Key Question 1
Should Clinicians Use Chloroquine or Hydroxychloroquine Alone or in Combination With Azithromycin for Prophylaxis Against COVID-19?
Key Question 2
Should Clinicians Use Chloroquine or Hydroxychloroquine Alone or in Combination With Azithromycin for Treatment of COVID-19?
Background
Using chloroquine or hydroxychloroquine, with or without azithromycin, to prevent coronavirus disease (COVID-19) after infection with novel coronavirus (SARS-CoV-2) or to treat COVID-19 began to receive attention following preliminary reports from in vitro (1) and human (2) studies. While multiple studies are planned or under way (3, 4), it is imperative to continually synthesize the results from the best available evidence to inform point-of-care decisions about the use of chloroquine or hydroxychloroquine. These practice points are based on a rapid and living systematic evidence review conducted by the University of Connecticut Health Outcomes, Policy, and Evidence Synthesis Group and will be updated as new evidence becomes available. The practice points development and update methods are included in the Appendix, available at Annals.org. This version of the practice points, based on an evidence review conducted on 17 April 2020, was approved by the American College of Physicians Board of Regents on 4 May 2020 and submitted to Annals of Internal Medicine on 6 May 2020.
Practice Points
The efficacy of chloroquine or hydroxychloroquine alone or in combination with azithromycin to prevent COVID-19 after infection with SARS-CoV-2 or to treat patients with COVID-19 is not established and future clinical trials are needed to answer these questions. There are known harms of these medications when used to treat other diseases (5, 6). Current evidence about efficacy and harms for use in the context of COVID-19 is sparse, conflicting, and from low quality studies, increasing the uncertainty and lowering our confidence in the conclusions of these studies when assessing the benefits or understanding the balance when compared with harms. These interim practice points are based on best available evidence. We will maintain these practice points as a living guidance document, updated as new evidence becomes available.
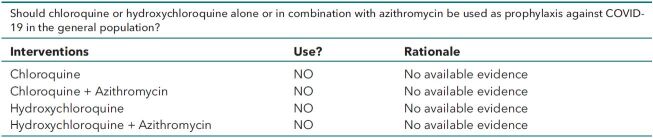
• Do not use chloroquine or hydroxychloroquine alone or in combination with azithromycin as prophylaxis against COVID-19 due to known harms and no available evidence of benefits in the general population.
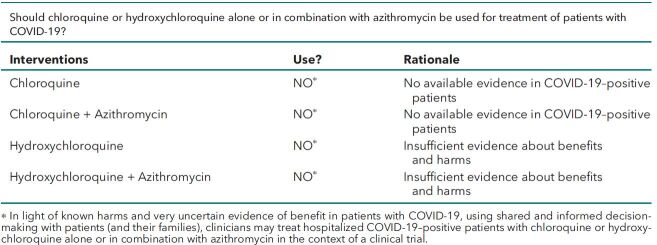
• Do not use chloroquine or hydroxychloroquine alone or in combination with azithromycin as a treatment of patients with COVID-19 due to known harms and no available evidence of benefits in patients with COVID-19.
• In light of known harms and very uncertain evidence of benefit in patients with COVID-19, using shared and informed decision making with patients (and their families), clinicians may treat hospitalized COVID-19–positive patients with chloroquine or hydroxychloroquine alone or in combination with azithromycin in the context of a clinical trial.
Should chloroquine or hydroxychloroquine alone or in combination with azithromycin be used as prophylaxis against COVID-19 in the general population?
Should chloroquine or hydroxychloroquine alone or in combination with azithromycin be used for treatment of patients with COVID-19?
Figure.
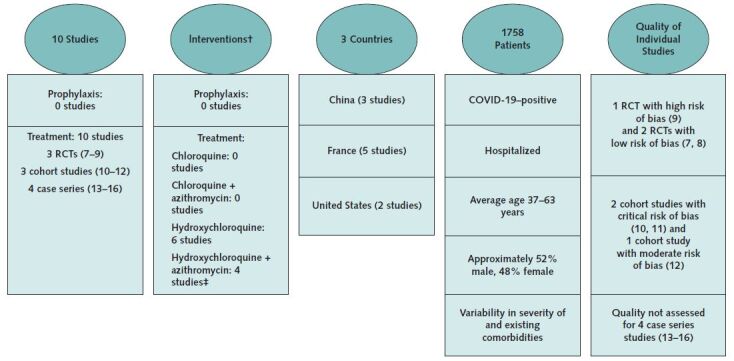
Evidence Description for COVID-19 Studies*.
COVID-19 = coronavirus disease 2019; RCT = randomized controlled trial.
* Evidence search was conducted by the University of Connecticut Health Outcomes, Policy, and Evidence Synthesis Group. Current search for evidence, completed on 17 April 2020, aimed to identify all studies about the use of chloroquine or hydroxychloroquine alone or in combination for prophylaxis or treatment of patients with COVID-19. (See Supplement, available at Annals.org.)
† The use and extent of parallel treatment interventions was difficult to determine. For example, in some studies, it was documented that patients received parallel interventions, whereas in other studies there was insufficient information to determine if patients did or did not receive parallel interventions.
‡ In 2 cohort studies (11, 12), the administration of azithromycin was not randomized, precluding judgment of efficacy
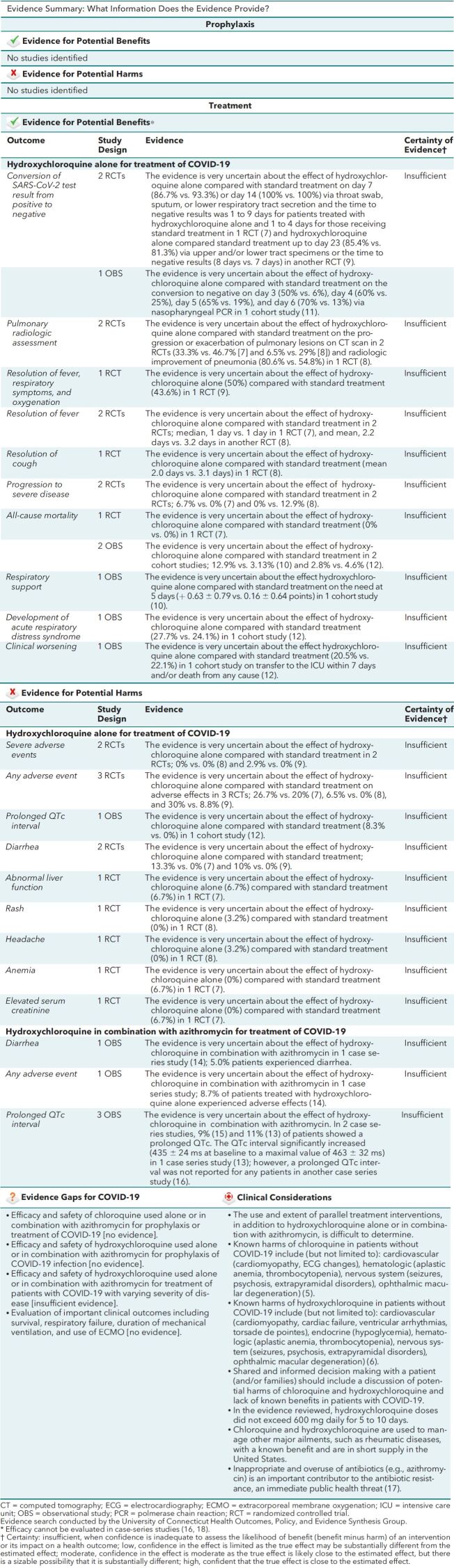
Evidence Summary: What Information Does the Evidence Provide?
Biography
Note: The Practice Points are developed by the Scientific Medical Policy Committee of the American College of Physicians. The Practice Points are “guides” only and may not apply to all patients and all clinical situations. All Practice Points are considered automatically withdrawn or invalid 5 years after publication, or once an update has been issued.
Acknowledgment: The Scientific Medical Policy Committee thanks Adrian V. Hernandez, MD, PhD; Yuani M. Roman, MD, MPH; Vinay Pasupuleti, MD, MS, PhD; Joshuan J. Barboza, MSc; and C. Michael White, PharmD, of the University of Connecticut Health Outcomes, Policy, and Evidence Synthesis Group for conducting the rapid evidence review that informed the development of these Practice Points.
Financial Support: Financial support for the development of the Practice Points comes exclusively from the ACP operating budget.
Disclosures: All financial and intellectual disclosures of interest were declared and potential conflicts were discussed and managed. A record of disclosures of interest and management of conflicts of is kept for each Scientific Medical Policy Committee meeting and conference call and can be viewed at https://www.acponline.org/about-acp/who-we-are/leadership/boards-committees-councils/scientific-medical-policy-committee/ disclosure-of-interests-and-conflict-of-interest-managementsummary-for-scientific-medical-policy. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-1998.
Corresponding Author: Amir Qaseem, MD, PhD, MHA, American College of Physicians, 190. N Independence Mall West, Philadelphia, PA 19106; e-mail, aqaseem@acponline.org. Current author addresses and author contributions are available at Annals.org.
Current Author Addresses: Dr. Qaseem: American College of Physicians, 190. N Independence Mall West, Philadelphia, PA 19106.
Dr. Yost: 800 Lancaster Avenue, Villanova, PA 19085.
Dr. Etxeandia-Ikobaltzeta: 1, Santa Margarita Hospital Street, Ground Floor 2,Office1,Room2,20303Irun, Gipuzkoa, Spain.
Dr. Miller: Penn Medicine Radnor, 250 King of Prussia Road, Radnor, PA 19087.
Dr. Abraham: 123 Summer Street, Suite 370, North Worcester, MA01608.
Dr. Obley: 3030 SW Moody, Suite 250, Portland, OR 97201.
Dr. Forciea: 3615 Chestnut Street, Philadelphia, PA 19104.
Dr. Jokela: 1405 West Park, #207, Champaign, IL 61801.
Dr. Humphrey: 3710 SW U.S. Veterans Hospital Road, Portland, OR 97201.
Author Contributions: Conception and design: I. Etxeandia- Ikobaltzeta, J.A.Jokela, M.Marcucci, A.Obley, A.Qaseem, J.Yost.
Analysis and interpretation of the data: L.L. Humphrey, J.A. Jokela, M.C. Miller, A. Qaseem, J. Yost.
Drafting of the article: G.M. Abraham, I. Etxeandia-Ikobaltzeta, J.A. Jokela, A. Qaseem, J. Yost.
Critical revision for important intellectual content: G.M. Abraham, E.A. Akl, R. Andrews, R.M. Centor, I. Etxeandia- Ikobaltzeta, M.A. Forciea, L.L. Humphrey, J.A. Jokela, D. Kansagara, M. Marcucci, A. Obley, A. Qaseem, J. Yost.
Final approval of the article: G.M.Abraham, E.A. Akl, R. Andrews, T.A. Bledsoe, R.M. Centor, I. Etxeandia-Ikobaltzeta, M.A. Forciea, R.A. Haeme, L.L. Humphrey, J.A. Jokela, D. Kansagara, M. Marcucci, M.C.Miller, A.Obley, A.Qaseem, J.Yost.
Statistical expertise: A. Qaseem.
Administrative, technical, or logistic support: A. Qaseem.
Correction: This article was corrected on 26 May 2020 to correct several errors, which are detailed in the Correction (www.annals.org/doi/10.7326/L20-0684).
Appendix: Practice Points Development Process
The Scientific Medical Policy Committee (SMPC), in collaboration with staff from ACP's Department of Clinical Policy, developed these Practice Points based on a rapid systematic evidence review conducted by the University of Connecticut Health Outcomes, Policy, and Evidence Synthesis Group. The SMPC comprises 11 internal medicine physicians representing various clinical areas of expertise and 1 public (nonclinician) member and includes members with expertise in epidemiology, healthy policy, and evidence synthesis. In addition to contributing clinical, scientific, and methodological expertise, Clinical Policy staff provided administrative support and liaised among the SMPC, evidence review funding entity and evidence team, and the journal. Clinical Policy staff and the SMPC reviewed and prioritized potential topic suggestions from ACP members, SMPC members, and ACP governance. A committee subgroup, including the chair of SMPC, worked with staff to draft the key questions and lead the development of the Practice Points. Clinical Policy staff worked with the subgroup and the evidence review team to refine the key question(s) and determine appropriate evidence synthesis methods for each key question. Via conference calls and e-mail, Clinical Policy staff worked with the committee subgroup to draft the Practice Points based on the results of the rapid systematic evidence review. The full SMPC reviewed and approved the final Practice Points. Before publication, ACP's Executive Committee of the Board of Regents also reviewed and approved the Practice Points on behalf of the ACP Board of Regents. The evidence review will be continually updated by the evidence review team. ACP will update the Practice Points based on the evidence review using the same process as for Version 1 (described above).
Footnotes
This article was published at Annals.org on 13 May 2020.
* This paper, written by Amir Qaseem, MD, PhD, MHA; Jennifer Yost, RN, PhD; Itziar Etxeandia-Ikobaltzeta, PharmD, PhD; Matthew C. Miller, MD; George M. Abraham, MD, MPH; Adam J. Obley, MD; Mary Ann Forciea, MD; Janet A. Jokela, MD, MPH; and Linda L. Humphrey, MD, MPH, was developed for the Scientific Medical Policy Committee of the American College of Physicians. Individuals who authored this work and served on the Scientific Medical Policy Committee from initiation of the project until its approval were Linda L. Humphrey, MD, MPH (Chair); Robert M. Centor, MD (Vice Chair); Elie A. Akl, MD, MPH, PhD; Rebecca Andrews, MS, MD; Thomas A. Bledsoe, MD; Mary Ann Forciea, MD; Ray Haeme†; Janet A. Jokela, MD, MPH; Devan L. Kansagara, MD, MCR; Maura Marcucci, MD, MSc; Matthew C. Miller, MD; and Adam Jacob Obley, MD. Approved by the ACP Board of Regents on 4 May 2020.
† Nonphysician public representative.
References
- 1. doi: 10.1038/s41422-020-0282-0. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro [Letter]. Cell Res. 2020;30:269-271. [PMID: 32020029] doi:10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed]
- 2. doi: 10.5582/bst.2020.01047. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [PMID: 32074550] doi:10.5582/bst.2020.01047. [DOI] [PubMed]
- 3. U.S. National Library of Medicine. ClinicalTrials.gov. Accessed at https://clinicaltrials.gov/ct2/home. on 3 April 2020.
- 4. Belhadi D, Peiffer-Smadja N, Lescure F-X, et al. A brief review of antiviral drugs evaluated in registered clinical trials for COVID- 19. Preprint. Posted 28 March 2020. medRxiv. doi:10.1101/ 2020.03.18.20038190.
- 5. Aralen Chloroquine Prescribing Information. Sanofi-Aventis; March 2013. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2013/006002s043lbl.pdf. on 3 April 2020.
- 6. Plaquenil Hydroxychloroquine Prescribing Information. Concordia Pharmaceuticals; January 2017. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf. on 3 April 2020.
- 7. doi: 10.3785/j.issn.1008-9292.2020.03.03. Chen J, Ping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). Journal of Zhejiang University (Medical Science). 2020;49. doi:10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed]
- 8. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. Preprint. Posted 10 April 2020. medRxiv. doi:10.1101/ 2020.03.22.20040758.
- 9. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. Preprint. Posted online 14 April 2020. medRxiv. doi:2020.04. 10.20060558.
- 10. Barbosa J, Kaitis D, Freedman R, et al. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. NEJM submission ID 20- 08882. 4 April 2020.
- 11. doi: 10.1016/j.ijantimicag.2020.105949. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. [PMID: 32205204] doi:10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed]
- 12. Mahevas M, Tran VT, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized forCOVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. Preprint. Posted online 14 April 2020. medRxiv. doi:10.1101/2020.04.10.20060699.
- 13. Chorin E, Dai M, Shulman E, et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/ azithromycin. Preprint. Posted 3 April 2020. medRxiv. doi:10.1101/ 2020.04.02.20047050.
- 14. doi: 10.1016/j.tmaid.2020.101663. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020:101663. [PMID: 32289548] doi:10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed]
- 15. doi: 10.1016/j.medmal.2020.03.006. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection [Letter]. Med Mal Infect. 2020. [PMID: 32240719] doi:10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed]
- 16. Lowe D. The latest hydroxychloroquine data, as of April 11. In the Pipeline blog. 11 April 2020. Accessed at https:// blogs.sciencemag.org/pipeline/archives/2020/04/11/the-latesthydroxychloroquine- data-as-of-april-11 on 12 April 2020.
- 17. doi: 10.7326/M15-1840. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425- 434. [PMID: 26785402] doi:10.7326/M15-1840. [DOI] [PubMed]
- 18. doi: 10.4103/0019-5413.30519. Petrisor B, Bhandari M. The hierarchy of evidence: levels and grades of recommendation. Indian J Orthop. 2007;41:11-15. [PMID: 21124676] doi:10.4103/0019-5413.30519. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.