Abstract
Monitoring the antibiotic resistance of H. pylori is an important step in the effective treatment of this bacterium, thus the aim of the present study was to assess the prevalence of antimicrobial resistance of H. pylori strains isolated from pediatric and adult patients with primary infections in 2016–2018. Antral biopsies from 334 treatment-naïve patients (126 children and 208 adults) were obtained. A total of 71 clinical H. pylori strains (22 from children and 49 from adults) were isolated and examined for amoxicillin (AMX), clarithromycin (CLR), metronidazole (MTZ), tetracycline (TET), and levofloxacin (LEV) susceptibility. The activity of the antibiotics was measured by E-tests. Strains were considered as resistant to antibiotics with minimum inhibitory concentrations (MICs) equal to ≥0.125 μg/mL (AMX), ≥0.5 μg/mL (CLR), ≥8 μg/mL (MTZ), and ≥1 μg/mL (TET and LEV). The highest prevalence of antibiotic resistance in H. pylori strains was observed for CLR and MTZ, at frequencies of 54.5% and 31.8% vs. 30.6% and 46.9% for children and adults, respectively. A much lower frequency of isolation of resistant strains was demonstrated for LEV and TET, this being 9.1% and 4.5% vs. 18.4% and 4.1% for pediatric and adult patients, respectively. The presence of AMX-resistant strains was not observed. The H. pylori strains isolated from Polish patients with primary infections showed a high level of antibiotic resistance to CLR and MTZ (>30%).
Keywords: Helicobacter pylori, antibiotic resistance, antibiotic susceptibility, clarithromycin, metronidazole, levofloxacin
1. Introduction
Infectious diseases have been a challenge for humanity since the dawn of time [1]. Improving sanitation and hygiene conditions has enhanced this situation, while the discovery and widespread introduction of antibiotics is considered a breakthrough moment in the fight against microbes. These compounds revolutionized medicine and significantly improved our quality of life [2]. Their use, however, is not without side effects, with the most important being the massive and progressive spread of antibiotic-resistant microorganisms [1,2,3,4]. The problem of modern antibiotic therapy is associated with the very rare introduction of new substances, with most currently available having been discovered in the mid-20th century [1,2]. To raise public awareness of the threat arising from the growing problem of antibiotic resistance, in 2017 WHO created a list of priority pathogens [5].
Among the catalog of the 12 families/genera of bacteria posing the greatest threat to human health, Helicobacter pylori was mentioned [5]. Classically, this bacterium is acquired during childhood and is capable of establishing lifelong colonization [6]. In most cases, such individuals remain asymptomatic, while clinical symptoms may include a wide range of gastropathies, including gastric ulcers and cancers [6,7,8]. Given the high prevalence of H. pylori (half of humanity) and the significant impact on health and mortality, proper assessment and effective treatment of infections caused by this bacterium seem to be of immense importance [8]. One of the most important problems in H. pylori therapy is the high heterogeneity of this microorganism, resulting from intensive horizontal gene transfer and high mutation frequency [6,7,9,10]. Therefore, constant monitoring of H. pylori resistance, especially the primary one indicating patients’ regional antibiotic intake profile, is an important process of limiting the prevalence of this bacterium and reducing its harmful effect on human health [7,8,10,11].
The aim of the present study was to determine the primary antibiotic resistance of H. pylori in pediatric and adult patients in Southern Poland in 2016–2018. In addition, a relationship between gender and age and the frequency of H. pylori resistance to specific antibiotics was also determined.
2. Materials and Methods
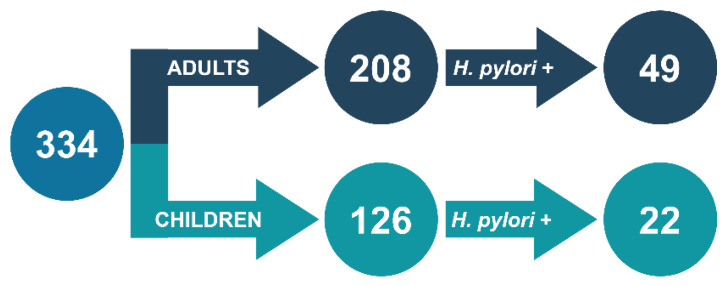
H. pylori strains were isolated at the Department of Microbiology, Wroclaw Medical University, Poland in 2016–2018 from biopsies obtained during routine gastroscopic examinations in four medical units in Wroclaw, Poland: Department and Clinic of Pediatrics, Gastroenterology and Nutrition; Department and Division of Surgical Didactics; Department of Gastroenterology and Hepatology; and J. Gromkowski Regional Specialist Hospital. Overall, 334 treatment-naïve patients were examined, including 126 children (0–15 years, 55 males and 71 females) and 208 adults (≥16 years, 68 males and 140 females). A total of 71 H. pylori strains from 22 pediatric (9 males and 13 females) and 49 adult (17 males and 32 females) patients were grown (Figure 1). Bacterial identification was made by confirming the positive reaction of urease, catalase, and oxidase, and the presence of spiral/curved Gram-negative rods. The research was approved by the bioethics commission of the Wroclaw Medical University (#111/2017 and #203/2019).
Figure 1.
Patient population and amount of H. pylori strains included in the study.
Bacterial cultures with a density of 3 McFarland scale units were sown on Mueller–Hinton (Becton Dickinson, Le Pont de Claix, France) media with 5% horse blood and incubated for 3–5 days under microaerophilic conditions and 37 °C. Determination of antibiotic sensitivity was performed using E-tests with amoxicillin (AMX, range: 0.016–256 µg/mL), clarithromycin (CLR, range: 0.016–256 µg/mL), metronidazole (MTZ, range: 0.016–256 µg/mL), tetracycline (TET, range: 0.016–256 µg/mL), and levofloxacin (LEV, range: 0.002–32 µg/mL), all from BioMerieux. H. pylori strains were considered resistant with the following limits: ≥0.125 µg/mL (AMX), ≥0.5 µg/mL (CLR), ≥1 µg/mL (TET and LEV) and ≥8 µg/mL (MTZ), in accordance with EUCAST 2019 recommendations [12].
Statistical analysis was performed using the STATISTICA program.
3. Results
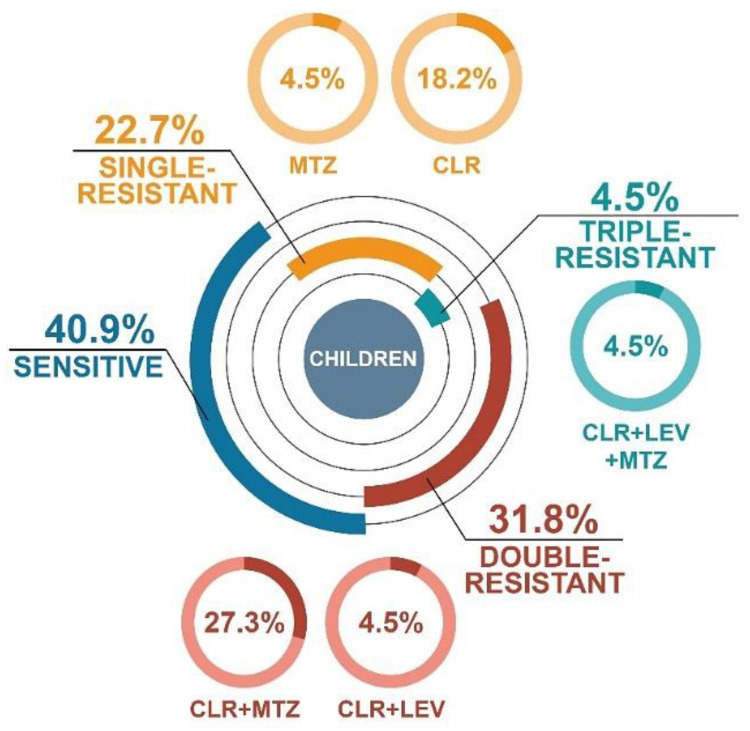
Among H. pylori isolates from pediatric patients, less than half of them were sensitive to the antibiotics tested (40.9%) (Figure 2). There were 22.7% single-resistant (18.2% CLR and 4.5% MTZ) and 31.8% double-resistant (27.3% CLR + MTZ and 4.5% CLR + LEV) strains. One isolate was triple-resistant (4.5%, CLR + LEV + TET). It is noteworthy that among thirteen resistant H. pylori strains obtained from children, eleven of them had monoresistance or co-resistance to CLR.
Figure 2.
Distribution of resistance profiles of H. pylori strains isolated from treatment-naïve pediatric patients from Poland during 2016–2018.
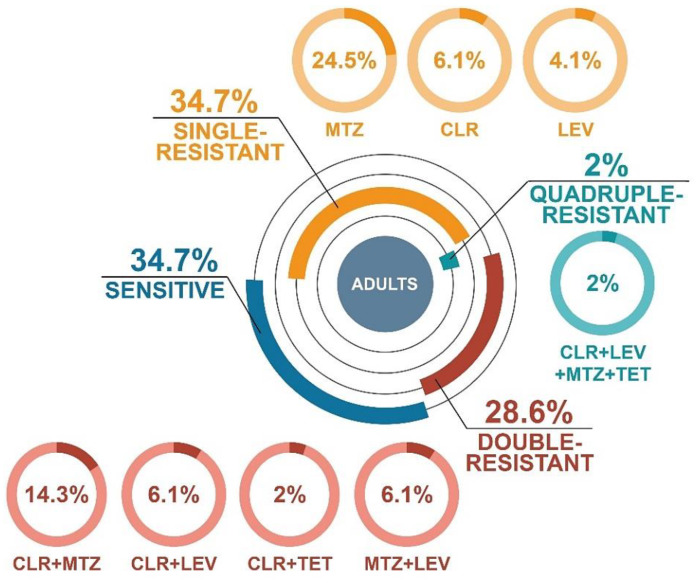
The resistance pattern in H. pylori isolates grown from adults had a similar distribution as in pediatric patients (Figure 3). In this case, the amount of both sensitive and single-resistant strains was 34.7% each. In the group of H. pylori strains with single resistance, MTZ resistance predominated (24.5%). Isolates with double resistance accounted for 28.6% (14.3% CLR + MTZ, 6.1% MTZ + LEV, 6.1% CLR + LEV, and 2% CLR + TET). One strain with quadruple resistance (2%, CLR + MTZ + TET + LEV) was observed.
Figure 3.
Distribution of resistance profiles of H. pylori strains isolated from treatment-naïve adults from Poland during 2016–2018.
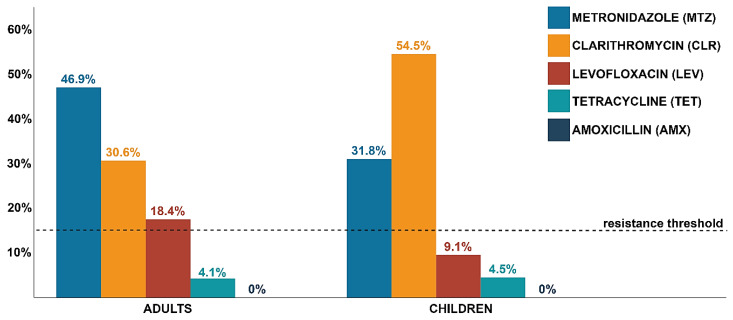
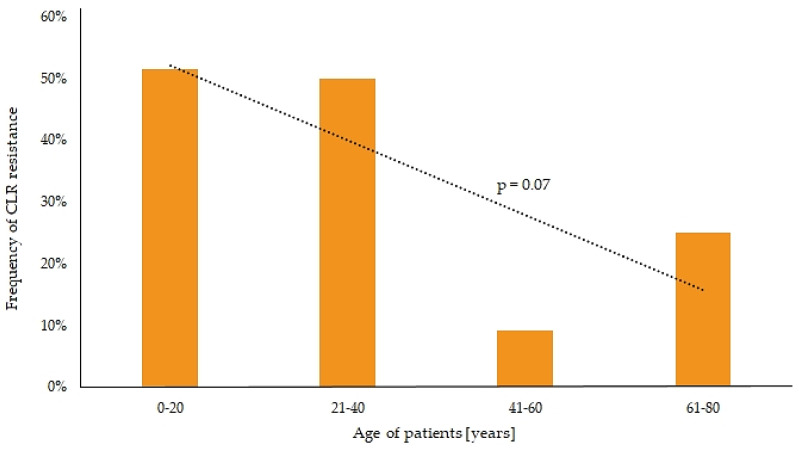
The highest frequencies of resistance among H. pylori strains were observed for CLR and MTZ, which were 30.6% and 54.5% and 46.9% and 31.8% in adults and children, respectively (Figure 4). Much lower prevalence was observed for LEV and TET, this being 18.4% and 9.1% and 4.1% and 4.5% in adult and pediatric patients, respectively. No AMX-resistant strains were seen in any group. Only for CLR-resistant H. pylori strains a statistically significant difference in the incidence between children and adults (p = 0.05) was observed (Figure 4). In addition, there was a negative tendency between increasing age and CLR resistance (p = 0.07) (Figure 5). The incidence of CLR-resistant H. pylori strains was around 50% in patients aged 0–40 years and it decreased in older age groups (9% and 25% in people aged 41–60 and 61–80 years, respectively). No relationship was seen between gender and resistance to any antibiotic (p > 0.05).
Figure 4.
The frequency of resistance to specific antibiotics among H. pylori strains isolated from treatment-naïve adult and pediatric patients from Poland during 2016–2018. The resistance threshold is equal to 15% and is considered as a contractual value above which the use of an antibiotic should not be taken into account in empirical therapies [13].
Figure 5.
Tendency between the frequency of clarithromycin (CLR) resistance among H. pylori strains and the age of patients.
4. Discussion
The crisis regarding antibiotic resistance is currently one of the most serious problems affecting humanity [3,14]. There are several factors responsible for this phenomenon, e.g., inadequate diagnostics (broad spectrum antibiotics, poorly selected antibiotics, antibiotic therapy for viral and fungal infections), inadequate dosage of drugs, and low vaccination frequency [14]. Analysis of global antibiotic consumption over the years 2000–2015 showed an increase in the use of these substances by 65%, and in the absence of appropriate programs controlling antibiotics consumption, in the next 15 years their intake will increase by 200% [15].
The use of antibiotics exerts selective pressure on microorganisms that colonize the human body [4]. Therefore, patients undergoing antibiotic therapies more often are exposed to a higher risk of infections with microbes resistant to antimicrobial substances. This is particularly important for bacteria colonizing their hosts for a prolonged period of time, such as H. pylori [6]. Exposure to monotherapy, aimed at eradicating other microorganisms, does not eliminate infection produced by H. pylori. Such treatment, however, can give the "bottleneck effect" on the population of this bacterium, contributing to the development of H. pylori populations resistant to specific groups of antibiotics.
The current article shows a high level of primary antibiotic resistance of H. pylori strains isolated from adults (30.6%, 46.9% and 18.4% for CLR, MTZ, and LEV, respectively) and children (54.5%, 31.8% and 9.1% for CLR, MTZ, and LEV, respectively). Reviewing reports from the last five years, it has been noticed that this prevalence is consistent with data from many European countries, including France [16], Italy [17,18], Spain [19,20] and Portugal [21], and concurrent with the multicenter study on H. pylori primary resistance in Europe from 2013 [11]. Comparison of our results with the study conducted 10 years ago in our research unit (Poland), regarding the primary resistance of H. pylori, indicates a persistent high level of CLR and MTZ resistance in both children and adults [22]. However, an increase in LEV resistance over the decade from 1.9% to 9.1% in pediatric patients and from 11.7% to 18.4% in adults has been observed. This is in line with reports from another research center in Poland, indicating an increasing resistance of H. pylori strains to LEV [23].
The alarming level of primary resistance of H. pylori is most likely correlated with the growing and uncontrolled intake of antibiotics used in the eradication of this bacterium [13]. This is confirmed by the global report on the consumption of antibiotics, which showed that the five most frequently consumed groups of these substances are β-lactams (penicillins and cephalosporins), macrolides, fluoroquinolones, tetracyclines, and trimethoprim [15]. Many studies have recognized the existence of a strong correlation between the intake of fluoroquinolones (urinary and respiratory tract infections), macrolides (respiratory tract infections), and nitroimidazoles (gynecological, parasitological and dental infections) in the treatment of other infections and the development of resistant H. pylori strains [11,24,25,26,27]. In the present study, the prevalence of CLR-resistant strains in children (54.5%) was almost twice as high as in adults (30.6%). Such a large discrepancy seems to be caused by a higher susceptibility of pediatric patients to respiratory infections and more frequent consumption of macrolides by this age group [28]. In addition, in the current study, a higher prevalence of LEV- and MTZ-resistant isolates was found in adults (18.4% and 46.9%, respectively) than pediatric patients (9.1% and 31.8%, respectively). These differences, although not statistically significant, correlate with reports of others, indicating a positive relationship between age of >50 years (higher exposure to urogenital infections) and the presence of H. pylori strains primarily resistant to LEV and MTZ [11,24,25]. The results obtained in this article seem to confirm the relationship between frequent consumption of antibiotics and the spread of H. pylori strains with primary resistance.
With the increase in resistance to antibiotics in H. pylori stains, appropriate attention should be paid to the choice of therapy (taking into account the local resistance profile and history of consumed antibiotics) [8]. The biggest challenge in the treatment of H. pylori in the world is resistance to CLR and LEV [9]. Resistance to MTZ although wide-spread can be overcome in vivo by extending the duration of therapy (up to 14 days) and increasing the dose (up to 1.5 g/day) [7,9,13]. Since the incidence of primary resistance to CLR and LEV in H. pylori strains isolated in Poland has exceeded 15%, the conventional threshold of acceptable prevalence [13], therapies with these antibiotics is discouraged. Based on this, according to the authors of the article, it is reasonable to use bismuth quadruple therapy (bismuth salts, MTZ, TET, and proton pumps inhibitor [PPIs]) as a first line treatment [7,29], because in this case resistance to MTZ does not significantly affect the therapeutic result [30,31]. It should be borne in mind, however, that this applies to strains with low resistance levels (8–32 µg/mL), and higher resistance (>32 µg/mL) may reduce the effectiveness of this therapy from >90% to 60% [32]. Very promising results in the Asian population have been obtained for high-dose double therapy (AMX and PPIs), i.e., eradication rate >90% and low level of side effects (<15%) [33,34]. Avoiding the use of antibiotics with high frequency of resistance among H. pylori strains and the use of AMX, to which no resistance is currently observed in Poland, give hope for a wider use of this therapy. Research on the European population is undoubtedly relevant.
5. Conclusions
The level of primary resistance to CLR and MTZ of H. pylori strains in both adults and children in Poland is high (>30%). The prevalence of CLR resistance is negatively correlated with increasing age and is almost twice as high in pediatric patients as in adults.
Author Contributions
Conceptualization, G.G. and F.M.; methodology, P.K., M.B., and G.G.; formal analysis, P.K.; investigation, P.K., M.B., and G.G.; samples collection, B.I., D.P., R.K., and K.L.; data curation, P.K. and G.G.; statistical analysis, Ł.Ł.; writing—original draft preparation, P.K.; writing—review and editing, P.K. and G.G.; visualization, P.K.; supervision, G.G. and F.M.; funding acquisition, P.K. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Wroclaw Medical University grant No: SUB.A130.19.021 and grant No: STM.A130.20.002. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.da Cunha B.R., Fonseca L.P., Calado C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics. 2019;8:45. doi: 10.3390/antibiotics8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson L.A. Understanding and Overcoming Antibiotic Resistance. PLoS Biol. 2017;15:e2003775. doi: 10.1371/journal.pbio.2003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venter H., Henningsen M.L., Begg S.L. Antimicrobial Resistance in Healthcare, Agriculture and the Environment: The Biochemistry Behind the Headlines. Essays Biochem. 2017;61:1–10. doi: 10.1042/EBC20160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cižman M., Plankar Srovin T. Antibiotic Consumption and Resistance of Gram-negative Pathogens (Collateral Damage) GMS Infect. Dis. 2018;6:Doc05. doi: 10.3205/id000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 6.Ailloud F., Didelot X., Woltemate S., Pfaffinger G., Overmann J., Bader R.C., Schulz C., Malfertheiner P., Suerbaum S. Within-host Evolution of Helicobacter pylori Shaped by Niche-specific Adaptation, Intragastric Migrations and Selective Sweeps. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori Infection—The Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 8.Guevara B., Cogdill A.G. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig. Dis. Sci. 2020 doi: 10.1007/s10620-020-06193-7. in press. [DOI] [PubMed] [Google Scholar]
- 9.Mégraud F. Antibiotic Resistance Is the Key Element in Treatment of Helicobacter pylori Infection. Gastroenterology. 2018;155:1300–1302. doi: 10.1053/j.gastro.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Thung I., Aramin H., Vavinskaya V., Gupta S., Park J.Y., Crowe S.E., Valasek M.A. Review article: The Global Emergence of Helicobacter pylori Antibiotic Resistance. Aliment. Pharmacol. Ther. 2016;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megraud F., Coenen S., Versporten A., Kist M., Lopez-Brea M., Hirschl A.M., Andersen L.P., Goossens H., Glupczynski Y. Study Group participants. Helicobacter pylori Resistance to Antibiotics in Europe and Its Relationship to Antibiotic Consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 12.EUCAST The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 9.0. [(accessed on 28 March 2020)];Eur. Comm. Antimicrob. Susceptibility Test. 2019 Available online: http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- 13.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha P., Cooper B.S., Coast J., Oppong R., Do Thi Thuy N., Phodha T., Celhay O., Guerin P.J., Wertheim H., Lubell Y. Enumerating the Economic Cost of Antimicrobial Resistance per Antibiotic Consumed to Inform the Evaluation of Interventions Affecting their Use. Antimicrob. Resist. Infect. Control. 2018;7:98. doi: 10.1186/s13756-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., Goossens H., Laxminarayan R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducournau A., Bénéjat L., Sifré E., Bessède E., Lehours P., Mégraud F. Helicobacter pylori Resistance to Antibiotics in 2014 in France Detected by Phenotypic and Genotypic Methods. Clin. Microbiol. Infect. 2016;22:715–718. doi: 10.1016/j.cmi.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Saracino I.M., Fiorini G., Zullo A., Pavoni M., Saccomanno L., Vaira D. Trends in Primary Antibiotic Resistance in H. pylori Strains Isolated in Italy between 2009 and 2019. Antibiotics. 2020;9:26. doi: 10.3390/antibiotics9010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorini G., Zullo A., Saracino I.M., Pavoni M., Vaira D. Antibiotic Resistance Pattern of Helicobacter pylori Strains Isolated in Italy during 2010–2016. Scand. J. Gastroenterol. 2018;53:661–664. doi: 10.1080/00365521.2018.1464596. [DOI] [PubMed] [Google Scholar]
- 19.Morilla A.M., Álvarez-Argüelles M.E., Duque J.M., Armesto E., Villar H., Melón S. Primary Antimicrobial Resistance Rates and Prevalence of Helicobacter pylori Infection in the North of Spain. A 13-year Retrospective Study. Gastroenterol. Hepatol. 2019;42:476–485. doi: 10.1016/j.gastrohep.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Macías-García F., Llovo-Taboada J., Díaz-López M., Bastón-Rey I., Domínguez-Muñoz J.E. High Primary Antibiotic Resistance of Helicobacter pylori Strains Isolated from Dyspeptic Patients: A Prevalence Cross-sectional Study in Spain. Helicobacter. 2017;22:e12440. doi: 10.1111/hel.12440. [DOI] [PubMed] [Google Scholar]
- 21.Almeida N., Romãozinho J.M., Donato M.M., Luxo C., Cardoso O., Cipriano M.A., Marinho C., Fernandes A., Calhau C., Sofia C. Helicobacter pylori Antimicrobial Resistance Rates in the Central Region of Portugal. Clin. Microbiol. Infect. 2014;20:1127–1133. doi: 10.1111/1469-0691.12701. [DOI] [PubMed] [Google Scholar]
- 22.Gościniak G., Biernat M., Grabińska J., Bińkowska A., Poniewierka E., Iwańczak B. The Antimicrobial Susceptibility of Helicobacter pylori Strains Isolated from Children and Adults with Primary Infection in the Lower Silesia Region, Poland. Polish J. Microbiol. 2014;63:57–61. doi: 10.33073/pjm-2014-008. [DOI] [PubMed] [Google Scholar]
- 23.Karczewska E., Klesiewicz K., Skiba I., Wojtas-Bonior I., Sito E., Czajecki K., Zwolińska-Wcisło M., Budak A. Variability in Prevalence of Helicobacter pylori Strains Resistant to Clarithromycin and Levofloxacin in Southern Poland. Gastroenterol. Res. Pract. 2012;2012:418010. doi: 10.1155/2012/418010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carothers J.J., Bruce M.G., Hennessy T.W., Bensler M., Morris J.M., Reasonover A.L., Hurlburt D.A., Parkinson A.J., Coleman J.M., McMahon B.J. The Relationship between Previous Fluoroquinolone Use and Levofloxacin Resistance in Helicobacter pylori Infection. Clin. Infect. Dis. 2007;44:e5–e8. doi: 10.1086/510074. [DOI] [PubMed] [Google Scholar]
- 25.McMahon B.J., Hennessy T.W., Bensler J.M., Bruden D.L., Parkinson A.J., Morris J.M., Reasonover A.L., Hurlburt D.A., Bruce M.G., Sacco F., et al. The Relationship among Previous Antimicrobial Use, Antimicrobial Resistance, and Treatment Outcomes for Helicobacter pylori Infections. Ann. Intern. Med. 2003;139:463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon C. Population-level Macrolide Consumption is Associated with Clarithromycin Resistance in Helicobacter pylori: An Ecological Analysis. Int. J. Infect. Dis. 2019;85:67–69. doi: 10.1016/j.ijid.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Boyanova L., Ilieva J., Gergova G., Davidkov L., Spassova Z., Kamburov V., Katsarov N., Mitov I. Numerous Risk Factors for Helicobacter pylori Antibiotic Resistance Revealed by Extended Anamnesis: A Bulgarian Study. J. Med. Microbiol. 2012;61:85–93. doi: 10.1099/jmm.0.035568-0. [DOI] [PubMed] [Google Scholar]
- 28.Megraud F. Helicobacter pylori and Antibiotic Resistance. Gut. 2007;56:1502. doi: 10.1136/gut.2007.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyssen O.P., Pérez-Aisa Á., Tepes B., Rodrigo-Sáez L., Romero P.M., Lucendo A., Castro-Fernández M., Phull P., Barrio J., Bujanda L. Helicobacter pylori First-line and Rescue Treatments in Patients Allergic to Penicillin: Experience from the European Registry on H pylori Management (Hp-EuReg) Helicobacter. 2020:e12686. doi: 10.1111/hel.12686. in press. [DOI] [PubMed] [Google Scholar]
- 30.Malfertheiner P., Bazzoli F., Delchier J.C., Celiñski K., Giguère M., Rivière M., Mégraud F. Helicobacter pylori Eradication with a Capsule Containing Bismuth Subcitrate Potassium, Metronidazole, and Tetracycline Given with Omeprazole versus Clarithromycin-based Triple Therapy: A Randomised, Open-label, Non-inferiority, Phase 3 Trial. Lancet. 2011;377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W., Chen Q., Liang X., Liu W., Xiao S., Graham D.Y., Lu H. Bismuth, Lansoprazole, Amoxicillin and Metronidazole or Clarithromycin as First-line Helicobacter pylori Therapy. Gut. 2015;64:1715–1720. doi: 10.1136/gutjnl-2015-309900. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.W., Kim N., Nam R.H., Lee S.M., Soo In C., Kim J.M., Lee D.H. Risk Factors of Rescue Bismuth Quadruple Therapy Failure for Helicobacter pylori Eradication. J. Gastroenterol. Hepatol. 2019;34:666–672. doi: 10.1111/jgh.14625. [DOI] [PubMed] [Google Scholar]
- 33.Yu L., Luo L., Long X., Liang X., Ji Y., Graham D.Y., Lu H. High-dose PPI-amoxicillin Dual Therapy with or without Bismuth for First-line Helicobacter pylori Therapy: A Randomized Trial. Helicobacter. 2019;24:e12596. doi: 10.1111/hel.12596. [DOI] [PubMed] [Google Scholar]
- 34.Yang X., Wang J.X., Han S.X., Gao C.P. High Dose Dual Therapy versus Bismuth Quadruple Therapy for Helicobacter pylori Eradication Treatment: A Systematic Review and Meta-Analysis. Medicine (United States) 2019;98:e14396. doi: 10.1097/MD.0000000000014396. [DOI] [PMC free article] [PubMed] [Google Scholar]