Abstract
Hypothesis:
Outer sulcus cell features and distribution are hypothesized to differ throughout regions of the human cochlea and between diseased and normal specimens.
Background:
Outer sulcus cells play a role in inner ear fluid homeostasis. However, their anatomy and distribution in the human are not well described.
Methods:
Temporal bone specimens with normal hearing (n = 10), Menière’s disease (n = 10), presbycusis with flat audiograms (n = 4), and presbycusis with sloping audiograms (n = 5) were examined by light microscopy. Outer sulcus cells were assessed quantitatively and qualitatively in each cochlear turn. One specimen was stained for tubulin immunofluorescence and imaged using confocal microscopy.
Results:
Outer sulcus cells interface with endolymph throughout the cochlea, with greatest contact in the apical turn. Mean outer sulcus cell counts in the upper apical turn(8.82) were generally smaller (all p < 0.05) than those of the upper basal (17.71), lower middle (18.99) upper middle(18.23), and lower apical (16.42) turns. Mean outer sulcus cell counts were higher (p < 0.05) in normal controls (20.1) than in diseased specimens (15.29). There was a significant correlation between mean cell counts and tonotopically expected hearing thresholds in the upper basal (r = −0.662, p = 0.0001), lower middle (r = −0.565, p = 0.0017), and upper middle (r = −0.507, p = 0.0136) regions. Other differences in cell morphology, distribution, or relationship with Claudius cells were not appreciated between normal and diseased specimens. Menière’s specimens had no apparent unique features in the cochlear apex. Immunofluorescence staining demonstrated outer sulcus cells extending into the spiral ligament in bundles forming tapering processes which differed between the cochlear turns in morphology.
Conclusion:
Outer sulcus cells vary throughout the cochlear turns and correlate with hearing status, but not in a manner specific to the underlying diagnoses of Menière’s disease or presbycusis.
Keywords: Cochlear outer sulcus cells, Menière’s disease, Otopathology, Presbycusis
The outer sulcus region of the cochlear duct is located on the lateral wall of the scala media between the organ of Corti and the spiral prominence. This area is populated by two main cell types, the Claudius cell and the outer sulcus cell. These outer sulcus cells were first described by Deiters (1) in 1860 based on their location and have also been called external sulcus cells. Boettcher (2) referred to the outer sulcus cells as root cells due to their numerous tentacle-like processes extending into the underlying spiral ligament, and this term is still frequently encountered. Outer sulcus cells can be distinguished from the adjacent Claudius cells by deeper hematoxylin and eosin staining and a characteristic appearance.
The outer sulcus cells have been proposed to play an important role in cochlear fluid homeostasis. The Hansen’s, Claudius, and outer sulcus cells are collectively coupled by gap junctions forming a component of the network involved in potassium ion recycling and homeostasis of the endolymph (3). As such, these cells have a unique bridging anatomic relationship between the perilymph and endolymph-containing compartments, a characteristic that differs throughout the turns of the cochlea. In animals, the outer sulcus cells in the apex of the cochlea are known to be exposed to the endolymph, but not in the basal region where they are covered by a layer of Claudius cells (4–6). Galić and Giebel (7) proposed that outer sulcus cells have varying metabolic profiles, which are suggested by two different appearing subtypes: “dark” outer sulcus cells with high metabolic activity and “light” outer sulcus cells with low metabolic activity. Furthermore, they noted the presence of clefts between outer sulcus cells and hypothesized that these are part a pump system that is responsive to high sound pressures by prompting the outflow of secretions through these clefts into the endolymphatic space.
More recently, the outer sulcus cells of the cochlear apex have been the subject of several studies due to their unique complimentary expression of the membrane-bound water channel proteins known as aquaporins (4–6). Specifically, aquaporin 5 has been shown to be expressed at the apex of the cell at the endolymph interface, while aquaporin 4 is expressed at the opposite pole of the cell at the basolateral membrane where perilymph is interfaced at the spiral ligament. Expression of aquaporin 5 seems to only be present in the cochlear apex where the outer sulcus cell endolymph interface exists. This unique anatomic relationship, aquaporin expression pattern, as well as osmotic water permeability coefficient measurements has led to the hypothesis that the outer sulcus cells in the cochlear apex may constitute a unique apparatus that acts as a “water shunt” within the endolymph–perilymph barrier (4–6).
Given the classic low-frequency sensorineural hearing loss pattern tonotopically correlated with the cochlear apex, which is known to occur in symptomatic cases of endolymphatic hydrops, and also the histologic and radiologic findings of early-stage hydropic ear disease occurring focally at the cochlear apex, a possible pathophysiologic role of this outer sulcus cell shunt in endolymphatic hydrops has been proposed (4–6,8). Considering this potential role of the outer sulcus cells in normal and pathologic cochlear function, a detailed accounting of their anatomic features and distribution throughout the human cochlea is desirable. To date, the overwhelming volume of data available relating to outer sulcus cells is derived from animal studies, mostly involving rodents (9–11) and a few in pigs (12,13), cats(10), and monkeys (10,14,15), while anatomic data regarding structure and function from human specimens is sparse (6). As such, this study sought to investigate the microscopic anatomy and distribution of the outer sulcus cells in human cochleae, with particular attention given to variations between the cochlear turns. Furthermore, given the potential role of outer sulcus cells in the pathophysiology of endolymphatic hydrops, we also sought to investigate whether outer sulcus cells differed between the normal cochlea versus those from individuals affected by Menière’s disease or presbycusis.
METHODS
Specimens were acquired and studied under Institutional Review Board approval at both participating institutions. Light microscopic examination of temporal bone specimens was undertaken, wherein specimens were selected from a large institutional collection that had been previously processed according to standardized published methodology (16,17). Ten bones from individuals with normal hearing and no history of ear disease were included. Furthermore, 19 abnormal temporal bones with the following characteristics were also included: 4 temporal bones with flat audiometric patterns of sensorineural hearing loss attributed to presbycusis but with no other known ear disease, 5 temporal bones with downward sloping audiometric patterns of sensorineural hearing loss attributed to presbycusis but with no other known ear disease, and 10 temporal bones from subjects with a classic clinical history of Menière’s disease and associated histopathologic findings consistent with endolymphatic hydrops in the affected ears. Associated premortem audiometry for each specimen had been performed within a few months before death.
Hematoxylin and eosin stained sections were evaluated using an Olympus (Center Valley, PA) light microscope at ×40 magnification. With the aid of an ocular grid, outer sulcus cell nuclei were counted in each cochlear turn in three mid-modiolar sections by a single investigator (F.B.C.). For each turn, the mean outer sulcus cell count from the three sections was calculated and used for data analysis. The relationship between the location of Claudius cells and outer sulcus cells in the outer sulcus region was noted for each cochlear turn. The criteria for distinguishing outer sulcus cells from adjacent Claudius and spiral prominence cells were based on cell shape, intensity of cytoplasmic staining, and nuclei characteristics such as location, shape, and the chro-matin staining pattern (9,18). Pairwise comparisons of outer sulcus cell mean counts at various cochlear turns between the four temporal bone types were performed using the Wilcoxon rank sum test. When possible, based on available audiologic data, correlation analysis between outer sulcus cell counts and expected associated tonotopic audiometric thresholds for each cochlear turn was undertaken based on an anatomical frequency scale (16,19) using the Spearman rank-order correlation. The tonotopic anatomical frequency scale assignments were as follows: upper basal = 2 kHz, 4 kHz, and 8 kHz; lower middle = 0.5 kHz and 1 kHz; upper middle = 0.25 kHz. Data was analyzed with using Stata Version 15 (StataCorp., College Station, TX). A result was considered statistically significant for p < 0.05.
Immunofluorescence in Celloidin Embedded Tissue Sections
Celloidin embedded sections from one temporal bone, a 50-year-old male with normal hearing, were stained with mouse monoclonal antibodies against acetylated tubulin, which is a cytoskeleton protein involved with en docytosis and intracellular transport. Detailed methods for celloidin removal and antigen retrieval have been described (20). Tissue sections were incubated at room temperature for 1 hour with a solution containing 1% bovine serum albumin fraction-V (Sigma, St. Louis, MO) and 0.5% Triton X-100 (Sigma) in PBS. At the end of the incubation, the blocking solution was removed and the primary monoclonal antibodies against acetylated tubulin (Sigma, Cat # T7452) were applied (1:1,000 in blocking solution). This monoclonal antibody recognizes an epitope located on the alpha3 isoform of Chlamydomonas and recognizes human and mouse tissue among other species. The diluted antibody was incubated 48 hours at 4°C in a humidity chamber. The secondary antibodies against mouse labeled with Alexa 594–red color- (1:1,000, Molecular Probes, Carlsbad, CA) were applied and incubated for 2 hours at room temperature in the dark. At the end of the incubation, sections were washed with PBS (3 × 10 min) and covered with Vectashield mounting media containing DAPI (Vector Labs, Burlingame, CA) to visualize all cell nuclei.
Controls
Mouse cochlea cryostat sections were used as positive controls. These sections were subjected to the same protocol as the immunofluorescence protocol of human cochlea sections. As negative controls, the primary antibody was omitted or pre-absorbed with the antigen as described previously (20) and the immunoreaction was performed as described above. No immunoreaction was detected in both types of negative controls.
Confocal Imaging
Digital laser confocal fluorescent images were obtained using a Leica (SP8) light sheet high-resolution microscope. Images were taken individually of acetylated tubulin (red channel), and the DAPI nuclei stain (Blue channel) for the same tissue cross-section at ×400 magnification. These images were then combined (merged) into a single image using computer software ImageJ to visualize the acetylated tubulin and DAPI simultaneously. Images were collected at the basal, middle, and apical portion of the cochlea. For each region a series of 30 digital images (0.5 μm thick) were collected for each channel and individual stacks were created. Final images were prepared using the Adobe Photoshop software program run on a Dell OptiPlex 3020 computer.
RESULTS
Qualitative Assessment
The outer sulcus region of the cochlear duct extends from the Hensen’s cells at the distal lateral margin of the organ of Corti to the spiral prominence and was noted to be populated by both Claudius cells and the outer sulcus cells. The Claudius cells, which are the more abundant subtype of the two, appeared as a single-cell layer that rested on the basilar membrane and the adjacent spiral ligament with their apices in contact with the endolymph of the scala media (Fig. 1). Claudius cells had a columnar morphology with a height that gradually diminished from the basal turn of the cochlea to the apex and their nuclei were located in close proximity to the base of the cell. Few cytoplasmic organelles were observed to be present within Claudius cells, resulting in a relatively clear appearance with light microscopy.
FIG. 1.
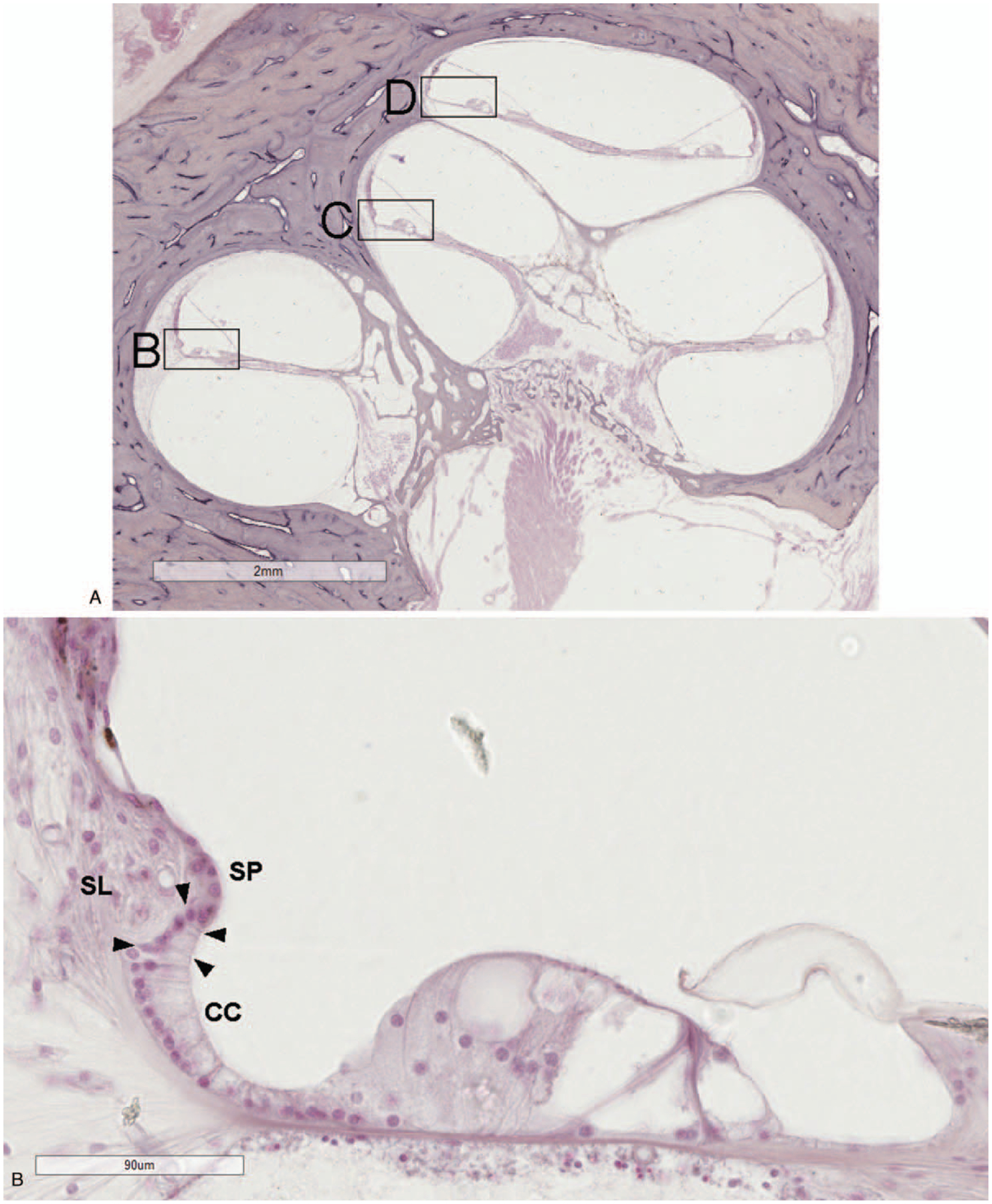
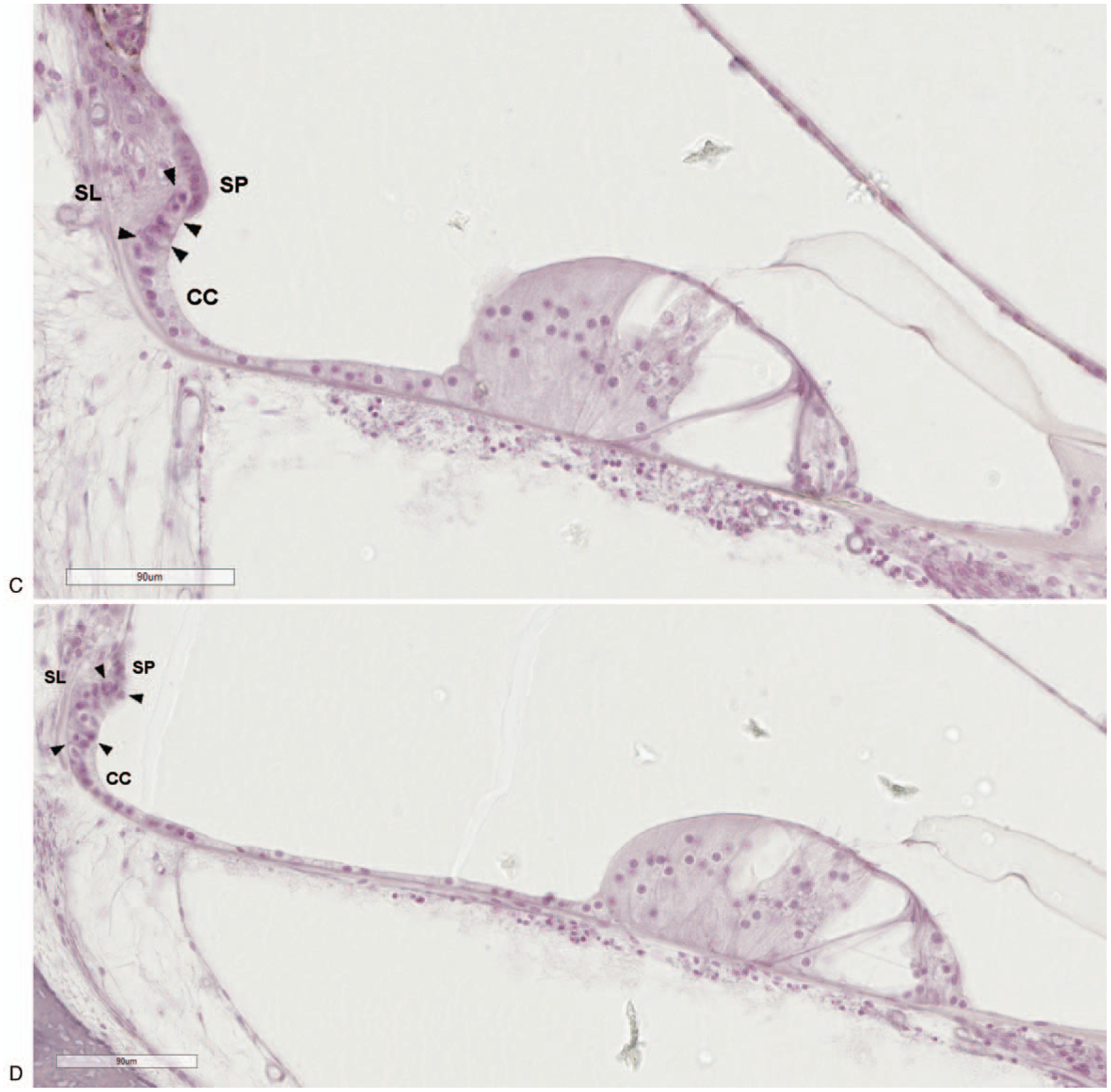
A, Light microscopy image of the human cochlea with boxes marking areas of high magnification. The outer sulcus region in the upper basal (B), upper middle (C), and upper apical (D) turns. The outer sulcus cells (arrowheads) are located between the last of the Claudius cells (CC) and the spiral prominence (SP). Their root-like processes extend into the adjacent spiral ligament (SL). (hematoxylin and eosin).
The outer sulcus cells were found between the last of the Claudius cells and the margin of the spiral prominence. In the basal turn of the cochlea, outer sulcus cells were generally found to be partially covered by Claudius cells, which limited their direct contact with the endolymph (Fig. 1). However, unlike the rodent cochlea, in which the outer sulcus cells are completely covered by the Claudius cells in the basal turn, the Claudius cell coverage in human basal cochlea was usually incomplete so that basal outer sulcus cells still maintained some contact with the endolymph (6,21).
In the basal turn, the outer sulcus cells are cuboid or columnar, whereas in the middle and upper turns they have a polygonal or round shape. The ovoid nuclei of the outer sulcus cells were typically visualized at one or two levels near the cell base throughout the basal turn, while appearing more clustered at multiple levels in the middle and upper turns. With hematoxylin and eosin staining, the cytoplasm was mildly granular and the unique root like extensions of these cells into the spiral ligament were at times difficult to visualize clearly. No differences in outer sulcus cell distribution, relationship with Claudius cells, or gross morphology were appreciated between cochleae from normal specimens versus cochleae from specimens with presbycusis or Menière’s disease. Nor were there obvious gross differences in these characteristics among Menière’s disease specimens based on severity of endolymphatic hydrops.
Immunofluorescence Staining of Outer Sulcus Cells Using Acetylated Tubulin
Acetylated tubulin immunofluorescence (AT-IF) was observed in the human cochlea in several cell types, among them the outer sulcus cells, the pillar cells in the organ of Corti, and spiral ganglia neurons. AT-IF confocal images (Fig. 2) allowed visualization of the outer sulcus cell cytoarchitecture including a delineation of the fusiform morphology and 3D organization, which was not fully apparent by light microscopy examination (Fig. 2—supplemental digital content with video of confocal stacks, http://links.lww.com/MAO/A910). In all three cochlear turns, AT-IF filaments were observed within the cytoplasm, running from the luminal portion to the basal portion of the outer sulcus cells. Filaments in outer sulcus cells adjacent to the spiral prominence were denser than those adjacent to the Claudius cells. In addition, the presence of filaments appeared to be progressively greater from the basal to the apical turn possibly suggesting greater transport activity apically. Outer sulcus cells extended into the spiral ligament and adjacent to the spiral prominence in bundles forming tapering phalangeal processes. These bundles contained a greater number of cells and extended more deeply into the spiral ligament in the basal turn. In the middle and apical turns each process contained fewer cells, was shorter in length, and projected in multiple directions. An idealized rendering of the observations made by both light and confocal microscopy is depicted in Figure 3.
FIG. 2.
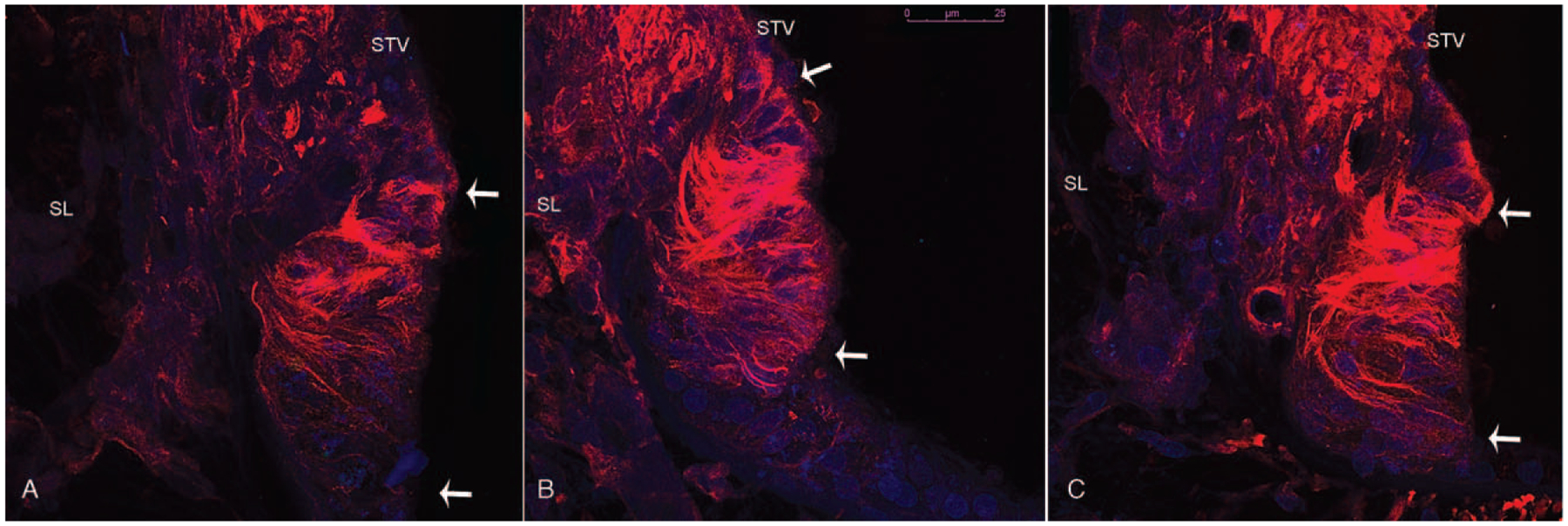
Acetylated tubulin immunofluorescence (red) in the outer sulcus cells of the basal (A), middle (B), and apical (C) turns of the human cochlea. White arrows mark the outer sulcus cell region adjacent to the stria vascularis (STV) (specifically the spiral prominence) and the spiral ligament (SL). The outer sulcus cell filaments run from the luminal portion to the basal portion of the cells and are progressively more prominent from the base to the apex of the cochlea. Scale bar—25 um.
FIG. 3.
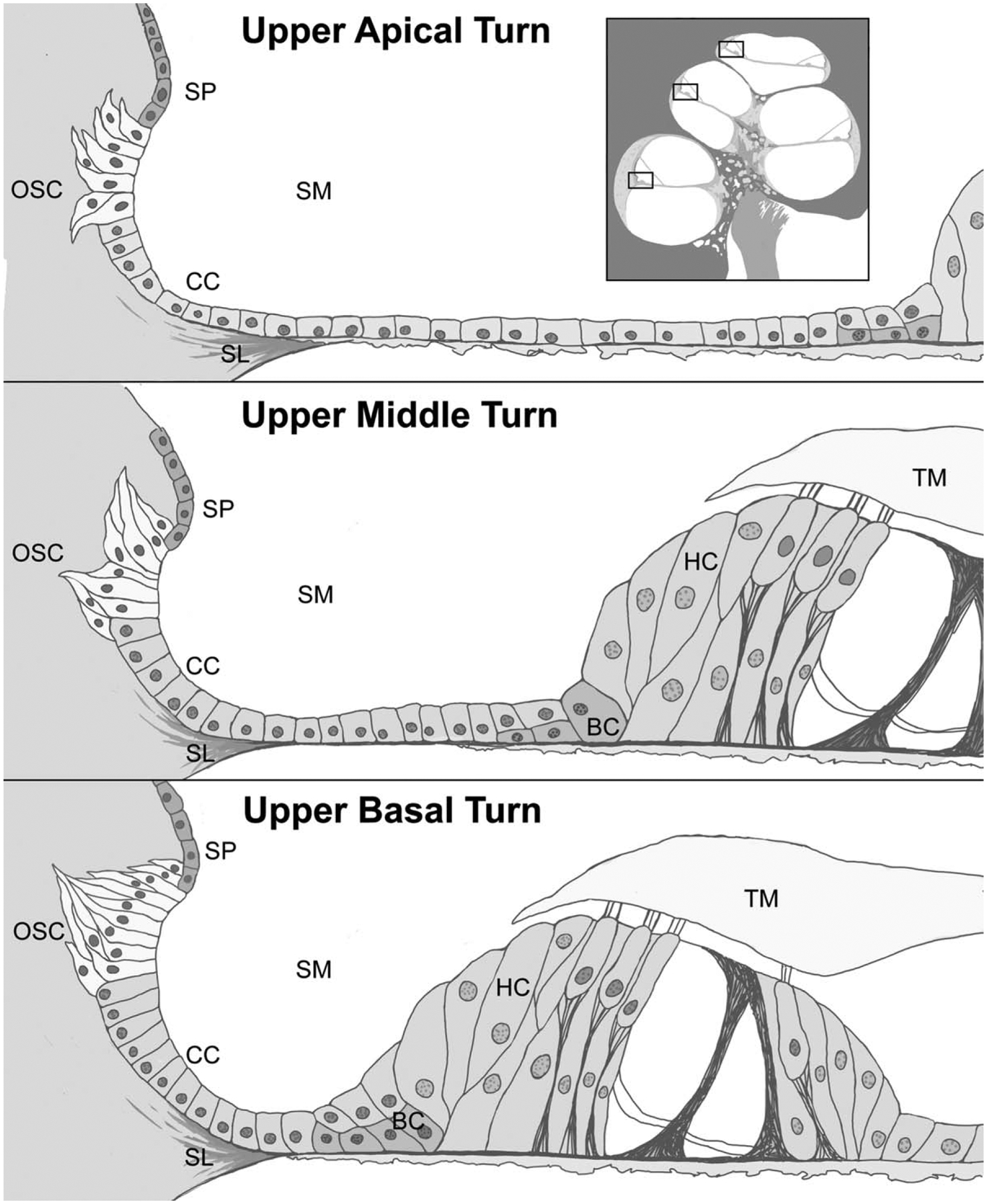
Idealized rendering of the cellular structures of the basal, middle, and apical turns of the human cochlea based on light and confocal microscopy findings. Outer sulcus cells extend into the spiral ligament in bundles forming tapering processes which differ between the cochlear turns in morphology. OSC indicates outer sulcus cell; SP, spiral prominence; CC, claudius cell; SL, spiral ligament; BC, Boettcher cell; HC, Hensen’s cell; TM, tectorial membrane; SM, scala media.
Quantitative Assessment
Mean outer sulcus cell counts in the upper apical turn (8.82) were generally smaller (all p < 0.05) than those of the upper basal (17.71), lower middle (18.99), upper middle (18.23), and lower apical (16.42) turns. Mean outer sulcus cell counts were higher (p < 0.05) in normal controls (20.1) than in diseased specimens (15.29). The mean outer sulcus cell count for all turns combined was19.8 for normal controls, 16.1 for Menière’s disease, 13.6 for downward sloping presbycusis, and 14.5 for flat presbycusis, as depicted in Figure 4. In the normal control-group there was a wider standard deviation of outer sulcus cell counts compared with the other groups (mean standard deviations: normal 7.4; Menière 4.9; downward sloping presbycusis 3.37; flat presbycusis3.27). Figure 4 also depicts mean outer sulcus cell counts at the various cochlear subsites for the four types of specimens.
FIG. 4.
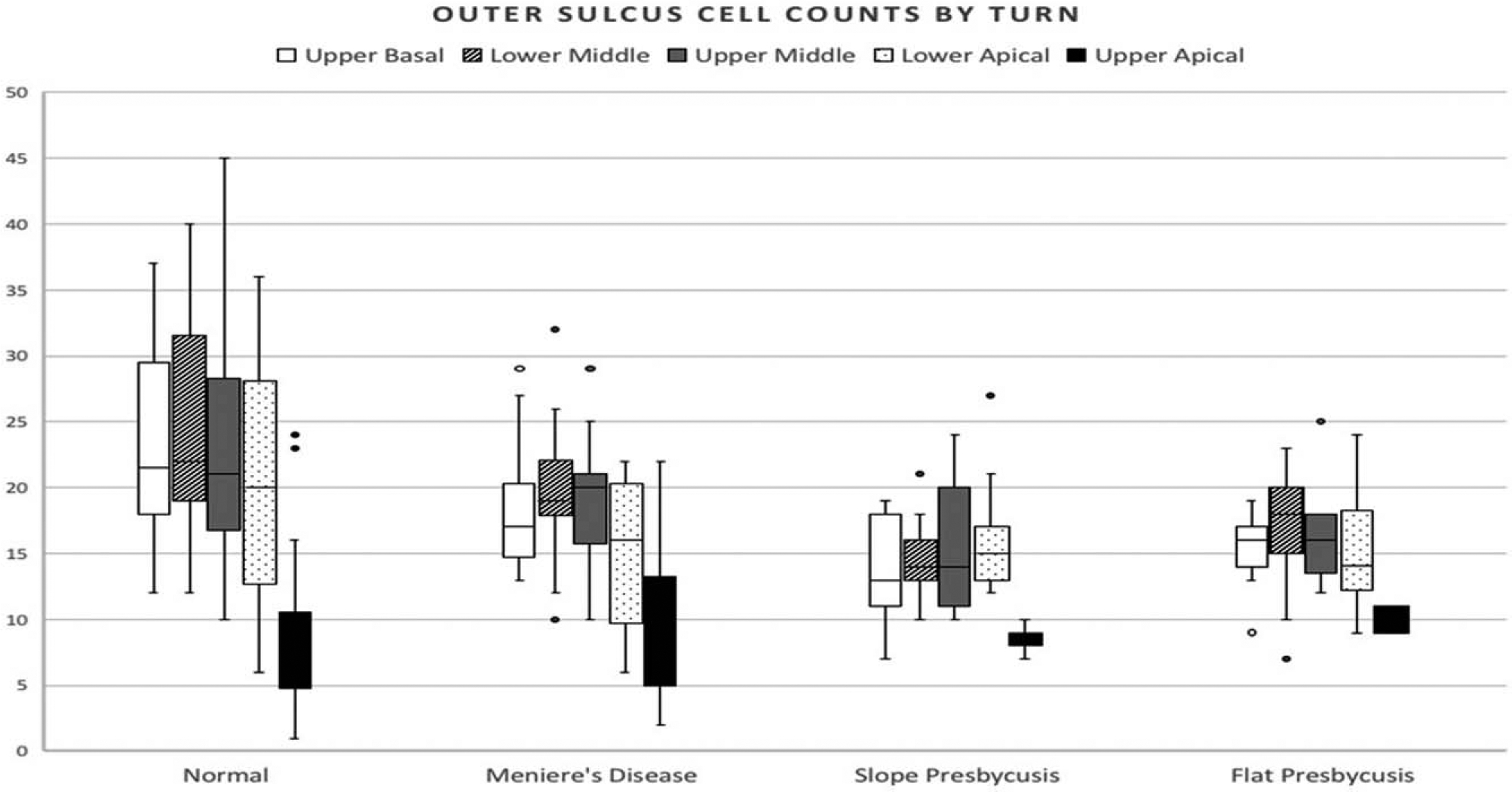
Outer sulcus cell counts for each cochlear turn from four different temporal bone types. Normal n = 10, Menière’s n 10, Slope n = 5, Flat n = 4. The box displays the upper and lower quartiles with the median range falling in between (interquartile range). The upper whisker shows 25% of the data which is higher and the lower whisker the 25% which is lower than the interquartile range. Outliers are displayed with a filled (known) or open (suspected) circle.
Pairwise comparisons of outer sulcus cell mean counts at various cochlear turns between the four temporal bone types are depicted in Table 1. The flat and sloped presbycusis groups were most similar with no significant difference in mean outer sulcus cell counts found in any of the cochlear turns; the flat presbycusis and Menière’s disease groups were also quite similar with a significant difference encountered only in the lower middle turn. There were no significant differences between the various temporal bone types with respect to mean outer sulcus cell counts in the upper apical turn and a difference in the lower apical turn only between normal and Menière’s disease specimens. The sites of greatest variability between temporal bone types were encountered in the upper basal and lower middle turns.
TABLE 1.
Pair-wise comparisons (Wilcoxon rank sum test) of mean outer sulcus cell counts at various cochlear turns in four different temporal bone types
| Turn | p Value | |||||
|---|---|---|---|---|---|---|
| Normal vs. Slope | Normal vs. Flat | Normal vs. Menière’s | Menière’s vs. Slope | Menière’s vs. Flat | Slope vs. Flat | |
| Upper Basal | 0.0000c | 0.0003c | 0.0007 | 0.0084b | 0.1554 | 0.4181 |
| Lower Middle | 0.0000c | 0.0041b | 0.0181a | 0.0001c | 0.0328a | 0.1223 |
| Upper Middle | 0.0031a | 0.0220a | 0.0825 | 0.0521 | 0.0835 | 0.4547 |
| Lower Apical | 0.1803 | 0.1285 | 0.0448a | 0.7904 | 0.6752 | 0.7293 |
| Upper Apical | 0.2624 | 0.2643 | 0.9767 | 0.2006 | 0.3134 | 0.1179 |
p < 0.05.
p < 0.01.
p < 0.001.
Pairwise comparisons between the cochlear turns for the various temporal bone types are shown in Table 2. There were statistically significant differences in all temporal bones between the upper apical region versus the upper basal, upper middle, and lower apical regions with the upper apical region having lower outer sulcus cell counts than these other sites. Cases with flat presbycusis had the least amount of variability in outer sulcus cell counts between the turns; whereas Menière’s specimens had the most variability between the turns. A significant association was observed between mean outer sulcus cell counts for all specimen types combined and tonotopically associated audiometric thresholds in the upper basal, lower middle, and upper middle cochlear turns (Table 3).
TABLE 2.
Pair-wise comparisons (Wilcoxon rank sum test) of mean outer sulcus cell counts derived from three mid-modiolar sections between five different cochlear locations for four patient groups
| Turn | p Value | |||
|---|---|---|---|---|
| Normal | Meniere | Slope | Flat | |
| Upper basal vs. lower middle | 0.6377 | 0.0314a | 0.6460 | 0.2755 |
| Upper basal vs. upper middle | 0.3698 | 0.2912 | 0.4170 | 1.0000 |
| Upper basal vs. lower apical | 0.0642 | 0.1610 | 0.3168 | 0.4559 |
| Upper basal vs. upper apical | 0.0000c | 0.0000c | 0.0018b | 0.0277a |
| Lower middle vs. upper middle | 0.2155 | 0.5716 | 1.0000 | 0.5393 |
| Lower middle vs. lower apical | 0.0262a | 0.0055b | 0.6732 | 0.3079 |
| Lower middle vs. upper apical | 0.0000c | 0.0000c | 0.0001c | 0.0597 |
| Upper middle vs. lower apical | 0.2392 | 0.0335a | 0.6168 | 0.4516 |
| Upper middle vs. upper apical | 0.0000c | 0.0000c | 0.0001c | 0.0157a |
| Lower apical vs. upper apical | 0.0000c | 0.0003c | 0.0001c | 0.0239a |
p < 0.05.
p < 0.01.
p < 0.001.
TABLE 3.
Correlation analysis between outer sulcus cell counts and tonotopically associated audiometric thresholds
| Turn | Spearman Coefficient | P Value |
|---|---|---|
| Upper basal | −0.662 | 0.0001 |
| Lower middle | −0.565 | 0.0017 |
| Upper middle | −0.507 | 0.0136 |
| All turns | −0.372 | 0.0514 |
DISCUSSION
Despite the presence of several animal studies in the scientific literature that investigate outer sulcus cell morphology, this is, to the authors’ knowledge, the first major histologic study of the outer sulcus cells in normal and diseased human specimens. Given the known variability in cochlear anatomy commonly encountered between mammalian species, we think that continued work in this area is important as some differences from what has been previously noted in rodents were encountered. For example, the coverage of outer sulcus cells by Claudius cells in the basal region of the cochlea seems less robust in humans than what has been described in rodents such that human outer sulcus cells seem to maintain some degree of interface with the endolymph of the basal turn, even if partially restricted.
With respect to the number of outer sulcus cells present in normal temporal bones versus diseased ears, we did encounter significantly higher counts in normal hearing controls as compared with all other diseased groups. Further, there seem to be significant correlations between the mean outer sulcus cell counts (all specimen types combined) of the upper basal, lower and upper middle turn and the expected tonotopic hearing thresholds at each of these sites. Yet, a central outcome of this study was that although the number of outer sulcus cells present seems to be correlated with the general hearing status of the specimen, it does not necessarily portray the tonotopic pattern predicted based on underlying disease. For example, outer sulcus cell counts are similarly decreased in both the Menière’s disease and presbycusis patient groups as compared with the normal hearing controls and these differences are most prominent in the basal and middle turns, which tonotopically code for high-frequency sounds. While perhaps this is not surprising for cases of downward sloping presbycusis wherein basal turn degenerative pathology might be expected, this finding was also present for Menière’s cases wherein low-frequency sensorineural hearing loss is more typical. Furthermore, while a few differences in outer sulcus cell counts between the various types of diseased ears were found in the basal turn and lower middle turn regions, there was overall less variability in this respect between the diseased groups than there was between each of the diseased groups and the normal controls. Thus, the lower outer sulcus cell counts found in diseased ears may be more of a reflection of the general health of the cochlea than an indicator of any focal pathologic process.
One noteworthy finding in this investigation was the status of the outer sulcus of the cochlear apex in the Menière’s disease cases given the hypothesized role of the apical outer sulcus cells as a “water shunt” between the endolymph and perilymph compartments (4–6). If prominent derangements of the apical outer sulcus in the Menière’s cases had been encountered, this may have signaled a possible pathophysiologic link between apical outer sulcus cell status and endolymphatic hydrops. Never-theless, no difference was in fact found qualitatively in the appearance of the outer sulcus region in Menière’s disease specimens versus the presbycusis cases or normal ears. Furthermore, there was no quantitative variation in the outer sulcus cell counts of the upper apex between any of the temporal bone subtypes. There was a significant difference between cell counts of the lower apex that was only present between Menière’s specimens and normal controls, yet given that this was an isolated finding, it is of unknown significance. It was also noted that there were significantly fewer outer sulcus cells present in the upper apex as compared with nearly all of the other cochlear subsites, yet this finding was not unique to Menière’s disease specimens as it was a common finding for all temporal bone types.
Another area of interest in this effort relates to presbycusis. No difference was noted in outer sulcus cell counts between flat and sloping presbycusis in any of the cochlear subsites, but these differed from normal controls in several respects. Nelson and Hinojosa (16) investigated individuals with flat audiometric patterns of hearing loss using a method to quantify the stria vascularis volume and found stria vascularis atrophy to be present in combination with inner hair cell and spiral ganglion cell degeneration. In a related study, Nelson and Hinojosa (17) also reported that individuals with downward sloping audiometric patterns of presbycusis exhibit degeneration of the stria vascularis, spiral ganglion cells, and inner/outer hair cells, while also demonstrating that these findings are correlated with the severity of hearing loss. In yet another study from the same institution, Gluth and Nelson (22) have further demonstrated a loss of vestibular ganglion cells in patients with presbycusis. Outer sulcus cell or other supporting cell type counts were not undertaken in any of these three investigations or other studies relating to the histopathology of presbycusis. Thus, the findings of the current study uniquely portray decline of yet another inner ear structure (outer sulcus cells) in ears affected by flat and sloping audiometric patterns of presbycusis, thereby supporting the concept that presbycusis is a multifactorial degenerative process that can simultaneously involve numerous cell types of the inner ear.
Although the work contained in this study has several unique and important attributes, there are several limitations that should be noted. First, because no other similar data exists in the relevant literature, there are no reference outer sulcus cell counts to which the present results could be compared. Second, as is the nature of archived temporal bone histopathologic specimens, although the tissues were preserved, processed, and stained in an identical fashion, the presence of mild postmortem degeneration rendered the judgment of distinguishing outer sulcus cells from the adjacent cells more challenging in some cases than others. Third, routine pure-tone audiometry does not test the full tonotopic spectrum of the cochlea, which inhibited our ability to check for correlations between hearing and cell counts throughout the entire cochlea. Lastly, given the rare nature of processed human temporal bone specimens having clinically documented pathologic conditions in addition to the tedious and time-consuming nature of the process of cell counting, the overall specimen sample size in this study is not particularly large, which may have potentially injected some degree of error.
CONCLUSION
In summary, a qualitative assessment of the outer sulcus cells did not demonstrate obvious morphologic differences between ears affected by Menière’s disease or presbycusis as compared with normal controls; however, a quantitative evaluation of outer sulcus cell counts did demonstrate significantly higher numbers of cells in normal ears as compared with all diseased subtypes and there did seem to be a correlation between mean cell counts and tonotopically expected hearing thresholds. In addition, there did not appear to be any prominent unique pathologic changes involving the outer sulcus cells of the cochlear apex of Menière’s disease specimens as compared with ears affected by presbycusis. Of the cochlear subsites examined, the upper apical turn had the fewest number of outer sulcus cells and this finding was consistent among all types of diseased and normal temporal bones that were studied.
Supplementary Material
Acknowledgments:
The authors appreciate Dr Matthew Schibler from the Advanced Microscopy Laboratory and Spectroscopy (AMLS) of California Nanosystems Institute at UCLA (CNSI) for allowing the use of SP8 Leica laser confocal high-resolution microscope. They also appreciate the assistance of Sang Mee Lee, PhD, at the University of Chicago with statistical analysis.
This work was supported by National Institute of Deafness and Other Communication Disorders Grant # U24DC015910.
Footnotes
The authors disclose no conflicts of interest.
Supplemental digital content is available in the text. DOI: 10.1097/MAO.0000000000002535
REFERENCES
- 1.Deiters O. Studies on the lamina spiralis membranacea: a contribution to the knowledge of the inner hearing organ. Bonn, Germany: Henry et Cohen; 1860. [Google Scholar]
- 2.Boettcher A. Regarding the development and construction of the labyrinth according to investigations on animals. Nova Acta Acad Cesariae 1869;35:1–203. [Google Scholar]
- 3.Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: Immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 1995;191:101–18. [DOI] [PubMed] [Google Scholar]
- 4.Eckhard A, Müller M, Salt A, Smolders J, Rask-Andersen H, Löwenheim H. Water permeability of the mammalian cochlea: Functional features of an aquaporin-facilitated water shunt at the perilymph-endolymph barrier. Pflugers Arch 2014;466:1963–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckhard A, Dos Santos A, Liu W, et al. Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflugers Arch 2015;467:2571–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirt B, Penkova ZH, Eckhard A, et al. The subcellular distribution of aquaporin 5 in the cochlea reveals a water shunt at the perilymph-endolymph barrier. Neuroscience 2010;168:957–70. [DOI] [PubMed] [Google Scholar]
- 7.Galić M, Giebel W. An electron microscopic study of the function of the root cells in the external spiral sulcus of the cochlea. Acta Otolaryngol Suppl 1989;461:1–15. [DOI] [PubMed] [Google Scholar]
- 8.Gürkov R, Jerin C, Flatz W, Maxwell R. Clinical manifestations of hydropic ear disease (Menière’s). Eur Arch Otorhinolaryngol 2019;276:27–40. [DOI] [PubMed] [Google Scholar]
- 9.Duvall AJ. The ultrastructure of the external sulcus in the guinea pig cochlear duct. Laryngoscope 1969;79:1–29. [DOI] [PubMed] [Google Scholar]
- 10.Iurato S. Submicroscopic Structure of the Inner Ear. London, UK: Pergamon Press; 1967. [Google Scholar]
- 11.Küçük B, Abe K. Scanning electron microscopy of the connective tissue along the lateral wall of the mouse cochlear duct with special reference to the external sulcus cells. Arch Histol Cytol 1990; 53:297–305. [DOI] [PubMed] [Google Scholar]
- 12.Shambaugh GE. On the structure and function of the epithelium in the sulcus spiralis externus. Arch Otol 1908;38:538–46. [Google Scholar]
- 13.Shambaugh GE. Cytology of the Inner Ear In: Cowdry’s Special Cytology. Vol 2 New York, NY: Paul B Hoeber, Inc; 1928. [Google Scholar]
- 14.Lawrence M. Structures of the spiral prominence and external sulcus and their relation to the organ of corti. Laryngoscope 1956;66:796–809. [DOI] [PubMed] [Google Scholar]
- 15.Kimura RS. Sensory and accessory epithelia of the cochlea In: Ultrastructural Atlas of the Inner Ear. London, UK: Butterworths; 1984:101–132. [Google Scholar]
- 16.Nelson EG, Hinojosa R. Presbycusis: A human temporal bone study of individuals with flat audiometric patterns of hearing loss using a new method to quantify stria vascularis volume. Laryngoscope 2003;113:1672–86. [DOI] [PubMed] [Google Scholar]
- 17.Nelson EG, Hinojosa R. Presbycusis: A human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope 2006; 116:1–12. [DOI] [PubMed] [Google Scholar]
- 18.Jagger DJ, Forge A. The enigmatic root cell—emerging roles contributing to fluid homeostasis within the cochlear outer sulcus. Hear Res 2013;303:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Merchant SN, Nadol JB. Schuknecht’s Pathology of the Ear., 3rd ed. Shelton, CT: People’s Medical Publishing House-USA; 2010. 42. [Google Scholar]
- 20.Lopez IA, Ishiyama G, Hosokawa S, et al. Immunohistochemical techniques for the human inner ear. Histochem Cell Biol 2016; 146:367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res 1996;100:80–100. [DOI] [PubMed] [Google Scholar]
- 22.Gluth MB, Nelson EG. Age-related change in vestibular ganglion cell populations in individuals with presbycusis and normal hearing. Otol Neurotol 2017;38:540–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


