Abstract
Objective
To test the hypotheses that hypertension and nocturnal blood pressure are related to white matter hyperintensity (WMH) volume, an MRI marker of small vessel cerebrovascular disease, and that WMH burden statistically mediates the association of hypertension and dipping status with memory functioning, we examined the relationship of hypertension and dipping status on WMH volume and neuropsychological test scores in middle-aged and older adults.
Methods
Participants from the community-based Maracaibo Aging Study received ambulatory 24-hour blood pressure monitoring, structural MRI, and neuropsychological assessment. Four hundred thirty-five participants (mean ± SD age 59 ± 13 years, 71% women) with available ambulatory blood pressure, MRI, and neuropsychological data were included in the analyses. Ambulatory blood pressure was used to define hypertension and dipping status (i.e., dipper, nondipper, and reverse dipper based on night/day blood pressure ratio <0.9, 0.9–1, and >1, respectively). Outcome measures included regional WMH and memory functioning derived from a neuropsychological test battery.
Results
The majority of the participants (59%) were hypertensive. Ten percent were reverse dippers, and 40% were nondippers. Reverse dipping in the presence of hypertension was associated with particularly elevated periventricular WMH volume (F2,423 = 3.78, p = 0.024) and with lowered memory scores (F2,423 = 3.911, p = 0.021). Periventricular WMH volume mediated the effect of dipping status and hypertension on memory (β = −4.1, 95% confidence interval −8.7 to −0.2, p < 0.05).
Conclusion
Reverse dipping in the presence of hypertension is associated with small vessel cerebrovascular disease, which, in turn, mediates memory functioning. These results point toward reverse dipping as a marker of poor nocturnal blood pressure control, particularly among hypertensive individuals, with potentially pernicious effects on cerebrovascular health and associated cognitive function.
The assessment of 24-hour ambulatory blood pressure allows more reliable measurement of blood pressure than what is typically measured clinically and the examination of blood pressure fluctuation over time.1–7 One indicator of fluctuation, nocturnal blood pressure decline or dipping, is a physiologically relevant effect associated with healthy blood pressure regulation.4,8 Nondipping, or a lack of nocturnal decline in blood pressure levels, is a frequent comorbidity with hypertension, with >40% of hypertensive older adults experiencing nondipping.9,10 Nondipping is associated with an increased risk of type 2 diabetes mellitus, reduced renal function, and left ventricular hypertrophy, and with severe vascular events such as coronary heart disease, stroke, and mortality.7,11–14
A lack of nocturnal blood pressure dipping is a potential risk factor for small vessel cerebrovascular disease, although this relationship remains unclear.2,4,15–17 Small vessel cerebrovascular disease is associated with lower cognitive functioning and higher risk for stroke, dementia, altered motor function, and mortality among older adults.18–26 White matter hyperintensities (WMH), which are regions of increased signal on T2-weighted brain MRI, are established markers of small vessel cerebrovascular disease.20,21,25,27 However, the factors by which peripheral vascular conditions can cause WMH accumulation remain unclear.18,28,29 Furthermore, there is some evidence that the causes of WMH might vary by their regional distribution, with periventricular WMH being particularly sensitive to hemodynamic changes, or aberrations of blood flow, while deep WMH are more sensitive to long-term ischemic changes.1,30,31
The purposes of this study were to examine the independent and interactive relationship of hypertension and dipping status on WMH volume and to determine whether WMH burden statistically mediates the effect of hypertension and dipping status on memory functioning in late middle-aged community-dwelling adults. We hypothesized that individuals with both hypertension and nondipping status would have the greatest WMH volume, which would, in turn, would account for poorer memory functioning.
Methods
Participants
Four hundred thirty-five participants from the Maracaibo Aging Study (MAS32) with available ambulatory blood pressure, MRI, and neuropsychological data were included in the study. The MAS began in 1998 in Maracaibo, Venezuela, as a prospective, longitudinal, community-based cohort study of cognitive aging and dementia.32 Initially, the MAS recruited participants who were living in the Santa Lucia community and were >55 years old (n = 2,349). The study was expanded in 2010 to include participants >40 years old and living in either Santa Lucia or the Santa Rosa de Agua community 6 miles away.33
Standard protocol approvals, registrations, and patient consents
The MAS was approved by Institutional Review boards of the University of Zulia and Columbia University. All participants in the MAS provided informed consent.
24-Hour blood pressure monitoring
The ambulatory blood pressure measurement devices (validated oscillometric 90207; SpaceLabs monitors, Redmond, WA) were programmed to obtain measurements every 15 minutes during the day (6 am–10:59 pm) and every 30 minutes at night (11 pm–5:59 am) over a 24-hour period. Ambulatory hypertension was defined as a 24-hour average of systolic blood pressure >130 mm Hg or diastolic blood pressure >80 mm Hg or the use of antihypertensive medication, following the guidelines of the European Society of Cardiology and European Society of Hypertension.34 All participants who did not meet these criteria were considered normotensive. As suggested in previous work,35 we used the ambulatory blood pressure measurements to calculate the night/day blood pressure ratio to identify the change in nocturnal blood pressure relative to daytime blood pressure. A participant's nocturnal dipping profile was defined as the ratio of systolic nighttime blood pressure to systolic daytime blood pressure. Dipping status was defined according to established criteria35 as follows: dipper, a normal decrease in nocturnal systolic blood pressure relative to diurnal blood pressure (i.e., night/day blood pressure <0.9); nondipper, a small or nonexistent decrease in nocturnal systolic blood pressure relative to diurnal blood pressure (i.e., night/day blood pressure between 0.9 and 1.0); and reverse dipper, an abnormal increase in nocturnal systolic blood pressure relative to diurnal blood pressure (i.e., night/day blood pressure >1.0).
MRI acquisition and processing
MRI scans were obtained on a 1.5T scanner (Optima MR360; GE Healthcare, Chicago, IL) and included T1-weighted (repetition time 7,904 milliseconds, echo time 2,460 milliseconds, field of view 256 × 256 mm with 1-mm contiguous slices) and T2-weighted fluid-attenuated inversion recovery (repetition time 8,000 milliseconds, echo time 123 milliseconds, inversion time 2,000 milliseconds, field of view 256 × 162 mm with 2-mm contiguous slices). Exclusion criteria for MRI scans were the presence of a pacemaker, aneurysm clip, neurostimulator, or cochlear implant or body weight >110 kg. In addition, potential participants were excluded if they had a history of major medical conditions or procedures thought to confound measurement of WMH such as multiple sclerosis, brain radiotherapy, brain surgery, implants for Parkinson disease, lupus, brain tumor (lymphoma), HIV, neurocysticercosis, neurosyphilis, brain tuberculosis, and brain trauma with loss of consciousness. All clinical and neuroimaging data were collected within a 3-week time interval.
Whole-brain and regional WMH volumes were quantified with previously described methods.36,37 Briefly, each participant's T2-weighted fluid-attenuated inversion recovery image was brain extracted, and a single gaussian curve was fit to voxel intensity values in the brain-extracted image. Intensity values >2.1 SD above the whole-brain mean intensity value were labeled as hyperintense voxels. The threshold was set by a trained operator who visually inspected the results. The resulting map was manually inspected and corrected for each participant, with any mislabeled WMH voxels removed. WMH volume, in cubic centimeters, was defined as the number of labeled voxels multiplied by voxel dimensions.
The WMH volumes were coregistered to the brain extracted T1-weighted volumes defined by FreeSurfer (version 6.0, surfer.nmr.mgh.harvard.edu), and the euclidean distance between each WMH-labeled voxel and the nearest ventricle was computed. Following previously defined criteria,30,31,38 these distances were used to define periventricular WMH volume, which comprised all WMH voxels lying 3 to 13 mm from the ventricular surface, and deep WMH volume, which comprised all WMH voxels lying >13 mm from the ventricular surface.
Neuropsychological evaluation
The neuropsychological evaluation consisted of a test battery,39 adapted to be applied in this community-dwelling sample, and the Cambridge Neuropsychological Test Automated Battery.40 We selected the total learning score from the Selective Reminding Test41 as the memory score for this study. As an exploratory analysis, we selected performance measures from Paired Associated Learning (PAL), Intra-Extra Dimensional Set Shift (IED), and Reaction Time (RTI) from the Cambridge Neuropsychological Test Automated Battery as secondary cognitive measures.
Patient health history
Physicians and nurses collected demographic and clinical information. Diabetes mellitus was defined by the criteria specified in the Seventh Report of the Joint National Committee42: a fasting glucose serum level >125 mg/dL or taking an antidiabetic drug. History of vascular disease encompassed stroke events, ischemic heart disease, and heart failure. History of smoking was defined as past and/or current smoking. Obesity was defined as a body mass index ≥30 kg.2,43 Diagnosis of dementia was assigned according to a Clinical Dementia Rating44 (CDR) score ≥1 and a consensus conference that included study physicians, experts in dementia diagnosis.
Statistical analysis
We used a general linear model to examine the association of hypertension and dipping status with WMH volume and cognition. We examined the main effects of hypertension and dipping status, as well as their interactions on WMH volume and cognition, adjusting for age and sex/gender. Analyses involving cognition additionally adjusted for number of years of education. We then examined the association between WMH volumes (periventricular and deep) and memory, adjusting for age, sex/gender, and years of education.
We used a moderated mediation model to test whether dipping status moderates the association of hypertension with WMH and with memory and whether WMH mediate the relationship of hypertension and dipping status with memory. This model allowed us to simultaneously test whether WMH volume mediates the relationship between dipping status and memory in both normotensive and hypertensive individuals, mirroring the interaction effects probed in our initial analyses. Moderated mediation analyses were conducted with PROCESS 3.1 (model 8) for SPSS.45 This approach to mediation modeling is based on bootstrapping, which resamples the sample population over many iterations (k = 5,000) to obtain a more robust estimate of the effect size (β) and confidence interval (CI) of each variable, without any assumption of how the variables are distributed.45,46
To confirm that our initial analyses were not due to quasi-arbitrary cut points to define categorical dipping classes, we used a linear regression model adjusted for age and sex/gender to test the association of continuously defined nocturnal dipping profile (i.e., ratio of systolic nighttime blood pressure to systolic daytime blood pressure) with WMH volume, both as a main effect and interacting with hypertension status. Another linear regression model, adjusted for age, sex/gender, and years of education, was used to test the association of nocturnal dipping profile with total learning score, both as a main effect and interacting with hypertension status. To ensure that our findings were not simply driven by constantly elevated blood pressure levels, we performed a linear regression to test the independent associations of 24-hour average systolic blood pressure and nocturnal dipping profile, adjusting for age and sex/gender, with periventricular WMH volume. Finally, because individuals with hypertension were reliably older than those without, we repeated the primary statistical analyses in an age-restricted subsample, selecting only individuals 50 to 70 years old to ensure that age did not confound our results.
Data availability
Requests for access to the data used in this study will be considered on a case-by-case basis.
Results
Participants
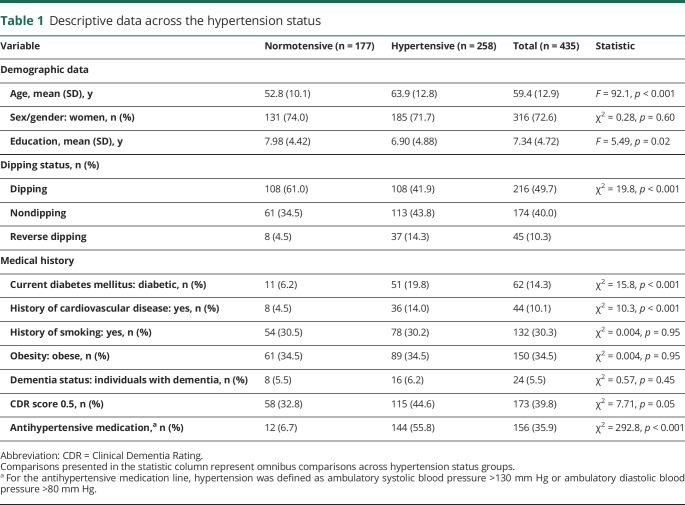
Descriptive statistics for demographic and blood pressure variables are presented in table 1. Hypertensive participants were older than normotensive participants and were more likely to be reverse dippers. Sex/gender distribution and the number of years of education did not differ between groups. The frequency of comorbid vascular conditions, including diabetes mellitus and history of cardiovascular disease, was higher in hypertensive participants than in normotensive participants. History of smoking and current obesity did not differ between groups. Diagnosis of dementia did not differ between groups, although a slightly higher proportion of individuals with hypertension had CDR scores of 0.5. Hypertensive participants were more likely to be taking hypertensive medication than normotensive participants.
Table 1.
Descriptive data across the hypertension status
Effects of hypertension and dipping profile by dipper categories
Hypertensive individuals had higher periventricular WMH volumes than those without hypertension (main effect of hypertension: F1,423 = 12.12, p = 0.001). This main effect was modified by an interaction between hypertension and dipping status (F2,423 = 3.78, p = 0.024); reverse dippers with hypertension had particularly elevated WMH volume compared with all other groups (figure 1A). Reverse dippers had greater periventricular WMH volume compared with dippers (p < 0.001) and nondippers (p < 0.001), while dippers and nondippers did not differ from each other (p = 0.89; main effect of dipping status F2,423 = 2.58, p = 0.08). Hypertensive individuals did not differ in deep WMH volume compared with all other groups (main effect of hypertension F1,423 = 2.39, p = 0.12, figure 1B). Dipping status did not moderate the relationship between hypertension and deep WMH volume (main effect of dipping status: F2,423 = 0.15, p = 0.86; interaction effect of dipping status by hypertension F2,423 = 0.85, p = 0.43).
Figure 1. WMH volume as a function of hypertension and dipping status.
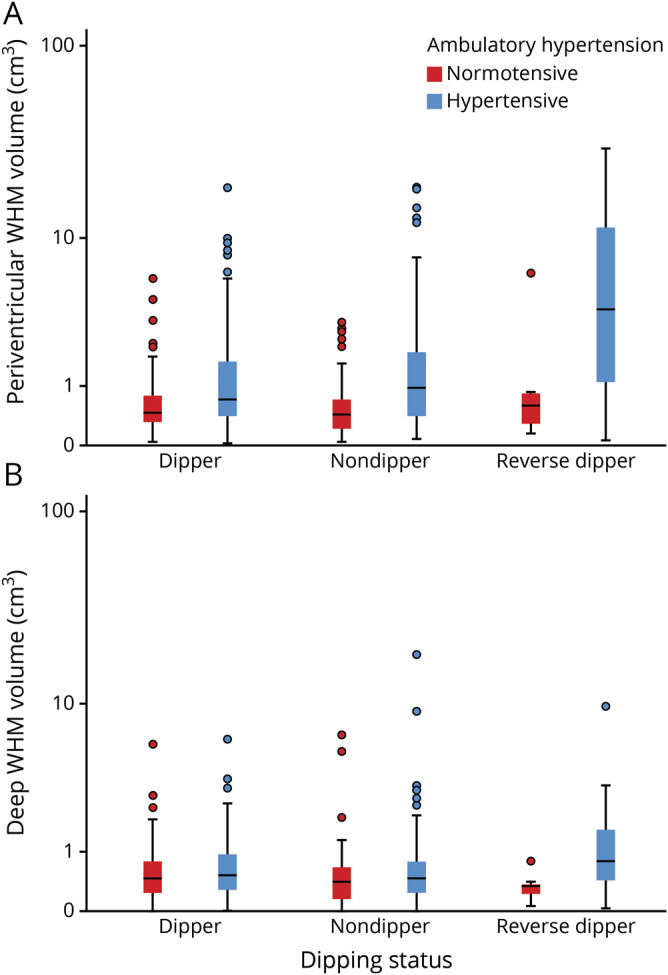
In each graph, the central bar represents the median, box marks the first and third quartiles, and bars extend 1.5 times the width of the box. Remaining points represent values outside this range. The y-axes are logarithmically scaled. (A) Reverse dipping in the presence of hypertension is associated with higher accumulation of periventricular white matter hyperintensity (WMH) volume at almost twice the rate of any other group. (B) There is no observed association among dipping status, hypertension, and deep WMH volume.
There was minimal effect of hypertension (F1,423 = 3.16, p = 0.08) and dipping status (F2,423 = 0.89, p = 0.41) on total learning score. However, dipping status moderated the effect of hypertension on total learning, with hypertensive reverse dippers having lower total learning scores than all other groups, paralleling the effects observed in periventricular WMH volumes (dipping status by hypertension interaction F2,423 = 3.91, p = 0.021; figure 2). Dipping status and hypertension were not associated with performance on other cognitive measures (PAL, IED, and RTI scores). There was a stronger relationship between periventricular WMH volume and total learning scores (β = −0.42, 95% CI −0.68 to −0.17, p = 0.001) than between deep WMH volume and total learning (β = −0.57, 95% CI −1.18 to 0.05, p = 0.073).
Figure 2. Total learning score as a function of hypertension and dipping status.
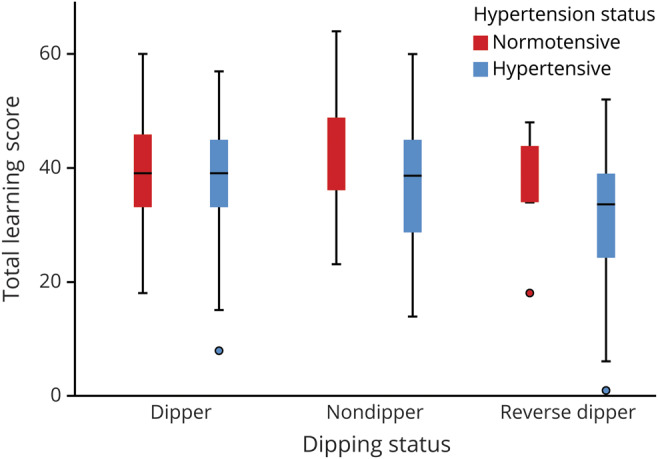
Reverse dipping in the presence of hypertension is associated with markedly lower memory function compared with all other groups. The central bar represents the median; box marks the first and third quartiles; and bars extend 1.5 times the width of the box. Remaining points represent values outside this range.
Mediation model
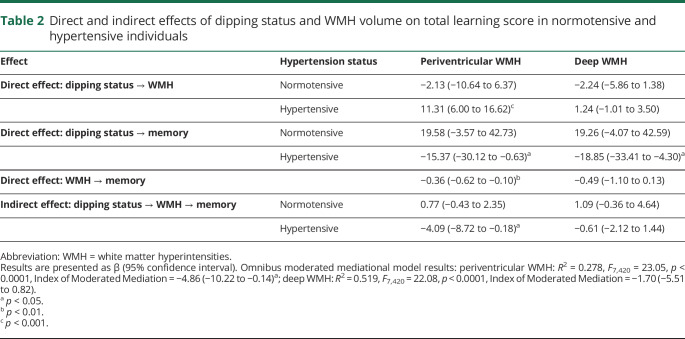
Table 2 presents the results of the moderated mediation model used to test the relationship among dipping magnitude, hypertension, WMH volume, and total learning score. In hypertensive individuals, greater dipping magnitude (i.e., a higher night/day blood pressure ratio) was associated with an increase in WMH volume and a decrease in total learning, while no such effects were observed in normotensive individuals. In hypertensive individuals, the effect of dipping magnitude on total learning was mediated by periventricular WMH volume (index −4.86, 95% CI −10.22 to −0.14, p < 0.05). A similar indirect effect mediated by deep WMH volume was not observed (index −1.70, 95% CI −5.51 to 0.82, p > 0.1).
Table 2.
Direct and indirect effects of dipping status and WMH volume on total learning score in normotensive and hypertensive individuals
Confirmatory analyses
Using dipping profile (i.e., the continuously defined night/day systolic blood pressure ratio) yielded results similar to those of the analyses that classified dipping status categorically. An increased dipping profile was associated with increased periventricular WMH volume (β = 7.98, 95% CI 3.47 to 12.5, p = 0.001) but not with deep WMH volume (β = 0.33, 95% CI −1.57 to 2.24, p = 0.73). The interaction of hypertension status with dipping profile was associated with increased periventricular WMH volume (β = 13.3, 95% CI 3.40 to 23.2, p = 0.009) but not with deep WMH volume (β = 3.43, 95% CI −0.79 to 7.65, p = 0.11). There was no observable relationship between dipping profile and total learning score (β = −7.45, 95% CI −19.8 to 4.87, p = 0.24). However, the interaction between hypertension status and dipping profile was strongly associated with a lower total learning score (β = −39.4, 95% CI −68.6 to −10.3, p = 0.008). Dipping profile and hypertension were not associated with other cognitive measures (PAL, IED, and RTI scores).
To ensure that the effects of dipping profile were not simply attributable to overall elevated blood pressure over a 24-hour period, we examined the simultaneous effects of average 24-hour systolic blood pressure and dipping profile on periventricular WMH volume and found that both 24-hour average blood pressure (β = 0.18, p < 0.001) and dipping profile (β = 0.10, p = 0.025) were independently associated with WMH volume. Although all primary analyses were adjusted for age, to ensure further that the relatively large age differences between hypertensive and normotensive individuals did not account for our primary findings, we conducted sensitivity analyses by restricting the sample to individuals ranging in age from 50 to 70 years (n = 216), which limited the age difference between groups to 2.4 years. In these supplementary analyses, the association between WMH volume and total learning score was slightly attenuated (within the CI of the association reported above), but our findings were otherwise the same.
Discussion
In this large community-based study with 24-hour ambulatory blood pressure monitoring, we examined the effects of 2 related vascular risk factors, hypertension and dipping status, on small vessel cerebrovascular disease and memory. Consistent with the results of previous studies,1,2,5,6,18,47 individuals with hypertension had elevated periventricular WMH volumes, but not deep WMH volumes, compared with individuals without hypertension. An increased nocturnal dipping profile was associated with an increase in WMH volume, particularly in hypertensive individuals. When defined categorically, dipping status did not show an independent relationship with WMH volume, as some previous studies reported.1,47 However, categorically defined dipping status did moderate the effect of hypertension: reverse dippers—those who experience a nocturnal increase in blood pressure—with hypertension as a group had a >2-fold increase in WMH volume compared with all other groups examined. The findings point to the relevance of nocturnal blood pressure status in cerebral vascular health.
We observed a similar relationship of hypertension and dipping status on cognition: reverse dippers with hypertension demonstrated lower cognitive test scores than dippers and nondippers. Although nondipping has not been linked to small vessel cerebrovascular disease,1,2,47 a growing body of literature ties reverse dipping to other vascular morbidities,7,17,48,49 particularly in the presence of hypertension.48,49 The results presented here suggest that the co-occurrence of reverse dipping and hypertension may greatly increase the likelihood or severity of small vessel cerebrovascular disease, indicating that dipping status can moderate the effects of hypertension and that reverse dipping can amplify the effects of hypertension on cerebrovascular health. Our results also indicate that reverse dipping may be particularly pernicious with respect to small vessel cerebrovascular disease and memory function.
We observed an interaction between hypertension and dipping status on memory, which was present in the absence of main effects of either hypertension or dipping status. This observation suggests that the co-occurrence of these conditions, rather than the presence of either one individually, promotes the effects of small vessel cerebrovascular disease on memory, a finding not characterized previously. We did not observe an effect of dipping status and hypertension on other cognitive domains. While cognitive impairment associated with vascular disease has historically been conceptualized to affect primarily executive functioning, there is also a well-established link with vascular brain health and memory functioning.4,50 Given our previous work linking WMH to risk and progression of Alzheimer disease, a disease typically characterized clinically by an amnestic syndrome, memory function was our a priori hypothesis and primary interest in the current study.
When we examined hypertensive and dipping profiles continuously rather than characterizing participants categorically, we found that a higher nocturnal dipping profile, indicating the presence of a nondipping or reverse dipping, was associated with an increase in WMH volume and a decrease in memory in hypertensive individuals. We also demonstrated that periventricular WMH volume mediated the relationship between the vascular variables and memory, supporting the causal pathway in which hypertension and dipping status interact to increase WMH accumulation, which, in turn, promotes memory decline. Given the cross-sectional design of our study, we are unable to prove the causal pathway we proposed. However, the results are consistent with our hypothesized causal model of hypertension and dipping status interacting to promote small vessel cerebrovascular disease and associated memory dysfunction. Longitudinal studies will help clarify the time course and more definitely establish causality.
All participants were middle-aged to older adults, so the relationships established in this study can be generalized only to this age stratum. Furthermore, although age was related to all outcomes studied, we adjusted for age and conducted additional sensitivity analyses with restricted age. We did not observe any effects of sex/gender, and observed dipping effects were independent of 24-hour blood pressure. Together, these analyses suggest that our observations were not attributable to age differences between those with and those without hypertension and that dipping status is associated with markers of cerebrovascular disease above and beyond blood pressure alone.
Our study is unique in that it is a large, community-based cohort of participants selected only on the basis of age, with a special focus on late middle age, an advantage shared by few other studies.2 A number of the other studies examining similar variables included participants from various clinical settings,6,17,48 and most studies examining WMH volume or cognition with respect to diurnal blood pressure variation enrolled participants because they had hypertension.1,4,11,16,47,48 In the current study, we examined the effects of dipping status and hypertension on WMH volume and memory in a large community-based sample of Venezuelan adults. The consideration of other vascular comorbidities is important because reverse dipping frequently occurs concurrently with hypertension. While the exact causes of nondipping and reverse dipping remain unknown, the collection of ambulatory blood pressure measurements allows better clinical monitoring and is gradually being adopted as a more accurate alternative to standard clinical measures.4,6,49
In this community-based cohort study, we examined the effects of dipping status and hypertension on small vessel cerebrovascular disease and memory. We found that an increased dipping profile, particularly reverse dipping, was associated with an increase in cerebrovascular disease and a poorer memory functioning in hypertensive participants. Our results point toward the importance of reverse dipping as a potentially pathognomonic driver of small vessel cerebrovascular disease, particularly in the presence of hypertension, and add to the mounting evidence showing the importance of vascular contributions to memory decline.
Glossary
- CDR
Clinical Dementia Rating
- CI
confidence interval
- IED
Intra-Extra Dimensional Set Shift
- MAS
Maracaibo Aging Study
- PAL
Paired Associated Learning
- RTI
Reaction Time
- WMH
white matter hyperintensities
Appendix. Authors
Footnotes
Podcast: NPub.org/89xwc1
Study funding
Supported by NIH (R01AG036469).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Associations of ambulatory blood pressure levels with white matter hyperintensity volumes in hypertensive patients. J Hypertens 2009;27:1446–1452. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi K, Jin Z, Homma S, et al. Night-time systolic blood pressure and subclinical cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Eur Heart J Cardiovasc Imaging 2019;20:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White WB, Wolfson L, Wakefield DB, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011;124:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano Y, Butler KR, Hall ME, et al. Associations of nocturnal blood pressure with cognition by self-identified race in middle-aged and older adults: the GENOA (Genetic Epidemiology Network of Arteriopathy) study. J Am Heart Assoc 2017;6:e007022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscari A, Faccioli L, Ghinelli M, et al. Hypertension and other determinants of white matter lesions in stroke patients. J Clin Hypertens 2016;18:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White WB, Marfatia R, Schmidt J, et al. INtensive versus standard ambulatory blood pressure lowering to prevent functional DeclINe in the ElderlY (INFINITY). Am Heart J 2013;165:258–265.e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WY, Melgarejo JD, Thijs L, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet 1988;2:397. [DOI] [PubMed] [Google Scholar]
- 9.Friedman O, Logan AG. Nocturnal blood pressure profiles among normotensive, controlled hypertensive and refractory hypertensive subjects. Can J Cardiol 2009;25:e312–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 2009;53:466–472. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Yan B, Gao Y, et al. Relationship between blood pressure reverse dipping and type 2 diabetes in hypertensive patients. Sci Rep 2016;6:25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens 2014;27:680–687. [DOI] [PubMed] [Google Scholar]
- 13.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 14.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Tabara Y, Igase M, et al. Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: the J-SHIPP study. Hypertens Res 2010;33:32–36. [DOI] [PubMed] [Google Scholar]
- 16.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 2008;26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Ohara T, Ozasa K. The relationship between 24-hour blood pressure readings, subcortical ischemic lesions and vascular dementia. Cerebrovasc Dis 2005;19:302–308. [DOI] [PubMed] [Google Scholar]
- 18.Abraham HM, Wolfson L, Moscufo N, Guttmann CR, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab 2016;36:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brickman AM, Honig LS, Scarmeas N, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol 2008;65:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol 2008;65:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000;14:224–232. [DOI] [PubMed] [Google Scholar]
- 23.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging 2009;30:450–456. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry 2005;76:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke 2007;38:2619–2625. [DOI] [PubMed] [Google Scholar]
- 26.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci (Lond) 2017;131:715–728. [DOI] [PubMed] [Google Scholar]
- 28.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 29.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126–135. [DOI] [PubMed] [Google Scholar]
- 30.Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008;64:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 2005;36:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maestre GE, Pino-Ramirez G, Molero AE, et al. The Maracaibo Aging Study: population and methodological issues. Neuroepidemiology 2002;21:194–201. [DOI] [PubMed] [Google Scholar]
- 33.Paz Reverol CL, Villalobos J, Moran Y, et al. Configuracion de las identidades de los “santaroseros” y su disposicion a participar en estudios con componente genetico. Revista Internacional de Salud, Bienestar y Sociedad 2014;1–11. [Google Scholar]
- 34.Mancia G, Fagard R, Narkiewicz K, et al. ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 35.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens 2009;23:645–653. [DOI] [PubMed] [Google Scholar]
- 36.Brickman AM, Sneed JR, Provenzano FA, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res 2011;193:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging 2015;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffanti L, Jenkinson M, Suri S, et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: a study in older adults. Neuroimage 2018;170:174–181. [DOI] [PubMed] [Google Scholar]
- 39.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol 1992;49:453–460. [DOI] [PubMed] [Google Scholar]
- 40.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 41.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974;24:1019–1025. [DOI] [PubMed] [Google Scholar]
- 42.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 43.Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004;24:e13–e18. [DOI] [PubMed] [Google Scholar]
- 44.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 45.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis, 2nd ed. New York: The Guilford Press; 2018. [Google Scholar]
- 46.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 2009;76:408–420. [Google Scholar]
- 47.van Boxtel MP, Henskens LH, Kroon AA, et al. Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. J Hum Hypertens 2006;20:5–13. [DOI] [PubMed] [Google Scholar]
- 48.Kwon HM, Lim JS, Kim YS, et al. Cerebral microbleeds are associated with nocturnal reverse dipping in hypertensive patients with ischemic stroke. BMC Neurol 2014;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan B, Peng L, Dong Q, et al. Reverse-dipper pattern of blood pressure may predict lacunar infarction in patients with essential hypertension. Eur J Neurol 2015;22:1022–1025. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018;21:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to the data used in this study will be considered on a case-by-case basis.