Abstract
The prevalence of major depressive disorder (MDD) is higher in women than men. Importantly, a differential behavioral response by sex to the antidepressant response to ketamine in rodents has been reported. Mechanistically, male depressed-like animals showed an increased spine density after ketamine treatment via restoration of synaptic protein levels while those proteins were not altered in female rats. In addition, preclinical studies indicate that the impairment of astrocytic plasticity is one of the contributing mechanisms in the pathophysiology of MDD. Accordingly, in this study, we determined the effect of sex on the rapid morphological alteration of hippocampal astrocytes and the serum level of BDNF one hour after S-ketamine injection. A single intraperitoneal dose of S-ketamine (15 mg/kg) or saline was injected to the male and female Flinders Sensitive Line (FSL) rats, a genetic animal model of depression and their brains were perfused one hour after treatment. The size of the GFAP positive astrocytes in the hippocampal subregions was measured. The volume of different hippocampal subregions was assessed using the Cavalieri estimator. Moreover, serum levels of BDNF were measured with enzyme-linked immunosorbent assay (ELISA) kits. The volume of hippocampal subregions significantly increased one hour after S-ketamine in both male and female FSL animals. However, a substantial alteration in the morphology of the hippocampal astrocytes was observed only in the female rats. Additionally, significantly increased serum BDNF levels in the female depressed rats were observed one hour after S-ketamine treatment. Our results indicate that the rapid effects of S-ketamine on the morphology of the hippocampal astrocytes and the serum level of BDNF are sex-dependent.
Keywords: Antidepressant, Astrocyte, BDNF, Ketamine, Hippocampus
1. Introduction
Major depressive disorder (MDD) is a serious neuropsychological disorder with a higher prevalence in women (8–10%) than in men (3–5%) (Kessler et al., 2005). Currently three main therapeutic strategies (pharmacotherapy, psychotherapy and electroconvulsive therapy) are used for MDD but none of them provides an optimal rapid and sustained clinical remission. The delay in response to conventional antidepressants, despite the rapid actions at the pharmacological target, leaves severely depressed patients at a risk of suicide. Consequently, there is an unmet need to develop more efficient drugs with a fast onset of antidepressant effect, minimal side effects and broader efficacy. The fast onset of action of glutamate receptor modulators, especially ketamine, is a promising feature for developing antidepressant drugs. In clinical studies, the antidepressant efficacy of ketamine in treatment-resistant depression has been reported between 25% and 100% with the onset of action within hours (Drewniany et al., 2015; Henter et al., 2017; Jaso et al., 2017; Murrough et al., 2013a, 2013b; Pereira et al., 2019; Sanacora et al., 2017). Preclinical studies have suggested that the rapid antidepressant effect of ketamine on the behavior in rodents is modulated differently in the prefrontal cortex (through mammalian target of rapamycin pathway, mTOR) and hippocampus (regulating eukaryotic elongation factor 2 (eEF2) kinase) (Zanos and Gould, 2018). Furthermore, the studies indicate that the release of brain-derived neurotrophic factor (BDNF) is required for the rapid antidepressant effect of ketamine (Autry et al., 2011; Huang et al., 2017; Lepack et al., 2015). In a different context, it has been reported that dysfunction of astrocytes results in the impairment of the synthesis of neurotrophic and angiogenic factors and glutamate metabolism (Acker et al., 2001; Erickson et al., 2001 ), and post-mortem studies have shown a reduction in the number of glia cells in the frontal cortex of depressed patients (Cotter et al., 2002; Rajkowska and Stockmeier, 2013). Crucially, it is not clear whether the structural alteration of astrocytes, including changes in their number and morphology, is the cause or the consequence of functional impairment of astrocytes in psychiatric disorders. Interestingly, female Sprague-Dawley rats respond to a lower dose of ketamine (2.5 mg/kg) (Carrier and Kabbaj, 2013), and the fast and sustained antidepressant-like effects of ketamine in the forced swim test (FST) are sex-dependent, suggesting that presynaptic release of glutamate in the hippocampus by ketamine could be sex-dependent (Franceschelli et al., 2015).
Therefore, the overriding aims of the present work were to examine possible effects of sex on the structural alterations of astrocytes in depression following ketamine, paired with investigations of the BDNF levels. Specifically, we examined (i) the morphological alteration of hippocampal astrocytes; and (ii) the serum levels of BDNF, one hour after S-ketamine treatment of male and female FSL rats, a genetic rat model of depression.
2. Experimental procedures
2.1. Animals
Adult male and female Flinders Sensitive Line (FSL) rats (male, n = 12; female, n = 10) with the average age of 90 days originating from the colonies at the Karolinska Institute were included in this study. Female rats housed in same cages/same conditions synchronize their estrous cycle (Jimenez-Vasquez et al., 2000; Saland and Kabbaj, 2018).
Animal care was carried out in accordance with the guidelines issued by the Danish committee on animal ethics (permission id 2012–15-2934–00254). The rats were housed in groups of two and the room temperature was maintained at (20–22 °C) with a normal 12 h light: dark cycle and free access to the food and water.
2.2. Treatments
Based on the treatment design, animals were assigned randomly to four groups (n 5–6/group) receiving intraperitoneally either a single (15 mg/kg) dose of the clinically used formulation of Sketamine HCI (Pfizer ApS, Denmark, ATC-code NOIAX14) (du Jardin et al., 2016; Muller et al., 2013) or saline as a vehicle group. The dose of ketamine administrated in this study was based on previous studies (Hunt et al., 2006; Liebenberg et al., 2015).
2.3. Tissue preparation
One hour after the S-ketamine injection, the animals were deeply anesthetized with an intraperitoneal injection of pentobarbital sodium/lidocaine (Unikem A/S, Copenhagen, Denmark) and perfused transcardially with heparinized (10 U/ml) 0.9% saline (pH=7.3) for 4 min, followed by ice cold 4% paraformaldehyde (pH=7.2–7.4) for 6 min. Following a random selection of the right or left hemisphere of the brains, the brains were embedded in 5% agar and were cut in 60-μm thick coronal sections on a vibratome 1200 S (Leica VT 1200 S vibrating blade microtome). The first section of each series was randomly chosen by using a random table.
Two sets of sections were selected based on a systematic sampling principle, and a section sampling fraction of 1/10. One set of sections was Nissl stained with a 0.25% thionin solution (thionin, Sigma T3387) and used for quantifying the hippocampal subfields volume. The second set of sections was stained for glial fibrillary acidic protein (GFAP), quantifying the size of GFAP positive astrocytes in the hippocampal subfields.
2.4. Immunohistochemistry for GFAP
Free-floating sections were washed in tris buffered saline (TBS) containing 0.1% Triton X-100 for 30 min followed by blocking endogenous peroxidase (30% H202 and methanol dissolved in TBS) for 30 min. Antigen retrieval prior to immunohistochemistry (IHC) was performed by rinsing sections in the boiled retrieval solution (Dako, Ref# Sl699, Denmark) dissolved in distilled water and heating in the microwave oven for 30 min. Thereafter, sections were washed 3 times in 1% BSA and 0.3% Triton-X-100 in TBS for 10 mim In the next step, sections were incubated with a polyclonal rabbit anti-GFAP (Dako, Ref# Z0334, Denmark) at 1 :500 dilutions in 1% BSA, TB buffer 50 rnM for 48 h at 4 0C. Subsequently, sections were washed in TBS with 0.1% BSA and Triton X-100, and then incubated in polyclonal secondary goat anti-rabbit lgG antibody/HRP (1 :200 dilution; Dako, Ref# P0448, Denmark) for 2 h followed by washing 3 times (10 min for each time) in TBS. The immunolabelling was done by using 3,3’diaminobenzidine (DAB) solution (Sigma, USA) for one min. Finally, the sections were mounted on the gelatin-coated slides, dried for 20 min, re-hydrated in demineralized water for 15 min, stained with 0.25% thionin solution (thionin, Sigma T3387), dehydrated through a graded series of alcohol (50%, 70%, 96%, 99%), cleared in xylene for 15 min and coverslipped.
2.5. Estimation of the hippocampal subfields volumes
The volumes of three hippocampal subfields (CAI stratum radiatum, granular cell layer and molecular layer of dentate gyrus) were quantified on Nissl stained sections by applying the Cavalieri estimator. Point counting (Gundersen et al., 1988) using the newCAST software (Visiopharm, Hørsholm, Denmark), an Olympus light microscope (Olympus BX50, Olympus, Denmark) modified for stereology with a digital camera (Olympus DP72, Olympus, Denmark), a motorized microscope stage (Prior H138 with controller H29, Cambridge, UK) and a 10 × objective lens (Olympus, Splan, N.A. 0.45) was performed.
The following formula was applied to measure the volume of hippocampal subregions:
where ΣP is the total number of the points hitting the region of interest, (a/p) is the area per point (0.007 mm2); T is the section thickness (60 μm) and SSF is the section sampling fraction (1/10)
2.6. Measurement of the size of the astrocytes
The quantification of the size of astrocytes was done by measuring the volume of astrocytes with a 3D nucleator and the longest diameter of astrocytes in two subregions of hippocampus: CAI.stratum radiatum (CAI.SR) and molecular layer of dentate gyrus (MDG) on GFAP stained sections. Delineation of CAI SR and MDG subregions were performed according to the rat brain atlas (Paxinos and Watson, 2006) using a 4 × objective lens (Olympus, Plan Apochromat, N.A. 0.20). Based on the division of granular cell layer of DG (GCL) along the transverse axis into the supra-pyramidal blade (located between the CA3 and CAI areas) and infra-pyramidal blade (located below the CA3 subfield) (Amaral et al., 2007), MDG was divided into supra-MDG and infra-MDG areas. By using 3D nucleator, the number of half-lines was set at 6 and the mode was vertical uniform random (VI-JR) based on the assumption of rotational symmetry of the astrocytes. Moreover, in this study, we did measure the size of the astrocytes by quantifying the longest diameter of the astrocytes.
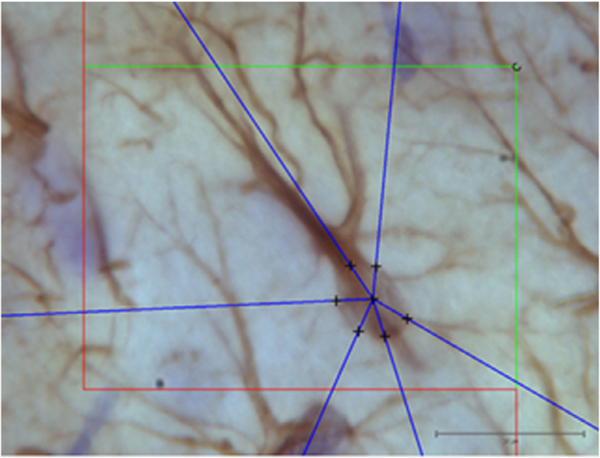
Volume and diameter of GFAP-immunopositive astrocytes were estimated with a 100 × oil-immersion objective lens (Olympus, Plan Apochromat, N.A. 1.25) (Fig 1). We sampled randomly 50–80 astrocytes per animal by using optical disector.
Fig. 1.
Illustration of the 3D nucleator tool for estimating the volume of GFAP positive astrocytes. Six half-lines (blue) were used under a 100 × objective lens. Scale bar is 20 μm.
2.7. Measurement of the serum levels of BDNF with an enzyme-linked immunosorbent assay (ELISA)
Blood was obtained directly from the heart during the transcardial perfusion procedure and collected in anticoagulant-free tubes with gel (Terumo, Venosafe™, VF-054SAS). The tubes were kept at room temperature for 1 h before centrifugation at 1500 g × 10 min at 4 °C, whereafter serum was collected and stored at −80 °C before quantification of the BDNF levels with commercially available ELISA kits (Quantikine BDNF ELISA kits, R&D Systems, Denmark). The standard curve (ranging from 17 to 1133 pg/ml) and the samples were run in duplicate. The samples were diluted 1:20. The determination was processed according to the manufacturer; specifications and the absorbance was immediately measured at 450 nm with wavelength correction set to 540 nm (EL 800 Universal Microplate reader, Bio-Tek instruments, INC).
2.8. Statistical analysis
Statistical analysis of data was performed by IBM SPSS Statistics 22 program and graphs were created with Sigmaplot 12.5 (SYSTAT, San Jose, CA). The rapid effect of S-ketamine treatment on the morphology of astrocytes and the serum levels of BDNF was examined by two-way analysis of variance (ANOVA) followed by a post hoc LSD test. The normal distribution of variables was tested before doing the one-way ANOVA, by generating Q-Q plots and histograms for each variable.
Two tailed Pearson analysis and r2 used to show the correlation between different structural parameters, with a two-tailed probability level of p<0.05 being used as the significance level.
3. Results
3.1. Volumes of the hippocampal subregions
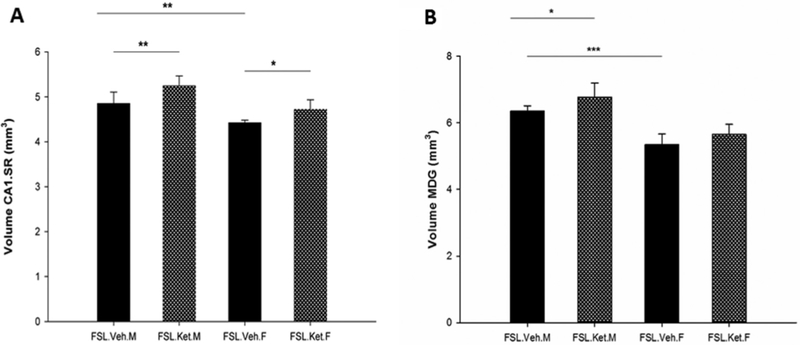
In the first set of observations, we examined the volumes of the hippocampal subregions. The volumes of the hippocampal subregions were notably influenced by sex F1,18 = 27.79 to 62.28; p = 0.000). Moreover, ketamine treatment had significant effects on the volumes of CAI.SR and MDG (F1,18 = 15.87, p = 0.001; F1.18 = 7.31, p = 0.01). We found a significant baseline difference in the volumes of the CAI.SR and MDG subfields of the hippocampus between depressed male-vehicle and female-vehicle rats (p = 0.002; p = 0.000, respectively), S-ketamine administration significantly increased the volume of the CAI.SR area in the female and male FSL rats (p = 0.032; p = 0.003, respectively, Fig. 2A). Regarding the volume of GCL, there was no difference between the vehicle and S-ketamine treatment groups in either male or female rats (p>0.05). In addition, S-ketamine significantly increased the volume of MDG In male rats (p = 0.031) but not in female rats (p>0.05) (Fig 2B).
Fig. 2.
Volume of the CAI.SR (A) and MDG (B) areas of the hippocampus in female and male FSL rats one hour after a single S-ketamine (15 mg/kg) or saline injection, *p<0.05, **p<O.01, ***p<0.001, F=female, M=male.
3.2. The size of the astrocytes in the hippocampal subregions
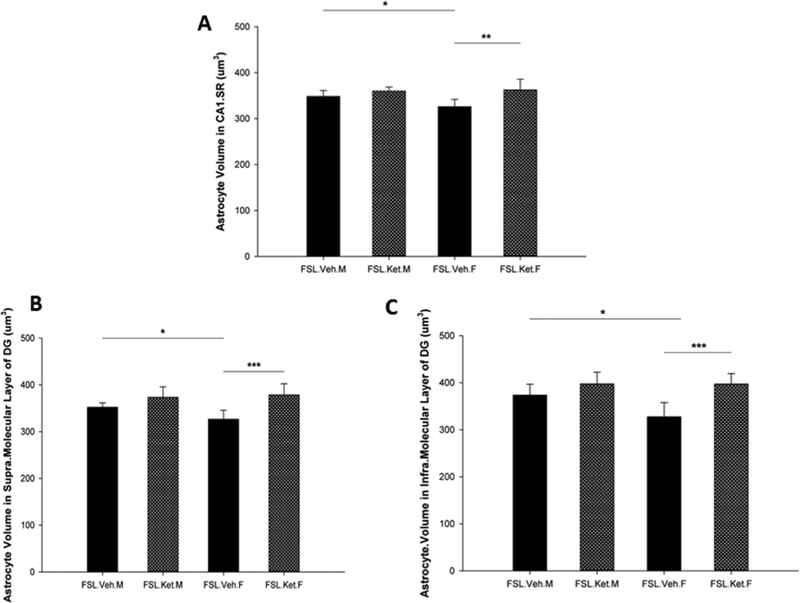
A significant effect of sex × treatment interaction was observed on the volume of astrocytes in the infra-pyramidal blade of DG F1,18 = p = 0.046). Moreover, a significant effect of ketamine treatment on the volume of astrocytes in the hippocampal subregions (CAI.SR, supra-and infrapyramidal blades of DG) was found F1,18 p = 0.002; p = 0.000; F1,18 = 18.88 p = 0.000). We found that the volume and diameter of the GFAP positive astrocytes in the CAI.SR area of the hippocampus were significantly larger in male-vehicle treated rats compared with the female-vehicle treated group (p = 0.03, The astrocyte size, including both volume and diameter of cell in the supra-and infra-molecular layers of DG, was significantly larger in the male-vehicle rats compared with the female-vehicle rats (Fig. 3B and, C).
Fig. 3.
Volume of the GFAP positive astrocytes in the CAI.SR (A), supra-molecular layer of DG (B) and infra-molecular layer of DG in the hippocampus in female and male FSL rats one hour after a single S-ketamine (15 mg/kg) or saline injection, *p<0.05 **p<0.01, F=female, M=male.
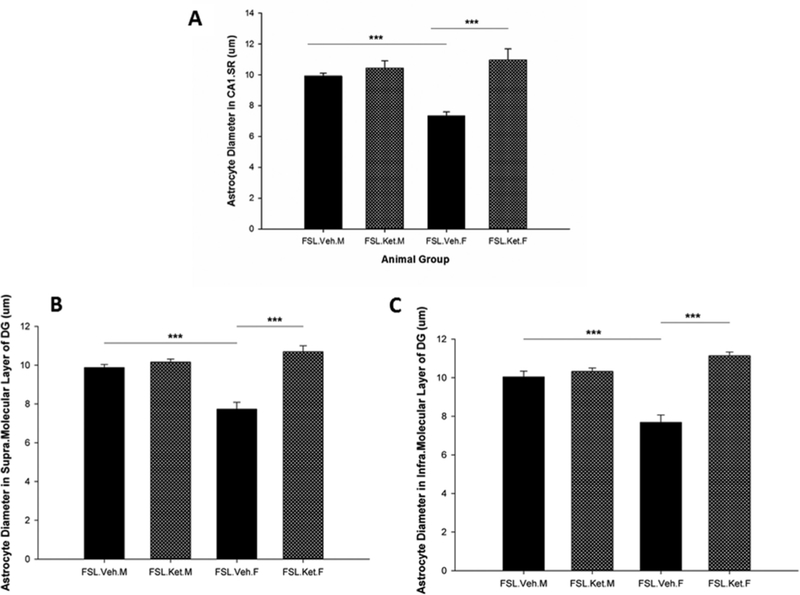
Regarding the effect of S-ketamine on the size of the astrocytes, the results showed a quantitative augmentation in the volume and diameter of astrocytes in the CAI.SR area of the hippocampus one hour after a single injection of S-ketamine in the female rats (p<0.05). In contrast, there was no statistical difference in the size of the astrocytes in the CAI SR subregion in the male-depressed rats after S-ketamine treatment (p>0.05) (Fig. 3A). Notably, our results demonstrated that astrocytes in the supraand infra-molecular layers of DG from female rats with S-ketamine treatment are significantly larger in comparison with female rats without treatment (p = 0.000; p = 0.000). However, in the male rats S-ketamine did not change the size of the astrocytes in the supra-and infra-molecular layers of DG, one hour after injection (p = 0.06; p = 0.12, 3B, and C). Importantly, we found the results of diameters of the astrocytes in the hippocampal subregions to be in line with the results of volume of astrocytes, in that significant changes in the diameter of astrocytes one hour after a single dose of S-ketamine in female FSL rats (p = 0.000) without significant effect in the male FSL rats (p>0.05, Fig. 4) were found. Pearson Correlation analysis and r2 showed a positive correlation between the volume of the CAI.SR subregion of the hippocampus and the size of the astrocytes in this area (r = 0.60, p = 0.003, R2 = 0.36). Additionally, we found a positive correlation between the volume of astrocytes in supra-and infra-molecular layers of DG and the total volume of MDG area (r = 0.46, p = 0.03, R2 = 0.21; r = 0.52, p = 0.01, R2 = 0.28), respectively.
Fig. 4.
Diameter of the GFAP positive astrocytes in the CAI.SR (A), supra.molecular layer of DG (B) and infra.molecular layer of DG (C) in female and male FSL rats one hour after a single S-ketamine (15 mg/kg) or saline injection, ***p<0.001.
3.3. The serum level of BDNF
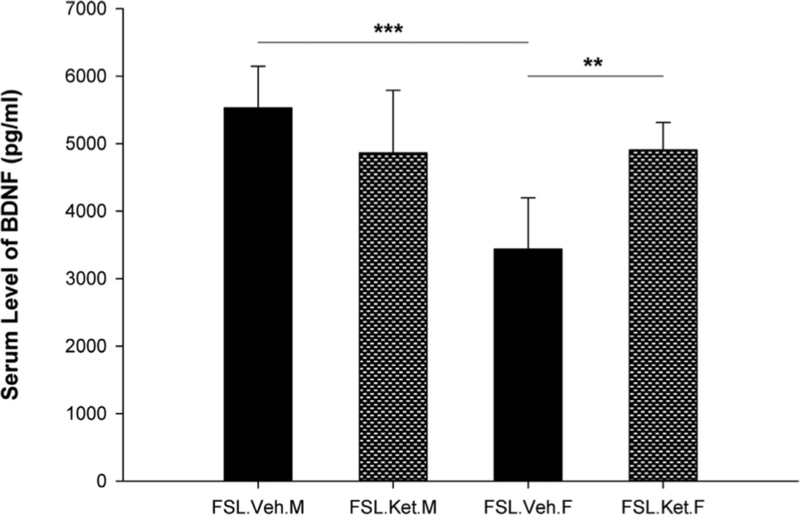
In the final set of observations, we assessed the serum levels of BDNF. Results of two-way ANOVA analysis showed significant effect of sex × treatment interaction on the serum levels of BDNF (F1,18 = 12.21; p = 0.003). We found that the serum BDNF levels were significantly higher in the male FSL-vehicle treated rats in comparison with female FSL-vehicle treated rats (p = 0.000). Moreover, one hour after a single dose of S-ketamine, an increase in the serum concentration of BDNF in the female FSL rats was observed (p = 0.004). However, in the male FSL rats no significant difference was observed between vehicle and S-ketamine treatment groups (p = 0.12) (Fig. 5).
Fig. 5.
Serum level of BDNF in the female and male FSL rats one hour after a single S-ketamine (15 mg/kg) or saline injection, **p<0.01, ***p<0.001, F=female, M=male.
4. Discussion
The main findings in the present study are the difference between sexes in response to S-ketamine and the rapid effect observed on the morphological levels, one hour following S-ketamine administration.
To test the hypothesis of sex-differentiated cellular and neurotrophic factor responses to S-ketamine, we compared the morphological alteration of the hippocampal astrocytes and the serum levels of BDNF one hour after a single dose of S-ketamine in male and female FSL rats. This question is relevant, as it has been reported that fast and sustained antidepressant-like effects of ketamine in the forced swim test (FST) are sex-dependent. Female stressed mice responded to lower doses of ketamine. However, ketamine (10 mg/kg) had a sustained (7 days) antidepressant effect in male but not in the female animals (Franceschelli et al., 2015). In another study, female rats responded behaviorally to a lower dose of ketamine (2.5 mg/kg) compared to the male rats. Of note, phosphorylation of mTOR or eEF2 pathways did not differ between the genders and consequently could not account for the sex difference (Carrier and Kabbaj, 2013). More recently, Thelen and co-workers examined the effect of sex on the behavioral, neurochemical, and synaptic molecular effects of repeated ketamine treatment (10 mg/kg; 21 days) in C57BL/6 J mice (Thelen et al., 2016) and found a significant sex difference in the synaptogenic response of hippocampus to ketamine in stress-naive mice suggesting that synaptic plasticity of hippocampus may be more important for females (Thelen et al., 2019),
The results of our study are in agreement with these observations, showing that the morphological changes of the hippocampal astrocytes one hour after S-ketamine treatment were sex-dependent, with significant increase in the size of the astrocytes only in female FSL rats. Moreover, according to a published paper from our group, the alterations in the depressive-like behavior and tryptophan (TRP) metabolite levels in the brain and plasma of the female FSL rats are independent of the estrous cycle (Eskelund et al., 2016). We previously reported that phase of the cycle does not modify NPY expression in maternally separated females (Jimenez-Vasquez et al., 2001), and more recently, it was shown that the concentrations of ketamine and norketamine were significantly higher over the first 30 min following treatment in both brain and plasma from females, due to the slower clearance rates and longer half-lives. Importantly, no significant differences in pharmacokinetic parameters were found between proestrus and diestrus female rats (Saland and Kabbaj, 2018).
One of the important brain structural changes in patients with MDD is the reduction of the hippocampal volume. Since, each subregion has a characteristic cytoarchitecture, it has been suggested that the alteration in the structural plasticity of the hippocampal areas could be different in response to depression and antidepressant treatment. For instance, the volumetric reduction of the hippocampus and prefrontal cortex of patients with MDD could be the consequence of the shrinkage of dendritic arbor together with the decrease of the glial cell number (Musazzi et al., 2012) The results of this study showed no significant rapid effect of ketamine on the volume of GCL in either male or female FSL rats. Therefore, it seems reasonable to conclude that changes in the neuronal density as the main mechanism leading to alteration of the volume of GCL needs extended period of time to occur.
Furthermore, findings from post-mortem studies indicate that changes in the number of glia cells are not identical among brain regions. For instance, it has been reported that the number of glial cells decreased in the dorsolateral prefrontal cortex and orbitofrontal cortex of patients with MDD (Cotter et al., 2002; Rajkowska et al., 1999), while an increase in the number of glial cells in the hippocampus of MDD patients has been observed (Stockmeier et al., 2004 Therefore, it is reasonable to suggest that changes in the morphology of glial cells (atrophy of branches) rather than changes in the glial cell density contribute to the hippocampal volume loss in depressed patients (Rajkowska and Miguel-Hidalgo, 2007)
The number of dendritic spines on the pyramidal cells of the CAI region of hippocampus is not immutable in female rats and monkeys and is influenced by the levels of circulating ovarian steroids (Cooke and Woolley, 2005; Hao et al., 2003; Leuner and Gould, 2010), In our previous studies, we have observed significant increases in the number of spine synapses in the CAI.SR area of hippocampus 1 h after a single injection of S-and the size of the astrocytes 24 h after a single dose of S-ketamin (Ardalan et al., 2017, 2016; Treccani et al., 2019), respectively. Remarkably, estrogen decreased the extracellular levels of glutamate by increasing the expression of astrocytic glutamate (Pawlak et al., 2005). Therefore, one possible explanation for higher sensitivity of the female depressed rats to the antidepressant effect of ketamine could be increased expression of astrocytic glutamate transporters in the hypertrophic activated astrocytes, or metabolic differences (Herzog et al., 2019; Leonard and Wegener, 2019) An other study demonstrated that the function of female astrocytes especially for clearing larger amounts of extracellular glutamate is independent of gonadal female hormones. Indeed, the suggested mechanism for regulating the function of female astrocytes is a cell-autonomous mechanism (Morizawa et al., 2012). Moreover, our recent results show that the alterations in the depressive-like behavior and tryptophan (TRP) metabolite levels in the brain and plasma of the female FSL rats are independent of the estrous cycle (Eskelund et al., 2016). Another finding of this study is that ketamine elicited a significant rapid change in the serum level of BDNF in the FSL female rats and this alteration followed the morphological alteration of hippocampal astrocytes. Previous studies indicated that the rapid antidepressant effect of ketamine is dependent on BDNF release (Liu et al., 2012). Moreover, a study on the cultured neurons demonstrated the rapid effect of incubation with ketamine on the release of BDNF in primary neuronal culture (15 min, 60 min, and 6 h) (Lepack et al., 2015). Since, astrocytes are one of the sources of BDNF (Althaus and Richter-Landsberg, 2000), the possible explanation for fast therapeutic effect of ketamine in females could be rapid activation of the astrocytes and subsequently the stimulation of BDNF synthesis by activated hypertrophic astrocytes. The critical issue in the given study is that the current changes in the serum level of BDNF are related to one hour after ketamine treatment and that the time trajectory of increased BDNF synthesis and release has not been elucidated. In the FSL male rats, ketamine did not affect the serum BDNF levels.
In contrast, ketamine increased the BDNF serum level in the female FSL indicating that the regulatory mechanism of rapid serum BDNF changes by ketamine is different between the female and male FSL rats; one possibility being a difference in ketamine metabolism (Zanos et al., 2016) Future studies are warranted to elucidate this issue in detail and understand the role of BDNF in the sex dependent antidepressant effect of ketamine and discover the possible association between BDNF release and the antidepressant effect of ketamine.
Regarding the method used for the astrocytic volumetric measurement, rotational symmetry around the vertical axis perpendicular to the hippocampal subregion was assumed. As discussed (Gundersen, 1988) calculation of unbiased measurements of the actual volume of astrocytes with the VUR nucleator requires sampling on the vertical uniform random (VUR) sections. However, due to technical limitations in the present work related to the complex structure of the hippocampal subregions, we could not fully guarantee fulfillment of this requirement. In our work, measurement on the coronal sections of hippocampus based on the rotational symmetry around the vertical axis was performed, which may introduce a potential bias in the cell volume measurement. However, since the same plane of cutting and the sampling method were used for all the four animal groups, it is expected that the bias would likely occur in all groups. Moreover, since we also estimated the size of the astrocytes by quantifying the longest diameter of astrocytes, yielding the same results, we believe that the technical obstacle for fulfilling the complete theoretical assumptions in the present work does not to constitute a problem for the main conclusions.
5. Conclusion
Our results indicate that S-ketamine induces alteration of the structural plasticity of hippocampus as well as morphological alteration of hippocampal astrocytes and affects the serum level of BDNF within one hour after a single administration in a sex-dependent manner. The translational validity of these findings in humans is currently unknown.
Supplementary Material
Acknowledgments
The paper is part of a PhD project by Maryam Ardalan, supervised by Gregers Wegener (animal models, design and pharmacological intervention) and Jens Randel Nyengaard (stereological design). We are grateful to Heidi Kaastrup Müller for her scientific consulting in this project.
Role of the funding source
This work was supported by the Lundbeck Foundation and the Aarhus University Research Foundation (AU-ldeas initiative (eMOOD)) and EU Horizon 2020 Maryam Ardalan (MA) was supported by the Lundbeck Foundation and Aleksander A. Mathe (AAM) by the Swedish Medical Research Council grant 10414, 2015–02966, 2016–02955. GW declares having received lecture fees from H. Lundbeck A/S, Servier SA, Astra Zeneca AB, Eli Lilly A/S, Sun Pharma Pty, and Pfizer Inc.
Funding sources had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2020.01.001.
References
- Acker T, Beck H, Plate KH, 2001. Cell type specific expression of vascular endothelial growth factor and angiopoietin-l and −2 suggests an important role of astrocytes in cerebellar vascularization. Mech. Dev 108, 45–57. [DOI] [PubMed] [Google Scholar]
- Althaus HH, Richter-Landsberg C, 2000. Glial cells as targets and producers of neurotrophins. Int. Rev. Cytol 197, 203–277. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P, 2007. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res 163, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardalan M, Rafati AH, Nyengaard JR, Wegener G, 2017. Rapid antidepressant effect of ketamine correlates with astroglial plasticity in the hippocampus. Br. J. Pharmacol 174, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardalan M, Wegener G, Rafati AH, Nyengaard JR, 2016. S-Ketamine rapidly reverses synaptic and vascular deficits of hippocampus in genetic animal model of depression. Int. J. Neuropsychopharmacol 20 (3), 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PE, Kavalali ET, Monteggia LM, 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS, 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. J. Neurobiol 64, 34–46. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP, 2002. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 12, 386–394. [DOI] [PubMed] [Google Scholar]
- Drewniany E, Han J, Hancock C, Jones RL, Lim J, Nemat Gorgani N, Sperry JK 3rd, Yu HJ, Raffa RB, 2015. Rapid-onset antidepressant action of ketamine: potential revoLution in understanding and future pharmacologic treatment of depression. J. Clin. Pharm. Ther 40, 125–130. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Muller HK, Elfving B, Sanchez C, Wegener G, 2016. Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology 233, 2813–2825. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Brosenitsch TA, Katz DM, 2001. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J. Neurosci.: Off. J. Soc. Neurosci 21, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelund A, Budac DP, Sanchez C, Elfving B, Wegener G, 2016. Female flinders sensitive line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 329, 337–348. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290, 49–60. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, 1988. The nucleator. J. Microsc 151, 3–21. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. , 1988. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol. Microbiol. Immunol. Scand 96, 857–881. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH, 2003. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol 465, 540–550. [DOI] [PubMed] [Google Scholar]
- Henter ID, de Sousa RT, Gold PW, Brunoni AR, Zarate CA Jr., Machado-Vieira R, 2017. Mood therapeutics: novel pharmacological approaches for treating depression. Expert Rev. Clin. Pharmacol 10, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog DP, Wegener G, Lieb K, Muller MB, Treccani G, 2019. Decoding the mechanism of action of rapid-acting antidepressant treatment strategies: does gender matter? Int. J. MOL. Sci 20 (4), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Lane HY, Lin CH, 2017. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017, 4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MJ, Raynaud B, Garcia R, 2006. Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol. Psychiatry 60, 1206–1214. [DOI] [PubMed] [Google Scholar]
- Jaso BA, Niciu MJ, ladarola ND, Lally N, Richards EM, Park M, Ballard ED, Nugent AC, Machado-Vieira R, Zarate CA, 2017. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr. Neuropharmacol 15, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Vasquez PA, Mathe AA, Thomas JD, Riley EP, Ehlers CL, 2001. Early maternal separation alters neuropeptide Y concentrations in selected brain regions in adult rats. Brain Res. Dev. Brain Res 131, 149–152. [DOI] [PubMed] [Google Scholar]
- Jimenez-Vasquez PA, Overstreet DH, Mathe AÅ, 2000. Neuropeptide Y in male and female brains of flinders sensitive line, a rat model of depression. Effects of electroconvulsive stimuli. J. Psychiatr. Res 34, 405–412. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Wegener G, 2019. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 32 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS, 2015. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychoph 18, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, 2010. Structural plasticity and hippocampal function. Annu. Rev. Psychol 61 (111–140), C111–C113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenberg N, Joca S, Wegener G, 2015. Nitric oxide involvement in the antidepressant-like effect of ketamine in the flinders sensitive Line rat model of depression. Acta Neuropsychiatr. 27, 90–96. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Lee F.s., Li XY, Bambico F, Duman RS, Aghajanian GK, 2012. Brain-derived neurotrophic factor va166met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry 71, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizawa Y, Sato K, Takaki J, Kawasaki A, Shibata K, Suzuki T, Ohta S, Koizumi S, 2012. Cell-autonomous enhancement of glutamate-uptake by female astrocytes. Cell. Mol. Neurobiol 32, 953–956. [DOI] [PubMed] [Google Scholar]
- Muller HK, Wegener G, Liebenberg N, Zarate CA Jr., Popoli M, Elfving B, 2013. Ketamine regulates the presynaptic release machinery in the hippocampus. J. Psychiatr. Res 47, 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, losifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013a. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, losifescu DV, 2013b. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Popoli M, 2012. Glutamate hypothesis of depression and its consequences for antidepressant treatments. Expert Rev. Neurother 12, 1169–1172. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C, 2005. Regulation of glutamate transporter glast and GLT-I expression in astrocytes by estrogen. Brain Res. Mol. Brain Res 138, 1–7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic Coordinates. Academic Press Hard Cover Edition. [Google Scholar]
- Pereira VS, Joca SRL, Harvey BH, Elfving B, Wegener G, 2019. Esketamine and rapastinel, but not imipramine, have antidepressant-like effect in a treatment-resistant animal model of depression. Acta Neuropsychiatr. 31, 258–265. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, 2007. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 6, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA, 1999. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 45, 1085–1098. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA, 2013. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, Kabbaj M, 2018. Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats. J. Pharmacol. Exp. Ther 367, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, 2017. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74, 399–405. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G, 2004. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 56, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis PM, 2019. Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience 398, 182–192. [DOI] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM, 2016. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res 312, 305–312. [DOI] [PubMed] [Google Scholar]
- Treccani G, Ardalan M, Chen E, Musazzi L, Popoli M, Wegener G, Nyengaard JR, Muller HK, 2019. S-Ketamine reverses hippocampal dendritic spine deficits in flinders sensitive line rats within 1h of administration. Mol. Neurobiol 56 (11), 7368–7379. [DOI] [PubMed] [Google Scholar]
- Zanos P, Gould TD, 2018. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer G.l., Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.