Abstract
Particularly in rural settings, there has been little research regarding the health impacts of fine particulate matter (PM2.5) during the wildfire season smoke exposure period on respiratory diseases, such as influenza, and their associated outbreaks months later. We examined the delayed effects of PM2.5 concentrations for the short-lag (1–4 weeks prior) and the long-lag (during the prior wildfire season months) on the following winter influenza season in Montana, a mountainous state in the western United States. We created gridded maps of surface PM2.5 for the state of Montana from 2009–2018 using spatial regression models fit with station observations and Moderate Resolution Imaging Spectroradiometer (MODIS) aerosol optical thickness data. We used a seasonal quasi-Poisson model with generalized estimating equations to estimate weekly, county-specific, influenza counts for Montana, associated with delayed PM2.5 concentration periods (short-lag and long-lag effects), adjusted for temperature and seasonal trend. Inconsistent with other studies, we did not detect an acute, short-lag PM2.5 effect nor short-lag temperature effect on influenza in Montana. Higher daily average PM2.5 concentrations during the wildfire season was positively associated with increased influenza in the following winter influenza season (expected 16% or 22% increase in influenza rate per 1 μg/m3 increase in PM2.5 based on two analyses, p = 0.04 or 0.008). This is one of the first observations of a relationship between PM2.5 during wildfire season and influenza months later.
Keywords: Air pollution, MODIS, PM2.5, Respiratory health, Influenza, Wildfire Smoke
1. Introduction
The last two decades have seen a dramatic increase in wildfire activity across much of the western United States (US), a trend that has been attributed to decreasing summer precipitation and increasing temperatures (Westerling et al., 2006; Abatzoglou and Williams, 2016; Holden et al., 2018). Communities impacted by smoke from nearby and distant wildfires experience high episodic exposures to fine particulate matter (aerodynamic diameter < 2.5 μm; PM2.5) with concentrations often exceeding 24-hour ambient air quality standards for extended periods (Liu et al., 2015). While recent studies have shown air quality improving for the contiguous US from the reduction of industrial and vehicular emissions (McClure and Jaffe, 2018; O’Dell et al., 2019), air pollution in wildfire-prone areas, particularly in the mountain west region of the US, has increased and is projected to further worsen due to climate-mediated increases in wildfire activity (Yue et al., 2013; Liu et al., 2016; Ford et al., 2018).
PM2.5 is widely known to have significant adverse effects on human health (US EPA 2009; Anderson et al., 2012; Kim et al., 2015), and several studies of PM2.5 during wildfires have found similar positive associations with respiratory effects, including increased hospitalization and medication use for asthma, increased urgent care visits for cardiopulmonary outcomes and increased mortality (for reviews see Liu et al., 2015; Reid et al., 2016; Adetona et al., 2016). Adverse respiratory outcomes associated with wildfire exposure also include hospitalizations and urgent care visits for respiratory infections, pneumonia and bronchitis (Reid et al., 2016). To date, all studies of wildfires have focused on acute health effects with analyses typically not extending beyond a few days lag period.
Wintertime influenza offers an opportunity to evaluate the potential for longer-lag delayed effects of PM2.5 associated with smoke from wildfire events. Traditionally, meteorological factors in temperate countries, such as low temperatures and humidity, have been shown to contribute to the risk of influenza outbreaks (Tamerius et al., 2013) and are well correlated with the seasonal changes in the US (Shaman et al., 2010). Recent studies have begun to investigate the associations of PM2.5 and influenza. For example, a study in Beijing, China, reported the association between the delayed impact of short-term exposure of PM2.5 and monthly influenza cases (Liang et al. 2014). A follow up study found correlations between PM2.5 exposure and daily influenza risk by age group in Beijing, China, suggesting a 1-day optimal lag effect (Feng et al., 2016). A more recent study showed consistently increased odds of healthcare encounters for influenza for elevated PM2.5 exposure estimates averaged across several lag periods, 0–28 days (Horne et al. 2018). However, to date and particularly in rural settings, there has been little research regarding the health impacts of PM2.5 during the wildfire season smoke exposure period on influenza occurrence months later.
While the burden of influenza can vary from season to season, it is estimated that between 9 and 49 million cases of influenza occur each year in the United States. Of these, an estimated 140,000–960,000 hospitalizations and up to 79,000 deaths due to influenza occur each year (www.cdc.gov/flu/about/burden/index.html). In addition, a 2007 review of the economic burden of influenza determined that direct medical costs average around $10.4 billion (Molinari et. al., 2007). In the western US state of Montana, approximately 10,000 cases of influenza are reported each season (approximately October – May; https://www.cdc.gov/flu/about/season/flu-season.htm), but it is likely that the actual number is higher as not all individuals who are infected will seek medical care (MT DPHHS data). In Montana, influenza is associated with approximately 900 hospitalizations and 60 deaths each year (www.cdc.gov/flu/about/burden/index.htm). Since influenza cases are monitored closely by state and federal agencies and the periods between wildfire activity and influenza transmission are offset by weeks to months, we have the opportunity to investigate the potential of PM2.5 exposure from wildfire season months to impart impacts weeks to months after exposure.
Wildfires have been identified as the dominant source of elevated surface PM2.5 across the Northern Rocky Mountain region (Idaho, Montana, and Wyoming) during the western US wildfire season (Liu et al., 2016; Brey et al., 2018). Here, we define wildfire season as July 1 - September 30. This time period accounts for >90% of annual wildfire emissions across the region (Urbanski et al., 2017; Urbanski et al., 2018). Inter-annual differences in wildfire activity and wildfire PM2.5 emissions result from variability within this time window. Even in the most active fire years, fire burned area and emissions outside July – September are a minimal fraction of the total in the Northern Rocky Mountain region (Urbanski et al., 2018). In Montana, wildfires are the primary PM2.5 emission source during the western US wildfire season (Urbanski et al., 2018). The dominant non-wildfire emissions of primary annual PM2.5 within Montana were dust from agriculture and unpaved roadways (53%) and prescribed fires (27%), while residential fuel combustion accounted for 2%, according to the Environmental Protection Agency (EPA) triennial National Emission Inventories (NEI) of 2011 and 2014 (www.epa.gov/air-emissions-inventories/national-emissions-inventory-nei).
The major contributor to short-lag PM2.5 exposures during flu season in Montana is biomass smoke exposure generated from wood stoves used for heating throughout the winter months. PM2.5 source apportionment modeling has identified wood smoke contributions to be between 56–77% of the ambient wintertime PM2.5 in multiple communities throughout western Montana (Ward and Lange 2010). Other contributions include dust (1–4%), ammonium nitrate from heavily fertilized agricultural fields and livestock waste (10–20%), sulfate (0–5%), diesel (0–5%), automobiles (0–4%), and unexplained sources (0–4%) (Ward and Lange 2010).
The objective of this study was to evaluate associations between PM2.5 and influenza counts at the county level in Montana. Specifically, we developed spatio-temporal PM2.5 maps to estimate PM2.5 effects for two different time frames: (1) PM2.5 exposure 1–4 weeks before influenza cases, hereafter referred to as short-lag PM2.5 and (2) PM2.5 during the wildfire season 1–10 months before influenza cases, hereafter referred to as long-lag PM2.5. We then evaluated associations between the delayed effects of PM2.5 for each time frame on influenza counts for counties in Montana for 2010–2018, adjusted for temperature and seasonal trend, in a quasi-Poisson model framework.
2. Methods
2.1. Influenza Data
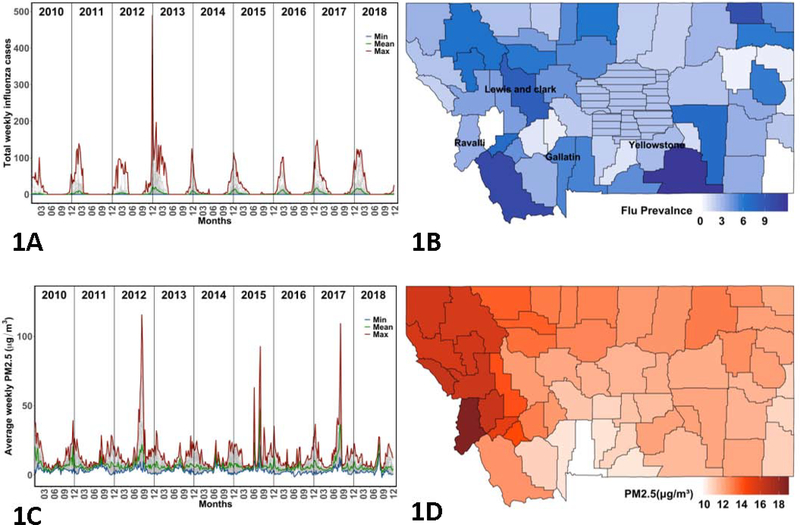
In this time-series analysis, influenza counts in Montana were provided by the Montana Department of Public Health and Human Services. The data used in this study are weekly county-level case counts of positive diagnoses of influenza from all reporting sources, including laboratory confirmations, hospitalizations, and clinical diagnoses. Influenza cases in those of all ages are reported. The Centers for Disease Control do not state the estimated under-reporting of influenza, but do acknowledge that it is largely under-reported (www.cdc.gov/flu/about/burden/how-cdc-estimates.htm). Six small population counties (Musselshell, Petroleum, Judith Basin, Wheatland, Golden Valley, and Fergus) were grouped into what is known as the ‘Central Montana Health District’ (CMHD). The CMHD and all 50 other Montana counties were included in this study for a total of 51 regions which will be referred to as counties for simplicity. In total, the influenza data for Montana produced 51 counties over 8 years or 408 ‘clusters’ of time series. Mean, minimum, and maximum case counts of each week for all counties are shown in Figure 1A. Figure 1B depicts flu incidence per 1,000 in each county for an example flu season period (October 1 2015 – April 30 2016) for each county in Montana.
Figure 1:
(A) Total weekly influenza cases plotted for all Montana counties, 2010–2018. (B) Flu season period prevalence (per 1,000) for each county in Montana, 2015–2016 with the ‘Central Montana Health District’ (CMHD) shown with dash. (C) Average weekly PM2.5 (ug/m3) plotted for each county in Montana, 2010–2018. (D) Average PM2.5 during the wildfire season (July 1 – September 30) for each county in Montana, 2015.
In temperate climates and during the northern latitude summer months (May-August), influenza counts are at their minimum, whereas winter months are the predominant season for infection due to cold temperatures, low humidity, and increased indoor crowding (Finkelman et al. 2007; Cauchemez et al. 2008; Tamerius et al. 2013). We considered two datasets for our modeling purposes. For our first dataset, we excluded flu counts from May 1 – August 30, as these primarily contained either unreported cases or 0 counts. This dataset hereafter referred to as the ‘complete’ dataset (n = 12,474) had an average flu season length of 31 weeks. Our second dataset accounted for the start and end of each flu season, further reducing the zero counts in the flu data. The second dataset hereafter referred to as the ‘reduced’ dataset (n = 6,308) was subset into vectors of flu counts with a single leading zero and trailing zero for each flu season within each year and each county. The reduced data set had an average flu season length of 15.5 weeks.
2.2. PM2.5 Model for Montana
We used daily PM2.5 measurements from air quality monitoring stations, combined with satellite retrievals of aerosol optical thickness from the Moderate Resolution Imaging Spectroradiometer (MODIS) to produce gridded maps of daily PM2.5 concentrations across the state of Montana from 2009 – 2018. Mean daily PM2.5 were retrieved from the EPA (https://www.epa.gov/outdoor-air-quality-data) and used as a response variable in the models. One hundred and seven stations within a domain bounded by −100 - −120 degrees longitude and 42 – 49 degrees latitude were used for model fitting. Counts of daily observations available for each station varied from 1% to 100%, with a mean of 21% and counts of the number of available observations by day ranged from 10 – 57, with a mean of 38. The 1 km MODIS aerosol optical thickness (AOT) product, developed using the MAIAC algorithm (Lyapustrin et al. 2018) was used as predictor. Quality assurance layers provided for each image were used to screen pixels containing snow or clouds, and only the highest quality observations were retained for analysis. Figure A.1 shows the correlation of the AOT data with the PM2.5 EPA station data. Use of remotely sensed products is particularly challenging in Montana, due to image contamination by clouds and snow. More than 90% of data were missing during winter months (Figure A.2). Therefore, satellite AOT data were only used in the models from June-October (but see comparison of models for November-May in Appendix). We infilled missing observations in the June-October AOT data using probabilistic Principal Components Analysis (Stacklies et al. 2007).
We used Bayesian spatial linear models implemented in the ‘spBayes’ package (spLM; Finley et al. 2015) in R version 3.5.3 (R Core Team 2019) to estimate daily PM2.5 concentrations. We fit a unique model for each day, and predicted the fitted model to a 12 km grid. Aerosol optical thickness was used as a predictor in this model along with an exponential covariance model. Uninformative priors were used for the regression parameters, a uniform prior was used for the spatial decay parameter with support for effective ranges between 5% and 90% of the maximum inter-point distance, and uninformative inverse gamma priors were used for the covariance sill and nugget (σ2 and τ2) parameters. For November-May days, we used thin plate spline regression (TPS) in the R package ‘fields’ (Nychka et al., 2017) to interpolate daily PM2.5 observations without any spatial covariates. This decision was made because of the high proportion of missing MODIS data in winter. Here, a spline regression model was fit for each date using the latitude and longitude of each station and predicted the model to a 12 km grid. We evaluated both the spLM and TPS models using a leave-one-out cross validation approach. Here, for every model date each observation was withheld. A model was fit using the remaining observations, and then predicted to the withheld observation. This procedure was repeated for each observation and each date and error statistics were retained for all days. The overall model fit for the summer wildfire season model was reasonably strong, with Mean Absolute Error of 2.79 (μg/m3) and r2 = 0.66 (Figure A.3A). The accuracy of the thin plate spline model, fit without the benefit of MODIS AOT data was not as strong as expected, with Mean Absolute Error of 3.11 (μg/m3) and r2 = 0.37 (Figure A.3B). Daily gridded PM2.5 estimates were combined into weekly grids using the mean, and then extracted for county in Montana also using the mean.
The resulting weekly PM2.5 time series for all counties is given in Figure 1C. For this study, we are primarily interested in evaluating two possible functions of PM2.5 in relation to influenza: (1) a long-lag effect experienced primarily from PM2.5 during wildfire season, and (2) a short-lag effect experienced primarily from biomass smoke exposure (e.g., Ward and Lange, 2010). We tested multiple such functions to express these different kinds of PM2.5 periods and effects as summarized in Table 1. For the long-lag effects, we used the average daily PM2.5 concentration for the months July 1 – September 30 preceding the flu-season, when PM2.5 density spikes due to wildfires, with the intent of estimating average PM2.5 concentrations during wildfire season. An example long-lag PM2.5 for each Montana county in 2015 is shown in Figure 1D. Figure A.4 shows how severe the wildfire seasons were each year in Montana using total area burned. For the short-lag effects, we looked at three different PM2.5 variables: (A) a lag in PM2.5 up to n weeks before current week of influenza, (B) a moving window daily average PM2.5 up to n weeks before current week of influenza, and (C) a daily average PM2.5 concentration over the entirety of flu-season.
Table 1:
Short-lag and long-lag PM2.5 variables considered.
| Variable Name | Description | ||
|---|---|---|---|
| PM2.5 Variables Tested | Long-lag Effects | Daily average PM2.5 during wildfire season | Total PM2.5 from months preceding flu-season (July 1 – September 30) divided by wildfire season total days (91 days) |
| Short-lag Effects | (A) n week lag (n = 1,2,3,4) | Lag PM2.5 up to n weeks before current week of influenza | |
| (B) n week moving window sum (n = 2,3,4) | Total PM2.5 up to n weeks before current week of influenza divided by n weeks | ||
| (C) Daily average PM2.5 during flu season | Total PM2.5 over entirety of flu-season (October 1 – April 30) divided by flu-season total days |
2.3. Statistical Analysis
The associations between weekly counts of influenza cases and the different PM2.5 effects were examined using the following quasi-Poisson regression model weighted by county population for all counties simultaneously:
| [1] |
where t is the week index, t = 1, 2, …, 435 weeks from 2010-Jan-03 to 2018-May-31, excluding weeks outside of the flu season, k is the county index, k = 1, 2, …, 51 counties as earlier defined, μt,k is the expected influenza count at time t in county k, assuming μt,k ~ Exponential Family(θ), Populationt,k is the population in county k in week t, entering the model as an offset allowing an influenza rate response, Temperaturet-1,k is the maximum daily temperature (in deg. Celsius) extracted using 250 m resolution gridded temperature data (Holden et al., 2018) in county k in week t-1, LongLagPM2.5t,k is the long-lag PM2.5 daily average from the previous wildfire season as described in the previous section for county k relative to week t, ShortLagPM2.5t,k is the short-lag PM2.5 effect as described in Table 1 for county k relative to week t, Fi(t,k) is the ith Fourier seasonal term (i = 1,2,3 for Sine and i = 4,5,6 for Cosine) for county k in week t, and Countyk is a county indicator (1 if county k and 0 otherwise). Notice that the first seven terms in the model (parameterized by β1, β 2, …, β 7) are all variables regularly associated with influenza dynamics (e.g., Imai et al., 2015).
A generalized estimating equation (GEE) was used to estimate the model parameters for this quasi-Poisson generalized linear model to address any residual temporal autocorrelation and uncertainty in the covariance structure of the flu counts. An autoregressive (AR(1)) covariance structure is assumed for the GEE to account for the weekly dependence in flu counts within each county-year cluster (51 counties x 8 years = 408 county-year clusters). The model for influenza rate (specified as influenza count with population as an offset) given in equation (1) was applied simultaneously to all counties in Montana and for the two datasets described in the previous section (complete and reduced), and basic statistical inference performed on the coefficients using Huber-White robust standard error estimates to account for uncertainty in the quasi-Poisson correlation structure. All analyses were performed using the geem and glm function in R (version 3.5.3; R Development Core Team) with a quasi-Poisson family to account for overdispersion, and each case weighted by the county population.
3. Results
3.1. Long-lag PM2.5 impacts on influenza
Average daily long-lag PM2.5 concentration (averaged over the period July 1 – September 30 during the previous wildfire season) was positively associated with increased influenza rate for both the complete and reduced datasets (p = 0.008 and p = 0.042, respectively) as shown in the model summary provided in Table 2. The estimated model coefficients are 0.1995 (SE = 0.0752) and 0.1479 (SE = 0.0724) for the complete and reduced datasets, respectively. For the complete dataset, we expect influenza incidence to increase by a factor of exp(0.1995) = 1.22 per 1 μg/m3 elevation in average daily wildfire season PM2.5 exposure (95% CI: (1.05, 1.41)). For the reduced dataset, we expect influenza incidence to increase by a factor of exp(0.1470) = 1.16 per 1 μg/m3 elevation in average daily wildfire season PM2.5 exposure (95% CI: (1.01, 1.33)).
Table 2:
Model summary for each variable and dataset.
| Complete Dataset (n = 12,474) | Reduced Dataset (n = 6,308) | ||||||
|---|---|---|---|---|---|---|---|
| Term | Estimate | Robust SE | pval | Term | Estimate | Robust SE | pval |
| Sine, Cosine | Varies | Varies | < 10−5 | Sine, Cosine | Varies | Varies | < 10−5 |
| Temperature Lag | −0.0014 | 0.0030 | 0.650 | Temperature Lag | 0.0001 | 0.0030 | 0.979 |
| Daily Long-Lag PM2.5 | 0.1995 | 0.0752 | 0.008 | Daily Long-Lag PM2.5 | 0.1470 | 0.0724 | 0.042 |
| Daily Short Lag PM2.5 | −0.0459 | 0.0464 | 0.323 | Daily Short Lag PM2.5 | −0.0407 | 0.0483 | 0.399 |
3.2. Short-lag PM2.5 impacts on influenza
Moving window daily average short-lag PM2.5 two weeks prior to the current week showed no association with influenza rate for the complete dataset (p = 0.323) and the reduced dataset (p = 0.399) (Table 2). Regardless of short-lag method chosen for the model (Table 1), no association was observed. Individual county-specific models indicated a positive association in 13–23 of the 51 counties, depending on which short-lag PM2.5 variable was used, but no overall effects were observed (Table A.1; Figures A.5–A.6).
3.3. Temperature impacts on influenza
The average maximum temperature (degrees Celsius) in the previous week showed no association with influenza rate for the complete dataset (p = 0.650) and the reduced dataset (p = 0.979) (Table 2). Individual county-specific models indicated that 39 out of 51 Montana counties show a negative albeit insignificant relationship between temperature and influenza counts (Figure A.7), the direction of which is consistent with all previous literature on this topic (e.g., Tamerius et al. 2013).
3.4. Residual Autocorrelation
There was strong evidence of temporal autocorrelation within the county-year clusters in the influenza count model residuals (Breusch-Godfrey test, p = 0.0004). To address this residual autocorrelation, robust Huber-White standard errors under an AR(1) covariance structure were used in the GEE modeling framework to assess the significance of model predictors.
4. Discussion
We found that higher average PM2.5 concentrations during the wildfire season positively associated with increased influenza in Montana counties in the following winter flu season. Individual county-specific models further support this result, showing long-lag PM2.5 positively associating with wintertime influenza in 50 out 51 counties (Table A.1; Figure A.5–A.7). Although there are studies that report the correlation between short-lag exposure of PM2.5 and influenza cases (Liang et al., 2014; Feng et al., 2016; Horne et al., 2018), our study suggests one of the longest lag associations observed for communities impacted by wildfires. Wildfire season for Montana (and much of the intermountain West) occurs between July – September with corresponding peak levels of PM2.5. Flu season spans October – April, with peak flu cases typically occurring in January. Thus, average daily PM2.5 concentrations during wildfire season months was observed to be positively associated with flu 1–10 months later, even after accounting for seasonal, temperature, and autocorrelative factors.
Past studies modeling the effects of PM2.5 during wildfire episodes on respiratory outcomes have typically looked at lagged associations of less than 5 days (Liu et al., 2015; DeFlorio et al. 2019). However, a recent study showed consistently increased odds of healthcare encounter for influenza for elevated PM2.5 exposure estimates averaged across several lag periods, 0–28 days (Horne et al. 2018). We also included a short-lag variable in our model using different methods to account for the effect of PM2.5 immediately preceding influenza rates (1 – 4 weeks; Table 1). Surprisingly, these results were less consistent than the long-lag PM2.5 variable, and we found little support for a short-lag PM2.5 effect on influenza. Individual county-specific models run with each different short-lag PM2.5 variable in Table 1 are compared in the Appendix (Table A.1; Figures A.5–A.7) and further corroborate this finding. Depending on which PM2.5 short-lag variable used, 13–23 of the 51 counties indicated a positive association with short-lag PM2.5 and influenza counts with at most 7 counties being significant (p < 0.05). The inability to separate out the various contributing factors to short-lag PM2.5 in the winter months (i.e., woodsmoke, other industrial pollutants) could be one reason our model was not able to find a short-lag PM2.5 association with flu. Several studies have evaluated specific PM components and cardio-respiratory outcomes, but findings have been inconsistent in linking isolated PM factors or sources to specific outcomes (Stanek et al., 2011). To our knowledge no such studies have evaluated PM component or PM source with respect to influenza, and this would be a potential area for further exploration.
Although our modeling was able to partially explain effects of long-lag PM2.5 concentrations on Montana’s counties, finer scale data could help reveal more spatially resolved details. Our modeling used PM2.5 maps produced at relatively coarse (12 km) spatial resolutions and aggregated to the county level. Future research should explore variation at finer resolution. Of particular concern in western rural states is the scarcity of air quality monitoring stations, which provide the data needed to deliver accurate respiratory health warnings and predictions to the public, as well as to provide the data to better understand the role air pollution has on respiratory diseases. In the intermountain west, the sparsity of air quality monitoring stations is further complicated by the region’s complex terrain which likely contributes to significant heterogeneity in air pollution levels across communities (Armstrong, 1998). Furthermore, it is likely that during the wildfire season inversions and drainage flows may lead to highly variable smoke exposure. Many areas of Montana and the intermountain west in the wintertime experience an increased risk of poor air quality due to cold-air inversions, trapping air pollutants in mountain valleys where most towns and residents are located (Ward and Lange 2010; Holden et al., 2011). Air quality monitoring stations are often located to represent worst-case exposures for the largest concentration of people or sited to capture background exposure. For example, in 2018 there were 19 sites in the Montana network that monitored PM2.5 (13 = Population Exposure, 5 = Background Exposure, and 1 = Source Impact; https://www3.epa.gov/ttnamti1/files/ambient/pm25/qa/vol2sec06.pdf). Regardless, it is unlikely that the single air monitor sites in many Montana communities provide an accurate representation of pollution exposure and could be missing much of the spatial patterning in PM2.5, as suggested by other urban area focused studies (Tunno et al. 2016). Thus, improved spatially resolved maps of PM2.5 would enhance understanding of particulate matter impacts on public health during both the winter and wildfire season. Such maps would also provide the spatial and topographically resolved data needed to identify fine scale PM2.5 effects on specific respiratory diseases, such as influenza.
There are several potential factors that are relevant to influenza risk that were not addressed in our study. For example, previous studies included sociodemographic factors and one study from Australia found that wealthier communities with lower levels of unemployment experienced greater flu activity than those less advantaged areas (Huang et al., 2017). Other studies have found that school calendars may play a role in influenza outbreaks, suggesting that closing schools could be effective in limiting the spread of influenza outbreaks (Chu et al., 2017), also contributing to the hypotheses on indoor crowding and increased person-to-person contact (Cauchemez et al., 2008). Furthermore, our model did not include vaccination rates (e.g., Baselga-Moreno et al., 2019), influenza strain, distance to airports (e.g., Hooten et al., 2010), and possible important determinants influenza in for rural states, such as healthcare access, or any other sociodemographic or economic variables, all of which could influence influenza transmission. Future studies could explore the interactions of virus-specific, climate, sociodemographic, and PM2.5 variables. Finally, we note that our models did not explicitly account for uncertainty in the particulate matter model. This uncertainty was higher in winter months, where satellite data were unavailable, and may have contributed to the lack of any relationship between short-lag PM exposure and influenza.
While our study supports a link between long-lag PM2.5 during wildfire season and wintertime influenza, the mechanisms underlying this relationship are complex, and beyond the scope of this study. Future work using in vivo and in vitro studies could be conducted to explore these underlying mechanisms for either viral etiology and/or host susceptibility. For example, some animal studies have looked at wood smoke particles’ sustained immune suppression effect (Samuelson et al, 2009; Migliaccio et al, 2013), showing this type of PM having a 24 hour sustained effect on respiratory bacterial infections. Other animal models suggest that the role of lagged PM exposure on influenza risk could occur via diminished capacity of pulmonary macrophages to secrete IL-6 and IFN-β (Ma et al. 2017). Ma et al. 2017 provide support for a 2-week exposure to outdoor PM2.5 from Shanghai, China, leading to decreased resistance to influenza via altered immune responses.
Predicting influenza outbreaks based on climatic and environmental factors, such as PM2.5, may be important for both short- and long-term public health planning. In the short-term, models may help predict outbreaks days to weeks in advance, giving public health officials an opportunity to target prevention messages and vaccine efforts. In the long-term, models linking climatic or environmental variables to influenza outbreaks may provide a picture for what populations can expect with ongoing climate change or extreme seasonal conditions. For example, Ford et al. (2018) projected change in PM2.5 based on prognostic land-fire models for the continental US with the worst areas in Montana forecasted to have a 5 μg/m3 increase in the annual average per decade. Moreover, identifying the predictors of influenza, such as long-lag PM2.5 effects, and improving upon the predictive models for influenza, will be important for the population health, as influenza is associated with approximately 900 hospitalizations and 60 deaths in Montana each year (www.cdc.gov/flu/about/burden/index.htm).
Supplementary Material
Highlights.
Prior studies of wildfire smoke have focused on acute cardiopulmonary health effects.
Novel analysis of wildfire season particulate matter and influenza occurrence months later.
Air pollution during wildfire seasons associated with influenza months later.
Air pollution during winter period not associated with influenza days later.
Acknowledgements
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health [Award Number P20GM103474]. MODIS Aerosol optical thickness and gridded PM2.5 data generated for this study is available at https://topofire.dbs.umt.edu/helmsdeep1/public_data/air_quality.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abatzoglou JT, Williams AP. 2016. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci 113(11770)LP–11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetona O, Reinhardt TE, Domitrovich J, Broyles G, Adetona AM, Kleinman MT, Ottmar RD, Naeher LP. 2016. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhal Toxicl 28(3):95–139. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. 2000. What constitutes an adverse health effect of air pollution? Official statement of the American Thoracic Society. Am J Respir Crit Care Med 161: 665–673. [DOI] [PubMed] [Google Scholar]
- Anderson JO, Thundiyil JG, Stolbach A. 2012. Clearing the air: a review of the effects of particulate matter air pollution on human health, J. Med. Toxicol 8:166e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BG 1998. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occupational and Environmental Medicine, 55(10), 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG, Forsberg B, Annesi-Maesano I, et al. 2009. Climate change and respiratory disease: European Respiratory Society position statement. Eur Respir J 34: 295–302. [DOI] [PubMed] [Google Scholar]
- Baselga-Moreno V, Trushakova S, McNeil S, Sominina A, Nunes MC, et al. 2019. Influenza epidemiology and influenza vaccine effectiveness during the 2016–2017 season in the Global Influenza Hospital Surveillance Network (GIHSN). BMC Public Health 19: 487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey SJ, Ruminski M, Atwood SA and Fischer EV. 2018. Connecting smoke plumes to sources using Hazard Mapping System (HMS) smoke and fire location data over North America, Atmos. Chem. Phys, 18(3), 1745–1761, doi: 10.5194/acp-18-1745-2018. [DOI] [Google Scholar]
- Cauchemez S, Valleron AJ, Boelle PY, Flahault A, Ferguson NM. 2008. Estimating the impact of school closure on influenza transmission from sentinel data. Nature 452:750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- Chu Y, Wu Z, Ji J, Sun J, Sun X, Qin G, Qin J, Xiao Z, Ren J, Qin D, Zheng X, Wang X. 2017. Effects of school breaks on influenza-like illness incidence in a temperate Chinese region: an ecological study from 2008 to 2015. BMJ 7; e013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraway JJ. 2016. Extending the Linear Model with R: Generalized Linear, Mixed Effects, and Nonparametric Regression Models, CRC Press, 2nd ed. [Google Scholar]
- Feng C, Li J, Sun W, Zhang Y, Wang Q. 2016. Impact of ambient fine particulate matter (PM2.5) exposure on the risk of influenza-like-illness: a time-series analysis in Beijing, China. Environmental Health 15:17: DOI 10.1186/s12940-016-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. 2007. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: Viral coexistence and latidudinal gradients. PLoS ONE 12:e1296 www.plosone.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley AO, Banerjee S, Gelfand A. 2015. spBayes for Large Univariate and Multivariate Point-Referenced Spatio-Temporal Data Models. Journal of Statistical Software 63(13):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B, Val Martin M, Zelasky SE, Fischer EV, Anenberg SC, Heald CL, Pierce JR. 2018. Future fire impacts on smoke concentrations, visibility, and health in the contiguous United States. GeoHealth. 2018;2: 10.1029/2018GH000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten MB, Anderson J, Waller LA. 2010. Assessing North American influenza dynamics with a statistical SIRS model. Spatial and Spatio-temporal Epidemiology 1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden ZA, Abatzoglou JT, Luce CH, Baggett LS. 2011. Empirical downscaling of daily minimum air temperature at very fine resolutions in complex terrain. Agricultural and Forest Meteorology 151:1066–1073. [Google Scholar]
- Huang X, Mengersen K, Milinovich G, Hu W. 2017. Effect of weather variability on seasonal influenza among different age groups in Queensland, Australia: A Bayesian spatiotemporal analysis. JID 215(11):1695–701. [DOI] [PubMed] [Google Scholar]
- Kim K-H, Kabir E, Kabir S. 2015. A review on the human health impact of airborne particulate matter. Environ Int 74:136–143, 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Liang Y, Fang L, Pan H, Zhang K, Kan H, Brook JR, Sun Q. 2014. PM2.5 in Beijing – temporal pattern and its association with influenza. Environmental Health 13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Mickley LJ, Sulprizio MP, Dominici F, Yue X, Ebisu K, Anderson GB, Khan RFA, Bravo MA, Bell ML. 2016. Particulate air pollution from wildfires in the Western US under climate change, Clim. Change, 138(3–4), 655–666, doi: 10.1007/s10584-016-1762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML. 2015. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res 136:120–132, 10.1016/j.envres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Wilson A, Mickley LJ, Dominici F, Ebisu K, Wang Y, et al. 2017. Wildfire-specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology 28(1):77–85, 10.1097/EDE.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen AC, Mubareka S, Steel J, Palese P. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens 3(10):e151 www.plospathogens.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen AC, Steel J. 2014. Roles of humidity and temperature in shaping influenza seasonality. Journal of Virology 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JC, Pereira G, Uhl SA, Bravo MA, Bell ML. 2015. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res. 0:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapustrin A, Wang Y, Korkin S, Huang D. 2018. MODIS Colliection 6 MAIAC algorithm. Atmospheric Measurement Techniques 11:5741–5765. [Google Scholar]
- Ma J-H, Song S-H, Guo M, Zuou J, Liu F, Peng L, Fu Z-R. 2017. Long-term exposure to PM2.5 lowers influenza virus resistnace via down-regulating pulmonary macrophage Kdm6a and mediates histones modificationin IL-6 and IFN-B promoter regions. Biochemical and Biophysical Research Communications 493:1122–1128. [DOI] [PubMed] [Google Scholar]
- McClure CD, Jaffe DA. 2018. US particulate matter air quality improves except in wildfire-prone areas. PNAS. 2018;www.pnas.org/cgi/doi/10.1073/pnas.1804353115S [DOI] [PMC free article] [PubMed]
- Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. 2013. Adverse effects of wood smoke PM(2.5) exposure on macrophage functions. Inhal Toxicol. 25(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NM et al. 2007. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 25(27):5086–5096 [DOI] [PubMed] [Google Scholar]
- Nychka D, Furrer R, Paige J, Sain S. 2017. Fields: Tools for spatial data R package version 9.8–6. University Corporation for Atmospheric Research, doi: 10.5065/D6W957CT. [DOI] [Google Scholar]
- Noonan C, Ward T, Navidi W, Sheppard L, Bergauff M, Palmer C. 2011. Assessing the impact of a wood stove replacement program on air quality and children’s health Research Report 162 Vol Boston, MA: Health Effects Institute; 2011. [PubMed] [Google Scholar]
- O’Dell K, et al. 2019. The contribution of wildland-fire smoke to US PM2.5 and its influence on recent trends, Environmental Science & Technology, 53, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Rom WN, Akpinar-Elci M, et al. 2012. An official American Thoracic Society workshop report: climate change and human health. Proc Am Thorac Soc 9: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Po JY, FitzGerald JM, Carlsten C. 2011. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 66(3):232–239. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reid CE, Brauer B, Johnston FH, Jerrett M, Balmes JR, Elliott CT. 2016. Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 124(9):1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson M, Cecilie Nygaard U, Lovik M. 2009. Particles from wood smoke and road traffic differently affect the innate immune system of the lung. Inhal Toxicol. 21(11):943–951. [DOI] [PubMed] [Google Scholar]
- Shaman J, Kandula S, Uang W, Karspeck A. 2017. The use of ambient humidity conditions to improve influenza forecast. PLoS Comput Biol. 13(11):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacklies W, Redestig H, Scholz M, Walther D, Selbig J. 2007. PCA Methods: a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23(9):1164–1167. [DOI] [PubMed] [Google Scholar]
- Stanek LW, Sacks JD, Dutton SJ, Dubois JJB. 2011. Attributing health effects to apportioned components and sources of particulate matter: An evaluation of collective results. Atmos Environ 45:5655–5663. [Google Scholar]
- Tamerius JD, Shaman J, Alonso WJ, Bloom-Feshbach K, Uejio CK, Comrie A, Viboud C. 2013. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLos Pathogens 9(3); 1–12. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunno BJ, Dalton R, Michanowicz DR, Chmool JLC, Kinnee E, Tripathy S, Cambal L, Clougherty JE. 2016. Spatial patterning in PM2.5 constituents under an inversion-focused sampling design across an urban area of complex terrain. Journal of Exposure Science and Environmental Epidemiology 26:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski SP, Reeves MC, Corley RE, Silverstein RP, Hao WM. 2018. Contiguous United States wildland fire emission estimates during 2003–2015. Earth Syst. Sci. Data, 10(4): 2241–2274, doi: 10.5194/essd-10-2241-2018. [DOI] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2009. Integrated Science Assessment (ISA) For Particulate Matter (Final Report). EPA/600/R-08/139F.Washington, DC:U.S. EPA. [Google Scholar]
- Ward T, Lange T. 2010. The impact of wood smoke on ambient PM2.5 in northern Rocky Mountain valley communities. Environ Pollut. 158:723–729. [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, Schirm AL, Lazar NA. 2019. Moving to a world beyond “p<0.05”. The American Statistician 73:1–19. [Google Scholar]
- Westerling AL, Hidalgo AH, Cayan DR, Swetnam TW. 2006. Warming and earlier spring increase western US forest wildfire activity. Science 313:940–943. [DOI] [PubMed] [Google Scholar]
- Yue X, et al. 2013. Ensemble projections of wildfire activity and carbonaceous aerosol concentrations over the western United States in the mid-21st century. Atmospheric Environment,77, 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.