Abstract
Little is known about the environmental conditions that drive the spatiotemporal patterns of SARS-CoV-2. Preliminary research suggests an association with meteorological parameters. However, the relationship with temperature and humidity is not yet apparent for COVID-19 cases in US cities first impacted. The objective of this study is to evaluate the association between COVID-19 cases and meteorological parameters in select US cities. A case-crossover design with a distributed lag nonlinear model was used to evaluate the contribution of ambient temperature and specific humidity on COVID-19 cases in select US cities. The case-crossover examines each COVID case as its own control at different time periods (before and after transmission occurred). We modeled the effect of temperature and humidity on COVID-19 transmission using a lag period of 7 days. A subset of 8 cities were evaluated for the relationship with meteorological parameters and 5 cities were evaluated in detail. Short-term exposure to humidity was positively associated with COVID-19 transmission in 4 cities. The associations were small with 3 out of 4 cities exhibiting higher COVID19 transmission with specific humidity that ranged from 6 to 9 g/kg. Our results suggest that weather should be considered in infectious disease modeling efforts. Future work is needed over a longer time period and across different locations to clearly establish the weather-COVID19 relationship.
Keywords: COVID-19 morbidity, Distributed lag non-linear model, Time-stratified case-crossover, Weather, Seasonality
Graphical abstract
1. Introduction
Experimental and observational studies demonstrate the influence of meteorological parameters on the seasonal transmission of influenza, human coronavirus (HCoV), and human respiratory syncytial virus (RSV), which are often characterized by distinct increases in incident cases and detection frequency in the winter months (Lowen and Steel, 2014; Tamerius et al., 2013; Tamerius et al., 2011; Midgley et al., 2017; Killerby et al., 2018; Landes et al., 2013; Morikawa et al., 2015). An accumulating evidence-base suggests that seasonal changes in indoor and outdoor environmental factors exert a modifying effect on both the transmission efficiency and viability of the respiratory virus and the host's airway immune defense (Moriyama et al., 2020). These environmental factors are then compounded by human behavior, social interactions, or hygiene practices that enhance viral transmission between individuals who are infected and those who are susceptible.
Like these seasonal viruses, SARS-CoV-2 can be transmitted through aerosols, large respiratory droplets, or direct contact with fomites (Lipsitch et al., 2020). SARS-CoV, responsible for the SARS outbreak in 2003, and SARS-CoV-2 responsible for COVID-19, rely on the same receptor-angiotensin-converting enzyme 2 (ACE2)-for infecting humans (Sun et al., 2020). Both made their debut in the winter months giving further credence to the role of the winter environment as an important contributor in transmission, particularly in temperate regions (Li et al., 2020; Paules et al., 2020; Kuiken et al., 2003; Peiris et al., 2003). Scientists conjecture that low humidity and temperature likely promote the viability of SARS-CoV-2 in respiratory droplets and it's plausible that airborne transmission is highly likely among COVID-19 cases with severe pneumonia. A recent population-based study examining the daily incidence of COVID-19 and daily temperature and relative humidity across Chinese provinces observed that in addition to dry and cold locations, locations with low absolute humidity also experienced increased virus transmission rates (Luo et al., 2020).
Little is known about the environmental conditions that drive the spatiotemporal patterns of SARS-CoV-2/COVID-19 and preliminary research suggests an association with meteorological parameters (Chen et al., 2020; Luo et al., 2020; Sajadi et al., 2020; Luo et al., 2020). However, the relationship between temperature and humidity is not yet apparent for COVID-19 cases in the US. As the US begins its public health response to COVID-19, the implementation of extensive public health interventions are needed at appropriate time scales to mitigate the health and economic impacts of the COVID-19 pandemic. Research on the seasonality and influence of meteorological parameters on COVID-19, such as temperature and specific humidity, can be used to inform the timing of effective interventions to mitigate SARS-CoV-2/COVID-19 transmission at the local scale to save countless lives and resources.
The objective of this research is to examine the association between meteorological variables and COVID-19 in US cities. Unlike previous studies, we will use a high-resolution spatiotemporal meteorological dataset to answer the following: Is there an association with meteorological parameters and COVID-19? If so, which meteorological parameters predict COVID-19 transmission? Is the association stronger after accounting for locally implemented social distancing measures? How does this relationship vary spatially across the US? By answering these questions, the knowledge gained on the contribution of environmental factors like temperature and humidity on transmission can be paired with other nonpharmaceutical interventions related to behavioral (e.g., wearing face mask, washing hands) factors that boost immunity or the timing of social distancing measures with seasonal spikes in influential environmental parameters to reduce transmission.
2. Methods
2.1. Study design and location
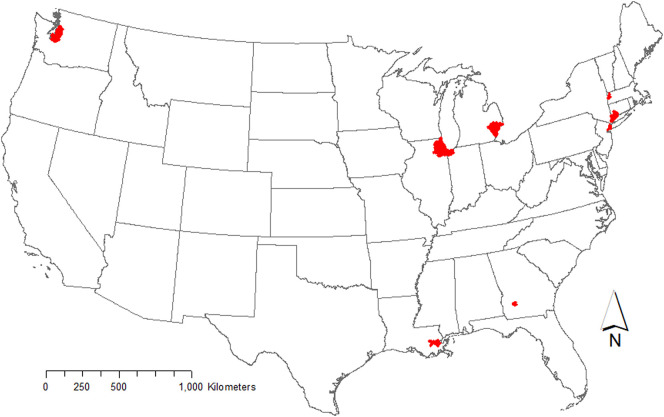
This retrospective case-crossover study examined the nonlinear and delayed association between environmental factors and COVID-19 transmission. We selected the following US locations that exhibited high relative caseloads of COVID-19 in the early stages of the pandemic for their underlying populations: Seattle, Washington; New York, NY; Albany GA; New Orleans, LA.; Bridgeport-Stamford-Norwalk, Conn.; Pittsfield, Mass; Detroit, MI; Chicago, IL. Fig. 1 is a map of the 8 study locations.
Fig. 1.
Study Area Locations, which include the counties that encompass the following cities: Seattle, Washington; New York, NY; Albany GA; New Orleans, LA.; Bridgeport-Stamford-Norwalk, Conn.; Pittsfield, Mass; Detroit, MI; Chicago, IL.
2.2. COVID-19 cases
The primary health outcome of interest was incident cases. Daily confirmed new cases of COVID-19 for all cities were abstracted from the Johns Hopkins Center for Systems Science and Engineering repository (source: https://github.com/CSSEGISandData/COVID-19). The repository continually assembles global COVID-19 cases from multiple sources including the World Health Organization, the Center for Disease Control, and the COVID-19 Tracking Project (Dong et al., 2020). We assumed at least a median incubation period of 5.2 days (Lauer et al., 2020). Case counts were log-transformed, and time series were created when cities had >2 new daily cases. Because deidentified and anonymized data on case morbidity were obtained from a publicly accessible data portal this research did not involve participant consent and institutional review was not warranted.
2.3. Environmental parameters
Meteorological data were derived from the European Center for Median Range Weather Forecast (ECMWF) atmospheric reanalysis dataset (ERA-5) (C3S, 2017). ERA-5 provides a suite of hourly weather parameters that may affect local COVID-19 transmissions at a 30-km spatial resolution. While not commonly used in environmental health studies, the advantage that ERA-5 data provides over individual weather station data is that spatial heterogeneity is more representative and the estimation of health effects of temperature and humidity can be derived in locations far from weather stations or without any station. Previous research has shown that reanalysis data and weather station data show similar health risk estimates (Royé et al., 2020). Daily average near-surface air temperature, specific humidity, and solar radiation were extracted from ERA-5 for each study location by a simple spatial average. Because relative humidity (RH) is highly correlated with temperature, we chose to instead include specific humidity (Q) as a predictor variable in the analysis.
2.4. Heat mapping
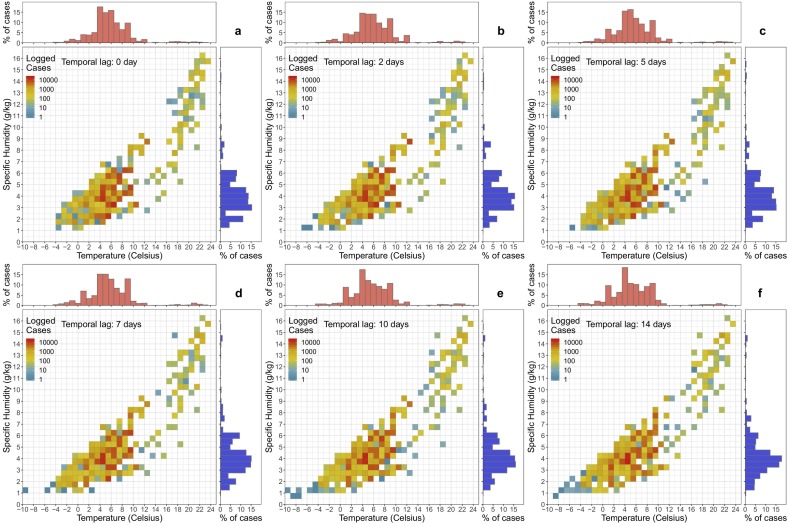
Preliminary studies have suggested that the combination of humidity and air temperature could affect the transmission of local COVID-19 cases (e.g., Sajadi et al., 2020; Lou et al., 2020; Oliveriros et al., 2020). We examined the association between local COVID-19 cases and air temperature and specific humidity using the density heatmap. To construct the density heatmap, the daily confirmed COVID-19 case reports were first separated based on their corresponding daily mean air temperature (every 1 °C) and mean specific humidity (every 0.5 g/kg). All daily confirmed case counts were classified into the same air temperature and specific humidity conditions (e.g., 0 °C < T air < 1 °C and 1 g/kg < Q < 1.5 g/kg) and evaluated together as a density measurement. This explanatory analysis was intended to demonstrate the association of COVID-19 cases with the combined effect of air temperature and specific humidity. The heatmap could identify the range of optimal meteorological conditions for local transmissions. Considering the incubation period of COVID-19, we applied the analysis to local weather data at different lead times (i.e., 0, 2, 5, 7, 10, 14 days).
2.5. Case-crossover distributed lag non-linear model
We applied a time-stratified case-crossover design that uses each individual COVID-19 case as their own control. A conditional Poisson regression was used in combination with the Distributed Lag Nonlinear model (DLNM). This approach is more flexible than conditional logistic regression (Armstrong et al., 2014) in that it allows for overdispersion. The application of the DLNM to the case-crossover design provides a means to assess the nonlinear and delayed effects, as well as the cumulative exposure-response between short-term daily average exposure to meteorological parameters and daily counts for COVID-19 cases. We performed separate analyses for our primary health outcome — COVID-19 morbidity — and each meteorological parameter relative to the median and quartiles (i.e., 50th versus 75th). This approach is suitable for studying the effects of time-varying exposures (e.g., intermittent changes in meteorological) on a rare, acute condition (i.e., COVID-19 transmission) (Armstrong et al., 2019; Malig et al., 2016; Guo et al., 2011). We relied on the following equation:
where t is the day of the observation; Y t is the observed daily case counts on day t; α is the intercept; T t,l is a matrix obtained by applying the DLNM to temperature or humidity, β is a vector of coefficients for T t,l, and l is the lag days. Stratat is a categorical variable of day (30 day time period) used to control for trends, and λ is a vector of coefficients. SDt is a binary variable that is “1” if day t was a social distancing order, and υ is the coefficient. Our model was adapted from similar work by Guo et al. (2011) who also employed a case-crossover design and DLNM to investigate the effects of temperature on mortality. Given that the incubation period between exposure and symptom occurrence is 2 to 14 days (Linton et al., 2020), we used a maximum 14-day lag period to explore the potential delayed association of temperature and humidity in our model for approximating the pre- and post-infection period for each case.
The DLNM utilizes the “cross-basis” function to flexibly model the lag and exposure components to account for cumulative effects in environmental exposure (Gasparrini et al., 2010). We first examined the association between temperature and humidity individually for our primary outcome. Final models included both temperature and humidity, to examine the contribution of temperature and humidity to COVID-19 transmission in US cities. But our assumption was that temperature would have a predominant effect followed by humidity based on emerging literature (e.g., Shi et al., 2020; Araujo et al., 2020; Wang et al., 2020; Oliveiros et al., 2020; Notari et al., 2020) and therefore humidity was included in the crossbasis term.
2.6. Sensitivity analysis
A sensitivity analysis was conducted to select degrees of freedom for the lag polynomial (2–8 degrees of freedom) and the response polynomial (2–8 degrees of freedom) for New Orleans, LA (data not shown). In addition, we changed the maximum lag to 14 and 20 days, which gave similar results (data not shown). Prior research has examined a 0 day, 3 day to 5 day lags for COVID-19 transmission (Ma et al., 2020; Wang et al., 2020) all the way to a lag period extending 7 to 14 days for meteorological parameters (Islam et al., 2020). For our initial examination of meteorological parameters independently, we compared the best model fit using quasi-Akaike Information Criterion (qAIC) to determine the optimal degrees of freedom and lag periods. q-AIC is a well-established technique for sensitivity analysis and was used to compare DLNM-only models and DLNM + Case-Crossover models to confirm the final model selection (Guo et al., 2011). Models were also examined for the adjustment for trends, such as the day of the week. Initially, we examined the influence of the month in the strata term for the DLNM + Case-Crossover models, but the qAIC values demonstrated the addition of these variables resulted in poor model fit. Likely due to the short time seriesand thus we selected the most parsimonious model that only included day in the strata. The “dlnm” package was used to create the DLNM model (Gasparrini, 2011) using R statistical software (R Core Team, 2020). We adopted the rare-disease assumption where our study hypothesis tested the association between weather exposure and a disease (i.e., COVID-19) characterized by low prevalence. Therefore, we assumed the odds ratio to approximate the relative risk. All relative risks (RR) were presented with corresponding 95% confidence intervals (95% CIs).
2.7. Attributable burden of COVID-19 transmission due to weather
In epidemiology, measures of potential impact are used to examine the expected impact of changing the distribution of one or more risk factors in a particular population (Kleinbaum et al., 1982, Szklo and Nieto, 2014). For example, the attributable risk, also known as the etiologic fraction, is used to examine the proportion of all new cases in a given time frame that is attributable (or causally associated) to the exposure of interests (Szklo and Nieto, 2014). Because the evidence-base linking COVID-19 transmission and weather is new and evolving, it is too early to assume a causal association. Therefore, we relied on the excess fraction (EF) as an analogous, but alternative measure to the attributable risks in our analysis, to approximate the excess caseload due to exposure. To examine the attributable burden of transmission for COVID-19 due to weather we calculated the percent excess fraction for humidity and temperature for individual cities. We used the following equation: % EF = b × (RRi − 1) / b × (RR − 1.0i) + 1.0), where b is the point prevalence of COVID-19 for each city. Point prevalence was calculated as the number of cases over the study period divided by the total population in a specific city. We adopted the modified version of this equation based on Gasparrini and Leone (2014) to extend the definition for the excess fraction.
3. Results
Our analysis included a total of 266,760 cases and 19,729 deaths across 8 cities (Table 1 ). The crude rate of COVID-19 per location was highest for New Orleans, LA (374 daily cases per 100,000 people), followed by New York City, NY (51 daily cases per 100,000 people), Albany, GA (42 daily cases per 100,000 people), and Bridgeport, CT (25 daily cases per 100,000 people). The lowest rates of COVID-19 cases were in Seattle, WA (4 daily cases per 100,000 people), and Pittsfield, MA (8 cases per 100,000 people). The highest crude death rates were observed in New York City, NY (6 daily deaths per 100,000 people), Albany GA (3 daily deaths per 100,000 people), New Orleans, LA (2 daily deaths per 100,000 people) and Detroit, MI (2 daily deaths per 100,000 people).
Table 1.
Distribution of key variables by city.
| City (county) | Latitude & longitude | Populationa | Study period (days) | Date of social distancing measures | # of total cases | # of deaths | Median daily temperature (°C) | Median daily specific humidity (g/kg) |
|---|---|---|---|---|---|---|---|---|
| Seattle, Washington (King) | 47.61° N 122.33° W | 745,000 | 2/29–4/23 (54) | 3/7 | 5532 | 385 | 2.19 | 3.98 |
| New York City, NY (New York) | 40.71° N 74.01° W | 8,336,817 | 3/5–4/11 (49) | 3/13 | 145,855 | 16,158 | 6.01 | 4.0 |
| Albany, GA (Dougherty) | 31.58° N 84.16° W | 75,200 | 3/14–4/23 (40) | 3/21 | 1479 | 107 | 18.78 | 9.88 |
| New Orleans, LA (Orleans) | 29.95° N 90.07° W | 1,670,000 | 3/11–4/23 (43) | 3/16 | 62,663 | 387 | 19.96 | 12.99 |
| Bridgeport-Stamford-Norwalk, Conn (Fairfield) | 41.18° N, 73.19° W | 943,823 | 3/12–4/23 (42) | 3/16 | 10,008 | 125 | 5.25 | 3.68 |
| Pittsfield, Mass (Berkshire) | 42.45° N, 73.25° W | 126, 348 | 3/10–4/23 (44) | 3/13 | 418 | 29 | 2.18 | 3.3 |
| Detroit, MI (Wayne) | 42.33° N, 83.05° W | 1,753,893 | 3/15–4/23 (39) | 3/16 | 14,994 | 1396 | 3.30 | 3.78 |
| Chicago, IL (Cook) | 41.88° N, 87.63° W | 2,710,000 | 3/10–4/23 (44) | 3/12 | 25,811 | 1142 | 3.475 | 3.91 |
Population data from ACS 2018 1-year estimate.
3.1. Density heatmaps
The density heatmap (Fig. 2 ) presents a descriptive explanatory analysis of the combined association of temperature and specific humidity on COVID-19 cases for the selected cities. Based on the heatmap, COVID-19 cases were more common in low specific humidity (2–6 g/kg) and low temperature (2–11 °C) conditions. This association was consistent when we consider different incubation times (lag 0–14 days).
Fig. 2.
The density heatmaps of COVID-19 cases in the selected cities in association with temperature and specific humidity at different time lags. The red histogram above each heatmap is the histogram of COVID-19 cases in relation to temperature while the blue histogram beside each heatmap is the one in relationship to specific humidity.
3.2. Distributed lag-non linear models
3.2.1. All locations
Table 2 shows the goodness of fit (qAIC) values across model types for all locations and parameters, which is a common validation and sensitivity technique (e.g., Gasparrini et al., 2010; Guo et al., 2011). In general, the humidity was the strongest predictor for COVID-19 cases, with a better model performance for humidity than temperature across all model types and study locations. Case crossover models performed higher in Seattle, WA, New York City, NY, Chicago, IL, and New Orleans, LA. The variation in the dose-response profile for humidity was negligible before and after adding temperature as a predictor into the model, indicating that humidity exhibited a robust association. Model performance was poor (indicated by high qAIC values) for Detroit, MI, Pittsfield, MA, and Bridgeport, CT. Results for these cities were insignificant and therefore not reported in the final results (Supplementary Fig. 1, Supplementary Fig. 2). Overall, the case-crossover + DLNM model outperformed the DLNM only model. However, select locations had better model fit for DLNM only (e.g., Albany, GA), although marginally better. Results were presented for the following cities: New Orleans, LA, Albany, GA, and Seattle, WA, and models were selected based on qAIC values. DLNM and case-crossover models were also constructed for these locations to analyze the effect of solar radiation (W/m2) on COVID incidence rates.
Table 2.
quasi-AIC values demonstrating model fit for DLNM and CCO models for each city.
| DLNM only |
DLNM + CCO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | Humidity | Humidity + temperature | Temperature | Humidity | Humidity + temperature | Humidity + SD | Humidity + temperature + SD | |
| Seattle, WA | 2.344 | 3.471 | 3.412 | 2.476 | 3.632 | 3.861 | 1.686 | 1.875 |
| Albany, GA | 2.609 | 2.522 | 2.343 | 3.016 | 2.862 | 2.676 | 3.123 | 2.899 |
| New York City, NY | 3.471 | 3.471 | 3.471 | 3.632 | 3.632 | 3.632 | 1.686 | 1.686 |
| Chicago, IL | 1.559 | 2.158 | 2.062 | 1.574 | 1.456 | 1.642 | 1.588 | 1.588 |
| Bridgeport, CT | 11.672 | 10.803 | 12.34 | 13.126 | 11.255 | 12.842 | 12.264 | 13.899 |
| Detroit, MI | 10.618 | 10.618 | 12.236 | 12.272 | 12.272 | 13.947 | 13.781 | 15.342 |
| Pittsfield, MA | 11.102 | 9.232 | 10.422 | 12.676 | 10.649 | 11.922 | 11.55 | 12.854 |
| New Orleans, LA | 3.022 | 3.182 | 3.528 | 2.296 | 2.094 | 2.331 | 2.285 | 2.525 |
3.2.2. New Orleans, LA
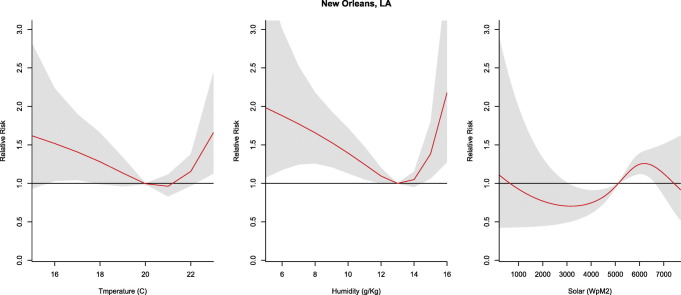
The relative risk for COVID-19 exhibited a U-shaped relationship with increases in cases at high and low humidity in New Orleans. With reference to the median humidity, relative risk peaked at minimum (5 g/kg, RR: 1.98, CI: 1.07–3.66) and maximum (16 g/kg, RR: 2.18, CI: 1.28–3.72) values. Similarly, temperature exhibited a U-shaped relationship with reference to the median and a significant relative risk at 16–17 °C (RR: 1.17–1.23; CI: 1.03–2.24) and at the maximum observed temperatures (23 °C; RR: 1.75, CI: 1.13–2.44). Solar values exhibited an inverted U-shaped relationship with a higher relative risk from 5200 to 6300 (W/m2) (Fig. 3 ).
Fig. 3.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature (far left), humidity (center), and solar radiation (far right) over a 30 day period in New Orleans, LA after adjusting for social distancing, 2020.
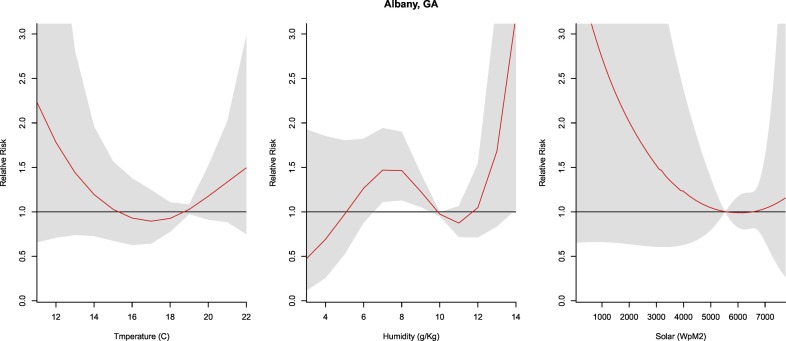
3.2.3. Albany, GA
Temperature and solar radiation were not significant predictors of COVID-19 cases. With reference to the median humidity, significant relative risk is observed from 6 to 9 g/kg (RR: 1.23–1.47, CI: 1.06–1.94). Due to a lower qAIC value and more robust results, unlike other cities, a DLNM-only model was applied to the humidity and the COVID-19 relationship for Albany, GA (Fig. 4 ).
Fig. 4.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature (far left), humidity (center), and solar radiation (far right) over a 30 day period in Albany, GA after adjusting for social distancing, 2020.
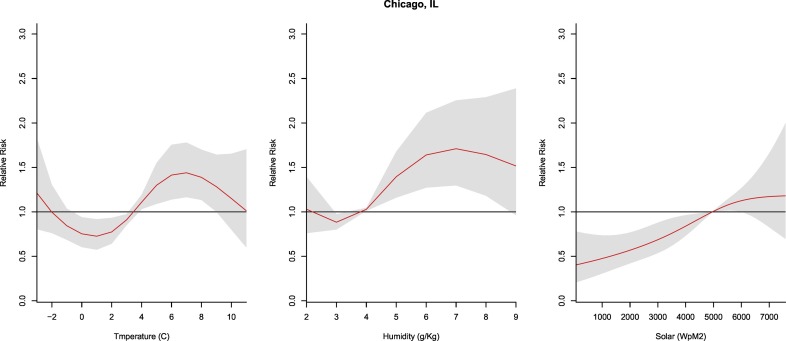
3.2.4. Chicago, IL
The relative risk for humidity values peaked at 7 g/kg (RR: 1.71, CI: 1.3–2.3) and was significant from 4 g/kg to 8 g/kg (RR: 1.03–1.71, CI: 1.01–2.23) in relation to the median. The relative risk for temperature was significant from 4 to 9 °C (RR: 1.11–1.44; CI: 1.03–1.78). Solar radiation demonstrated a linear relationship with lower solar values resulting in lower COVID-19 risk (Fig. 5 ).
Fig. 5.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature (far left), humidity (center), and solar radiation (far right) over a 30 day period in Chicago, IL after adjusting for social distancing, 2020.
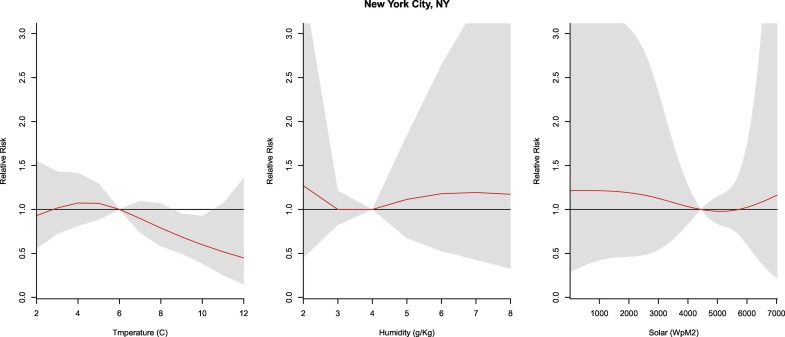
3.2.5. New York City, NY
Temperature exhibited a linear association with the COVID-19 incidence that revealed a protective effect from 9 to 10 °C (RR: 0.60–0.69, CI: 0.39–0.95), whereas no relationship was observed between humidity and solar radiation and COVID-19 cases in NYC (Fig. 6 ).
Fig. 6.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature (far left), humidity (center), and solar radiation (far right) over a 30 day period in New York City, NY after adjusting for social distancing, 2020.
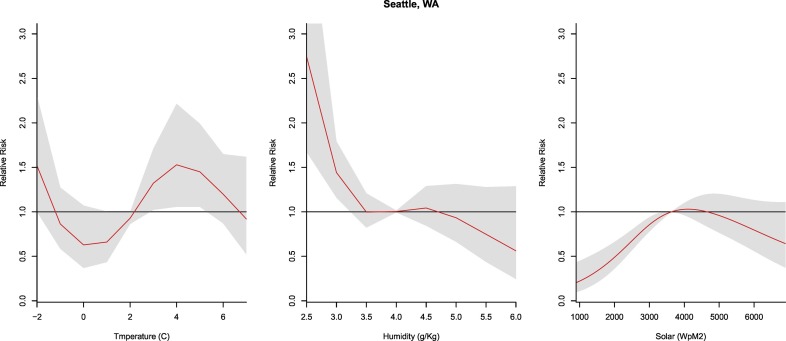
3.2.6. Seattle, WA
The temperature was significant from 3 to 5 °C (RR: 1.59–1.95, CI: 1.22–2.65). However, the humidity was significant at the lowest values with an increased risk of transmission occurring at <3 g/kg (RR: 1.44, CI: 1.16–1.80). Lower risk of transmission was observed for the lowest values of solar radiation (i.e., <3200 W/m2, RR: 0.93, CI: 0.89–0.97) (Fig. 7 ).
Fig. 7.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature (far left), humidity (center), and solar radiation (far right) over a 30 day period in Seattle, WA after adjusting for social distancing, 2020.
3.2.7. The excess burden of new COVID-19 cases due to weather
Overall, the attributable burden of excess COVID-19 cases associated with exposure to humidity and temperature was low for each city (Table 3 ). The excess fraction was the highest for New Orleans, with 3.7 to 4.5% of new cases occurring within the humidity range of 5 g/kg to 16 g/kg and 6.8 to 9.1% occurring within the temperature range of 16(°C) to 23(°C).
Table 3.
The % excess fraction of COVID-19 cases attributable to humidity and temperature exposure in select US cities.
| Prevalence | Humidity |
Temperature |
|
|---|---|---|---|
| EF% | EF% | ||
| Seattle, WA | 5532/745,000 (0.01) | 0.44 | 0.58 to 0.94 |
| Albany, GA | 1479/75,200 (0.02) | 0.46 to 0.93 | – |
| New Orleans, LA | 62,663/1,670,000 (0.04) | 3.78 to 4.51 | 6.75 to 9.11 |
| Chicago, IL | 25,811/2,710,000 (0.01) | 0.03 to 0.70 | 0.11 to 0.44 |
4. Discussion
In this study, we examined whether daily meteorological patterns in humidity, temperature, and solar radiation were associated with the transmission of COVID-19 in U.S. cities that emerged as early hot spots for infection. We applied the DLNM to a case-crossover design to assess the nonlinear and delayed effects of meteorological parameters on COVID-19 incident cases. To our knowledge, this study is the first to assess the effects of meteorological variables on COVID-19 morbidity using a robust distributed lag nonlinear model and case-crossover design. We observed a weak but statistically significant relationship between COVID and meteorological parameters for select locations including Albany, GA, New Orleans, LA, New York City, NY, and Chicago, IL and no relationship for other locations like Pittsfield, MA, Detroit, MI and Bridgeport, CT. Spatially, we found a weaker or insignificant relationship with meteorological variables in the northeastern US (e.g., Pittsfield, MA, Bridgeport, CT, and New York City, NY). In contrast, all southern cities (e.g., Albany, GA, and New Orleans, LA) exhibited a stronger association with meteorological variables. This difference could in part be due to the time period (March–April) where weather daily fluctuations are more prominent depending on the origin of air masses resulting in greater temperature and humidity ranges for southern locations. Although this analysis is based on selected cities in the United States only, this result is similar to results derived from selected cities worldwide with community transmission (Sajadi et al., 2020).
Humidity was observed as the best predictor for the coronavirus outbreak followed by temperature and solar radiation. The majority of cities included in this study demonstrated a nonlinear dose-response relationship between a range of specific humidity conditions and sustained COVID-19 transmission. More specifically, 3 of the 4 cities were characterized by a significant relationship between COVID-19 transmission and humidity (e.g., Albany, GA, New Orleans, LA, and Chicago, IL). Humidity in the range of 6 to 9 g/kg (analogous to an Absolute Humidity range of 7.56–11.37 g/m3) was a significant predictor of COVID-19 cases and resulted in an up to two-fold increased risk of transmission in some areas. Early research in China and other international locations reported a similar relationship between the variability in relatively humid conditions and transmission of COVID-19 (Lou et al., 2020; Shi et al., 2020; Oliveiros et al., 2020; Bukhari et al., 2020; Rahman et al., 2020; Islam et al. 2020). Our results for specific humidity are higher than those reported by Sajadi et al. (2020) who reported optimal transmission at low specific humidity levels (3–6 g/kg) for locations outside of the US.
Temperature and solar radiation did not exhibit a strong association with COVID-19 incidence in our study locations. Our results for New York City, NY support and extend previous research on COVID-19 and meteorological parameters in New York City that found a significant association with temperature using simple correlation coefficients (Bashir 2020). Bashir et al. observed a direct association with higher temperatures predicting higher COVID-19 cases (2020). Conversely, our research found a protective effect at higher temperatures and is corroborated by earlier studies (Qi et al., 2020; Wang et al., 2020) and other respiratory viruses (Moriyama et al., 2020). These mixed findings on the influence of temperature on COVID-19 transmission highlight the need for more analysis across a variety of geographic locations and over a longer time series.
4.1. Future studies
The modeling approach used in this research study can be used to expand upon the evidence-base with the addition of social determinants of health (e.g., age, sex, race, and ethnicity, occupation, income status) to examine the joint and independent effects of social and environmental drivers of COVID-19 transmission. The transmission of respiratory viruses, like COVID-19, is likely to be impacted by a number of factors including meteorological conditions, population density, testing capacity, and geographic disparities in access to and quality of medical care (Dalziel et al., 2018). These factors should be considered in future studies to fully understand the contextual influence of meteorological effects on COVID-19 transmission.
4.2. Strengths and limitations
The main strength of this study was the case-crossover design. This design is used in observational studies to capture short-term effects of exposures and removes the effects of seasonal and secular trends by allowing each COVID-19 case to serve as their own control (e.g., Armstrong et al., 2019; Malig et al., 2016; Guo et al., 2011). This design was particularly advantageous given the limited information available on cases and the short time series under investigation. While the current evidence base is newly emerging, the majority of published studies to date have only examined the relationship between meteorological factors and transmission using descriptive correlation statistics or simple linear regression. One important advantage to the DLNM method is that it not only allows the model to maintain a detailed time course of the non-linear exposure-response relationship, but it also generates an estimate for the overall effect of an exposure on a health outcome in the midst of changes in the effect over different lagged or delay periods (Gasparrini et al., 2010). Unlike previous studies examining the influence of meteorological factors on COVID-19 transmission, an additional strength of our study is the adjustment for social distancing measures (Sajadi et al., 2020).
Most environmental health research includes either a variable for relative humidity (RH) and/or absolute humidity (AH). However, specific humidity, the metric included in our study, is more conservative and less susceptible to changes in pressure and temperature compared to AH. Further, in addition to the confounding influence of humidity and temperature, RH is typically not useful as a stand-alone humidity variable in environmental health or epidemiological research. Our results are comparable to a few recent studies examining the association between COVID-19 and specific humidity (e.g., Ma et al., 2020; Sajadi et al., 2020).
Recent research has demonstrated the linkage between poor air quality and COVID-19 mortality (Wu et al., 2020); however, we did not adjust for background air quality measures as a potential confounding factor in our study. While our modeling strategy did adjust for social distancing measures, our estimates do not account for underreporting of case counts (Lachmann, 2020), demographic data on cases, changes in testing capacity, or the date of onset of COVID-19 symptoms. This study did not include information on the type or amount of testing at each location, as this data was not available at the time of publication. There is currently a void of publicly accessible COVID-19 testing data at the local level. While efforts are underway to capture this data at the state-level, there are a number of inconsistencies relating to reliance on multiple data sources, the timing of the release of these data, and changes in the ways in which states are counting negative and positive test results. However, future research studies should consider including daily testing, as well as other contexutal social and environmental parameters, as control variables for examining the association between meteorological variables and COVID-19 cases.
5. Conclusion
Meteorological factors may influence COVID-19 transmission and spread in the US. The influence of meteorological parameters on COVID-19 was modest and not uniform throughout select study locations. Humidity was the best predictor of COVID-19 transmission compared to solar radiation and temperature in US cities presenting as early emergers in the pandemic. The case-crossover design was an enhancement to the application of DLNM. As an emerging infection, future research is needed to fully understand the impact of environmental conditions on COVID-19 transmission.
The following are the supplementary data related to this article.
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature over a 30 day period in select U.S. Cities after adjusting for social distancing, 2020.
Summary of cumulative exposure-response curves for COVID-19 morbidity for humidity over a 30 day period in select U.S. Cities after adjusting for social distancing, 2020.
CRediT authorship contribution statement
Jennifer D. Runkle: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Margaret M. Sugg: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Ronald D. Leeper: Investigation, Writing - review & editing. Yuhan Rao: Investigation, Writing - review & editing. Jessica L. Mathews: Formal analysis, Methodology, Writing - review & editing. Jared J. Rennie: Investigation, Writing - review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the NCICS COVID-19 Modeling team at the North Carolina Institute for Climate Studies, North Carolina State University for their input during the manuscript development process. We also thank Stella R. Harden for her assistance in the sensitivity analysis and calculation of quasi-AIC values.
Editor: SCOTT SHERIDAN
References
- Araujo M.B., Naimi B. Spread of SARS-CoV-2 Coronavirus likely to be constrained by climate. medRxiv. 2020 [Google Scholar]
- Armstrong B.G., Gasparrini A., Tobias A. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC Med. Res. Methodol. 2014;14(1):122. doi: 10.1186/1471-2288-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B., Sera F., Vicedo-Cabrera A.M., Abrutzky R., Åström D.O., Bell M.L.…Diaz M.H. The Role of Humidity in Associations of High Temperature with Mortality: A Multicountry, Multicity Study. Environmental health perspectives. 2019;127(9):097007. doi: 10.1289/EHP5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari Q., Jameel Y. 2020. Will coronavirus pandemic diminish by summer? Available at SSRN 3556998. [Google Scholar]
- Chen B., Liang H., Yuan X., Hu Y., Xu M., Zhao Y.…Zhu X. medRxiv; 2020. Roles of Meteorological Conditions in COVID-19 Transmission on a Worldwide Scale. [Google Scholar]
- Copernicus Climate Change Service (C3S) ERA5: fifth generation of ECMWF atmospheric reanalyses of the global climate. Copernicus Climate Change Service Climate Data Store (CDS), 2020-04-29. 2017. https://cds.climate.copernicus.eu/cdsapp#!/home
- Dalziel B.D., Kissler S., Gog J.R., Viboud C., Bjørnstad O.N., Metcalf C.J.E., Grenfell B.T. Urbanization and humidity shape the intensity of influenza epidemics in US cities. Science. 2018;362(6410):75–79. doi: 10.1126/science.aat6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J. Stat. Softw. 2011;43(8):1–20. http://www.jstatsoft.org/v43/i08/ URL. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A., Leone M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014;14(1):55. doi: 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models. Stat. Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Barnett A.G., Pan X., Yu W., Tong S. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag nonlinear model. Environ. Health Perspect. 2011;119(12):1719–1725. doi: 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam N., Shabnam S., Erzurumluoglu A.M. Temperature, humidity, and wind speed are associated with lower Covid-19 incidence. medRxiv. 2020 [Google Scholar]
- Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum D.G., Kupper L.L., Morgenstern H. John Wiley & Sons; 1982. Epidemiologic Research: Principles and Quantitative Methods. [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., Van Amerongen G., van Riel D.…Ling A.E. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. The Lancet. 2003;362(9380):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A. medRxiv; 2020. Correcting Under-reported COVID-19 Case Numbers. [Google Scholar]
- Landes M.B., Neil R.B., McCool S.S., Mason B.P., Woron A.M., Garman R.L., Smalley D.L. The frequency and seasonality of influenza and other respiratory viruses in T Tennessee: two influenza seasons of surveillance data, 2010–2012. Influenza Other Respir. Viruses. 2013;7(6):1122–1127. doi: 10.1111/irv.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R.…Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of internal medicine. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J. Clin. Med. 2020:9. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N. Engl. J. Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- Lowen A.C., Steel J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014;88(14):7692–7695. doi: 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Majumder M., Liu D., Poirier C., Mandl K., Lipsitch M., Santillana M. 2020. The Role of Absolute Humidity on Transmission Rates of the COVID-19 Outbreak. Published online February, 17, 2020-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S.…Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Science of The Total Environment. 2020:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malig B.J., Pearson D.L., Chang Y.B., Broadwin R., Basu R., Green R.S., Ostro B. A time-stratified case-crossover study of ambient ozone exposure and emergency department visits for specific respiratory diagnoses in California (2005–2008) Environ. Health Perspect. 2016;124(6):745–753. doi: 10.1289/ehp.1409495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C.M., Haynes A.K., Baumgardner J.L., Chommanard C., Demas S.W., Prill M.M.…Gerber S.I. Determining the seasonality of respiratory syncytial virus in the United States: the impact of increased molecular testing. The Journal of infectious diseases. 2017;216(3):345–355. doi: 10.1093/infdis/jix275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Kohdera U., Hosaka T., Ishii K., Akagawa S., Hiroi S., Kase T. Seasonal variations of respiratory viruses and etiology of human rhinovirus infection in children. J. Clin. Virol. 2015;73:14–19. doi: 10.1016/j.jcv.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Ann. Rev. Virol. 2020;7 doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Notari A. Temperature dependence of COVID-19 transmission. MedRrxiv. 2020 doi: 10.1016/j.scitotenv.2020.144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveiros B., Caramelo L., Ferreira N.C., Caramelo F. Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. medRxiv. 2020 [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W.…Cheng V.C.C. Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W.…Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Science of The Total Environment. 2020:138778. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.A., Hossain M.G., Singha A.C., Islam M.S., Islam M.A. 2020. A Retrospective Analysis of Influence of Environmental/Air Temperature and Relative Humidity on SARS-CoV-2 Outbreak. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- Royé D., Íñiguez C., Tobías A. Comparison of temperature–mortality associations using observed weather station and reanalysis data in 52 Spanish cities. Environmental Research. 2020;183:109237. doi: 10.1016/j.envres.2020.109237. [DOI] [PubMed] [Google Scholar]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. 2020. Temperature and Latitude Analysis to Predict Potential Spread and Seasonality for COVID-19. (Available at SSRN 3550308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P., Dong Y., Yan H., Li X., Zhao C., Liu W.…Xi S. medRxiv; 2020. The impact of temperature and absolute humidity on the coronavirus disease 2019 (COVID-19) outbreak-evidence from China. [Google Scholar]
- Sun Z., Thilakavathy K., Kumar S.S., He G., Liu S.V. Potential factors influencing repeated SARS outbreaks in China. Int. J. Environ. Res. Public Health. 2020;17(5):1633. doi: 10.3390/ijerph17051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklo M., Nieto F.J. Jones & Bartlett Publishers; 2014. Epidemiology: Beyond the Basics. [Google Scholar]
- Tamerius J., Nelson M.I., Zhou S.Z., Viboud C., Miller M.A., Alonso W.J. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ. Health Perspect. 2011;119(4):439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J.D., Shaman J., Alonso W.J., Bloom-Feshbach K., Uejio C.K., Comrie A., Viboud C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;(3):9. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. 2020. High Temperature and High Humidity Reduce the Transmission of COVID-19. (Available at SSRN 3551767) [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. medRxiv; 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of cumulative exposure-response curves for COVID-19 morbidity for temperature over a 30 day period in select U.S. Cities after adjusting for social distancing, 2020.
Summary of cumulative exposure-response curves for COVID-19 morbidity for humidity over a 30 day period in select U.S. Cities after adjusting for social distancing, 2020.