Abstract
Objective
To assess whether informant-reported apneas during sleep (witnessed apneas) in cognitively unimpaired (CU) elderly persons are associated with higher levels of brain tau.
Methods
From the population-based Mayo Clinic Study of Aging, we identified 292 CU elderly ≥65 years of age with both AV-1451 tau-PET and Pittsburgh compound B (PiB)-PET scans and whose bed partners and close relatives had completed a questionnaire that assessed whether participants had witnessed apneas during sleep. For this cross-sectional analysis, we selected the entorhinal and inferior temporal cortices as our regions of interest (ROIs) because they are highly susceptible to tau accumulation. PET signal was scaled to the cerebellum crus to calculate standardized uptake value ratio (SUVR). We fit linear models to assess the association between regional tau and witnessed apneas while controlling for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global PiB.
Results
Forty-three participants (14.7%) were found to have witnessed apneas during sleep. The report of witnessed apneas was associated with higher tau-PET SUVR elevation in our ROIs: 0.049 SUVR (95% confidence interval [CI] 0.010–0.087, p = 0.015) in the entorhinal cortex and 0.037 SUVR (95% CI 0.006–0.067, p = 0.019) in the inferior temporal cortex after controlling for confounders.
Conclusion
We identified a significant association between witnessed apneas in CU elderly and elevated tau-PET signal in tau-susceptible brain regions. These results suggest a plausible mechanism that could contribute to cognitive impairment and the development of Alzheimer disease. Longitudinal observations are necessary to determine direction of causality.
Obstructive sleep apnea (OSA) is characterized by frequent stop breathing events during sleep.1 OSA is prevalent in the elderly (5%–73%).2 It increases with aging2–4 until it plateaus in frequency at ≈65 years of age.5,6 It has been associated with an increased risk of cognitive decline7,8 and mild cognitive impairment (MCI)/dementia.9–11 A meta-analysis of prospective studies estimated that individuals with sleep-disordered breathing are 26% more likely to develop cognitive impairment,12 but the mechanisms behind this association have not been elucidated.9–11
In Alzheimer disease (AD), both neuritic plaques composed of β-amyloid (Aβ) fibrils and neurofibrillary tangles composed of hyperphosphorylated tau (p-tau) accumulate in the brain and can contribute to neurodegeneration.13,14 Sleep disruption has been associated with increased AD pathology15–18 by affecting Aβ19–21 and tau22–25 regulation. We have shown that self-reported sleep disturbance, as measured by excessive daytime sleepiness (EDS), is associated with global cortical thinning (implying neurodegeneration)26 and longitudinal amyloid accumulation.18 Individuals with EDS had a higher frequency of stop breathing events during sleep (witnessed apneas),18,26 suggesting underlying OSA. However, the relationship between tau accumulation and OSA remains unclear.27–29
Deposition of 3R/4R tau in AD is ubiquitous in the temporal regions, suggesting a regional susceptibility to AD pathophysiologic processes at early stages.30–34 Temporal tau-PET level with AV1451, particularly in the entorhinal cortex, is a good predictor of cognitive changes in individuals with preclinical AD and cognitively unimpaired (CU) individuals,33,35 making it a useful neuroimaging biomarker of AD. In this study, we hypothesized that CU elderly participants with witnessed apneas would have higher tau-PET levels in tau-susceptible brain regions.
Methods
Participants
The Mayo Clinic Study of Aging (MCSA) is a large population-based cohort of community-dwelling residents from Olmsted County (Minnesota). Further details of the original MCSA design, including participant eligibility and enrollment, have been published elsewhere.36 For this cross-sectional study, we initially identified all CU participants ≥65 years old who had at least a single tau-PET, Pittsburgh compound B (PiB)-PET, and structural brain MRI available and completed core questions of our sleep assessment from April 2015 to April 2018. Of 308 participants who met these criteria, we excluded 16 (5%) who had a history of a comorbid neurologic disorder. A total of 292 participants were included in this study.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. Written informed consent was obtained from all participants.
Cognitive assessment
As part of the MCSA protocol, the participants underwent a comprehensive cognitive evaluation, including detailed history, neurologic examination, and neuropsychological battery assessing 4 cognitive domains (executive, language, memory, and visuospatial). The CU status was based on a collective agreement among the MCSA study coordinator, neuropsychologist, and examining physician, as previously described,36–38 taking into account education, prior occupation, and visual or hearing deficits.
Sleep symptoms assessment
The Mayo Sleep Questionnaire39 was used to collect core sleep-related symptoms from participants and informants (bed partners, spouses, and close relatives). To screen for OSA, informants were asked, “Does the patient ever seem to stop breathing during sleep?” Informants were also asked whether participants appeared to act out in their dreams (dream enactment behavior). The participants answered questions regarding changes in sleep duration and sleepiness. Daytime sleepiness was assessed with the Epworth Sleepiness Scale.40 EDS was defined as an Epworth Sleepiness Scale score ≥10 to remain consistent with our previous work18,26 and other published literature with samples of similar age groups.41,42
Clinical assessment
Trained nurses obtained the history of cardiovascular and medical comorbid conditions from the Rochester Epidemiology Project medical records-linkage system.43,44 Body mass index (BMI) was obtained by direct weight and height measurements, and history of tobacco use was acquired from the structured interview of the participants.
Neuroimaging assessment
Tau-PET was performed with flortaucipir (18F-AV-1451).45 Imaging was acquired between 80 and 100 minutes after injection. PiB-PET was performed with (11)C-PiB46 between 40 and 60 minutes after injection. Tau-PET and PiB-PET were analyzed following similar methodology published previously by our group31 with our in-house fully automated image processing pipeline. Briefly, the tau-PET and PiB-PET data were processed after these scans were registered to the participant's 3T MRI scan. Each PET voxel was divided by a magnetic resonance–based probabilistic tissue segmentation to generate a partial volume–corrected PET image,47 which was then masked to include only gray matter (tau-PET) or gray and white matter (PiB-PET), respectively, to exclude voxels that were more likely to contain CSF.
We computed regional tau-PET and PiB-PET standardized uptake value ratio (SUVR) by estimating the voxel number–weighted average of the median uptake in each region of interest (ROI) and dividing by the uptake in the cerebellar crus gray matter.48 For tau-PET data analysis, we chose the entorhinal cortex and inferior temporal cortex as our ROIs because they are highly susceptible regions for tau accumulation in AD.30–32 These 2 regions are widely used to quantify tau-PET signal in preclinical AD. Global PiB estimation was computed to optimize the linear regression models because global PiB is highly associated with tau accumulation.30–32 For this estimation, we calculated the weighted average PiB SUVR in the following ROIs: prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus. Global PiB (or amyloid) positivity was defined by an SUVR ≥1.48. This cutoff is equivalent to our previously defined cutoff of 1.42 based on the reliable worsening method,48 after adjustment for updated processing pipelines and reference region (cerebellar hemisphere to cerebellar crus). This cutoff selects participants in whom amyloid accumulation is more like likely to occur,48,49 corresponding to Thal amyloid phases of at least 1 to 2.50
Statistical analysis
Participants with and without a history of witnessed apneas were compared regarding their baseline demographic, clinical, and imaging characteristics. Continuous variables were compared with either the Student t test or the Mann-Whitney U test, according to data frequency distribution and homoscedasticity. The normality assumption was tested by visual inspection of variable histograms and by means of the Kolmogorov-Smirnov test. Categorical data were compared by use of a χ2 test or Fisher exact test. For a sensitivity analysis of group differences using pairwise tests (paired t test, Wilcoxon signed-rank test, and McNemar test, as applicable), participants with witnessed apneas (cases) were randomly matched (1:1 ratio) to participants without witnessed apneas (controls) on age (with maximum tolerance of 2 years of difference) and global PiB (with maximum tolerance of 0.06 SUVR difference) because these were the only other variables associated with tau levels in our sample. Case-control matching was performed with a Python extension for SPSS (Fuzzy command) with random case order when drawing matches and giving priority to exact matches. PiB SUVR tolerance at 0.06 was considered sufficient for appropriate matching considering that PiB-PET test-retest variability is ≈3% to 10%.e1
A multiple linear regression model was fit to test for an association between a positive report of witnessed apneas (independent variable) and tau SUVR (dependent variable) in the entorhinal and inferior temporal cortices. To control for potential confounders, the following variables were included as covariates: age, sex, years of education, BMI, hypertension, hyperlipidemia, diabetes mellitus, reduced sleep, EDS, and global PiB SUVR. The α level was set at 0.05 for 2-tailed tests. Statistical analyses were performed with IBM SPSS Statistics for Windows Version 24 (IBM Corp, Armonk, NY).
Data availability
Data that support the findings of this study are available for sharing with qualified investigators on reasonable request.
Results
Demographic and clinical characteristics
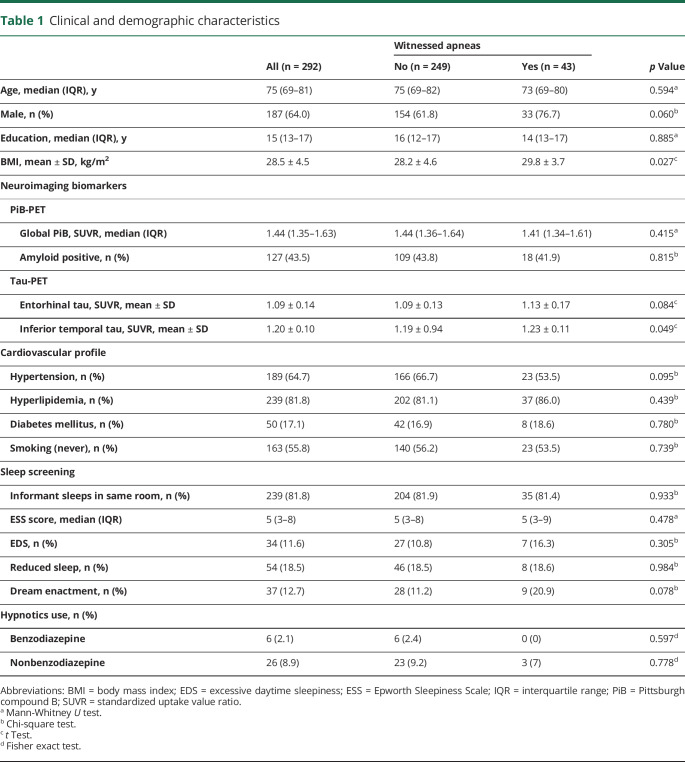
Of the 292 participants included in our study, 43 (14.7%) were reported to have witnessed apneas during sleep. Participants with reported witnessed apneas had higher BMI and trended toward a more frequent male predominance. Participants with and without witnessed apneas had a similar profile of comorbid cardiovascular disease, hypnotic use, and other sleep-related symptoms. Global PiB levels and amyloid positivity status were also similar (table 1).
Table 1.
Clinical and demographic characteristics
Tau-PET imaging
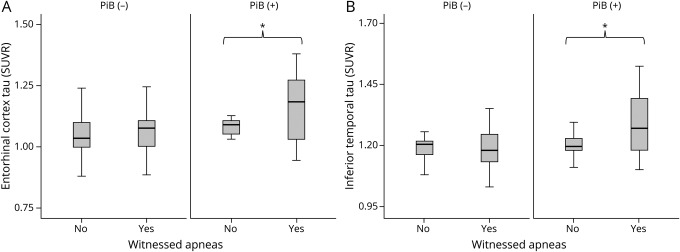
When unadjusted tau SUVRs were compared between participants with and without witnessed apneas, no significant differences were found in the entorhinal cortex (1.13 ± 0.17 vs 1.09 ± 0.13, p = 0.084). However, tau SUVR was higher in the inferior temporal cortex of participants with vs without witnessed apneas (1.23 ± 0.11 vs 1.19 ± 0.94, p = 0.049) (table 1). A sensitivity analysis using age- and global PiB–matched participants with and without witnessed apneas showed differences that were significant in amyloid-positive (PiB+) participants (figure 1 and tables e-1 and e-2, doi.org/10.5061/dryad.gb5mkkwm2). In this subsample, both entorhinal and inferior temporal SUVRs were elevated in participants with witnessed apneas (1.20 ± 0.22 vs 1.11 ± 0.11, p = 0.036; and 1.29 ± 0.13 vs 1.21 ± 0.74, p = 0.029, respectively) (figure 2). Further demographic and clinical characterization of amyloid-positive subsamples did not reveal additional differences (table e-2). Despite the use of a 0.06 global PiB SUVR cutoff for case-control matching, a statistically significant difference in global PiB SUVR was detected between amyloid-negative groups in a smaller scale (mean difference 0.019), with higher amyloid levels in participants without witnessed apneas (table e-2). Although this finding could have theoretically obscured differences in tau SUVR between groups, the size effect noted is within PiB-PET test-retest variability,e1 and the difference was not confirmed in amyloid-positive participants, suggesting doubtful significance. In amyloid-negative participants, nonspecific uptake, increased signal inhomogeneity, and low signal-to-noise ratios are expected and could lead to spurious associations. Restricting the global PiB cutoff for case-control matching at such a low level was not performed because it would lead to an unjustified loss in statistical power that would preclude this sensitivity analysis.
Figure 1. Tau SUVR according to report of witnessed apneas and amyloid status.
Box-plot representation of tau standardized uptake value ratio (SUVR) in the (A) entorhinal and (B) inferior temporal cortices of participants with and without witnessed apneas according to amyloid (Pittsburgh compound B [PiB]) status. *p < 0.05.
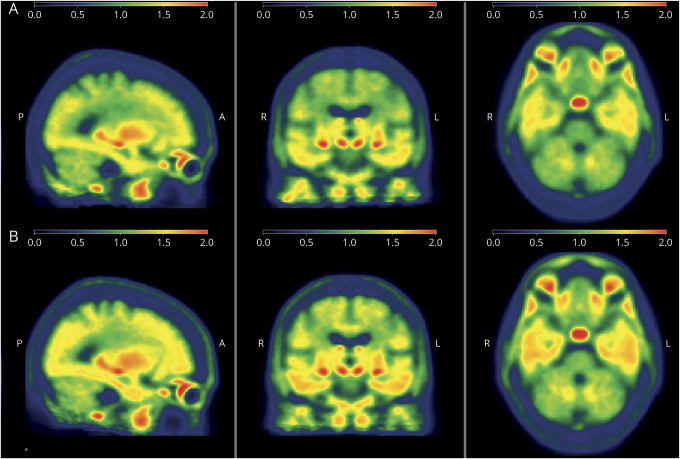
Figure 2. Tau-PET imaging according to report of witnessed apneas.
Average tau-PET signal in age- and global Pittsburgh compound B–matched amyloid-positive participants (A) without and (B) with witnessed apneas.
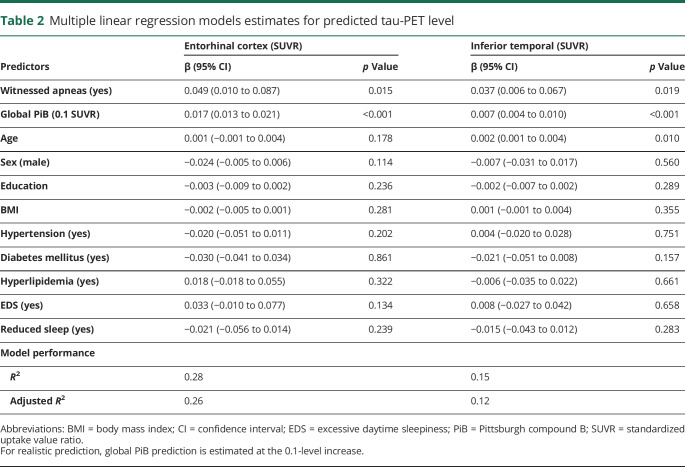
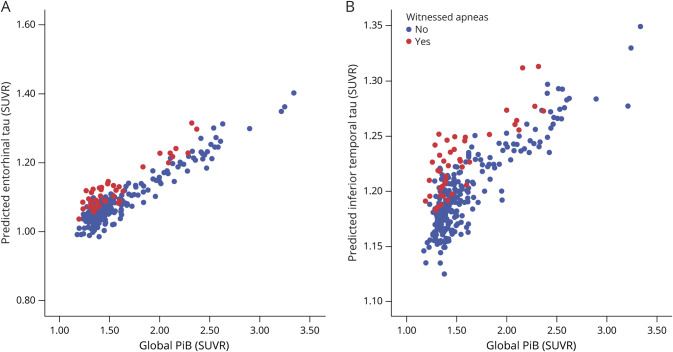
We performed linear regression models to assess for an association between witnessed apneas and tau SUVR in our ROIs while controlling for multiple confounders. For this analysis, the whole sample was included. The models revealed that the presence of witnessed apneas was associated with an average 0.049 elevation in the entorhinal tau SUVR (95% confidence interval 0.010–0.087, p = 0.015) and 0.037 elevation in the inferior temporal tau SUVR (95% confidence interval 0.006–0.067, p = 0.019). Compared to the average tau level in those participants without witnessed apnea, this mean elevation represented a 4.5% and 3.1% increase, respectively. Both entorhinal and inferior temporal tau SUVRs were highly associated with global PiB (table 2). Figure 3 illustrates the independent effect of witnessed apnea in predicted tau SUVR according to global PiB levels. To rule out overfitting of the data, we also performed a sensitivity analysis using a regression model with restricted covariates (age, global PiB, sex, and witnessed apnea). The results were very similar to the full model (table e-3, doi.org/10.5061/dryad.gb5mkkwm2).
Table 2.
Multiple linear regression models estimates for predicted tau-PET level
Figure 3. Model estimation of tau-PET signal.
Model estimates of predicted tau standardized uptake value ratio (SUVR) according to global Pittsburgh compound B (PiB) in the entorhinal (A) and (B) inferior temporal cortices of participants without (blue) and with (red) witnessed apneas.
Discussion
We found that CU elderly individuals who had witnessed apneas during sleep had higher tau-PET levels in the entorhinal and inferior temporal cortices. This association was independent of age and global PiB burden and was more pronounced in amyloid-positive participants. Although a causal relationship cannot be established with our cross-sectional design, our findings corroborate the hypothesis that OSA in the elderly could be associated with an increased susceptibility to tau accumulation, which may be related to an increased risk for AD dementia.
Sleep appears to play an important role in promoting downscaling of synaptic connections to renormalize synaptic strength and to improve synaptic efficiency,e2,e3 which seems to be mediated to some extent by slow-wave activity (SWA).e3,e4 SWA also promotes a decline in cerebral metabolism,e5 which may contribute to the restorative effect of sleep. On the other hand, sleep appears to facilitate the clearance of toxic metabolic byproducts through the glymphatic pathway,20,23 which correlates with increased SWA, lower heart rate, and decreased sympathetic activity.20,e6
Apneas could adversely affect sleep-dependent synaptic homeostatic processes in multiple ways. As air flow obstruction occurs, increased ventilatory effort, intermittent hypoxia, and hypercapnia ensue, which often increase sympathetic activity,e7 arousal,e8 and subsequent sleep fragmentation,e9 resulting in decreased SWA and increased brain metabolic demand.e10,e11 In addition, intermittent hypoxia causes increased oxidative stress and inflammation, altered gene regulation, decreased mitochondrial activity, metabolic dysfunction, and decreases in the cellular and molecular substrates of synaptic plasticity.e12,e13 Increased respiratory effort during apneas may also affect the glymphatic system.e14 Overall, apnea-related sleep disruption could erode sleep-related restorative processes, possibly causing synaptic/network overload.
The link we found between witnessed apneas and tau deposition has substantial support in the literature. Synaptic activity modulates extracellular tau release,e15,e16 which may contribute to tau pathology.e17 Tau release decreases during sleep, mostly as a function of reduced brain activity,24 but possibly influenced by clearance through the glymphatic pathway.20,23
In predominantly CU elderly, increased CSF p-tau/Aβ42 ratio and a composite tau-PET level were associated with reduced SWA after controlling for apnea-hypopnea index (AHI).25 Similarly, in older adults without dementia, higher CSF p-tau was associated with increased sleep fragmentation and increased metabolic demand (as measured by CSF lactate).29 In addition, an autopsy study in elderly individuals without dementia found an inverse association between antemortem sleep consolidation (or less fragmentation) and neurofibrillary tangle pathology.22 Recently, a prospective study showed a longitudinal increase in p-tau in patients with OSA compared to patients without OSA after 2 to 3 years.27 This is consistent with evidence from animal models suggesting that intermittent hypoxia may contribute to tau pathology.e18–e20 In contrast, a single study using regional tau-PET imaging in older veterans showed no associations between tau-PET level and a diagnosis of OSA by history.28 A possible explanation may be that the control group did not exclude individuals with undiagnosed or high-risk OSA.
A key strength of our study is the investigation of witnessed apneas in a population-based cohort using tau-PET. CU elderly participants who had witnessed apneas during sleep had higher tau-PET levels compared to those without apneas. Because amyloid deposition is the most significant predictor of tau deposition in the preclinical stages of AD30–32 and is associated with sleep disruption,15–18 we adjusted for amyloid when we tested for these associations (table 2) and performed sensitivity analyses by matching on amyloid deposition (figures 2 and 3). In both sets of analyses, we found a significant association between witnessed apneas and tau-PET signal. While our results investigated regional findings as primary outcomes, we cannot exclude a widespread effect on tau deposition.
It is difficult to determine the clinical significance of tau level increase predicted by witnessed apneas alone because the amount of cognitive decline predicted by tau in these regions also depends on the age group, cortical thickness, and amyloid burden.33 Multiple other factors also contribute to cognitive dysfunction in OSA besides AD pathology, particularly when it is untreated (e.g., fragmented sleep, recurrent hypoxia, and sleepiness).e21,e22 However, when we compared amyloid-positive CU elderly individuals (≥65 years old) (n = 204) with those with MCI (n = 37) from our original MCSA database,30,32 we found a mean tau-PET SUVR difference of 0.178 in the entorhinal cortex (p < 0.001) and 0.136 in the inferior temporal cortex (p < 0.001). Therefore, a mean tau-PET level SUVR elevation of 0.049 in the entorhinal cortex and 0.037 in the inferior temporal cortex as predicted by witnessed apneas in our model could hypothetically represent 27% of a difference of the size seen between CU and MCI, suggesting that the size effect estimated by our models is within a similar scale of potentially clinically relevant differences.
Overall, our work corroborates the previous findings suggesting that increased tau levels are associated with either sleep fragmentation (with reduced SWA), intermittent hypoxia, or OSA diagnosis. The available literature does not allow a proper assessment of the independent effect of each of these variables, which would be a challenging endeavor because a high level of multicollinearity and interactions would be expected when modeling tau levels on the basis of sleep apnea–related features. Moreover, the direction of causality cannot be clearly established on the basis of the majority of previous studies. A growing body of evidence suggests a bidirectional association between sleep disruption and Aβ.15–17 The same could be expected for tau, particularly in light of the direct effects of tau in sleep of a tau transgenic mice model,e23 associations between increased tau and impaired sleep spindle dynamics,e24 and early tau accumulation in areas modulating sleep-wake cycle before involving cortical regions.e25–e27 It is possible that cortical tau burden could be a surrogate for tau-dependent neurodegeneration of brain stem nuclei, which could theoretically contribute to decreased arousal threshold, abnormal compensatory neuromuscular reflexes and central sleep apneas. In fact, the prevalence of central sleep apneas appears to increase in older adults,4,e28 but they represent a minority of cases of sleep-disordered breathing in this age group4 and are often associated with other comorbid medical conditions.e28
The literature has shown strong correlations between amyloid and tau levels, supporting the hypotheses that amyloid (or amyloid-related processes) facilitates tau accumulation at a molecular level.13 OSA has been associated with longitudinal amyloid accumulation,e29 which may further disrupt slow-wave sleep activitye30 and thereby contribute to tau accumulation also via sleep disruption.25 In our previous work, we have shown that EDS was associated with longitudinal amyloid accumulation.18 Although an independent association between witnessed apneas and amyloid accumulation was not seen in a sensitivity analysis of that study, the only symptom of sleep-related disturbance that was different between participants with and without EDS was witnessed apneas, suggesting underlying OSA.18 Substantial evidence supports that OSA with EDS may characterize a different phenotype of OSA that has increased vulnerability to sleep disruption, its cardiovascular complications,e31 and possibly AD pathology. However, our sample was not powered to test for such interactions. It is also possible that the relationship between amyloid accumulation and OSA is dose dependent. This is corroborated by previous work demonstrating associations between amyloid levels and OSA severity, as measured by AHI or hypoxemia,29,e32,e33 but not with self-reported sleep-disordered breathing symptoms.e34
It is clear that the relationship between OSA and dementia goes beyond tau and Aβ accumulation. The effects of sleep fragmentation and hypoxemia on oxidative stress, mitochondrial activity, cardiovascular function, and metabolism are remarkablee12,e13 and can contribute to neuroinflammation and neurodegeneration,e35,e36 which are nonspecific but share pathways with AD.e37,e38 It is probable that the association between OSA and dementia depend on both AD-related pathophysiologic processes and vascular abnormalities from endothelial dysfunction.e39 It is also notable that a relatively high frequency of dream enactment behavior (20.9%) was noted in the group with witnessed apnea. Although severe OSA can mimic REM behavior sleep disorder,e40 it is possible that OSA could contribute to mixed pathology with coexistent synuclein deposition.e41,e42
Due to the epidemiologic nature of the MCSA, sleep studies were not part of the study protocol. We did not systematically confirm the diagnosis of OSA, its severity, degree of sleep fragmentation, and treatment status. We used witnessed apneas as a surrogate for OSA diagnosis, as seen in previous literature.e43,e44 In the STOP-Bang questionnaire for OSA screening,e45 the component observed apnea alone, which is similar to our assessment, was found to have 71.9% sensitivity and 69% specificity for the diagnosis of OSA (as defined by AHI score >5) in predominantly middle-aged patients referred to the sleep clinic due to sleep complaints.e46 Multiple other studies showed that observed/witnessed apneas had higher sensitivity (73%–83%) when OSA diagnosis was defined by higher AHI cutoff points (AHI score ≥10 or ≥15), at the expense of lower specificity (33%–51%). The low specificity, however, is equivalent to that found in well-validated OSA screening questionnaires such as the STOP-Bang (27%–44% for AHI≥15).e47 Moreover, we relied on an informant-reported as opposed to self-reported symptom (as in the STOP-Bang), which may have better diagnostic performance, because elderly individuals may lack awareness of OSA symptoms,e48 particularly those occurring during sleep. Therefore, our assessment was deemed suitable for selecting participants at a higher risk for OSA. This is corroborated by the higher BMI and a trend toward more male participants in the group with witnessed apneas because both are independent risk factors for OSA.e45,e49,e50 The presence of apneas during sleep might have been missed by some informants because nearly 20% of them did not sleep in the same room. However, the percentage of informants sleeping in the same room was not different between groups, and >90% of informants in both groups were spouses who lived with the participant at the time of the interview (table e-4, doi.org/10.5061/dryad.gb5mkkwm2). In addition, the 14.7% frequency of participants with witnessed apneas in our study is likely an underestimation of the true prevalence of the disease in similar populations (5%–56%).2 This is due to not only limitations of our assessment but also the fact that we included participants without cognitive impairment or other comorbid neurologic disorders (e.g., stroke). Although we did not find significant differences in hypertension and EDS frequency between groups, they are not consistent features of OSA in the elderly.42,e51 It is also notable that OSA may be associated with central apneas, and some participants may have predominantly central or mixed (central and obstructive) apneas,4,e50 which may have different pathophysiologic implications beyond the scope of this work. Moreover, OSA can co-occur with other sleep or circadian rhythm disorders not specifically investigated in our study, which could contribute to its deleterious effects. Lastly, our study included only CU participants, precluding the assessment of potential interactions between cognitive status (CU vs MCI vs dementia) and tau accumulation. It is possible that later stages of AD are associated with an increased vulnerability to sleep disturbance, resulting in greater tau accumulation. These limitations might have resulted in underestimated group differences.
Our work provides further evidence supporting the association between OSA and tau levels in elderly individuals before the onset of MCI or dementia. Future prospective studies should assess the direction of this association to clarify whether these individuals are more susceptible to tau accumulation and whether sleep-related interventions such as continuous positive airway pressure could slow down this process.
Acknowledgment
The authors thank all the study participants and staff in the MCSA, Mayo Alzheimer's Disease Research Center, and Aging Dementia Imaging Research laboratory at the Mayo Clinic for making this study possible. They also thank AVID Radiopharmaceuticals, Inc, for support in supplying AV-1451 precursor, chemistry production advice and oversight, and Food and Drug Administration regulatory cross-filing permission and documentation needed for this work.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- AHI
apnea-hypopnea index
- BMI
body mass index
- CU
cognitively unimpaired
- EDS
excessive daytime sleepiness
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- OSA
obstructive sleep apnea
- p-tau
hyperphosphorylated tau
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- SWA
slow-wave activity
Appendix. Authors
Study funding
This work was supported by NIH grants U01 AG006786 (principal investigator [PI]: R.C. Petersen), R01 NS097495 (PI: P. Vemuri), R01 AG056366 (PI: P. Vemuri), P50 AG016574 (PI: R.C. Petersen), R37 AG011378 (PI: C.R. Jack), R01 AG041851 (PIs: C.R. Jack and D.S. Knopman), and R01 AG034676 (Rochester Epidemiology Project PI: W.A. Rocca); a Gerald and Henrietta Rauenhorst Foundation grant, the Millis family, the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation, the Alzheimer's Association (Zenith Fellows Award), the Liston Award, the Elsie and Marvin Dekelboum Family Foundation, the Schuler Foundation, and Opus building NIH grant C06 RR018898.
Disclosure
D.Z. Carvalho reports no disclosures. E. St. Louis serves on the Adverse Events Adjudication committees and Data Safety Monitoring Board for Inspire, Inc and has received research support from Mayo Clinic Center for Clinical and Translational Science, NIH, the Michael J. Fox Foundation, and Sunovion, Inc. C.G. Schwarz received research funding from the NIH. V.J. Lowe consults for Bayer Schering Pharma, Piramal Life Sciences, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, and AVID Radiopharmaceuticals. B.F. Boeve has served as an investigator for clinical trials sponsored by GE Healthcare, FORUM Pharmaceuticals, and C2N Diagnostics. He receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge Medicine, 2009). He serves on the Scientific Advisory Board of the Tau Consortium. He has consulted for Isis Pharmaceuticals. He receives research support from the NIH (U01 AG045390, U54 NS092089, P50 AG016574, UO1 AG006786, RO1 AG041797) and the Mangurian Foundation. S.A. and A. Reddy report no disclosures. M.M. Mielke served as a consultant to Eli Lilly and received unrestricted research grants from Biogen and Lundbeck. D.S. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network (DIAN) study and is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. R.C. Petersen consults for Roche, Inc, Merck, Inc, Genentech, Inc, and Biogen, Inc, and GE Healthcare and receives royalties from Oxford University Press for the publication of Mild Cognitive Impairment. C.R. Jack consults for Lily and serves on an independent data monitoring board for Roche, but he receives no personal compensation from any commercial entity. P. Vemuri receives research funding from NIH (National Institute on Aging and National Institute of Neurological Disorders and Stroke). Go to Neurology.org/N for full disclosures.
References
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 2011;86:549–554; quiz 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillan A, Morrell MJ. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheff) 2016;12:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep 1991;14:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men, I: prevalence and severity. Am J Respir Crit Care Med 1998;157:144–148. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med 2001;2:511–516. [DOI] [PubMed] [Google Scholar]
- 6.Sforza E, Hupin D, Pichot V, Barthelemy JC, Roche F. A 7-year follow-up study of obstructive sleep apnoea in healthy elderly: the PROOF cohort study. Respirology 2017;22:1007–1014. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2015;63:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin MS, Sforza E, Roche F, Barthelemy JC, Thomas-Anterion C; PROOF Study Group. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the PROOF-Synapse cohort. Sleep 2015;38:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One 2013;8:e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017;74:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 2014;71:505–508. [DOI] [PubMed] [Google Scholar]
- 14.Jeong S. Molecular and cellular basis of neurodegeneration in Alzheimer's disease. Mol Cells 2017;40:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol 2014;10:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer's disease. Sleep Med Rev 2017;31:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci 2016;39:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho DZ, St Louis EK, Knopman DS, et al. Association of excessive daytime sleepiness with longitudinal beta-amyloid accumulation in elderly persons without dementia. JAMA Neurol 2018;75:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucey BP, Hicks TJ, McLeland JS, et al. Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Ann Neurol 2018;83:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013;70:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014;34:16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holth JK, Fritschi SK, Wang C, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019;363:880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucey BP, McCullough A, Landsness EC, et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med 2019;11:eaau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho DZ, St Louis EK, Boeve BF, et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med 2017;32:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bubu OM, Pirraglia E, Andrade AG, et al. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 2019;42:zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias A, Cummins T, Tyrrell R, et al. Risk of Alzheimer's disease in obstructive sleep apnea syndrome: amyloid-beta and tau imaging. J Alzheimers Dis 2018;66:733–741. [DOI] [PubMed] [Google Scholar]
- 29.Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer's disease biomarkers changes. Sleep 2017;40:szx001. [DOI] [PubMed] [Google Scholar]
- 30.Vemuri P, Lowe VJ, Knopman DS, et al. Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst) 2017;6:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack CR Jr, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain 2018;141:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe VJ, Wiste HJ, Senjem ML, et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer's dementia. Brain 2018;141:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS, Lundt ES, Therneau TM, et al. Entorhinal cortex tau, amyloid-beta, cortical thickness and memory performance in non-demented subjects. Brain 2019;142:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 35.Lowe VJ, Bruinsma TJ, Wiste HJ, et al. Cross-sectional associations of tau-PET signal with cognition in cognitively unimpaired adults. Neurology 2019;93:e29–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 2012;78:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 2013;9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 41.Hayley AC, Williams LJ, Kennedy GA, et al. Excessive daytime sleepiness and falls among older men and women: cross-sectional examination of a population-based sample. BMC Geriatr 2015;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sforza E, Pichot V, Martin MS, Barthelemy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med 2015;16:981–986. [DOI] [PubMed] [Google Scholar]
- 43.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz AJ, Yu P, Miller BB, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 2016;139:1539–1550. [DOI] [PubMed] [Google Scholar]
- 46.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 47.Meltzer CC, Leal JP, Mayberg HS, Wagner HN Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr 1990;14:561–570. [DOI] [PubMed] [Google Scholar]
- 48.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jack CR Jr, Wiste HJ, Lesnick TG, et al. Brain beta-amyloid load approaches a plateau. Neurology 2013;80:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain 2015;138:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data available from Dryad (additional eReferences): 10.5061/dryad.gb5mkkwm2 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available for sharing with qualified investigators on reasonable request.