Abstract
Objective
We sought to assess the prevalence, correlates, and consequences of periodic limb movements of sleep (PLMS) in persons with obstructive sleep apnea (OSA) and the effect (worsening or improvement) of continuous positive airway pressure (CPAP) therapy on PLMS in a large prospective multicenter randomized controlled trial.
Methods
We performed retrospective analyses of data from the Apnea Positive Pressure Long-term Efficacy Study, a prospective multicenter randomized controlled trial. A total of 1,105 persons with OSA enrolled in this study underwent a polysomnographic investigation at baseline, another one for CPAP titration, and another study 6 months after randomization to either active CPAP or sham CPAP.
Results
Of all participants, 19.7% had PLM index (PLMI) ≥10/hour, 14.8% had PLMI ≥15/hour, 12.1% had PLMI ≥20/hour, 9.3% had PLMI ≥25/hour, and 7.5% had PLMI ≥30/hour. The odds of having a PLMI ≥10 were higher in older participants (odds ratio [OR] 1.03, p < 0.001), men (OR 1.63. p = 0.007), those using antidepressants (OR 1.48. p = 0.048), and those with higher caffeine use (OR 1.01, p = 0.04). After controlling for OSA and depression, PLMS were associated with increased sleep latency, reduced sleep efficiency, and reduced total sleep time. No significant relationships were noted between PLMS frequency and subjective sleepiness (Epworth Sleepiness Scale score) or objective sleepiness (Maintenance of Wakefulness Test). There was no differential effect of CPAP in comparison to sham CPAP on PLMS after 6 months of therapy.
Conclusions
PLMS are common in patients with OSA and are associated with a significant reduction in sleep quality over and above that conferred by OSA. Treatment with CPAP does not affect the severity of PLMS.
Periodic limb movements of sleep (PLMS) are characterized by stereotypical limb movements that are more likely to occur in the lower extremities. The movements may be associated with arousals from sleep and the condition may be associated with reduced sleep quality and hypertension.1–3 Retrospective studies suggest a high prevalence of comorbid PLMS in patients with obstructive sleep apnea (OSA).1,4
Although PLMS are a potential sleep disruptor, their clinical significance is unclear. They are often seen in association with other disorders. When in conjunction with restless legs syndrome (RLS), the latter is typically treated to allow better sleep. More complex is the association of PLMS and OSA. The 2 conditions frequently coexist but it is uncertain whether PLMS change with treatment of OSA. A study of 84 patients examined the prevalence of PLMS with different levels of OSA severity and found that PLMS decreased after continuous positive airway pressure (CPAP) treatment in 20 participants, particularly those with mild OSA.5 Others have hypothesized that OSA and PLMS occur concurrently and that treatment with positive airway pressure (PAP) simply unmasks the PLMS.6 However, these and most other studies assessing the effect of PAP therapy on evolution of PLMS suffered from a lack of control group. Finally, the presence of PLMS during a PAP titration study has been suggested to be an indication of incomplete resolution of OSA with treatment.7
The current study involves retrospective analyses of data from the Apnea Positive Pressure Long-term Efficacy Study (APPLES), a prospective multicenter randomized controlled trial in persons with OSA. The primary objective of this study was to assess the effect of CPAP therapy on neurocognitive outcomes after 6 months of active CPAP or sham CPAP. Polysomnography was obtained at baseline, for CPAP or sham CPAP titration, as well as after 4 and 6 months of therapy, providing information about OSA, PLMS, and sleep quality. Utilizing this large database, we sought to confirm the prevalence of PLMS in OSA and assess the clinical and polysomnographic variables associated with PLMS in persons with OSA. We also aimed to test whether the presence of PLMS in patients with sleep-disordered breathing may be an indication of incomplete resolution of breathing abnormalities with CPAP therapy and whether treatment has an effect (worsening or improvement) on PLMS.
Methods
Study design
A detailed description of the APPLES study protocol is available in an earlier study.8 Briefly, participants were recruited at 5 clinical centers and those who met the eligibility criteria underwent diagnostic polysomnography. Those with apnea-hypopnea index (AHI) of 10 or greater but without significant hypoxemia (defined as O2 saturation below 75% for more than 10% of the duration of the study) were randomly assigned to receive either active CPAP therapy (REMStar Pro, Phillips Respironics, Murrysville, PA) or sham CPAP for 6 months. The sham CPAP appeared identical to the actual CPAP units and provided only a trivial amount of pressure (1–2 cm H2O). The active therapy and the sham CPAP group underwent an actual or a sham titration, respectively, in the sleep laboratory. Polysomnography consisted of monitoring of the EEG (C3-A2 or C4-A1, O2-A1 or O1-A2), electro-oculogram (ROC-A1, LOC-A2), chin and anterior tibialis EMG, heart rate by 2-lead ECG, snoring intensity (anterior neck microphone), nasal pressure (nasal cannula), thoracic and abdominal movement (inductance plethysmography bands), and oxygen saturation (pulse oximetry). The anterior tibialis EMG from both legs were used to score PLMS utilizing American Academy of Sleep Medicine (AASM) guidelines. (The minimum duration of a leg movement event was 0.5 seconds, the maximum duration was 10 seconds, and the minimum amplitude was an 8 μV increase in EMG voltage above resting EMG. The leg movements during a period from 0.5 seconds preceding an apnea or hypopnea event to 0.5 seconds following an apnea or hypopnea event were excluded). PLM index (PLMI) was defined as the total number of PLMS divided by the total sleep time (TST). An arousal was defined as an abrupt shift in the EEG frequency for at least 3 seconds after at least 10 continuous seconds of sleep. Arousal index was defined as the total number of arousals divided by the TST. The AHI, TST, sleep efficiency, sleep onset latency (SOL), arousal index, and PLMI were computed. The change in PLMI from the titration study to the 6-month follow-up study was compared between the CPAP and sham control groups to assess whether CPAP therapy worsens PLMI.
Participants
A total of 1,516 participants were screened and 1,105 were ultimately randomized (558 to active CPAP and 547 to sham CPAP) after excluding 268 participants due to exclusion criteria and 143 participants who withdrew from the study for diverse reasons.8 Data were available for 849 participants at the 6-month period (443 in the CPAP group and 406 in the sham group). People using medications likely to affect neurocognitive function or alertness (e.g., hypnotics, sedating antihistamines, anticonvulsants, stimulants) were excluded from the study.
Study measurements
The Hamilton Rating Scale for Depression (HAM-D)9 was administered at the baseline and 6-month follow-up visits and a score ≥8 was considered indicative of presence of depression. The Epworth Sleepiness Scale (ESS)10,11 was also administered at baseline and at 6-month visits and a score >10 suggested excessive daytime sleepiness. Finally, a maintenance of wakefulness test (MWT)12 was considered at the baseline and the 6-month visits and consisted of four 20-minute trials in the daytime. The mean sleep latency of all 4 trials was used for these analyses. The data coordinating center ensured quality assurance and quality control procedures for these data.8 Encore Pro SmartCards (Phillips Respironics) were used to monitor adherence to the therapy.
Statistical analyses
Data from all 1,105 participants were used to analyze the factors associated with PLMS at baseline. Student unpaired t test was used to test relationships between continuous variables and χ2 test for categorical variables. Linear regression was used with PLMI as the dependent to analyze the relationship between PLMI scores and demographic and polysomnographic characteristics at baseline and at 6 months. Logistic regression was used to assess odds for PLMI ≥10. Data were expressed as mean ± SD and the statistical significance level was set at p < 0.05 (2-tailed) for all tests. SPSS v 20.0 for Windows (SSPS, Chicago, IL) was used for statistical analyses.
Standard protocol approvals, registrations, and patient consent
A detailed description of the APPLES study protocol is available in an earlier study.8 The participants were recruited from 5 sites after institutional review board approval at all sites to enroll human participants. All participants provided written consent to participate in the study.
Data availability
The article includes analysis of data from APPLES (ClinicalTrials.gov identifier: NCT00051363). Researchers can request data from the APPLES coordination committee. Information on the particular methods used in the study can be provided by the authors at the request of qualified researchers.
Results
The mean age of the 1,105 participants was 51.6 ± 12 years (range 18–83 years), the mean body mass index was 32.2 ± 7.1 kg/m2, and the mean AHI was 40.1 ± 25.2 (range 6–156/hour). The participants included 723 men (65.4%) and 382 women (34.6%).
Prevalence of PLMS
Of all participants, 19.7% had PLMI ≥10/hour, 14.8% had PLMI ≥15/hour, 12.1% had PLMI ≥20/hour, 9.3% had PLMI ≥25/hour, and 7.5% had PLMI ≥30/hour. The 75th percentile PLMI was 5.5, 80th percentile was 9.3, 90th percentile was 24.1, and 95th percentile was 37.2.
Clinical and polysomnographic variables associated with PLMI
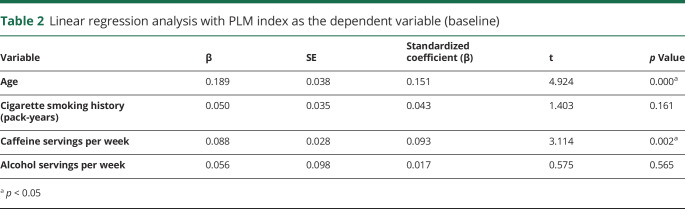
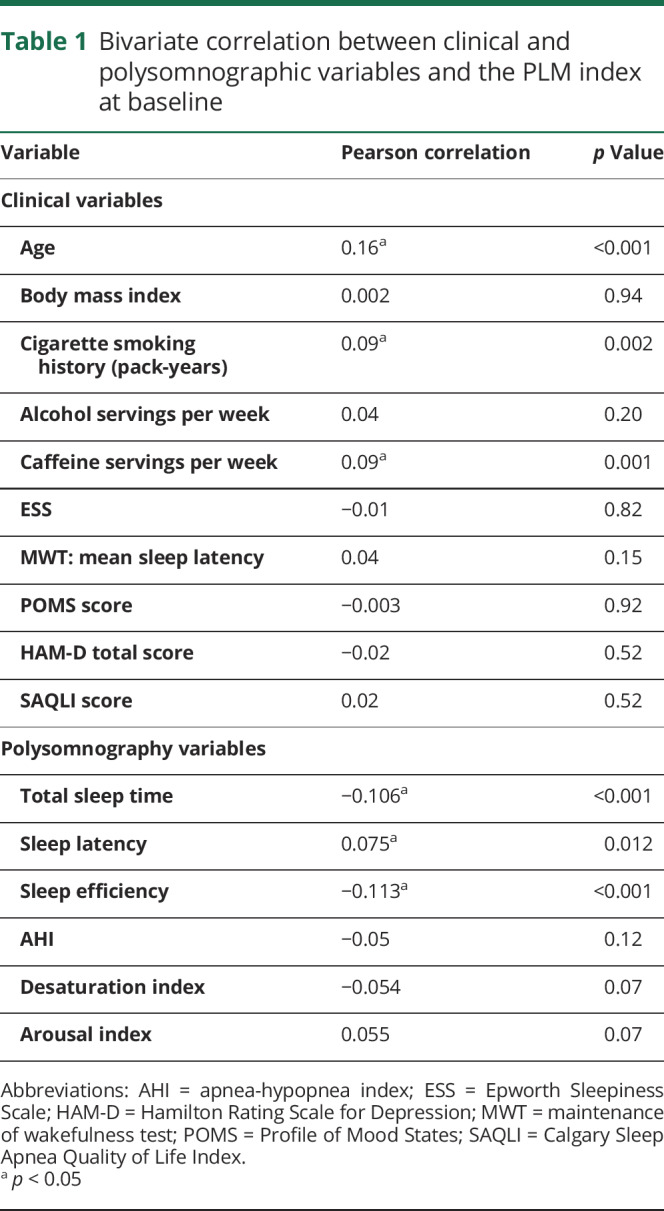
Correlation analyses revealed significant correlation between age, cigarette smoking history (pack-years), and caffeine servings per week with PLMI (table 1). A higher proportion of men than women had PLMI ≥10/hour (22.0% vs 15.4%, p = 0.01) or PLMI ≥25/hour (10.7% vs 6.8%, p = 0.04). No correlation was seen between PLMI and HAM-D score, Profile of Mood States, or Sleep Apnea Quality of Life Index scores. PLMI was also associated positively with sleep onset latency and inversely with sleep efficiency and TST on polysomnography. A positive correlation with arousal index approached statistical significance as well. Linear regression models showed that the association between PLMI and these sleep variables was independent of AHI and depression (HAM-D score). There was no significant correlation between PLMI and AHI, ESS scores, or MWT sleep latency.
Table 1.
Bivariate correlation between clinical and polysomnographic variables and the PLM index at baseline
Clinical predictors in multivariate models
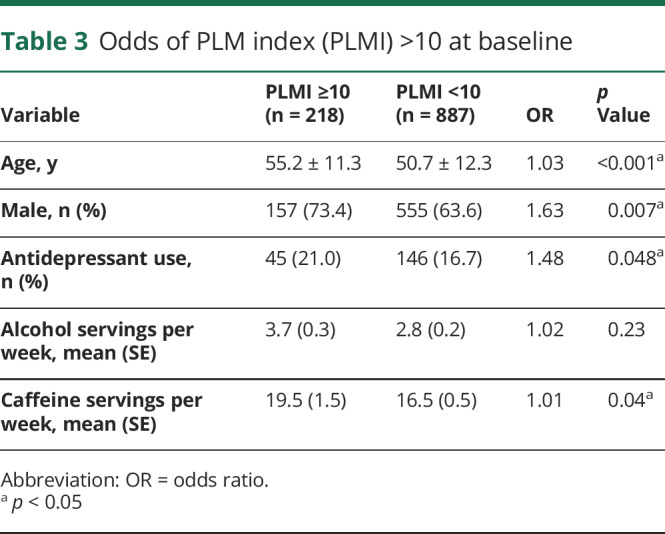
A linear regression model showed increasing age and total caffeine servings per week to be independent predictors of PLMI (table 2). Logistic regression analyses revealed higher odds of PLMI >10 with older age (odds ratio [OR] 1.03, p < 0.001), male sex (OR 1.63. p = 0.007), antidepressant use (OR 1.48. p = 0.048), and caffeine servings (OR 1.01. p = 0.04) (table 3).
Table 2.
Linear regression analysis with PLM index as the dependent variable (baseline)
Table 3.
Odds of PLM index (PLMI) >10 at baseline
Effect of PLMS on sleep quality
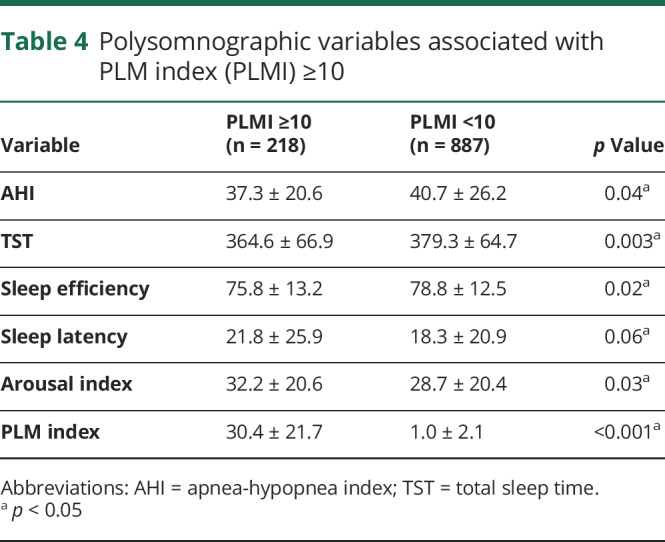
When compared with participants with PLMI <10/hour at baseline, those with PLMI ≥10/hour had slightly lower AHI (table 4). Despite the lower AHI, the PLMI ≥10/hour group demonstrated lower sleep efficiency and TST, higher arousal index, and a tendency towards higher sleep latency (table 4).
Table 4.
Polysomnographic variables associated with PLM index (PLMI) ≥10
Analysis of the results from the titration studies revealed no significant correlation between PLMI and residual AHI on the titration study (R = −0.07, p = 0.45). PLMI correlated negatively with TST (R = −0.43, p < 0.001) and sleep efficiency (R = −0.42, p < 0.001) and positively with SOL (R = 0.20, p = 0.04) and arousal index (R = 0.27, p = 0.006) on polysomnography. Similarly, specifically in participants randomized to CPAP, there was no correlation between PLMI and the residual AHI on the titration study (R = −0.008, p = 0.85) but correlated negatively with TST (R = −0.20, p < 0.001) and sleep efficiency (R = −0.20, p < 0.001) and positively with arousal index (R = 0.24, p < 0.001). PLMI was an independent predictor of sleep efficiency (β = −1.13, p = 0.001) on this study when controlled for age and residual AHI.
We also assessed the relationship between the residual AHI and PLMS on the titration study in participants with PLMI >10/hour at baseline to evaluate whether the presence of PLMS on this study may be an indication of an incomplete resolution of breathing abnormalities with CPAP therapy. There was no correlation between PLMI and residual AHI on the titration study (R = −0.07, p = 0.45). The PLMS persisted despite adequate titration based on AASM recommendations. In this group as well, a linear regression model showed that PLMI (β = −0.33, p < 0.001) and increasing age (β = −0.28, p = 0.003), but not AHI (β = −0.03, p = 0.98), were negatively associated with sleep efficiency.
Evaluating the 6-month studies in participants on CPAP therapy, a linear regression model showed that age (β = −0.27, p < 0.001), residual AHI (β = −0.14, p = 0.02), and PLMI (β = −0.07, p = 0.01) were independent negative predictors of sleep efficiency.
Effect of PAP therapy on PLMI
The CPAP and the sham groups did not differ significantly in the baseline PLMI (7.2 ± 16.6 vs 6.4 ± 13.7, p = 0.4) or the mean change in PLMI from baseline to 6 months (3.0 ± 16.6 vs 2.2 ± 15.9, p = 0.5). The PLMI in the CPAP group at 6 months (n = 443) was similar to that in the sham group (n = 406) (9.7 ± 19.0 vs 8.7 ± 17.0, p = 0.4). The prevalence of PLMI ≥25/hour was similar in the CPAP and the sham groups at baseline (9.5% vs 9.1%, p = 0.9) and at 6 months (14.7% vs 11.3%, p = 0.15). In the CPAP group, 52.2% had the same or higher PLMI at 6 months compared to baseline vs 47.8% in the sham group (p = 1.0). Among those with a baseline PLMI ≥10/hour, there was a similar decline in PLMI in both CPAP and sham groups at 6 months (−4.2 ± 25.4 vs −4.8 ± 25.0, p = 0.9). In addition, the decline in PLMI at 6 months among those with a baseline PLMI ≥10/hour was nonsignificant between the CPAP and the sham groups when only the participants with average PAP adherence >4 hours/night were analyzed (−4.3 ± 25.8 vs −8.3 ± 25.1, p = 0.4). We also looked at the PLMI change from the titration study to the polysomnography done 6 months later. Age, sex, and PLMI at the titration study were significantly associated with change in PLMI 6 months later in a linear regression model, but randomization to the CPAP or the sham group was not a significant predictor of this change. A multiple regression model in the participants randomized to CPAP demonstrated no difference in the mild OSA group and the moderate to severe OSA group in the change in PLMI with therapy (p = 0.13). Specifically looking at the participants with mild OSA, there was no change in the PLMI in the CPAP or the sham groups.
Discussion
In this study, we evaluated the prevalence and predictors of PLMS in patients with OSA and whether treatment with CPAP affects the severity of PLMS. We found that increasing age, smoking, and caffeine use were associated with more severe PLMS. The odds of having a PLMI ≥10 were higher in older participants, in men, in those using antidepressants, and with higher caffeine use. After controlling for OSA and depression, PLMS were significantly associated with a reduction in sleep quality as defined by increased sleep latency, reduced sleep efficiency, and reduced TST. This association was seen on the baseline and the titration studies as well as on the 6-month polysomnogram despite adequate treatment of OSA. No significant relationships were noted between PLMS frequency and subjective or objective sleepiness. Finally, treatment with CPAP did not affect the severity of PLMS.
The prevalence of PLMS at baseline was 15%, which is significantly higher than that reported in the general population. Estimates of PLMS prevalence vary widely in the literature, in part related to the minimum frequency of events considered to be significant. To clarify this issue, the current study provides prevalence data based on several different cutoffs to allow easy comparison between the past and any future studies. For example, one retrospective case series of 798 consecutive patients screened for OSA with polysomnography estimated a 47% prevalence of PLMS6; the prevalence in patients with comorbid OSA was 48%. However, in this study, PLMS was defined as a periodic leg movement arousal index ≥5/hour. The population was similar to our study in that participants had a high prevalence of comorbid OSA. In contrast, a community-based sample of approximately 592 participants, using PLMI >15/hour to define PLMS, estimated a prevalence of 7.6%.7 In the current study, the prevalence of PLMI ≥15/hour was 14.8%, roughly twice the prevalence reported in the aforementioned study. Finally, another population-based study of 2,162 men and women in Switzerland estimated a 28.6% prevalence of PLMS using PLMI >15/hour.13 Notably, the participants in this study were significantly older (age range 35–75 years, mean 58 years) than the APPLES participants. Information on any prevalent OSA was not provided in the earlier 2 studies. Nevertheless, while several studies including these latter 2 have used a PLMI cutoff of 15/hour, the current study demonstrates that a lower cutoff of 10/hour was equally discriminative for worse sleep quality as well as the common predictors of PLMS.
Our results confirm prior findings in the literature that prevalence of PLMS is higher in men and with increasing age, including studies with and without comorbid OSA6,7,13 The other clinical predictors of PLMS in the current study include the number of caffeine servings and antidepressant use. Haba-Rubio et al.13 studied 2,162 Swiss men and women with PLMS and also found that antidepressant use was independently associated with PLMI >15/hour. This sample did not include information on OSA, thus the current study remains the largest to date to confirm these clinical predictors of PLMS in participants with known OSA.
We found that PLMS were independently associated with worse sleep quality independent of AHI at baseline, titration, and 6 months post-CPAP treatment. Whether and how this worse sleep quality translates into clinical consequences is not clear. Some studies have suggested an association between PLMS and an increased risk of cardiovascular disorders.2,14,15 Other studies have demonstrated a correlation between PLMI and affective symptoms and fatigue,16 cognitive decline (particularly executive function) in older men without dementia,17 and lower quality of life in children.18 One study even suggested a possibility of higher mortality in those with PLMI >15/hour.19 Future studies should be designed to assess the effect of PLMS therapy on clinical outcomes in persons with OSA. If indeed the therapy of PLMS is beneficial in patients with OSA, polysomnography may appear to be the preferential diagnostic study for OSA as it allows making a concomitant diagnosis of PLMS, an ability lacking in most home sleep apnea test devices. The negative association seen between PLMS and sleep quality in the current study is consistent with the past studies that have demonstrated that PLMS result in sleep disruption.16,20 The current study is among the largest and demonstrates that in persons with OSA, who already have a reduced quality of sleep, PLMS significantly worsen sleep quality further, independent of the severity of sleep apnea.
We did not find an association between the PLMI and subjective or objective excessive daytime sleepiness. This finding is consistent with that from an earlier study that found no significant relation between PLMS and sleepiness.4 Similar to that study, the current study has a large sample size and includes both subjective and objective evaluation of sleepiness. The current study utilized MWT while the cited study utilized MSLT. Furthermore, we assessed the effect of PLMS on residual sleepiness in patients with OSA after 6 months of CPAP therapy and did not find a correlation. An earlier study from the APPLES database suggests younger age, more severe sleep apnea, and depression to be independent predictors of sleepiness in persons with OSA.21 Hence, adequate treatment of OSA and depression should be the primary focus in patients with sleepiness.
While prior studies have shown that PLMS and OSA are commonly comorbid, there are conflicting data regarding whether treatment with CPAP influences the presence or severity of PLMS. It has been suggested that treatment of OSA with CPAP may unmask PLMS.5,6 Another study observed an increase in PLMS 6 months postoperatively in children with OSA treated by adenotonsillectomy.22 However, these studies have generally lacked a control group. We did not find any correlation between residual AHI and PLMI on the titration study or the follow-up studies. To our knowledge, the current study is the first large study to evaluate the effect of CPAP treatment on periodic limb movements compared to controls with sham CPAP. While changes were noted over a period of 6 months, they were similar in the CPAP and the sham group. Studies lacking a control group may erroneously attribute such changes over time to PAP therapy.
According to the AASM Clinical Practice Guidelines and based on the International Classification of Sleep Disorders, 2nd Edition, PLMS are regarded as an incidental finding when coexistent with sleep-related breathing disorders or RLS. In such instances, treating the underlying sleep disorder (which is regarded as the primary sleep disorder) is recommended as the first step in therapy. It is suggested that the treatment for PLMS should only be considered if symptoms persist after treatment of OSA. However, the current study did not demonstrate any beneficial effect of CPAP on reducing the severity of PLMS. It suggests that the 2 disorders may coexist independently and clinicians should consider concurrent treatment of the 2 disorders for patients with symptoms suggestive of a reduction in sleep quality.
The strengths of this study include a large sample size, use of a control group with sham CPAP, and use of standardized, objective measurement tools including polysomnography and MWT, thus reducing measurement error and reporting biases. The participants underwent repeat polysomnography for titration as well as after 6 months, providing a reasonably long follow-up.
The study has several limitations that may constrain potential interpretations. First, while the study assessed PLMS in participants with OSA, the data regarding presence or change in symptoms of RLS over time or with PAP therapy are not available. It is unclear if the PLMS are isolated and discovered because of polysomnography or whether RLS symptoms would have predicted several of these cases. Second, the study did not report PLMS arousal index. However, we found that even without specifically taking PLMS arousal index into account, the PLMI was significantly associated with worse sleep efficiency. Third, in the absence of a standard definition of significant PLMI, we chose an index of PLMI ≥10/hour. A higher cutoff of 15/hour has been used in several studies but our results suggest that even a lower cutoff was indicated with significantly inferior sleep quality. We performed sensitivity analyses with the 15/hour cutoff and the results were generally similar to those reported herein. Fourth, several other factors including chronic kidney disease and medications other than antidepressants may also be important in pathophysiology of PLMS, but were not evaluated in depth in the current study. Fifth, our analyses can only be generalized to persons with both OSA and PLMS, and not to those without concomitant OSA. Finally, the current study does not inform whether the therapy of PLMS would have additive benefit over just the therapy of OSA with PAP therapy.
To our knowledge, this is the largest randomized controlled analysis evaluating the effect of PAP treatment on PLMS. Our results show a high prevalence of PLMS in patients with OSA compared to those reported historically in the general population, and confirm that male sex, increasing age, caffeine use, and antidepressant use are significant independent predictors of PLMS in this group, as in the general population. The study did not show a differential effect of CPAP in comparison to sham CPAP on PLMS after 6 months of therapy. However, PLMS had a negative effect on the objective sleep quality independent of AHI. These results suggest that sleep quality could improve if both OSA and PLMS were to be treated concurrently. This is contrary to the current AASM guidelines that recommend that treatment of comorbid PLMS should be considered only after treatment of OSA. Given the adverse health risks associated with untreated PLMS suggested in prior studies and worse sleep quality despite CPAP therapy found in this study, further investigations of concurrent therapy are warranted. Specifically, trials evaluating the effect of treatment on diverse clinical measures including symptoms, blood pressure, mood changes, subjective improvement in sleep, and quality of life, as well as objective polysomnographic findings such as improvement in sleep continuity, sleep architecture, and reduction in arousal index, will provide further insights into the potential benefit of treating PLMS in concurrence with OSA.
Acknowledgment
The APPLES investigators thank Dr. Sylvan Green, who died before the results of this trial were analyzed, for help with the design and conduct of the study. Administrative core: Clete A. Kushida, MD, PhD; Deborah A. Nichols, MS; Eileen B. Leary, BA, RPSGT; Pamela R. Hyde, MA; Tyson H. Holmes, PhD; Daniel A. Bloch, PhD; William C. Dement, MD, PhD. Data coordinating center: Daniel A. Bloch, PhD; Tyson H. Holmes, PhD; Deborah A. Nichols, MS; Rik Jadrnicek, Microflow; Ric Miller, Microflow; Usman Aijaz, MS; Aamir Farooq, PhD; Darryl Thomander, PhD; Chia-Yu Cardell, RPSGT; Emily Kees; Michael E. Sorel, MPH; Oscar Carrillo, RPSGT; Tami Crabtree, MS; Booil Jo, PhD; Ray Balise, PhD; Tracy Kuo, PhD. Clinical coordinating center: Clete A. Kushida, MD, PhD; William C. Dement, MD, PhD; Pamela R. Hyde, MA; Rhonda M. Wong, BA; Pete Silva; Max Hirshkowitz, PhD; Alan Gevins, DSc; Gary Kay, PhD; Linda K. McEvoy, PhD; Cynthia S. Chan, BS; Sylvan Green, MD. Clinical centers: Stanford University: Christian Guilleminault, MD; Eileen B. Leary, BA, RPSGT; David Claman, MD; Stephen Brooks, MD; Julianne Blythe, PA-C, RPSGT; Jennifer Blair, BA; Pam Simi; Ronelle Broussard, BA; Emily Greenberg, MPH; Bethany Franklin, MS; Amirah Khouzam, MA; Sanjana Behari Black, BS, RPSGT; Viola Arias, RPSGT; Romelyn Delos Santos, BS; Tara Tanaka, PhD. University of Arizona: Stuart F. Quan, MD; James L. Goodwin, PhD; Wei Shen, MD; Phillip Eichling, MD; Rohit Budhiraja, MD; Charles Wynstra, MBA; Cathy Ward; Colleen Dunn, BS; Terry Smith, BS; Dane Holderman; Michael Robinson, BS; Osmara Molina, BS; Aaron Ostrovsky; Jesus Wences; Sean Priefert; Julia Rogers, BS; Megan Ruiter, BS; Leslie Crosby, BS, RN. St. Mary Medical Center: Richard D. Simon Jr., MD; Kevin Hurlburt, RPSGT; Michael Bernstein, MD; Timothy Davidson, MD; Jeannine Orock-Takele, RPSGT; Shelly Rubin, MA; Phillip Smith, RPSGT; Erica Roth, RPSGT; Julie Flaa, RPSGT; Jennifer Blair, BA; Jennifer Schwartz, BA; Anna Simon, BA; Amber Randall, BA. St. Luke's Hospital: James K. Walsh, PhD; Paula K. Schweitzer, PhD; Anup Katyal, MD; Rhody Eisenstein, MD; Stephen Feren, MD; Nancy Cline; Dena Robertson, RN; Sheri Compton, RN; Susan Greene; Kara Griffin, MS; Janine Hall, PhD. Brigham and Women's Hospital: Daniel J. Gottlieb, MD, MPH; David P. White, MD; Denise Clarke, BSc, RPSGT; Kevin Moore, BA; Grace Brown, BA; Paige Hardy, MS; Kerry Eudy, PhD; Lawrence Epstein, MD; Sanjay Patel, MD. The authors thank Sleep Health Centers for the use of their clinical facilities to conduct this research. Consultant teams: Methodology Team: Daniel A. Bloch, PhD; Sylvan Green, MD; Tyson H. Holmes, PhD; Maurice M. Ohayon, MD, DSc; David White, MD; Terry Young, PhD. Sleep-Disordered Breathing Protocol Team: Christian Guilleminault, MD; Stuart Quan, MD; David White, MD. EEG/Neurocognitive Function Team: Jed Black, MD; Alan Gevins, DSc; Max Hirshkowitz, PhD; Gary Kay, PhD; Tracy Kuo, PhD. Mood and Sleepiness Assessment Team: Ruth Benca, MD, PhD; William C. Dement, MD, PhD; Karl Doghramji, MD; Tracy Kuo, PhD; James K. Walsh, PhD. Quality of Life Assessment Team: W. Ward Flemons, MD; Robert M. Kaplan, PhD. APPLES Secondary Analysis–Neurocognitive Team: Dean Beebe, PhD; Robert Heaton, PhD; Joel Kramer, PsyD; Ronald Lazar, PhD; David Loewenstein, PhD; Frederick Schmitt, PhD. National Heart, Lung, and Blood Institute: Michael J. Twery, PhD; Gail G. Weinmann, MD; Colin O. Wu, PhD. Data and Safety Monitoring Board: 7-year term: Richard J. Martin, MD (Chair); David F. Dinges, PhD; Charles F. Emery, PhD; Susan M. Harding, MD; John M. Lachin, ScD; Phyllis C. Zee, MD, PhD. Other terms: Xihong Lin, PhD (2 years); Thomas H. Murray, PhD (1 year).
Glossary
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- APPLES
Apnea Positive Pressure Long-term Efficacy Study
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- HAM-D
Hamilton Rating Scale for Depression
- MWT
maintenance of wakefulness test
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PLMI
PLM index
- PLMS
periodic limb movements of sleep
- RLS
restless legs syndrome
- SOL
sleep onset latency
- TST
total sleep time
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was funded by contract 5UO1-HL-068060 from the National Heart, Lung and Blood Institute. The APPLES pilot studies were supported by grants from the American Academy of Sleep Medicine and the Sleep Medicine Education and Research Foundation to Stanford University and by the National Institute of Neurologic Disorders and Stroke (N44-NS-002394) to SAM Technology.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med 2006;2:281–287. [PubMed] [Google Scholar]
- 2.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 2009;32:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S; Osteoporotic Fractures in Men Study Group. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation 2011;124:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med 2001;164:1454–1458. [DOI] [PubMed] [Google Scholar]
- 5.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep 2003;26:717–720. [DOI] [PubMed] [Google Scholar]
- 6.Hedli LC, Christos P, Krieger AC. Unmasking of periodic limb movements with the resolution of obstructive sleep apnea during continuous positive airway pressure application. J Clin Neurophysiol 2012;29:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo WH, Guilleminault C. Periodic leg movement, nasal CPAP, and expiratory muscles. Chest 2012;142:111–118. [DOI] [PubMed] [Google Scholar]
- 8.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med 2006;2:288–300. [PubMed] [Google Scholar]
- 9.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standard. J Sleep Res 2000;9:5–11. [DOI] [PubMed] [Google Scholar]
- 12.Doghramji K, Mitler MM, Sangal RB, et al. A normative study of the maintenance of wakefulness test (MWT). Electroencephalogr Clin Neurophysiol 1997;103:554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol 2016;79:464–474. [DOI] [PubMed] [Google Scholar]
- 14.Cassel W, Kesper K, Bauer A, et al. Significant association between systolic and diastolic blood pressure elevations and periodic limb movements in patients with idiopathic restless legs syndrome. Sleep Med 2016;17:109–120. [DOI] [PubMed] [Google Scholar]
- 15.Boulos MI, Murray BJ, Muir RT, et al. Periodic limb movements and white matter hyperintensities in first-ever minor stroke or high-risk transient ischemic attack. Sleep 2016;40:zsw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy De Buisseret FX, Mairesse O, Newell J, Verbanck P, Neu D. While isolated periodic limb movement disorder significantly impacts sleep depth and efficiency, co-morbid restless leg syndrome mainly exacerbates perceived sleep quality. Eur Neurol 2017;77:272–280. [DOI] [PubMed] [Google Scholar]
- 17.Leng Y, Blackwell T, Stone KL, Hoang TD, Redline S, Yaffe K. Periodic limb movements in sleep are associated with greater cognitive decline in older men without dementia. Sleep 2016;39:1807–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Herzeele C, Dhondt K, Roels SP, et al. Periodic limb movements during sleep are associated with a lower quality of life in children with monosymptomatic nocturnal enuresis. Eur J Pediatr 2015;174:897–902. [DOI] [PubMed] [Google Scholar]
- 19.Choi JW, Song JS, Lee YJ, Jeong DU. Periodic limb movements in sleep is associated with increased mortality. Psychiatry Investig 2017;14:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri R, Rundo F, Zucconi M, et al. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. Sleep 2015;38:919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budhiraja R, Kushida CA, Nichols DA, et al. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur Respir J 2017;50:pii: 1700348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chervin RD, Chung S, O'Brien LM, et al. Periodic leg movements during sleep in children scheduled for adenotonsillectomy: frequency, persistence, and impact. Sleep Med 2014;15:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The article includes analysis of data from APPLES (ClinicalTrials.gov identifier: NCT00051363). Researchers can request data from the APPLES coordination committee. Information on the particular methods used in the study can be provided by the authors at the request of qualified researchers.