This cohort study assesses overall survival by comparing 4 reduced-intensity conditioning and nonmyeloablative conditioning regimens in allogeneic hematopoietic cell transplantation treatment for patients with non-Hodgkin lymphoma.
Key Points
Question
Are reduced-intensity conditioning and nonmyeloablative conditioning (RIC-NMAC) regimens at a higher spectrum of intensity associated with higher nonrelapse mortality and inferior overall survival compared with RIC-NMAC regimens at a lower spectrum of intensity in patients with non-Hodgkin lymphoma undergoing allogeneic transplant?
Findings
In this cohort study of registry data from 1823 adult patients with non-Hodgkin lymphoma, the most commonly used RIC-NMAC regimen, fludarabine-melphalan 140, was associated with the highest nonrelapse mortality risk and inferior overall survival compared with the other regimens.
Meaning
The findings suggest that use of a more intense RIC-NMAC regimen, such as fludarabine-melphalan 140, for allogeneic transplant in patients with non-Hodgkin lymphoma should be considered with caution.
Abstract
Importance
Reduced-intensity conditioning and nonmyeloablative conditioning (RIC-NMAC) regimens are frequently used in allogeneic hematopoietic cell transplant (HCT) for non-Hodgkin lymphoma. However, the optimal RIC-NMAC regimen in allogeneic HCT for non-Hodgkin lymphoma is not known.
Objective
To investigate whether RIC-NMAC regimens at a higher end of the intensity spectrum are associated with increased nonrelapse mortality and lower overall survival compared with RIC-NMAC regimens at the lower end of the intensity spectrum in patients with non-Hodgkin lymphoma undergoing allogeneic HCT.
Design, Setting, and Participants
This cohort study used data from 1823 adult patients with non-Hodgkin lymphoma in the Center for International Blood and Marrow Transplant Research registry. Included patients underwent allogeneic HCT using matched related or unrelated donors between January 2008 and December 2016. Statistical analysis was performed from June 1, 2019, to February 10, 2020.
Interventions
Patients received 1 of 4 RIC-NMAC regimens: fludarabine-intravenous busulfan (Flu-Bu), approximately 6.4 mg/kg (n = 458); fludarabine-melphalan (Flu-Mel140), 140 mg/m2 (n = 885); fludarabine-cyclophosphamide (Flu-Cy) (n = 391); or Flu-Cy with 2 Gy total body irradiation (Flu-Cy-2GyTBI) (n = 89).
Main Outcomes and Measures
The primary outcome was overall survival. Secondary outcomes were nonrelapse mortality, incidence of relapse, progression-free survival, and the incidence of acute and chronic graft-vs-host disease (GVHD).
Results
Of 1823 patients, 1186 (65%) were male, with a mean (SD) age of 54.8 (9.9) years. The 4-year adjusted OS was 58% in the Flu-Bu cohort, 67% in the Flu-Cy-2GyTBI cohort, 49% in the Flu-Mel140 cohort, and 63% in the Flu-Cy cohort (P < .001). After adjustment for age, Karnofsky performance score, HCT comorbidity index, NHL subtype, remission status at HCT, and the use of antithymocyte globulin or alemtuzumab, the regression analysis showed a significantly higher mortality risk associated with Flu-Mel140 compared with Flu-Bu (hazard ratio [HR], 1.34; 95% CI, 1.13-1.59; P < .001). Compared with the Flu-Cy cohort, the Flu-Mel140 cohort had a higher risk of chronic GVHD (HR, 1.38; 95% CI, 1.15-1.65; P < .001). The Flu-Mel140 regimen was associated with a higher nonrelapse mortality risk (HR, 1.78; 95% CI, 1.37-2.31; P < .001) compared with the Flu-Bu regimen.
Conclusions and Relevance
The findings suggest that use of the more intense RIC-NMAC regimen, Flu-Mel140, may have a negative association with overall survival and may be associated with higher nonrelapse mortality. The Flu-Bu and Flu-Cy regimens with or without 2GyTBI regimens appeared to provide comparable overall survival.
Introduction
Reduced-intensity conditioning and nonmyeloablative conditioning (RIC-NMAC) regimens have significantly expanded the access to allogeneic hematopoietic cell transplant (HCT) in the treatment of hematologic malignant neoplasms.1,2,3,4 Although RIC-NMAC regimens generally are associated with lower nonrelapse mortality compared with myeloablative conditioning regimens,5,6 disease relapse remains the most common cause of treatment failure among patients with non-Hodgkin lymphoma (NHL) undergoing allogeneic HCT with such lower-intensity platforms.7,8,9 The various RIC-NMAC regimens commonly used in practice differ in their immunosuppressive and cytoreductive effects, but to our knowledge, no prospective randomized trials are available to define an optimal regimen among patients with NHL.
A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis compared HCT outcomes among patients with lymphoma who underwent either a total body irradiation (TBI)–based regimen or a non–TBI-containing NMAC regimen allogeneic HCT.10 The study found a higher risk of graft-vs-host disease (GVHD) associated with TBI-based approaches but no difference in survival. A retrospective North American and Spanish study (n = 136) showed inferior overall survival (OS) associated with use of fludarabine-melphalan, 140 mg/m2 (Flu-Mel140) compared with fludarabine-busulfan (Flu-Bu).11 This survival difference was largely attributed to a higher risk of acute grade 2 to 4 GVHD associated with Flu-Mel140. That study was, however, limited because of its small sample size, inclusion of Hodgkin lymphoma with NHL histologic findings, and the absence of comparison with other commonly used RIC-NMAC regimens (eg, fludarabine-cyclophosphamide [Flu-Cy]–based approaches).
We hypothesized that within the RIC-NMAC spectrum, patients with NHL receiving a higher-intensity regimen, such as Flu-Mel140, would have higher nonrelapse mortality and inferior OS compared with those receiving lower-intensity regimens, such as Flu-Bu, Flu-Cy, or Flu-Cy with 2 Gy total body irradiation (Flu-Cy-2GyTBI).
Methods
Data Source
This cohort study used data from the CIBMTR, a working group of more than 500 transplantation centers worldwide that contributed detailed data on HCT to a statistical center at the Medical College of Wisconsin, Milwaukee. Participating centers were required to report all transplants consecutively, and compliance was monitored by onsite audits. Computerized checks for discrepancies, physicians' review of submitted data, and onsite audits of participating centers ensured data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Patients provided written informed consent for research. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
The CIBMTR collects data at 2 levels: Transplant Essential Data and Comprehensive Report Form data. Transplant Essential Data include disease type, age, sex, pre-HCT disease stage and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, posttransplant disease progression and survival, development of a new malignant neoplasm, and cause of death. All CIBMTR centers contribute Transplant Essential Data.
The CIBMTR uses a weighted randomization scheme to select a subset of patients for Comprehensive Report Form reporting with more details about disease and pretransplant and posttransplant clinical information. Both Transplant Essential Data– and Comprehensive Report Form–level data are collected before HCT, 100 days after HCT, 6 months after HCT, and annually thereafter or until death. Data for the current analysis were retrieved from CIBMTR Transplant Essential Data and Comprehensive Report Form (eTable 7 in the Supplement).
Patients
This analysis included adults (aged ≥18 years) with NHL who underwent their first RIC-NMAC regimen allogeneic HCT between January 2008 and December 2016. Eligible donors included either HLA-identical sibling donors or unrelated donors matched at the allele-level at HLA-A, HLA-B, HLA-C, and HLA-DRB1. Graft source was limited to peripheral blood. GVHD prophylaxis was limited to calcineurin inhibitor–based approaches. The study cohort was divided into 4 RIC-NMAC regimens (Flu-Bu, Flu-Cy-2GyTBI, Flu-Mel140, and Flu-Cy). Patients in the Flu-Bu cohort received a uniform intravenous busulfan dose of approximately 6.4 mg/m2. The Flu-Mel140 cohort was limited to a melphalan dose of 140 mg/m2. Allogeneic HCT recipients could have received in vivo T-cell depletion with antithymocyte globulin or alemtuzumab. Patients receiving ex vivo graft manipulation were not included.
Definitions and End Points
The intensity of allogeneic HCT conditioning regimens was assessed using consensus criteria.12 Disease response at the time of HCT was assessed using the International Working Group criteria in use during the era of this analysis.13 The primary end point was OS; death from any cause was considered an event, and surviving patients were censored at the last follow-up. Secondary outcomes included nonrelapse mortality, progression or relapse, GVHD, progression-free survival (PFS), and time to neutrophil and platelet recovery. Nonrelapse mortality was defined as death without evidence of lymphoma progression or relapse; relapse was considered a competing risk.
Progression or relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a complete remission; nonrelapse mortality was considered a competing risk. For PFS, a patient was considered to experience treatment failure at time of progression or relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at the last follow-up. Acute GVHD and chronic GVHD were graded using established clinical criteria.14,15 Probabilities of PFS and OS were calculated using the Kaplan-Meier estimates.
Neutrophil recovery was defined as the first of 3 successive days with an absolute neutrophil count of 500/μL after posttransplant nadir. Platelet recovery was considered to have occurred on the first of 3 consecutive days with a platelet count of 20 000/μL or higher in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical Analysis
The study compared 4 RIC-NMAC regimens: Flu-Bu vs Flu-Cy-2GyTBI vs Flu-Mel140 vs Flu-Cy. The χ2 test for categorical variables and Kruskal-Wallis test for continuous variables were used to compare baseline characteristics. Completeness of follow-up was assessed as described previously16 and was at least 90% in all groups at 4 years, consistent with low rates of loss to follow-up. Statistical analysis was performed from June 1, 2019, to February 10, 2020.
Cumulative incidences of hematopoietic recovery, GVHD, relapse, and nonrelapse mortality were calculated to accommodate for competing risks. Associations among patient-, disease-, and transplant-associated variables (eTable 1 in the Supplement) and outcomes of interest were evaluated using Cox proportional hazards regression for PFS and OS; a cause-specific hazard model for chronic GVHD, relapse, and nonrelapse mortality to account for competing risks; and logistic regression for acute GVHD. Forward stepwise selection was used to identify covariates associated with outcomes. Covariates with 2-sided P < .01 were considered to be statistically significant. The proportional hazards assumption for Cox regression and the cause-specific hazards function were tested by adding a time-dependent covariate for each risk factor and each outcome. Center effect was examined by the score test for chronic GVHD, relapse, nonrelapse mortality, PFS, and OS and testing a random association from the generalized linear mixed model for acute GVHD.17 No center effect was seen for any outcomes. Interactions between the main effect and significant covariates were examined. Unadjusted probabilities were calculated for neutrophil recovery, platelet recovery, and GVHDs. Adjusted probabilities were calculated based on the final model for relapse, nonrelapse mortality, PFS, and OS.18,19
We also confirmed the final model for relapse, nonrelapse mortality, PFS, and OS by comparing it with the model from multiple imputation and propensity score matching. We imputed missing covariates using multiple imputation and obtained 10 imputed data sets.20 Then, multinomial logistic regression was used to obtain propensity scores for each person using Rubin rules.21 Persons were matched using estimated propensity scores with nearest neighbor caliper matching.22 We fitted the marginal Cox proportional hazards regression model for PFS and OS, the marginal cause-specific hazards model for relapse, and nonrelapse mortality23 for each imputed data set to account for matched pairs. The final model for each outcome was summarized using Rubin rules. To account for multiple pairwise tests, the false discovery rate24 was used with a 0.05 cutoff, and Q values (adjusted P values from the false discovery rate) were reported. We further confirmed the analysis results by conducting a subset analysis for Flu-Bu vs Flu-Mel140 with multiple imputation and propensity score matching.
Results
Baseline Characteristics
The patient population (N = 1823) was divided into 4 cohorts: Flu-Bu (n = 458), Flu-Cy-2GyTBI (n = 89), Flu-Mel140 (n = 885), and Flu-Cy (n = 391). The baseline patient-, disease-, and transplant-associated characteristics are shown in eTable 2 in the Supplement. Of the 1823 patients, 1186 (65%) were male, with a mean (SD) age of 54.8 (9.9) years. There were no significant differences between the 4 cohorts in terms of patient sex, remission status at HCT, and history of autologous HCT. Significantly more patients in the Flu-Bu (187 of 458 [41%]) and Flu-Cy-2GyTBI (34 of 89 [38%]) cohorts were older than 60 years compared with the Flu-Mel140 (282 of 885 [32%]) and Flu-Cy (123 of 391 [32%]) cohorts (P < .001). Significantly more patients in the Flu-Bu cohort had a Karnofsky performance score of less than 90, HCT comorbidity index of at least 3, and self-identified white race (eTable 2 in the Supplement) compared with the other cohorts. Aggressive lymphomas (diffuse large B-cell lymphoma and T-cell NHL) were more frequent in the Flu-Bu (262 [57%]) and Flu-Mel140 (557 [63%]) cohorts (P < .001).
Overall Survival
The 4-year adjusted OS was 58% in the Flu-Bu cohort, 67% in the Flu-Cy-2GyTBI cohort, 49% in the Flu-Mel140 cohort, and 63% in the Flu-Cy cohort (P < .001) (Table and Figure). After adjustment for age, Karnofsky performance score, HCT comorbidity index, NHL subtype, remission status at HCT, and the use of antithymocyte globulin or alemtuzumab, the regression analysis showed a significantly higher mortality risk associated with Flu-Mel140 compared with Flu-Bu (hazard ratio [HR], 1.34; 95% CI, 1.13-1.59; Q < .001), Flu-Cy-2GyTBI (HR, 1.82; 95% CI, 1.23-2.69; Q = .01), and Flu-Cy (HR, 1.59; 95% CI, 1.29-1.95; Q < .001) (eTable 3 in the Supplement). There was no significant difference in OS among Flu-Bu, Flu-Cy-2GyTBI, and Flu-Cy. The inferior OS associated with Flu-Mel140 was also found with propensity score matching (HR, 1.43; 95% CI, 1.20-1.71; Q < .001) (eTable 4 in the Supplement).
Table. Engraftment, GVHD, and Adjusted Transplant Outcomes.
| Outcome | Flu-Bu (n = 458) | Flu-Cy-2GyTBI (n = 89) | Flu-Mel140 (n = 885) | Flu-Cy (n = 391) | P valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| Evaluated, No. | Probability, % (95% CI) | Evaluated, No. | Probability, % (95% CI) | Evaluated, No. | Probability, % (95% CI) | Evaluated, No. | Probability, % (95% CI) | ||
| Neutrophil recovery at 30 d | 454 | 99 (98-100) | 87 | 99 (96-100) | 871 | 96 (94-97) | 389 | 98 (97-99) | <.001 |
| Platelet recovery at 100 d | 451 | 97 (95-98) | 87 | 97 (92-99) | 862 | 90 (88-92) | 362 | 99 (97-100) | <.001 |
| Acute GVHD stage 2-4 at 6 mo | 435 | 40 (35-45) | 78 | 29 (20-40) | 840 | 38 (35-42) | 380 | 34 (29-39) | .12 |
| Acute GVHD stage 3 or 4 at 6 mo | 432 | 13 (10-16) | 78 | 10 (5-18) | 832 | 15 (12-17) | 378 | 15 (12-19) | .42 |
| Chronic GVHD at 1 y | 433 | 41 (36-46) | 81 | 32 (23-43) | 813 | 43 (39-46) | 370 | 42 (37-48) | .29 |
| Adjusted nonrelapse mortality | |||||||||
| Overall | 457 | NA | 89 | NA | 879 | NA | 389 | NA | NA |
| 1 y | NA | 11 (8-14) | NA | 9 (3-15) | NA | 17 (14-19) | NA | 8 (5-11) | <.001 |
| 4 y | NA | 16 (13-20) | NA | 17 (9-25) | NA | 26 (23-30) | NA | 17 (12-21) | <.001 |
| Adjusted progression and/or relapse | |||||||||
| Overall | 457 | NA | 89 | NA | 879 | NA | 389 | NA | NA |
| 1 y | NA | 38 (33-42) | NA | 28 (18-38) | NA | 30 (28-33) | NA | 40 (35-44) | .002 |
| 4 y | NA | 47 (42-51) | NA | 33 (23-44) | NA | 37 (33-40) | NA | 50 (45-55) | <.001 |
| Adjusted progression-free survival | |||||||||
| Overall | 457 | NA | 89 | NA | 879 | NA | 389 | NA | NA |
| 1 y | NA | 52 (47-56) | NA | 63 (53-74) | NA | 53 (50-56) | NA | 51 (46-56) | .18 |
| 4 y | NA | 38 (34-43) | NA | 51 (40-61) | NA | 39 (36-43) | NA | 35 (30-40) | .07 |
| Adjusted overall survival | |||||||||
| Overall | 458 | NA | 89 | NA | 885 | NA | 391 | NA | NA |
| 1 y | NA | 73 (69-77) | NA | 78 (69-86) | NA | 69 (66-72) | NA | 78 (74-83) | .002 |
| 4 y | NA | 58 (54-63) | NA | 67 (57-77) | NA | 49 (46-53) | NA | 63 (58-68) | <.001 |
Abbreviations: Bu, busulfan; Cy, cyclophosphamide; Flu, fludarabine; GVHD, graft-vs-host disease; Mel, melphalan; NA, not applicable; TBI, total body irradiation.
Overall P values for a given year from the Wald test.
Figure. Adjusted Outcomes of Patients With Non-Hodgkin Lymphoma Undergoing Reduced-Intensity Conditioning Allogeneic Transplant.
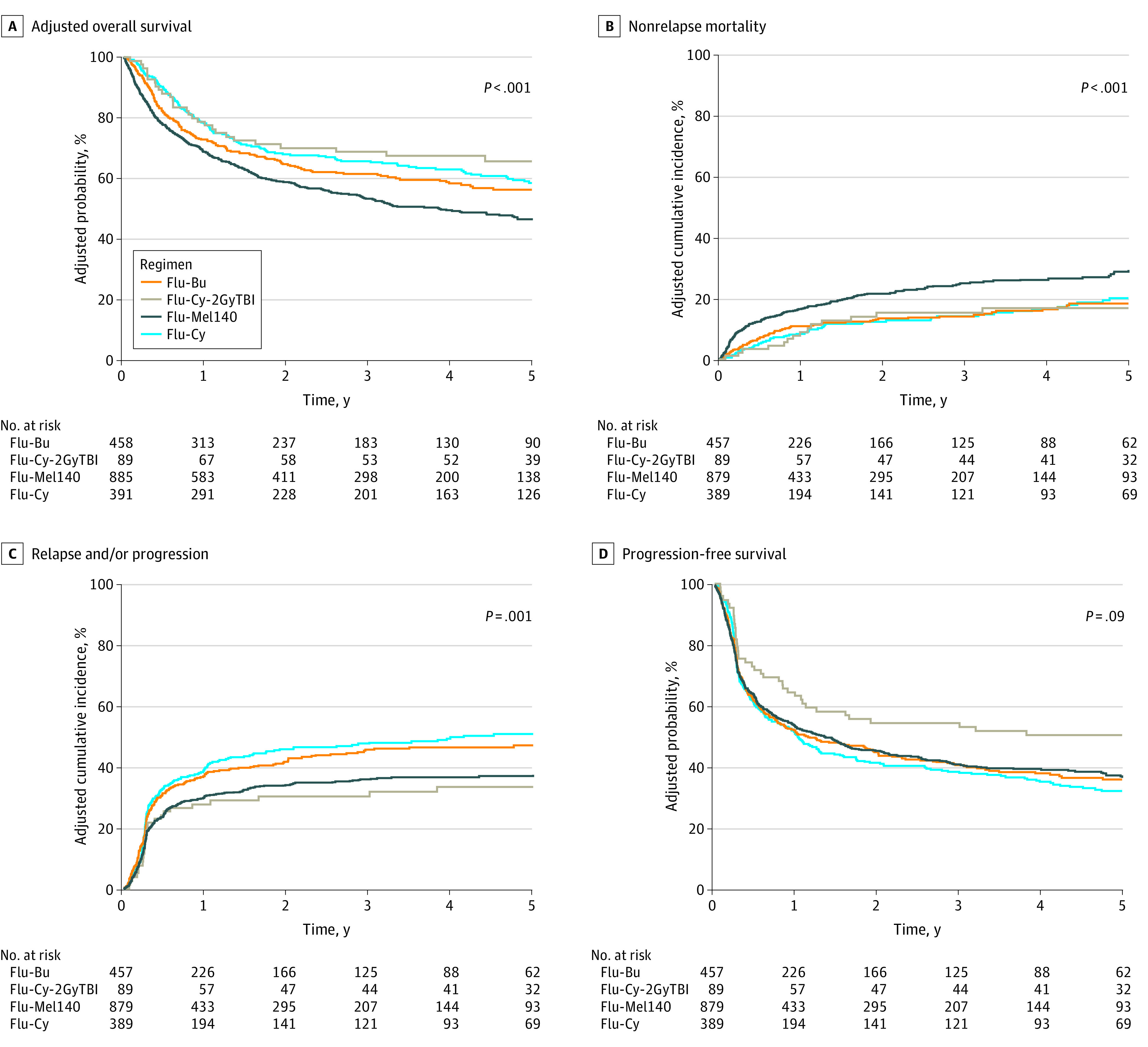
Bu indicates busulfan; Cy, cyclophosphamide; Flu, fludarabine; Mel, melphalan; and TBI, total body irradiation.
Nonrelapse Mortality
The 4-year adjusted cumulative incidence of nonrelapse mortality was 16% in the Flu-Bu cohort, 17% in the Flu-Cy-2GyTBI cohort, 26% in the Flu-Mel140 cohort, and 17% in the Flu-Cy cohort (P < .001) (Table and Figure). After adjustment for age, Karnofsky performance score, HCT comorbidity index, history of autologous HCT, and use of antithymocyte globulin or alemtuzumab in the regression analysis, the use of the Flu-Mel140 regimen was associated with a significantly higher risk of nonrelapse mortality compared with the Flu-Bu regimen (HR, 1.78; 95% CI, 1.37-2.31; Q < .001) (eTable 3 in the Supplement). Compared with the Flu-Bu regimen, the Flu-Cy and Flu-Cy-2GyTBI regimens were not associated with a higher risk of nonrelapse mortality (eTable 3 in the Supplement). The higher nonrelapse mortality associated with Flu-Mel140 was also found with propensity score matching (HR, 1.91; 95% CI, 1.48-2.46; Q < .001) (eTable 4 in the Supplement).
Relapse and PFS
The adjusted cumulative incidence of relapse or progression at 4 years was 47% in the Flu-Bu cohort, 33% in the Flu-Cy-2GyTBI cohort, 37% in the Flu-Mel140 cohort, and 50% in the Flu-Cy cohort (P < .001) (Table and Figure). After adjustment for remission status at HCT, NHL subtype, donor type, and the use of antithymocyte globulin or alemtuzumab, the regression analysis showed that the Flu-Mel140 cohort did not have significantly lower risk of relapse or progression compared with the Flu-Bu cohort (HR, 0.79; 95% CI, 0.66-0.94; Q = .07) (eTable 3 in the Supplement). Compared with the Flu-Bu regimen, the Flu-Cy and the Flu-Cy-2GyTBI regimens were not associated with a significantly lower risk of relapse or progression (eTable 3 in the Supplement). The lower relapse risk associated with the Flu-Mel140 compared with the Flu-Cy regimens was also found with propensity score matching (HR, 0.75; 95% CI, 0.62-0.91; Q = .04) (eTable 4 in the Supplement).
The 4-year adjusted PFS was 38% in the Flu-Bu cohort, 51% in the Flu-Cy-2GyTBI cohort, 39% in the Flu-Mel140 cohort, and 35% in the Flu-Cy cohort was (P = .07) (Table and Figure). After adjustment for Karnofsky performance score, remission status at HCT, NHL subtype, and the use of antithymocyte globulin or alemtuzumab, the regression analysis did not show a significantly improved PFS associated with the Flu-Mel140 (HR, 1.03; 95% CI, 0.89-1.19), the Flu-Cy-2GyTBI (HR, 0.70; 95% CI, 0.51-0.98) or the Flu-Cy (HR, 1.07; 95% CI, 0.89-1.29) regimen compared with the Flu-Bu regimen (overall P = .09) (eTable 3 in the Supplement). Propensity score matching validated these findings (eTable 4 in the Supplement).
Hematopoietic Recovery
The cumulative incidence of neutrophil recovery at day 30 was significantly lower in the Flu-Mel140 cohort (96%) compared with the Flu-Bu (99%), the Flu-Cy-2GyTBI (99%), and the Flu-Cy (98%) cohorts (P < .001) (Table). The day 100 cumulative incidence of platelet recovery was also significantly lower in the Flu-Mel140 cohort (90%) compared with the Flu-Bu (97%), the Flu-Cy-2GyTBI (97%), and the Flu-Cy (99%) cohorts (P < .001) (Table).
Acute and Chronic GVHD
The univariate cumulative incidence rates of grade 2 to 4 and 3 to 4 acute GVHD across 4 cohorts are shown in the Table. After adjustment for GVHD prophylaxis, the multivariate analysis (eTable 3 in the Supplement) showed no statistically significant difference in the risk of grade 3 to 4 acute GVHD in patients who received the Flu-Cy-2GyTBI (HR, 0.81; 95% CI, 0.37-1.77), the Flu-Mel140 (HR, 1.42; 95% CI, 0.99-2.02), and the Flu-Cy (HR, 1.34; 95% CI, 0.9-2.02) regimens compared with the Flu-Bu regimens.
On univariate analysis, the cumulative incidence of chronic GVHD at 1 year was 41% in the Flu-Bu cohort, 32% in the Flu-Cy-2GyTBI cohort, 43% in the Flu-Mel140 cohort, and 42% in the Flu-Cy cohort (Table). After adjusting for donor type, GVHD prophylaxis, and antithymocyte globulin or alemtuzumab use in conditioning, the multivariate analysis (eTable 3 in the Supplement) showed no significant difference in risk of chronic GVHD in patients who received Flu-Cy-2GyTBI (HR, 0.96; 95% CI, 0.68-1.34), Flu-Mel140 (HR, 1.12; 95% CI, 0.94-1.33), and Flu-Cy (HR, 0.81; 95% CI, 0.67-0.99) compared with Flu-Bu. However, the Flu-Mel140 cohort had a significantly higher risk of chronic GVHD when compared with the Flu-Cy cohort (HR, 1.38; 95% CI, 1.15-1.65; P < .001; Q = .006).
Subset Analysis
A subset analysis comparing Flu-Bu vs Flu-Mel140 also showed inferior outcomes associated with Flu-Mel140 (eTable 5 in the Supplement). No interaction between NHL histologic findings and the main effect (ie, conditioning regimen type) was seen for any of the following outcomes at a significance level of 0.01: acute grade 3 to 4 GVHD, chronic GVHD, nonrelapse mortality, relapse or progression, PFS, and OS.
Causes of death
The most common cause of death in all the cohorts was recurrent or progressive lymphoma (53% [103 of 194] in the Flu-Bu cohort, 37% [11 of 30] in the Flu-Cy-2GyTBI cohort, 38% [164 of 428] in the Flu-Mel140 cohort, and 42% [60 of 143] in the Flu-Cy cohort). GVHD and infectious complications were the other common causes of death (eTable 6 in the Supplement).
Discussion
We report the findings of, to our knowledge, the largest registry analysis comparing the outcomes of patients with NHL undergoing allogeneic HCT after RIC-NMAC regimens with Flu-Bu, Flu-Cy-2GyTBI, Flu-Mel140, or Flu-Cy and made several important observations: (1) hematopoietic recovery was slower after the Flu-Mel140 regimen; (2) there were no significant differences in grade 3 to 4 acute GVHD; (3) compared with Flu-Mel140, Flu-Cy was associated with a lower risk of chronic GVHD; and (4) Flu-Mel140 was associated with a significantly higher risk of nonrelapse mortality and with inferior OS compared with the other regimens.
The importance of conditioning regimen intensity for patients with NHL undergoing allogeneic HCT has been debated. A CIBMTR analysis for patients with chemosensitive diffuse large B-cell lymphoma showed no difference in 5-year PFS and OS between the myeloablative conditioning regimen and the RIC-NMAC regimens.25 Similarly, even among patients with refractory NHL, intensity of conditioning regimens (myeloablative conditioning vs RIC-NMAC) was not associated with survival outcomes.5,6 Although previous CIBMTR studies5,6 have questioned the use of myeloablative conditioning regimens among patients with lymphoma, there are limited comparative data published on the relative efficacy and toxic effects of the individual RIC-NMAC regimens commonly used in NHL.
A few reports11,26 have compared the outcomes associated with Flu-Mel and Flu-Bu in patients with lymphoma, albeit with limited sample size. Kekre et al11 examined the outcomes in 136 patients with lymphoma, and Yerushalmi et al26 studied 144 patients; in both of these studies,11,26 Flu-Mel was associated with a higher risk of acute GVHD, nonrelapse mortality, and inferior OS. Unlike with NHL, for myeloid malignancies, several studies27,28 have reported that Flu-Mel was associated with a lower relapse rate, higher nonrelapse mortality, and equivalent or inferior survival compared with Flu-Bu.
There are several important differences between our study and previously published reports.11,26 First, the large number of patients in our study improved on the limited power of smaller studies by allowing us to match propensity scores. Second, Flu-Cy and Flu-Cy-2GyTBI regimens were not included in previous comparisons.11,26
In addition, previous studies11,26,27,28 were either limited to myeloid disorders or pooled patients with Hodgkin lymphoma and NHL, but these patients had different demographic characteristics. Patients who underwent allogeneic HCT for a diagnosis of Hodgkin lymphoma were younger29 (median age, 35 years), more likely to have received a previous autologous transplant, and often had fewer comorbid conditions and thus may have been able to tolerate higher-intensity regimens (eg, Flu-Mel140), unlike the significantly older cohort with NHL in this analysis.
Limitations
We acknowledge the limitation that our study included a variety of NHL subtypes (which had varying degrees of relapse risk). However, we found the association of conditioning regimen did not vary according to NHL histologic findings, and this justified inclusion of different NHL subtypes in this analysis.
Busulfan dose intensity in the Flu-Bu regimen ranges from RIC dosage (Flu-Bu2, 2 days of busulfan at 4 mg/kg per day orally or 3.2 mg/kg per day intravenously) to myeloablative conditioning (Flu-Bu3/Flu-Bu4, 3 or 4 days of busulfan at 4 mg/kg per day orally or 3.2 mg/kg per day intravenously) dosage. Previous reports30 on NHL have shown no advantage of using a myeloablative conditioning Flu-Bu regimen vs an RIC Flu-Bu regimen.
Similarly, melphalan dose varied from 100mg/m2 to 140mg/m2 across Flu-Mel regimens, and no differences in outcomes have been noted between the 2 doses.31 To reduce the heterogeneity of the regimens, we used the most common RIC Flu-Bu dose (total busulfan dose of approximately 6.4 mg/kg intravenous) and Flu-Mel140 dose (melphalan dose of 140 mg/m2) for our analysis.
Our study included patients who received grafts from only matched and related or matched and unrelated donors, and GVHD prophylaxis was limited to calcineurin inhibitor–based approaches. Patients who underwent haploidentical HCT with posttransplant cyclophosphamide had similar survival compared with patients receiving grafts from HLA-matched donors.32,33 Haploidentical HCT recipients were excluded from our study because this donor source is associated with the Flu-Cy-2GyTBI regimen.
Similar to other registry-based analyses, there are important caveats to be considered. Unlike a randomized clinical trial, this was a cohort comparison based on registry data. We therefore used propensity score matching to balance the distributions of significant covariates between the groups and to reduce the association of confounding variables. Transplant center practices can be associated with survival outcomes. Therefore, we examined center effect and found none in this analysis.
Patients in this study had various NHL subtypes. However, a lack of interaction between the main effect and NHL subtypes argues against a differential effect of RIC-NMAC regimens across different NHL subtypes.
Conclusions
In this cohort study, we found that the high-intensity RIC-NMAC regimen Flu-Mel140 was associated with significantly higher nonrelapse mortality risk and inferior OS compared with other regimens despite patients who received this regimen having fewer comorbidities at baseline. The other regimens evaluated provided comparable survival outcomes. The results of our study are potentially practice changing and caution against the use of more intense conditioning regimens in the treatment of NHL.
eTable 1. Variables Tested in Cox Proportional Hazards Regression Model for NHL
eTable 2. Baseline Characteristics of NHL Patients Receiving RIC/NMA Conditioning Regimens Followed by allo-HCT
eTable 3. Adjusted Risk of GVHD, NRM, and Progression/Relapse, by Conditioning Regimen
eTable 4. Propensity Score Matching Results
eTable 5. Subset Analysis Results by Conditioning Regimen
eTable 6. Causes of Death
eTable 7. CIBMTR Data Collection Forms
References
- 1.Epperla N, Ahn KW, Ahmed S, et al. . Rituximab-containing reduced-intensity conditioning improves progression-free survival following allogeneic transplantation in B cell non-Hodgkin lymphoma. J Hematol Oncol. 2017;10(1):117. doi: 10.1186/s13045-017-0487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenske TS, Ahn KW, Graff TM, et al. . Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174(2):235-248. doi: 10.1111/bjh.14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klyuchnikov E, Bacher U, Kröger NM, et al. . Reduced-intensity allografting as first transplantation approach in relapsed/refractory grades one and two follicular lymphoma provides improved outcomes in long-term survivors. Biol Blood Marrow Transplant. 2015;21(12):2091-2099. doi: 10.1016/j.bbmt.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NN, Ahn KW, Litovich C, et al. . Outcomes of Medicare-age eligible NHL patients receiving RIC allogeneic transplantation: a CIBMTR analysis. Blood Adv. 2018;2(8):933-940. doi: 10.1182/bloodadvances.2018018531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamadani M, Saber W, Ahn KW, et al. . Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: a cohort analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2013;19(4):625-631. doi: 10.1016/j.bbmt.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamadani M, Saber W, Ahn KW, et al. . Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19(5):746-753. doi: 10.1016/j.bbmt.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avivi I, Canals C, Vernant J-P, et al. ; EBMT Lymphoma Working Party . Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplant. 2014;49(5):671-678. doi: 10.1038/bmt.2014.4 [DOI] [PubMed] [Google Scholar]
- 8.Dreger P, Sureda A, Ahn KW, et al. . PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3(3):360-369. doi: 10.1182/bloodadvances.2018027748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epperla N, Ahn KW, Litovich C, et al. . Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: a CIBMTR analysis. J Hematol Oncol. 2019;12(1):6. doi: 10.1186/s13045-018-0696-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S, Le-Rademacher J, Artz A, McCarthy PL, Logan BR, Pasquini MC. Comparison of non-myeloablative conditioning regimens for lymphoproliferative disorders. Bone Marrow Transplant. 2015;50(3):367-374. doi: 10.1038/bmt.2014.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kekre N, Marquez-Malaver FJ, Cabrero M, et al. . Fludarabine/busulfan versus fludarabine/melphalan conditioning in patients undergoing reduced-intensity conditioning hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transplant. 2016;22(10):1808-1815. doi: 10.1016/j.bbmt.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. doi: 10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. doi: 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, et al. . Chronic graft-versus-host syndrome in man. a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. doi: 10.1016/0002-9343(80)90380-0 [DOI] [PubMed] [Google Scholar]
- 16.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309-1310. doi: 10.1016/S0140-6736(02)08272-7 [DOI] [PubMed] [Google Scholar]
- 17.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145-156. doi: 10.1007/BF00985764 [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. doi: 10.1016/j.cmpb.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87-93. doi: 10.1016/j.cmpb.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlett JW, Seaman SR, White IR, Carpenter JR; Alzheimer’s Disease Neuroimaging Initiative* . Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res. 2015;24(4):462-487. doi: 10.1177/0962280214521348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 22.Liu Y, Nickleach D, Lipscomb J Propensity score matching for multiple treatment comparisons in observational studies. In: Proceedings of the 59th ISI World Statistics Congress; August 25-30, 2013; Hong Kong. Session CPS108. [Google Scholar]
- 23.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis. State of the Art. NATO Science. Series E: Applied Sciences. Vol 211 Springer; 1992. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 24.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165-1188. [Google Scholar]
- 25.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. ; Lymphoma Working Committee of the CIBMTR . Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256-4262. doi: 10.1182/blood-2012-06-436725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerushalmi R, Shem-Tov N, Danylesko I, Avigdor A, Nagler A, Shimoni A. Fludarabine and treosulfan compared with other reduced-intensity conditioning regimens for allogeneic stem cell transplantation in patients with lymphoid malignancies. Bone Marrow Transplant. 2015;50(12):1526-1535. doi: 10.1038/bmt.2015.174 [DOI] [PubMed] [Google Scholar]
- 27.Baron F, Labopin M, Peniket A, et al. . Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2015;121(7):1048-1055. doi: 10.1002/cncr.29163 [DOI] [PubMed] [Google Scholar]
- 28.Shimoni A, Hardan I, Shem-Tov N, et al. . Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21(10):2109-2116. doi: 10.1038/sj.leu.2404886 [DOI] [PubMed] [Google Scholar]
- 29.Ahmed S, Kanakry JA, Ahn KW, et al. . Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide-based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25(9):1859-1868. doi: 10.1016/j.bbmt.2019.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bourgeois A, Labopin M, Blaise D, et al. ; Société Francophone de Greffe de Moelle et de Thérapie Cellulaire . Reduced-intensity versus reduced-toxicity myeloablative fludarabine/busulfan-based conditioning regimens for allografted non-Hodgkin lymphoma adult patients: a retrospective study on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Ann Oncol. 2017;28(9):2191-2198. doi: 10.1093/annonc/mdx274 [DOI] [PubMed] [Google Scholar]
- 31.Saini NY, Saliba RM, Rondon G, et al. . Impact of donor type and melphalan dose on allogeneic transplantation outcomes for patients with lymphoma. Biol Blood Marrow Transplant. 2019;25(7):1340-1346. doi: 10.1016/j.bbmt.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh N, Karmali R, Rocha V, et al. . Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34(26):3141-3149. doi: 10.1200/JCO.2015.66.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. . Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938-947. doi: 10.1182/blood-2015-09-671834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Variables Tested in Cox Proportional Hazards Regression Model for NHL
eTable 2. Baseline Characteristics of NHL Patients Receiving RIC/NMA Conditioning Regimens Followed by allo-HCT
eTable 3. Adjusted Risk of GVHD, NRM, and Progression/Relapse, by Conditioning Regimen
eTable 4. Propensity Score Matching Results
eTable 5. Subset Analysis Results by Conditioning Regimen
eTable 6. Causes of Death
eTable 7. CIBMTR Data Collection Forms


