Abstract
Objectives
We investigated performance of antenatal pelvic floor muscle training (PFMT) among Chinese pregnant women, to explore its effects on postpartum stress urinary incontinence (SUI).
Methods
We conducted a prospective cohort study in Shenzhen, China among 815 singleton pregnant women age ≥18 years, who were continent before pregnancy. Telephone follow-up was conducted at 6 weeks postpartum. Logistic univariable and multivariable regression analyses were used to estimate effects of antenatal PFMT (frequency and duration) on SUI postpartum among subgroups defined by SUI during pregnancy. The interactions of antenatal PFMT and PFMT duration on SUI postpartum were tested.
Results
Among 798 women included in the analysis, 127 (15.91%) had SUI at 6 weeks postpartum. Only 157 (19.67%) women performed antenatal PFMT, none under supervision. After adjusting potential confounders, neither frequency (odds ratio (OR) = 1.08, 95% confidence interval (CI) 0.89–1.32) nor duration (OR = 1.03, 95% CI 0.87–1.23) of antenatal PFMT was a significant factor in postpartum SUI. No interactions of antenatal PFMT and PFMT duration on SUI postpartum were found in any participants or subgroups.
Conclusion
No effect of self-reported, unsupervised, self-initiated antenatal PFMT on SUI 6 weeks postpartum was found. Low doses and no supervision may have contributed to the negative results.
Keywords: Pelvic floor muscle training, stress urinary incontinence, pregnancy, postpartum-associated factors, antenatal care, China
Introduction
Urinary incontinence is defined as the complaint of involuntary loss of urine.1 Although urinary incontinence is a non-fatal condition, it seriously affects patients’ quality of life, as well as sexual function and marital relationships.2,3 Pregnancy and delivery are strong risk factors for urinary incontinence. Physiological changes during pregnancy and the delivery process have a damaging effect on the structure and function of the pelvic floor complex, which can result in a wide range of symptoms of pelvic floor dysfunction including urinary incontinence.4 A review by Thom and Rortveit5 reported that during the first 3 months postpartum, the pooled prevalence of urinary incontinence was 33%.
Stress urinary incontinence (SUI) is the most common type of postpartum urinary incontinence, accounting for about 70% of all cases of incontinence.6 The reported prevalence of SUI is nearly 19% shortly after delivery (around 6 weeks postpartum).7,8 Follow-up studies have shown that the onset of SUI during pregnancy or shortly after delivery significantly increased the risk of persistent symptoms at 12 to 15 years postpartum.9,10 Therefore, SUI during or after pregnancy requires close attention and must be improved.
The only conservative intervention currently considered effective for the treatment of SUI is pelvic floor muscle training (PFMT).11 The mechanisms responsible for the efficacy of PFMT are believed to be that PFMT can change neuromuscular function and morphology to enable automatic pelvic floor muscle contraction.12 However, two problems exist with use of PFMT to treat urinary incontinence. First, although PFMT has been shown to be effective in treating postpartum urinary incontinence,13 its effectiveness is dependent on the right approach and good compliance. In randomized controlled trials (RCTs), PFMT is supervised by a physiotherapist, who usually conducts intensive intervention for at least 8 to 12 weeks.14 However, in clinical practice, because of limited conditions and resources, instruction in PFMT is usually conducted via verbal education and written manuals. Therefore, the effect of unsupervised antenatal PFMT in the clinical environment needs further verification. Second, few studies have focused on the performance of antenatal PFMT among Chinese pregnant women, and the current research and guidance lack evidence in the Chinese population. Therefore, the aims of this study were to 1) investigate the performance of antenatal PFMT among Chinese pregnant women and 2) explore the association of antenatal PFMT and postpartum SUI. Findings from this study are expected to provide real-world evidence regarding the value of unsupervised antenatal PFMT for postpartum SUI, to help in decision making regarding perinatal pelvic floor health services.
Methods
Participants and design
In the present prospective cohort study, pregnant women attending prenatal clinics were recruited conveniently at Shenzhen Maternal and Child Healthcare Hospital from July 2016 to July 2017 and from Shenzhen Hospital of Southern Medical University from January 2017 to July 2017. Inclusion criteria were: (a) women with a singleton pregnancy and (b) age 18 years or above. Exclusion criteria were: (a) urinary incontinence before pregnancy; (b) history of abdominal or vaginal surgery; (c) diabetes or hypertension, and (d) placenta previa, threatened abortion, amniotic fluid abnormalities, fetal growth restriction, or vaginal bleeding.
On arrival at the clinic, eligible participants were verbally invited to participate in the study. Invited participants received an explanation of the research. Written informed consent was obtained from all women who wish to participate in the study. Prior to data collection, ethical approvals were obtained from the Ethics Committees of ShenZhen Hospital of Southern Medical University (no. NYSZYYEC20170014) and ShenZhen Maternal and Child Health Care Hospital (no. 2016-30).
Data collection
Pregnancy-related data were collected at the time of inclusion using a self-report questionnaire (questionnaire 1), including maternal age, height, pre-pregnancy weight, number of pregnancies, prior abortions/miscarriages, and delivery history. Delivery information, including gestational weeks, the mode of delivery, episiotomy, and neonatal weight were obtained by checking the electronic medical records system after delivery. Women who experienced abortion or miscarriage in their previous pregnancy were not followed up further. Telephone follow-up was performed at 6 weeks postpartum by a trained midwife who was not involved in this study. Structured interviews were conducted using another self-reported questionnaire (questionnaire 2), to obtain information including current weight, constipation during the previous pregnancy, performance of PFMT during the previous pregnancy, and SUI during or after the previous pregnancy. SUI during or after the previous pregnancy was present if the woman answered affirmatively to the question “During or after your previous pregnancy, did you experience involuntary loss of urine on effort or physical exertion (e.g., sporting activities) or on sneezing or coughing?”1 Performance of antenatal PFMT was assessed using structured questions: “Did you exercise the pelvic floor muscles (muscles around the urethra, vagina, and rectum) during your previous pregnancy?”15 If the woman answered yes, she was asked three further questions: “Was antenatal PFMT performed under supervision of a health care professional?”, “What was the frequency of antenatal PFMT?”, and “How long did you perform antenatal PFMT”. We invited six experts (one obstetrician, three midwives, two physical therapists, and one research nurse) to evaluate the appropriateness and validity of questionnaires 1 and 2. Content validity was assessed using the item content validity index (I-CVI). Every I-CVI of questionnaire 1 was 1.00, and each I-CVI of questionnaire 1 was above 0.80, indicating good expert validity.16
Data analysis
Descriptive statistics are shown as mean ± SD or frequency and percentage. Comparisons between groups by frequency and duration were analyzed using a Student t-test for continuous variables and chi-squared test for categorical data. Univariable analysis was conducted to identify potential confounders of the associations between antenatal PFMT and postpartum SUI. Variables that were significant in the univariable analyses were entered into a multivariable logistic regression model with backward elimination to identify independent associations between antenatal PFMT (frequency and duration) and SUI postpartum. The odds ratios (ORs) and 95% confidence intervals (95%CIs) of antenatal PFMT on SUI at 6 weeks postpartum according to PFMT duration were estimated for all participants, and for subgroups as defined by SUI during pregnancy. The stratification of PFMT duration depended on the data distribution. Interactions of antenatal PFMT and PFMT duration with SUI at 6 weeks postpartum were also tested.
The frequency and duration of antenatal PFMT were presented using Microsoft Excel. R software version 3.4.3 (The R Project for Statistical Computing, Vienna, Austria) and EmpowerStats (X&Y Solutions, Inc., Boston, MA, USA) were used for all other statistical analyses. All statistical tests were two-tailed, and the level of significance was set at 0.05.
Results
Participant selection
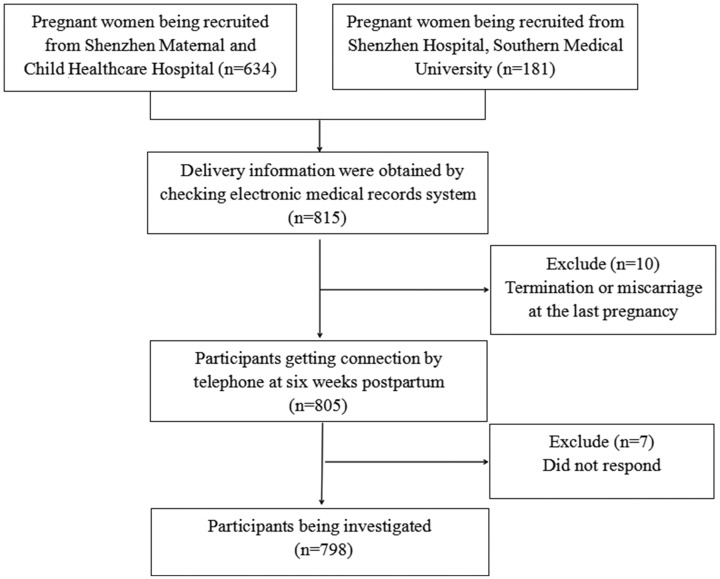
We enrolled 634 women from Shenzhen Maternal and Child Healthcare Hospital, and 181 women from Shenzhen Hospital of Southern Medical University. A final total of 798 participants were included in the analysis. Figure 1 shows the participant selection flowchart. Mean age of the study group was 30.4 ± 4.1 years (range 20 to 46 years), among which 250, 260, and 288 were in their first, second, and third trimester, respectively.
Figure 1.
Flow chart of participant inclusion.
Frequency and duration of reported practice of antenatal PFMT
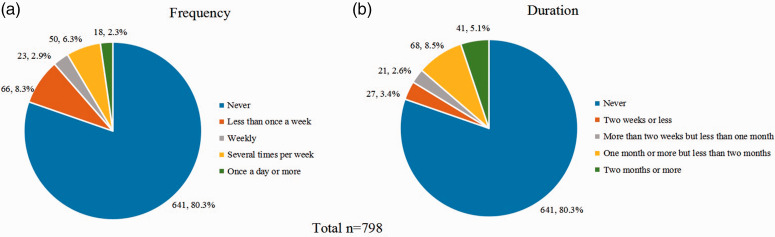
Only 19.67% (157/798) of pregnant women reported practicing antenatal PFMT, none of whom did so under supervision. The frequency and duration of reported practice of antenatal PFMT are presented in Figure 2. Only 19.67% (157/798) of women reported that they performed PFMT during pregnancy, among which 26.1% (41/157) did so for 2 months or more.
Figure 2.
Frequency and duration of reported practice of antenatal pelvic floor muscle training (PFMT).
Comparison of pregnant women according to PFMT performance
Table 1 shows a comparison of participant characteristics according to whether they performed antenatal PFMT. No significant differences were found among the background variables of pregnant women in this study.
Table 1.
Comparison of characteristics between pregnant women who did and did not perform PFMT (N = 798).
| Variables | Total (n = 798) |
Performed antenatal PFMT |
||
|---|---|---|---|---|
| No (n = 641) | Yes (n = 157) | P-value | ||
| Maternal age (years) | 30.42 ± 4.15 | 30.39 ± 4.03 | 30.56 ± 4.62 | 0.642a |
| Education | 0.536b | |||
| Junior high school or less | 288 (36.09) | 228 (35.57%) | 60 (38.22%) | |
| Senior high school or more | 510 (63.91) | 413 (64.43%) | 97 (61.78%) | |
| Household income per month (RMB) | 0.198b | |||
| Less than 10000 | 160 ( 20.05) | 136 (21.22%) | 24 (15.29%) | |
| 10000–20000 | 395 (49.50) | 316 (49.30%) | 79 (50.32%) | |
| More than 20000 | 243 (30.45) | 189 (29.49%) | 54 (34.39%) | |
| Pre-pregnancy BMI (kg/m2) | 20.96 ± 3.05 | 20.90 ± 2.75 | 21.21 ± 4.07 | 0.257a |
| Number of pregnancies | 1.99 ± 1.07 | 2.00 ± 1.07 | 1.92 ± 1.07 | 0.358a |
| History of delivery | 0.906b | |||
| Nulliparous | 431 (54.01) | 344 (53.67%) | 87 (55.41%) | |
| Cesarean delivery | 122 (15.29) | 98 (15.29%) | 24 (15.29%) | |
| Vaginal delivery | 245 (30.70) | 199 (31.05%) | 46 (29.30%) | |
| History of abortion/miscarriage (yes) | 279 (34.96) | 231 (36.04%) | 48 (30.57%) | 0.198b |
| Constipation during pregnancy (yes) | 205 (25.69) | 159 (24.80%) | 46 (29.30%) | 0.248b |
| SUI during pregnancy (yes) | 267 (33.46) | 212 (33.07%) | 55 (35.03%) | 0.641b |
Qualitative values are presented as frequency (%). Data presented as mean with standard deviation (SD) and number (n) with percentage (%).
t. bχ2.
Abbreviations: BMI, body mass index; SUI, stress urinary incontinence; PFMT, pelvic floor muscle training.
Analyses of antenatal PFMT frequency and duration for postpartum SUI
SUI prevalence was 15.91% (127/798) at 6 weeks postpartum. Results of univariable analyses for postpartum SUI are shown in Table 2. Maternal age (P < 0.001), number of pregnancies (P < 0.001), vaginal delivery compared with nulliparous women(P < 0.001), history of abortion or miscarriage (P < 0.001), constipation during pregnancy (P = 0.007), SUI during pregnancy (P < 0.001), and current vaginal delivery (P < 0.001) were found to be predictors of postpartum SUI in univariable analyses. Neither frequency (OR = 1.08, 95%CI, 0.89–1.32) nor duration (OR = 1.03, 95%CI, 0.87–1.23) of antenatal PFMT was found to be a significant factor for SUI at 6 weeks postpartum.
Table 2.
Univariable and multivariable logistic regression for SUI at 6 weeks postpartum (N=798).
| Variables |
Univariable analysis |
Multivariable analysisa |
||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Maternal age (years) | 1.08 (1.03, 1.13) | <0.001 | N/A | N/A |
| Education (senior high school or more) | 1.05 (0.71, 1.55) | 0.814 | ||
| Household income per month (RMB) b | 0.95 (0.74, 1.21) | 0.655 | ||
| Pre-pregnancy BMI (kg/m2) | 1.06 (1.00, 1.12) | 0.057 | N/A | N/A |
| Number of pregnancies | 1.47 (1.25, 1.73) | <0.001 | N/A | N/A |
| History of delivery | ||||
| Nulliparous | 1.0 | |||
| Cesarean delivery | 1.23 (0.67, 2.26) | 0.498 | N/A | N/A |
| Vaginal delivery | 2.89 (1.91, 4.38) | <0.001 | N/A | N/A |
| History of abortion/miscarriage | 2.15 (1.47, 3.16) | <0.001 | N/A | N/A |
| Constipation during pregnancy | 1.75 (1.17, 2.63) | 0.007 | N/A | N/A |
| SUI during pregnancy | 5.21 (3.47, 7.81) | <0.001 | N/A | N/A |
| Obstetrics-related variable | ||||
| Gestational age at delivery (weeks) | 0.97 (0.84, 1.11) | 0.610 | N/A | N/A |
| Delivery mode (VD) | 2.26 (1.43, 3.55) | <0.001 | N/A | N/A |
| Episiotomy | 1.33 (0.79, 2.24) | 0.282 | N/A | N/A |
| Neonatal weight (kg) | 1.00 (1.00, 1.00) | 0.759 | N/A | N/A |
| Antenatal PFMT | ||||
| Frequency | 1.10 (0.91, 1.31) | 0.329 | 1.08 (0.89, 1.32) | 0.450 |
| Duration | 1.04 (0.89, 1.21) | 0.659 | 1.03 (0.87, 1.23) | 0.703 |
Adjusted for maternal age, pre-pregnancy BMI, delivery history, number of pregnancies, abortion/miscarriage history, constipation and SUI during pregnancy, and mode of delivery at this birth.
Less than 10,000 RMB used as reference.
Abbreviations: BMI, body mass index; CI, confidence interval; N/A, unavailable; OR, odds ratio; SUI, stress urinary incontinence; PFMT, pelvic floor muscle training; VD, vaginal delivery.
Effects of antenatal PFMT frequency and duration on postpartum SUI
We divided PFMT frequency into two categories: weekly or less, and more than weekly. PFMT duration was classified as training for less than 1 month, and 1 month or more. There was no evidence that antenatal PFMT with a frequency of more than weekly has an influence on SUI at 6 weeks postpartum, even in pregnant women who practiced for more than 1 month. No interactions of antenatal PFMT (weekly or less vs. more than weekly) and PFMT duration (less than 1 month vs. 1 month or more) on SUI at 6 weeks postpartum were found, for all participants and for subgroups defined according to experience of SUI during pregnancy (Table 3).
Table 3.
Effects of antenatal PFMT on SUI at 6 weeks postpartum stratified by PFMT duration for all participants, and subgroups as defined by SUI during pregnancy.
| No. participants | OR (95%CI)a | P-value | P for interactionb | |
|---|---|---|---|---|
| All participants (n = 798) | ||||
| PFMT duration | 0.632 | |||
| Less than 1 month | 689 | 1.44 (0.54, 3.82) | 0.468 | |
| One month or more | 109 | 0.70 (0.16, 3.14) | 0.644 | |
| SUI during pregnancy (yes) (n = 267) | ||||
| PFMT duration | 0.516 | |||
| Less than 1 month | 230 | 2.37 (0.67, 8.35) | 0.180 | |
| One month or more | 37 | 0.28 (0.01, 5.54) | 0.401 | |
| SUI during pregnancy (no) (n = 531) | ||||
| PFMT duration | 0.967 | |||
| Less than 1 month | 459 | 0.59 (0.07, 5.26) | 0.637 | |
| One month or more | 72 | 0.00 (0.00, Inf)§ | 0.998 | |
For all participants, adjusted for confounders of maternal age, pre-pregnancy BMI, delivery history, number of pregnancies, abortion/miscarriage history, constipation and SUI during pregnancy, and mode of delivery at this birth. For subgroups, adjusted for the abovementioned confounders except SUI during pregnancy.
Interactions of antenatal PFMT (weekly or less vs. more than weekly) and PFMT duration (less than 1 month vs. 1 month or more) on SUI at 6 weeks postpartum were tested.
The model failed because of the small sample size.
Abbreviations: CI, confidence interval; OR, odds ratio; SUI, stress urinary incontinence; PFMT, pelvic floor muscle training.
Discussion
In the current study, the prevalence of SUI at 6 weeks postpartum was 15.91% (127/798). Several studies in China have focused on SUI or urinary incontinence in the postpartum period. The current findings were consistent with those of a study in Taiwan17 reporting an SUI prevalence of 12.5% (70/560) at 12 months postpartum. A study investigating primiparous women in Hong Kong7 reported an 18.6% (61/328) prevalence of SUI at 8 weeks postpartum. In the current study, 10.9% (47/431) of primiparous women reported that they experienced SUI after delivery at 6 weeks postpartum. The prevalence rates of postpartum urinary incontinence reported in limited studies among primiparous women on the Chinese mainland ranged from 5.0% to 8.0% between 6 weeks to 6 months postpartum,6,18 which are lower than the data in our study. In addition, we found that 21.8% (80/367) of multiparous women in this study experienced SUI at 6 weeks after delivery. Specifically, in our study, multiparous women who previously underwent vaginal delivery are more likely to develop SUI at 6 weeks postpartum than primiparous women (OR = 2.89, 95%CI, 1.91–4.38). Moreover, the risk of SUI at 6 weeks postpartum among women who experienced SUI during pregnancy was 5.21 times that of women who did not experience SUI during pregnancy. The present results demonstrated that SUI during pregnancy is a significant risk factor for SUI postpartum, which is comparable with the results of previous studies.9,10,19,20 Other studies6,7,17,18 have reported that maternal age, pre-pregnancy BMI, abortion or miscarriage history, constipation, and vaginal delivery are also predictors of postpartum SUI; therefore, these should be treated as confounding factors when investigating the effect of antenatal PFMT on postpartum SUI.
The National Institute for Clinical Excellence recommends that all women practice PFMT during their first pregnancy to prevent SUI (Grade A).21 Many studies have explored the effect of antenatal PFMT;22–26 however, to the best of our knowledge, little empirical work has been published regarding the performance of antenatal PFMT among Chinese pregnant women. The present study findings revealed that only 19.67% (157/798) of pregnant women conducted PFMT. This percentage was 68.9% (494/717) in a study conducted in England during 2001,27 23.6% (81/343) in Norway in 2007,28 and 54.0% (156/289) in Northeast Scotland in 2007.29 Performance of PFMT with a frequency of more than once a week has been considered an adequate level according to the literature;30 however, only 8.5% (68/798) of pregnant women conducted adequate PFMT. The percentage of women with this frequency of PFMT was reported to be 38.5% (276/717) by Mason et al.,27 54.5% (392/720) by Chiarelli et al.31 and 42.6% (123/289) by Whitford et al.29 In contrast, performance of antenatal PFMT among Chinese pregnant women is quite unsatisfactory. In addition, we did not find any significant association between the characteristics of pregnant women and the frequency/duration of antenatal PFMT implementation. This implies that the frequency and duration of PFMT performed by pregnant women is not related to age, parity, or whether SUI is experienced during pregnancy. These findings have also been reported by Bø et al.28
Although contradicting the evidence reported by Wilson et al.,32 the results of our study indicate that unsupervised antenatal PFMT has no effects on postpartum SUI. There are several possible explanations for this result. The most likely is that antenatal PFMT in this study did not reach the expected effective dose. In the current study, 2.26% (18/798) of pregnant women performed daily PFMT, but only seven women (0.87%, 7/798) conducted daily PFMT for more than 1 month. This finding was supported by a pragmatic trial22 that failed to demonstrate significant effectiveness of antenatal PFMT for preventing urinary incontinence 1 year after the first delivery. The author of the study22 explained that the reason for the negative result may be because adherence (only 5% of women did daily PFMT) in the antenatal PFMT group was low. Moreover, the reported antenatal PFMT in this study was performed without supervision, meaning that there was no way to guarantee the correctness of pelvic floor muscle contraction. A previous study23 also observed no effect of PFMT taught in a general fitness class for pregnant women without individual instruction for correct pelvic floor muscle contraction. Furthermore, there is insufficient evidence to support the effect of antenatal PFMT on postpartum urinary incontinence.33 Although a meta-analysis13 found that antenatal PFMT had preventive effects for postpartum urinary incontinence in pregnant women, this conclusion was based on studies with supervised PFMT and high adherence. In our study, however, we were unable to demonstrate an association between unsupervised antenatal PFMT at low doses and postpartum SUI, for women either with or without SUI during pregnancy.
Strengths and limitations
The strengths of our study include the following: 1) the observational design adopted in this study may closely reflect real-world practice; 2) examination of stratified and interaction effects between frequency and duration of antenatal PFMT on postpartum SUI has not been previously reported. Our study also has several limitations. First, the initial intention was to analyze the effect of daily practice for 2 months or more (compared with pregnant women did not practice PFMT at all); however, the proportion who practiced PFMT with this frequency was too small to be able to make comparisons. Only five pregnant women did daily PFMT for 2 months or more, accounting for 0.63% of the total sample. Therefore, frequency was grouped into weekly or less, and more than weekly, and the practice duration was classified as training less than 1 month, and 1 month or more. Even so, the sample sizes for stratified and interaction analyses were still limited, resulting in failure to be able to test the effect of antenatal PFMT on SUI in pregnant women who practiced PFMT for 1 month or more. Moreover, in the current study, we made no attempt to determine why a minority of pregnant women performed daily PFMT. One possible reason may be that the benefits of antenatal PFMT are not explained to pregnant women in routine clinical settings. Barriers and facilitators to antenatal PFMT should be further studied. Second, the data regarding antenatal PFMT and SUI during pregnancy were collected retrospectively; a risk of recall bias was introduced, which may weaken our conclusions. Third, because of the limited study period, we followed up SUI at 6 weeks postpartum and failed to assess the long-term effects of antenatal PFMT. A longer period of observation is needed to identify the possible effects of antenatal PFMT on postpartum SUI. Finally, we only looked at the frequency and duration of antenatal PFMT; effects of the volume and intensity of reported PFMT could not be analyzed.
Conclusion
Despite the abovementioned limitations, this study indicated that 1) performance of antenatal PFMT among Chinese pregnant women was quite unsatisfactory, and 2) no effect of self-reported, unsupervised, and self-initiated antenatal PFMT on SUI at 6 weeks postpartum could be found, based on retrospectively collected data. Nevertheless, the role of antenatal PFMT should not be dismissed simply because the effect observed in RCTs24–26 has not been observed in real-world settings. In the future, real-world observational studies should be conducted under high-quality service conditions, such as effective instruction with supervision, standardized training programs with set frequency and duration, and a high level of adherence, to test the effect of antenatal PFMT on postpartum SUI.
Acknowledgements
We wish to warmly thank all the pregnant women who participated in this study, nurses and managers who made this study possible, and Dr. Jennifer Barrett, for his help with the English language.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Sanming Project of Medicine in ShenZhen, China (No. SZSM201612018), the Seedling Program of Shenzhen Hospital of Southern Medical University (No. 2018MM03), and the National Natural Science Foundation of China (No. 71904075). The funding body had no role in the study design, data collection, data analysis, interpretation, or manuscript writing.
ORCID iDs
Ling Chen https://orcid.org/0000-0002-4637-250X
Wenzhi Cai https://orcid.org/0000-0002-2354-5199
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29: 4–20. [DOI] [PubMed] [Google Scholar]
- 2.MacArthur C, Glazener CM, Wilson PD, et al. Persistent urinary incontinence and delivery mode history: a six-year longitudinal study. BJOG 2006; 113: 218–224. [DOI] [PubMed] [Google Scholar]
- 3.Ternent L, Vale L, Buckley B, et al. Measuring outcomes of importance to women with stress urinary incontinence. BJOG 2009; 116: 719–725. [DOI] [PubMed] [Google Scholar]
- 4.Herbert J. Pregnancy and childbirth: the effects on pelvic floor muscles. Nurs Times 2009; 105: 38–41. [PubMed] [Google Scholar]
- 5.Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand 2010; 89: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Li L, Lang JH, et al. Prevalence and risk factors for peri- and postpartum urinary incontinence in primiparous women in China: a prospective longitudinal study. Int Urogynecol J 2012; 23: 563–572. [DOI] [PubMed] [Google Scholar]
- 7.Chan SS, Cheung RY, Yiu KW, et al. Prevalence of urinary and fecal incontinence in Chinese women during and after their first pregnancy. Int Urogynecol J 2013; 24: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 8.Hantoushzadeh S, Javadian P, Shariat M, et al. Stress urinary incontinence: pre-pregnancy history and effects of mode of delivery on its postpartum persistency. Int Urogynecol J 2011; 22: 651–655. [DOI] [PubMed] [Google Scholar]
- 9.Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol 2006; 108: 248–254. [DOI] [PubMed] [Google Scholar]
- 10.Dolan LM, Hosker GL, Mallett VT, et al. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG 2003; 110: 1107–1114. [PubMed] [Google Scholar]
- 11.Fritel X, Fauconnier A, Bader G, et al. Diagnosis and management of adult female stress urinary incontinence: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol 2010; 151: 14–19. [DOI] [PubMed] [Google Scholar]
- 12.Bo K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int Urogynecol J 2004; 15: 76–84. [DOI] [PubMed] [Google Scholar]
- 13.Woodley SJ, Boyle R, Cody JD, et al. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev 2017; 12: D7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morkved S, Bo K. Effect of pelvic floor muscle training during pregnancy and after childbirth on prevention and treatment of urinary incontinence: a systematic review. Br J Sports Med 2014; 48: 299–310. [DOI] [PubMed] [Google Scholar]
- 15.Bo K, Fleten C, Nystad W. Effect of antenatal pelvic floor muscle training on labor and birth. Obstet Gynecol 2009; 113: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 16.Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health 2006; 29: 489–497. [DOI] [PubMed] [Google Scholar]
- 17.Lin YH, Chang SD, Hsieh WC, et al. Persistent stress urinary incontinence during pregnancy and one year after delivery; its prevalence, risk factors and impact on quality of life in Taiwanese women: an observational cohort study. Taiwan J Obstet Gynecol 2018; 57: 340–345. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Zhang HX, Yu HY, et al. The prevalence of fecal incontinence and urinary incontinence in primiparous postpartum Chinese women. Eur J Obstet Gynecol Reprod Biol 2010; 152: 214–217. [DOI] [PubMed] [Google Scholar]
- 19.Jelovsek JE, Chagin K, Gyhagen M, et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am J Obstet Gynecol 2018; 218: 221–222. [DOI] [PubMed] [Google Scholar]
- 20.Geraerts I, Van Kampen M. Twelve-year follow-up of conservative management of postnatal urinary and faecal incontinence and prolapsed outcomes: randomised controlled trial. BJOG 2014; 121: 1741–1742. [DOI] [PubMed] [Google Scholar]
- 21.Urinary incontinence in women: management. Guideline 171.: National Institute for Health and Clinical Excellence (NICE); 201. 3 [cited accessed 30 October 2015]. Available from: [www.nice.org.uk/guidance/cg171/ resources/urinary incontinence-in-women-management-35109747194821].
- 22.Fritel X, de Tayrac R, Bader G, et al. Preventing urinary incontinence with supervised prenatal pelvic floor exercises: a randomized controlled trial. Obstet Gynecol 2015; 126: 370–377. [DOI] [PubMed] [Google Scholar]
- 23.Bo K, Haakstad LA. Is pelvic floor muscle training effective when taught in a general fitness class in pregnancy? A randomised controlled trial. Physiotherapy 2011; 97: 190–195. [DOI] [PubMed] [Google Scholar]
- 24.Sut HK, Kaplan PB. Effect of pelvic floor muscle exercise on pelvic floor muscle activity and voiding functions during pregnancy and the postpartum period. Neurourol Urodyn 2016; 35: 417–422. [DOI] [PubMed] [Google Scholar]
- 25.Sangsawang B, Serisathien Y. Effect of pelvic floor muscle exercise programme on stress urinary incontinence among pregnant women. J Adv Nurs 2012; 68: 1997–2007. [DOI] [PubMed] [Google Scholar]
- 26.Mørkved S, Bø K, Schei B, et al. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: a single-blind randomized controlled trial. Obstet Gynecol 2003; 101: 313–319. [DOI] [PubMed] [Google Scholar]
- 27.Mason L, Glenn S, Walton I, et al. Do women practise pelvic floor exercises during pregnancy or following delivery? Physiotherapy 2001; 87: 662–670. [DOI] [PubMed] [Google Scholar]
- 28.Bo K, Haakstad LAH, Voldner N. Do pregnant women exercise their pelvic floor muscles? Int Urogynecol J 2007; 18: 733–736. [DOI] [PubMed] [Google Scholar]
- 29.Whitford HM, Alder B, Jones M. A cross-sectional study of knowledge and practice of pelvic floor exercises during pregnancy and associated symptoms of stress urinary incontinence in North-East Scotland. Midwifery 2007; 23: 204–217. [DOI] [PubMed] [Google Scholar]
- 30.Chiarelli P, Cockburn J. Promoting urinary continence in women after delivery: randomised controlled trial. BMJ 2002; 324: 1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiarelli P, Murphy B, Cockburn J. Women’s knowledge, practises, and intentions regarding correct pelvic floor exercises. Neurourol Urodyn 2003; 22: 246–249. [DOI] [PubMed] [Google Scholar]
- 32.Wilson PD, Herbison RM, Herbison GP. Obstetric practice and the prevalence of urinary incontinence three months after delivery. Br J Obstet Gynaecol 1996; 103: 154–161. [DOI] [PubMed] [Google Scholar]
- 33.Soave I, Scarani S, Mallozzi M, et al. Pelvic floor muscle training for prevention and treatment of urinary incontinence during pregnancy and after childbirth and its effect on urinary system and supportive structures assessed by objective measurement techniques. Arch Gynecol Obstet 2019; 299: 609–623. [DOI] [PubMed] [Google Scholar]