Abstract
Objectives
We investigated the predictors of functional outcome in young patients enrolled in a multiethnic study of intracerebral hemorrhage (ICH).
Methods
The Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study is a prospective multicenter study of ICH among adult (age ≥18 years) non-Hispanic white, non-Hispanic black, and Hispanic participants. The study recruited 1,000 participants per racial/ethnic group. The present study utilized the subset of ERICH participants aged <50 years with supratentorial ICH. Functional outcome was ascertained using the modified Rankin Scale (mRS) at 3 months. Logistic regression was used to identify factors associated with poor outcome (mRS 4–6), and analyses were compared by race/ethnicity to identify differences across these groups.
Results
Of the 3,000 patients with ICH enrolled in ERICH, 418 were studied (mean age 43 years, 69% male), of whom 48 (12%) were white, 173 (41%) were black, and 197 (47%) were Hispanic. For supratentorial ICH, black participants (odds ratio [OR], 0.42; p = 0.046) and Hispanic participants (OR, 0.34; p = 0.01) had better outcomes than white participants after adjustment for other factors associated with poor outcome: age, baseline disability, admission blood pressure, admission Glasgow Coma Scale score, ICH volume, deep ICH location, and intraventricular extension.
Conclusions
In young patients with supratentorial ICH, black and Hispanic race/ethnicity is associated with better functional outcomes, compared with white race. Additional studies are needed to identify the biological and social mediators of this association.
There is limited information about spontaneous nontraumatic intracerebral hemorrhage (ICH) in young patients. Because of the increase in ICH incidence with age, risk factors and outcomes for older patients with this condition have been extensively characterized. Studies that describe risk factors and outcomes in patients under age 50 with ICH have been limited to small, single-center, retrospective studies.1–4
There is a disparity in risk factor burden for ICH among racial/ethnic groups.5 Hypertension, which has been estimated to account for 45%–60% of nonlobar ICH cases,6 is disproportionately prevalent and poorly controlled among the black community and is extensively untreated among the Hispanic community.7,8 However, the actual case–fatality rate in minority populations has been shown to be significantly lower than in the white population.9 Black and Hispanic patients tend to present with ICH at younger ages,9–11 which may explain the lower case–fatality rates.12
Given the lack of data regarding ICH in the young and the differences observed in post-ICH functional outcomes across race/ethnicity among older adults, we hypothesized that post-ICH functional outcomes differ by race/ethnicity in the young. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study is a prospective multicenter study of ICH among adult (age ≥18 years) non-Hispanic white, non-Hispanic black, and Hispanic patients. In the present study, we utilized the subset of ERICH cases under the age of 50 years with supratentorial ICH to identify predictors of poor outcome in this understudied population.
Methods
Study design
The ERICH study is a prospective, multicenter, longitudinal study of primary nontraumatic ICH in adults older than 18 years.
Standard protocol approvals, registrations, and patient consents
The protocol was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from each patient or each patient's guardian.
Case ascertainment
Between 2011 and 2015, the ERICH study prospectively enrolled 1,000 cases each among self-reported non-Hispanic black, non-Hispanic white, and Hispanic patients with nontraumatic primary ICHs across 42 recruitment sites. Sites were selected based on the demographic distribution of the minority African American and Hispanic American populations in the United States with a target to have equal power across race/ethnicities for analyses. Cases were enrolled via hot pursuit in order to minimize survival bias. Once each demographic was completed, recruitment was halted for that group. Cases remained in follow-up for 1 year, and data collection was completed by February 2016.13 ICH was defined by the onset of acute neurologic symptoms within a 24-hour period with intraparenchymal blood identified on admission neuroimaging. Consistent with prior studies, cases of “ICH in the young” were defined as age <50 years.1,14 Given the different underlying pathophysiologies and outcomes of infratentorial and primary intraventricular hemorrhage (IVH), patients with infratentorial ICH (n = 77) and primary IVH (n = 7) were excluded from analysis. Patients with missing data points for 3-month functional outcome or admission ICH volume (n = 94) were also excluded from the analysis. A total of 418 cases were ultimately included in analysis. In a secondary analysis, we used the carry-forward method to include cases with missing modified Rankin Scale (mRS) scores at 3 months in the multivariable logistic regression modeling. Missing mRS scores at 3 months were replaced with the mRS score at 30 days poststroke or at final discharge.
Outcome ascertainment
We utilized the mRS to evaluate the functional outcome of young patients with ICH included in the study. The primary outcome was the mRS score 3 months after the ICH, assessed by phone interview and ascertained using a standardized mRS questionnaire. Consistent with previous studies in ICH, poor outcome was defined as mRS ≥4.15–17
Neuroimaging
All participants had neuroimaging confirmation of the ICH. In order to limit individual biases, a centralized neuroimaging core reviewed all imaging to confirm the diagnosis of spontaneous nontraumatic ICH, determine location (lobar, deep, or infratentorial), and quantify ICH and IVH volumes by planimetric analysis using Alice software (Paraxel Corporation, Waltham, MA). IVH extension was recorded if present on the baseline CT scan. ICH expansion was measured only for patients who had at least 2 CT scans and was defined as a 33% increase in ICH volume between the first 2 serial scans.18 Further details on neuroimaging diagnostics are included in the ERICH study protocol.13
Variable definition
The ERICH study collected data pertaining to details of the acute hospitalization for ICH on an extensive chart abstraction form. Among the items collected were length of stay, prestroke mRS, first recorded systolic and diastolic blood pressures in the emergency department, initial Glasgow Coma Scale (GCS) score, initial laboratory values (e.g., serum blood sugar, white blood count, international normalized ratio [INR]), ICH score, cocaine abuse, external ventricular drain (EVD) placement, craniotomy, do-not-resuscitate order, and withdrawal of care (comfort care order or discharge to hospice).13 All participants were also administered a standardized baseline interview. Both the chart abstraction and baseline interview collected data on medical history and medication use prior to the ICH; medical history items (e.g., hypertension, diabetes, hypercholesterolemia, coronary artery disease, atrial fibrillation) and medication use (e.g., anticoagulants, antiplatelets, statins) were considered positive if reported on either the chart abstraction or the baseline interview. Hypertension was considered untreated if no antihypertensive medication appeared on the patient's medication list. Current smoker status was recorded on the baseline interview. For the analysis, premorbid disability was defined as a prestroke mRS score ≥2, and initial INR was dichotomized by ≤1.7 or >1.7.
Statistical analysis
Data are presented as counts (percentages) for discrete variables and mean (SD) or median (interquartile range [IQR]) for continuous variables, as appropriate.
Exposures
Due to reported differences in risk factors, severity, and functional recovery by ICH location,19 association analyses were compared between lobar and nonlobar ICH. We also compared analyses based on the race/ethnicity of the patients.
Distribution of covariates across race/ethnicity
In order to identify differences in covariate distribution across non-Hispanic black, non-Hispanic white, and Hispanic young patients with ICH, the Pearson χ2 test was used to compare the frequency distribution of categorical variables. For any analyses that had fewer than 5 counts, the Fisher exact test was reported. The Kruskal-Wallis test was used to compare medians for ordinal variables or for continuous variables that were not normally distributed, or that were heteroscedastic.
Logistic regression analysis
We used univariable and multivariable logistic regression to model the unadjusted and adjusted odds, respectively, of poor outcome (mRS 4–6) at 3 months across levels of the covariates of interest. Multivariable model building proceeded as follows: first, covariates with p < 0.1 in univariable analyses were included in the model; second, universal confounders (age and sex) were forced into the model; third, covariates with p < 0.1 were backward eliminated; fourth, collinear covariates, as expressed by a variance inflation factor >5, were identified and 1 covariate was removed from the model. After transforming ICH volume to its natural log, all variables in the model fulfilled the assumption for linearity of logits. In a secondary analysis, we conducted an interaction analysis between race/ethnicity and ICH volume by introducing interaction terms into the multivariable logistic regression model.
Distribution of covariates between missing and nonmissing cases
In a secondary analysis, the frequency distribution of discrete covariates between missing and nonmissing cases was compared using the Pearson χ2 or Fisher exact test, when appropriate. For non-normally distributed continuous variables and ordinal variables, the Mann-Whitney U test was used.
For all statistical analyses, a 2-sided p value of 0.05 was set as the significance threshold and 95% confidence intervals (CIs) were reported for all odds ratios (ORs). SPSS Statistics software (IBM Corp., Armonk, NY) was used for all analyses.
Data availability
The authors certify that the data, methods, and materials used to conduct the present research have been thoroughly documented. Anonymized data will be shared by request from any qualified investigator through the ERICH study principal investigator (woodlu@ucmail.uc.edu).
Results
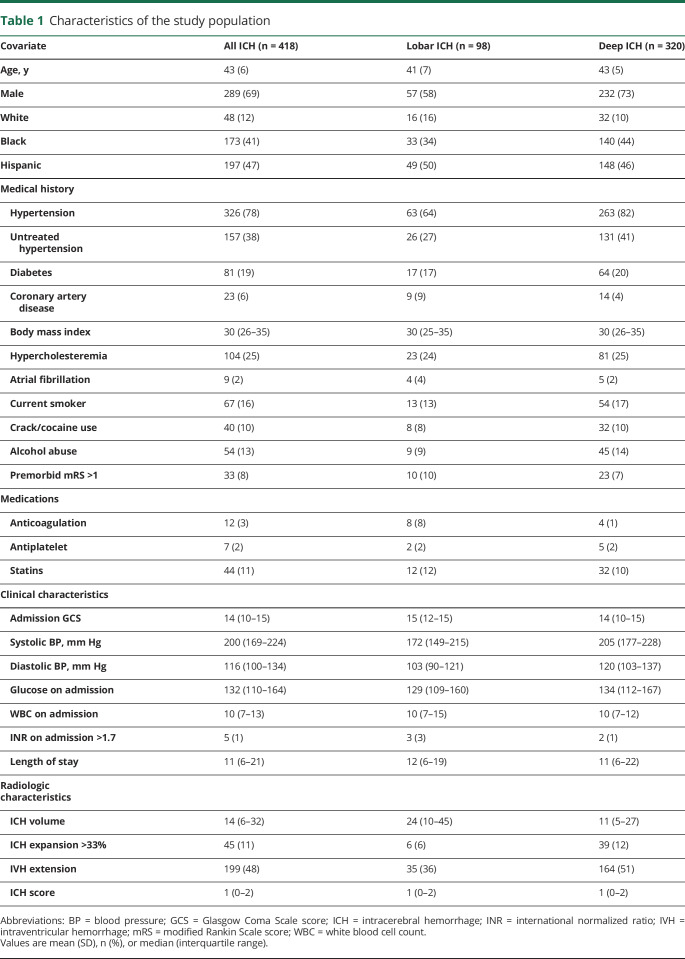
Of the 3,000 patients with ICH enrolled in the ERICH study, 596 patients aged <50 years were considered for the present analysis. After excluding infratentorial ICH, primary IVH, cases with missing admission ICH volume, and cases with missing 3-month mRS scores, 418 young patients with ICH (mean [SD] age, 43 [6] years; 69% male) were included in the present analysis (figure). Within this young cohort, 173 (41%) were non-Hispanic black, 48 (12%) were non-Hispanic white, and 197 (47%) were Hispanic. Distribution of age, sex, GCS score on admission, ICH location, EVD placement, withdrawal of care, and medical history of untreated hypertension, diabetes, high cholesterol, or premorbid disability were similar between those included in our analysis and those excluded due to missing data. Compared with our analyzed cases, cases with missing data included a greater frequency of white patients, a lesser frequency of Hispanic patients, smaller ICH volumes, and less IVH extension (table e-4, doi.org/10.5061/dryad.6kb10h4). White patients with missing outcomes had smaller median ICH volumes compared with white patients included in the study (4 mL, IQR 3–10; 24 mL, IQR 8–42, p = 0.001). Given the differing underlying pathophysiologic mechanisms between lobar and deep ICH, we compared patient and ICH characteristics between these 2 groups. Within our cohort, 98 (23%) patients presented with lobar bleeds and 320 (77%) presented with nonlobar bleeds. The median ICH volumes for deep and lobar bleeds were 11 mL (IQR, 5–27 mL) and 24 mL (IQR, 10–45 mL), respectively (p < 0.001) (table 1).
Figure. Selection of participants from the Ethnic/Racial Variations of Intracerebral Hemorrhage study for the present analysis.
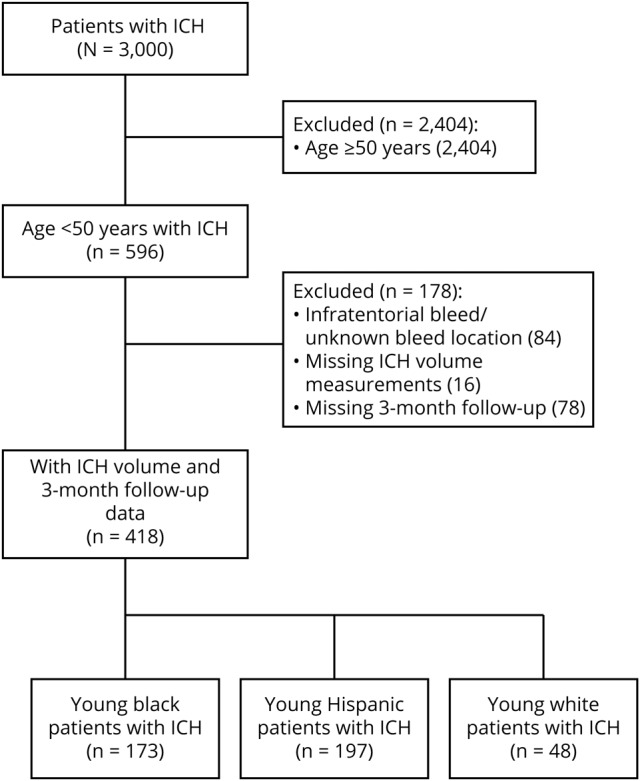
ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage.
Table 1.
Characteristics of the study population
Functional outcome at 3 months
Three months after the ICH, 146 (35%) young patients had a poor functional outcome, as represented by an mRS of 4–6. Specifically, 73 (18%) were unable to walk without assistance and attend to their own bodily needs (mRS 4); 32 (8%) were bedridden, incontinent, and required constant nursing care and attention (mRS 5); and 41 (10%) had died (mRS 6) (table e-5, doi.org/10.5061/dryad.6kb10h4). Among the latter, 35 (85%) died as inpatients. Of those who died in the hospital, 8 (23%) patients died in the first 48 hours of hospital arrival. There was a greater distribution of poor outcome at 3 months among young patients who had presented with deep ICH (38% vs 26%, p = 0.046), but no difference in 3-month mortality between these 2 groups (p = 0.88). At 6 and 12 months after the ICH, 119 (29%) and 111 (27%) young patients had an mRS score of 4–6, respectively.
Race/ethnicity and poor functional outcome
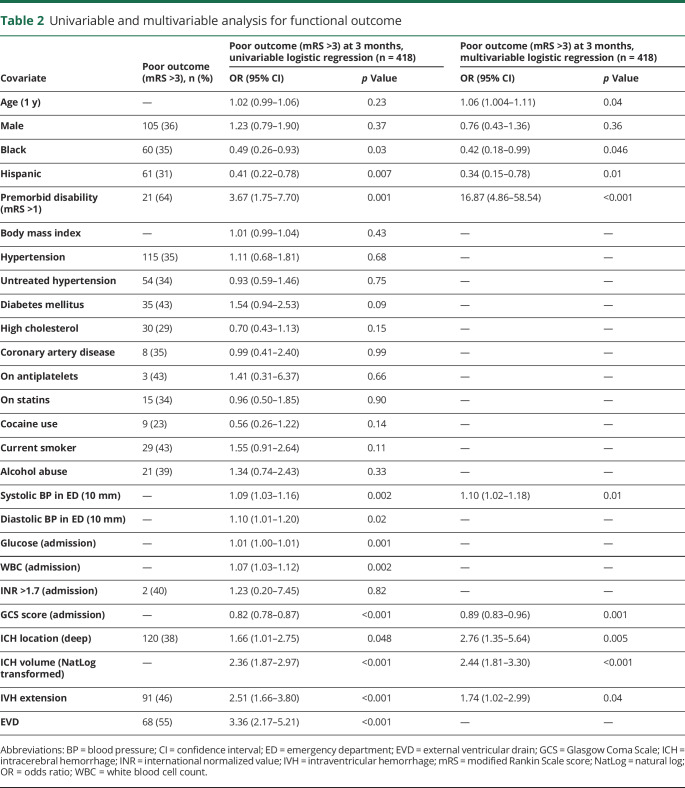
Functional outcome after ICH differed by race/ethnicity. The proportion of young patients with ICH with a 3-month mRS of 4–6 across race/ethnic categories was 52%, 35%, and 31% for white, black, and Hispanic patients, respectively. In univariable analysis, compared with white patients, black patients had a significant 51% reduction in odds of poor outcome at 3 months (p = 0.03), and Hispanic patients had a significant 59% reduction in these same odds (p = 0.007) (table 2). Compared to black patients, Hispanic patients had a 15% reduction in the odds for poor outcome (p = 0.45) that was not statistically significant. The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, IVH extension, systolic blood pressure, and GCS score on admission. In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months (OR, 0.42; 95% CI, 0.18, 0.99; p = 0.046) and Hispanic patients had a 66% reduction in the same odds (OR, 0.34; 95% CI, 0.15, 0.78; p = 0.01) (table 2). An interaction analysis between black patients and ICH volume was not statistically significant. Secondary analysis including patients with missing 3-month mRS scores (n = 496) did not significantly alter the results of the multivariable model: black patients had a 55% reduction in the odds of poor functional outcome at 3 months (OR, 0.45; 95% CI, 0.22, 0.94; p = 0.032) and Hispanic patients had a 64% reduction in the odds of poor functional outcome at 3 months (OR, 0.36; 95% CI, 0.18, 0.74; p = 0.005).
Table 2.
Univariable and multivariable analysis for functional outcome
Characterization of ICH by race/ethnicity
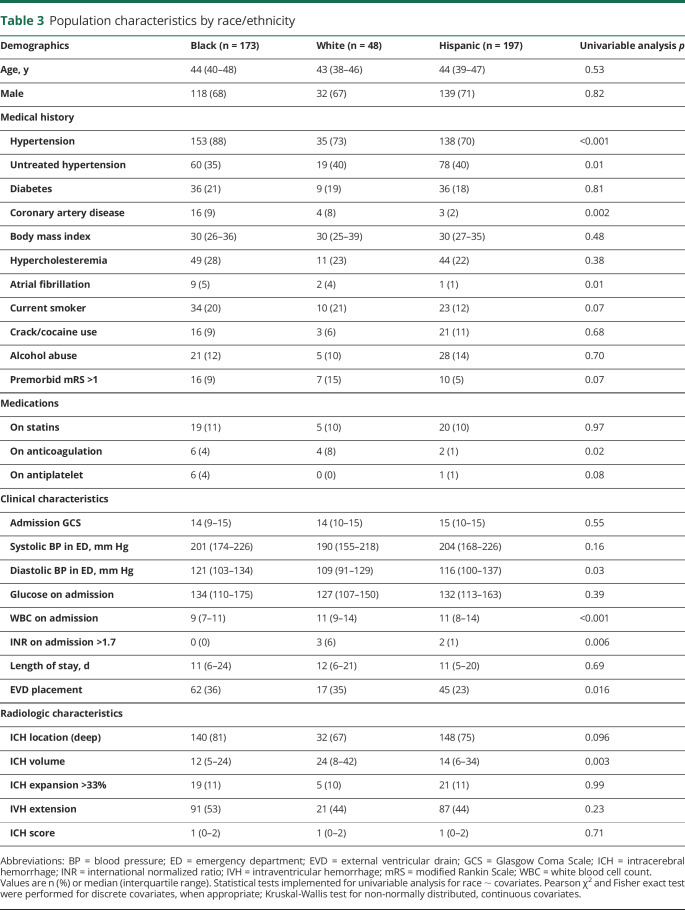
We completed multiple secondary analyses to explore potential mechanisms for the observed difference in functional outcomes across race/ethnicity. First, we evaluated the volume of the ICH upon admission to the hospital across these strata and found that hematomas were larger in white patients (median 24 mL) compared with black patients (median 12 mL) and Hispanic patients (median 14 mL) (table 3). The difference in volume was significant between white and black patients (p = 0.002), although not statistically significant between white and Hispanic patients (p = 0.10), likely due to the variability in volumes among young Hispanic patients. Second, we compared the baseline and clinical characteristics of these ethnic groups, finding that white and Hispanic patients were more likely to have untreated hypertension, white patients were more likely to receive oral anticoagulation treatment, and black and white patients were more likely to have coronary artery disease, atrial fibrillation, and to undergo EVD placement (table 3). There were no differences in discharge mortality, defined as inpatient death or discharge to hospice, among young black, white, and Hispanic patients (p = 0.20).
Table 3.
Population characteristics by race/ethnicity
Other predictors of poor functional outcome
We confirmed that several factors known to influence functional outcome in older patients with ICH also play an important role in young patients with this condition. The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients. In addition, age, premorbid conditions, deep location of the bleed, and presence of IVH were all found to be significantly associated with poor functional outcome in multivariable analysis (table 2). As expected, placement of an EVD also significantly predicted poor outcome, although this instance likely represents a downstream clinical marker of severity rather than a true factor associated with poor outcome. Of note, IVH presence and EVD placement were likely collinear in multivariable modeling (variance inflation factor 1.255), and IVH presence was kept in the final model.
Discussion
The present study investigated the outcomes of young patients with ICH enrolled in the ERICH study, a large, ethnically diverse, multicenter, prospective study. From a cohort of 596 patients with ICH under the age of 50, we studied 418 young patients who presented with supratentorial bleeds and had complete data on ICH volume and 3-month outcomes. Consistent with previous studies on ICH among all age groups, GCS score on admission, age, systolic blood pressure on admission, ICH volume, deep ICH location, and IVH presence were significant independent predictors of poor outcome at 3 months for ICH in the young.20–25 Furthermore, we show that, compared with their white counterparts, young black and Hispanic patients have a significant reduction in odds for poor outcome at 3 months.
Consistent with the hypertensive profile of our study population, the majority of the young patients with ICH presented with deep hemorrhages,26,27 with only a quarter presenting with lobar hemorrhages. Prior studies have reported that the risk of lobar hemorrhage with advanced age is strongly associated with the increased incidence of cerebral amyloid angiopathy (CAA).28,29 As CAA is rare in young adults under age 50,30 the lobar bleeds identified in our cohort are likely to be related to high blood pressure. In line with this argument, approximately 4 out of 5 young patients with ICH presented with a history of diagnosed hypertension, of which nearly half were untreated. After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse. In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.
Our results expand upon a previous study from the Get with the Guidelines–Stroke registry that explored a similar question in all age groups. This study found that black and Hispanic patients with ICH had a 21% and 27% reduction in risk of in-hospital mortality, respectively, compared with white patients with ICH. Of note, these findings persisted after adjustment for available data points on baseline characteristics.9,31 However, because the study did not collect data on well-established predictors of functional outcomes, such as ICH location, ICH volume, IVH extension, and GCS score on admission, it is unclear the extent to which differences in these unmeasured covariates account for the observed differences in mortality by race/ethnicity. In the present study, we show that even when accounting for these strong, and most widely regarded, predictors of functional outcome, black race and Hispanic ethnicity bear a significant association with short-term functional outcomes after ICH.
Our results also provide important information about the factors that may mediate the observed difference across ethnic groups. Admission hematoma volume32 and hematoma expansion33 are the most potent predictors of clinical outcome in ICH. Hematoma volume is measured on the first CT scan upon admission to the hospital, before any medical intervention is implemented. Consequently, it reflects the initial, unmodifiable brain injury caused by direct damage inflicted by blood spilled into the brain parenchyma. Hematoma expansion, on the other hand, is a delayed, secondary process amenable to treatment, and the target of most therapeutic interventions evaluated in ICH in recent years.34–36 In this study, white patients with ICH had larger hematoma volumes on admission (median size 24 mL) compared to black (median size 12 mL) and Hispanic (median size 14 mL) patients. Furthermore, an interaction analysis between black patients and ICH volume was not statistically significant, suggesting that the lower ICH volumes in black patients did not contribute to their better outcomes at 3 months. Importantly, the proportion of hematoma expansion was similar across ethnic groups. In combination, these findings indicate that the observed differences in clinical outcome are most likely driven by biological, social, and treatment factors related to risk of ICH, rather than by differences in the early management after admission to the hospital. Further research is needed to determine the reasons for the improved outcomes which could lead to changes in management for all patients.
Our results also point to treatment with oral anticoagulants (OAC) before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes. Specifically, white patients had higher prevalence of OAC use (n = 4, 8%) when compared to black and Hispanic patients (n = 6, 4% and n = 2, 1%, respectively) and this difference was reflected by a higher proportion of white patients having INR > 1.7 on admission (n = 3, 6%). OAC treatment is a known risk factor for ICH and an established predictor of poor outcome in this condition.37 However, because only a small proportion of enrolled young patients with ICH were on OAC prior to presentation, these results should be further validated by future studies.
The greatest strengths in our study are the large number of young patients with ICH we included in our analysis, the homogenous recruitment of ICH cases with hypertensive etiologies, and the emphasis on minority enrollment. Patient history was constructed from chart abstraction and case interview responses, creating a robust picture of the patient's health prior to hospitalization. Radiologic diagnostics, including ICH location and hematoma volume, were centrally performed in a standardized fashion by a panel of experts to limit individual biases and discrepancies in measurement. However, there are also some limitations to our study. Because we used a broad categorization of racial/ethnic groups, we likely lost the more nuanced differences in ICH presentation and recovery among more specifically categorized cultural and racial subgroups. Second, because younger patients were more likely to be black or Hispanic and less likely to be white, our results may be limited by reduced statistical power. Third, we were unable to assess the importance of ICH stability on outcome due to the unstandardized timing and number of CT scans. Fourth, a significant proportion of cases excluded due to missing data included young white patients with ICH with smaller ICH volumes, which may limit our analysis to a subset of young white patients with ICH with more severe bleeds and worse outcomes. Finally, despite our large cohort of young patients with ICH, it may still not be large enough to capture the differences among the racial/ethnic subgroups.
We conducted the largest investigation on spontaneous supratentorial ICH in patients under the age of 50, using cases from the ERICH study. We show that young black and young Hispanic patients have better short-term outcomes, independent of medical history, radiologic characteristics, and hospital characteristics, after spontaneous supratentorial ICH. Although presenting ICH severity varied by race/ethnicity among young patients, it did not fully account for the differences in functional outcome at 3 months. Furthermore, despite reports that minority patients fare better due to an average younger age at ICH onset, our results demonstrate that differences in functional recovery by race/ethnicity persist even when studying a young adult population. This may indicate distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury across different racial/ethnic groups. Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population. Future studies will also be required to describe the presentation and characteristics of infratentorial hemorrhage in the young.
Glossary
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
- OAC
oral anticoagulants
- OR
odds ratio
Appendix. Authors
Study funding
The ERICH study is supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS: U-01-NS069763). This report does not represent the official view of NINDS, the NIH, or any part of the US Federal Government. No official support or endorsement of this article by NINDS or NIH is intended or should be inferred.
Disclosure
L. Miyares reports no disclosures relevant to the manuscript. G. Falcone is supported by the National Institute on Aging (grant K76AG059992), the American Heart Association (grant 18IDDG34280056), the Yale Pepper Scholar Award (grant P30AG021342), and the Neurocritical Care Society Research Fellowship. A. Leasure, O. Adeoye, F. Shi, S. Kittner, C. Langefeld, A. Vagal, and K. Sheth report no disclosures relevant to the manuscript. D. Woo was supported by a grant from NINDS (U-01-NS069763). Go to Neurology.org/N for full disclosures.
References
- 1.Koivunen RJ, Satopaa J, Haapaniemi E, et al. Predictors of early mortality in young adults after intracerebral hemorrhage. Stroke 2014;45:2454–2456. [DOI] [PubMed] [Google Scholar]
- 2.Go, Park H, Lee CH, Hwang SH, Han JW, Park IS. The outcomes of spontaneous intracerebral hemorrhage in young adults: a clinical study. J Cerebrovasc Endovasc Neurosurg 2013;15:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Sandoval JL, Romero-Vargas S, Chiquete E, et al. Hypertensive intracerebral hemorrhage in young people: previously unnoticed age-related clinical differences. Stroke 2006;37:2946–2950. [DOI] [PubMed] [Google Scholar]
- 4.Lai SL, Chen ST, Lee TH, Ro LS, Hsu SP. Spontaneous intracerebral hemorrhage in young adults. Eur J Neurol 2005;12:310–316. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics: 2016 update. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 6.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage. Stroke 2002;33:1190. [DOI] [PubMed] [Google Scholar]
- 7.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the Reasons for Geographic and Racial Differences in Stroke study. Stroke 2006;37:1171–1178. [DOI] [PubMed] [Google Scholar]
- 8.Viruell-Fuentes EA, Ponce NA, Alegria M. Neighborhood context and hypertension outcomes among Latinos in Chicago. J Immigr Minor Health 2012;14:959–967. [DOI] [PubMed] [Google Scholar]
- 9.Xian Y, Holloway RG, Smith EE, et al. Racial/ethnic differences in process of care and outcomes among patients hospitalized with intracerebral hemorrhage. Stroke 2014;45:3243–3250. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the Northern Manhattan Stroke Study. Stroke 2002;33:2789–2793. [DOI] [PubMed] [Google Scholar]
- 11.Trimble B, Morgenstern LB. Stroke in minorities. Neurol Clin 2008;26:1177–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radholm K, Arima H, Lindley RI, et al. Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing 2015;44:422–427. [DOI] [PubMed] [Google Scholar]
- 13.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, et al. Clinical characteristics and outcome of intracerebral hemorrhage in young adults. J Neurol 2014;261:2143–2149. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern LB, Zahuranec DB, Sanchez BN, et al. Full medical support for intracerebral hemorrhage. Neurology 2015;84:1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta VP, Garton ALA, Sisti JA, et al. Prognosticating functional outcome after intracerebral hemorrhage: the ICHOP score. World Neurosurg 2017;101:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke 2014;9:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowlatshahi D, Demchuk A, Flaherty M, Ali M, Lyden P, Smith E. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011;76:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sreekrishnan A, Dearborn JL, Greer DM, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care 2016;25:384–391. [DOI] [PubMed] [Google Scholar]
- 20.Rathor MY, Rani MFA, Jamalludin AR, Amran M, Shahrin TCA, Shah A. Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. J Res Med Sci 2012;17:1056–1062. [PMC free article] [PubMed] [Google Scholar]
- 21.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage. Stroke 2008;39:2304. [DOI] [PubMed] [Google Scholar]
- 22.Delcourt C, Sato S, Zhang S, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 2017;88:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score. Stroke 2001;32:891. [DOI] [PubMed] [Google Scholar]
- 24.Dandapani BK, Suzuki S, Kelley RE, Reyes-Iglesias Y, Duncan RC. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke 1995;26:21. [DOI] [PubMed] [Google Scholar]
- 25.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 2004;35:1364–1367. [DOI] [PubMed] [Google Scholar]
- 26.Jackson CA, Sudlow CLM. Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry 2006;77:1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012;79:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilar MI, Brott TG. Update in intracerebral hemorrhage. Neurohospitalist 2011;1:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer PHC, Jolink WMT, Kappelle LJ, Algra A, Klijn CJM. Risk factors for lobar and non-lobar intracerebral hemorrhage in patients with vascular disease. PLoS One 2015;10:e0142338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 2011;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke. Stroke 2005;36:374. [DOI] [PubMed] [Google Scholar]
- 32.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 33.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis 2013;35:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016;375:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 37.Pezzini A, Grassi M, Paciaroni M, et al. Antithrombotic medications and the etiology of intracerebral hemorrhage: MUCH-Italy. Neurology 2014;82:529–535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors certify that the data, methods, and materials used to conduct the present research have been thoroughly documented. Anonymized data will be shared by request from any qualified investigator through the ERICH study principal investigator (woodlu@ucmail.uc.edu).