Abstract
Cartilage is a specialized tissue that has a relatively homogenous endogenous cell population but a diverse extracellular matrix (ECM) with depth-dependent mechanical properties. Cartilage repair remains an elusive clinical goal, with biological interventions preferred to arthroplasty in younger patients. Osteochondral transplantation (OCT) has emerged for the treatment of cartilage defects and osteoarthritis. Fresh allografts stored at 4 °C for periods of time have been utilized, though matrix and cell viability loss remained an issue. To address this, several studies have developed media formulations to maintain cartilage explants in vitro. One promising factor for these applications is sprifermin, a human-recombinant fibroblast growth factor-18 which stimulates chondrocyte proliferation and matrix synthesis and is in clinical trials for osteoarthritis treatment. We hypothesized that addition of sprifermin during storage would maintain the unique depth-dependent mechanical profile of cartilage explants, a feature of cartilage not often evaluated. In this study, cartilage explants were maintained for up to 6 weeks with or without a weekly 24-hour exposure to sprifermin (100 ng/ml) and the compressive modulus was assessed. Results showed that sprifermin treated samples maintained their depth-dependent mechanical profile through 3 weeks, whereas untreated samples lost their mechanical integrity over 1 week of culture. Sprifermin also affected ECM balance by maintaining levels of extracellular collagen and suppression of matrix metalloproteinase production. These findings support the use of sprifermin as a medium additive for OCT allografts during in vitro storage, and present a potential mechanism where sprifermin may impact a functional characteristic of cartilage in repair strategies.
Keywords: cartilage, fibroblast growth factor-18, mechanical properties
Introduction
The normal function of cartilage is to resist compressive loads generated with normal activity. In order to accomplish this task, over a lifetime of use, the tissue has evolved to possess complex mechanical properties, including biphasic interactions between the solid and fluid phases of the tissue (Ateshian et al., 1994; Mow et al., 1980), tension-compression non-linearity, imbued by the high concentration and direction-dependent organization of collagen (Huang et al., 2003; Mow et al., 1992; Park et al., 2003; Soltz and Ateshian, 2000; Sophia Fox et al., 2009), and depth-dependent compressive properties, largely dictated by the high concentration and distribution of proteoglycans through the depth (Chen et al., 2001; Korver et al., 1990). In degenerative conditions, this complex distribution of ECM is disrupted, and mechanical function is compromised, becoming more isotropic and less specialized.
Despite its complex function over a lifetime of normal use, cartilage function can be compromised by acute and localized injury. For instance, a recent review revealed that treatment of focal cartilage lesions has increased 5 % annually, with an incidence rate of 90 per 10,000 patients (McCormick et al., 2014). When symptomatic, these focal injuries can be treated with chondroplasty, cell-based strategies, such as ACI, MACI, and microfracture, or may be treated with osteochondral transfer (OCT), wherein an entire osteochondral unit is transferred from one location to another (Bartlett et al., 2005; Basad et al., 2010; Brittberg, 2008; Brittberg, 2010; Horas et al., 2003; Knutsen et al., 2007; Knutsen et al., 2004; Mithoefer et al., 2005; Peterson et al., 2010; Steadman et al., 2001). OCT can be performed in an autologous fashion (where living donor tissue is moved from one location to another in an individual) or in an allogeneic fashion (wherein fresh cadaveric tissue is the source the donor segment) (Aubin et al., 2001; Bugbee and Convery, 1999; Chow et al., 2004; Chu et al., 1999; Hangody et al., 2010; Hangody and Fules, 2003; Hangody et al., 2008; Marcacci et al., 2007; Williams et al., 2007). While early versions of this procedure used fresh-frozen and devitalized cadaveric tissue, recent studies have suggested that outcomes for allogeneic OCT are better when a living implant is used (Malinin et al., 2006; McCarty et al., 2010).
While promising as a therapeutic, donor osteochondral units must first be screened for communicable disease prior to implantation, necessitating storage of tissues for several weeks. Several studies have examined long-term storage of allografts at freezing (−70 °C), refrigerated (4 °C), and body (37 °C) temperatures, with the most common primary outcome being chondrocyte viability (Allen et al., 2005; Ball et al., 2004; Ohlendorf et al., 1996; Pallante-Kichura et al., 2013; Pallante et al., 2009; Pallante et al., 2012; Williams et al., 2004; Williams et al., 2003). Storage of allografts in hypothermic conditions may maintain the integrity of extracellular matrix, but does so at the expense of chondrocyte viability. While maintaining viability is readily achieved over such a time period when storing grafts at body temperature, this storage and screening process is detrimental to the mechanical properties of the osteochondral unit, potentially limiting its efficacy upon implantation.
Indeed, in vitro culture or storage of living cartilage results in the rapid loss of mechanical function (Bian et al., 2008). This occurs as a consequence of the rapid loss and or degradation of the dense extracellular matrix (ECM) (Hascall et al., 1983b). Once removed from the load-bearing synovial environment, both mechanical and biochemical cues that would normally promote tissue homeostasis are lost, and so the tissue begins to degrade. There have been numerous attempts to preserve or promote the cartilage phenotype during in vitro culture of living cartilage, with varying degrees of success. Early studies in this field, employing mechanical loading systems and supplementation of media with growth factors, demonstrated an anabolic effect and have explicated many of the key factors that regulate cartilage homeostasis (Fitzgerald et al., 2004; Guilak et al., 1994; Hall et al., 1991; Hascall et al., 1983a; Luyten et al., 1988; Sah et al., 1994; Sah et al., 1989). Towards the practical application of preserving implant properties prior to OCT procedures, these media formulations have been increasingly well defined, with some making their way to clinical and commercial application (Mickevicius et al., 2015; Teng et al., 2008). For instance, based on a chemically defined media formulation containing transforming growth factor-beta3, the Missouri Osteochondral Preservation System enables the transfer of very large and highly viable osteochondral segments for total joint restoration (Garrity et al., 2012; Kuroki et al., 2017; Stoker et al., 2017; Stoker et al., 2018).
Despite this progress in the field, it is not yet clear whether such preservation systems fully retain the graded and refined mechanical properties of native tissue. It is also not clear whether the current media formulations represent the optimal, and whether other molecules may have a preservative effect. To that end, we and others have recently shown that fibroblast growth factor 18 (FGF18) stimulates chondrocyte proliferation and matrix production in vitro, and reduces cartilage degeneration and increases de novo matrix formation by osteoarthritic cartilage in vivo (Ellsworth et al., 2002; Moore et al., 2005). A recombinant version of this protein, known as sprifermin, is currently a non-approved drug candidate and in clinical development for osteoarthritis, also has positive effects in vitro, in vivo, and in several recent pre-clinical and clinical trials in humans (Dahlberg et al., 2016; Gigout et al., 2017; Lohmander et al., 2014; Mori et al., 2014; Power et al., 2014; Reker et al., 2017). Sprifermin has decreases collagen type I expression in monolayer culture, and decreases collagen type II expression at dosing concentrations >100 ng/mL. With regards to cell morphology, Sprifermin results in chondrocytes taking on a more rounded morphology, with a loss of elongated shape and stress fibres (Gigout et al., 2017). In this study, we tested whether the addition of sprifermin (recombinant human FGF18) to standard media formulations could maintain the refined mechanical properties of articular cartilage during long-term in vitro culture, with a particular focus on the characteristic depth-dependent compressive properties of the native tissue.
Materials and Methods
Cartilage Explant Harvest and Culture
Full thickness articular cartilage explants were harvested from the trochlear groove of juvenile (3–6 month old) bovine stifle joints (Research 87, Boylston, MA) using aseptic technique and a sterile 4 mm diameter biopsy punch. Explants were washed three times in sterile phosphate buffered saline (PBS) supplemented with 200 units/mL penicillin, 200 μg/mL streptomycin and 0.5 μg/mL Amphotericin B (Gibco by Life Technologies, Carlsbad, CA). Following washing, explants were sharply dissected to remove non-cartilaginous tissues (subchondral bone) and cultured overnight in complete medium consisting of Dulbecco’s Modified Eagle Medium (Gibco by Life Technologies, Carlsbad, CA) with 10 % by volume fetal bovine serum, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 2.5 μg/mL of Amphotericin B (Gibco by Life Technologies, Carlsbad, CA), 1x MEM Vitamins (Corning Cellgro, Manassas, VA), 25 mM HEPES Buffer (Gibco by Life Technologies, Carlsbad, CA), and 50 μg/ml L-Ascorbic Acid 2-Phosphate (Sigma-Aldrich, St. Louis, MO). Following overnight culture, samples were randomly assigned into treatment and time groups per the study design. Samples were maintained fully submerged in 1 mL complete medium per sample and cultured at 37 °C and 5 % CO2. Media were changed three times per week, with 1 ml collected at each media change frozen at −80 °C for later analysis. The initial study was planned for a duration of 3 weeks with follow up studies planned for end points between 3 and 6 weeks. The data presented was gleaned from all samples at the same time points over multiple experiments. Replicate numbers are provided with each figure in addition to the summarized data in Table 1. Note that the reduction in sample numbers for MMP and media GAG assessments was the result of sequential tissue removal at each weekly time point.
Table 1:
N for various assays
| Collagen | GAG | Solid Vol | MMP | Media GAG | Normalized Bulk Modulus | Local Mechanics | ||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Control | 23 | 23 | 19 | 23 | 15 | ||
| Week 1 | Control | 26 | 26 | 17 | 25 | 24 | 21 | 16 |
| Sprifermin | 24 | 25 | 15 | 25 | 25 | 19 | 14 | |
| Week 2 | Control | 26 | 26 | 15 | 21 | 21 | 20 | 15 |
| Sprifermin | 27 | 27 | 17 | 21 | 21 | 21 | 16 | |
| Week 3 | Control | 30 | 30 | 20 | 17 | 17 | 25 | 16 |
| Sprifermin | 31 | 31 | 21 | 17 | 17 | 24 | 17 | |
| Week 4 | Control | 19 | 19 | 19 | 13 | 13 | 19 | 15 |
| Sprifermin | 19 | 19 | 19 | 13 | 13 | 17 | 13 | |
| Week 5 | Control | 19 | 19 | 19 | 10 | 10 | 18 | 14 |
| Sprifermin | 19 | 19 | 19 | 10 | 10 | 17 | 13 | |
| Week 6 | Control | 23 | 23 | 18 | 7 | 7 | 21 | 12 |
| Sprifermin | 24 | 24 | 19 | 7 | 7 | 24 | 15 |
Recombinant Human FGF18 (rhFGF18) Treatment
Recombinant human fibroblast growth factor 18 (rhFGF18, alias sprifermin, Merck KGaA, Darmstadt, Germany) was added to complete medium at a concentration of 100 ng/ml. The concentration was chosen as low to medium based on previous in vitro assays demonstrating a dose dependency in a wide range of concentrations (Gigout et al., 2017). As established by previous studies, a treatment duration of 24 hours each week was chosen to enhance the ‘hit and run’ effect that Sprifermin has been shown to operate by, where a short term exposure and then remove elicits the greatest anabolic response. After treatment, media was then replaced with fresh complete medium (Gigout et al., 2017). Controls were treated similarly, with exchange of complete medium after the identical 24 hours. Thereafter, all cultures were changed into fresh medium every 3 days.
Mechanical Testing
For unconfined compression testing, samples were tested using a custom-built device (Figure 1) (Mauck et al., 2000; Mauck et al., 2006). Briefly, a 0.02 N creep load was applied at a velocity of 10 μm/s and held for 300 seconds, followed by 10 % compressive strain applied over 200 seconds followed by a 1000 second hold. Equilibrium modulus was calculated by dividing the measured load at the end of the 1000 second hold by the cross-sectional area and applied strain. To assay the depth-dependent mechanical properties of the cartilage explants, samples were tested in unconfined compression using a custom micrometer-driven inverted microscope mounted apparatus (Farrell et al., 2012). The deep zone of each sample was trimmed on a freezing stage microtome to ensure parallel surfaces for mechanical testing, while still preserving the superficial zone. The sample was then measured with digital calipers and cut diametrically to produce a semi-cylinder. While this may cause some fiber discontinuity in the sample, all specimens were tested in the same fashion. One half was fixed in 4 % paraformaldehyde for histology and the other half was stained with Hoechst 33342 (1:100 dilution) to identify cell nuclei that would later be used as fiducial markers. The tissue was then placed cut side down into the device and the loading platens were brought into contact with the sample. Fluorescent images were taken on an inverted microscope (Nikon Eclipse TE2000-U) and equilibrium load readings were recorded at initial contact, and following each of five 4 % strain increments, up to 20 % strain. Samples were loaded manually using a micrometer-driven linear stage at approximately 1% strain per second and held for 800 seconds. Image correlation software (Vic2d, Correlated Solutions) was then used to compute a 2D strain field with a resolution of 16.25 microns throughout the depth of the tissue using the nuclei as fiducial markers (Farrell et al., 2012). The data was further analyzed using a custom MATLAB program to calculate the average strain the direction of loading in 50 μm bins across the depth of the sample, starting at the cartilage surface and continuing through the depth of the sample. Strain data from the first and last 100 microns of each sample was discarded due to edge effects. The equilibrium modulus for each bin was calculated by dividing the equilibrium load by the measured cross-sectional area and bin strain. This results in a depth-dependent profile of the compressive equilibrium modulus. To compare these local data to the tissue-scale modulus data acquired using the test described above, the average strain across the whole tissue was calculated. This data was then normalized by dividing the measured modulus at each time point by average modulus at the start of each experiment. This was done to account for differences in the testing modalities and biological variability between studies. This data was then included in the analysis of the tissue-scale mechanics.
Fig. 1.
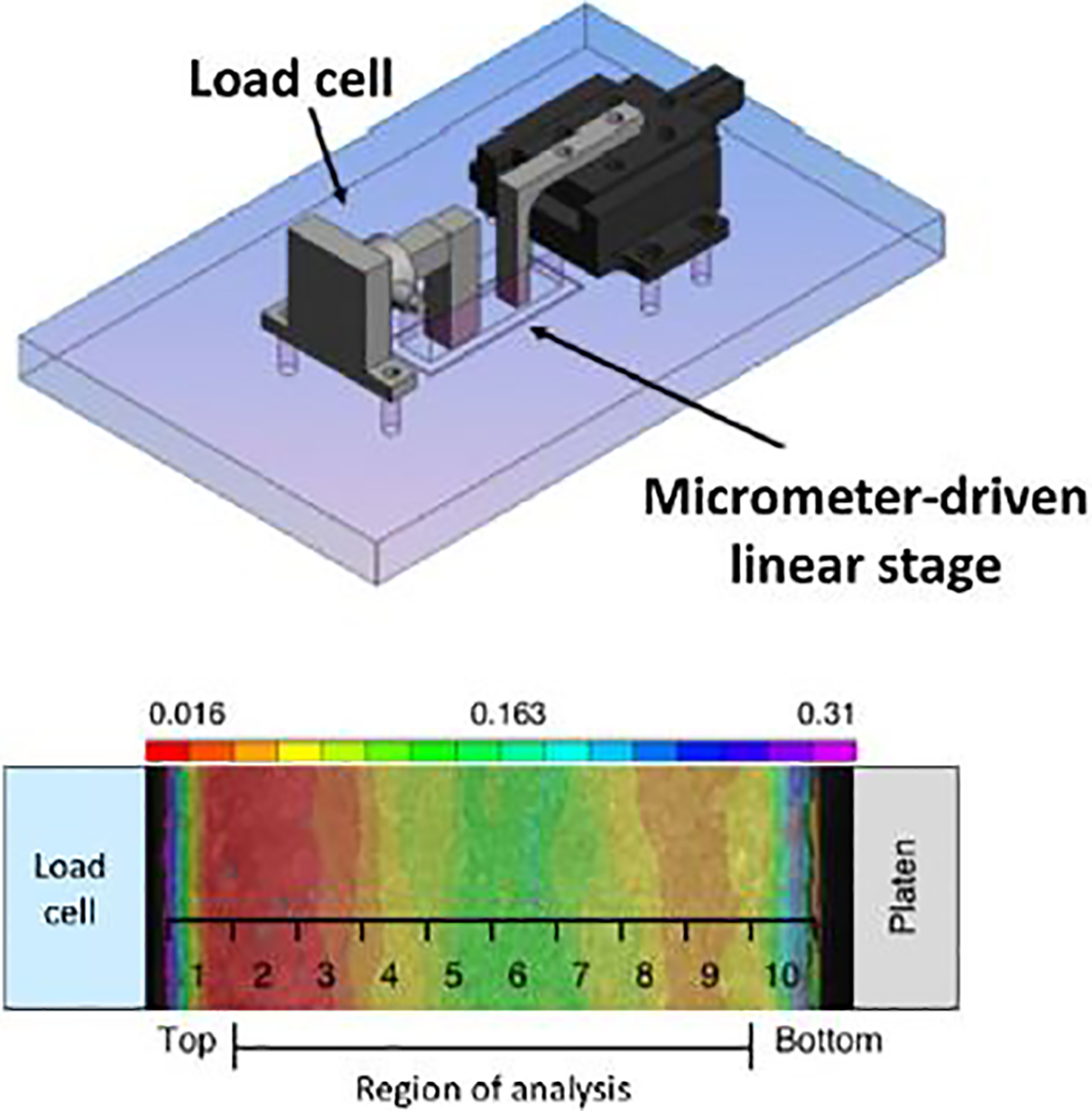
Schematic of custom compression device mounted on an inverted fluorescent microscope and heat map of strain mapped to ten distinct regions of analysis from the superficial to deep zone. Adapted from Farrell et al. (2012), used with permission.
Biochemical Assays
Sulfated glycosaminoglycan (GAG) content was quantified via the colorimetric 1,9-dimethylmethylene blue assay (Farndale et al., 1986). Collagen content was quantified using the orthohydroxyproline assay and a conversion factor 7.6 (Stegemann and Stalder, 1967). Matrix metalloproteinase (MMP) activity levels in the media were quantified with the SensoLyte Fluroimetric assay (AnaSpec) using a fluorometric assay reading at ex/em 490 nm/520 nm. This Generic MMP Assay Kit is designed to detect MMP-1, 2, 3, 7, 8, 9, 12, 13, and 14, making it ideal for high throughput screen and detecting generic MMP activity. Wet and dry weights were recorded before samples were digested in a Proteinase K digestion buffer for 18 hours at 60 °C with frequent mixing (50 units/ml in 100 mM Tris-HCl, Worthington, Lakewood NJ). GAG and collagen content were all quantified on digested samples. GAG and MMP levels in the medium were quantified on a weekly basis. Solid volume fraction was calculated as the quotient of the dry weight divided by the wet weight of each sample. From this, the change in water content was expressed as a percentage by calculating the difference in solid volume fraction at each week relative to the mean week 0 value.
Data Analysis and Statistics
For mechanical testing data analysis, studies were normalized to mean time zero values and combined. GAG and collagen content were normalized to wet weight to account for variability in the size of each individual tissue segment. Error bars in figures represent the standard error of the mean. Statistical significance was determined using a 1-way or 2-way ANOVA with Bonferroni’s post-hoc test, as appropriate.
Results
Cartilage Explant Tissue-Scale Mechanics with FGF18 Treatment
The equilibrium modulus of cartilage explants decreased with in vitro culture over six weeks, regardless of sprifermin treatment (Figure 2). Samples cultured in control medium (Figure 2, white bars) had significantly lower equilibrium moduli compared to week 0 values at every week following one week of culture (n=12–23, p<0.05). Conversely, the equilibrium modulus of samples treated with rhFGF18 (black bars) did not become significantly lower until week 6 (n=17–29, p<0.05). Similarly, the tissue-scale equilibrium modulus of the explants treated with rhFGF18 was higher than control explants at time points of 2, 3, and 4 weeks (n=12–29, p<0.05).
Fig. 2.
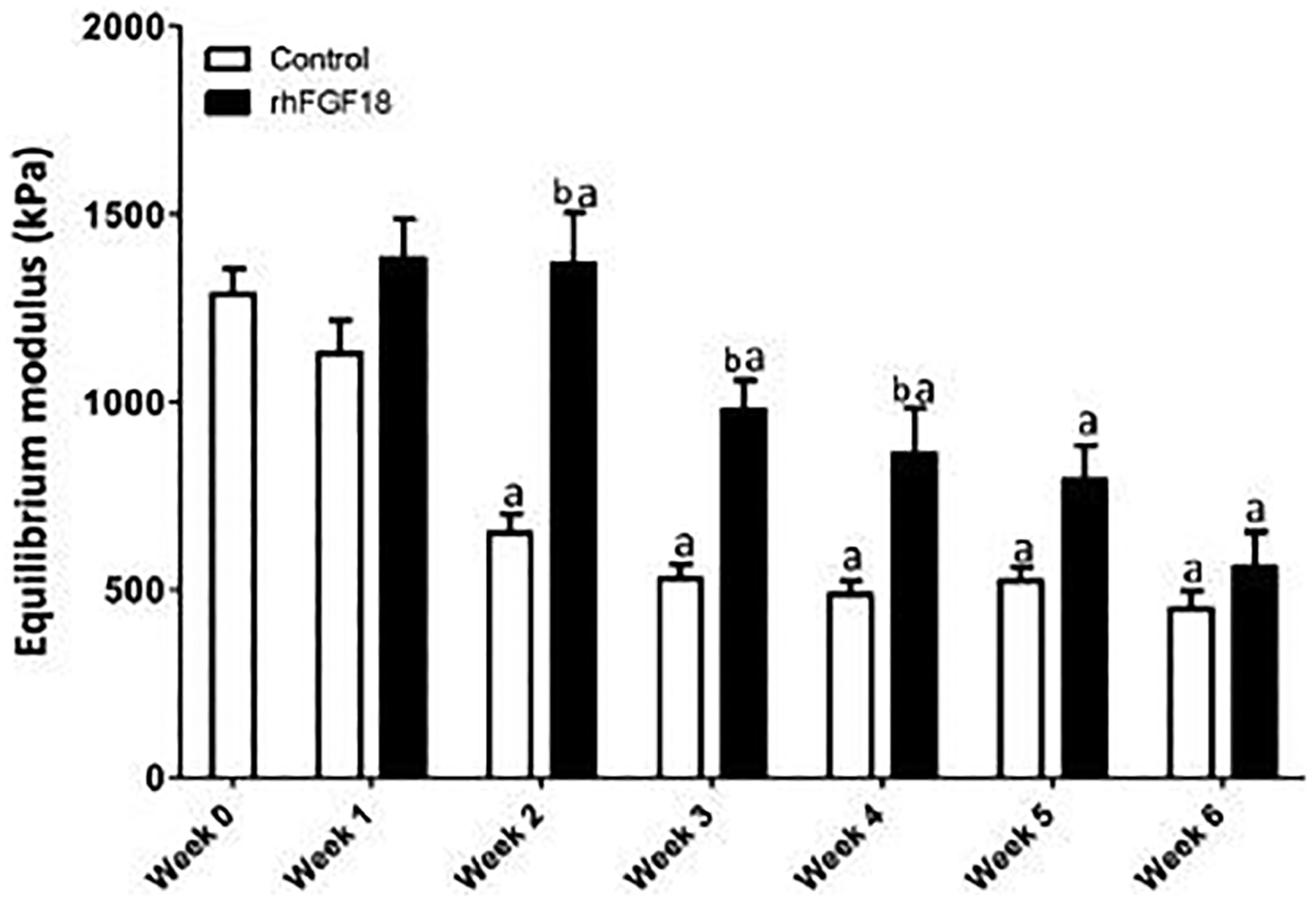
Equilibrium modulus of AC on the tissue scale. Samples were cultured in vitro for up to 6 weeks ± 100 ng/mL rhFGF18. Treated explants did not differ from baseline modulus throughout 5 weeks. Additionally, the modulus of the treated explants was significantly higher than that of the control at weeks 2, 3 and 4 (a p < 0.05 vs. week 0, b p < 0.05 vs. control; n = 17–25).
Cartilage Explant Local Mechanics with Sprifermin Treatment
To better understand these differences in tissue-scale compressive mechanics, each sample was tested using a custom device to assay tissue modulus in 50 μm increments from the superficial zone through the deep zone. Baseline, or week 0, depth-dependent mechanics showed an increase in modulus as function of distance from the cartilage surface (Figure 3, grey), consistent with previous findings (Schinagl et al., 1996; Wang et al., 2003; Wang et al., 2002). Control samples (dashed lines, n=12–17) had moduli that were below baseline levels throughout the depth of the cartilage explants. These differences became more pronounced with culture duration. Additionally, control samples quickly lost the depth-dependence characteristics of native cartilage, seen here as a flattening of the modulus profile. In contrast, sprifermin treated samples (solid colored lines, n=12–17) retained a depth dependent profile matching that of the baseline week 0 control through five weeks of in vitro culture, with modulus increasing as a function of distance from the cartilage surface. Further investigation of this data were assessed in the 100–1000 micron region of the tissue, in order to determine differences in the superficial and lower/mid zone of the cartilage samples, rather than the full depth presented in Figure 3. This layer spanning 1mm thickness represents the layer of cartilage that will remain in the adult. These trends were quantified as average moduli in regions near the surface (100–150 microns), in the middle (450–500 microns), and in the deep (950–1000 microns) zones of the tissue as a function of culture duration in Figure 4. At these distances from the cartilage surface, the control samples (dashed lines) showed a decreased modulus by week 3 near the surface and in the middle zone, and by week 4 in the deep zone. The rhFGF18 treated samples (solid lines) showed no differences in the deep zone, a decrease at week 4 in the middle zone, and decreases at weeks 4 and 5 near the surface. Overall, control samples showed a decrease in modulus in 11 of 18 samples (61 %) whereas sprifermin samples had differences in only 3 of 18 samples (17 %).
Fig. 3.
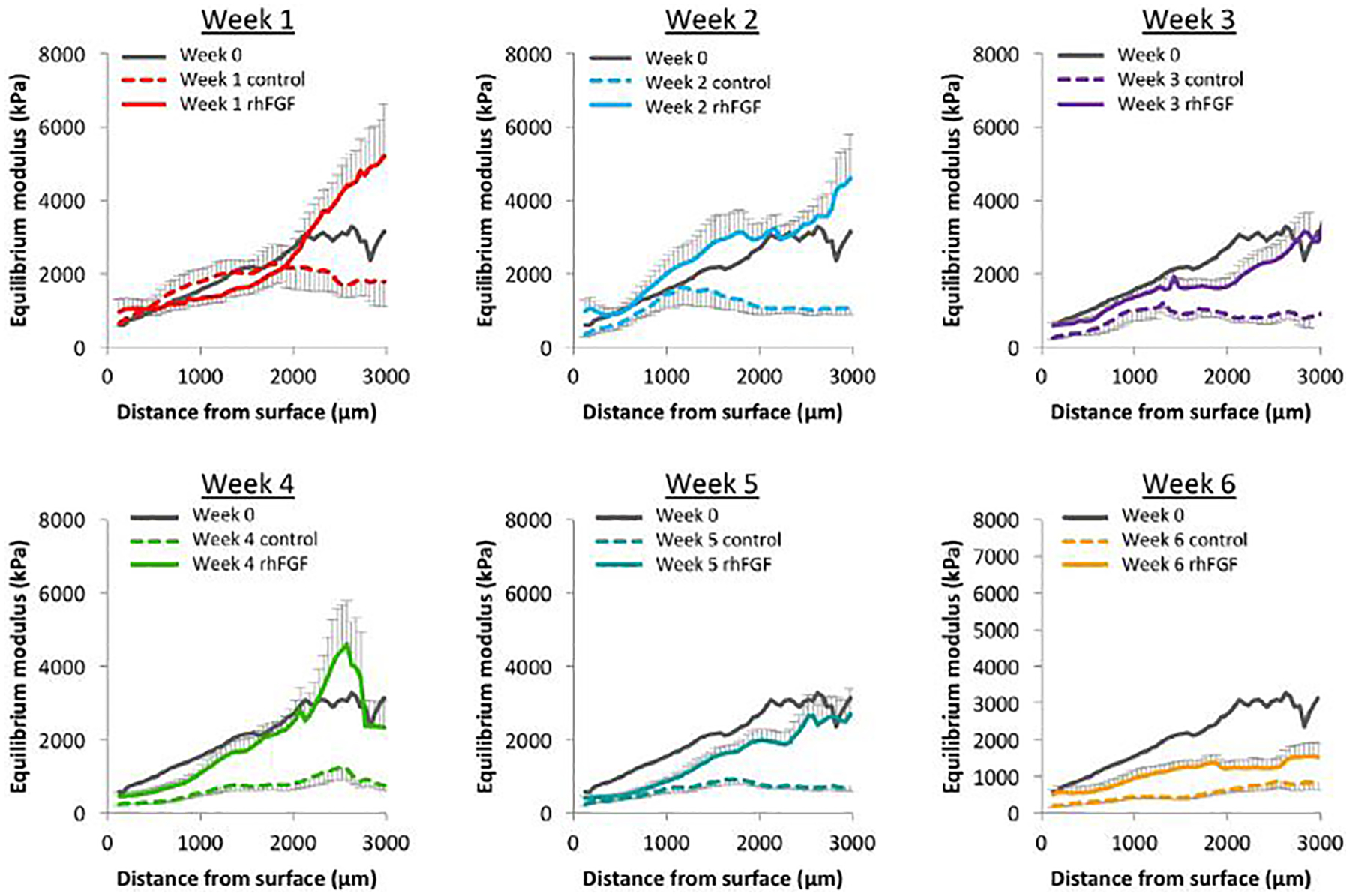
Depth-dependent equilibrium modulus of AC explants cultured in intro for 0–6 weeks. Baseline modulus through the depth is shown in grey (n = 12–17).
Fig. 4.
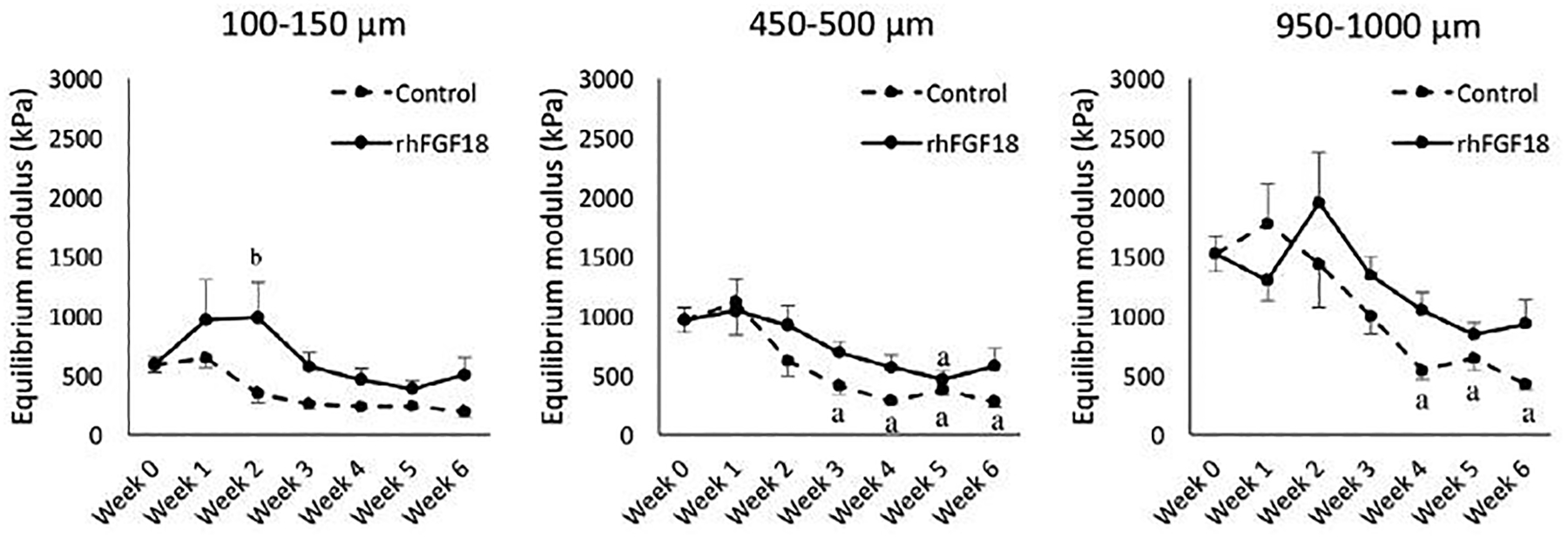
Mean equilibrium modulus of AC explants at distances of 100–150,450–500 and 950–1,000 pm from the AC surface over the course of 6 weeks of in vitro culture. a p < 0.05 vs. week 0, b p < 0.05 vs. control.
Explant Biochemical Content with Sprifermin Treatment
Following mechanical testing, the biochemical composition of the tested samples was determined. The biochemical composition was determined on the half that was mechanically tested. The adjacent hemi-cylinder was fixed in 4 % para-formaldehyde. Total collagen content, as a percentage of wet weight, decreased significantly in control samples after one week of culture (Figure 5A, white bars) compared to week 0 levels (n=19–30, p<0.05). In comparison, sprifermin treated explants (solid bars) had significantly lower collagen content after only 4 and 6 weeks of in vitro culture (n=19–30, p<0.05). Additionally, sprifermin treated explants had higher collagen content compared to controls at all time points after week 2. GAG content of explants was also measured as a percentage of wet weight with time (Figure 5B). There was a significant decrease in GAG content for control samples starting at week 2 (white bars), while for sprifermin treated explants these decreases were not apparent until week 3. Only at week 2 was there any detectable difference in GAG content between groups.
Fig. 5.
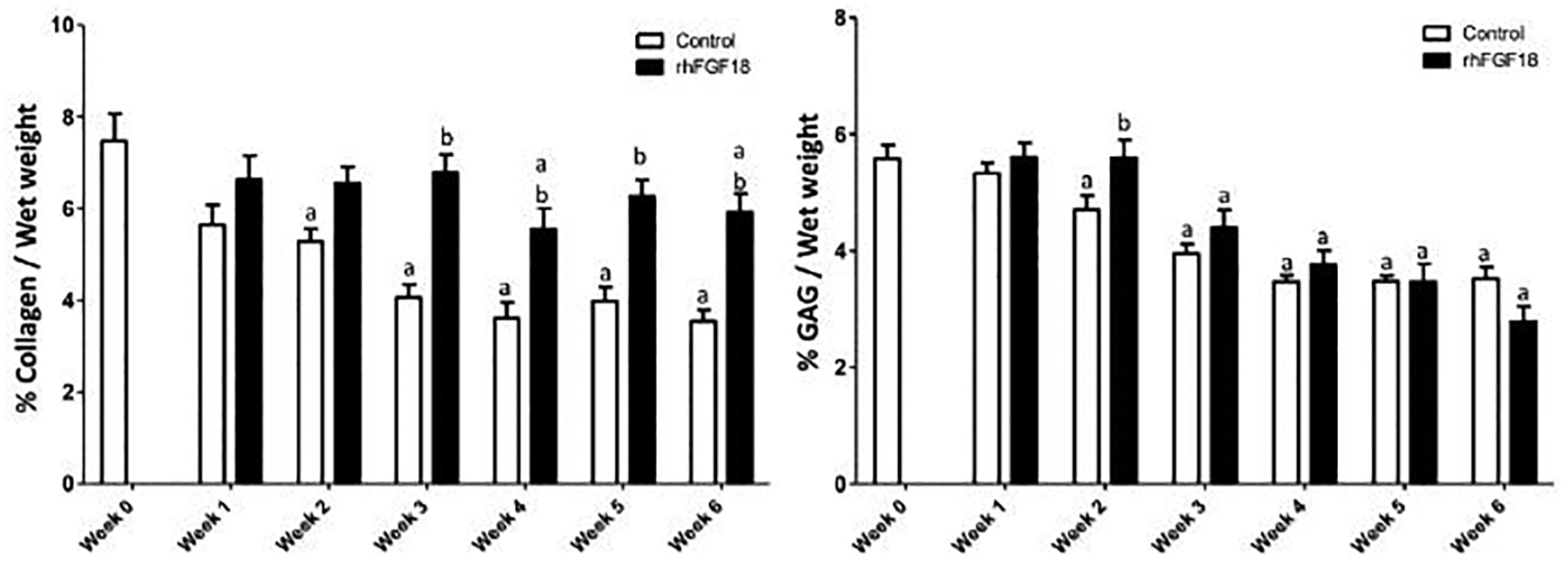
Collagen content as measured by the orthohydroxyproline assay and sulphated-GAG contented measured by the 1,9-dimethylmethylene blue assay for AC explants cultured in vitro for 0–6 weeks. Data were normalised to wet weight. a p < 0.05 vs. week 0 control, b p < 0.05 vs. control at same time point (n = 19–31).
Explant Macroscopic Appearance and Water Content with Sprifermin Treatment
Through six weeks, explants in control conditions appeared to visibly swell and distort from their original cylindrical shape, whereas sprifermin treated explants maintained their size and shape. Both control and sprifermin treatment groups showed a significant difference in solid volume fraction after one week of culture (Figure 6A). However, the solid volume fraction with sprifermin treatment was higher than controls at each week following. This can be expressed as a change in water content (Figure 6B), where there was a significant increase in water content in control samples at all time points. For treated explants, while there was an increase at after week 1, this change was attenuated compared that seen in control conditions.
Fig. 6.
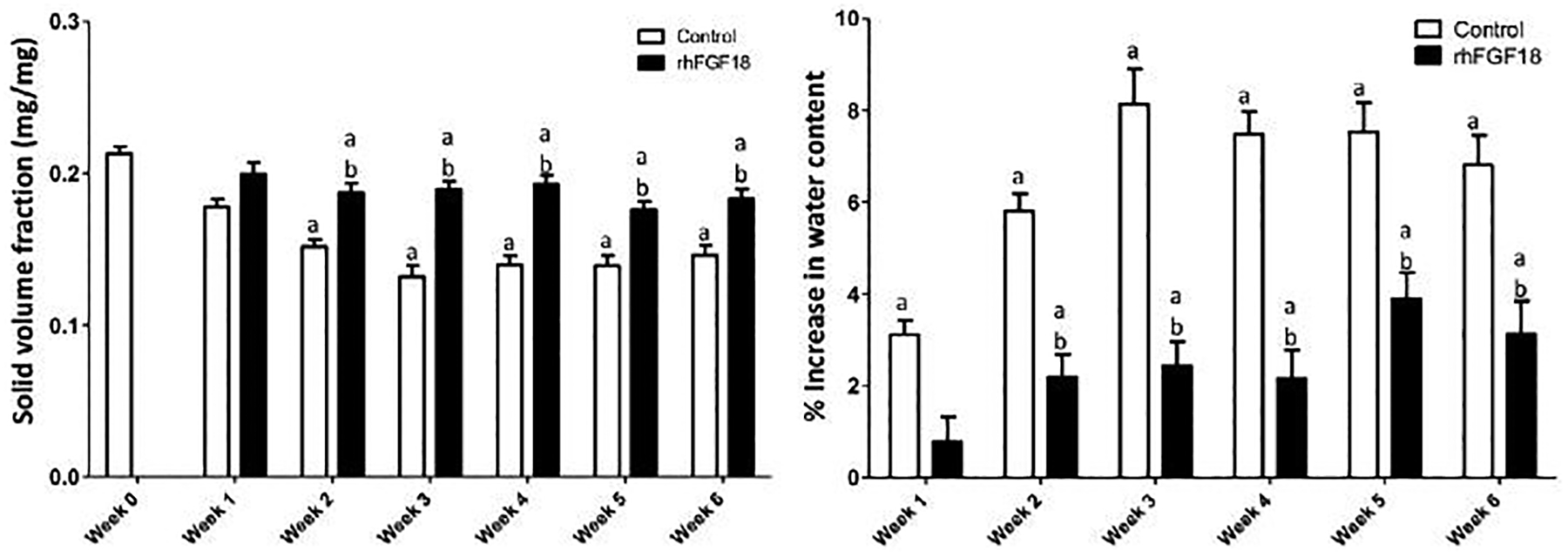
Increase in water content is attenuated with sprifermin treatment. a p < 0.05 vs. week 0 control, b p < 0.05 vs. control at same time point (n = 19–31).
Matrix Loss from Explants with Sprifermin Treatment
Assays on the medium were also performed to determine GAG and MMP release from the tissue. Although there were some minor differences in weekly GAG released into the medium, none of these differences reached the level of significance (Figure 7A). Similarly, cumulative GAG release into the medium did not differ between treatment groups (Figure 7B). Control samples also showed a steady level of MMP released to the medium each week. Conversely, sprifermin treated samples showed a marked reduction in MMP in the medium through the first three weeks of culture (Figure 8).
Fig. 7.
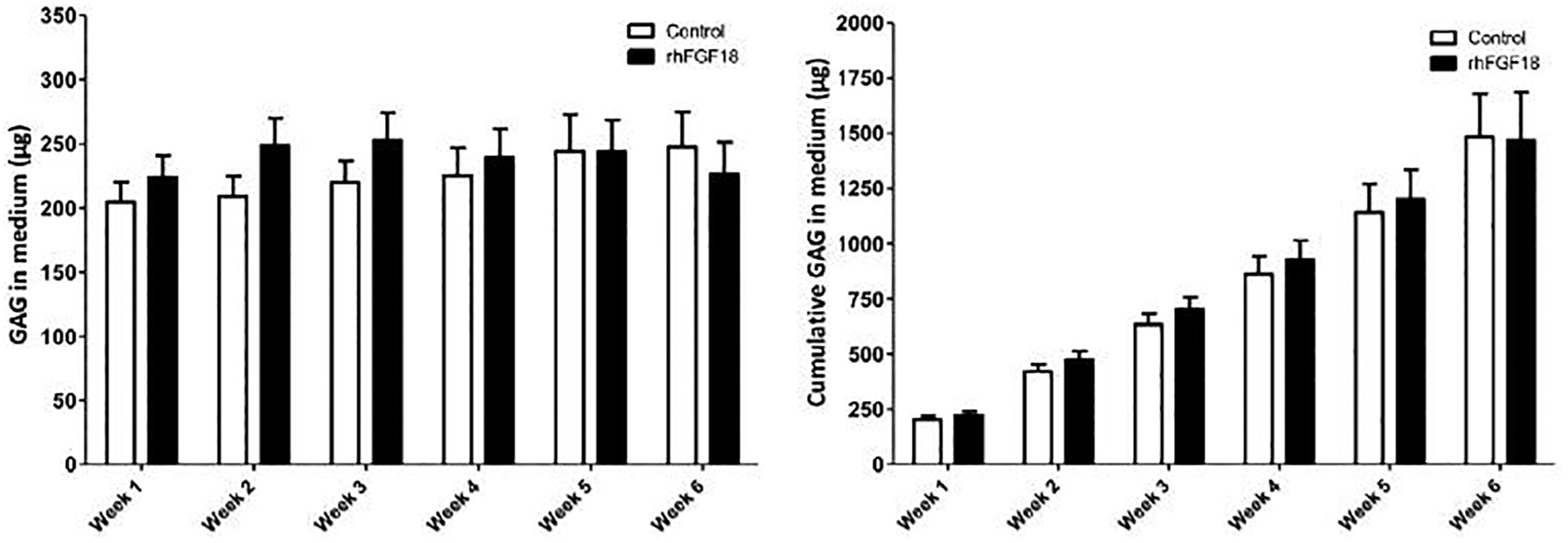
GAG released into the culture medium per explant and cumulative GAG in the medium over the course of 6 weeks of in vitro culture (n = 7–25).
Fig. 8.
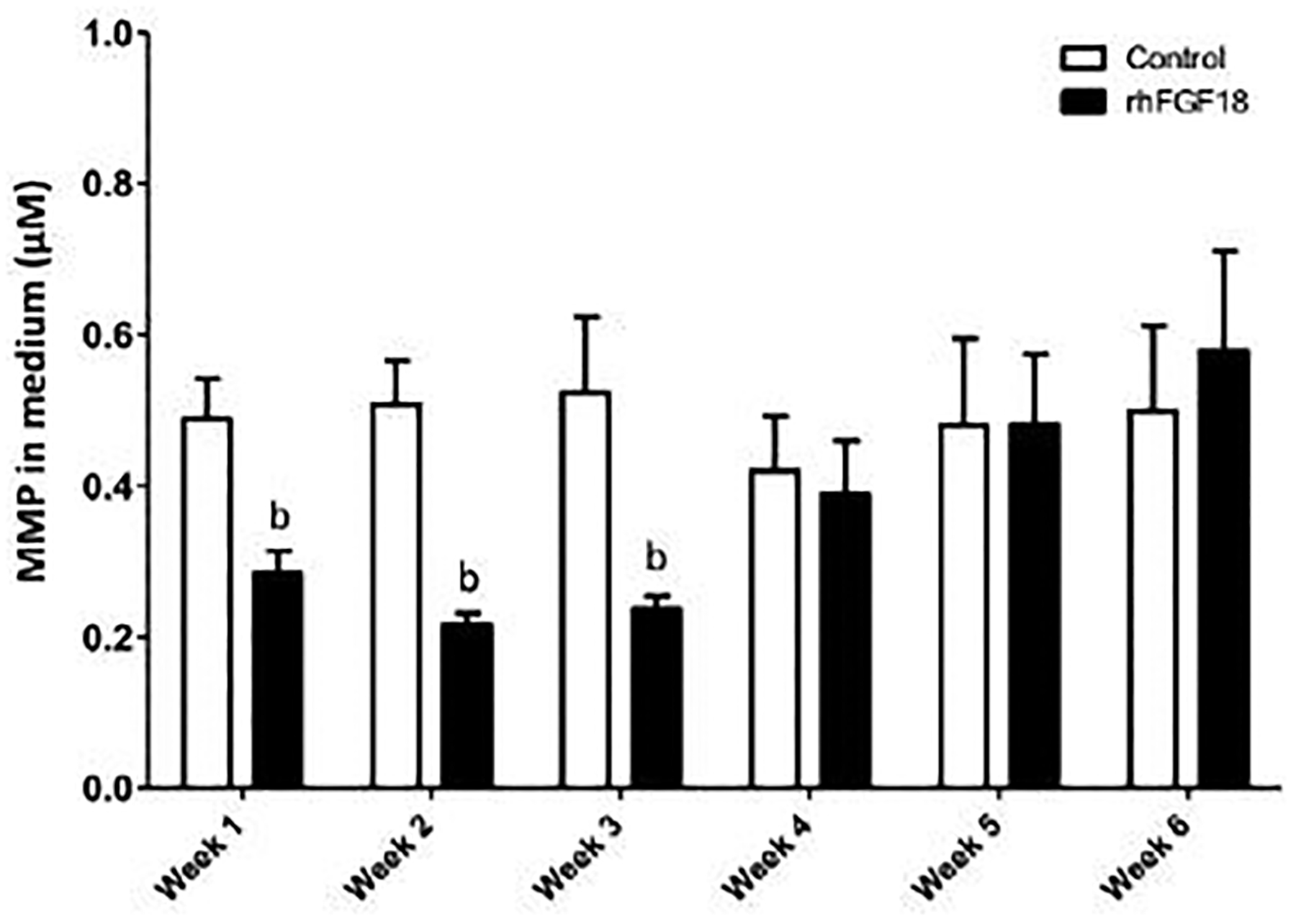
MMP concentration in the medium. MMP release into the medium was significantly suppressed with weekly sprifermin treatments through the first 3 weeks of exposure (bp < 0.05 vs. control at same time point, n = 7–25).
Discussion
In this study, we demonstrated the efficacy of sprifermin as storage media additive to preserve articular cartilage during extended in vitro storage. At the tissue level, sprifermin had a significant effect on the equilibrium modulus. Control samples quickly decreased modulus starting after week 2, while sprifermin treated samples showed no change in modulus through five weeks of in vitro culture. Additionally, the sprifermin treatment group had a higher modulus when compared to control samples at weeks 2 through 4 of culture. These findings were replicated using two different measurement techniques, while not directly comparable the differences between treated and untreated were reproducibly similar. To visualize the order of magnitude of these changes, the results from one study are shown in Figure 2. In this replicate study, sprifermin maintained or increased the tissue-scale modulus from week 0 through week 2 of culture, whereas control explants decreased in modulus at all time points.
To further examine and identify changes, we utilized a technique to track strain (and so calculate modulus) throughout the depth of the cartilage tissue, allowing us to determine the depth-dependent mechanical properties of each sample. This is an important feature, given that the native surrounding tissue shows such depth dependence, and so functional integration of the implanted osteochondral unit might be compromised if the implant does not match native tissue values. This high-resolution method resulted in a plot of modulus in 50 micron increments. As early as one week of in vitro culture, there was a divergence in mechanical properties between the control (Figure 3, dashed lines) and sprifermin samples (Figure 3, solid lines), where the former decreased from 2000 to 3000 microns from the cartilage surface and the latter increased over initial values at the same depth. This divergence point moved closer to the cartilage surface as the duration of culture increased, indicating that control samples were losing their mechanical properties in the deep zones faster than in the superficial zones. This pattern results in control samples that have lost their intrinsic inhomogeneity, whereas samples from the sprifermin treatment group maintained a depth-dependent modulus profile through five weeks of culture.
The largest differences in local mechanical properties were observed in the deepest zones of our samples, furthest from the cartilage surface. The samples used in this study are from immature bovine cartilage, where the cartilage thickness will be much less when the animals fully mature. Thus, it was important to examine changes in this tissue closer to the cartilage surface, in regions that will likely remain as mature, permanent cartilage and not transition into bone. In these more superficial regions, sprifermin also preserved the mechanical properties of the cartilage for at least three weeks, as shown in Figure 4. This finding demonstrates that sprifermin is able to preserve the depth-dependent mechanical characteristics of articular cartilage in an ex vivo environment for an extended duration. Given that this three-week time window is sufficient for screening of implants for communicable diseases, this finding may improve preservation methods for allogeneic OCT procedures.
The mechanical testing carried out in this study to determine both the tissue-scale and local properties of the tissue consisted of uniaxial compression. This is a sensible first assay, given that the native tissue functions in compression and testing of the tissue in this way is necessary to understand the change in mechanical properties (Korhonen et al., 2003). There are two principle extracellular matrix components that regulate the mechanical properties of cartilage, proteoglycans (PGs) and collagen (Julkunen et al., 2007). Although the PG content is known to resist compressive loads through charge-charge repulsion (via the fixed negative charges on the PGs) and water retention and interstitial fluid pressurization (Khoshgoftar et al., 2013) (via the Donnon osmotic effect and the frictional drag of fluid flowing through the small pores), it appears that PGs are not the central player in the action of sprifermin. Rather, the preservation of cartilage mechanical integrity with weekly sprifermin treatment appears to be due to maintenance of the collagen network. While collagen acts to resist tensile loads, it is also a key contributor to the mechanical response in compression by resisting the outward expansion of the cartilage tissue (the Poisson effect). The equilibrium Poisson ratio of native cartilage is quite low (on the order of 0.1–0.2), due in large part to the high tensile properties in the plane of the tissue from the high collagen content (Wang et al., 2003). Explants treated with rhFGF18 had higher collagen levels when compared to control samples at all time points from week 3 to 6. Additionally, treated samples did not differ from baseline levels until week 4. These significant differences were not seen in either the GAG levels in the tissue itself, or the GAG measured in the medium. Given that the mechanical response of the tissue is largely governed by these two components, we conclude that sprifermin maintained native cartilage mechanical properties through preservation of the collagen network.
Further evidence of the importance of collagen and the ability of sprifermin to maintain this crucial network is in the solid volume fraction or swelling of the tissues during in vitro culture. Solid volume fraction and collagen content are directly related (Bank et al., 2000). The change in solid volume fraction can also be expressed as the influx of water. Control samples were unable to resist the imbibition of water into the tissue, and so the tissue swelled substantially. We hypothesize that since control samples are unable to resist this expansion, they may also lose their ability to undergo interstitial fluid pressurization during loading, resulting in reduced mechanical functionality. Cartilage samples from the sprifermin treatment group were better able to resist swelling as the collagen network was better preserved. The changes in the collagen content are likely due to differential expression and activity of MMPs. We measured the MMP activity in the culture medium as an indicator of MMP expression and activation during in vitro culture. Sprifermin treatment significantly suppressed MMP levels for three weeks, while control samples had a higher, steady concentration of MMPs in the media. This is the same period over which we determined that sprifermin treatment maintained tissue-scale mechanical properties, local depth-dependent mechanical properties, and collagen content. The mechanism of action of FGF18 is to induce cell proliferation, which is in the articular joint context the specific proliferation of chondrocytes because of the FGF-receptor subtype distribution. The chondrocytes need space for proliferation and therefore this process is started with the expression of matrix dissolving proteases, e.g. MMP13. Chondrocytes that recently proliferated are producing more matrix (more, because the cartilage is populated by more chondrocytes and more, because of an increased matrix production rate per cell) by activation of the ERK pathway (Gigout et al., 2017). In 3D culture, FGF18 increases the number of matrix-producing chondrocytes, improves the type II:I collagen ratio, and enables chondrocytes to produce a hyaline extracellular matrix. Furthermore, FGF18 displays a ‘hit and run’ mode of action, which is a common phenomenon for growth factors (Gigout et al., 2017). The chondrocyte survival time in cartilage explants seems to be increased in in-vitro experiments, however, cell viability was not evaluated in this current study.
The findings of this study suggest that sprifermin may be used as a medium supplement during storage or extended culture of viable cartilage tissue has significant clinical application. Currently, donor tissue for allograft procedures is refrigerated while it is screened prior to transplant. This practice maintains some of the mechanical properties of the native tissue, but also results in low chondrocyte viability. Using sprifermin under normal culture and storage conditions, we showed that cartilage mechanical inhomogeneity can be maintained over a similar time course. In this study, we used cartilage from multiple bovine sources of approximately the same age. The advantage to this was in reducing the number of variables, namely age of the cartilage. However, in future work and for clinical applications, the maturity of the cartilage will be an important factor to consider as it has a great effect on the tissue homeostasis and activity. Studies to address this issue and to replicate these findings in human sourced materials are now underway.
Conclusions
Sprifermin preserved the intrinsic depth-dependent mechanical properties of articular cartilage during long-term in vitro culture/storage conditions through maintenance of extracellular collagen and suppression of matrix metalloproteinase production. This methodology may be applied in a clinical scenario to improve on current transplantation practice, where prior to surgery the tissue is stored during screening, compromising its mechanical properties. Furthermore, this study also presents potential mechanisms for sprifermin mode of action in protecting the integrity of cartilage and ultimately impact a functional characteristic of articular cartilage in restorative cartilage strategies.
Acknowledgements
This work was in part supported by a grant from Merck KGaA of which one of the authors (H.G.) is a paid employee. Support was also provided by the Department of Veteran’s Affairs (I01 RX001213) and the NIH via the Penn Center for Musculoskeletal Diseases Histology and Biomechanics Cores (P30 AR069619).
References
- Allen RT, Robertson CM, Pennock AT, Bugbee WD, Harwood FL, Wong VW, Chen AC, Sah RL, Amiel D (2005) Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med 33: 1479–1484. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Lai WM, Zhu WB, Mow VC (1994) An asymptotic solution for the contact of two biphasic cartilage layers. J Biomech 27: 1347–1360. [DOI] [PubMed] [Google Scholar]
- Aubin PP, Cheah HK, Davis AM, Gross AE (2001) Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res 391 Suppl: S318–327. [DOI] [PubMed] [Google Scholar]
- Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD (2004) The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res 418: 246–252. [DOI] [PubMed] [Google Scholar]
- Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM (2000) The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum 43: 2202–2210. [DOI] [PubMed] [Google Scholar]
- Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 87: 640–645. [DOI] [PubMed] [Google Scholar]
- Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J (2010) Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc 18: 519–527. [DOI] [PubMed] [Google Scholar]
- Bian L, Lima EG, Angione SL, Ng KW, Williams DY, Xu D, Stoker AM, Cook JL, Ateshian GA, Hung CT (2008) Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech 41: 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittberg M (2008) Autologous chondrocyte implantation--technique and long-term follow-up. Injury 39 Suppl 1: S40–49. [DOI] [PubMed] [Google Scholar]
- Brittberg M (2010) Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med 38: 1259–1271. [DOI] [PubMed] [Google Scholar]
- Bugbee WD, Convery FR (1999) Osteochondral allograft transplantation. Clin Sports Med 18: 67–75. [DOI] [PubMed] [Google Scholar]
- Chen AC, Bae WC, Schinagl RM, Sah RL (2001) Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech 34: 1–12. [DOI] [PubMed] [Google Scholar]
- Chow JC, Hantes ME, Houle JB, Zalavras CG (2004) Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy 20: 681–690. [DOI] [PubMed] [Google Scholar]
- Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D (1999) Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res 36: 159–168. [PubMed] [Google Scholar]
- Dahlberg LE, Aydemir A, Muurahainen N, Gühring H, Fredberg Edebo H, Krarup-Jensen N, Ladel CH, Jurvelin JS (2016) A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol 34: 445–450. [PubMed] [Google Scholar]
- Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren HP, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD (2002) Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage 10: 308–320. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883: 173–177. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Comeau ES, Mauck RL (2012) Mesenchymal stem cells produce functional cartilage matrix in three-dimensional culture in regions of optimal nutrient supply. Eur Cell Mater 23: 425–440. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ (2004) Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem 279: 19502–19511. [DOI] [PubMed] [Google Scholar]
- Garrity JT, Stoker AM, Sims HJ, Cook JL (2012) Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med 40: 2542–2548. [DOI] [PubMed] [Google Scholar]
- Gigout A, Guehring H, Froemel D, Meurer A, Ladel C, Reker D, Bay-Jensen AC, Karsdal MA, Lindemann S (2017) Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage 25: 1858–1867. [DOI] [PubMed] [Google Scholar]
- Guilak F, Meyer BC, Ratcliffe A, Mow VC (1994) The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage 2: 91–101. [DOI] [PubMed] [Google Scholar]
- Hall AC, Urban JP, Gehl KA (1991) The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res 9: 1–10. [DOI] [PubMed] [Google Scholar]
- Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I (2010) Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med 38: 1125–1133. [DOI] [PubMed] [Google Scholar]
- Hangody L, Fules P (2003) Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A Suppl 2: 25–32. [DOI] [PubMed] [Google Scholar]
- Hangody L, Vásárhelyi G, Hangody LR, Sükösd Z, Tibay G, Bartha L, Bodó G (2008) Autologous osteochondral grafting--technique and long-term results. Injury 39 Suppl 1: S32–39. [DOI] [PubMed] [Google Scholar]
- Hascall VC, Handley CJ, McQuillan DJ, Hascall GK, Robinson HC, Lowther DA (1983a) The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys 224: 206–223. [DOI] [PubMed] [Google Scholar]
- Hascall VC, Morales TI, Hascall GK, Handley CJ, McQuillan DJ (1983b) Biosynthesis and turnover of proteoglycans in organ culture of bovine articular cartilage. J Rheumatol Suppl 11: 45–52. [PubMed] [Google Scholar]
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R (2003) Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85-A: 185–192. [DOI] [PubMed] [Google Scholar]
- Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA (2003) Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng 125: 84–93. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Kiviranta P, Wilson W, Jurvelin JS, Korhonen RK (2007) Characterization of articular cartilage by combining microscopic analysis with a fibril-reinforced finite-element model. J Biomech 40: 1862–1870. [DOI] [PubMed] [Google Scholar]
- Khoshgoftar M, Wilson W, Ito K, van Donkelaar CC (2013) Influence of tissue- and cell-scale extracellular matrix distribution on the mechanical properties of tissue-engineered cartilage. Biomech Model Mechanobiol 12: 901–913. [DOI] [PubMed] [Google Scholar]
- Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89: 2105–2112. [DOI] [PubMed] [Google Scholar]
- Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A: 455–464. [DOI] [PubMed] [Google Scholar]
- Korhonen RK, Laasanen MS, Toyras J, Lappalainen R, Helminen HJ, Jurvelin JS (2003) Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech 36: 1373–1379. [DOI] [PubMed] [Google Scholar]
- Korver GH, van de Stadt RJ, van Kampen GP, van der Korst JK (1990) Composition of proteoglycans synthesized in different layers of cultured anatomically intact articular cartilage. Matrix 10: 394–401. [DOI] [PubMed] [Google Scholar]
- Kuroki K, Stoker AM, Stannard JP, Bozynski CC, Cook CR, Pfeiffer FM, Cook JL (2017) Biologic Joint Repair Strategies: The Mizzou BioJoint Story. Toxicol Pathol 45: 931–938. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, Eckstein F (2014) Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 66: 1820–1831. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH (1988) Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys 267: 416–425. [DOI] [PubMed] [Google Scholar]
- Malinin T, Temple HT, Buck BE (2006) Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am 88: 762–770. [DOI] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S (2007) Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med 35: 2014–2021. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122: 252–260. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Yuan X, Tuan RS (2006) Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14: 179–189. [DOI] [PubMed] [Google Scholar]
- McCarty WJ, Pallante AL, Rone RJ, Bugbee WD, Sah RL (2010) The proteoglycan metabolism of articular cartilage in joint-scale culture. Tissue Eng Part A 16: 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, Wilsen H, Bach B Jr, Cole B (2014) Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy 30: 222–226. [DOI] [PubMed] [Google Scholar]
- Mickevicius T, Pockevicius A, Kucinskas A, Gudas R, Maciulaitis J, Noreikaite A, Usas A (2015) Impact of storage conditions on electromechanical, histological and histochemical properties of osteochondral allografts. BMC Musculoskelet Disord 16: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer K, Williams RJ, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG (2005) The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 87: 1911–1920. [DOI] [PubMed] [Google Scholar]
- Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL (2005) Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage 13: 623–631. [DOI] [PubMed] [Google Scholar]
- Mori Y, Saito T, Chang SH, Kobayashi H, Ladel CH, Geuhring H, Chung UI, Kawaguchi H (2014) Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J Biol Chem 289: 10192–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG (1980) Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng 102: 73–84. [DOI] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A, Poole AR (1992) Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13: 67–97. [DOI] [PubMed] [Google Scholar]
- Ohlendorf C, Tomford WW, Mankin HJ (1996) Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res 14: 413–416. [DOI] [PubMed] [Google Scholar]
- Pallante-Kichura AL, Chen AC, Temple-Wong MM, Bugbee WD, Sah RL (2013) In vivo efficacy of fresh versus frozen osteochondral allografts in the goat at 6 months is associated with PRG4 secretion. J Orthop Res 31: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallante AL, Bae WC, Chen AC, Gortz S, Bugbee WD, Sah RL (2009) Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts. Am J Sports Med 37 Suppl 1: 24S–32S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallante AL, Gortz S, Chen AC, Healey RM, Chase DC, Ball ST, Amiel D, Sah RL, Bugbee WD (2012) Treatment of articular cartilage defects in the goat with frozen versus fresh osteochondral allografts: effects on cartilage stiffness, zonal composition, and structure at six months. J Bone Joint Surg Am 94: 1984–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Krishnan R, Nicoll SB, Ateshian GA (2003) Cartilage interstitial fluid load support in unconfined compression. J Biomech 36: 1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L, Vasiliadis HS, Brittberg M, Lindahl A (2010) Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 38: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Power J, Hernandez P, Guehring H, Getgood A, Henson F (2014) Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J Orthop Res 32: 669–676. [DOI] [PubMed] [Google Scholar]
- Reker D, Kjelgaard-Petersen CF, Siebuhr AS, Michaelis M, Gigout A, Karsdal MA, Ladel C, Bay-Jensen AC (2017) Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. J Transl Med 15: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, Trippel SB (1994) Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys 308: 137–147. [DOI] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD (1989) Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7: 619–636. [DOI] [PubMed] [Google Scholar]
- Schinagl RM, Ting MK, Price JH, Sah RL (1996) Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Ann Biomed Eng 24: 500–512. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA (2000) A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng 122: 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophia Fox AJ, Bedi A, Rodeo SA (2009) The basic science of articular cartilage: structure, composition, and function. Sports Health 1: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391 Suppl: S362–369. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K (1967) Determination of hydroxyproline. Clin Chim Acta 18: 267–273. [DOI] [PubMed] [Google Scholar]
- Stoker AM, Stannard JP, Cook JL (2017) Chondrocyte Viability at Time of Transplantation for Osteochondral Allografts Preserved by the Missouri Osteochondral Preservation System versus Standard Tissue Bank Protocol. J Knee Surg 31: 772–780. [DOI] [PubMed] [Google Scholar]
- Stoker AM, Stannard JP, Kuroki K, Bozynski CC, Pfeiffer FM, Cook JL (2018) Validation of the Missouri Osteochondral Allograft Preservation System for the Maintenance of Osteochondral Allograft Quality During Prolonged Storage. Am J Sports Med 46: 58–65. [DOI] [PubMed] [Google Scholar]
- Teng MS, Yuen AS, Kim HT (2008) Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res 466: 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chahine NO, Hung CT, Ateshian GA (2003) Optical determination of anisotropic material properties of bovine articular cartilage in compression. J Biomech 36: 339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Deng JM, Ateshian GA, Hung CT (2002) An automated approach for direct measurement of two-dimensional strain distributions within articular cartilage under unconfined compression. J Biomech Eng 124: 557–567. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Dreese JC, Chen CT (2004) Chondrocyte survival and material properties of hypothermically stored cartilage: an evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med 32: 132–139. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Ranawat AS, Potter HG, Carter T, Warren RF (2007) Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am 89: 718–726. [DOI] [PubMed] [Google Scholar]
- Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD (2003) Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am 85-A: 2111–2120. [DOI] [PubMed] [Google Scholar]


