Abstract
Introduction
As non-communicable disease (NCD) burden rises worldwide, community-based programmes are a promising strategy to bridge gaps in NCD care. The HealthRise programme sought to improve hypertension and diabetes management for underserved communities in nine sites across Brazil, India, South Africa and the USA between 2016 and 2018. This study presents findings from the programme’s endline evaluation.
Methods
The evaluation utilises a mixed-methods quasi-experimental design. Process indicators assess programme implementation; quantitative data examine patients’ biometric measures and qualitative data characterise programme successes and challenges. Programme impact was assessed using the percentage of patients meeting blood pressure and A1c treatment targets and tracking changes in these measures over time.
Results
Almost 60 000 screenings, most of them in India, resulted in 1464 new hypertension and 295 new diabetes cases across sites. In Brazil, patients exhibited statistically significant reductions in blood pressure and A1c. In Shimla, India, and in South Africa, country with the shortest implementation period, there were no differences between patients served by facilities in HealthRise areas relative to comparison areas. Among participating patients with diabetes in Hennepin and Ramsey counties and hypertension patients in Hennepin County, the percentage of HealthRise patients meeting treatment targets at endline was significantly higher relative to comparison group patients. Qualitative analysis identified linking different providers, services, communities and information systems as positive HealthRise attributes. Gaps in health system capacities and sociodemographic factors, including poverty, low levels of health education and limited access to nutritious food, are remaining challenges.
Conclusions
Findings from Brazil and the USA indicate that the HealthRise model has the potential to improve patient outcomes. Short implementation periods and strong emphasis on screening may have contributed to the lack of detectable differences in other sites. Community-based care cannot deliver its full potential if sociodemographic and health system barriers are not addressed in tandem.
Keywords: diabetes, hypertension, public health
Key questions.
What is already known?
Cardiovascular disease and diabetes represented 17.4% of the global burden of disease in 2017, however, only 2% of overall development assistance for health was dedicated to combating non-communicable disease (NCD) in that same year.
Prior research suggests that community-based interventions can be both cost saving and associated with improved outcomes among patients with hypertension and diabetes in countries and communities across a broad range of socioeconomic settings.
What are the new findings?
Patients affiliated with HealthRise programmes in Brazil and the USA showed progress in meeting diabetes and hypertension treatment targets and declining blood pressure and haemoglobin A1c since programme enrolment.
In India and South Africa, no detectable differences in blood pressure or A1c levels were observed between patients being served by facilities involved in the HealthRise programme and facilities that were not affiliated with HealthRise.
Existing health systems infrastructure and social determinants of health limit the potential effect of community-based programmes aimed at improving the detection, treatment and care of hypertension and diabetes.
What do the new findings imply?
Continued work is needed to understand which community-based NCD interventions may work best given local contexts and needs.
Health system strengthening, increased financing for NCDs and a locally driven focus on how interventions and community factors together, may contribute to improving health for individuals.
Introduction
Hypertension and diabetes account for increasingly more early death and illness worldwide,1–3 particularly in places where rapid sociodemographic changes have spurred shifts in diet, physical activity and other key risk factors for non-communicable diseases (NCDs). Despite cardiovascular diseases and diabetes resulting in 17.4% of the global disease burden in 2017,2 these conditions and NCDs more broadly remain severely underfunded by national governments and donors alike, with only 2% of overall development assistance for health dedicated to NCDs in 2017.4
Challenges in implementing models of care that address chronic diseases persist in low-income and middle-income countries (LMICs),5–7 where many health systems are not equipped to reliably diagnose and treat NCDs,8–11 and also in high-income countries, where medication affordability and health insurance can be major obstacles to high-quality care.12 13 As a result, sizeable gaps observed along the cascade of care—diagnosing, treating and controlling disease—emphasise the need to better reach patients, retain them in care and promote effective interventions for disease management.5 14–19
Community-based programmes, which bridge communities and health systems and include a range of interventions such as education and outreach, self-management and home-based care, have emerged as a promising approach to filling gaps in access.20–22 Past research suggests community-based interventions focused on hypertension and diabetes detection and case management can be both cost saving and associated with improved outcomes.23–26 Specifically, results from LMICs include lower blood pressure (BP) associated with mobile technology-supported primary healthcare interventions in Indonesia27; decreased BP related to a community health worker (CHW)-led chronic disease programme in rural Uganda28; reduced BP and blood glucose associated with Iran’s rural Behvarz system29; better hypertension control for patients receiving CHW home visits in Gauteng, South Africa30 and higher odds of hypertension or diabetes control related to CHW-led interventions in Chiapas, Mexico.31 Some studies indicate community-based screening for hypertension and diabetes can be effective,32–35 but how well these activities identify undiagnosed or at-risk individuals across underserved communities is less well known.
To strengthen the evidence base for community-based NCD interventions, HealthRise was developed to implement and evaluate pilot programmes aimed to improve screening, diagnosis, management, and control of hypertension and diabetes among underserved communities.36 37 HealthRise was launched in 2014 and pilot programmes were implemented in nine communities in Brazil, India, South Africa and the USA between 2016 and 2018 and were composed of interventions tailored to local needs and contexts. A prospective evaluation of these initiatives at each site sought to assess whether these community-based programmes could increase the proportion of patients meeting treatment targets for diabetes and hypertension compared with usual care. This study presents the main cross-country findings, aiming to provide insights for community-based NCD programmes targeting underserved populations globally.
Methods
Study design
A global evaluation framework was designed prior to intervention implementation at all sites and is presented in online supplementary file 1 and described elsewhere.36 37 Briefly, we used a mixed-methods quasi-experimental approach, drawing from process indicators to assess programme implementation; quantitative data to measure changes over time and/or differences in patient outcomes associated with programme participation and qualitative data to contextualise patients, providers and stakeholders’ experiences with HealthRise. Process and endline evaluation methods and indicators ultimately varied across sites due to data availability, resource constraints and government regulations; however, each location’s study design aligned with the global evaluation framework.
bmjgh-2019-001959supp001.pdf (1.7MB, pdf)
HealthRise programme
HealthRise involved several partners for each site in Brazil (Teófilo Otoni and Vitória da Conquista), India (Udaipur and Shimla), South Africa (uMgungundlovu and Pixley ka Seme) and the USA (Minnesota’s Rice, Hennepin and Ramsey Counties) to support programme implementation and evaluation. Online supplementary file 2 details the programme organisational structure and additional detail also can be found elsewhere.36 37 Countries were selected by the Medtronic Foundation, the funder of HealthRise, prior to programme implementation and evaluation onset, and pilot sites were determined by a combination of factors (ie, range of underserved populations with existing health service gaps, high NCD burden, interest of government and nongovernmental partners). A summary description of the health system in each of the selected countries is presented in online supplementary file 3. Programme implementation and monitoring duration varied by site, as shown in online supplementary file 4, with India and USA having the longest implementation periods (2016–2018), and Brazil and South Africa having the shortest (2017–2018). The Institute for Health Metrics and Evaluation (IHME) conducted the evaluation, and was not involved in pilot programme design or implementation.
bmjgh-2019-001959supp002.pdf (375.8KB, pdf)
bmjgh-2019-001959supp003.pdf (181.7KB, pdf)
bmjgh-2019-001959supp004.pdf (212.7KB, pdf)
Table 1 summarises each site’s target population, health demands and challenges (as identified by baseline needs assessments conducted by IHME38–41), and programme implementation dates and main interventions; greater detail on each intervention can be found elsewhere.36 Intervention composition and implementation inherently varied by HealthRise site, as they sought to explicitly address gaps and barriers identified through the needs assessments. Across sites, interventions were implemented alongside usual care and services provided previously, and involved healthcare worker training, health education, patient empowerment activities and regular monitoring of programme enrollees. Individual sites often had unique interventions and implementation approaches (eg, interventions focusing on lifestyle modification included building three outdoor public gyms at primary healthcare units in Vitória da Conquista and building a full-service grocery store with an in-house interdisciplinary wellness team in Hennepin County), as well as main locations of implementation. While most sites’ interventions occurred at primary care clinics or units, others extended to secondary care (eg, increased availability of specialised tests in Teófilo Otoni) and the workplace (eg, workplace-based screening in Vitória da Conquista and uMgungundlovu).
Table 1.
Overview of interventions by HealthRise site
| Site | Implementation | Key characteristics/challenges of communities served by HealthRise | Key HealthRise interventions and activities |
| Brazil | |||
| Teófilo Otoni region (comprises 10 municipalities), Minas Gerais State | May 2017 to December 2018 |
|
|
| Vitória da Conquista, Bahia State | March 2017 to December 2018 |
|
|
| India | |||
| Udaipur, Rajasthan | June 2016 to November 2018 |
|
|
| Shimla, Himachal Pradesh | June 2016 to November 2018 |
|
|
| South Africa | |||
| Pixley ka Seme, Northern Cape | March 2017 to August 2018 |
|
|
| uMgungundlovu, KwaZulu-Natal | February 2017 to August 2018 |
|
|
| USA | |||
| Hennepin County, Minnesota | July 2016 to September 2018 |
|
|
| Ramsey County, Minnesota | June 2016 to September 2018 |
|
|
| Rice County, Minnesota | September 2016 to October 2018 |
|
|
More detailed descriptions of HealthRise interventions in each country, as provided by grantees and compiled by Abt Associates, are published elsewhere.36
CHW, community health worker; DM, diabetes mellitus; HTN, hypertension; NCD, non-communicable disease.
Case definitions
The following case definitions were used across all sites: for hypertension, (1) prevalent cases were patients with a reported or documented diagnosis of hypertension based on country-specific guidelines, or those without a previous or current diagnosis but had systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg at the time of data collection; (2) diagnosed cases were patients with a reported or documented diagnosis of hypertension and (3) patients meeting treatment targets were prevalent cases with SBP <140 mm Hg and DBP <90 mm Hg. For diabetes, (1) prevalent cases were patients with a reported or documented diagnosis of diabetes based on country-specific guidelines, or those without a previous diagnosis but had haemoglobin A1c ≥6.5% at the time of data collection; (2) diagnosed cases were patients with a reported or documented diagnosis of diabetes and (3) patients meeting treatment targets were prevalent cases with A1c <8%.
Data collection
All survey instruments were designed by IHME with input from local evaluation partners and translated to the appropriate languages and are available at http://www.healthdata.org/healthrise-evaluation/data-collection-tools
Process indicators (eg, screening, health workers training indicators) and endline quantitative and qualitative data collection varied by programme site, a result of the variability in site-specific programme activities, intervention populations, and existing data infrastructure. In brief, de-identified patient or medical record extractions were available for Brazil and the USA while patient exit surveys were performed at facilities in Shimla and South Africa. Where possible, comparison group data were collected or drawn from available databases on comparable patient populations. Details on site-specific endline data collection processes and sources are available in online supplementary file 5, including descriptions of sampling procedures for exit surveys. Qualitative data consisted of key informant interviews with facility administrators, clinic- and home-based providers, and policymakers as well as focus group discussions with patients, which were facilitated by either IHME (US only) or independent local data collection professionals contracted by IHME (Brazil, India and South Africa). Endline data for Udaipur, India are not currently available due to delays in government approval for data collection. Table 2 summarises data availability and sample sizes by site and data type.
Table 2.
Endline data availability and patient sample sizes by HealthRise site and for intervention and comparison patients
| Data collection | Brazil | India | South africa | Minnesota, United States | ||||
| Teófilo otoni | Vitória da conquista | Shimla | Pixley ka seme | uMgungundlovu | Hennepin county | Ramsey county | Rice county | |
| HealthRise (intervention) | ||||||||
| Quantitative | Baseline, endline | Baseline, endline | Endline | Endline | Endline | Baseline, endline | Baseline, endline | Baseline, endline |
| Total patients (interviewed or enrolled)* | 4210 | 2610 | 277 | 62 | 88 | 121 | 78 | 217 |
| Health facilities for patient interviews | – | – | 18 | 7 | 7 | – | – | – |
| Total patients enrolled at endline | – | – | – | – | – | 104 | 53 | 217 |
| Patients with hypertension | 3992 | 2443 | 224 | 45 | 74 | 82 | 21 | 72 |
| Patients with hypertension and biometric data | 1169 | 1095 | 222 | 38 | 67 | 77 | 21 | 71 |
| Patients with diabetes | 1028 | 1052 | 110 | 38 | 42 | 76 | 46 | 120 |
| Patients with diabetes and biometric data | 176 | 68 | 110 | 37 | 38 | 60 | 41 | 92 |
| Qualitative | ||||||||
| Total interviews and focus groups | 22 | 15 | 19 | 5 | 10 | 5 | 9 | 6 |
| Patient focus groups | 5 | 4 | 5 | – | 4 | – | – | – |
| Community health workers (CHWs) and frontline health workers† | 10 | 7 | 3 | – | 3 | 2 | 3 | 2 |
| Facility-based or clinic-based providers | – | – | 3 | 3 | 2 | 1 | 3 | 2 |
| Facility or clinic managers and administrators | 5 | 3 | 4 | 2 | 1 | 2 | 3 | 2 |
| Policy-makers | 2 | 1 | – | – | – | 3 | ||
| Other‡ | – | – | 4 | – | – | – | – | – |
| Comparison | ||||||||
| Quantitative | – | – | Endline | Endline | Endline | Baseline, endline | Baseline, endline | Baseline, endline |
| Total patients (interviewed or enrolled)* | – | – | 230 | 123 | 149 | – | – | – |
| Health facilities for patient interviews | – | – | 12 | 12 | 14 | – | – | – |
| Total patients enrolled at endline | – | – | – | – | – | 107 | 99 | 311 |
| Patients with hypertension | – | – | 165 | 107 | 125 | 84 | 58 | 178 |
| Patients with hypertension and biometric data | – | – | 160 | 91 | 122 | 84 | 58 | 172 |
| Patients with diabetes | – | – | 109 | 62 | 76 | 28 | 77 | 303 |
| Patients with diabetes and biometric data | – | – | 109 | 62 | 73 | 13 | 71 | 296 |
| Qualitative | – | – | – | |||||
| Total interviews and focus groups | 12 | 8 | 13 | 7 | 11 | – | – | – |
| Patient focus groups | 3 | 2 | 4 | – | 2 | – | – | – |
| CHWs and frontline health workers† | 6 | 4 | 3 | – | 6 | – | – | – |
| Facility-based or clinic-based providers | – | – | 3 | 3 | – | – | – | – |
| Facility or clinic managers and administrators | 3 | 2 | 3 | 4 | 3 | – | – | – |
| Policy-makers | – | – | – | – | – | – | – | – |
| Other | – | – | – | – | – | – | – | – |
Total patients for Shimla, India and South Africa reflect patients surveyed at facilities as part of the endline evaluation, and not those formally enrolled in HealthRise. Patients with hypertension or diabetes and biometric data for Brazil and US sites are prevalent cases of hypertension or diabetes and had at least two biometric measure.
*For Shimla and South Africa sites, total patients are those surveyed at facilities as part of endline evaluation data collection, not those enrolled in HealthRise.
†For Shimla, household-based health workers (eg, ASHAs and outreach workers) were included in the CHW and front-line healthworker category.
‡For Shimla, other included HealthRise master trainers and a HealthRise grantee official from MAMTA.
ASHAs, accredited social health activists.
bmjgh-2019-001959supp005.pdf (255.1KB, pdf)
Endline evaluation analysis
Quantitative data
We used two main outcome indicators for both diabetes and hypertension across sites: (1) the proportion of HealthRise patients (Brazil and USA) or those surveyed at facilities (Shimla and South Africa) meeting defined treatment targets and (2) average change in biometric measures among enrolled patients (Brazil and USA). We limited all analyses to patients who were prevalent cases and had corresponding biometric data.
For Brazil, at least two biometric readings were needed: one at HealthRise enrolment (baseline) and the most recent measure by endline. We ran paired-sample t-tests to assess whether statistically significant changes in hypertension and diabetes measures—per cent meeting treatment targets and average biometric readings—occurred for HealthRise patients from baseline to endline readings.
For the USA, where we had biometric readings at baseline and endline for HealthRise patients and for comparison groups at each site, we conducted difference-in-difference analyses to quantify the potential effect of intervention exposure. We first ran an unadjusted model, only including binary variables for HealthRise status and timing (ie, baseline or endline) and then an interaction term for HealthRise at endline to capture the effect of HealthRise over time. We also considered an adjusted model, including the following covariates to account for potential systematic differences in US HealthRise and comparison patients: sex (female, male); age (<50 years, 50 years or older); time elapsed from baseline to endline (<12 months, 12 months or more) and comorbidities at baseline (prevalent case of only hypertension or diabetes, prevalent case of both hypertension and diabetes).
In Shimla and both sites in South Africa, we used the average of up to three SBP and DBP measures taken as overall BP status for each patient. Patients also reported treatment status, ever and current (ie, taken medication for hypertension or diabetes as prescribed by a healthcare provider in the last 2 weeks), allowing us to assess cascades of care from diagnosis to meeting treatment targets among prevalent cases. We ran Welch’s t-tests (ie, assuming unequal variance between groups) to evaluate whether statistically detectable differences were observed between patients presenting at facilities in implementation and comparison areas.
Qualitative data
Key informant interviews and focus group discussions were audio recorded, and as necessary, translated and transcribed in English. Each transcription was read (or audio file was listened to) multiple times by a single researcher who assessed open-ended questionnaire responses using thematic analysis.42 Themes were identified at the semantic level. Data were entered into excel templates for analysis with a focus on data patterns associated with overarching research questions. Data codes were collated to generate themes by site. Qualitative data analysis presented in this study is limited to common themes across intervention sites only.
Patient and public involvement
Patients were not involved in the design or the development of the HealthRise evaluation.
Results
Across Brazil, India and South Africa sites, 59 342 people without a previous hypertension diagnosis and 56 642 individuals not previously diagnosed with diabetes were screened at public screening events, home visits or at health clinics through HealthRise programmes (table 3). The majority of screenings were conducted in India (78.8% for hypertension and 69.7% for diabetes). Of those screened for hypertension, 6439 surpassed diagnostic thresholds for elevated BP and were referred to health facilities for diagnostic confirmation, of which 1464 were newly diagnosed with hypertension. Of those with no previous diabetes, 2563 exceeded diagnostic thresholds for elevated blood glucose and were referred for further testing, resulting in 295 new diabetes diagnoses.
Table 3.
HealthRise screening and diagnosis outputs for programme sites in Brazil, India and South Africa
| Data collection | Across HealthRise sites | Brazil | India | South africa | |||
| Teófilo otoni | Vitória da conquista | Udaipur | Shimla | Pixley ka seme | uMgungun-dlovu | ||
| Hypertension | |||||||
| Individuals screened* | 59 342 | 3129 | 2315 | 26 144 | 20 606 | 2366 | 4782 |
| Individuals screened above threshold† | 6439 | 871 | 626 | 835 | 2214 | 677 | 1216 |
| Individuals screened and newly diagnosed | 1464 | 190 | 233 | 264 | 555 | 93 | 129 |
| Diabetes | |||||||
| Individuals screened* | 56 642 | 5396 | 3609 | 17 994 | 21 482 | 3570 | 4591 |
| Individuals screened above threshold‡ | 2563 | 125 | 499 | 839 | 900 | 71 | 129 |
| Individuals screened and newly diagnosed | 295 | 40 | 44 | 107 | 56 | 24 | 24 |
*Individuals with no previously reported diagnosis of hypertension or diabetes and participated in a HealthRise-supported screening activity.
†SBP ≥140 mm Hg or DBP ≥90 mm Hg.
‡Random blood glucose (RBG) measure of ≥140 mg/dL in Vitória da Conquista and both India sites; fasting glucose (FG) ≥126 mg/dL or blood glucose of 200 mg/dL following a glucose tolerance test in both South Africa sites; and a RBG reading of ≥200 mg/dL with at least one classical diabetes symptom (polyuria, polydipsia or polyphagia) or a FG ≥126 mg/dL following a cardiovascular risk assessment (patients with a body mass index ≥25; or age ≥45, or at least moderate cardiovascular disease risk were referred to get a FG test at the health facility) in Teófilo Otoni.
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Across all nine sites, 3637 local healthcare professionals were trained on diabetes and hypertension-related topics through HealthRise programmes: 979 in Brazil, 1847 in India, 778 in South Africa and 33 in the USA. CHWs comprised 60.7% of all healthcare professionals trained. A summarised country-specific flow chart of HealthRise participants and additional process evaluation indicators, by site, are in online supplementary file 6.
bmjgh-2019-001959supp006.pdf (4MB, pdf)
Endline quantitative findings
Differences or changes in hypertension and diabetes metrics varied across sites. In Brazil and the USA, where patient-level changes since programme enrolment could be tracked, HealthRise patients generally saw progress in reducing biometric measures and meeting treatment targets. In Vitória da Conquista, more patients met treatment targets for hypertension (45.9% (43.0%–48.9%)) and diabetes (61.8% (49.4%–72.7%)) at endline than at baseline (35.4% (32.6%–38.6%), p<0.001; and 36.8% (26.0%–49.1%), p<0.001, respectively), and patients showed declines for SBP (ie, an average decrease of 4.2 mm Hg (3.1–5.2); p<0.001) and A1c (ie, an average reduction of 0.9 (0.5–1.4); p<0.001) since programme enrolment. Teófilo Otoni HealthRise patients also recorded reductions in SBP (ie, an average decrease of 1.9 mm Hg (0.7–3.1); p<0.01) and A1c (ie, an average decline of 0.6 for A1c (0.4–0.9); p<0.001), with more patients meeting hypertension treatment targets at endline (52.2% (49.3–55%)) compared with baseline (48.3% (45.5–51.2%); p<0.05). For diabetes, more patients met treatment targets at endline (59.7% (52.3–67.0%)) than at baseline [49.4% (42.0–56.9%); p<0.01). Detailed changes in hypertension and diabetes metrics in both Brazilian sites are presented in online supplementary file 7.
bmjgh-2019-001959supp007.pdf (298.6KB, pdf)
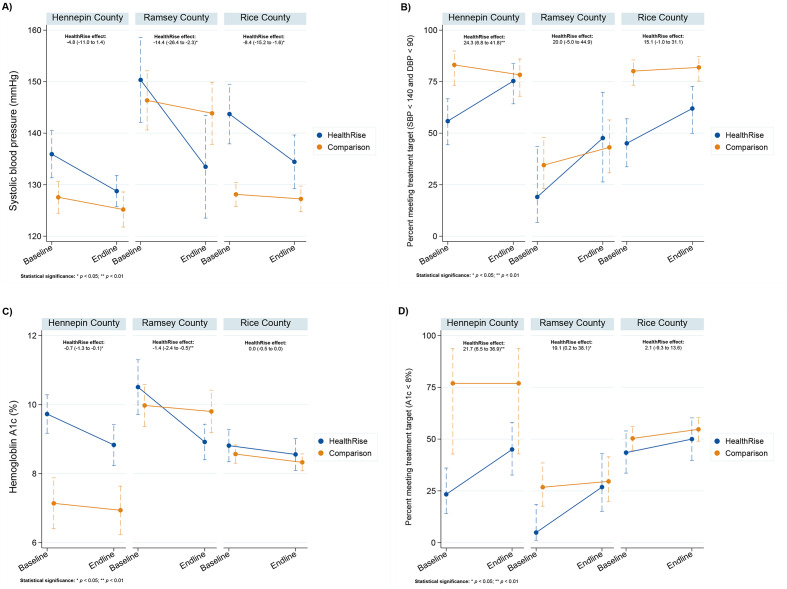
In the USA, unadjusted difference-in-difference model results (figure 1; all model results are included in online supplementary file 8) show that HealthRise programme participation was associated with reductions in SBP compared with comparison patients in Ramsey (14.4 mm Hg decline (2.3–26.4); p<0.05) and Rice (8.4 mm Hg decrease (1.6–15.2); p<0.05) counties. In only Hennepin County, HealthRise participation was associated with an increase in the percent of hypertension patients meeting treatment targets (24.3% point increase (6.8–41.8); p<0.01) relative to comparison patients. For patients with diabetes, HealthRise participation was associated with declines in A1c relative to comparison groups in Hennepin (0.7 decrease in A1c (0.1–1.3); p<0.05) and Ramsey (1.4 decrease in A1c (0.5–2.4); p<0.01) counties. The percentage of HealthRise patients with diabetes meeting treatment targets increased compared with comparison patients in Hennepin (21.7% point rise (6.5–36.9; p<0.01) and in Ramsey county (19.1% point increase ([0.2–38.1; p<0.05) but not among patients in Rice county. Unadjusted model results corresponded with adjusted results across outcome measures in all sites except for change in patients meeting treatment targets for diabetes in Ramsey county (19.1% point increase (0.0–38.0; p=0.05)).
Figure 1.
Differences among US HealthRise and comparison patients from baseline to endline for systolic blood pressure (A), percentage of hypertension patients meeting disease treatment targets (B), haemoglobin A1c (C) and percentage of diabetes patients meeting disease treatment targets (D). Included us patients are limited to prevalent cases of hypertension or diabetes at baseline with at least two biometric measures. Treatment targets were <140 mm Hg SBP and <90 mm Hg DBP for hypertension, and <8% A1c for diabetes. The effect of HealthRise was quantified with a difference-in-difference analysis; the effect of HealthRise by endline is reported for each site, with statistical significance denoted by *P<0.05, **P<0.01. DBP, diastolic blood pressure; SBP, systolic blood pressure.
bmjgh-2019-001959supp008.pdf (308.7KB, pdf)
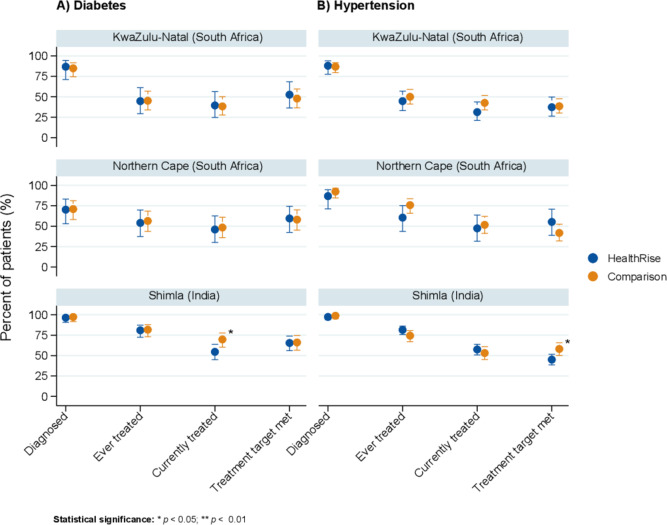
For Shimla, India and South African sites, endline analyses focused on differences between patients presenting at facilities in HealthRise implementation and comparison areas (figure 2). In Shimla, patients with hypertension and diabetes at facilities in HealthRise areas generally did not differ from patients in comparison areas in terms of the cascade of care metrics; exceptions were meeting treatment targets for hypertension and percent currently on treatment for diabetes, where the patients in the comparison areas showed higher rates than patients in HealthRise facilities. In Pixley ka Seme and uMgungundlovu, patients attending facilities in HealthRise areas did not differ from those in comparison areas along hypertension and diabetes cascades of care.
Figure 2.
Cascade of care for diabetes (A) and hypertension (B) based on patient interviews at facilities located in HealthRise implementation and comparison areas in Shimla, India and in South Africa sites. Included patients are limited to prevalent cases of hypertension or diabetes with biometric measures corresponding with prevalent conditions. Treatment targets were <140 mm Hg SBP and <90 mm Hg DBP for hypertension, and <8% A1c for diabetes. Statistical significance was determined by Welch's t-test, and is denoted by *P<0.05 and **P<0.01. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Endline qualitative findings
Qualitative analysis identified key themes relevant across sites (table 4). First, respondents expressed positive views of the overarching intervention model—an international programme focused on community-based interventions for NCDs—and opportunities to test new services or structures at their sites. In Brazil, providers specifically identified reorganising patient flows and health unit routines as supportive of more structured care delivery. New training opportunities, intensified group activities for patients, increased availability of some specialised tests, and using tablets to aid in patient record-keeping and care decisions were also viewed as positive developments. In the USA, enabling clinical staff to work with in-home providers for the first time was viewed as beneficial for patient care.
Table 4.
Summary of key themes and sample quotes from qualitative data collected across all HealthRise sites
| HealthRise thematic area components and contexts | Sample thematic quotes |
| Global and cross-cutting | |
|
‘E-clinics give the same care as hospitals and time is saved’—Administrator, India |
|
‘Many of the diagnoses we were seeing were directly related to social determinants of health, particularly healthy food access and access to affordable and culturally appropriate clinical care.’—Administrator, US |
|
‘All health-related programmes that were given the ASHA workers, there have been advantages due to it.’—Clinic provider, India |
|
‘Before the programme, there was not much communication with the doctors and the nurses. Our work is usually with people from the community only. The HealthRise programme has helped us in increasing our communication with officials at the clinic…Our rapport with doctors and nurses has improved.’—CHW, India |
| |
|
‘As you can see, we got here at the clinic at 6am but here we are still waiting for assistance.’—Patient, South Africa |
| Brazil | |
|
‘I think the EMR resulted in a better way of communicating about the patient…any professional can now access the information stored in there’—Frontline health worker |
|
‘A more intense multidisciplinary approach…I missed that a lot. A psychologist, a nutritionist…so that we could discuss the cases together.’—Frontline health worker |
|
‘I really like eating rice, but we can’t. But I eat it anyway.’—Patient |
| India | |
|
‘You feel it—sleepy, dizzy, irritable, can't control yourself.’—Patient |
|
‘From MAMTA for the past 2 years they are coming continuously in our village. They give us information and also tell us precautions about what to do. They do medical check-ups also every month after the health centre was made. Have given us cards as well.’—Patient |
|
‘Initially people think, 'what do they know, they are just freshly appointed ASHAs,' but they bring people to us, mobilise people; people do listen to them.’—Clinic-based provider |
|
‘Life is too fast paced, people pay more attention to electronics, social media and not nutrition and exercise.’—Patient |
| South Africa | |
|
‘Outside the facility, we have adherence club where chronic patients are being taught about exercises and adherence in the community. Then we have collection points where patients fetch their medication, the collection point are at scheduled halls or education institutions. That has helped us…because we take the medication to the people.’—Frontline health worker |
|
‘It’s not good and it’s also not bad, it’s in between. There are some patients who understanding our working conditions that maybe we have shortage of medication at that particular time and maybe we are busy because this is the only clinic in the community, but there are some patients who do not understand, they would say that we are slow or do not care about the patients.’—Facility manager |
|
‘If one doesn’t have cash, he can’t get to the hospital. We then use the services of prophets and traditional healers.’—Patient |
|
‘(Facilities) need to employ more staff and equipment, increase the resources needed and focus on each and every chronic condition….our government is trying but it is not enough.’—Frontline health worker |
| USA | |
|
‘The global aspect is quite unique…utilising similar strategies in different countries with very different health systems but with a similar population focus and similar workforce approaches…. I'm not aware of other projects that have attempted that across a set of different jurisdictions and landscapes.’—Policymaker |
|
‘We've learnt that a lot of the hurdle we have to get past is educating other healthcare providers on what we do…what is a CP and how can we be part of their team and help to better serve their patients…the ones who do now understand our role…they are our champions, they get so excited…we definitely see resistance in the beginning.’—Community paramedic |
|
‘The home visits contributed to more rational use of clinic time…and improved care on my end. From listening to CHWs, I have a better understanding of what's going on in people's lives.’—Clinic-based provider |
CHW, community health worker; NCD, non-communicable disease.
Second, social determinants of health contributing to the risk, onset and management of hypertension and diabetes were consistently reported as substantial challenges. Barriers to healthcare and better health outcomes, including poverty, low levels of health education and limited access to affordable and nutritious food were reported across all sites.
Third, local front-line health workers such as CHWs, ASHAs and community caregivers were viewed by patients and health facility staff and administrators as vital to programmes, as they supported patients facing substantive linguistic, cultural and geographic barriers. For instance, in Shimla, clinic-based providers found CHWs valuable in providing counselling services beyond what is possible within the time constraints of typical clinical appointments.
Care coordination (ie, linkages between different types of providers; homes, communities and clinics; and myriad information systems) also was viewed as critical for supporting more efficient and effective care. Overall, integrated care was viewed positively by participants; however, substantive challenges also emerged, many of which were related to incorporating in-home providers in care teams in the USA. For example, some clinical providers showed initial scepticism about the added value of in-home providers, and most administrators did not have prior experience managing CHWs and community paramedics. In Brazil, interviewees often indicated preferences for health professionals beyond doctors and nurses to be more involved in their care.
Some patients and providers also reported improved patient empowerment through knowledge gained about NCDs and greater confidence in managing these conditions. Further, as highlighted by CHWs in Shimla, providers also learnt more about NCDs and disease management practices. In Brazil, health providers requested more regular opportunities for in-person training, particularly in-depth technical trainings on measuring blood glucose and BP during home visits.
Finally, interviewees across sites stressed the importance of strong health systems, particularly since interventions relied on the availability of necessary staff, facility capacities and services. For instance, in Brazil, Shimla and South Africa, patients and providers identified several long-standing challenges related to core health system functions, particularly adequate medication supplies, sufficient staffing and reliable referral processes. Many of these challenges were outside the scope of HealthRise intervention; nonetheless, they played a role in how interviewees viewed the implementation strengths and issues of the programme.
Discussion
The prospective evaluation of the multisite, global HealthRise programme, a community-based programme seeking to improve hypertension and diabetes care among underserved populations, demonstrates the complexity of assessing community-based interventions across diverse settings and variable data environments. The programme’s implementation and evaluation faced many challenges, including some associated with establishing and maintaining monitoring efforts, particularly within pre-existing systems in underserved communities. Yet, process evaluation findings indicate that more than 56 000 and 59 000 individuals were screened for diabetes and hypertension, respectively, in Brazil, India and South Africa; however, a much smaller proportion of patients received new diagnoses and were referred to care, highlighting potential limitations of large-scale screening programmes. While Brazil and most US sites showed patient-level progress, with increasingly more hypertension and patients meeting disease control targets since programme enrolment and substantial declines in patients’ A1c and SBP decreased since baseline, improved disease management was not detected among patients in HealthRise implementation areas compared with those in comparison areas in Shimla and South Africa. A relatively short implementation period in some sites, alongside the inherent tension between demonstrating impact within time constraints and properly capturing the often slower or complex changes of health behaviours and systems, may underlie these findings. Limited data availability on intervention adherence and fidelity precludes further assessments of the programmes’ implementation quality and its impact on endline findings in each site.
Qualitative data highlighted some positive views of integrating home-based health workers in NCD care to bridge geographical, linguistic and cultural divides, and the importance of effective care coordination across provider types, data platforms and between facilities and communities. Nonetheless, long-standing gaps in system infrastructure likely contributed to continued challenges with care provision and social determinants of health continued to play roles in patient abilities’ to access treatment and manage their conditions. In combination, these findings demonstrate the potential for community based, and particularly, CHW led, interventions to improve NCD outcomes, but also underscore how their reach and effectiveness can be hindered by broader health system, infrastructure and policy constraints. Irrespective of their increasingly vital role for underserved populations, community-based programmes cannot fully remedy inadequate prioritisation or investments in strong, well-coordinated primary care and NCD services.
Successes and challenges for HealthRise and broader community-based NCD interventions
HealthRise programmes referred thousands of screened individuals to care, yet relatively few new diagnoses occurred. Low yields from population-based screening activities are not uncommon,43 44 and these findings support guidelines recommending more selective screening of high-risk groups to improve cost-effectiveness.45 A primary focus on screening may also have contributed minimal community-level effects in India and South Africa. Interventions in Brazil and the USA were more oriented towards improving access to care and medication adherence, which could more quickly affect health outcomes than screening activities, which only initiate the process of bringing patients into care.
In Brazil, both HealthRise sites exhibited notable progress; nevertheless, since comparison patient data were not collected for Brazil sites, we cannot ascribe these patient-level patterns to HealthRise participation. Despite these positive trends, qualitative data indicated poor adherence to medication for both Brazil sites, as well as patient-reported sociocultural tensions around adopting dietary changes and health system-level obstacles to accessing multidisciplinary care and reliably stocked medication at health facilities. In the USA, HealthRise participation was associated with reductions in A1c or SBP and increases in patients meeting treatment targets at some sites relative to comparison patients. Several factors may have contributed to observable impacts at US sites, which had among the longer programme implementation durations and the most robust evaluation, relative to other sites. Interventions in the USA were targeted to address specific barriers to keeping patients in care; the number of patients reached was small, so each person received substantial focus; and the US health system is better organised and equipped to deal with NCDs and therefore did not face the same health system challenges experienced in other HealthRise sites. However, as demonstrated by recent analyses of ‘superutiliser’ patients with medically and socially complex conditions,46 replicating intervention impact to larger populations may be difficult, especially if the resource intensity and patient attention provided through the US HealthRise programmes are not feasible or sustainable. This is particularly relevant given some of the reported difficulties in early-stage programme implementation in the USA, such as recruiting and retaining CHWs and ensuring all providers could access and update electronic medical records.
Additional data and contextual information are needed to better understand why programme impact was not found in India and South Africa. Unlike the Brazil sites, where HealthRise interventions were incorporated into longstanding community-based healthcare structures and CHW-led service provision, India and South Africa HealthRise grantees often had to build systems—both physical and administrative—from the ground-up to support NCD care coordination, medication logistics and community engagement. This challenge, in combination with relatively short implementation periods, and screening being higher programmatic priorities in these sites, may underlie the negligible community-level effects on outcomes. Further, we could not fully account for other local or national initiatives to expand NCD care in both HealthRise and comparison areas; for instance, India launched national guidelines for NCD screening activities through CHWs and community platforms in 2017,47 48 potentially spurring the scale-up of broader community-based NCD programming throughout India after HealthRise began in Shimla. Other studies, including an evaluation of a CHW-managed intervention for patients in India with high cardiovascular disease risk,49 indicate patient-level barriers to care like cost, transport and medication availability could affect intervention impact as well. Substantial drop-offs in care cascades also emphasise the need for locally relevant mechanisms for coordinated care.50 These findings correspond with larger-scale assessments of diabetes care cascades in India and South Africa,14 18 both of which stressed the importance of strengthening NCD case detection and management for more rural, underserved communities.
Additional challenges were highlighted in qualitative analyses. Despite some indication of heightened patient empowerment, as measured by self-reported knowledge and confidence in at least some sites, adherence to recommended dietary and physical activity behaviour changes emerged as a source of tension between patients and providers, highlighting the difficulty of enacting meaningful cognitive and behaviour change amid strong social and environmental influences. Additional challenges that could negatively affect the adoption and scale-up of community-based NCD interventions in resource-constrained settings included the availability and quality of technologies for care coordination; minimal experience managing or working with in-home providers as members of care teams; and challenges in securing long-term funding for community-based NCD programmes, as well as broader health system capacities for NCD care (eg, functional diagnostic equipment, reliable stocking of NCD pharmaceuticals, accessible primary care services). Without greater prioritisation of NCDs in health financing—from government sources to development partners alike—the potential impact of community-based NCD programmes could be hindered by the lack of underlying infrastructure and resources.4 9
Potential implications for community-based NCD interventions
Building off of previous work, the present study offers some programmatic considerations, including facility-level and community priorities, as well as for national agendas on NCD prevention and treatment. In Brazil, India and South Africa, pre-existing health system challenges, ranging from medication stock-outs to long travel times to reach health facilities, posed obstacles to patients and providers. To more effectively treat the rising burden of NCDs, it is critical to address deficiencies in facility infrastructure, transportation, staffing and supplies. In the USA, many providers and administrators had limited previous exposure to home-based providers, which made programme implementation challenging at times, especially during the early stages of intervention. Identifying processes and supportive technologies by which care teams may incorporate home-based care more seamlessly, particularly in terms of sharing patient data and informing facility staff about findings from home visits, is likely to be beneficial. Finally, especially for LMICs, there is an urgent need for development partners to dedicate more funding to NCD care and strengthening health systems more broadly.4 Without a greater emphasis on these health financing areas, many communities in LMICs will remain ill equipped to provide effective NCD care.9
Limitations
Our study’s findings should be interpreted in light of its limitations. First, while HealthRise sites were selected to represent a range of underserved populations worldwide, findings are not generalisable to all underserved communities seeking to improve NCD care. Continued work is needed to understand which community-based NCD interventions may work best given local contexts and needs. Second, despite being incorporated into the initial process evaluation framework, comprehensive information on intervention reach and fidelity (ie, the degree to which interventions were implemented per protocol) were not available across sites and thus could not be included in the present study. To better understand intervention impact, ongoing and future community-based programmes could greatly benefit from ensuring adequate funding, personnel and infrastructure to establish and maintain data collection for evaluation indicator monitoring. Third, in the USA, comparison groups were constructed retrospectively by each grantee, using available patient record information and were not selected by random assignment. While efforts were made to ensure that included comparison patients generally represented individuals who would have been eligible for HealthRise enrolment, they may have differed from individuals who enrolled. Fourth, in Shimla, India and South Africa, we were limited to cross-sectional patient data at endline, and thus could not directly assess potential differences in cascades of care from baseline to endline among HealthRise implementation and comparison areas. Not being able to explicitly account for pre-intervention differences in these areas and how they changed over time may contribute to some findings in Shimla (ie, patients presenting at facilities in comparison areas having somewhat higher levels of current diabetes treatment and meeting treatment targets for hypertension than patients in HealthRise implementation areas). Also, based on our sampling strategy, we cannot rule out cross-contamination in Shimla and South Africa (ie, patients presenting at facilities in comparison areas engaged in HealthRise activities and/or patients in HealthRise implementation areas were not exposed to HealthRise programming). Fifth, the global evaluation team could not verify monitoring data accuracy for sites in India and South Africa, as only aggregated data were provided by grantees due to government regulations governing data use outside the country. Organisations were assigned by the government in each country to check the validity of data before it was transmitted to IHME. Sixth, for Brazil and the USA, we only included patients who remained enrolled at endline in the endline analyses; by taking this ‘as treated’ analytical approach, which provides insights into programme effects closer to full adherence, these patients may not represent all potential target populations for HealthRise interventions and results may be positively biased. Seventh, while results varied by site, the relative lack of differences for several indicators between patient groups and over time could be related to factors beyond programme effectiveness. It is possible that, on average, HealthRise programme implementation and patient duration in the programme was not long enough to detect positive effects; this may be particularly relevant for sites where community-based care and CHW networks had not previously been longstanding models of service provision and therefore required substantial time to establish. Changes in clinical outcomes also can lag behind intervention exposure and thus improvements may not have been fully realised by endline. Eight, information on the existence of preintervention services or programmes were not available across sites; subsequently, it was not possible to ascertain the potential effects of this factor on endline evaluation results.
Conclusion
The global HealthRise programme involved multisite, locally tailored community-based pilot interventions focused on NCD care among underserved populations and incorporated a prospective evaluation by an independent party—all important steps towards strengthening the knowledge base of what works and what does not for improving NCD service delivery at local levels. Across the nine sites in four countries, some progress on patient-level indicators occurred; nonetheless, constraints on both implementation and evaluation periods and variable comparison groups across sites emphasise the need for longer-term evaluations of community-based NCD programmes in the future. Biological, behavioural and sociocultural factors all contribute to the risk for and development of NCDs, requiring multifaceted approaches to optimally support patients and families coping with these complex conditions. Achieving notable impacts on NCDs will not only require broader health system strengthening and increased financing for NCDs, but also a more locally driven focus on how interventions and community factors together contribute to improving health for all individuals.
Acknowledgments
We would like to acknowledge the support and insight of local data collection teams Social Surveys Africa, GfK Mode, IGMC Shimla, Development Solutions, Dinamica Cursos, Tesla Gestao and Press Consultoria and implementation partners Expectra Health Solutions, Project HOPE, Catholic Health Association of India, MAMTA Health Institute for Mother and Child, Pillsbury United Communities, Regions Hospital Foundation, HealthFinders Collaborative Inc, and the HealthRise teams at Universidade Federal dos Vales do Jequitinhonha e Mucuri, Telehealth Network of Minas Gerais, Universidade Federal de Minas Gerais, Universidade Federal de Bahia, Universidade Estadual do Sudoeste da Bahia, Servico Social da Industria, and Associacao de Apoio ao Portador de Diabetes de Vitoria da Conquista who provided the data for this study. Without them, this work would not be possible. We also acknowledge the state and local governments in HealthRise areas for their cooperation and support with HealthRise intervention and evaluation activities. We thank Kelsey Bannon, Erin Palmisano, and Laura Di Giorgio for assisting in the management and execution of the HealthRise evaluation. We also thank Gopal Chauhan for his sincere efforts to support and strengthen the HealthRise programme in India and Maria de Fátima Marinho and Ceres Almeida for their support which was fundamental to the success and sustainability of the HealthRise programme in Brazil. Last, we thank all individuals who participated in this study.
Footnotes
Handling editor: Valery Ridde
Contributors: LSF, KC, NF and EG contributed to the analysis of the data, produced tables and figures, and wrote the initial draft of the manuscript. LSF, NF and EG finalised the manuscript based on reviewer feedback. LSF, SW, MB, JNC, DVC, KC, HCD, NF, RG, TG, KPH, CRM, VM, MN, BP, MBR, GR, BT, AW and EG developed survey instruments, collected data, verified and managed data, contributed to analyses or some combination of the aforementioned contributions. EG conceptualised and managed the evaluation. PB, NC, JD and MTUB provided guidance on the HealthRise project as a whole. AB, CCh, CCi, HCN, MLC, VEK, PE, MALB, JMa, CM, MSM, SMa, DSdM, SMi, JMu, JAQO, MGO, VP, TR, ALR, DR, GS, DAS, TT, HT and SV developed, managed or implemented HealthRise intervention activities or data monitoring systems, or some combination of the aforementioned activities. VC and TPN managed data collection activities. All authors read and approved the final manuscript.
Funding: Funding for the HealthRise project came from the Medtronic Foundation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. JAQO received doctoral scholarship from CNPq/CAPES/IATS, Brazil. ALR was supported in part by CNPq (grant 310679/2016–8), and Instituto de Avaliação de Tecnologia em Saúde - IATS, grant 465518/2014–1) and by FAPEMIG (Programa Pesquisador Mineiro, PPM-00 428–17).
Competing interests: PB, NC, JD and MTUB are employees of the Medtronic Foundation. AB, CCh, CCi, MLC, VEK, PE, MALB, JMa, CM, MSM, SMa, DSdM, SMi, JMu, HCN, JAQO, MGO, VP, TR, ALR, DR, GS, DAS, TT, HT and SV are recipients of HealthRise grants from the Medtronic Foundation to implement HealthRise interventions. LSF, SW, MB, JNC, DVC, KC, HCD, NF, RG, TG, KPH, CRM, VM, MN, BP, MBR, GR, BT, AW, VC, TPN, and EG are recipients of funding from grants from the Medtronic Foundation to evaluate HealthRise interventions.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethical approval for this study was obtained from the institutional review board of the University of Washington, as well as the local data collection agencies and government entities for each site. This research conforms to the principles embodied in the Declaration of Helsinki. All personal identifiers were removed prior to the data being sent to IHME for analysis; only de-identified data were analysed.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. The datasets generated and/or analysed during the current study are available, when possible, in the GHDx data repository, http://ghdx.healthdata.org/series/healthrise-evaluation
References
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1923–94. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AY, Cowling K, Micah AE, et al. Past, present, and future of global health financing: a review of development assistance, government, out-of-pocket, and other private spending on health for 195 countries, 1995–2050. The Lancet 2019;393:2233–60. 10.1016/S0140-6736(19)30841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med 2019;16:e1002751. 10.1371/journal.pmed.1002751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control. Circulation 2016;134:441–50. 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinesh N, Yijing F, Kunihiro M, et al. Abstract P119: gap between need and supply for hypertension care in low- and middle-income countries. Circulation 2019;139:AP119. [Google Scholar]
- 8.Atun R, Jaffar S, Nishtar S, et al. Improving responsiveness of health systems to non-communicable diseases. Lancet 2013;381:690–7. 10.1016/S0140-6736(13)60063-X [DOI] [PubMed] [Google Scholar]
- 9.Bollyky TJ, Templin T, Cohen M, et al. Lower-Income countries that face the most rapid shift in noncommunicable disease burden are also the least prepared. Health Aff 2017;36:1866–75. 10.1377/hlthaff.2017.0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockers PC, Laing RO, Ashigbie PG, et al. Effect of Novartis access on availability and price of non-communicable disease medicines in Kenya: a cluster-randomised controlled trial. Lancet Glob Health 2019;7:e492–502. 10.1016/S2214-109X(18)30563-1 [DOI] [PubMed] [Google Scholar]
- 11.Vialle-Valentin CE, Serumaga B, Wagner AK, et al. Evidence on access to medicines for chronic diseases from household surveys in five low- and middle-income countries. Health Policy Plan 2015;30:1044–52. 10.1093/heapol/czu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine (US) Committee on Health Insurance Status and Its Consequences America’s Uninsured Crisis: Consequences for Health and Health Care. Washington (DC): : National Academies Press (US), 2009. Available: http://www.ncbi.nlm.nih.gov/books/NBK214966/ [Accessed 6 Apr 2019]. [PubMed]
- 13.Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability Working group: conclusions and recommendations. Diabetes Care 2018;41:1299–311. 10.2337/dci18-0019 [DOI] [PubMed] [Google Scholar]
- 14.Prenissl J, Jaacks LM, Mohan V, et al. Variation in health system performance for managing diabetes among states in India: a cross-sectional study of individuals aged 15 to 49 years. BMC Med 2019;17:92. 10.1186/s12916-019-1325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda N, Sapienza D, Guerrero R, et al. Control of hypertension with medication: a comparative analysis of national surveys in 20 countries. Bull World Health Organ 2014;92:10–19. 10.2471/BLT.13.121954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MK, Bullard KM, Gregg EW, et al. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014;161:681. 10.7326/M14-0019 [DOI] [PubMed] [Google Scholar]
- 17.Berry KM, Parker W-A, Mchiza ZJ, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011-2012. BMJ Glob Health 2017;2:e000348. 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokes A, Berry KM, Mchiza Z, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: evidence from the SANHANES-1, 2011-2012. PLoS One 2017;12:e0184264. 10.1371/journal.pone.0184264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gakidou E, Mallinger L, Abbott-Klafter J, et al. Management of diabetes and associated cardiovascular risk factors in seven countries: a comparison of data from national health examination surveys. Bull World Health Organ 2011;89:172–83. 10.2471/BLT.10.080820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry HB, Zulliger R, Rogers MM. Community health workers in low-, middle-, and high-income countries: an overview of their history, recent evolution, and current effectiveness. Annu Rev Public Health 2014;35:399–421. 10.1146/annurev-publhealth-032013-182354 [DOI] [PubMed] [Google Scholar]
- 21.Martinez J, Ro M, Villa NW, et al. Transforming the delivery of care in the post-health reform era: what role will community health workers play? Am J Public Health 2011;101:e1–5. 10.2105/AJPH.2011.300335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med 2007;32:435–47. 10.1016/j.amepre.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med 2017;53:S121–30. 10.1016/j.amepre.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kangovi S, Mitra N, Grande D, et al. Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial. Am J Public Health 2017;107:1660–7. 10.2105/AJPH.2017.303985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K, Choi JS, Choi E, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health 2016;106:e3–28. 10.2105/AJPH.2015.302987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeet G, Thakur JS, Prinja S, et al. Community health workers for non-communicable diseases prevention and control in developing countries: evidence and implications. PLoS One 2017;12:e0180640. 10.1371/journal.pone.0180640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A, Praveen D, Maharani A, et al. Association of multifaceted mobile Technology–Enabled primary care intervention with cardiovascular disease risk management in rural Indonesia. JAMA Cardiol 2019;4:978–86. 10.1001/jamacardio.2019.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neil DS, Lam WC, Nyirangirimana P, et al. Evaluation of care access and hypertension control in a community health worker driven non-communicable disease programme in rural Uganda: the chronic disease in the community project. Health Policy Plan 2016;31:878–83. 10.1093/heapol/czw006 [DOI] [PubMed] [Google Scholar]
- 29.Farzadfar F, Murray CJL, Gakidou E, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet 2012;379:47–54. 10.1016/S0140-6736(11)61349-4 [DOI] [PubMed] [Google Scholar]
- 30.Ndou T, van Zyl G, Hlahane S, et al. A rapid assessment of a community health worker pilot programme to improve the management of hypertension and diabetes in Emfuleni sub-district of Gauteng Province, South Africa. Glob Health Action 2013;6:19228. 10.3402/gha.v6i0.19228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman PM, Franke MF, Arrieta J, et al. Community health workers improve disease control and medication adherence among patients with diabetes and/or hypertension in Chiapas, Mexico: an observational stepped-wedge study. BMJ Glob Health 2018;3:e000566. 10.1136/bmjgh-2017-000566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snella KA, Canales AE, Irons BK, et al. Pharmacy- and community-based screenings for diabetes and cardiovascular conditions in high-risk individuals. J Am Pharm Assoc 2006;46:370–7. 10.1331/154434506777069598 [DOI] [PubMed] [Google Scholar]
- 33.Ploylearmsang C, Sookaneknun P, Poophalee T, et al. Diabetes and hypertension screening by pharmacy students in Thai communities. Am J Pharm Educ 2013;77:56. 10.5688/ajpe77356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung VQ, Morley K, O'Neil E, et al. Evaluation of a hypertension screening programme in independence, Belize. West Indian Med J 2005;54:130–4. 10.1590/S0043-31442005000200009 [DOI] [PubMed] [Google Scholar]
- 35.Gaziano TA, Abrahams-Gessel S, Denman CA, et al. An assessment of community health workers' ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health 2015;3:e556–63. 10.1016/S2214-109X(15)00143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abt Associates HealthRise final report: expanding access to chronic disease care through community approaches in four countries, 2020. [Google Scholar]
- 37.Institute for Health Metrics and Evaluation (IHME) HealthRise evaluation final report. Seattle, WA, USA: IHME Forthcoming. [Google Scholar]
- 38.Flor LS, Phillips B, McNellan C, et al. HealthRise needs assessment, Brazil: Vitória dA Conquista and Teófilo Otoni. Seattle, WA, USA: Institute for Health Metrics and Evaluation, 2016. [Google Scholar]
- 39.Gabert R, Ng M, Sogarwal R, et al. Identifying gaps in the continuum of care for hypertension and diabetes in two Indian communities. BMC Health Serv Res 2017;17:846. 10.1186/s12913-017-2796-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollum A, Gabert R, McNellan CR, et al. Identifying gaps in the continuum of care for cardiovascular disease and diabetes in two communities in South Africa: baseline findings from the HealthRise project. PLoS One 2018;13:e0192603. 10.1371/journal.pone.0192603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabert R, Thomson B, Gakidou E, et al. Identifying high-risk neighborhoods using electronic medical records: a population-based approach for targeting diabetes prevention and treatment interventions. PLoS One 2016;11:e0159227. 10.1371/journal.pone.0159227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun V, Clarke V. Thematic analysis. in: APA Handbook of research methods in psychology, vol 2: research designs: quantitative, qualitative, neuropsychological, and biological. Washington, DC, US: American Psychological Association, 2012: 57–71. [Google Scholar]
- 43.Janssen PGH, Gorter KJ, Stolk RP, et al. Low yield of population-based screening for type 2 diabetes in the Netherlands: the addition Netherlands study. Fam Pract 2007;24:555–61. 10.1093/fampra/cmm052 [DOI] [PubMed] [Google Scholar]
- 44.Toscano CM, Zhuo X, Imai K, et al. Cost-Effectiveness of a national population-based screening program for type 2 diabetes: the Brazil experience. Diabetol Metab Syndr 2015;7:95. 10.1186/s13098-015-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Diabetes Association Screening for diabetes. Diabetes Care 2002;25:S21–4. 10.2337/diacare.25.2007.S21 [DOI] [PubMed] [Google Scholar]
- 46.Finkelstein A, Zhou A, Taubman S, et al. Health Care Hotspotting - A Randomized, Controlled Trial. N Engl J Med 2020;382:152–62. 10.1056/NEJMsa1906848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministry of Health and Family Welfare Operation guidelines prevention, screening and control of common non-communicable diseases: hypertension, diabetes and common cancers (oral, breast, cervix). New Delhi, India: Government of India, 2017. [Google Scholar]
- 48.Mondal S, Van Belle S. India's ncd strategy in the SDG era: are there early signs of a paradigm shift? Global Health 2018;14:39. 10.1186/s12992-018-0357-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peiris D, Praveen D, Mogulluru K, et al. SMARThealth India: a stepped-wedge, cluster randomised controlled trial of a community health worker managed mobile health intervention for people assessed at high cardiovascular disease risk in rural India. PLoS One 2019;14:e0213708. 10.1371/journal.pone.0213708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffy M, Ojikutu B, Andrian S, et al. Non-Communicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Health 2017;22:926–37. 10.1111/tmi.12901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001959supp001.pdf (1.7MB, pdf)
bmjgh-2019-001959supp002.pdf (375.8KB, pdf)
bmjgh-2019-001959supp003.pdf (181.7KB, pdf)
bmjgh-2019-001959supp004.pdf (212.7KB, pdf)
bmjgh-2019-001959supp005.pdf (255.1KB, pdf)
bmjgh-2019-001959supp006.pdf (4MB, pdf)
bmjgh-2019-001959supp007.pdf (298.6KB, pdf)
bmjgh-2019-001959supp008.pdf (308.7KB, pdf)