Abstract
Importance:
Improved screening methods for women with dense breasts are needed because of their increased risk of breast cancer and of failed early diagnosis by screening mammography.
Objective:
To compare the screening performance of abbreviated breast MRI (AB-MR), and digital breast tomosynthesis (DBT) in women with dense breasts.
Design, Setting, and Participants:
Cross-sectional study with longitudinal follow-up at 48 academic, community hospital, and private practice sites in the US and Germany, conducted between December 2016 and November 2017, that included average-risk women aged 40–75 years with heterogeneously dense or extremely dense breasts undergoing routine screening. Follow up ascertainment of cancer diagnoses was complete through September 12th, 2019.
Exposure:
All women underwent screening by both DBT and AB-MR, performed in randomized order and read independently to avoid interpretation bias.
Main outcome measures:
The primary endpoint was the invasive cancer detection rate. Secondary outcomes included sensitivity, specificity, the additional-imaging-recommendation-rate, and positive predictive value (PPV) of biopsy, using invasive cancer and DCIS to define a positive reference standard. All outcomes are reported at the participant level. Pathology of core or surgical biopsy was the reference standard for cancer detection rate and PPV; interval cancers reported until the next annual screen were included in the reference standard for sensitivity and specificity.
Results:
Among 1516 enrolled women, 1444 (median age 54, range 40–75) completed both examinations and were included in the analysis. The reference standard was positive for invasive cancer with or without DCIS in 17 women, and for DCIS alone in another 6. No interval cancers were observed during follow-up. AB-MR detected all 17 women with invasive cancer, and 5/6 women with DCIS. DBT detected 7/17 women with invasive cancer, and 2/6 women with DCIS. The invasive-cancer-detection-rate was 11.8 per 1000 women [95% CI 7.4–18.8] for AB-MR versus 4.8 per 1000 women [95% CI 2.4–10.0] for DBT, a difference of 7 per 1000 women [95% CI for the difference 2.2–11.6] (exact McNemar p=0.002). For detection of invasive cancer and DCIS, for AB-MR versus DBT, sensitivity was 95.7% [95% CI 79.0–99.2] versus 39.1% [95% CI 22.2–59.2] (p=0.001). Specificity was 86.7% [95% CI 84.8–88.4] versus 97.4% [95% CI 96.5–98.1] (p<0.001). Additional-imaging-recommendation-rate was 7.5% [95% CI 6.2–9.0] versus 10.1% [95% CI 8.7–11.8] (p=0.02). PPV was 19.6% [95% CI 13.2–28.2] versus 31.0% [95% CI 17.0–49.7] (p=0.15).
Conclusion and relevance:
Among women with dense breasts undergoing screening, AB-MR, compared with DBT, was associated with a significantly higher rate of invasive breast-cancer detection. Further research is needed to better understand the relationship between screening methods and clinical outcome.
Trial registration:
NCT02933489, ECOG-ACRIN (EA1141).
Introduction
Dense fibroglandular tissue represents an important reason for failed early diagnosis in women who participate in mammographic screening, and increases a woman’s likelihood of being diagnosed with interval and/or advanced breast cancer.1–3 Approximately half of the screening-relevant age group have dense breasts.4
Whole-breast ultrasound is often used for supplemental screening in women with dense breasts, but requires substantial human resources to perform, only moderately increases sensitivity, and is associated with high false-positive and short-term follow-up rates.5–7
Digital breast tomosynthesis (DBT) is a recent improvement to conventional mammography that generates quasi-3-dimensional images of the breast, improving mammographic sensitivity and specificity.8–9 While initially only used as a supplement to digital mammography, DBT is increasingly used as a replacement.9
Breast magnetic resonance imaging (MRI) offers the highest cancer-detection-rate of all breast imaging modalities. Although most evidence exists for using MRI to screen the small proportion of women at very high risk of breast cancer,9–13 there is accumulating evidence that the higher sensitivity of MRI is also seen in women at average risk.14 However, the use of conventional, full-protocol breast-MRI to screen the large cohort of average-risk women with dense breasts will neither be practical nor cost-effective.
Abbreviated breast-MRI (AB-MR) has been introduced to reduce the complexity and cost of MRI by reducing image acquisition and interpretation time, in order to improve access to breast-MRI.15 Multiple studies have confirmed equivalent diagnostic accuracy of AB-MR with full MRI protocols.16 These observations have led to the consideration of utilizing AB-MR to screen women with dense breasts.
This study compared the diagnostic performance of AB-MR and DBT for screening average-risk women with dense breasts in a mixture of academic, community hospital, and private institutions. The primary objective was to compare the respective invasive cancer detection rates at the participant level.
Methods
Study design and participants
This multicenter intra-individual comparative cross-sectional study with longitudinal follow-up was conducted at 48 different institutions in the United States (47/48) and Germany (1/48), including academic institutions (24/48), and community hospitals or private practices (24/48). The National Cancer Institute Central Institutional Review Board (NCI CIRB) approved the protocol and was the IRB of record for 40 institutions; the remaining 8 institutions used their institutional IRB. Written informed consent was obtained from all participants.
Accrual started in December 2016 and was completed in November 2017. Follow up ascertainment of cancer diagnoses was complete through September 12th, 2019.
Per protocol (eSupplement 1), clinically asymptomatic women aged 40–75 scheduled to undergo routine breast cancer screening with DBT were enrolled if they had dense breasts as reported on their most recent screening mammogram. Women with a history of benign breast biopsy, remote history of treated breast cancer, or family history of breast cancer were eligible. Women were excluded if they had a screening breast ultrasound within the past 12 months, or ever had a breast-MRI, a molecular breast imaging study, or a contrast-enhanced mammogram, or would qualify for full-protocol breast-MRI based on American Cancer Society guidelines.17
The individual risk scores of study participants who met criteria for the Breast Cancer Surveillance Consortium (BCSC) risk calculator (i.e., no personal history of breast cancer and aged < 75 years) were determined.23
Self-reported information on race and ethnicity using fixed categories was obtained to compare the sociodemographic composition of the trial to the US population.
Participants underwent imaging with both DBT and AB-MR at study baseline and after one year, and will be followed for two additional years. Central randomization (in a 1:1 ratio using permuted blocks of size 4 without replacement) was used to determine the order of the imaging exams. Imaging studies were interpreted independently by two different board certified breast radiologists who remained blinded to the results of the other modality. DBT and AB-MR were performed within a single 24-hour period.
After a positive AB-MR study, additional imaging is not considered useful to further support or refute the indication to biopsy. Hence, AB-MR is not associated with call-back. In women with a biopsy recommendation, no biopsy was performed until both studies were completed and interpreted. All suspicious findings on DBT or AB-MR were biopsied regardless of the final interpretation of the other modality. If both modalities were positive in the same breast, the site radiologist determined whether the same or different lesions were seen.
Characteristics of invasive cancers and DCIS were described using standard histopathologic and immuno-histochemical features, as well as size (largest diameter) and lymph node status in accordance with the criteria from the American College of Pathology.19
For follow-up, women were contacted by phone at 6 months (± 1 month) and at 11–13 months after the study baseline and prior to the second screening round. Women were asked whether they received any breast imaging since the study baseline screen, and whether they were diagnosed with breast cancer, and, if so, how the breast cancer was discovered. Pathology of core or surgical biopsy was the reference standard for cancer detection rate and PPV of biopsy; in addition, interval cancers reported during 11–13 months of follow-up, until the next annual screen, were included in the reference standard for sensitivity and specificity.
Imaging technique and interpretation
All AB-MR studies had to be completed with a total acquisition time of less than 10 minutes, and included a T2-weighted acquisition, and a T1-weighted acquisition before and after bolus injection of contrast [0.1 mml/kg body-weight gadobenate-dimeglumine (Multihance, Bracco Monroe/NJ)]. All studies were interpreted according to the Breast Imaging Reporting and Data System (BI-RADS);18 further information is provided in eMethods 1 in eSupplement 2.
Endpoints
The primary endpoint was the rate of invasive cancer detection by each modality on the study baseline-screening at the participant level, defined as the fraction of participants in whom an invasive cancer was detected by the modality at the site of the imaging abnormality as verified by pathology (core biopsy or surgical excision).
Four secondary endpoints are included in this report: sensitivity, specificity, additional-imaging-recommendation-rate (i.e. call-back plus recommendation for short-term follow-up), and positive predictive value of biopsy (PPV). The presence of Invasive cancer and/or DCIS was defined as a positive reference standard for these end points. Additional secondary endpoints of the study that are not included in this report comprise participant-reported outcomes, tumor biology by genomic profiles, PPV and additional-imaging-recommendation rates of the second screening round, and the 3-year incident cancer rate. Data collection for these endpoints continues.
Additional exploratory endpoints included the overall cancer detection rate (invasive cancers and DCIS), the interval cancer rate, lesion-level estimates of PPV, stage of cancers at diagnosis according to AJCC 8.019, and characteristics of cancers based on histopathological and immune-histochemical features.
The following post hoc endpoints were not pre-specified according to the Statistical Analysis Plan (eSupplement 1): the proportion of women with invasive cancer who had a positive screening result; the proportion of women without invasive cancer, but possibly with DCIS, who had a negative screening result and the proportion of women with a positive screening result who were shown to have invasive cancer. These estimates should be interpreted with caution because all imaging studies had been interpreted with readers expected to classify as positive not only findings consistent with invasive cancer, but also findings consistent with DCIS. Point estimates and confidence intervals by modality are supplied for these post-hoc, unplanned endpoints.
Adverse events reporting
The study required expedited adverse event reporting using the Cancer Therapy Evaluation Program’s Adverse Event Reporting System (CTEP-AERS). All adverse events were recorded, regardless of attribution, according to the Common Terminology Criteria for Adverse Events.
Statistical considerations
The analysis cohort consisted of all participants who received both screening examinations. The projected sample size of 1450 participants was chosen to provide 90% power to detect a difference in the invasive cancer detection rate of 9/1000 women between the modalities using a two-sided McNemar test of level 0.05 (eMethods 2b in eSupplement 2); sample size estimation was challenging because at the time of protocol development, no published studies existed on AB-MR screening of women at average risk in comparison to DBT; thus, the limited evidence on cancer detection rates for MRI screening of average risk women in comparison with digital mammography were used that reported additional invasive cancer detection between 6 and 11 per 1000.15;20;
The invasive cancer detection rate was estimated as the fraction of participants with invasive cancer and a positive test result (BI-RADS 3–5) at the location of the cancer indicated by core or surgical biopsy. PPV was estimated as the fraction of participants undergoing biopsy that resulted in invasive cancer or DCIS. Sensitivity was estimated as the fraction of participants with cancer (invasive or DCIS), for whom the imaging modality result was positive (BI-RADS 3–5) for a location that matched the location of the cancer indicated by the reference standard. Specificity was estimated as the fraction of participants without cancer (invasive or DCIS) by the reference standard, for whom the imaging modality result was negative (BI-RADS 1–2).
Wilson confidence intervals are reported for estimates of binomial proportions. The Wald interval with Bonett-Price Laplace adjustment is reported for the difference in invasive cancer detection rates.21 Because of the paired design, detection rates, sensitivities, specificities and additional-imaging-recommendation-rates were compared using the exact McNemar’s test. Generalized estimating equation (GEE) regression was used to compare PPV, with the p-value reported from the corresponding score test.22 All reported p-values are two-sided. A post hoc Bonferroni adjustment was used for multiple comparisons of the primary and the four secondary endpoints (total of 5 comparisons), with p-values below 0.01 considered statistically significant.
Per protocol, endpoints were analyzed using aggregate data over all institutions. Post-hoc sensitivity analyses examining clustering by institution and using multiple imputation for missing reference standard are described in eMethods2c in eSupplement 2.
Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC) and R version 3.4.4 software (R project; http://www.r-project.org/). The BCSC risk score was computed using publicly available software.23
Results
Of the 1516 enrolled participants (Figure 1), 757 were randomized to undergo DBT first, and 759 to undergo AB-MR first. Six participants (0.4%) were ineligible for reasons specified in Figure 1. Of the remaining 1510 eligible women, 1444 (96%) received both exams and comprise the analysis set. Protocol adherence for the AB-MR scan time was achieved in 97% (1394/1444) of women, with a mean scan time of 8.0 (±1.3) minutes, median: 7.9; interquartile range: 7.2–9.0 minutes.
Figure 1:
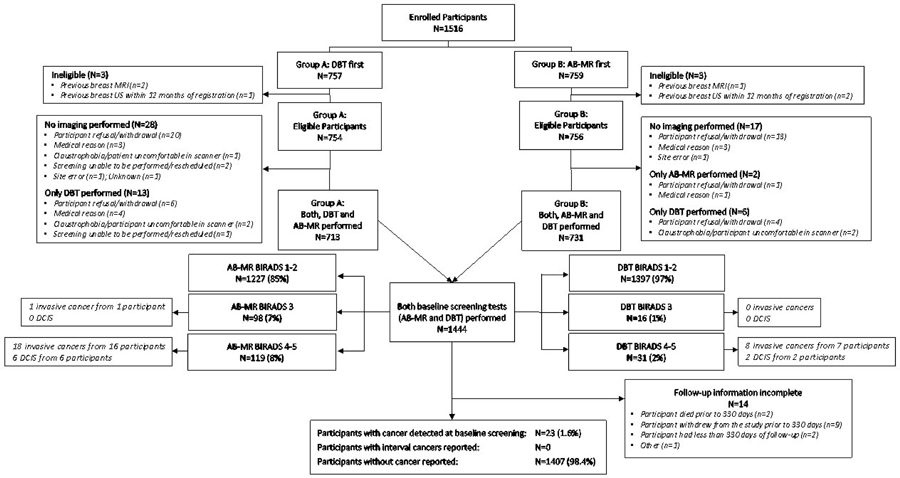
Study Flow Chart
Table 1 and eTable 1 (eSupplement 2) summarize the demographic characteristics and risk distributions of the analysis cohort of 1444 women. 77% had heterogeneously dense and 15% had extremely dense breasts; 8% had dense breasts on the most recent prior mammogram used to determine study inclusion, but were non-dense at the time of the study. The BCSC risk score yielded a median 5-year risk of invasive breast cancer of 1.6% (range 0.3%–7.8%).
Table 1 –
Baseline demographics and risk characteristics
| Eligible subjects with both scans completed (N =1444) | |
|---|---|
| N (%) | |
| Age (years) | 54.9 (8.5) |
| Mean (SD) | |
| Median (Range) | 54 (40, 75) |
| Race | N=1361 |
| White | 1233 (91%) |
| Black/African American | 61 (4%) |
| Asian | 57 (4%) |
| Mixed race | 5 (<1%) |
| American Indian/Alaskan Native | 3 (<1%) |
| Native Hawaiian/Pacific Islander | 2 (<1%) |
| Hispanic or Latino | 39/1356 (3%) |
| Menopausal status | N=1443 |
| Pre-menopausal | 440 (30%) |
| Peri-menopausal | 94 (7%) |
| Naturally post-menopausal | 669 (46%) |
| Surgically post-menopausal | 240 (17%) |
| Breast density from year 0 DBT | N=1444 |
| ACR-A: Almost entirely fat | 2 (<1%) |
| ACR-B: Scattered fibroglandular densities | 115 (8%) |
| ACR-C: Heterogeneously dense | 1108 (77%) |
| ACR-D: Extremely dense | 219 (15%) |
| History of at least one 1st degree relative with breast cancer | 271/1438 (19%) |
| History of at least one 1st degree relative with ovarian cancer | 30/1438 (2%) |
| Prior benign biopsy with atypias | 10/1423 (1%) |
| BCSC 5-year risk a, b | N=1385 |
| Mean (SD) | 1.8 (0.8) |
| Median (Range) | 1.6 (0.3, 7.8) |
| BCSC 10-year risk a | N=1385 3.7 (1.6) |
| Mean (SD) | |
| Median (Range) | 3.5 (0.9, 14.2) |
DBT: Digital Breast Tomosynthesis; AB-MR: Abbreviated breast Magnetic Resonance Imaging; BCSC: Breast Cancer Surveillance Consortium
ACR-A-D: Categorization of breast density from non-dense to dense. ACR-A: Almost entirely fat (<25% fibroglandular); ACR-B: Scattered fibroglandular densities (25–50% fibroglandular); ACR-C: Heterogeneously dense breasts (51–75% fibroglandular); ACR-D: Extremely dense breasts (>75% fibroglandular). Note that women were included based on the density of the respective last screening mammogram and could have undergone involution since that last screening mammogram.
BCSC risk scores could not be calculated for a total of 12 randomized subjects due to age >74 years (n=4) or previous diagnosis of breast cancer (n=8). In addition, 47 randomized subjects with either unknown or unavailable data on prior history of breast cancer were also excluded from the BCSC score summaries.
The Breast Cancer Surveillance Consortium 5-year Risk Score distinguishes between low risk (< 1.0), average risk (1.0 to ≤ 1.66), increased risk (1.67 to < 6.0), and high risk (≥ 6.0).
Primary Endpoint:
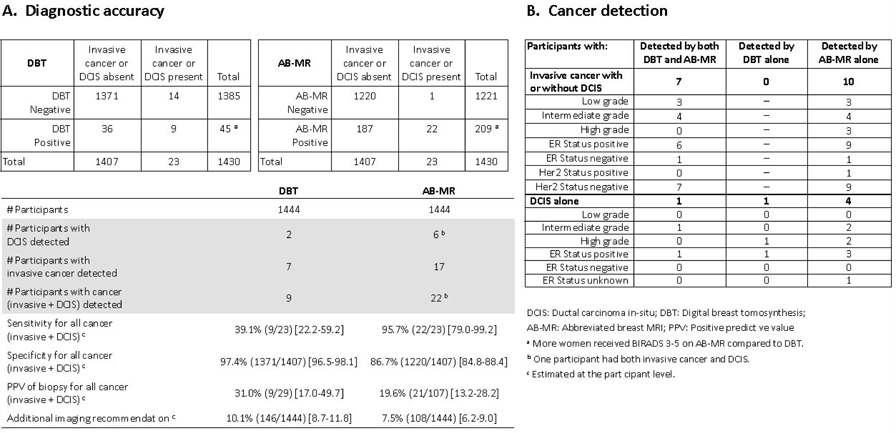
19 invasive cancers were detected in 17 women on the study baseline screening round (Table 2; Figure 2). Cancer status was known in all participants for the detection analysis. AB-MR detected the invasive cancer in all 17 women, and DBT in 7 women, yielding an invasive-cancer-detection-rate of 11.8 per 1000 women [95% CI 7.4–18.8] for AB-MR versus 4.8 per 1000 women [95% CI 2.4–10.0] for DBT, a difference of 7 per 1000 women [95% CI for the difference 2.2–11.6] (p=0.002).
Table 2 –
Screen-detected cancers
| Obs a | Case | Lesion detected at AB-MR b | Lesion detected at DBT b | Breast density | Age | Type of cancer | Largest diameter c (mm) | Maximum reported grade c | ER c | PR c | HER2 c | BCSC 5-year risk score | BCSC 10-year risk score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | + | + | ACR-C | 62 | DCIS | 15 | 2 | Positive | Positive | Not performed | 2.79 | 5.62 |
| 2 | 2 | + | ACR-C | 69 | DCIS | 20 | 2 | Positive | Positive | Not performed | 3.24 | 6.17 | |
| 3 | 3 | + | ACR-C | 68 | DCIS | 10 | 2 | Positive | Negative | Not performed | 4.64 | 8.82 | |
| 4 | 4 | + | ACR-C | 71 | DCIS | 10 | 3 | Positive | Negative | Not performed | 2.10 | 3.95 | |
| 5 | 5 | + | ACR-C | 68 | DCIS | 5 | 3 | Not performed | Not performed | Not performed | 2.08 | 4.02 | |
| 6 | 6a | + | ACR-D | 63 | DCIS | 30 | 2 | Positive | Not performed | Not performed | 3.40 | 6.77 | |
| 7 | 7d | +d | ACR-D | 40 | DCIS | 70 | 3 | Positive | Positive | Not performed | 1.25 | 3.13 | |
| 8 | 8 | + | ACR-B | 62 | IDC | 4 | 1 | Positive | Positive | Negative | 2.04 | 4.13 | |
| 9 | 9 | + | ACR-B | 65 | ILC | 16 | 2 | Positive | Negative | Negative | 1.50 | 2.97 | |
| 10 | 10a | + | + | ACR-C | 62 | IDC | 11 | 1 | Positive | Positive | Negative | 1.92 | 3.88 |
| 11 | 10b | + | + | ACR-C | 62 | IDC | 9 | 2 | Positive | Positive | Negative | 1.92 | 3.88 |
| 12 | 11 | + | ACR-C | 72 | IDC | 8 | 2 | Positive | Positive | Negative | N/A e | N/A e | |
| 13 | 12 | + | ACR-C | 67 | ILC | 14 | 2 | Positive | Positive | Negative | 3.14 | 6.08 | |
| 14 | 13a | + | + | ACR-C | 56 | ILC | 25 | 2 | Positive | Positive | Negative | 2.41 | 5.07 |
| 15 | 13b | + | ACR-C | 56 | ILC | 25 | 2 | Not available | Not available | Not available | 2.41 | 5.07 | |
| 16 | 14 | + | + | ACR-C | 64 | IDC | 18 | 2 | Negative | Negative | Negative | 2.89 | 5.73 |
| 17 | 15 | + | + | ACR-C | 48 | IDC | 10 | 1 | Positive | Positive | Negative | 1.07 | 2.43 |
| 18 | 16 | + | ACR-C | 47 | IDC | 10 | 1 | Positive | Positive | Negative | 0.99 | 2.21 | |
| 19 | 17 f | + | + | ACR-C | 44 | IDC | N/A f | 2 | Positive | Positive | Negative | 1.39 | 3.28 |
| 20 | 18 | + | ACR-C | 52 | IDC | 24 | 3 | Negative | Negative | Positive | 1.92 | 4.19 | |
| 21 | 19 | + | + | ACR-C | 46 | Mixed IDC/ILC | 48 | 1 | Positive | Positive | Negative | 0.93 | 2.16 |
| 22 | 20 | + | ACR-C | 51 | IDC | 10 | 3 | Positive | Negative | Negative | 2.01 | 4.41 | |
| 23 | 21 | + | ACR-C | 70 | IDC | 10 | 2 | Positive | Negative | Negative | 4.80 | 8.96 | |
| 24 | 22 | + | + | ACR-C | 41 | IDC | 10 | 1 | Positive | Positive | Negative | 0.61 | 1.50 |
| 25 | 23 | + | ACR-D | 53 | IDC | 10 | 1 | Positive | Positive | Negative | 4.83 | 10.21 | |
| 26 | 6b | + | ACR-D | 63 | IDC | 14 | 3 | Positive | Positive | Negative | 3.40 | 6.77 |
AB-MR: Abbreviated breast Magnetic Resonance Imaging; DBT: Digital Breast Tomosynthesis; ER: Estrogen receptor status; PR: Progesterone receptor status; HER2: Human epidermal growth factor receptor 2; DCIS: Ductal carcinoma in situ; IDC: Invasive ductal cancer; ILC: Invasive lobular cancer; ACR-B: Scattered fibroglandular densities; ACR-C: Heterogeneously dense breasts; ACR-D: Extremely dense breasts;
‘Obs’ signifies a count of each individual cancer, and ‘Case’ signifies each unique participant, where participants with more than one cancer have separate cancers distinguished by ‘a’ and ‘b’ (e.g. 6a, 6b).
A ‘+’ denotes that the cancer was detected by the modality; a blank cell denotes that the cancer was not detected by the modality.
Grade shown is the highest reported grade across the core and surgical biopsy. For ER, PR, HER2, if either the core or surgical biopsy reported positivity, the value is reported as positive. Largest diameter, ductal carcinoma pattern and invasive pattern are from the surgical pathology. In one case (no. 9), the histologic description of a lobular-invasive cancer corresponded to intermediate grade, although no specific grading information was provided
DBT reported 2 lesions which were both of the same pathological lesion (a single 70 mm DCIS).
BCSC risk scores could not be computed for this participant due to a previous diagnosis of breast cancer.
N/A, not available. This case had invasive disease (IDC) by vacuum assisted core biopsy, but there was no residual cancer in the surgical biopsy specimen which contained only benign changes with atypia. Thus, tumor size data from surgical pathology are not available; however, the lesion size was 11 mm by AB-MR and 9 mm by DBT.
Figure 2:
Summary of relevant study findings
Secondary Endpoints:
Sensitivity and specificity
According to the reference standard information, 23 women had cancer (invasive or DCIS) at the study baseline screen, and 1421 had remained cancer-free. Of those, 1407 had complete follow-up until the next screening date. None had breast cancer diagnosed during follow up. No cancer was reported in the remaining 14 participants, but follow-up information was incomplete; accordingly, the reference standard status was considered missing (Figure 1).
The sensitivity of AB-MR for invasive cancer or DCIS was significantly higher than that of DBT, at 95.7% (22/23) [95% CI 79.0–99.2] versus 39.1% (9/23) [95% CI 22.2–59.2] (p=0.001) (Figure 2). Specificity of AB-MR was significantly lower than that of DBT, at 86.7% (1220/1407) [95% CI 84.8–88.4] versus 97.4% (1371/1407) [95% CI 96.5–98.1] (p<0.001) (Figure 2; eFigure 2 in eSupplement 2).
Positive predictive value
PPV of biopsy for AB-MR was 19.6% (21/107) [95% CI 13.2–28.2], not statistically significantly different compared with DBT, at 31.0% (9/29) [95% CI 17.0–49.7] (p=0.15) (Figure 2).
Additional-imaging-recommendation-rate
The call-back rate for screening DBT was 10.1% (146/1444) [95% CI: 8.7–11.8] and 0% for AB-MRI. Short-term follow-up (BI-RADS 3) was recommended for DBT in 1.2% of women (18/1444) [95% CI 0.8–2.0], all of whom also had a call-back, and for AB-MR in 7.5% of women (108/1444) [95% CI 6.2– 9.0]. Thus, additional imaging (either call-back or short-term follow-up) was required in 7.5% (108/1444) [95% CI 6.2–9.0] of women for AB-MR, and in 10.1% (146/1444) [95% CI 8.7–11.8] of women for DBT; this difference was not statistically significant after Bonferroni adjustment (p=0.02) (Figure 2).
Exploratory and post hoc endpoints
Overall cancer detection
In addition to the 19 invasive cancers observed in 17 women, 6 women were diagnosed with pure DCIS; one woman with invasive cancer also had DCIS, for a breast cancer prevalence of 23 of 1444 women, or 15.9 per 1000 women. AB-MR identified cancer in 22 of the 23 women with cancer, DBT in 9 women, for an overall cancer detection rate of 15.2 per 1000 women (22/1444) [95% CI 10.1–23.0] for AB-MR versus 6.2 per 1000 women (9/1444) [95% CI 3.3–11.8] for DBT (p=0.001) (Figure 2).
Interval cancer rate
No interval cancers were reported, leading to an estimated interval-cancer rate of 0% (0/1407) [95% CI 0%–0.27%].
Cancer characteristics by method of detection
Table 2 provides details of the invasive cancers and DCIS, including the method of detection. The median size of invasive cancer was 10.5 mm, range 4–48 mm. One of the 23 participants with cancer had positive nodes, for a node-negative rate of 96%. Figure 2 summarizes the histopathological and immuno-histochemical characteristics of cancers stratified by method of detection. Of the 7 participants with DBT-detected invasive cancers, cancer grade was low-grade in 3/7 and intermediate-grade in 4/7. These same cancers were also detected by AB-MR. Of the 10 additional participants with invasive cancer detected by AB-MR alone, cancer grade was low-grade in 3/10, intermediate-grade in 4/10, and high-grade in 3/10.
Lesion level PPV:
The PPV of biopsy on the lesion level was 19.0% (24/126) [95% CI 12.6 – 27.7] for AB-MR versus 35.5% (11/31) [95% CI 19.5 – 55.5] for DBT (p=0.08).
Additional, non-pre-specified post-hoc analyses:
A post-hoc analysis of the detection rate intended to adjust for clustering by site produced qualitatively similar results as in the primary analysis (eResults 1 in eSupplement 2). A post-hoc analysis of sensitivity and specificity using mixed modeling with random effects for institution and multiple imputation for missing reference standard produced qualitatively similar results as in the primary analysis (eResults 2 in eSupplement 2).
The post-hoc estimates of the analogues of sensitivity, specificity, and PPV using only invasive cancer as the reference were 100.0% [95% CI 81.6 – 100.0], 86.4% [95% CI 84.5 – 88.1], and 15.0% [95% CI 9.4 – 23.0], respectively, for AB-MR, and 41.2% [95% CI 21.6 – 64.0], 97.3% [95% CI 96.3 – 98.0], and 24.1% [95% CI 12.0 – 42.7], respectively, for DBT, and are similar to the estimates using invasive cancer or DCIS as the reference standard (eFigure 1 in eSupplement 2).
Adverse events
A total of 13 adverse events were reported in 12 women within 1 year of registration; the majority were grade 1 or less (8/13, 62%). A detailed description of reported adverse events is in eTable 2 (eSupplement 2). The most common adverse events were mild allergic reactions (3 events) and anxiety (2 events).
Discussion
In this study on the performance of AB-MR for routine breast-cancer screening of average risk women with dense breasts, AB-MR was associated with a significantly higher invasive-breast-cancer detection rate compared with DBT, also referred to as 3D-mammography. The significantly higher sensitivity of AB-MR was associated with a reduced specificity, but with a PPV that was not significantly different to that of DBT. Women and referring physicians should be aware that having a screening AB-MR, especially a baseline examination, may lead to additional benign biopsies, 6 month follow up recommendations, or both. On the other hand, DBT, but not AB-MR, may require further imaging after initial screen-detected abnormalities.
Although there is a close correlation between early diagnosis and breast-cancer survival, and although systematic mammographic screening has been used to improve early diagnosis for several decades, breast cancer continues to represent a leading cause of cancer death in women.24–25 Apart from variable attendance rates, the effect of mammographic screening on breast-cancer mortality is mitigated by overdiagnosis of biologically inert, prognostically insignificant cancers,26 but also by underdiagnosis of potentially lethal disease.3, 27–28 Due to the masking effect of fibroglandular tissue, such underdiagnosis is especially likely in women with dense breasts, leading to persistently high rates of interval and advanced-stage cancers in these women.1–3 Women with dense breasts are therefore underserved with regular, mammographic screening. Therefore, there is a substantial clinical need for methods that reduce underdiagnosis (interval cancers and/or diagnosis of late-stage disease) in these women.23 The passage of state and federal breast density legislation, requiring women to be informed about their breast density and its diagnostic and prognostic implications, has contributed to this need.29 The results of this study demonstrate that AB-MR improved breast-cancer detection in women with dense breasts; the fact that no interval cancers were observed during follow-up further supports this conclusion.
Improved early detection is an important means to reduce breast-cancer mortality in women with dense breasts. This study does not provide evidence regarding mortality, or on the degree of possible overdiagnosis. Collecting such evidence requires much larger randomized clinical trials (RCT) with long-term follow-up of at least 15–20 years. RCT on surrogate endpoints for breast-cancer mortality such as tumor-stage-at-diagnosis and/or interval cancer rates may shorten the time required to conduct definitive randomized trials.
To help gain some insight into the rate of desirable detection of relevant cancers versus undesirable detection of inconsequential disease, the characteristics of cancers detected were reported as an established proxy for their prognostic importance/likelihood of progression. Based on the distribution of nuclear grades and receptor status, the invasive cancers detected by AB-MR did not differ from those also detected by DBT; however, the 3 high-grade invasive cancers in this study were only AB-MR detected, and missed by DBT. This is consistent with prior observations regarding the tumor characteristics of MR-only detected cancers.30 Beyond these classifications based on standard histopathological and immuno-histochemical results, further analyses on genomic features (PAM50, Oncotype-DX) on study-detected invasive cancers and DCIS are underway. Since the prognostic importance of DCIS is controversial, the primary objective of this study was invasive cancer detection. The fact that AB-MR increased detection of pure DCIS as it increased detection of invasive cancers could be considered as possible evidence of overdiagnosis until more information is available.31
The study design allowed the determination of the diagnostic performance of AB-MR as an independent screening method for women with dense breasts. The results suggest that in women undergoing AB-MR, the contribution of mammography or DBT is limited; in this cohort, none of the invasive cancers, but one high-grade DCIS were only detected by DBT. This is consistent with existing evidence on the limited contribution to cancer detection of mammography in women undergoing MRI for screening. 12,14,30,32,33 Future studies are necessary to determine whether AB-MR alone could be used to screen women with dense breasts, given that mammography/DBT would add additional cost and exposure to ionizing radiation with no or limited added benefit.34
Uniformity across study sites was established by standardizing the AB-MR protocol and the contrast agent used (Multihance), and by providing the Society of Breast MRI interpretation algorithms. As a result, despite the fact that 47 of the 48 participating sites did not have prior practical experience with AB-MR, the cancer-detection-rate was similar to levels published by skilled MRI practices on full-protocol MRI, 10–13;30–33 and the PPV of AB-MR did not differ significantly from that of DBT. Still, at 19.6%, the PPV associated with AB-MR was on the lower end of PPV-levels published for full-protocol MRI, and the quality assurance benchmarks for full-protocol MRI screening.35 However, these benchmarks were established for women at high risk, whereas this study included women at average risk only; the lower breast-cancer prevalence in average versus high-risk screening will per se reduce the PPV. In addition, per study inclusion criteria, all participants had to have a prior mammogram to determine breast density, but could not have had a prior breast MRI. Therefore, all of the DBT-studies, but none of the AB-MR-studies were interpreted with prior imaging for comparison – a fact that introduces a systematic bias for test specificity and PPV in favor of DBT.36–37 In view of the well-established effect of prior imaging on reader performance, it is likely that PPV of AB-MR will further increase in subsequent screening rounds, i.e. with availability of prior AB-MR studies, and with increasing reader expertise.
AB-MR was well tolerated. Protocol adherence was high, and accrual was completed a year ahead of schedule. Participant-reported data on the acceptability of AB-MR will be reported separately.
AB-MR takes less than 10 minutes examination time; it requires the i.v. injection of a gadolinium-based contrast agent, but does not involve ionizing radiation or breast compression. It does not require new equipment beyond existing equipment for regular, full-protocol breast MRI, and does not require specific radiologist training beyond the level required by the American College of Radiology (ACR) breast MRI accreditation. AB-MR, just as ultrasound or other imaging methods considered as supplemental or alternate screening methods for women with dense breasts, is currently not reimbursable for women at average risk. Recent studies have reported gadolinium deposition in individuals following administration of so-called linear gadolinium-based contrast agents.38 Although to date this deposition is not known to have any clinical significance, studies are currently underway to better understand this phenomenon. With macrocyclic gadolinium-based contrast agents, gadolinium deposition is not observed; temporary retention of very small (nano-molar) amounts of the injected dose does occur, but is subsequently excreted without de-chelation.39–40
Limitations
This study has several limitations. First, this study does not provide evidence of the association between AB-MR and breast cancer mortality. Women with dense breasts who consider AB-MR as a screening option should be informed of this limitation. However, this lack of evidence also exists for all other existing, supplemental, or alternate screening options, including digital mammography, whole-breast ultrasound, and DBT.
Second, the cost-effectiveness of AB-MR relative to DBT was not evaluated. Since about half of women in the screening-relevant age range exhibit dense breast tissue, further risk stratification is needed, to better tailor the use of supplemental or advanced screening tests such as AB-MR. Third, although AB-MR does not require specific additional equipment beyond what is used for regular breast MRI, given the current limited availability of breast MRI in general for screening the relatively small number of women at high risk of breast cancer, the ability of centers to offer AB-MR may be limited until more MRI units are added. Fourth, since the eligibility criteria required a prior mammogram to assess breast density, the study compared an incidence DBT screen to a prevalence AB-MR screen. Fifth, the study found that AB-MR detected an additional 7 invasive cancers per 1000 women rather than 9 per 1000, an effect size the study had 90% power to detect. This estimate was based on preliminary studies of AB-MR and MRI screening for women with average risk; 15,20 all these prior studies had compared AB-MR or standard MRI with digital mammography, but not with DBT. This may account for the lower incremental cancer detection rate observed in the comparison between AB-MR and DBT. 10,12,14,15,20,32,33
Conclusion:
Among women with dense breasts undergoing screening, AB-MR, compared with DBT, was associated with a significantly higher rate of invasive-breast-cancer detection. Further research is needed to better understand the relationship between screening methods and clinical outcome.
Supplementary Material
KEY POINTS:
Question:
What is the invasive-breast-cancer detection rate of abbreviated breast magnetic resonance imaging (AB-MR) compared to digital breast tomosynthesis (DBT) in women with dense breasts undergoing routine screening?
Findings:
This cross-sectional study with longitudinal follow up included 1444 women who underwent both AB-MR and DBT, interpreted independently, AB-MR detected significantly more invasive cancers (17 patients; 11.8 per 1000 women) than DBT (7 patients; 4.8 per 1000 women). No invasive cancer was identified by DBT alone, or as interval cancer during follow-up.
Meaning:
Among women with dense breasts undergoing screening, AB-MR was associated with a significantly higher rate of invasive-cancer-detection than DBT.
Acknowledgement
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA189828, CA180790, CA180791, CA180795, CA180828, CA180847, CA180868, CA189819, CA180836, CA189860, CA189956. For the conduct of this study, ECOG/ACRIN received funding from Bracco, Monroe/NJ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
The authors would like to acknowledge Dr Janice S. Sung, MD, Memorial Sloan Kettering Cancer Center, for her additional contribution in study design and manuscript preparation.
The authors would like to acknowledge the help of Dr. Robert Smith, PhD, Vice President, Cancer Screening Program, American Cancer Society, for his help during manuscript revision.
The authors would like to express their gratitude to all women who were and are willing to participate in this study, as well as to those who considered participating.
None of the persons listed in the acknowledgement received a compensation for their contribution.
Role of the funding source:
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Data sharing statement
Data presented in this manuscript can be made available to interested researchers within a year from the publication of the manuscript. For additional information, interested researchers can contact the office of the Biostatistics and Data Management Center of ECOG-ACRIN, located at the Center for Statistical Sciences, Brown University School of Public Health. This data sharing statement can be found in eSupplement 3
Conflict of interest disclosures:
Dr. Kuhl, Dr. Bergin, Dr. Gareen, Dr. Holt, Dr. Miller, Dr. Newstead, Dr. Nicholson, Dr. Prather, Mr. Snyder, Dr. Sung and Dr. Ward have nothing to report. Dr Comstock reports grants from ECOG-ACRIN during the conduct of the study; personal fees from Bracco Diagnostics and personal fees from Bayer Inc outside the submitted work. Dr Gareen reports grants from National Cancer Institute and grants from Bracco Imaging during the conduct of the study. Dr Gatsonis reports grants from EA Cancer Research Group during the conduct of the study. Dr Harvey reports other from Hologic, Inc and non-financial support and other from Volpara Solutions, LLC outside the submitted work. Dr Rahbar reports grants from GE Healthcare outside the submitted work. Dr Schnall reports grants from National Cancer Institute and grants from Bracco Diagnostics during the conduct of the study; grants from Siemens Healthineers outside the submitted work.
References
- 1.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36 [DOI] [PubMed] [Google Scholar]
- 2.Kerlikowske K, Cook AJ, Buist DS et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holm J, Humphreys K, Li J et al. Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol. 2015;33:1030–7 [DOI] [PubMed] [Google Scholar]
- 4.Sprague BL, Gangnon RE, Burt V et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohuchi N, Suzuki A, Sobue T et al. ; J-START investigator groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Blume JD, Cormack JB et al. ; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprague BL, Stout NK, Schechter C et al. Benefits, Harms, and Cost-Effectiveness of Supplemental Ultrasonography Screening for Women With Dense Breasts. Ann Intern Med 2015;162:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedewald SM, Rafferty EA, Rose SL et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499–507 [DOI] [PubMed] [Google Scholar]
- 9.Zackrisson S, Lång K, Rosso A et al. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018. 19:1493–1503. [DOI] [PubMed] [Google Scholar]
- 10.Warner E, Plewes DB, Hill KA et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–25 [DOI] [PubMed] [Google Scholar]
- 11.Kriege M, Brekelmans CT, Boetes C et al. Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–37 [DOI] [PubMed] [Google Scholar]
- 12.Kuhl C, Weigel S, Schrading S et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450–7 [DOI] [PubMed] [Google Scholar]
- 13.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology. 2017;283:361–370 [DOI] [PubMed] [Google Scholar]
- 15.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first post-contrast subtracted images and maximum-intensity projection-a novel approach to breast-cancer screening with MRI. J Clin Oncol. 2014;32:2304–10. [DOI] [PubMed] [Google Scholar]
- 16.Kuhl CK. Abbreviated Magnetic Resonance Imaging (MRI) for Breast Cancer Screening: Rationale, Concept, and Transfer to Clinical Practice. Annu Rev Med. 2019;70:501–519. [DOI] [PubMed] [Google Scholar]
- 17.Saslow D, Boetes C, Burke W et al. American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89 [DOI] [PubMed] [Google Scholar]
- 18.D’Orsi CJ, Sickles EA, Mendelson EB et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013 [Google Scholar]
- 19.Amin MB, Edge SB, Greene FL, et al. (Eds.) AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017 [Google Scholar]
- 20.Schrading S, Kuhl- C. MRI screening of women at average risk of breast cancer. J Clin Oncol. 2013;31:(suppl 26; abstr 1). [Google Scholar]
- 21.Fagerland MW, Lydersen S, Laake P. Recommended tests and confidence intervals for paired binomial proportions. Statist. Med. 2014, 33 2850–2875 [DOI] [PubMed] [Google Scholar]
- 22.Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics. 2000;56:345–351. [DOI] [PubMed] [Google Scholar]
- 23.Vachon CM, Pankratz VS, Scott CG et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 2015;107(5). pii: dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RA, Andrews KS, Brooks D et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297–316 [DOI] [PubMed] [Google Scholar]
- 25.Njor SH, Schwartz W, Blichert-Toft M, Lynge E. Decline in breast cancer mortality: how much is attributable to screening? J Med Screen. 2015;22:20–7 [DOI] [PubMed] [Google Scholar]
- 26.Puliti D, Duffy S, Miccinesi G et al. Overdiagnosis in mammographic screening for breast-cancer in Europe: a literature review. J Med Screen 2012. 19: 42. [DOI] [PubMed] [Google Scholar]
- 27.Kirsh VA, Chiarelli AM, Edwards SA et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103:942–50 [DOI] [PubMed] [Google Scholar]
- 28.Porter PL, El-Bastawissi AY, Mandelson MT et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91:2020–8 [DOI] [PubMed] [Google Scholar]
- 29.Keating NL, Pace LE. New Federal Requirements to Inform Patients About Breast Density: Will They Help Patients? JAMA 2019;321:2275–2276.. [DOI] [PubMed] [Google Scholar]
- 30.Sung JS, Stamler S, Brooks J et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology. 2016;280:716–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology. 2009;253(2):281–3. [DOI] [PubMed] [Google Scholar]
- 32.Lo G, Scaranelo AM, Aboras H, et al. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology. 2017;285:36–43 [DOI] [PubMed] [Google Scholar]
- 33.van Zelst JCM, Mus RDM, Woldringh G et al. Surveillance of Women with the BRCA1 or BRCA2 Mutation by Using Biannual Automated Breast US, MR Imaging, and Mammography. Radiology 2017;285:376–388 [DOI] [PubMed] [Google Scholar]
- 34.Ali RMK, England A, McEntee MF, Mercer CE, Tootell A, Hogg P. Effective lifetime radiation risk for a number of national mammography screening programmes. Radiography (Lond).2018;24:240–246. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Ichikawa L, Valencia E et al. Performance Benchmarks for Screening Breast MR Imaging in Community Practice. Radiology. 2017;285:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frankel SD, Sickles EA, Curpen BN, Sollitto RA, Ominsky SH, Galvin HB. Initial versus subsequent screening mammography: comparison of findings and their prognostic significance. AJR Am J Roentgenol. 1995;164:1107–9. [DOI] [PubMed] [Google Scholar]
- 37.Vreemann S, Gubern-Mérida A, Schlooz-Vries MS et al. Influence of Risk Category and Screening Round on the Performance of an MR Imaging and Mammography Screening Program in Carriers of the BRCA Mutation and other Women at Increased Risk. Radiology. 2018;286:443–451 [DOI] [PubMed] [Google Scholar]
- 38.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–841 [DOI] [PubMed] [Google Scholar]
- 39.Jost G, Frenzel T, Boyken J, Lohrke J, Nischwitz V, Pietsch H. Long-term Excretion of Gadolinium-based Contrast Agents: Linear versus Macrocyclic Agents in an Experimental Rat Model. Radiology. 2019;290:340–348. [DOI] [PubMed] [Google Scholar]
- 40.Tibussek D, Rademacher C, Caspers J et al. Gadolinium Brain Deposition after Macrocyclic Gadolinium Administration: A Pediatric Case-Control Study. Radiology. 2017;285:223–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


