Abstract
Membrane filtration is one of the most reliable methods for water treatment. However, wider application is limited due to biofouling caused by accumulation of microorganisms on the membrane surface. This report details a heatable carbon nanotube composite membrane with self-cleaning properties for sustainable recovery from biofouling. Microfiltration polycarbonate/carbon-nanotubes hybrid membranes were fabricated using drawable nanotubes that maintained the porosity and provided electrical conductivity to the membrane. Less than 25 V potential and 2–3 W power increase membrane temperature to 100°C in ~10 s. This temperature is above what most microbial life, bacteria and viruses can handle. When this membrane was employed, filtered Escherichia coli collected on its surface were successfully annihilated within 1 min. Ohmic heating of this membrane could be an effective solution to combat biofouling and complications associated with membrane-based filtration. This is a novel and highly desirable approach to combat biofouling, due to its simplicity and economic advantage.
Keywords: Carbon nanotubes, drawable and spinnable CNT, membranes, ohmic heating, biofouling, water and wastewater treatment, Escherichia coli
Introduction
Membrane filtration can provide effective separation of microorganisms such as bacteria and viruses from water and wastewater. However, biofouling of membranes is a major hurdle to wider applications of the membrane technology (Liao et al. 2004; Meng et al. 2009; Mansouri et al. 2010; Guo et al. 2012; Deng et al. 2016). The occurrence is a result of naturally arising microorganisms and bacteria aggregating to form a protective environmental biofilm. Within a biofilm, microorganism colonies can often thrive unharmed, shielded from the surrounding chemical environment. As a result, the protected population grows with increasing biofilm thickness. The three major categories of microorganisms involved, as listed by the US Environmental Protection Agency in accordance with the National Primary Drinking Water Regulations, are protozoan parasites (Cryptosporidium, Giardia lamblia), bacteria (Legionella) and viruses (USEPA 2009).
Biofouling of membranes is the result of continuous biofilm accumulation on the membrane surface. It is caused by many factors, including membrane surface properties such as roughness, surface charge and hydrophobicity (Vanysacker et al. 2014; Zhao et al. 2015). Biofouling is also affected by the operating conditions, such as flow velocity and applied pressure, as well as the chemical nature of the water, pH, ionic strength, multivalent cations and organic foulants present in the water (Zhao et al. 2015). Furthermore, the surface properties of the cells, such as surface charge and hydrophobicity, have significant impact in the dynamics of biofouling (Zhao et al. 2015). As a result of biofilm accumulation, membrane flux declines; therefore, feed pressure and energy consumption increases. These factors ultimately raise the cost of operating membranes.
Traditionally, keeping the filtration device in operation requires routine membrane cleaning using chemicals, which causes premature membrane degradation (Nguyen et al. 2012). However, the frequent use of cleaning chemicals increases costs (Srijaroonrat et al. 1999; Pidou et al. 2009) and shortens the life-time of polymeric membranes (Puspitasari et al. 2010). Minimizing biofouling can help alleviate the intensity and duration of cleaning processes. Examples of such processing include surface treatments, micro-texturing, grooming, sloughing and coating (Sondi and Salopek-Sondi 2004; Chae et al. 2009; Aslam et al. 2017).
Engineered nanomaterials (ENMs) will find numerous applications that will improve environmental technologies and help protect public health. These applications will include industrial separations, potable water supply, chemical synthesis, energy generation and transmission, ground water remediation and air quality control to name a few. In particular, carbon nanotubes (CNTs) are electrically conducting, large aspect ratio molecules that can be assembled into a filter format. Additionally, CNTs are chemically stable to 450°C temperatures in air and mechanically stronger than metals. By engineering long CNTs into a cross-hatch pattern and assembling them onto a polymeric membrane, a heatable composite membrane was made. This report details a heatable composite membrane that merges a microfiltration polycarbonate (PC) membrane with a porous carbon nanotube film. The polycarbonate (PC) membrane having average pore size of 1 μm and the heatable CNT film was made of a five-layer-thick screen of 450 μm long-aligned CNTs. The five layers were laid in a way that alternated their direction by 90°. The CNT film can reach surface temperatures upwards of 140°C within 10 s under < 25 V of potential and consuming 2–3 W of power. The PC membrane was able to reject bacteria efficiently and the CNT film was capable of destroying microbial life on the surface of the PC membrane via resistive heating without the use of any chemicals.
Experimental
Fabrication of porous carbon nanotube films
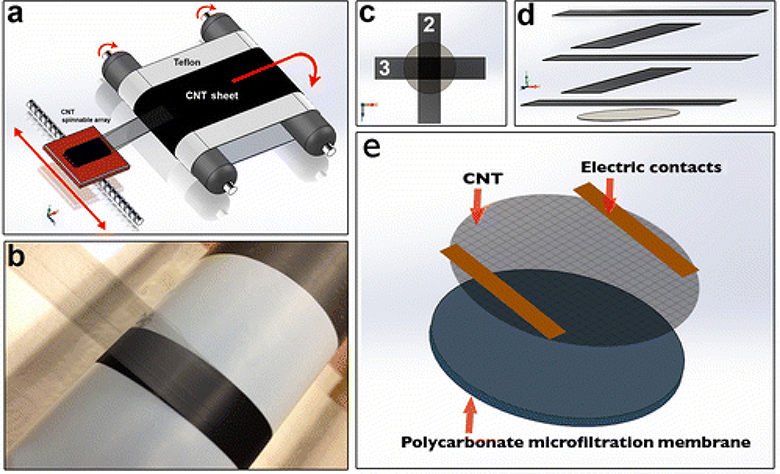
CNTs from vertically aligned, spinnable (also referred to as drawable) arrays were employed for fabricating the resistive heating membrane layer on the PC supporting membrane. Drawable CNTs have the ability to assemble into a porous film with the CNTs aligned in the direction of the drawing and can be continuously collected on a drum or belt as shown in Figure 1a (Alvarez et al. 2015; Wang et al. 2015). During the assembly process, porous CNT film was collected into a drum and the aligned CNT porous film was a transparent ribbon (Figure 1b). Catalyst-free drawable CNTs were synthesized using chemical vapor deposition (CVD); details of the method are published elsewhere (Alvarez et al. 2015). Drawable arrays were densely packed with vertically aligned CNT of ~0.45 mm long that self-assembled into films when drawn without the use of binders or additional chemicals. The CVD synthesis process uses a thin film catalyst of iron and cobalt, sputtered over a 5 nm aluminum oxide buffer layer of a 10.6 cm silicon wafer. Substrata with catalyst were loaded into a modified commercial CVD reactor ET3000 from CVD Equipment Corporation (Central Islip, NY, USA), and the growth process took place at 740 Torr and 750°C (Alvarez et al. 2015). The synthesis of vertically aligned drawable CNTs took 20 min, and they were about 450 μm thick. Typically, CNT film drawing starts at one edge of the CNT array and continues until the array is consumed at the opposite edge. Approximately each linear millimeter of CNT array allows a linear meter of ribbon to be drawn. The ribbon or film assembly processes, shown in Figure 1a, was done manually, although it could also be assembled via machine at speeds of up to 16 m s−1 by using collector drums connected to an electrical motor (Alvarez et al. 2015). In a similar manner shown in Figure 1b where the CNT thin film drawing was collected onto a Teflon™ layered drum, the CNT thin film was assembled onto a porous PC membrane.
Figure 1.
CNT/PC membrane assembly process: (a) scheme that shows CNT sheet being drawn from a spinnable array, collected on a Teflon™ belt. This process can be conducted at drawing speeds of 16 m s−1; (b) picture of the CNT sheet assembly and film collection on a Teflon™ drum; (c) arrangement and (d) assembly of five alternating perpendicular layers of CNT ribbon drawn and laid onto the surface of a polycarbonate microfiltration membrane (1 μm pore size). Three layers run along the conductive axis of alignment and complete the resistive heating circuit, while the other two layers run along the supportive axis and maintain the structure. Two pieces of copper tape served as electrodes and were placed on top; (e) graphic representation of CNT membrane assembly and components.
Composite membrane assembly
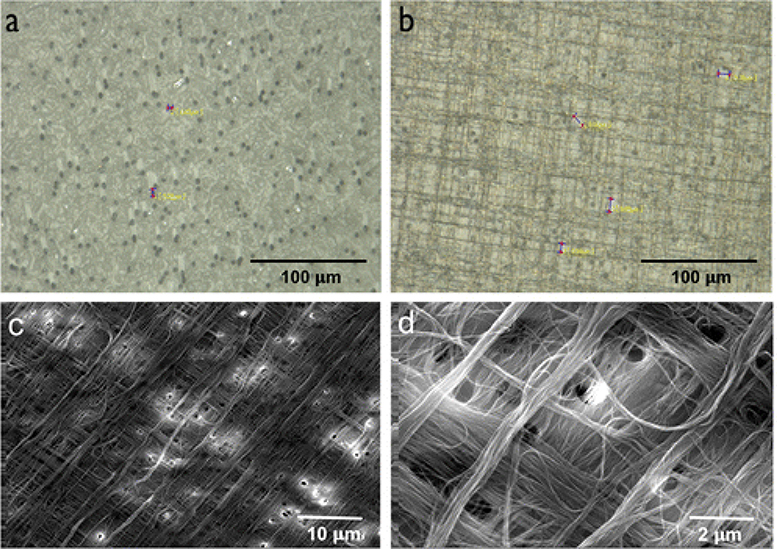
To build the resistive heating PC/CNT membrane, five layers of CNT film were drawn and placed manually on top of a polycarbonate microfiltration membrane (pore diameters of 1 μm) in a 90° cross-hatch pattern, as seen in Figure 1c. Each layer was laid perpendicular to the one below it, so that three layers ended up running along the resistive heating electrodes (direction of conduction) and two ran parallel to the electrodes (perpendicular) (Figure 1d). The cross-hatch patterned CNTs were assembled on the PC membrane surface as seen in Figure 1e. As a final step of the fabrication process, the entire hybrid membrane was wetted with ethanol in order to increase the adsorption of CNTs to the PC and improve densification, which occurred after solvent evaporation. Copper tape electrodes were spaced 20 mm apart at either end of the active heating membrane area (Figure 1e). After placing the electrodes, the membrane was trimmed to the active heating area, 20 mm × 16 mm, and the additional conductive CNT material added ~0.25 μm in thickness to the entire membrane device. A picture of the PC membrane at 500 × magnification is shown in Figure 2a, and its corresponding PC/CNT membrane assembly is displayed in Figure 2b. Low and high magnification SEM micrographs of ethanol densified membranes are shown in Figure 2c and Figure 1d, where the cross-hatch pattern is seen for the CNT bundles. In parallel assemblies, CNT surfaces were subjected to oxygen plasma (100 W and O2 flow rate 0.2 l min−1) to enhance the hydrophilicity of the CNT surface, as previously described (Malik et al. 2016). Hydrophilic PC membranes (PCT1047100, Sterlitech, Kent, WA, USA) were used as support substrata for the composite hybrid membrane. The original membranes were 47 mm in diameter, with average pore sizes of 1 μm, and allowed a maximum operating temperature of 140°C.
Figure 2.
Optical microscope images taken from the surface of the polycarbonate filtration membrane (1 μm pore size) as the alternating five CNT layers were applied perpendicularly and densified. Following a clockwise arrangement: (a) the original membrane surface; (b) after the first two layers were applied; (c) low and (d) high magnification SEM micrographs of ethanol densified membrane with five layers on a cross-hatch pattern employed as self-heating membranes.
Bacterial culture
Escherichia coli (ATCC 25922) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and rehydrated with 5 ml of tryptic soy broth (TSB) (Becton Dickinson, Franklin Lakes, NJ, USA). The rehydrated culture was inoculated onto tryptic soy agar (TSA) plates (Becton Dickinson). The plates were incubated overnight at 36.5 ± 1°C. Single colonies were transferred to 30 ml of TSB, and incubated overnight at 35°C at 100 rpm in a shaking incubator. The culture was centrifuged at 3,000 rpm for 5 min and the pellet was re-suspended with 30 ml of phosphate buffered saline (PBS) (Sigma-Aldrich). This culture and transfer processing is a standard procedure published elsewhere (APHA et al. 2012).
Resistive heating membrane application
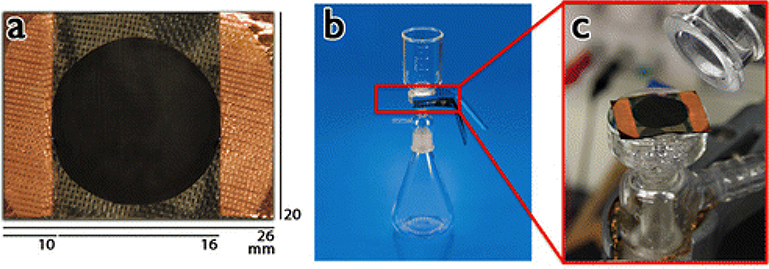
The membrane was assembled for filtering E. coli as shown in Figure 3a, which displays the components of the PC/CNT hybrid membrane including its contacts. The glass microfiltration membrane setup is shown in Figure 3b, and at higher magnification in Figure 3c. The E. coli suspension with a cell concentration of 1017 cc−1 was pipetted onto the surface of the membrane. Vacuum was applied to aid in the filtering process. Once the majority of water was filtered, the membrane was removed from the assembly and placed on a polylactic acid (PLA) 3-D-printed platform to facilitate drying and subsequent handling. In addition, copper tape was employed on the lid underside to make electrical contact with the CNT membrane. Alligator clips were used to provide power from an HP E3612A DC bench top power supply (120 V, 500 mA, 30 W).
Figure 3.
CNT/PC membrane filtration process: (a) membrane dimensions; (b) membrane location and E. coli filtering setup; (c) closer view of the CNT/PC membrane in the filtering setup.
Once the lid was in place, the power supply was turned on and the temperature was monitored using a FLIR T-640 infrared (IR) camera. Voltages of 20–22 V (varied slightly between samples) were applied for 60 s. The intention was to reach temperatures > 100°C to test the lethality of a 60-s application.
Bacterial viability
After heat treatment, the PC/CNT membranes were stained using the Live/Dead BacLight Bacterial viability kits (Life Technologies, Eugene, OR, USA). Briefly, equal aliquots of SYTO 9 and propidium iodide were applied onto the filters to stain the cells directly. The filters were incubated in the dark at room temperature for 15 min and then filtered under vacuum to remove the stain solution. The filters were mounted on a pre-cleaned microscope slide and a coverslip was placed over the BacLight mounting oil drop. The viability of cells was examined within 6 h using a fluorescence microscope (Olympus I × 51 inverted microscopy system, Olympus, Waltham, MA, USA) with green and red filters.
Results and discussion
Membrane characterization and performance
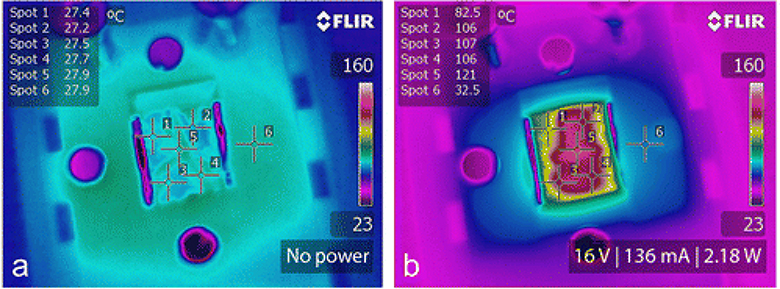
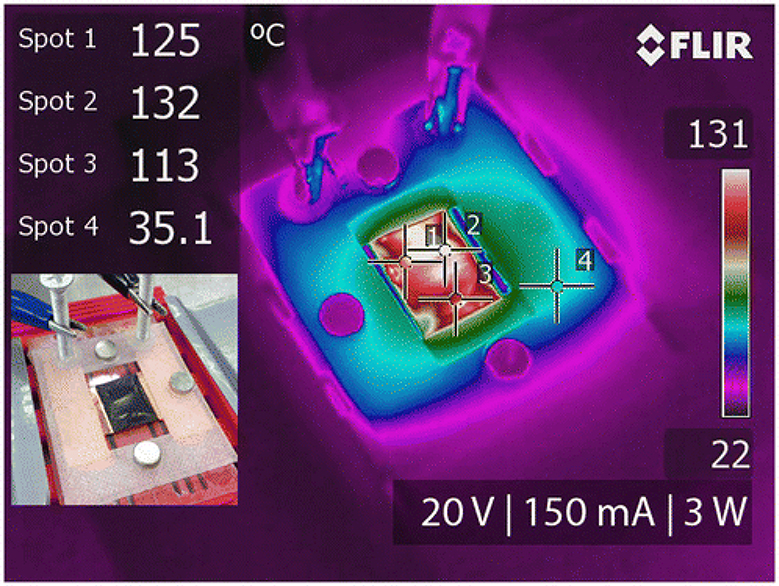
Application of long CNTs on membranes has its own advantages. The main benefit of drawable CNT arrays is that they facilitate the pore assembly of catalyst-free porous aligned CNTs films. They are well known for their chemical inertness at ambient temperature and can remain stable up to 450°C without oxidation. The synthesis and assembly process ensures a higher level of control over the purity of the electrically conducting material. The PC membranes were chosen for stability at high temperatures, and the level of adhesion that CNTs display on them. CNT cross-hatch patterns could be applied on other materials besides PC. An IR image of a dry membrane before applying electrical potential demonstrated that the surface temperature was equivalent to the ambient (Figure 4a). After 16 V were applied to the CNT/PC membrane, surface temperatures in the range of 105–121°C were reached (Figure 4b). At that voltage, the dry membrane drew 136 mA of current, consumed 2.18 W of power and demonstrated a resistivity of 3.66 × 10−5 Ωm. Several different membranes were assembled and tested with varying layers of CNT films, in order to determine the minimum number of CNT layers needed to provide lethal temperatures to E. coli while still allowing liquid to pass through. Membranes with 3–5 layers were tested, and higher temperatures were achieved with five layers while maintaining the same voltage (14 V) (Table 1). This observation can be explained by the reduction of electrical resistance as the number of layers increased. In addition, membranes after functionalization typically displayed higher resistivity, therefore even though the same voltage was applied, hydrophilic membranes reached lower temperatures compared to their hydrophobic counterparts. Although the performance varied slightly due to the extremely thin geometry of the five-layered CNT porous sheet, when 20 V were applied to the filter, it drew 150 mA, had a resistivity of ~4.16 × 10−5 Ωm and consumed 3.0 W of power, which was enough to bring the surface temperature up to 110–135°C within the first 10 s. During bacterial testing, the wetted PC/CNT membrane was powered for 60 s and resulted in surface temperatures ranging from 110 to 140°C, as shown in the IR image in Figure 5.
Figure 4.
IR photography of PC/CNT composite microfiltration membrane, comparing dry surface temperature before (a) and after (b) the application of 16 V, which drew 136 mA and consumed 2.18 W of power to generate surface temperatures > 100°C within 10 s of turning on power. Six different spots were measured to evaluate the uniformity and compare to non-heated surface.
Table 1.
Characterization of three, four and five CNT-layer membranes comparing the temperatures achieved as a function of membrane thickness (number of layers) and the functionalization where hydrophobic refers to the membrane in its pristine state while hydrophilic refers to a similar membrane after plasma functionalization.
| CNT layers | Type of CNTs | Current (mA) | Power (W) | Temp (°C) |
|---|---|---|---|---|
| 3 | Hydrophobic | 79 | 1.11 | 94 |
| Hydrophilic | 74 | 1.04 | 74 | |
| 4 | Hydrophobic | 125 | 1.75 | 108 |
| Hydrophilic | 89 | 1.25 | 87 | |
| 5 | Hydrophobic | 121 | 1.69 | 132 |
Note: Current between the electrodes and power consumed increased with the number of layers.
Figure 5.
IR photography of the PC/CNT composite microfiltration membrane in operation. After application of aqueous E. coli suspension, 20 V applied drew 150 mA, consumed 3 W and reached surface temperatures in the range of 113–132°C as shown by temperatures measured at spots 1 to 4. The power was applied for 60 s.
Composite membrane effectiveness
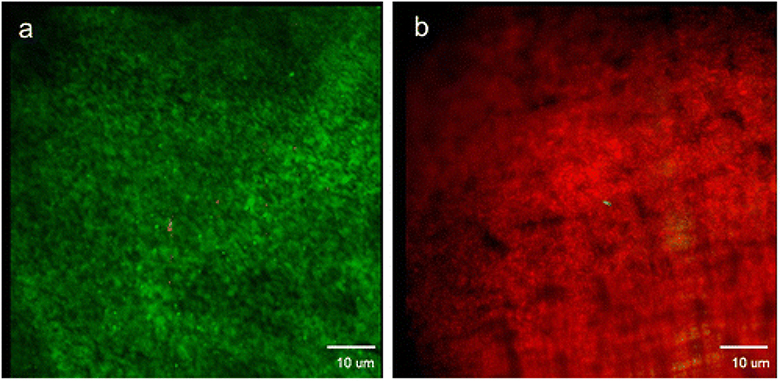
As displayed in Figure 6a, E. coli on the CNT/PC membrane were mostly alive before applying the ohmic heating. However, after ohmic heating for 60 s, most E. coli cells on the CNT-enhanced membrane were no longer viable (Figure 6b). This test confirmed the significance and effectiveness of the membrane in eliminating biofilms.
Figure 6.
Live/Dead fluorescent staining microscopy of E.coli. Green appears where bacterial outer membranes remained intact (live) and red appears where outer membrane damage has occurred (dead). E. coli on the PC/CNT composite membrane before heating (a) and E. coli on the PC/CNT composite membrane after operating the resistive heating circuit for 30 s (b).
The results demonstrated that the hybrid membrane provided an effective method for combating biofouling. The five-layered CNT film between the two copper electrodes allowed an electric current to run across the membrane and to power a resistive heater that was capable of completely destroying the accumulated E. coli. The membrane surface temperature reached > 100°C within 10 s. Final temperatures of 100–140°C were recorded with the IR camera after 60 s of operation. Reaching these temperatures consumed 2.6–3.0 W of power, when applying 20–22 V potential. The approach described could be successfully applied to current filtration technology to combat biofouling, which would avoid flushing the water treatment systems with harsh chemical cleaners. In addition to heat treatments, the electrical conductivity of the CNT material could be used to help absorb/discharge metal ion contaminants in the water by applying a slight potential across the membrane.
The work presented a successful prototype of heatable CNT composite membrane with self-cleaning properties for recovery from biofouling. There are further opportunities to optimize porosity of the membrane as a function of the CNT layers, membrane connection design, and surface functionalization of the CNT side of hybrid membrane. Plasma functionalization is a surface treatment method that can potentially help create active groups such as carboxyl, hydroxyl, and/or amine. However, these initial results showed very distinctly that CNTs can be used to help eliminate E. coli quickly and efficiently. A plasma functionalization step was applied to make the CNT self-heating membrane more hydrophilic, particularly when the number of CNT layers was high. Hydrophilicity helped the CNT filter to be well-wetted by passing fluid, thus allowing intimate contact between the CNT and the microorganisms in the processed water. The latter was important for heat transfer and self-cleaning when the filter heating mode was applied.
Effect of membrane and CNTs on microbial life
Despite the advantages of CNTs, their potential toxicity must also be considered in the design of CNT membranes. CNTs are classified as toxic to aquatic organisms, including crustaceans, bacteria, algae, and ciliates, with half maximal effective concentrations (EC50) or median lethal concentrations (LC50) of 1.04–167 mg l−1 for single-walled CNTs (SWCNT) and 8.7–500 mg l−1 for multi-walled CNTs (MWCNT) (Kahru and Dubourguier 2010). In this study, the CNTs were confined on the PC membrane surface, thus preventing leaching. This is expected due to their large aspect ratio and Van der Waals attraction among the long CNTs (~450 μm) employed in the enhanced composite membrane. Additionally, CNTs ran across many pores on the PC membrane, which probably created enough friction to make it unlikely for a 450 μm long CNT to be driven into a 1 μm diameter pore. That is not the case for powdered CNT, as reported in the literature, where the effect on human health remains unclear and CNTs are typically dispersed in solution. Cognizant of these concerns, even though no visible contaminant precipitates were observed, ecotoxicity data guidelines should be followed to evaluate the potential of CNT leaching into the water stream (Lin and Xing 2008; Cerrillo et al. 2015). Ultimately, a more vigorous fabrication method that eliminates any CNT leaching is needed; further research would include developing a robust attachment that can permanently anchor or ‘fix’ CNTs onto polymeric membrane substrata.
Besides destroying harmful microbial contaminants and biofilms, due to the large surface area of the CNT, the PC/CNT membranes have the potential to capture heavy metals as well as emerging contaminants including pharmaceutical and personal care products (PPCPs) and endocrine disrupting compounds (EDCs). While toxic heavy metals have long been considered targets for water treatment, occurrences of ‘emerging’ contaminants (eg EDCs and PPCPs) have not been of concern until recent advances in sensitive analytical techniques were established and relevant toxicology information obtained. Their continued persistence in the public water supply requires new technologies to help remove them, and these aspects are under investigation.
Conclusions
A resistive PC/CNT hybrid membrane is a simple approach to eliminating biofouling on membrane surfaces. Different CNT assembly parameters were studied, such as the number of CNT ribbon layers, the voltage and the current required to generate enough heat for eradicating bacteria. E. coli cells filtered through the PC/CNT hybrid membrane were exposed to temperatures > 100°C to evaluate their viability. The PC/CNT membrane, also called heatable membrane with self-cleaning properties, successfully eliminated an estimated 99% of the E. coli in about 60 s, which allowed this membrane to be re-used without significant filtering system downtime. The process offers reduced operation costs, minimizes the use of chemical cleaners, and, most importantly, contributes to membrane technology applications. Effective temperatures exceeding 100°C lethal to E. coli were achieved at a low voltage of 20–25 V and a power consumption of 2.6–3 W.
Acknowledgements
The US Environmental Protection Agency, through its Office of Research and Development, partially funded and managed the research described herein. This work has been subjected to the agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the agency; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use. We also thank Natalie Ochman for her proof reading assistance.
Funding
This work is supported by the Ohio Water Resources Center (WRC) [grant number 2017OH518B]. This work was also partially funded by the National Science Foundation (NSF) [grant numbers CMMI-0727250, SNM-1120382, ERC-0812348]. The authors appreciate the support of [grant number ONR DURIP N00014-15-1-2473], ARMY AMC W911NF-16-2-0026, UC strategic collaborative funding for sensor development, UC Interdisciplinary Faculty Collaboration Awards and NASA NNX13AF46A grants and The US Environmental Protection Agency, through its Office of Research and Development.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Alvarez NT, Miller P, Haase M, Kienzle N, Zhang L, Schulz MJ, Shanov V. 2015. Carbon nanotube assembly at near-industrial natural-fiber spinning rates. Carbon. 86:350–357. doi: 10.1016/j.carbon.2015.01.05810.1016/j.carbon.2015.01.058 [DOI] [Google Scholar]
- 2.[APHA] American Public Health Association, AWWA, WEFW. 2012. Standard methods for the examination of water and wastewater. 22nd ed. Washington (DC): APHA. [Google Scholar]
- 3.Aslam M, Charfi A, Lesage G, Heran M, Kim J. 2017. Membrane bioreactors for wastewater treatment: a review of mechanical cleaning by scouring agents to control membrane fouling. Chem Eng J. 307:897–913. doi: 10.1016/j.cej.2016.08.14410.1016/j.cej.2016.08.144 [DOI] [Google Scholar]
- 4.Cerrillo C, Barandika G, Igartua A, Areitioaurtena O, Marcaide A, Mendoza G. 2015. Ecotoxicity of multiwalled carbon nanotubes: standarization of the dispersion methods and concentration measurements. Environ Toxicol Chem. 34:1854–1862. doi: 10.1002/etc.v34.810.1002/etc.v34.8 [DOI] [PubMed] [Google Scholar]
- 5.Chae S, Wang S, Hendren ZD, Wiesner MR, Watanabe Y, Gunsch CK. 2009. Effects of fullerene nanoparticles on Escherichia coli K12 respiratory activity in aqueous suspension and potential use for membrane biofouling control. J Membr Sci. 329:68–74. doi: 10.1016/j.memsci.2008.12.02310.1016/j.memsci.2008.12.023 [DOI] [Google Scholar]
- 6.Deng LJ, Guo WS, Ngo HH, Zhang HW, Wang J, Li JX, Xia SQ, Wu Y. 2016. Biofouling and control approaches in membrane bioreactors. Bioresour Technol. 221:656–665. doi: 10.1016/j.biortech.2016.09.10510.1016/j.biortech.2016.09.105 [DOI] [PubMed] [Google Scholar]
- 7.Guo WS, Ngo HH, Li JX. 2012. A mini-review on membrane fouling. Bioresour Technol. 122:27–34. doi: 10.1016/j.biortech.2012.04.08910.1016/j.biortech.2012.04.089 [DOI] [PubMed] [Google Scholar]
- 8.Kahru A, Dubourguier HC. 2010. From ecotoxycology to nanoecotoxycology. Toxycology. 269:105–119. [DOI] [PubMed] [Google Scholar]
- 9.Liao BQ, Bagley DM, Kraemer HE, Leppard GG, Liss SN. 2004. A review of biofouling and its control in membrane separation bioreactors. Water Environ Res. 76:425–436. doi: 10.2175/106143004X15152710.2175/106143004X151527 [DOI] [PubMed] [Google Scholar]
- 10.Lin D, Xing B. 2008. Tannin acid adsorption and its role for stabilizing carbon nanotube suspensions. Environ Sci Technol. 42:5917–5923. doi: 10.1021/es800329c10.1021/es800329c [DOI] [PubMed] [Google Scholar]
- 11.Malik R, McConnell C, Alvarez NT, Haase M, Gbordzoe S, Shanov V. 2016. Rapid, in situ plasma functionalization of carbon nanotubes for improved CNT/epoxy composites. RSC Adv. 6:108840–108850. doi: 10.1039/C6RA23103A10.1039/C6RA23103A [DOI] [Google Scholar]
- 12.Mansouri J, Harrisson S, Chen V. 2010. Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem. 20:4567–4586. doi: 10.1039/b926440j10.1039/b926440j [DOI] [Google Scholar]
- 13.Meng FG, Chae SR, Drews A, Kraume M, Shin HS, Yang FL. 2009. Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res. 43:1489–1512. doi: 10.1016/j.watres.2008.12.04410.1016/j.watres.2008.12.044 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T, Roddick FA, Fan L. 2012. Biofouling of water treatment membranes: a review of the underlying causes, monitoring techniques and control measures. Membranes. 2:804–840. doi: 10.3390/membranes204080410.3390/membranes2040804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pidou M, Parsons SA, Raymond G, Jeffrey P, Stephenson T, Jefferson B. 2009. Fouling of a membrane coupled photocatalytic process treating greywater. Water Res. 43:3932–3939. doi: 10.1016/j.watres.2009.05.03010.1016/j.watres.2009.05.030 [DOI] [PubMed] [Google Scholar]
- 16.Puspitasari V, Granville A, Le-Clech P, Chen V. 2010. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep Purif Tech. 72:301–308. doi: 10.1016/j.seppur.2010.03.00110.1016/j.seppur.2010.03.001 [DOI] [Google Scholar]
- 17.Sondi I, Salopek-Sondi B. 2004. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 275:177–182. doi: 10.1016/j.jcis.2004.02.01210.1016/j.jcis.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 18.Srijaroonrat P, Julien E, Aurelle Y. 1999. Unstable secondary oil/water emulsion treatment using ultrafiltration: fouling control by backflushing. J Membr Sci. 159:11–20. doi: 10.1016/S0376-7388(99)00044-710.1016/S0376-7388(99)00044-7 [DOI] [Google Scholar]
- 19.[USEPA] U.S. Environmental Protection Agency. 2009. National primary drinking water regulation table. EPA 816-F-09–004. Washington (DC): Office of Ground Water and Drinking Water; Available from: https://www.epa.gov/sites/production/files/2015-11/documents/howeparegulates_mcl_0.pdf [Google Scholar]
- 20.Vanysacker L, Boerjan B, Declerck P, Vankelecom IFJ. 2014. Biofouling ecology as a means to better understand membrane biofouling. Appl Microbiol Biotechnol. 98:8047–8072. doi: 10.1007/s00253-014-5921-210.1007/s00253-014-5921-2 [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Zhao D, Alvarez N, Shanov VN, Heineman WR. 2015. Optically transparent carbon nanotube film electrode for thin layer spectroelectrochemistry. Anal Chem. 87:9687–9695. doi: 10.1021/acs.analchem.5b0178410.1021/acs.analchem.5b01784 [DOI] [PubMed] [Google Scholar]
- 22.Zhao F, Xu K, Ren H, Ding L, Geng J. 2015. Combined effects of organic matter and calcium on biofouling of nanofiltration membranes. J Membr Sci. 486:177–188. doi: 10.1016/j.memsci.2015.03.03210.1016/j.memsci.2015.03.032 [DOI] [Google Scholar]