protective equipment and tracheostomy care
Infection prevention guidance in the UK advises the use of PPE based on risk assessment and the level of SARS‐CoV‐2 transmission present in the community at the time. With the current high community prevalence, patients admitted to hospital with existing tracheostomies should be tested immediately and isolated if possible until infection is excluded. Patients who are non‐infectious should be managed in safe cohort locations, separate from infectious or recovering patients, and standard infection prevention precautions must continue.
Appropriate use of PPE is only one part of a system to reduce the risks of contamination and infection to healthcare workers [62]. Extensive advice on infection control related to COVID‐19 is published by Public Health England [30, 63]. Minimal PPE includes the use of gloves, aprons, eye protection and a fluid repellent surgical face mask for direct contact. Enhanced PPE is required for aerosol‐generating procedures and includes the additional use of a long‐sleeved fluid‐repellent gown/coveralls and an FFP3 or N95 respirator or PAPR. Eye protection is recommended for all tracheostomy care. Respiratory PPE choice is influenced by the likelihood of aerosol‐generation, the requirement for positive‐pressure ventilation and whether the patient will be managed with a deflated or uncuffed tube (Table 2). Specific considerations for children with tracheostomies and their family/carers are detailed elsewhere (www.tracheostomy.org.uk).
Table 2.
Summary of aerosol‐generating procedures and recommended personal protective equipment for staff caring for patients with confirmed or suspected COVID‐19.
| Procedure | Example | |
|---|---|---|
| Non‐aerosol‐generating procedure | General contact with no aerosol‐generating procedure |
Entry into COVID‐19 cohort open area, closed bay or single‐occupancy room. Care, procedures or assessments delivered with no aerosol generating procedures. |
| Closed suction | For those not receiving positive‐pressure ventilation. Use the Kelley circuit. | |
| Cuff down with no ventilator | Patient wears a surgical facemask and tube covering or trachy‐mask during staff contact. | |
| Cleaning | Cleaning of equipment. | |
| Aerosol‐generating procedure | Airway management | Tracheostomy insertion, decannulation, emergency management, direct or indirect laryngoscopy, nasogastric tube insertion or adjustment. |
| Tube care | Open suction, cleaning, dressing, ties or tapes, cuff management, inner tube changes. | |
| Ventilator care | Change of ventilator circuit or HME filter. | |
| Endoscopy | Fibreoptic endoscopic evaluation of swallowing. | |
| Speech and language therapy assessment | Close contact (< 2 m), procedures or assessment with risk of coughing or secretion exposure. | |
| Physiotherapy | Close contact (< 2 m), procedures or assessment with risk of coughing or secretion exposure. | |
| High‐risk aerosol environment | General contact during any aerosol generating procedure | Positive‐pressure ventilation or frequent aerosol‐generating procedure locations (ICUs, closed bays, single‐occupancy room). |
| Cuff down with a ventilator | Cuff deflation trials receiving positive‐pressure ventilation |
For patients receiving positive‐pressure ventilation or pressure support, a cuff‐inflated system with closed ‘in‐line’ suction will minimise aerosolisation. However, the positive pressure makes aerosolisation from general and tracheostomy care more likely, rendering these environments high‐risk. Aerosol generating procedures still occur in non‐ventilated patients due to coughing and contact with respiratory or oral secretions. A ‘Kelley’ circuit allows closed suction to remain connected, with an expiratory viral HME filter in situ [64]. This may be helpful for non‐ventilated patients. The requirement for oxygen, humidification and frequency of inner tube care should be reviewed daily. Patients with deflated cuffs should be asked to wear a fluid‐resistant surgical mask.
As patients recover and become non‐infectious, tracheostomy care and PPE use will continue based on risk assessment, returning to infection prevention standard precautions in line with individual patient need. Healthcare professionals should remain cautious until it is clear that this transition has occurred.
Conclusion
The recommendations made in this multidisciplinary, multi‐specialty guidance are based on reports from previous epidemics, early experience of countries affected by the current pandemic and on expert consensus opinion. The effectiveness of these recommendations will only be realised through comprehensive data collection and analysis. The Global Tracheostomy Collaborative (GTC) database (www.globaltrach.org) is freely available for sites to collect comprehensive patient‐level data, harm metrics and surrogates for the quality of care, such as time to vocalisation, oral intake and decannulation. Implementation of the GTC Quality Improvement programme has recently demonstrated rapid improvements in the quality and safety of clinical care, using the GTC database to benchmark against the 7000 global cases currently registered in the dataset. Recognising the urgency of data collection, the surgical stakeholders have collaborated to develop a registry of patients undergoing tracheostomy during the pandemic (www.entuk.org; www.laryngology.uk; www.baoms.org.uk).
Data about infectivity and the persistence of viral RNA in critically ill patients and the transmission potential of various aerosol‐generating procedures are lacking and future research may help to inform optimal timing, performance and management of tracheostomy. The challenge of providing safe, high quality care to high volumes of initially infectious complex patients is upon us. Stakeholders can support, guide and protect staff with measured, collaborative and consistent guidance founded upon best practices and emerging information.
Acknowledgements
The authors thank following individuals who have contributed to this manuscript and the associated resources: S Papworth‐Heidel; P Wastell; J Hamilton; K Whittle; W Stobbs; A Cooper; C Crocker; P Openshaw; L Vassiliou; D Broderick; P Kyzasa; K Sanders; C Katre; A Sawyer; J Collier; C Schilling; D Dhariwal; J Osher; A Smith; S McGowan; A Ginnelly; A‐L Sutt; C Mills; C Iezzi; G Jones; R Gallagher; S Dickson; S Laha; ST Webb; N Tolley; T Tatla; and A Arora. No external funding or competing interests declared.
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Journal of the American Medical Association 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Intensive Care National Audit and Research Centre report on COVID‐19 in critical care. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 24/04/2020).
- 4. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. Journal of the American Medical Association 2020; 323: 1545–6. [DOI] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with sars‐cov‐2 in Singapore. Journal of the American Medical Association 2020; 323: 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doherty C, Atkinson D, Bruce I, et al. Targeted training using paediatric tracheostomy emergency algorithm improves performance in simulated scenarios. British Journal of Anaesthesia 2015; 114: 1015–24. [Google Scholar]
- 9. Mehta AB, Syeda SN, Bajpayee L, et al. Trends in tracheostomy for mechanically ventilated patients in the United States. American Journal of Respiratory and Critical Care Medicine 2015; 192: 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahn JM, Benson NM, Appleby D, et al. Long‐term acute care hospital utilization after critical illness. Journal of the American Medical Association 2010; 303: 2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas M, Sutherasan Y, Brunetti I, et al. Mortality and long‐term quality of life after percutaneous tracheotomy in Intensive Care Unit: a prospective observational study. Minerva Anestesiologica 2018; 84: 1024–31. [DOI] [PubMed] [Google Scholar]
- 12. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Intensive Care Medicine 2020; 46: 854–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay JK, Khoo ML‐C, Loh WS. Surgical considerations for tracheostomy during the COVID‐19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngology–Head and Neck Surgery 2020; 146: 517–8. [DOI] [PubMed] [Google Scholar]
- 14. Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE 2012; 7(4): e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu J, Yang N, Wei Y, et al. Clinical Characteristics of 54 medical staff with COVID‐ 19: a retrospective study in a single center in Wuhan, China. Journal of Medical Virology 2020; 92: 807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hui DS. Severe acute respiratory syndrome (SARS): lessons learnt in Hong Kong. Journal of thoracic disease 2013; 5(Suppl 2): S122–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID‐19) in China. Journal of Hospital Infection 2020; 105: 100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan W‐j, Ni Z‐y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. New England Journal of Medicine 2020; 382: 1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clinical Infectious Diseases 2020. Epub 28 March. 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Yan L‐M, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. The Lancet Infectious Diseases 2020; 20: 656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respiratory Medicine 2020; 8: 506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 24. Rumbak MJ, Graves AE, Scott MP, et al. Tracheostomy tube occlusion protocol predicts significant tracheal obstruction to air flow in patients requiring prolonged mechanical ventilation. Critical Care Medicine 1997; 25: 413–7. [DOI] [PubMed] [Google Scholar]
- 25. Young D. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: The tracman randomized trial. Journal of the American Medical Association 2013; 309: 2121–9. [DOI] [PubMed] [Google Scholar]
- 26. Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta‐analysis. PLoS ONE 2014; 9: e92981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andriolo BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Cochrane Database of Systematic Reviews 2015; 1: CD007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta‐analysis. Lancet Respiratory Medicine 2015; 3: 150–8. [DOI] [PubMed] [Google Scholar]
- 29. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371: 126–34. [DOI] [PubMed] [Google Scholar]
- 30. Cook TM, El‐Boghdadly K, McGuire B, et al. Consensus guidelines for managing the airway in patients with COVID‐19. Anaesthesia 2020; 75: 785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID‐19 complicating airway management. Anaesthesia 2020; 75: 972. [DOI] [PubMed] [Google Scholar]
- 32. Brodsky MB, Levy MJ, Jedlanek E, et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Critical Care Medicine. 2018; 46: 2010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lake MA. What we know so far: COVID‐19 current clinical knowledge and research 2020. Clinical Medicine 2020; 20: 124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou T, Zhang HP, Chen WW, et al. Cuff‐leak test for predicting postextubation airway complications: a systematic review. Journal of Evidence‐Based Medicine 2011; 4: 242–54. [DOI] [PubMed] [Google Scholar]
- 35. Cook TM. Personal protective equipment during the COVID‐19 pandemic – a narrative review. Anaesthesia 2020; 75: 920–7. [DOI] [PubMed] [Google Scholar]
- 36. Chao T, Martin N, Chalian AA, et al. Tracheotomy in ventilated patients with COVID‐19. Annals of Surgery 2020. Epub 5 May. 10.1097/SLA.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broderick D, Kyzas P, Sanders K, et al. Surgical tracheostomies in COVID‐19 patients: important considerations and the "5Ts" of safety. British Journal of Oral and Maxillofacial Surgery 2020; 58: 585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Public Health England . Environmental decontamination, in COVID‐19: infection prevention and control guidance. 2020. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control (accessed 14/04/2020).
- 39. Surgery C. Novel NYU Langone percutaneous tracheostomy technique. 2020. https://youtu.be/26ToEI21isE (accessed 14/4/2020).
- 40. Tien HC, Jogeklar A, Cooper AB, Brenneman F. Elective and emergency surgery in patients with severe acute respiratory syndrome (SARS). Canadian Journal of Surgery 2005; 48: 71–4. [PMC free article] [PubMed] [Google Scholar]
- 41. Chee VW, Khoo MF, Lee SF, et al. Infection control measures for operative procedures in severe acute respiratory syndrome‐related patients. Anesthesiology 2004; 100: 1394–8. [DOI] [PubMed] [Google Scholar]
- 42. Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID‐19 pandemic. JAMA Otolaryngology–Head and Neck Surgery 2020; 146: 579–84. [DOI] [PubMed] [Google Scholar]
- 43. Chan JYK, Wong EWY, Lam W. Practical aspects of otolaryngologic clinical services during the 2019 novel coronavirus epidemic. JAMA Otolaryngology–Head & Neck Surgery 2020; 146: 519–20. [DOI] [PubMed] [Google Scholar]
- 44. British Laryngological Association . Tracheostomy guideline ‐ COVID‐19. 2020. https://www.britishlaryngological.org/news/tracheostomy‐guideline‐covid‐19 (accessed 14/4/2020).
- 45. ENTUK . COVID‐19 Tracheostomy Guidance. 2020. https://www.entuk.org/covid‐19‐tracheostomy‐guidance‐t‐jacob‐et‐al (accessed 14/4/2020).
- 46. Salna M, Tipograf Y, Liou P, et al. Tracheostomy is safe during extracorporeal membrane oxygenation support. ASAIO Journal 2020; 66: 652–6. [DOI] [PubMed] [Google Scholar]
- 47. Haddad S. Tachyphylaxis to cisatracurium – case reports and literature review. Middle East Journal of Anaesthesiology 2008; 19: 1079–92. [PubMed] [Google Scholar]
- 48. Tschida SJ, Graupe KJ, Hoey LL, Vance‐Bryan K. Resistance to nondepolarizing neuromuscular blocking agents. Pharmacotherapy 1996; 16: 409–18. [PubMed] [Google Scholar]
- 49. McGrath BA, Bates L, Atkinson D, Moore JA. National Tracheostomy Safety Project: multidisciplinary guidelines for the management of tracheostomy and laryngectomy airway emergencies. Anaesthesia 2012; 67: 1025–41. [DOI] [PubMed] [Google Scholar]
- 50. Doherty C, Neal R, English C, et al. Multidisciplinary guidelines for the management of paediatric tracheostomy emergencies. Anaesthesia 2018; 73: 1400–17. [DOI] [PubMed] [Google Scholar]
- 51. Ng FK, Wallace S, Coe B, et al. From smartphone to bed‐side: exploring the use of social media to disseminate recommendations from the National Tracheostomy Safety Project to front‐line clinical staff. Anaesthesia 2019; 75: 227–33. [DOI] [PubMed] [Google Scholar]
- 52. McGrath B. By the patient, for the patient. Determining key quality of care measures for improving tracheostomy care. Medical Research Archives 2019; 7: 1–23. [Google Scholar]
- 53. Hall A, Bates J, Ifeacho S, et al. Implementation of the TRACHE care bundle: improving safety in paediatric tracheostomy management. Archives of Disease in Childhood 2017; 102: 563–5. [DOI] [PubMed] [Google Scholar]
- 54. Zhonghua J. Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Respiratory Care Committee of Chinese Thoracic Society 2020. Epub 20 Feb. 10.3760/cma.j.issn.1001-0939.2020.0020. [DOI] [PubMed] [Google Scholar]
- 55. Harrison L, Ramsden J, Winter S, et al. Guidance for surgical tracheostomy and tracheostomy tube change during the COVID‐19 pandemic. ENTUK 2020. https://www.entuk.org/tracheostomy‐guidance‐during‐covid‐19‐pandemic (accessed 14/04/2020). [Google Scholar]
- 56. Brusasco C, Corradi F, Vargas M, et al. In vitro evaluation of heat and moisture exchangers designed for spontaneously breathing tracheostomized patients. Respiratory Care 2013; 58: 1878. [DOI] [PubMed] [Google Scholar]
- 57. Medicines and Healthcare products Regulatory Agency . Risk of using different airway humidification devices simultaneously. NHS/PSA/W/2015/012. 2015. https://www.england.nhs.uk/patientsafety/wp‐content/uploads/sites/32/2015/12/psa‐humidification‐devices.pdf (accessed 13/04/2020).
- 58. McGrath BA, Lynch J, Wilson M, et al. Above cuff vocalisation: a novel technique for communication in the ventilator‐dependent tracheostomy patient. Journal of the Intensive Care Society 2016; 17: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ten Hoorn S, Elbers PW, Girbes AR, Tuinman PR. Communicating with conscious and mechanically ventilated critically ill patients: a systematic review. Critical Care 2016; 20: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. National Tracheostomy Safety Project . Consideration for tracheostomy in the Covid‐19 outbreak. 2020. http://www.tracheostomy.org.uk/storage/files/NTSP%20COVID_19%20tracheostomy%20guidance%2031_3_20.pdf (accessed 04/04/2020).
- 61. de Mestral C, Iqbal S, Fong N, et al. Impact of a specialized multidisciplinary tracheostomy team on tracheostomy care in critically ill patients. Canadian Journal of Surgery. 2011; 54: 167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheung JCH, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID‐19 in Hong Kong. Lancet Respiratory Medicine 2020; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Public Health England . COVID‐19: infection prevention and control guidance. 2020. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/wuhan‐novel‐coronavirus‐wn‐cov‐infection‐prevention‐and‐control‐guidance#mobile‐healthcare‐equipment (accessed 12/04/2020).
- 64. Kelley J, Steele A. The Kelley Circuit: a solution for the management of in‐hospital self‐ventilating tracheostomy patients, providing humidification and filtration, with closed‐ circuit suctioning. http://www.tracheostomy.org.uk/storage/files/The%20Kelley%20Circuit%20For%20Tracheostomy.pdf (accessed 13/04/2020).


