Abstract
During the past two decades the field of nanomedicines has experienced significant progress. To date, over sixty nanoparticle (NP) formulations have been approved in the US and EU while many others are in clinical or preclinical development, indicating a concerted effort to translate promising bench research to commercially viable pharmaceutical products. The use of NPs as novel drug delivery systems, for example, can improve drug safety and efficacy profiles and enable access to intracellular domains of diseased cells, thus paving the way to previously intractable biological targets. However, the measurement of their physicochemical properties presents substantial challenges relative to conventional injectable formulations. In this perspective, we focus exclusively on particle size, a core property and critical quality attribute of nanomedicines. We present an overview of relevant state-of-the-art technologies for particle sizing, highlighting the main parameters that can influence the selection of techniques suitable for a specific size range or material. We consider the increasing need, and associated challenge, to measure size in physiologically relevant media. We detail the importance of standards, key to validate any measurement, and the need for suitable reference materials for processes used to characterize novel and complex NPs. This perspective highlights issues critical to achieve compliance with regulatory guidelines and to support research and manufacturing quality control.
Keywords: Physicochemical characterization; Particle sizing; Reference materials/standards, Critical quality attributes, Physiological conditions
INTRODUCTION
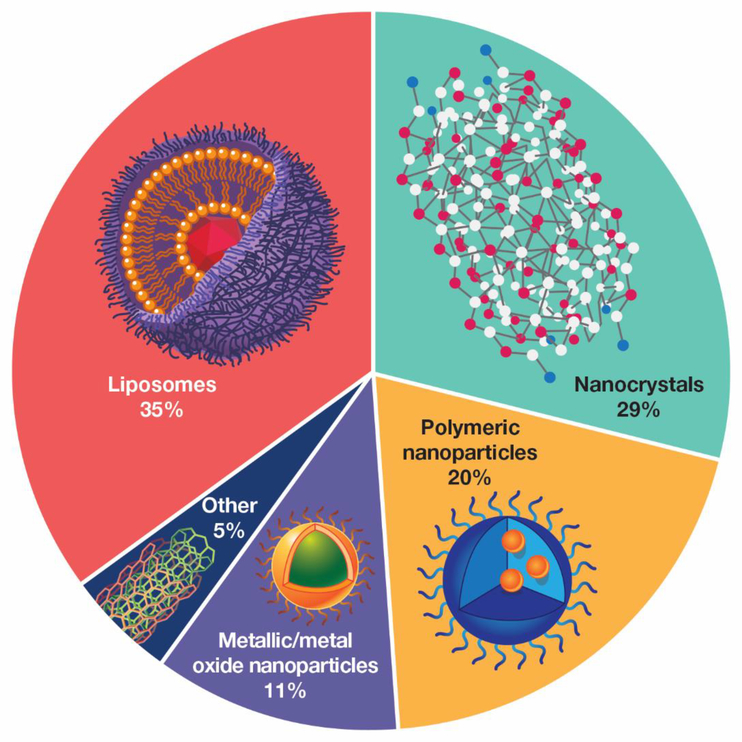
Nanomedicine can be defined as the field in which nanotechnologies are applied for the diagnosis, prevention and treatment of disease (1). There are different types of nanotechnologies used in nanomedicines, however for the purposes of this perspective, we are focusing on nanoparticle-based products ranging in the nanometer size from 1–1000 nm. The size range associated with nanotechnology varies according to different definitions and organizations. The Organization for Standardization (ISO) and the US National Nanotechnology Initiative and European Commission define “nanoscale” as having dimensions between roughly 1 nm and 100 nm, whereas this perspective uses the broader definition applied commonly by industry. These nanotechnologies (Figure 1) can include nano-delivery systems that contain encapsulated, dispersed, adsorbed or conjugated drugs and/or imaging agents (2). The nanomedicines field has grown and evolved substantially, as shown both by the number of peer reviewed publications in nanomedicine related areas (>22,000 in the past 10 years (Web of Science Boolean search, refer to Supplementary Material for details)), and the increasing number of clinical trials (about 200 ongoing (PharmaCircle search, refer to Supplementary Material for details) involving nanoparticles (NPs). The interest in this field, driven by the potential for targeting new cell compartments and improving half-life and biodistribution of drugs, has also attracted the attention of regulatory agencies and underscored the need to define critical quality attributes (CQAs) specific to nanomedicine (3). Amongst CQAs, particle size analysis with accuracy and precision is a principal physicochemical property, necessary to define the quality of nanomedicines, from both an academic (research) and an industrial (development and manufacturing) perspective. Particle size analysis in NP-based products is important for understanding how size may change with time, thus indicating product shelf life and NP stability, and differences in drug loading. Size can also determine a NP’s ability to accumulate in selective tissues (e.g., tumors) via, for example, the enhanced permeation and retention effect. Unlike small molecule drugs and proteins, NP-based drug delivery systems can span a wide range of physical sizes in the nanometer range and are typically polydisperse. Additionally, these systems are generally dispersed in a complex aqueous medium where they require treatment to ensure colloidal stability. Often, there are agglomerates present that must also be detected and characterized for purposes of manufacturing quality control, efficacy and safety. This presents substantial challenges in terms of reliable and reproducible measurements, critical issues that have been raised recently by the scientific community (4).
Figure 1.
Pie chart showing relative distribution of nanomaterials used in drug products that were submitted to the FDA from 2010–2015 (Adapted from D’Mello et al.(5)). The chart has been simplified by grouping related materials. For example, polymeric NPs includes iron-polymer complexes, micelles, dendrimers, and polymeric NPs.
In 2017, the authors formed a particle size focused subteam under the auspices of the Nanomedicines Alliance, jointly with the European Technology Platform for Nanomedicine. This group had representatives from industry (AstraZeneca, NanoCarrier US and Pfizer), government (FDA-CDER, NCL and NIST) and academia (Trinity College Dublin). The Nanomedicines Particle Size Team (NPST) focused on disseminating the scientific and regulatory aspects and policy needs of nanomedicines with an emphasis on size measurement. NPST organized three symposia focused on particle size, with the objective of attracting strong leadership and expertise in the particle size analysis field to share their diverse and applied knowledge. The result, a symposium on “Particle Sizing of Nanoparticles: From Regulatory & Metrology Aspects to Application & Analysis” was organized through the American Chemical Society during the August 2018 meeting in Boston, MA. Two additional symposia followed at the European Foundation for Clinical Nanomedicine meeting (CLINAM 2018) in Basel, Switzerland.
Three common themes resonated throughout the particle size symposia at these two separate international events: 1) Selection of appropriate analytical techniques for particle sizing and the importance of orthogonal analysis, 2) Selection of appropriate media for relevant particle size measurements and 3) Reference materials and standards for nanomedicine applications. This perspective aims to discuss and provide insight into these three themes by highlighting the various analytical sizing techniques available, the importance of particle sizing under physiological conditions, and the need for metrologically defined standards. As one of the fundamental CQAs for preclinical and clinical product development, size has a pivotal role in quality control (QC) where it is a key acceptance criterion for manufactured NPs and NP-based drugs. It can be used to define their shelf life and handling. Size is also a critical measurement in early design of nanomedicines to explore effects of different sized NPs in the context of their in vivo performance, such as prolonging plasma circulation, tissue penetration, and intracellular uptake, for example. The focus of this perspective will be on the QC aspects and its impact on injectable NPs drug products.
During the aforementioned meetings, a definitive need for novel and orthogonal characterization techniques was highlighted. There are several requirements within each sizing technique that needs to be taken into account in order to correctly interpret the implications of what is measured for the CQA. Some requirements are basic for any analytical technique, such as measurements need to be reproducible, true and precise. Other requirements are very specific such as the composition of NPs, the importance of assessing a broad range of concentrations under diverse handling and storage conditions (e.g., to cover a clinical dose range), and the capacity to measure a broad size range, including smaller NPs (for example dendrimers in the sub 20 nm region) emerging as new drug delivery systems (6, 7) as well as aggregates and agglomerates. Lastly, as it relates to a high throughput setting, the ease of sample handling and automation is valuable to industry. More recently, orthogonal analysis, particularly with respect to size measurement, has been recognized as critical to reliable/reproducible characterization of NPs in biomedical applications (8). Diverse sizing techniques are commercially available and instrumentation improvements taking into account NP shape, polydispersity, dispersing media or solvent, concentration, particle composition and interactions with the measuring system, are expanding to provide a range of options. For instance, industry would benefit from online measurements compared to offline or “batch sampling”. The adoption of cost-effective solutions, for instance when separation and characterization are achieved in a single analysis, can potentially reduce the development costs for nanomedicines.
Measurement Technologies – A Brief Overview
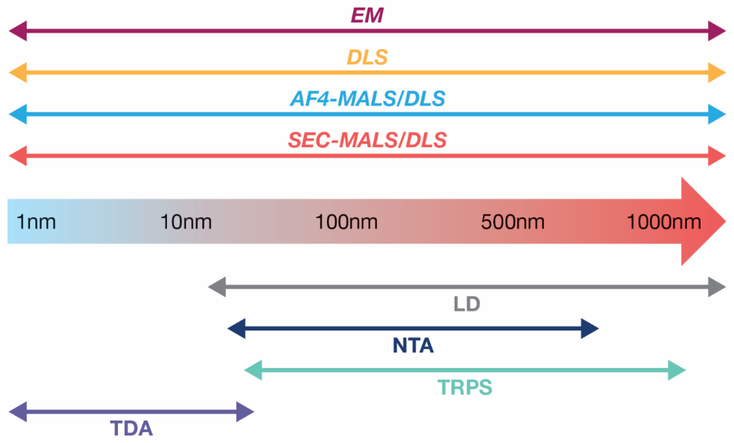
There are a considerable number of analytical sizing techniques (and instrumentation) available to the researcher and selecting the appropriate method can be challenging. Choosing the appropriate technique depends on many parameters such as NP type, size, and concentration, to name a few. Since it is impossible to cover every sizing technique, we have decided to focus on the more commonly used and available sizing techniques (Figure 2). The more traditional techniques that are currently widely used in research and industry are dynamic light scattering (DLS), laser diffraction (LD), and electron microscopy (EM). This is followed by a discussion on the application of separation, namely SEC (size exclusion chromatography) and FFF (field flow fractionation), coupled with light scattering to help with resolution issues. Finally, a discussion on relatively new sizing techniques (for example, nanoparticle tracking analysis (NTA), tunable resistive pulse sensing / resistive pulse sensing (TRPS/RPS), and Taylor dispersion analysis (TDA)), which do not require a separation process but can still analyze polydispersed samples, is given.
Figure 2.
Schematic representation comparing the applicable size (diameter) range for different sizing techniques of nanoparticles. Note that these ranges are approximate and can depend highly on other material or matrix properties.
DLS is the most widely available and extensively used analytical technique for sizing in the nanoscale region. It is a popular technique used by both academics and industry because the instrumentation is relatively inexpensive and simple to use (5). Despite the reliance on DLS for routine particle sizing, this technique is impacted by several limitations mainly around the three themes previously mentioned, therefore the use of orthogonal sizing techniques from the very early stages of product development is highly recommended. One of the drawbacks of DLS and, more generally, of techniques based on light scattering, is that the amount of light scattered by a particle is strongly correlated with particle size. This is a concern for smaller NPs (diameter < 10 nm), since the presence of a small number of large particles can scatter much more than a large population of small particles, which would then contribute marginally to the total signal.
Laser diffraction (LD) is a well-established sizing technique in the pharmaceutical industry. The intensity of the scattered light as a function of angle is used to calculate the size of a volume-equivalent sphere based on Mie theory. LD has been historically associated with analysis of large particles, i.e., sub-micrometer to millimeter size range, but advanced optics extend measurement into the nanometer range. As a result, LD has a wide dynamic range and is a relatively fast measurement. However, the optical properties, namely the refractive index and the imaginary component, of the particles must be known. In the absence of this information, modelling is performed to determine the optical properties which can in turn lead to errors (9).
Another traditional and widely used sizing technique is electron microscopy, e.g., transmission and scanning electron microscopy (TEM and SEM), which is an imaging method applied generally to dry deposited samples. EM has been used for decades to measure particle size and define morphology simultaneously. One of the main limitations of this technique is sample preparation, which requires drying the sample on a solid substrate, and often negative staining (for organic components). Both procedures can potentially create artefacts due to aggregation. Additionally, to obtain a statistically significant representation of a sample, multiple image acquisitions are needed, requiring software to help analyze the images and to reduce human bias. The introduction of Cryo-EM has further reduced artefacts generated due to staining and drying, as the sample is vitrified, and, with highly ordered samples, the resolution can be angstrom level (10). Unfortunately, the expensive instrumentation required, the need for a skilled operator, maintenance costs and the turnaround time of analyses (especially for Cryo-EM) render the use of this technique prohibitive in terms of routine batch QC on manufactured NP batches. However, this is a prominent technique in the product discovery phase to evaluate not only size, but other CQA’s such as morphology.
The techniques discussed up to this point are “batch mode” methods. Examples of flow mode methods include SEC and FFF, which, when coupled with a series of different detectors, may improve resolution even at the smallest size range (11, 12). These techniques are coupled at the front end with a liquid chromatography system, which can be easily automated making the sample handling efficient and enabling for high throughput analysis. However, in contrast to most batch techniques, separation-based techniques generally require method development for each type of NP platform to ensure robust and accurate measurements, thus they can be more time consuming, at least initially. For sizing applications, both SEC and FFF are typically coupled to at least two detectors: for example, UV-Vis, refractive index (RI) or multi-angle light scattering (MALS). Additionally, the inclusion of on-line DLS with SEC or FFF can overcome the polydispersity issue associated with DLS batch measurements. The multi-detector approach has the capacity to provide mass concentration, composition, molar mass, size and shape of each detected NP population in a single experiment. Nevertheless, to obtain information on size and shape from combined MALS and DLS results, the NPs must be larger than about 10 nm (i.e., for small isotropic scatterers, the radius of gyration, Rg, cannot be determined by MALS). In order to overcome this limitation, some instruments have been equipped with a viscometer, which provides intrinsic viscosity data and allows for size measurement of isotropic scatterers. On the other hand, on-line DLS allows size measurements over the entire nanometric range. FFF is a separation technique without a solid phase that can be used to fractionate samples in complex mixtures and covers a wide size range. There are several typologies of FFF on the market that use different forces for separation of which Asymmetric-Flow Field-Flow Fractionation (AF4) is the most widely used. SEC and FFF share some aspects (they are both liquid based separations) and the choice of using one technique over the other is highly dependent on the target analyte; FFF offers more flexibility with respect to sample type and mobile phase, however method development can be time consuming and challenging (11, 13). That said, the adoption of FFF by the pharmaceutical and nanotechnology industries is rapidly accelerating. In recognition of this increasing awareness and utilization of FFF measurement technology, the International Organization for Standardization (ISO) recently published the first international standard on the application of FFF for nanoparticles (14).
Generally, batch techniques can struggle with the analysis of polydisperse samples, although there are some techniques that are able to resolve multiple populations in a single system, for instance NTA and TRPS (15–17). NTA (also referred to more generally as particle tracking analysis) is based on light scattering detection, but with the addition of a charge-coupled device camera and advanced software that can track the trajectory of individual NPs. NTA provides an improvement in resolution of different size particles compared with DLS, nonetheless NTA has a low-end size limit (tens of nm) that is above DLS due to the tracking technology (16, 18). An added feature of NTA is the simultaneous measurement of size and particle concentration. An extension to NTA, multispectral advanced nanoparticle tracking analysis (MANTA) incorporates three lasers at different wavelengths which allows for the simultaneous analysis of polydispersed samples and a wider dynamic size range. In TRPS which is based on impedance due to a particle passing through a nanopore, yields three properties simultaneously: particle size, particle concentration and zeta potential. In contrast to the other techniques mentioned in this section, which are based on first principles, TRPS analysis requires calibration, which adds time to the measurement procedure, but also improves traceability. This can be both a detriment and a benefit depending on the application. Nevertheless, NTA and TRPS share a common limitation: the smallest size that can be accurately analyzed is several tens of nanometers, excluding a large portion of drug delivery systems that do not meet this size cutoff.
Taylor Dispersion Analysis (TDA) is based on dispersion of a solute plug in a capillary under laminar Poiseuille flow (19). Recently, an automated characterization system based on TDA with UV detection became commercially available, and as a result TDA is gaining a renewed interest in industry. For instance, at AstraZeneca several small NP products have been screened for size using this novel instrumentation, producing comparable data to other orthogonal techniques. In this instrument, a microcapillary flow system is coupled with a UV-based detector, enabling mass-weighted sizing measurements that are not adversely affected by the presence of a small amount of large species in solution and producing more robust results at the very small scale (0.3 nm to 20 nm) (20). Nevertheless, each technique has limitations; TDA can be biased by the presence of chromophoric impurities in the sample and interactions between solutes and capillary walls that can impact results; additionally, this is still a batch-mode technique and polydisperse samples cannot be effectively analyzed.
Looking forward, the ideal instrumentation would have the ability to characterize not just NP size, but also NP concentration and perhaps surface charge and shape properties at the single NP level in a wide range of media and relevant NP concentrations. Nevertheless, no such “ideal” instrument yet exists, and therefore orthogonal measurements are necessary in most cases to obtain the required size and size related information for nanomedicine applications. The shear diversity and inherent complexity of nanomaterials require careful selection and application of appropriate techniques. The recent emergence of new technologies and the continuing effort to develop and expand the sizing tool box for nanotechnology is extremely encouraging.
Measurement Media
The second focus or theme of this perspective is the selection of appropriate media to use for particle sizing measurements and the resulting factors that must be considered. This is critical and is dictated primarily by the context of the measurement (i.e., purpose). For instance, measuring size under storage conditions versus use conditions (e.g., physiological media, buffers or dispersant). The type of media used in both the storage and physiological conditions can greatly impact particle size measurement. Depending on the storage condition, an aqueous buffer solution (e.g., phosphate buffer saline (PBS), saline) containing a lyoprotectant (e.g., 10 % sucrose) could be used if the NP were stored at −80°C or below. In the case of physiological conditions, whole blood would be the ideal medium, but typically serum or plasma is used instead.
The choice of which aqueous buffer solution is used for particle sizing measurements depends on the type of NP and its stability in that medium. For example, at a minimum, particle sizing in the storage medium (i.e., native dispersing medium) should be made at stock concentration (depending on what sizing technique is employed) and at several dilutions. These types of measurements assess the NP storage or shelf-life stability (21). Additional aqueous solutions should be tested such as those used for zeta potential measurements (typically low salt concentration media) and in vitro drug release studies (if performed using non-protein containing media). Measurements in PBS are often used to mimic physiological conditions in the body and the in vitro drug release media is typically PBS. While PBS is a “clean and ideal” dispersant solution, the lack of protein-content makes it a non-indicative solution to be used when assessing the potential NP-protein interactions in a physiological environment. Regardless of the aqueous buffer solution chosen, currently available sizing instrumentation is equipped to handle these types of measurements.
However, a word of caution is needed regarding measurement conditions and analysis which are often technique-dependent. Measurements at stock concentration are ideal but often impractical. For example, multiple scattering can occur for light scattering methods (i.e., DLS) or clogging of pores for single-particle counting methods (i.e., TRPS). Techniques that measure size indirectly through the measurement of diffusion coefficients require knowing the viscosity of the sample. Thus, the dependence of viscosity on sample concentration would be required for accurate size measurements. As a result of these issues, samples are often diluted. This raises a question: how much can a sample be diluted to address the above-mentioned issues while maintaining both good instrument signal and practical relevance? Some techniques such as TRPS may require the addition of surfactant to the dispersing medium for the method to work properly; the effect of such an addition on NP integrity would be better elucidated through measurement using orthogonal techniques.
Particle sizing assessment under physiological conditions is more relevant and more challenging to measure (22). For nanomedicines that would eventually be administered intravenously, the particle size in a more appropriate physiological medium such as whole blood would represent a more accurate descriptor in vivo. This size would contain plasma proteins that have bound to the surface of the NP, which is often referred to as the protein corona. This larger size and new surface chemistry will ultimately dictate the NP’s biological performance. However, due to the large micrometer sizes of red and white blood cells (RBCs and WBCs) relative to the nanoformulations, it is not a trivial matter to simply measure in whole blood. In addition to their size, the relatively high concentrations of both the RBCs and WBCs would dominate the sample. Moreover, blood is a complex mixture of proteins, RBCs and WBCs, platelets, and co-factors among other things making it very viscous and impractical to run in most instruments without dilution. Finally, human blood is regarded as a biohazard and potentially infectious thus making it non-ideal from a safety and logistical perspective.
As an alternative, plasma or serum is used instead. The RBCs and WBCs are removed in plasma while serum is plasma with the clotting factors removed. Thus, the large blood cells, which limited the size measurements, are now removed, and we are left primarily with the plasma proteins. However, the protein concentration in plasma and serum is still relatively high. The proteins, namely albumins, globulins, and fibrinogen, can be present at concentrations up to 50 mg/mL. Using DLS as an example, size measurements on either plasma or serum alone results in a multimodal-size distribution (intensity-weighted distribution) with hydrodynamic sizes ranging from 7 nm to 100 nm making NP sizing difficult (J.D. Clogston, unpublished results). This limitation is overcome by measuring in diluted plasma or serum, typically 10%(v/v) (9, 23). Diluting the plasma and/or serum reduces the protein concentration and its contribution/interference in sizing measurements. However, this may still not represent a true physiological assessment. From a logistical instrument measurement point of view, the reduced protein concentration minimizes the potential swamping of signal due to high protein concentrations, fouling/clogging of the instrument, and reduced viscosity. The latter is important in sizing methods that require the viscosity to be known; as noted earlier, plasma and/or serum is complex and by diluting it, its viscosity approaches that of the dispersing medium. However, caution is still needed when working with diluted plasma and/or serum. For example, batch-mode DLS (off-line measurements made without any separation process) may require adjusting the NP concentration accordingly to have sufficient signal relative to the protein. Flow-mode DLS analysis (measurements made after separation such as SEC or AF4) is often better suited as the plasma proteins can be separated from the NPs.
Despite the limitations and considerations needed when making measurements under physiological conditions, techniques such as AF4-MALS/DLS, NTA, and TRPS have been used successfully to measure nanoformulations in plasma and/or serum (24). Sizing of colloidal gold NPs (25), polymeric micelles, and iron oxide NPs (26) in serum has been accomplished using AF4-DLS/MALS. PEGylated gold NPs (27, 28) and silica NPs (29) have been sized in the presence of serum proteins using NTA. TRPS has been used to measure the size and size distribution of silica NP (30) and nickel NPs (31) in the presence of serum.
While the FDA does not specify the medium in which to measure size, FDA does mention that the nanomaterial should be adequately characterized in a relevant media (32). Regarding particle sizing, it is beneficial to make measurements in both aqueous buffer solutions and in the presence of plasma and/or serum. The former will give information on shelf-life/storage stability, while the latter on how proteins will influence the NP size. If the goal is the development of a nanomedicine, measurements made in the appropriate aqueous buffer solutions will be critical in designing the nanomaterial, whereas measurements in plasma/serum address the translatable and feasibility aspects. With the emergence of new technologies, making reliable and reproducible measurements in any aqueous buffer solution and under physiological conditions will become possible.
Size Standards
As the complexity of nanomedicines increase with the demands on delivery of novel active pharmaceutical ingredients (API) beyond the conventional small molecule drugs, an increase in the demands for traceably characterizing these nano-delivery systems exists. This is the final focus of the perspective. There is a general lack of relevant standards for characterizing nanomedicine products, including physicochemical endpoints, which could in turn result in failure at the clinical stage (24). As a result, there is an urgent need to develop new standards or to adapt existing ones to ensure the quality and safety of emerging nanomedicine products. In this context, we should consider measurement standards as enabling tools; they provide a commonly accepted point of reference for measurements and measurement data from product development to manufacturing to regulatory oversight. Put simply, the primary role of standards is to instill confidence in the resulting data or quality system, which in turn helps ensure the safety and efficacy of the product and its efficient regulatory review.
Consensus documentary standards (e.g., test methods, guides, and specifications issued by international standards development organizations such as ISO and ASTM International) and artifact standards (i.e., reference materials – RMs – defined by ISO Guide 30 (33) as materials sufficiently homogeneous and stable with respect to one or more specified properties, which have been established to be fit for their intended use in a measurement process) play related but different roles. Certified RMs (CRMs) are generally issued by authoritative bodies such as the US National Institute of Standards and Technology, the European Commission Joint Research Centre and the German Federal Institute for Material Research and Testing (BAM), among others. Commercial sources also exist for RMs with varying levels of metrological quality, including CRMs that meet the ISO requirements. Unfortunately, a searchable compendium of available RMs for size measurement currently does not exist to our knowledge, though BAM maintains a partial list of size-based RMs for NPs (34). Note, the BAM database has not been updated in several years.
From a regulatory perspective, the use of documentary standards reduces the number of review cycles and accelerates the entry of quality products into the market (35). Similarly, RMs and CRMs serve to validate methods used in submissions, to assess laboratory performance and to underpin manufacturing quality control/quality assurance (QC/QA). From a regulatory viewpoint, if a measurement method is used to quantify size as a CQA, which is specified for a certain value or range of values, it is necessary to then demonstrate that the method can reliably measure in that size range. In this context, analytical method validation is a key component of the regulatory process (36), and RMs can provide the necessary metrological traceability for that purpose.
Size and size distribution have been identified as CQAs for all nanomaterial-containing products (5, 32); it is therefore necessary to have standards that support the metrological traceability of size measurements in the relevant size range and containing relevant materials and preferably under use conditions. In this context, “fit for use” is both the driving force and the principal limiting factor for relevant standards needed for nanomedicine applications. For instance, the importance of size measurements in complex physiological media, and standards to support those measurements, has been frequently identified as a high priority for the nanomedicine field (3, 24, 37); however, to our knowledge, currently no formally validated standards are available for this purpose. Similarly, most existing RMs suitable for size measurements in the nanometric range are dispersed in aqueous media, are not stable in physiological media and/or have not been certified or evaluated in complex media. So, their use for nanomedicine related purposes must be demonstrated and documented (i.e., validated). Interference from other species coexisting with the target NP in complex media is a substantial issue for most measurement methods, as previously discussed in this perspective article. This issue also limits the development of standard methods and RMs that are relevant and fit for use under conditions associated with the actual administration and therapeutic action of nanomedicines (e.g., intravenous or subcutaneous delivery).
There are a number of published size measurement standards covering a wide variety of techniques (including some previously mentioned, e.g., DLS). Many of these standards have been published by ISO Technical Committee 24/Subcommittee 4 (Particle Characterization), ISO Technical Committee 229 (Nanotechnologies) and ASTM Committee E56 (Nanotechnology). Only a few of these standards were developed specifically for NPs or nano-objects and only one for nanomedicine applications; most are generally applicable, and there is no direct link to physiological media. More importantly, the vast majority of these standards are not specific to materials that are relevant to the most common nanomaterials used in drug products (Figure 1). There is clearly a growing need for nano-specific size standards and for standard methods that address both complex media and specific nanomaterials used for therapeutic or diagnostic purposes. Unfortunately, the consensus standards development process itself is laborious and subject to a limited common pool of willing experts; as a result, the process is not always favorable for rapidly emerging technologies like nanomedicine. In place of standards, several organizations (e.g., NIST (38), US Nanotechnology Characterization Laboratory (39), EU Nanomedicine Characterization Laboratory (40)) are developing, evaluating and publishing standard operating procedures, assays and protocols, which provide an opportunity for dialogue between industry, standards development organizations and government agencies leading to the advancement of formally recognized and validated standard methods.
We anticipate that standards development efforts geared towards nanomedicines will substantially increase as regulatory agencies continue to engage directly with industry and standards organizations in the nanomedicine sector. The FDA has an active interagency Nanotechnology Task Force on standards, and the FDA’s Center for Drug Evaluation and Research (CDER) has recently released draft guidance for industry on their proposed program for recognition of consensus standards related to pharmaceutical quality (41). Similarly, the European Medicines Agency (EMA) has released reflection papers, which emphasize the measurement of particle size for NP containing product submissions in order to ensure their quality (42, 43). Furthermore, regulations generally require that “analytical procedures used in testing medical products meet appropriate standards of accuracy, sensitivity, specificity, and reproducibility and are suitable for their intended purpose” (FDA 21 CFR Sections 211.165 and 211.194). Analytical procedures include size measurement, where standard methods and RMs can provide the necessary validation to meet this requirement. Therefore, an appropriate standard method for size measurement must still be validated for the intended purpose as part of the submission process.
The inherent complexity of innovative nanomedicines is itself a complicating factor for establishing regulatory information requirements, which are increasingly based on identification and characterization of CQAs NPs used in medical products are frequently hybrid in nature, containing multiple chemical components and physical structures. Unlike traditional molecular drugs, nano-drugs also contain varying degrees of polydispersity in size, shape and composition, and this can affect their performance and biodistribution, among other endpoints (44). As mentioned previously, “size” is universally identified as a CQA for nano-enabled medical products as it relates to regulatory assessment of quality, safety and bioequivalence for generics. However, the diversity and complexity of nanometric formulations is a challenge for establishment of standardized methods and relevant RMs for size measurement.
Standards will also need to address limits of applicability (fit for purpose), QC analysis and reporting of data. For instance, FDA has observed that DLS size measurements have been used in nearly 50% of all nano-enabled drug submissions (5). Yet the way in which data are analyzed and reported is inconsistent at best (i.e., not standardized). Also, the frequency with which DLS is used outside of acceptable limits is problematic and a concern from a regulatory perspective. Yet international standards for DLS (e.g., ISO 22412–2017) already exist and provide substantial guidance for sample preparation, measurement, analysis and reporting. So why is the use of DLS in submissions still a problem? The answer is two-fold. First, existing DLS standards were not developed with nanomedicines in mind; they are generic in nature and this leaves room for interpretation, particularly for guidance standards that do not recommend a specific course of action. Second, the language of these standards does not specifically address regulatory needs or requirements (which may differ from R&D needs), again leaving room for interpretation by users.
RMs present many issues relevant to nanomedicine size measurements. For instance, their availability is limited, though NP RMs certified for size have existed in one form or another since at least the early 1990s (e.g., NIST SRM 1963 – 100 nm polystyrene spheres) (45). Also, the cost and time necessary to produce a CRM is a significant obstacle; the RM production cycle is material and application dependent, but typically requires 2 to 3 years to complete once the candidate CRM has been identified and the required methodology has been metrologically established. On a positive note, production of new RMs can be greatly facilitated by direct industry/stakeholder involvement in the prioritization, funding, development and/or certification processes. An example of this type of “collaboration” is the expedited production of three citrate-stabilized gold NP RMs (RMs 8011, 8012 and 8013) by NIST in cooperation with the US National Cancer Institute in 2007. These RMs have proven to be greatly impactful, with nearly 50 % of units being purchased by industry, along with their application in the early development and validation of new methods and instrumentation. Post-production can be complicated by inherent stability issues that limit shelf-life for many NPs over extended time periods and the availability of appropriate source materials. Also, nanometric RMs are primarily used to calibrate or qualify the operational performance of a sizing instrument or method; from a practical standpoint, they will not likely cover the full range of sizes and nanomaterial types considered relevant for nanomedicine regulatory validations.
For these reasons, it is likely that the development of new RMs for nanomedicine size measurements will continue to lag behind the development of documentary (methodology) standards. In this case, alternatives to certified RMs must be found, including commercially available and in-house prepared representative test materials. Most instrument vendors will provide test materials that are intended to check the appropriate performance of their sizing instruments; however, these materials generally do not meet the criteria for certified RMs (e.g., with respect to traceability), and have not been tested on other measurement platforms or through interlaboratory comparison. A glaring gap also exists for standards that support the accurate measurement of number-based size distributions in various media; currently no RMs exist that are certified to validate NP concentration measurements, a necessity to meet the EU based regulatory definition of a nanomaterial (46).
SUMMARY AND FUTURE OUTLOOK
Nanomedicine development is rapidly progressing, with more and more NP-based therapeutic concepts translating from the benchtop of academic laboratories to preclinical and clinical studies driven by industry. With the establishment of this field, the requirement for specific quality controls has risen; herein, the focus was set on discussing the state-of-the-art and addressing challenges for a key CQA, namely particle sizing.
As discussed in the previous sections, the shear diversity and inherent complexity of NPs require careful selection of the appropriate techniques for particle sizing, as each technique presents a range of optimal uses and limitations. The employment of orthogonal measurements is a tenant of good practices and has been encouraged by regulatory agencies. Applying orthogonal techniques and interpreting what their results mean – as they might not yield the same value – is an additional challenge for regulators and industry alike and is a direct consequence of the inherent complexity and diversity of NPs relevant to nanomedicine.
Additionally, particle sizing assessment, in media that more closely mimic physiological conditions, is garnering increased attention. As highlighted by the regulatory agencies, size in complex media is relevant from a transferability prospective, as it provides a more accurate descriptor of NP behavior in vivo; nevertheless, these measurements are also more challenging as described above. With the emergence of new technologies, making reliable and accurate measurements in any aqueous buffer solution and under physiological conditions is increasingly becoming a real possibility.
Standards are a fundamental aspect of sizing, enabling confidence in the results obtained. In the case of nanomedicines, existing standards can be applied in some situations, but validation is necessary to prove they are fit for purpose (beyond instrument operational verification). Furthermore, standards will increasingly need to address how to rationalize dissimilar results obtained from orthogonal sizing techniques applied to the same nano-enabled product and how to cope with measurements in complex media.
The field of nanomedicine has created novel but challenging opportunities for the development of techniques and new standards that address size measurement. There are still gaps in the analytical methodologies and instrumentation development as well as the definition of requirements to be met for nanomedicines. However, the recent approvals of nanomedicine drug products, pharmaceutical development interest in NPs from large pharmaceutical companies, the continued and growing interest in nanomedicine from the academic community, and the increased interaction between regulatory agencies, industry, and standards and RM developers, suggest to the authors that the nanomedicine field is primed for increased standardization efforts and new measurement technology developments.
Supplementary Material
ACKNOWLEDGEMENTS AND DISCLOSURES
J.D.C., V.A.H., A.P-M, S.P. and P.L.S. would like to thank all of the speakers who participated in the ACS symposium on “Particle Sizing of Nanoparticles: From Regulatory & Metrology Aspects to Application & Analysis” in Boston, MA and the speakers who participated in the two symposia organized at CLINAM 2018 in Basel, Switzerland. The authors thank Joseph Meyer, Leidos Biomedical Research, Inc., for graphic illustrations.
ABBREVIATIONS
- CDER
FDA Center for Drug Evaluation and Research
- EMA
European Medicine Agency
- FDA
US Food and Drug Administration
- ISO
International Organization for Standardization
- JRC
European Commission Joint Research Centre
- NIST
National Institute of Standards and Technology
- US NCL
US National Cancer Institute, Nanotechnology Characterization Laboratory
- EU NCL
European Union NanoMedicine Characterization Laboratory
- SRM
Standard Reference Material (NIST trademarked product)
- RM
Reference Material
Footnotes
The authors confirm that this article has no conflict of interest to declare in the contents of the manuscript. The identification of any commercial product or trade name does not imply endorsement or recommendation by the National Institute of Standards and Technology, the Nanotechnology Characterization Lab, AstraZeneca or Pfizer.
REFERENCES
- 1.Freitas RA Jr. What is nanomedicine? Nanomedicine. 2005;1(1):2–9. [DOI] [PubMed] [Google Scholar]
- 2.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine. 2005;1(3):193–212. [DOI] [PubMed] [Google Scholar]
- 3.Halamoda-Kenzaoui B, Calzolai L, Urban P, Zuang V, Baconnier S, Boisseau P, Bastogne T, Bazile D, Borchard G, Di FT, Borgos SE, Cederbrant K, Di FG, Dobrovolskaia MA, Gaspar R, Gracia B, Hackley VA, Leyens L, Liptrott N, Park M, Patri A, Roebben G, Roesslein M, Thurmer R, Bremer-Hoffmann S. Bridging communities in the field of nanomedicine. Regul Toxicol Pharmacol. 2019;106:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faria M, Bjornmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR, Boyd BJ, Thordarson P, Whittaker AK, Stevens MM, Prestidge CA, Porter CJH, Parak WJ, Davis TP, Crampin EJ, Caruso F. Minimum information reporting in bio-nano experimental literature. Nat Nanotechnol. 2018;13(9):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Mello SR, Cruz CN, Chen M-L, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12(6):523–529. [DOI] [PubMed] [Google Scholar]
- 6.Evans ER, Bugga P, Asthana V, Drezek R. Metallic nanoparticles for cancer immunotherapy. Mater Today (Oxford, U K). 2018;21(6):673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svenson S The dendrimer paradox - high medical expectations but poor clinical translation. Chem Soc Rev. 2015;44(12):4131–4144. [DOI] [PubMed] [Google Scholar]
- 8.Tsai D-H, Lu Y-F, Del Rio FW, Cho TJ, Guha S, Zachariah MR, Zhang F, Allen A, Hackley VA. Orthogonal analysis of functional gold nanoparticles for biomedical applications. Anal Bioanal Chem. 2015;407(28):8411–8422. [DOI] [PubMed] [Google Scholar]
- 9.Clogston Jeffrey D., Crist Rachael M., McNeil SE. Physicochemical Characterization of Polymer Nanoparticles: Challenges and Present Limitations In: Vauthier Christine, Ponchel G, editors. Polymer Nanoparticles for Nanomedicines A Guide for their Design, Preparation and Development. Switzerland: Springer, Cham; 2016. p. 641. [Google Scholar]
- 10.Renaud J-P, Chari A, Ciferri C, Liu W-t, Remigy H-W, Stark H, Wiesmann C. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat Rev Drug Discovery. 2018;17(7):471–492. [DOI] [PubMed] [Google Scholar]
- 11.Gigault J, Pettibone JM, Schmitt C, Hackley VA. Rational strategy for characterization of nanoscale particles by asymmetric-flow field flow fractionation: A tutorial. Anal Chim Acta. 2014;809:9–24. [DOI] [PubMed] [Google Scholar]
- 12.Pitkanen L, Striegel AM. Size-exclusion chromatography of metal nanoparticles and quantum dots. TrAC, Trends Anal Chem. 2016;80:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zattoni A, Roda B, Borghi F, Marassi V, Reschiglian P. Flow field-flow fractionation for the analysis of nanoparticles used in drug delivery. J Pharm Biomed Anal. 2014;87:53–61. [DOI] [PubMed] [Google Scholar]
- 14.ISO. Nanotechnologies -- Analysis of nano-objects using asymmetrical-flow and centrifugal field-flow fractionation. ISO Technical Specification 21362:2018. In.; 2018. p. 38.
- 15.Weatherall E, Willmott GR. Applications of tunable resistive pulse sensing. Analyst (Cambridge, U K). 2015;140(10):3318–3334. [DOI] [PubMed] [Google Scholar]
- 16.Maguire CM, Prina-Mello A, Maguire CM, Prina-Mello A, Rosslein M, Wick P. Characterisation of particles in solution - a perspective on light scattering and comparative technologies. Sci Technol Adv Mater. 2018;19(1):732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maas SLN, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, Schiffelers RM, Wauben MHM, Broekman MLD, Nolte-’t Hoen ENM. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J Controlled Release. 2015;200:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caputo F, Clogston J, Calzolai L, Rosslein M, Prina-Mello A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J Controlled Release. 2019;299:31–43. [DOI] [PubMed] [Google Scholar]
- 19.Taylor G Dispersion of soluble matter in solvent flowing slowly through a tube. Proc R Soc London, Ser A. 1953;219:186–203. [Google Scholar]
- 20.Cottet H, Martin M, Papillaud A, Souaied E, Collet H, Commeyras A. Determination of Dendrigraft Poly-L-Lysine Diffusion Coefficients by Taylor Dispersion Analysis. Biomacromolecules. 2007;8(10):3235–3243. [DOI] [PubMed] [Google Scholar]
- 21.Clogston JD, Patri AK. Importance of Physicochemical Characterization Prior to Immunological Studies In: Dobrovolskaia MA, McNeil SE, editors. Handbook of Immunological Properties of Engineered Nanomaterials: World Scientific; 2013. p. 720. [Google Scholar]
- 22.Grossman JH, Crist RM, Clogston JD. Early Development Challenges for Drug Products Containing Nanomaterials. AAPS J. 2017;19(1):92–102. [DOI] [PubMed] [Google Scholar]
- 23.Wolfram J, Suri K, Yang Y, Shen J, Celia C, Fresta M, Zhao Y, Shen H, Ferrari M. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf, B. 2014;114:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gioria S, Caputo F, Urban P, Maguire CM, Bremer-Hoffmann S, Prina-Mello A, Calzolai L, Mehn D. Are existing standard methods suitable for the evaluation of nanomedicines: some case studies. Nanomedicine (London, U K). 2018;13(5):539–554. [DOI] [PubMed] [Google Scholar]
- 25.Capomaccio R, Ojea Jimenez I, Colpo P, Gilliland D, Ceccone G, Rossi F, Calzolai L. Determination of the structure and morphology of gold nanoparticle-HSA protein complexes. Nanoscale. 2015;7(42):17653–17657. [DOI] [PubMed] [Google Scholar]
- 26.Ashby J, Schachermeyer S, Pan S, Zhong W. Dissociation-Based Screening of Nanoparticle-Protein Interaction via Flow Field-Flow Fractionation. Anal Chem (Washington, DC, U S). 2013;85(15):7494–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spadavecchia J, Movia D, Moore C, Maguire CM, Moustaoui H, Casale S, Volkov Y, Prina-Mello A. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: from synthesis to proof-of-concept in vitro studies. Int J Nanomed. 2016;11:791–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moustaoui H, Saber J, Djeddi I, Liu Q, Movia D, Prina-Mello A, Spadavecchia J, Lamy de la Chapelle M, Djaker N. A protein corona study by scattering correlation spectroscopy: a comparative study between spherical and urchin-shaped gold nanoparticles. Nanoscale. 2019;11(8):3665–3673. [DOI] [PubMed] [Google Scholar]
- 29.Gollwitzer C, Bartczak D, Goenaga-Infante H, Kestens V, Krumrey M, Minelli C, Palmai M, Ramaye Y, Roebben G, Sikora A, Varga Z. A comparison of techniques for size measurement of nanoparticles in cell culture medium. Anal Methods. 2016;8(26):5272–5282. [Google Scholar]
- 30.Sikora A, Shard AG, Minelli C. Size and ζ-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir. 2016;32(9):2216–2224. [DOI] [PubMed] [Google Scholar]
- 31.Pal AK, Aalaei I, Gadde S, Gaines P, Schmidt D, Demokritou P, Bello D. High resolution characterization of engineered nanomaterial dispersions in complex media using tunable resistive pulse sensing technology. ACS Nano. 2014;8(9):9003–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA US. Drug Products, Including Biological Products, that Contain Nanomaterials - Guidance for Industry- Draft Guidance Document. In: CDER, editor.; December 2017. p. 29. [Google Scholar]
- 33.ISO. Reference materials -- Selected terms and definitions. ISO Guide 30: 2015. In.; 2015. p. 8.
- 34.German Federal Institute for Material Research and Testing (BAM), Nanoscaled Reference Materials. Available from: http://www.nanorefmat.bam.de/en/category_10_nanoobjects_nanoparticles_nanomaterials.htm.
- 35.Tyner K Regulatory considerations for drug products containing nanomaterials: US FDA perspective In.Seminar presented at the US National Institute of Standards and Technology; Gaithersburg, MD; January 17, 2017. [Google Scholar]
- 36.FDA US. Analytical Procedures and Methods Validation for Drugs and Biologics- Guidance for Industry. In: CDER, editor.; July 2015. p. 18. [Google Scholar]
- 37.Report of the 2016 Global Summit on Regulatory Science (GSRS16) Nanotechnology Standards and Applications. In. Bathesda, Maryland: U.S. National Institutes of Health; 2016. p. 35. [Google Scholar]
- 38.National Institute of Standards and Technology (NIST). Available from: https://www.nist.gov/.
- 39.Nanotechnology Characterization Laboratory (NCL) webpage. Available from: https://ncl.cancer.gov/.
- 40.EU Nanomedicine Characterisation Laboratory (EU NCL). Available from: http://www.euncl.eu/.
- 41.FDA US. CDER’s Program for the Recognition of Voluntary Consensus Standards Related to Pharmaceutical Quality- Draft Guidance Document. In: CDER, editor.; February 2019. p. 10. [Google Scholar]
- 42.European Medicines Agency. Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product EMA/CHMP/806058/2009/Rev. 02. In. United Kingdom: Committee for Human Medicinal Product (CHMP); 2013. [Google Scholar]
- 43.European Medicines Agency. Reflection paper on the data requirements for intravenous iron-based nano-colloidal products developed with reference to an innovator medicinal product EMA/CHMP/SWP/620008/2012. In. Amsterdam: Committee for Medicinal Products for Human Use; 2015. [Google Scholar]
- 44.Kaga S, Truong NP, Esser L, Senyschyn D, Sanyal A, Sanyal R, Quinn JF, Davis TP, Kaminskas LM, Whittaker MR. Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly. Biomacromolecules. 2017;18(12):3963–3970. [DOI] [PubMed] [Google Scholar]
- 45.Mulholland GW, Bryner NP, Croarkin C. Measurement of the 100 nm NIST SRM 1963 by differential mobility analysis. Aerosol Sci Technol. 1999;31(1):39–55. [Google Scholar]
- 46.Rauscher H, Roebben G, Mech A, Gibson N, Kestens V, Linsinger TPJ, Riego Sintes J. JRC Science for Policy Report. An overview of concepts and terms used in the European Commission’s definition of nanomaterial In. Luxembourgh: Publications Office of the European Union; 2019. p. 44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.