Abstract
Heterochromatin suppresses repetitive DNA, and a loss of heterochromatin has been observed in aged cells of several species, including humans and Drosophila. Males often contain substantially more heterochromatic DNA than females, due to the presence of a large, repeat-rich Y chromosome, and male flies generally have shorter average life spans than females. Here we show that repetitive DNA becomes de-repressed more rapidly in old male flies relative to females, and repeats on the Y chromosome are disproportionally mis-expressed during aging. This is associated with a loss of heterochromatin at repetitive elements during aging in male flies, and a general loss of repressive chromatin in aged males away from pericentromeric regions and the Y. By generating flies with different sex chromosome karyotypes (XXY females; X0 and XYY males), we show that repeat de-repression and average lifespan is correlated with the number of Y chromosomes. This suggests that sex-specific chromatin differences may contribute to sex-specific aging in flies.
Introduction
The chronic deterioration of chromatin structure has been implicated as one of the molecular signatures of aging1–3, and an overall loss of heterochromatin and repressive histone marks is observed in many old animals4–6. Heterochromatin is enriched at repetitive DNA, and its loss can result in de-repression and mobilization of silenced transposable elements (TEs)7–13. The amount of repetitive DNA can differ substantially between sexes, due to the presence of a highly repetitive (and normally poorly assembled) Y or W chromosome in the heterogametic sex. In the fruit fly Drosophila melanogaster, for example, males contain a ~40-Mb large completely repetitive Y chromosome14,15, while the pericentromeric heterochromatin on the X only amounts to ~10–20-Mb (depending on the strain). Thus, this implies that a substantially larger fraction of the male genome is heterochromatic compared to the female genome14. Males have a shorter average lifespan in many taxa, including humans and most Drosophila species16–18. Indeed, the genetic sex determination system predicts adult sex ratios in tetrapods, with the heterogametic sex being less frequent19. Lower survivorship of the sex with the repetitive Y or W chromosome may suggest a link between sex-specific mortality, chromatin and sex chromosomes.
Here, we test for an association between sex-specific heterochromatin loss and a de-repression of repetitive DNA during aging, by assaying chromatin and gene expression profiles in young and aged individuals of D. melanogaster. We further create flies with different sex chromosome karyotypes (that is, X0 and XYY males and XXY females), to assess the influence of the Y chromosome on longevity and sex-specific changes in repeat expression.
Results
Drosophila males and females differ in repeat content and longevity.
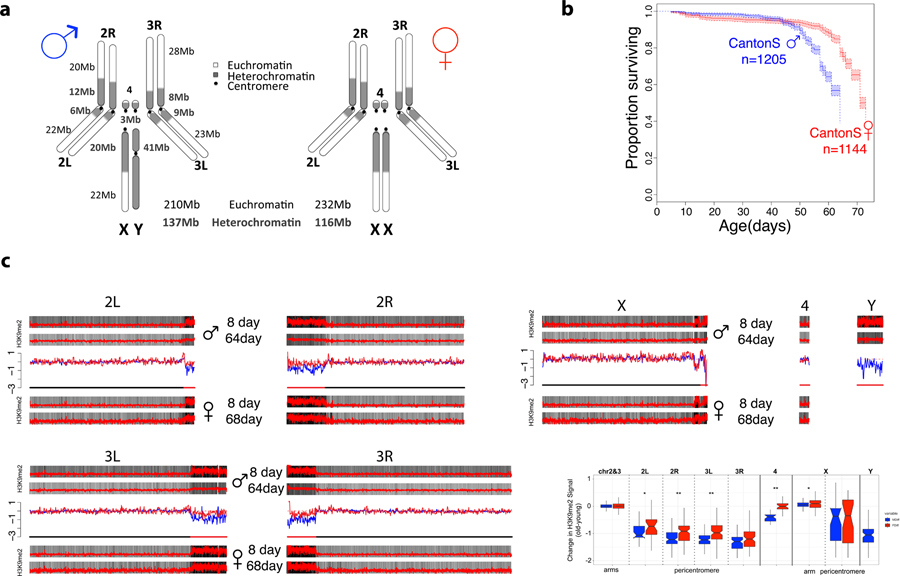
We chose D. melanogaster to investigate the contribution of the Y chromosome to sex-specific aging, since a substantial fraction of its heterochromatin, including its Y chromosome, has been assembled20. In addition, the availability of mutant strains allows us to generate flies that differ in their sex chromosome configuration. Figure 1A shows a schematic overview of the karyotype for D. melanogaster males and females, and the approximate size and position of large heterochromatic segments14. Previous studies have shown that males have approximately 20-Mb more heterochromatin per cell than females14 (Figure 1A), and flow cytometry estimates verify that the Canton-S strain used here shows similar differences in repeat content between the sexes (Table 1). Thus, this confirms that male Canton-S flies contain significantly more repetitive DNA than female.
Figure 1. Aging and the sex-specific chromatin landscape in Drosophila.
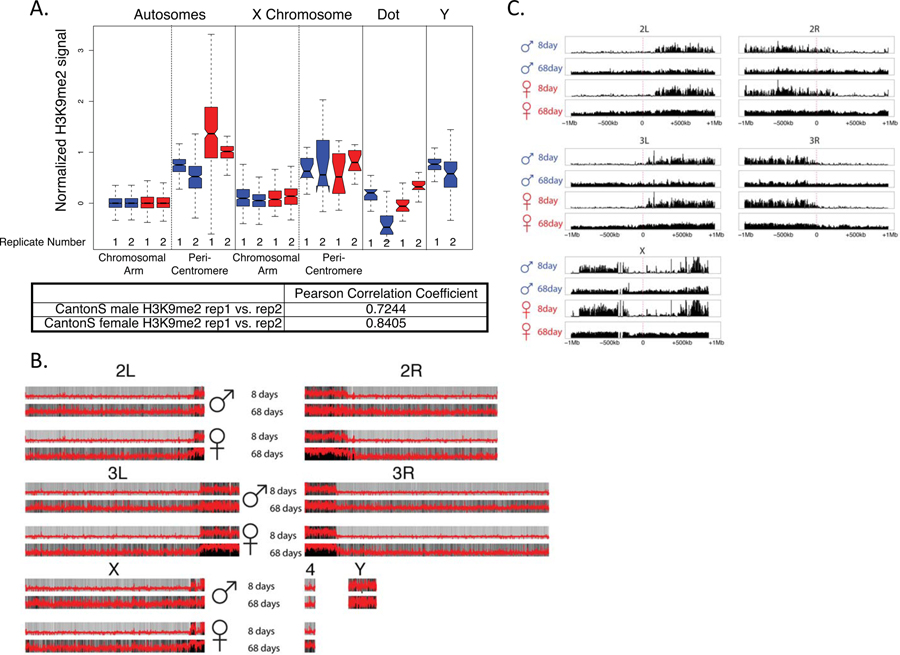
A. Kaplan-Meier survivorship curves63 for Canton-S males (blue) and females (red), with the shaded region indicating the upper and lower 95% confidence interval calculated from the Kaplan-Meier curves. Karyotypes of male and female D. melanogaster are shown, with heterochromatic regions indicated in blue, and euchromatic regions in gray. The number of flies counted for each sex (n) to obtain the survivorship curves is indicated. B. Genome-wide enrichment of H3K9me2 for young (8 days) and old (64 or 68 days) D. melanogaster males and females along the different chromosome arms. Enrichment in 5kb windows is shown in red lines (normalized ratio of ChIP to input, see Materials & Methods), and the enrichment in 20kb windows is shown in gray scale according to the scale in the lower right of the panel, with the darkest gray corresponding to the highest 5% of values across all windows from all samples, and the lightest gray corresponding to the lowest 10% of values across all windows from all samples. Subtraction plots show the absolute difference in signal of 50kb windows between young and aged flies along the chromosome arms, with each sample further smoothed by subtracting out the median autosomal euchromatin signal, with females in red and males in blue, and the pericentromeric region of each chromosome indicated by the red segment of the line beneath each chromosome. C. Box plot showing the smoothed ChIP signal for all 50kb windows in different chromosomal regions (* p<0.05, ** p<1e-6, *** p<1e-12, Wilcoxon test) for males (blue) and females (red), with pericentromere boundaries defined by the Release 6 version of the D. melanogaster genome. Boxes represent 25th and 75th percentile, and whiskers show the most extreme values within 1.5x the inter-quartile range.
Table 1.
Flow cytometry estimates of the mean diploid genome size
| Strain | Genotype | Sex | Flow cytometry 2n (Mb) | Heterochromatin (Mb) |
|---|---|---|---|---|
| Canton-S | XX | female | 356.8 | 123 |
| 2549 | X^X | female | 346.3 | 112.5 |
| 4248 | X^X | female | 352.3 | 118.5 |
| 100950 | X^X | female | 356.3 | 122.5 |
| Canton-S | XY | male | 357.6 | 145.7 |
| 2549 | XŶ | male | 344.7 | 132.8 |
| 4248 | XŶ | male | 344.4 | 132.5 |
| 100950 | XŶ | male | 350.5 | 138.6 |
| 2549/Canton-S | X^XY | female | 389.5 | 155.7 |
| 4248/Canton-S | X^XY | female | 398.7 | 164.9 |
| 100950/Canton-S | X^XY | female | 404.8 | 171 |
| 2549/Canton-S | X0 | male | 319.3 | 107.4 |
| 4248/Canton-S | X0 | male | 321.6 | 109.7 |
| 100950/Canton-S | X0 | male | 317.6 | 105.7 |
| 2549/Canton-S | XŶY | male | 394.9 | 183 |
| 4248/Canton-S | XŶY | male | 388.5 | 176.6 |
| 100950/Canton-S | XŶY | male | 390.2 | 178.3 |
Flow cytometry estimates of the mean diploid genome size of the different karyotypes investigated (based on 3 replicate measures). Canton-S and 2549 measure are from reference22.
The approximate heterochromatin content for the strains investigated is indicated, assuming that the euchromatic size is constant for all chromosomes (i.e. 233.8Mb for flies with 2 X chromosomes, and 211.9Mb for flies with a single X chromosome, see Fig. 1A).
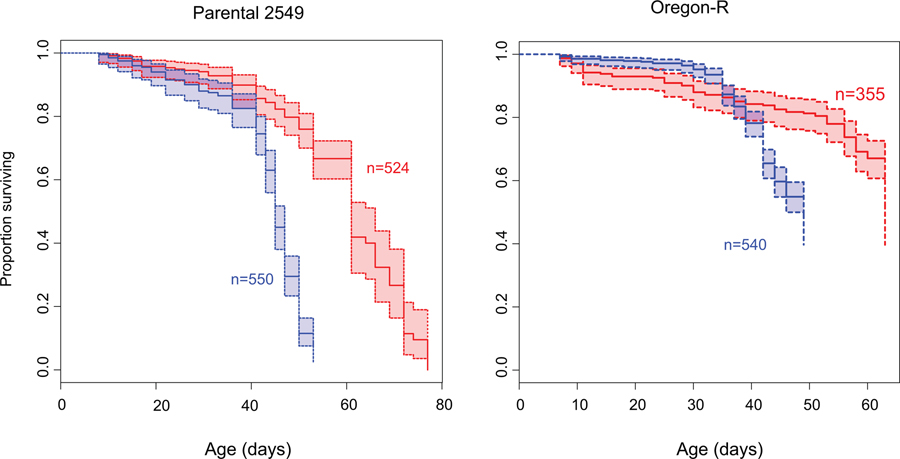
Previous work in Drosophila showed that for the vast majority of species, male flies live significantly shorter than female flies16. In particular, a study surveying longevity in 68 species (and 89 strains) of Drosophila found statistically significant differences in longevity between the sexes for 55 strains. Females had higher longevity in 53 strains, and males lived longer in only 2 strains16. We determined longevity for males and females of two standard lab strains from D. melanogaster (Canton-S and Oregon-R), and the 2549 strain from the Bloomington Stock Center, which has a compound metacentric X chromosome (that is, two X chromosomes fused at the centromere) and a hetero-compound X-Y chromosome (that is, an X chromosome inserted between the two arms of the Y chromosome). Lifespan assays confirm that males live significantly shorter than females, for both wildtype strains and the 2549 strain (Figure 1B, see Extended Data Fig. 1 for lifespan assays in Oregon-R and 2549). Increased longevity of females is consistent with multiple studies on sex-specific lifespan in Drosophila16,17.
Heterochromatin loss differs between sexes.
We gathered replicate ChIP-seq data for a repressive histone modification typical of heterochromatin (H3K9me2) from young 8-day and old 64–68-day D. melanogaster males and females (Canton-S), to test for sex-specific heterochromatin loss during aging. We used a ‘spike in’ normalization method to compare the genomic distribution of chromatin marks across samples21,22. Specifically, we spiked-in a fixed amount of chromatin from D. miranda to each D. melanogaster chromatin sample prior to ChIP and sequencing. We chose this species to serve as an internal standard for our D. melanogaster ChIPs, since they are sufficiently diverged from each other that there is very little ambiguity in the assignment of reads to the correct species. We employed a previously described normalization strategy21, where the relative recovery of D. melanogaster ChIP signal vs. D. miranda ChIP signal, normalized by their respective input counts, was used to quantity the relative abundance of the chromatin mark in D. melanogaster. We also used a linear regression model to estimate the relative recovery of D. melanogaster ChIP signal vs. D. miranda ChIP signal, normalized by their respective input counts23. Overall enrichment patterns and differences between sexes and ages are quantitatively similar between the two methods, showing that our inferences are robust to our normalization strategy (Figure S1A).
Repetitive regions pose a challenge for mapping with short reads, since one cannot be sure that a particular locus is generating the reads in question if they map to multiple positions. Our study is concerned with the overall behavior of repetitive regions in the genome during aging, and not focused on any particular locus. Thus, analyzing all reads (including those mapping to multiple locations) is most appropriate for our purpose. However, we repeated our analysis using only uniquely mapping reads, which confirms that our inferences are robust when only considering uniquely mapping reads (Figure S1B).
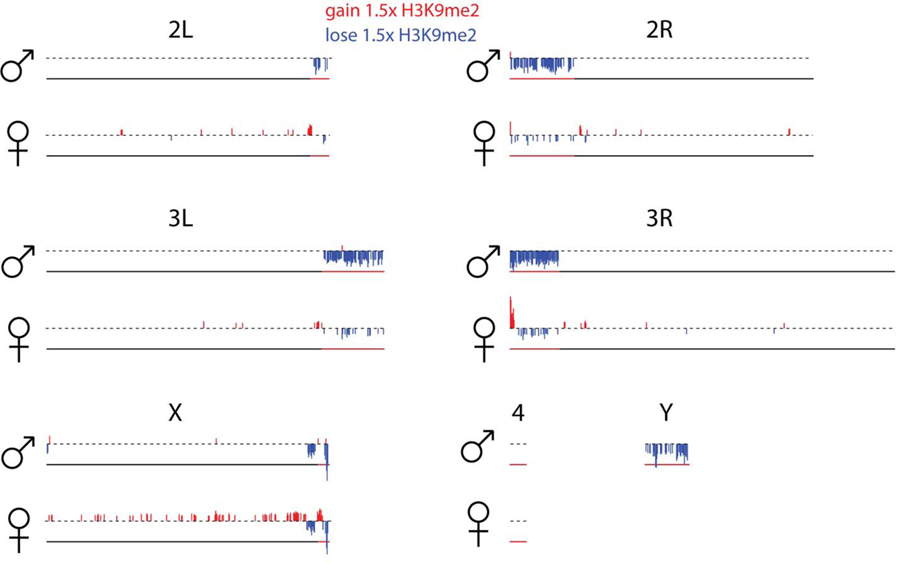
Figure 1C shows the genomic distribution of the repressive histone modification H3K9me2 for young and old male and female flies. As expected, heterochromatin is enriched at repetitive regions, including pericentromeres, the small dot chromosome and the repeat-rich Y. While the genomic distribution of H3K9me2 looks similar between young males and females22, heterochromatin enrichment changes dramatically in old male but less so in old female flies. In particular, we see a general loss of heterochromatin at repetitive regions in aged males (Figure 1C; Extended Data Fig. 2 for biological replicate), and males show significantly more regions that lose H3K9me2 signal (1.5-fold or more) during aging compared to females (232 vs. 73, p<2.2e-16, Fisher’s exact test; Extended Data Fig. 3). Almost all regions that lose heterochromatin are located within the pericentromere or the Y chromosome (Extended Data Fig. 3, Figure S2). On the other hand, fewer regions in males gain H3K9me2 signal (1.5-fold or more) during aging relative to females (6 vs. 120, p<2.2e-16, Fisher’s exact test; Extended Data Fig. 3). Genomic regions that gain H3K9me2 signal are enriched on the X of females (p<2.2e-16, Fisher’s exact test; Extended Data Fig. 3, Figure S2), and tend to be located close to the pericentromeric boundary (p=0.04 Fisher’s exact test; Figure S3, Extended Data Fig. 3, Figure S2). This suggests that heterochromatin / euchromatin boundaries are less efficiently maintained in old flies, resulting in spreading of heterochromatin from the repeat-rich pericentromere into neighboring regions. Thus, our results show that male flies lose heterochromatin marks more rapidly than female flies in D. melanogaster and our results are reproducible using different mapping and normalization strategies, and independent biological replicates.
Mis-expression of heterochromatic and lamina-associated genes in old flies.
Sex-specific chromatin changes during aging are associated with sex-specific expression changes. To study gene expression during aging, we gathered replicated stranded RNA-seq data from young and old flies (Figure S4, Table S3, S4). We find that genes located in pericentromeric regions change their expression more during aging compared with genes in chromosomal arms, in both sexes (Figure S5). While heterochromatin typically has a repressive effect on gene expression, genes located in normally heterochromatic regions (such as the pericentromere) are known to depend on this repressive chromatin environment for proper transcription24. Indeed, the global loss of heterochromatin in pericentromeric regions is associated with reduced expression levels of pericentromeric genes in aged males and females (Figure S5). Genes that gain the H3K9me2 mark during aging tend to decrease in expression (Figures S6, S7).
Overall, we find that gene expression during aging differs between males and females. Of the top 1000 genes that are differentially expressed during aging in males and females, 39.4% show expression changes in both sexes (Figure S8, Table S3). Chromosomal location does not appear to be the main determinant influencing gene expression during aging; of the 394 genes that are most differentially expressed in both males and females, only 12 are located inside or within 1Mb of the pericentromere (we expect 20 by chance). The most differentially expressed genes affect similar functional categories in males and females (including several GO categories associated with immune responses, Figure S9, S10), but many GO terms are unique to one sex (Table S4). Consistent with previous work25–27, genes involved in immune system function and regulation were significantly differentially expressed as a function of age (Figure S4, Table S3, S4). Immunity genes are enriched in lamin-associated heterochromatin domains in Drosophila28, and age-associated lamin-B reduction in fat body cells was shown to contribute to heterochromatin loss and de-repression of genes involved in immune responses28 and deregulation of transposable elements29. Lamin-B loss has also been observed in the brain of old flies30, and we find that genes residing in lamin-associated domains in the central brain of Drosophila31 change their expression more during aging compared to genes not associated with lamin (Figure S11). The rDNA locus has also been shown to be mis-expressed during aging5; we generated rDNA-depleted libraries, which precludes us from studying rDNA expression.
Repeat de-repression in old male flies.
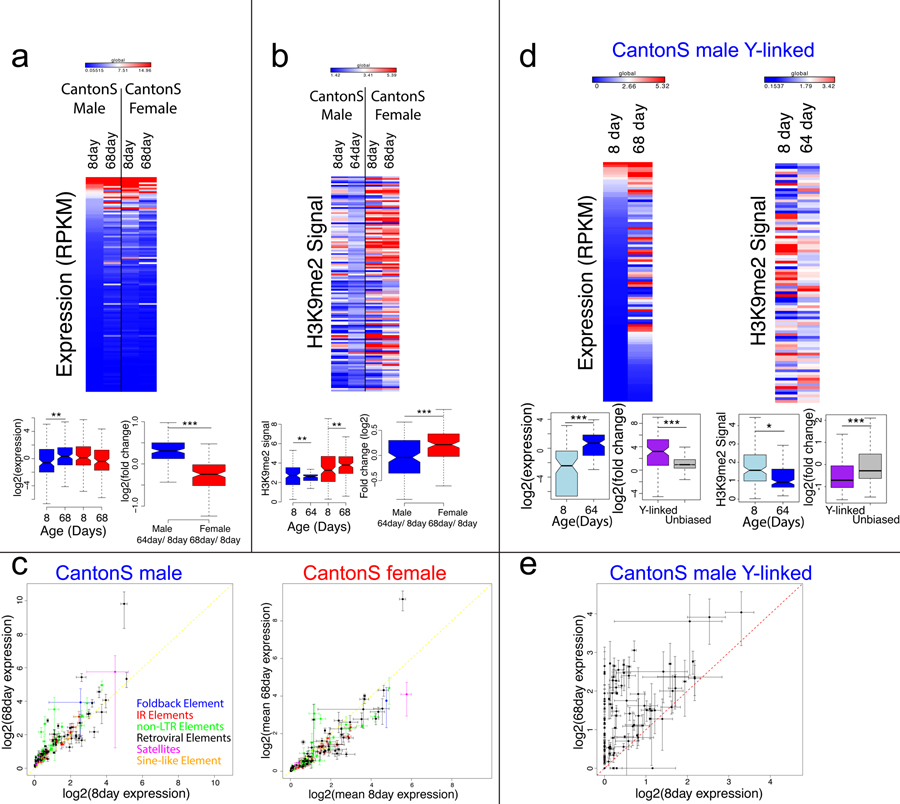
In addition, we find sex-specific differences in repeat reactivation during aging. We mapped our transcriptome data to the consensus repeat library of D. melanogaster and detect low levels of expression of repetitive elements in young male and female flies (Figure 2A). Aged females maintain efficient repression of TEs, while expression for the major classes of annotated TEs increases during aging for males (Figure 2A,C). De-repression of TEs is more pronounced in males both in terms of the number of individual elements that show a significant increase in expression during aging, as well as the fraction of the transcriptome that consists of repetitive transcripts across all repetitive elements. Overall, we find that in females, 6 repetitive elements show a significant increase in expression during aging and 14 a significant decrease (Figure 2C), but the total fraction of transcripts derived from repeats increases during aging (the fraction of repetitive reads in all RNA-seq reads is 2.0% at 8 days, vs. 4.6% at 68 days, Table S1, Figure S12). The increase in repeat expression is much more pronounced in males, with 32 repetitive elements showing a significant increase in expression during aging and 4 showing a significant decrease (Figure 2C, Figure S12), and the total fraction of repetitive reads increases from 1.6% to 5.8% (Table S1). The TE showing the highest level of de-repression in both sexes is copia, which is expressed 28-fold more in old versus young males, and expressed 15-fold more in old versus young females (Figure 2C). H3K9me2 profiles at TE families show that there is a general enrichment of this repressive mark in young male and female flies (Figure 2B). Consistent with genome-wide expression profiles showing overall efficient silencing of repeats in old females, there is no global loss of the repressive chromatin mark at repetitive elements in 68-day old females (in fact, there is a slight increase, Figure 2B). However, aged D. melanogaster males undergo a general loss of the H3K9me2 histone modification in repetitive elements (Figure 2B). Thus, chromatin and gene expression profiles show that TEs lose their epigenetic silencing and become mis-expressed in old male flies.
Figure 2. Sex-specific silencing and expression of repeats during aging.
A. Expression of all repeats from FlyBase consensus library from Release 6 of the D. melanogaster genome in young (8day) and old (~68day) male and female Canton-S, averaged across replicates, with significance values calculated using the Wilcoxon test (* p< 0.05, ** p<0.01, *** p<1e-5). Heatmaps are visualized globally according to the scale, with dark red corresponding to the top 5% of all values across all samples and dark blue corresponding to the bottom 5% of all values across all samples. B. H3K9me2 enrichment in repeats from FlyBase consensus library in young (8day) and old (64 or 68day) male and female Canton-S, averaged across replicates, with significance values calculated using the Wilcoxon test (* p<0.05, ** p<0.01, *** p<1e-5). The heatmap is scaled in the same manner as in (A.). C. Expression for repeat families for old and young males and females, with lines indicating the standard deviation for each estimate of expression across replicates and colors indicating the class of repetitive element. D. Expression and H3K9me2 signal in putatively Y-linked repeats in young (8day) and old (64 or 68day) Canton-S males, with significance values calculated using the Wilcoxon test (* p<0.01, ** p<1e-4, *** p<1e-10). Heatmaps are scaled in the same manner as in (A). E. Expression of putatively Y-linked repeats for old and young Canton-S males, with lines indicating the standard deviation for each estimate of expression across replicates. Boxes represent 25th and 75th percentile, and whiskers show the most extreme values within 1.5x the inter-quartile range.
Males have approximately 20% more repetitive sequence than females, due to the repeat-rich Y chromosome. The sex-specific increase in repeat expression may be triggered by the presence of the heterochromatic Y chromosome in males, and the Y indeed shows a dramatic loss of heterochromatin during aging (Figure 1C). To see if Y-linked repeats are especially prone to mis-regulation during aging, we used de novo assembled male-specific and male-biased (putatively Y-linked) repetitive sequences22 (Figure S13). Indeed, we find that in males, putatively Y-linked repeats are up-regulated more strongly during aging, relative to repeats present in both sexes (p=5.9e-12, Wilcoxon test, Figure 2D,E). Overall, we find that 42 Y-linked repeats show a significant increase in expression during aging (and only one a significant decrease; Figure 2D,E, Figure S14), and the total fraction of transcripts derived from Y-linked repeats increases more than 9-fold in old males (Table S2). Additionally, putatively Y-linked repeats disproportionately lose the repressive histone modification H3K9me2 during aging compared to other repeats (p=3.4e-11, Wilcoxon test, Figure 2D). Thus, male-biased and male-specific repeats, i.e. repeats that are located on the Y chromosome, are especially prone to de-repression during aging in males.
Flies with additional Y chromosomes have decreased lifespan.
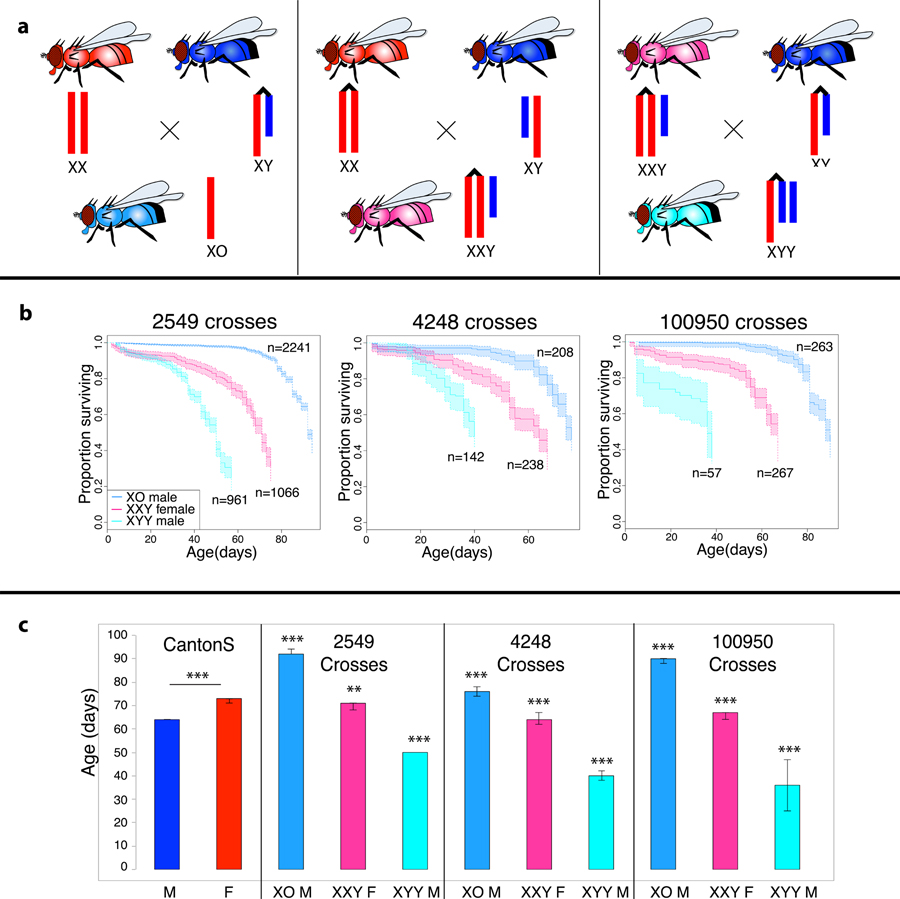
In Drosophila, sex is determined by the ratio of X chromosomes vs. autosomes, independently of the presence of the Y chromosome32. This allowed us to test whether the Y chromosome contributes to sex-specific TE de-repression and aging, by generating D. melanogaster females containing a Y chromosome (XXY flies), and males with either zero or two Y chromosomes (X0 and XYY flies). We crossed Canton-S flies to different strains with attached-X and attached-X-Y chromosomes to generate XXY females and X0 and XYY males (Figure 3A, see Methods for strain information and Table 1 for genome size estimates). Note that X0 males and XXY females of a given cross have the same autosomal background, but differ in their genomic background from other crosses and from Canton-S, which can contribute to lifespan variation among strains (Figure 3B,C). Utilizing different strains to generate flies with aberrant sex chromosomes, however, should allow us to control for genomic background effects to some extent, by randomizing across different backgrounds. Indeed, we find qualitatively identical results using three independent strains to generate XO/XXY/XYY flies, suggesting that differences in longevity are not due to genomic background, but caused by the presence or absence of the Y. Note, however, that we cannot formally exclude background effects using these crosses.
Figure 3. Survivorship of XXY females and X0 and XXY males.
A. Schematic crossing scheme used to generate flies with aberrant sex chromosomes, with Canton-S used as the wild-type (wt) males and females for all crosses, and various lines with C(1)RM and C(1;Y) indicated by the attached X/XY karyotypes. Crosses between wt females and attached XY males result in X0 flies (left), crosses between attached X females and wt males result in XXY females (middle), and crosses between XXY females and attached XY males result in XYY males (right). B. Kaplan-Meier survivorship curves for flies with aberrant sex chromosome karyotype, generated with various C(1)RM and C(1;Y) lines as indicated at the top of each survivorship curve (stock 2549 and 4248 were obtained from the Bloomington Stock Center, and stock 100950 was obtained from Kyoto). The crosses to obtain the XO, XXY and XYY flies are shown in panel A. Shaded areas indicate the upper and lower 95% confidence interval calculated from the Kaplan-Meier curves. C. Median lifespan for each of the different karyotypes measured, with error bars indicating the upper and lower 95% confidence intervals (estimated by the Kaplan-Meier curves). Significance is compared to the wild-type Canton-S of the same sex for each aberrant karyotype, and was calculated using the survdiff package in R (* p<0.01, ** p<1e-6, *** p<1e-12).
In particular, we compared sex-specific lifespans of wildtype Canton-S D. melanogaster flies, and XXY female and X0 and XYY male resulting from (back)crosses to the three different attached-X and attached-X-Y strains (Figure 3B,C). Cumulative survival probabilities show that life span of females that contain a Y chromosome (XXY females) is reduced relative to wildtype females or males that lack a Y chromosome (X0 males) for all crosses assayed (Figure 3B,C). Indeed, X0 males show a dramatic increase in life span relative to wildtype males, and even outlive wildtype females (Figure 3B,C). X0 males are sterile and have the least amount of repetitive DNA of all karyotypes investigated (~10Mb less than Canton-S females and ~40Mb less than Canton-S males; Table 1); both of these factors may contribute to increased lifespan. Males with two Y chromosomes (XYY), in contrast, live the shortest (Figure 3B,C), and their lifespan is reduced considerably relative to wildtype males, despite both karyotypes being fertile. Thus, survivorship data are consistent with the hypothesis that the number of Y chromosomes influences organismal survival in Drosophila.
Mis-expression of Y genes and repeats in flies with additional Y chromosomes.
Gene expression changes during aging in the aberrant karyotypes show many of the same patterns as wildtype flies, with similar networks of GO terms enriched in both X0 and XYY males, and XXY females, including “reproduction” or “immune response” (Figure S9). Overall, we find that 101 of the top 10% of genes mis-expressed during aging are shared among all 5 karyotypes (p<<1e-5, permutation test). These genes do not show any enrichment for a particular GO term, and only 6 of them are located inside or within 1Mb of the pericentromere (expect 4.8 genes).
Genomic location also influences gene expression changes during aging in flies with aberrant karyotypes. As in wildtype males and females, genes located in the pericentromere show a decrease in expression during aging in XXY females and X0 males (Figure S15; XYY flies show no significant expression change at pericentromeric genes). Y-linked genes in wild-type males are expressed almost exclusively in male reproductive tissues33, and we do not detect any expression of Y-linked genes even in very old XY male heads (Figure S15). In contrast, we find that Y-linked genes are inefficiently silenced in heads of XXY females and XYY males and are becoming de-repressed even more during aging (6 Y genes are expressed in old XXY females, and 14 in old XYY males, Figure S15).
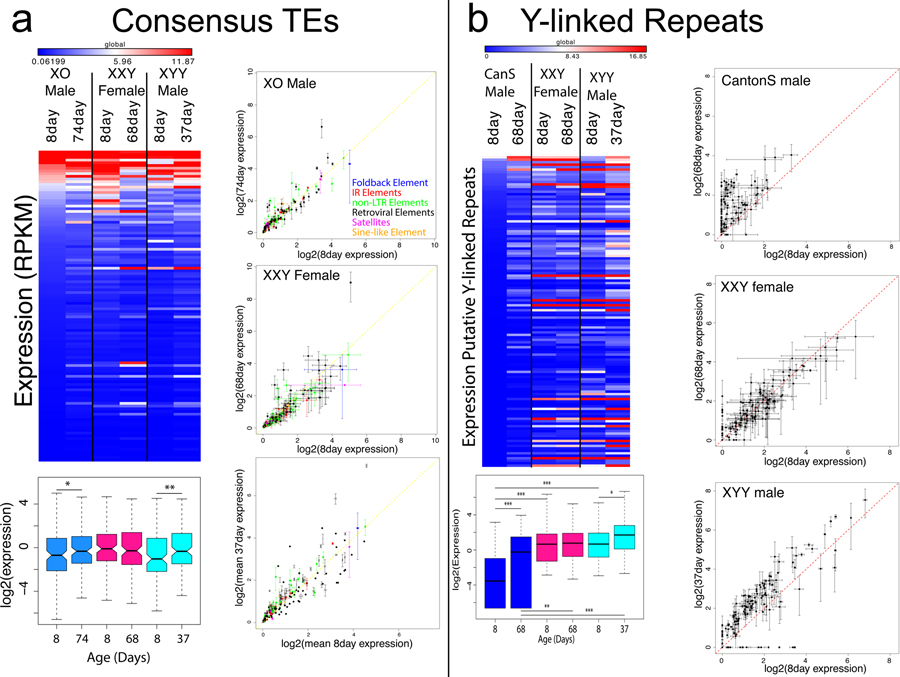
Thus, wildtype males maintain efficient silencing at their Y-linked genes during aging, despite global heterochromatin loss on the Y chromosome and mis-expression of Y-linked repeats. Silencing mechanisms on the Y chromosome of XXY and XYY flies, on the other hand, appear to be generally compromised, even in young individuals. Indeed, we previously showed that the Y chromosome affects global heterochromatin integrity22. Young flies with additional Y chromosomes (XXY females or XYY males) show lower levels of H3K9me2 enrichment at their TEs and a de-repression of Y-linked repeats relative to wildtype flies, while X0 flies showed increased levels of H3K9me2 at repeats22. Expression profiles in XXY females and XYY/X0 males suggest that the absence or presence of the Y chromosome modulates expression of TEs during aging (Figure 4). Expression profiles from aged flies with aberrant sex chromosome karyotypes confirm our expectation that X0 males show less de-repression of TEs during aging relative to wildtype males (Figure 4A). XXY females, on the other hand, show more mis-expression of repeats during aging compared to wildtype females. In XXY females, 7 elements show a significant increase in expression during aging and 3 elements show a significant decrease in expression (Figure 4A, compared to 6 /14 elements that increase/decrease expression in wild-type females), and the fraction of repetitive transcripts increases more during aging for XXY females (3.1-fold increase in XXY females vs. 2.2-fold in wildtype females, Table S1). XYY males show the greatest number of repeats with significantly increased expression during aging (33 elements, Figure 4A), but not the highest fold change in total fraction of repetitive reads (Table S1), partly because young XYY males already show the highest expression of repeats of any of the 5 karyotypes (Table S1), and partly because old XYY males are approximately 30 days younger than the other karyotypes (Figure 3B).
Figure 4. Expression of repetitive elements in XXY females and X0 and XYY males during aging.
A. Expression of all repeats from FlyBase consensus library from the Release 6 of the D. melanogaster genome. The heatmap shows averaged expression across replicates, with significance values calculated using a Wilcoxon test (* p<0.1, ** p<0.05, *** p<0.01). The scatterplots show expression of all repeats from the FlyBase consensus library in young and old X0 males, XXY females, and XYY males, with lines indicating the standard deviation of each expression value calculated from replicates, and color indicating the class of repetitive element. B. Expression for putatively Y-linked (male-specific) repeats in karyotypes with a Y chromosome, averaged across replicates, with significance calculated using the Wilcoxon test (* p<0.05, ** p<0.001, *** p<1e-5) Scatterplots like in (A.) for putatively Y-linked repeats. Boxes represent 25th and 75th percentile, and whiskers show the most extreme values within 1.5x the inter-quartile range.
Mis-expression of repetitive elements in XXY females and XYY males is especially pronounced for repeats found on the Y chromosome. Y-linked repeats show reduced silencing even in young XXY females and XYY males relative to wildtype males22 (Figure 4B), and become de-repressed even more during aging in XXY and XYY individuals (Figure 4B). Overall, wildtype males express 64 putatively Y-linked repeats during their lifespan, while XXY females express 86 and XYY males express 102. In XXY females, 13 repeats significantly increase in expression during aging (and 5 decrease), and 71 repeats significantly increase in expression in XYY males (and 8 decrease; Figure S14). Indeed, even at just 37 days old, XYY males already show higher, presumably harmful expression of Y-linked repeats compared to 68-day-old wild-type males (Figure 4B).
Discussion
Heterochromatin loss during aging has been observed in a large variety of species, ranging from yeast, to worms, flies and mammals4–6. Here, we show that sex-specific heterochromatin loss might contribute to sex-specific cellular aging in Drosophila. In particular, we find that increased heterochromatin loss in old male flies is associated with increased expression of repeats, and especially of repeats located on the Y chromosome. TE activation in old flies can lead to mutagenic insertions and genomic instability8–11. We further show that the absence or presence of a Y chromosome correlates with increased/reduced lifespan in flies.
The molecular mechanisms underlying heterochromatin loss during aging are poorly understood, and our study does not allow us to address the causative cellular mechanisms underlying sex-specific heterochromatin loss and aging. One prominent heterochromatic structure that has been directly implicated in aging is the nucleolus5,34–36. The nucleolus, the site of ribosome assembly, is formed at the tandemly repeated ribosomal DNA (rDNA) and is embedded in heterochromatin in most eukaryotes37. In Drosophila, the rDNA cluster is located on the X and Y chromosome, each containing a few hundred rDNA transcriptional units in tandem repeats5,36. Nucleolar size and activity have been mechanistically implicated in aging and longevity38,39. In yeast, rDNA instability (i.e. reduction of rDNA copy number and associated accumulation of extrachromosomal rDNA circles) is a major cause of replicative aging34,35. Destabilization and loss of rDNA has been shown during aging of male germline stem cells in Drosophila, which manifests cytologically as atypical morphology of the nucleolus36. In contrast, no such defects of nucleolar morphology were found in young and old female germline stem cells36. The rDNA locus is highly transcribed, and possible collusions between the replication and transcription machinery are thought to contribute to genomic instability of the rDNA40. In male Drosophila, transcription of rDNA is normally restricted to the Y chromosome (nucleolar dominance41), and transcriptionally active Y-linked rDNA copies are preferentially lost in aging germline stem cells36. Flies with only a single rDNA cluster (i.e. X0 flies) live the longest, while flies with extra Y chromosomes (and thus more rDNA copies; see Figure S15) live shorter (Figure 3). This suggests a more complex picture on how the nucleolus may contribute to sex-specific aging, and future detailed functional experimentation is necessary to understand the molecular basis of the sex-specific differences in heterochromatin loss in Drosophila.
Aging is modulated by both genetic and environmental factors, including diet, temperature or mating status of flies, some of which show sex-biased effects on longevity42. Dietary restriction, an intervention known to extend life span, has been shown to prevent TE activation in old flies11, and it will be of great interest to study how manipulations that are sex-biased in their effects on aging will influence sex-specific heterochromatin loss and repeat activation in aged flies. To conclude, our data support the hypothesis that the repeat-rich Y chromosome may decrease life span in Drosophila. Loss of heterochromatin in repetitive regions during aging is more pronounced in male flies, and is accompanied by a de-repression of TEs. Y-linked repeats disproportionally lose their repressive marks and become reactivated, and analysis of flies with aberrant sex chromosome configurations is consistent with the notion that the Y has a direct influence on organismal survival. Age-related heterochromatin loss on the repetitive, sex-limited Y or W-chromosome and repeat re-activation could contribute to lower survivorship of the heterogametic sex across taxa19, including humans. Y chromosomes of Drosophila and humans are known to harbor structural polymorphism in heterochromatic sequences and copy-number variation in repeats43,44, and polymorphism on the D. melanogaster Y has been shown to affect lifespan45, the formation of heterochromatin46, and the regulation of TEs and 100s of genes genome-wide47,48. More generally, individual humans and flies show extensive variation in their repeat content49,50, and our results raise the question whether natural variation in repetitive sequences can contribute to genetic variation in longevity among individuals.
Materials & Methods
Drosophila strains.
Fly strains were obtained from the Bloomington Stock Center and the Kyoto Stock Center. The following strains were used: Canton-S; Oregon-R; 2549 (C(1;Y),y1cv1v1B/0 & C(1)RM,y1v1/0); 4248 (C(1)RM, y1 pn1 v1 & C(1;Y)1, y1 B1/0; svspa-pol) from the Bloomington Stock Center, and 100950 (0/C(1)RM, y1wstr/C(t;Y)1, y1 y+ ac1 sc1 w1) from the Kyoto Stock Center. The crossing scheme used to obtain X0 and XYY males and XXY females is depicted in Fig. 3A. For chromatin and gene expression analyses, flies were grown in incubators at 25°C, 60% relative humidity, and 12h light for the indicated number of days following eclosion, and were then flash-frozen in liquid nitrogen and stored at −80°C. See Fig. S17 for exact crossing scheme.
Genome size estimation.
We estimated genome size of the 5 karyotypes of interest using flow cytometry methods similar to those described in ref. 51. Briefly, samples were prepared by using a 2mL Dounce to homogenize one head each from an internal control (D. virilis female, 1C=328 Mb) and one of the 5 karyotypes in Galbraith buffer (44mM magnesium chloride, 34mM sodium citrate, 0.1% (v/v) Triton X-100, 20mM MOPS, 1mg/mL RNAse I, pH 7.2). After homogenizing samples with 15–20 strokes, samples were filtered using a nylon mesh filter, and incubated on ice for 45 minutes in 25 ug/mL propidium iodide. Using a BD Biosciences LSR II flow cytometer, we measured 10,000 cells for each unknown and internal control sample. We ran samples at 10–60 events per second at 473 voltage using a PE laser at 488 nm. Fluorescence for each D. melanogaster karyotype was measured using the FACSDiva 6.2 software and recorded as the mode of the sample’s fluorescent peak interval. We calculated the genome size of the 5 karyotypes by multiplying the known genome size of D. virilis (328 Mb) by the ratio of the propidium iodide fluorescence in the unknown karyotype to the D. virilis control.
Lifespan assays.
Lifespan data was collected for all karyotypes in the same rearing conditions as described above. The lifespan assays were conducted as described in ref. 52. Briefly, synchronized embryos were collected on agar plates, mobilized with a cotton swab, washed 3 times with PBS pH 7.4, and 10µl of embryos were pipetted to a fresh vial of standard molasses fly medium (0.68% agar, 2.7% yeast, 6.67% cornmeal, 0.456% propionic acid, 1.6% sucrose, 0.76% of 95% ethanol, 0.09% Tegosept, 8.2% molasses, 0.0625% CaCl2, 0.75% Na Tartrate). Adult flies were collected over 2 days, and were allowed to mate for 2 more days. Flies were then sexed quickly in batches under light CO2 to minimize exposure, and 30 flies were counted into each vial. Vials were then flipped, without using CO2, every 2–3 days, and fly deaths were recorded. Throughout the experiments, flies were grown in incubators at 25°C, 60% relative humidity, and 12h light. Flies that were observed escaping the vial were censored. To collect samples for the RNA-seq and ChIP-seq experiment, we censored the entire lifespan experiment once it reached 50% survivorship and flash-froze the remaining flies in liquid nitrogen. In total, 8,829 flies in 297 vials were counted for the lifespan assays reported here.
Chromatin Immunoprecipitation and Sequencing.
We performed ChIP-seq experiments using a standard protocol adapted from ref. 53. Briefly, approximately 2ml of adult flash-frozen flies were dissected on dry ice, and heads and thoraces were used to fix and isolate chromatin. Following chromatin isolation, we spiked in 60µl of chromatin prepared from female Drosophila miranda larvae (approximately 1µg of chromatin). We then performed immunoprecipitation using 4µl of the H3K9me2 (Abcam ab1220) antibody. After reversing the cross-links and isolating DNA, we constructed sequencing libraries using the BIOO NextFlex sequencing kit. Sequencing was performed at the Vincent J. Coates Genomic Sequencing Laboratory at UC Berkeley, supported by NIH S10 Instrumentation Grants S10RR029668 and S10RR027303. We performed 50bp single-read sequencing for our input libraries, and 100bp paired-end sequencing for H3K9me2 libraries, due to their higher repeat content.
We collected replicate datasets for H3K9me2 enrichment in aged males and females to confirm differences seen between the sexes and between young and old samples (Extended Data Fig. 2; Figure S18). Replicate H3K9me2 data for young flies are from ref. 22.
RNA extraction and RNA-seq.
We collected replicate RNA samples for aged individuals of all five karyotypes of interest; replicate RNA data for young flies are from ref. 22. Additionally, we collected 3 replicate samples for aged male Canton-S, aged XXY females, and aged XYY males, and 4 replicate samples for aged female Canton-S and aged X0 males. After flash-freezing in liquid nitrogen, we dissected and pooled 5 heads from each sample, extracted RNA, and prepared stranded total RNA-seq libraries using Illumina’s TruSeq Stranded Total RNA Library Prep kit with Ribo-Zero ribosomal RNA reduction chemistry, which depletes the highly abundant ribosomal RNA transcripts (Illumina RS-122–2201). We performed single-read sequencing for all total RNA libraries at the Vincent J. Coates Genomic Sequencing Laboratory at UC Berkeley.
Mapping of sequencing reads and data normalization.
For all D. melanogaster alignments, we used Release 6 of the genome assembly and annotation20. For all ChIP-seq datasets, we used Bowtie254 to map reads to the genome, using the parameters “-D 15 –R 2 –N 0 –L 22 –i S,1,0.50 --no-1-mm-upfront”, which allowed us to reduce cross-mapping to the D. miranda genome to approximately 2.5% of 50bp reads, and 1% of 100bp-paired end reads. We also mapped all ChIP-seq datasets to the D. miranda genome assembly55 to calculate the proportion of each library that originated from the spiked-in D. miranda chromatin versus the D. melanogaster sample.
To calculate the ChIP signal we first calculated the coverage across 5kb windows for both the ChIP and the input, and then normalized by the total library size, including reads that map to both D. melanogaster and the D. miranda spike. We then calculated the ratio of ChIP coverage to input coverage for each of the 5kb windows, and normalized by the ratio of D. melanogaster reads to D. miranda reads in the ChIP library, and then by the ratio of D. melanogaster reads to D. miranda reads in the input, to account for differences in the ratio of sample to spike present before immunoprecipitation. We describe the validation of this normalization method in ref. 22. Note that this normalization strategy accounts for differences in ploidy levels of sex chromosomes (see Figure S7 in ref. 22).
Gene expression analysis.
For each replicate of RNA-seq data, we first mapped RNA-seq reads to the ribosomal DNA scaffold in the Release 6 version of the D. melanogaster genome, and removed all reads that mapped to this scaffold, as differences in rRNA transcript abundance are likely to be technical artifacts from the total RNA library preparation, which aims to remove the bulk of rRNA transcripts. We then mapped the remaining reads to the Release 6 version of the D. melanogaster genome using STAR56, using default parameters. We then counted reads mapping to each transcript using the FeatureCounts module of Subread57. Gene counts were imported into DESeq2 for differential expression analysis58, using the two replicates for each karyotype to calculate log fold change p-value estimates. GO analysis was performed using GOrilla, using ranked lists of differentially expressed genes59. The GO terms that were enriched and had a p-value less than 10−5 were visualized using the software Revigo60. Gene expression changes during aging for all five karyotypes are given in Table S3.
Repeat libraries.
We used two approaches to quantify expression of repeats. Our first approach was based on consensus sequences of known repetitive elements that were included in the Release 6 version of the D. melanogaster genome and are available on FlyBase. These included consensus sequences for 125 TEs and the 3 largest satellites (359, dodeca, and responder).
Our second approach aimed to specifically assess the repeat content of the Y chromosome. Since the Y chromosome is poorly assembled and repetitive elements on the Y are not annotated, we previously assembled repetitive elements de novo from male and female genomic DNA reads using RepARK and identified 101 male-specific repeats comprising 13.7kb of sequence, based on male-specific coverage analysis22,61.
To assess expression of repetitive elements, we mapped RNA-seq reads to each of the repeat libraries (consensus TEs and putatively Y-linked repetitive elements) using Bowtie254 and the parameters “-D 15 –R 2 –N 0 –L 22 –i S,1,0.50 --no-1-mm-upfront”. We then calculated the mean coverage across each repetitive element using Bedtools62, and normalized the coverage by the number of uniquely-mapping reads in the sequencing library. We made this calculation independently for each replicate for each time sample and karyotype, and then calculated both the average expression value as well as the standard deviation, to assess statistical significance and reproducibility (Figure S19).
To assess H3K9me2 signal in repetitive elements, we took a similar approach as we did for calculating ChIP enrichment profiles across the genome. First, we mapped both ChIP and input sequencing reads to each of the repeat libraries using Bowtie2 and the parameters “-D 15 –R 2 –N 0 –L 22 –i S,1,0.50 --no-1-mm-upfront”. We then calculated the mean coverage across each repetitive element using Bedtools62, and normalized the coverage by the total library size, including reads that mapped to both the D. melanogaster and D. miranda genomes. We then calculated the ratio of ChIP coverage to input coverage for each repetitive element, and then normalized by the ratio of D. melanogaster reads to D. miranda reads in the ChIP library, and then by the ratio of D. melanogaster reads to D. miranda reads in the input, as described above and in ref. 22. This method accounts for differences in copy number of the repetitive elements by dividing the ChIP coverage by each repeat’s coverage in the input.
Extended Data
Extended Data Fig. 1. Survivorship curves of additional D. melanogaster strains.
Shown are Kaplan-Meier survivorship curves for line 2549 males and females ((C(1;Y),y1cv1v1B/0 & C(1)RM,y1v1/0) and Oregon-R wild-type males and females.
Extended Data Fig. 2. Genome-wide enrichment of H3K9me2 for replicate young and old D. melanogaster males and females along the different chromosome arms.
Pearson correlation coefficients for replicate H3K9me2 datasets for old males and females, and boxplots of normalized enrichment values for the replicates. Genome-wide plots were generated using biological replicate data as in Figure 1B and 1D.
Extended Data Fig. 3. Loss and gain of heterochromatin during aging.
Shown are chromosomal locations of 50kb windows that gain (red) or lose (blue) at least 1.5-fold H3K9me2 signal during aging for males and females. Pericentromeric regions are indicated by the red portion of the line beneath each chromosome.
Supplementary Material
Acknowledgements:
D.B. was funded by NIH grants (nos. R01GM076007, GM101255 and R01AG057029).
Footnotes
Data availability: All RNA-seq and ChIP-seq reads are deposited on NCBI under BioProject ID PRJNA594556.
Competing interests: The authors declare no competing interests.
References
- 1.O’Sullivan RJ & Karlseder J The great unravelling: chromatin as a modulator of the aging process. Trends Biochem Sci 37, 466–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood JG et al. Chromatin remodeling in the aging genome of Drosophila. Aging Cell 9, 971–978 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsurumi A & Li WX Global heterochromatin loss: a unifying theory of aging? Epigenetics 7, 680–688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160–1163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson K et al. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet 8, e1002473 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haithcock E et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci U S A 102, 16690–16695 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W et al. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci 16, 529–531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Cecco M et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12, 247–256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cecco M et al. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging 5, 867–883, doi:100621 [pii] (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood JG & Helfand SL Chromatin structure and transposable elements in organismal aging. Frontiers in genetics 4, 274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JG et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc Natl Acad Sci U S A, 113(40):11277–11282. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Meter M et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nature Communications 5, 5011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsner D, Meusemann K & Korb J Longevity and transposon defense, the case of termite reproductives. Proc Natl Acad Sci U S A 115, 5504–5509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskins RA et al. Heterochromatic sequences in a Drosophila whole-genome shotgun assembly. Genome Biol 3, RESEARCH0085 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CH & Larracuente AM Heterochromatin-Enriched Assemblies Reveal the Sequence and Organization of the Drosophila melanogaster Y Chromosome. Genetics 211, 333–348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon JS, Gagen KP & Zhu DL Longevity of 68 species of Drosophila. . Ohio. J. Sci 90, 16–32. (1990). [Google Scholar]
- 17.Tower J & Arbeitman M The genetics of gender and life span. Journal of Biology 8, 38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehtovaara A, Schielzeth H, Flis I & Friberg U Heritability of life span is largely sex limited in Drosophila. The American naturalist 182, 653–665 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Pipoly I et al. The genetic sex-determination system predicts adult sex ratios in tetrapods. Nature 527, 91–94 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Hoskins RA et al. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome research 25, 445–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XY, Harrison MM, Villalta JE, Kaplan T & Eisen MB Establishment of regions of genomic activity during the Drosophila maternal to zygotic transition. eLife 3, e03737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown EJ, Nguyen AH & Bachtrog D The Drosophila Y chromosome affects heterochromatin integrity genome-wide bioRxiv 156000. doi: 10.1101/156000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonhoure N et al. Quantifying ChIP-seq data: a spiking method providing an internal reference for sample-to-sample normalization. Genome Research 24, 1157–1168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu BY, Emtage PC, Duyf BJ, Hilliker AJ & Eissenberg JC Heterochromatin protein 1 is required for the normal expression of two heterochromatin genes in Drosophila. Genetics 155, 699–708 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson KA et al. Genome-Wide Gene Expression in relation to Age in Large Laboratory Cohorts of Drosophila melanogaster. Genetics research international 2015, 835624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garschall K & Flatt T The interplay between immunity and aging in Drosophila. F1000Research 7, 160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pletcher SD et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Current Biology 12, 712–723 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Zheng X & Zheng Y Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell 159, 829–843 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Zheng X, Xiao D & Zheng Y Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence. Aging Cell 15, 542–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran JR, Chen H, Zheng X & Zheng Y Lamin in inflammation and aging. Current Opinion in Cell Biology 40, 124–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pindyurin AV et al. The large fraction of heterochromatin in Drosophila neurons is bound by both B-type lamin and HP1a. Epigenetics & Chromatin 11, 65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salz HK & Erickson JW Sex determination in Drosophila: The view from the top. Fly 4, 60–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho AB, Koerich LB & Clark AG Origin and evolution of Y chromosomes: Drosophila tales. Trends in Genetics 25, 270–277 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganley AR & Kobayashi T Ribosomal DNA and cellular senescence: new evidence supporting the connection between rDNA and aging. FEMS yeast research 14, 49–59 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sinclair DA, Mills K & Guarente L Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 277, 1313–1316 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Lu KL, Nelson JO, Watase GJ, Warsinger-Pepe N & Yamashita YM Transgenerational dynamics of rDNA copy number in Drosophila male germline stem cells. eLife 7, doi: 10.7554/eLife.32421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng JC & Karpen GH H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nature cell biology 9, 25–35 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchwalter A & Hetzer MW Nucleolar expansion and elevated protein translation in premature aging. Nature Communications 8, 328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiku V et al. Small nucleoli are a cellular hallmark of longevity. Nature communications 8, 16083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helmrich A, Ballarino M, Nudler E & Tora L Transcription-replication encounters, consequences and genomic instability. Nature Structural & Molecular biology 20, 412–418 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Greil F & Ahmad K Nucleolar dominance of the Y chromosome in Drosophila melanogaster. Genetics 191, 1119–1128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tower J Sex-Specific Gene Expression and Life Span Regulation. Trends in Endocrinology and Metabolism: TEM 28, 735–747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyckegaard EM & Clark AG Ribosomal DNA and Stellate gene copy number variation on the Y chromosome of Drosophila melanogaster. Proc Natl Acad Sci U S A 86, 1944–1948 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repping S et al. Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet 35, 247–251 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Griffin RM, Le Gall D, Schielzeth H & Friberg U Within-population Y-linked genetic variation for lifespan in Drosophila melanogaster. Journal of Evolutionary biology 28, 1940–1947 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Lemos B, Branco AT & Hartl DL Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc Natl Acad Sci U S A 107, 15826–15831 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemos B, Araripe LO & Hartl DL Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science 319, 91–93 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Sackton TB, Montenegro H, Hartl DL & Lemos B Interspecific Y chromosome introgressions disrupt testis-specific gene expression and male reproductive phenotypes in Drosophila. Proc Natl Acad Sci U S A 108, 17046–17051 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewing AD & Kazazian HH Jr. High-throughput sequencing reveals extensive variation in human-specific L1 content in individual human genomes. Genome research 20, 1262–1270 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosco G, Campbell P, Leiva-Neto JT & Markow TA Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 177, 1277–1290 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis L et al. Intrapopulation genome size variation in D. melanogaster reflects life history. PLoS Genet 10, e1004522 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linford NJ, Bilgir C, Ro J & Pletcher SD Measurement of lifespan in Drosophila melanogaster. J Vis Exp, doi: 10.3791/50068 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alekseyenko A, Larschan E, Lai W, Park P & Kuroda M High-resolution ChIP-chip analysis reveals that the Drosophila MSL complex selectively identifies active genes on the male X chromosome. Genes Dev 20, 848–857 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langmead B & Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellison CE & Bachtrog D Dosage compensation via transposable element mediated rewiring of a regulatory network. Science (New York, N.Y.) 342, 846–850 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao Y, Smyth GK & Shi W featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eden E, Navon R, Steinfeld I, Lipson D & Yakhini Z GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supek F, Bošnjak M, Škunca N & Šmuc T REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch P, Platzer M & Downie BR RepARK--de novo creation of repeat libraries from whole-genome NGS reads. Nucleic acids research 42, e80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quinlan AR BEDTools: The Swiss-Army Tool for Genome Feature Analysis. Current Protocols in Bioinformatics 47, 11 12 11–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan EL & Meier P Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn 53, 457–481 (1958). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.