Abstract
Objectives
Infant anthropometric growth varies across socioeconomic factors, including maternal education and income, and may serve as an indicator of environmental influences in early life with long-term health consequences. Previous research has identified sociodemographic gradients in growth with a focus on the first year and beyond, but estimates are sparse for growth before 6 months. Thus, our objective was to examine the relationship between sociodemographic factors and infant growth patterns between birth and 5 months of age.
Design
Prospective cohort study.
Settings
Low-income to middle-income neighbourhoods in Santiago, Chile (1991–1996).
Participants
1412 participants from a randomised iron-deficiency anaemia preventive trial in healthy infants.
Main outcome measures
Longitudinal anthropometrics including monthly weight (kg), length (cm) and weight-for-length (WFL) values. For each measure, we estimated three individual-level growth parameters (size, timing and velocity) from SuperImposition by Translation and Rotation models. Size and timing changes represent vertical and horizontal growth curve shifts, respectively, and velocity change represents growth rate shifts. We estimated the linear association between growth parameters and gestational age, maternal age, education and socioeconomic position (SEP).
Results
Lower SEP was associated with a slower linear (length) velocity growth parameter (−0.22, 95% CI –0.31 to –0.13)—outcome units are per cent change in velocity from the average growth curve. Lower SEP was associated with later WFL growth timing as demonstrated through the tempo growth parameter for females (0.25, 95% CI 0.05 to 0.42)—outcome units are shifts in days from the average growth curve. We found no evidence of associations between SEP and the weight size, timing or velocity growth rate parameters.
Conclusion
Previous research on growth in older infants and children shows associations between lower SEP with slower length velocity. We found evidence supporting this association in the first 5 months of life, which may inform age-specific prevention efforts aimed at infant length growth.
Keywords: epidemiology, public health, community child health
Strengths and limitations of this study.
The sample includes monthly anthropometric measures in the first 5 postnatal months—not available in any study to date and allowing better fitting growth models.
We used the Graffar Index, a detailed measure of socioeconomic position (SEP) specific to low-income to middle-income groups, an understudied population, which may reduce misclassification of SEP.
As the sample was low-to-middle income, these results may not generalise to groups with even lower or higher income or SEP.
Introduction
Interest in early life infant growth has grown as evidence accumulates that it is associated with the development of adult disease, sometimes decades later. Some chronic disease outcomes associated with infant growth characteristics include obesity, endothelial dysfunction and metabolic syndrome.1–3 Explanations for these associations include early infancy as a critical window of time for susceptibility to environmental exposures for chronic disease risk factors.4 Socioeconomic position (SEP) is one such exposure. SEP is associated with child growth patterns, in particular, length5–12 and weight.13–16 In these studies, lower SEP is generally associated with faster weight gain during childhood, while the inverse holds true for length. These socioeconomic gradients in growth appear to emerge in early life7 and persist.5
Gaps remain in our understanding regarding sociodemographic predictors of growth during infancy and childhood. One such gap relates to the earliest period of infant growth. Most studies to date include three or fewer observations before 6 months,5–8 10 11 13 14 16 preventing non-linear specifications between weight or height spanning this time. However, curvilinear models of growth with more than three observations offer better model fit for early infancy growth. Growth during the first 6 months in the human lifespan is characterised by accelerated growth at the outset and levelling off at around 6 months.17 Given these unique features, early infant growth may yield unique associations with predictors not influential during later periods of growth. Understanding the relationship between early infant growth and sociodemographic factors may yield new information that highlight the potential for earlier interventions to promote optimal health.
Identifying novel associations in this age range can better pinpoint the timing and influence of sociodemographic factors. Given the sparsity of information in the literature focusing on these points, our aim in this study is to examine sociodemographic predictors of infant weight, length and weight-for-length (WFL) growth from 0 to 5 months in an infancy cohort of over 1400 healthy Chilean children. Based on prior research in middle-income to high-income countries applied to a wider range of ages in childhood that is described above, we expected that SEP will be inversely associated with weight gain and positively associated with length growth.
Methods
Study sample
The data in this study are drawn from the Santiago Longitudinal Study, a cohort study from low-income to middle-income neighbourhoods in Santiago, Chile. Between 1991 and 1996, infants were recruited for an infancy iron-deficiency anaemia preventive trial18 or neuromaturation study.19 Inclusion criteria for the infancy studies included full-term infants (greater than or equal to 37 weeks gestational age (GA)) with birth weight 3.0 kg, vaginal birth, no major health problems for the infant, and, for the preventive trial, no iron-deficiency anaemia present at 5–6 months. Those with iron-deficiency anaemia and the next non-anemic control were invited to participate in the neuromaturation study and are not considered here. Participant eligibility and follow-up information have previously been reported.18
We characterised the growth period prior to treatment randomisation, which occurred at 6 months. Anthropometric measures prior to study enrolment were obtained from the medical chart. The total sample size included 1657 infants who completed the preventive trial.
Outcome and sociodemographic measures
Anthropometric measurements included weight (kg), length (cm) and WFL (g/cm). Weight was measured to the nearest 0.01 kg on an electronic scale at local public health clinics. Length was measured on a recumbent board to the nearest 0.1 cm. GA, obtained from the medical chart, was among the set of variables included in the models as a covariate.
Sociodemographic measures were self-reported by the mother, including maternal age (years), total years of education and the modified Graffar Index,20 an index of SEP used in lower income countries.21 The modified Graffar Index represents a sum of 10 measures regarding education, family composition and housing characteristics, which are summed to create a scale with higher values indicating lower social class (online supplementary Appendix Table 1). Mothers self-reported breastfeeding characteristics from birth, including date of first bottle and age at weaning if weaned. From this information, we created variables for breast feeding as the sole source of milk and mixed breast and bottle feeding at 5 months.
bmjopen-2019-033695supp001.pdf (214.3KB, pdf)
Statistical analyses
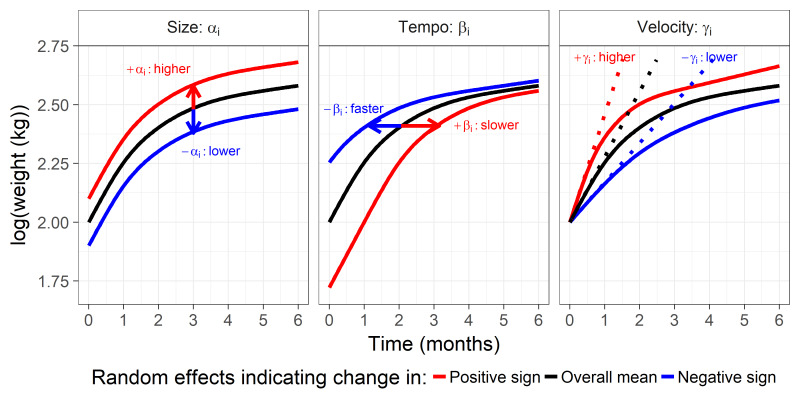
Summary statistics included median and IQRs for continuous variables and per cent with counts for categorical variables. All summary statistics were stratified by child sex. We used two steps to assess the association between infant growth and sociodemographic predictors: (1) SuperImposition by Translation and Rotation (SITAR) approach22 to estimate infant weight, length and WFL growth characteristics from birth to 5 months followed by (2) linear regression to estimate the relationship between sociodemographic predictors and these growth characteristics. We used a non-linear mixed effects model23 to estimate the growth characteristics with the R nlme package.24 Each model produces up to three different SITAR growth parameters per individual, which have been named ‘size’, ‘tempo’ and ‘velocity’22 (figure 1). Size indicates a shift of the growth curve up and down for an individual relative to the average growth curve. Tempo indicates a shift of the growth curve to the left or right on the age scale for an individual relative to the average growth curve. Lastly, velocity indicates a transformation of the age scale in the non-linear model, shrinking or enlarging the age scale for an individual relative to the average growth curve. These three parameters are noted as having biologically meaningful interpretations, which are difficult to obtain with other growth models.23 Unless otherwise noted, any references to size, tempo and velocity refer to these parameters from the SITAR construct applied to early infant growth.
Figure 1.
Type of change in random effects relative to the sample mean trajectory in weight growth curve trajectories following a shape-invariant model.
The results from the second step analyses are reported. In addition to including males and females and adjusting for sex of the child (in the pooled analyses), sex-stratified analyses were also used for all three anthropometric outcomes, as some estimated associations between SITAR growth parameters and SEP indicators differed by sex of the child.
The adjusted models in the second step started with four covariates: GA, maternal age, total years of maternal education and Graffar Index.20 We removed covariates from the model based on the least absolute shrinkage and selection operator (lasso) approach.25 This approach has better performance than conventional model selection methods with a univariate approach26 such as stepwise methods.27 The lasso approach assists in selecting predictors with the strongest coefficients28 while balancing bias and variation in the model. We used the glmnet package in R29 to estimate shrunken parameters and the selectiveInference package30 to provide inference via statistical tests and CIs. Each set of comparisons by outcome, that is, weight, length or WFL was considered separately. Multiple comparisons increase the possibility of statistically significant study findings by chance alone. Therefore, we controlled for multiple comparisons using a Bonferroni correction at an alpha level of 0.05. A coefficient for the predictor of a weight size growth parameter outcome in the second step indicates a change in log(kg) for a one-unit change in the predictor; we multiply this coefficient by 100 to make a symmetric percentage difference on a modified percentage scale.31 32 Similarly, a one-unit change in the predictor corresponds to a symmetric percentage change in the velocity growth parameter. Time (days) is not log transformed and the coefficient for this outcome corresponds to a shift in the time scale in days.
For analyses, we used a complete case data set, that is, all participants with non-missing covariates. The proportion of missing data was less than 1% for all variables except the Graffar Index, which had less than 3% missing. The median number of non-missing outcome (anthropometric) values was six out of six monthly measures (birth to 5 months). The per cent of missing outcome values at each time point ranged from 9% at months 1 and 2 to 0.2% at birth. In a post hoc data analysis, we used logistic regression models to estimate associations between SEP (the Graffar Index) as a continuous variable, and binary breastfeeding status outcomes—any or exclusive—at 5 months.
Patient and public involvement
Participants were mothers and infants recruited for research. The mothers were not involved in setting the study design, research questions or outcome measures for this study.
Results
Participants (n=1412) were 53% male and 47% female. Median GA (Q1, Q3) was 40 weeks (39, 40). Median maternal age (Q1, Q3) was 26 years (22, 31), and mothers had a median (IQR) of 10 (8–12) years of education at the time of their infant’s birth (table 1). For the six monthly anthropometric measurements prior to 6 months, each infant had at least two observations, and 72% had measures at all six time points.
Table 1.
Descriptive statistics of sociodemographic characteristics, median (IQR)
| Characteristic | Male | Female | Total |
| n | 747 | 665 | 1412 |
| Gestational age (weeks) | 40.0 (39.0–40.0) | 40.0 (39.0–40.0) | 40.0 (39.0–40.0) |
| Graffar Index | 27.0 (23.0–33.0) | 27.0 (23.0–33.0) | 27.0 (23.0–33.0) |
| Maternal age (years) | 26.0 (21.8–30.9) | 25.5 (21.7–30.3) | 25.8 (21.8–30.8) |
| Maternal education (years) | 10.0 (8.0–12.0) | 10.0 (8.0–12.0) | 10.0 (8.0–12.0) |
We assessed the best model fit for each anthropometric measure via the lowest Bayesian information criterion for growth independent of any covariates. After evaluating all possible combinations of SITAR models from one to three parameters for each of the three anthropometric measures, the best fit (online supplementary Appendix Table 2) models included: (1) all three growth parameters for weight, that is, size, tempo and velocity, (2) sex-specific growth trajectories with tempo and velocity parameters for length and (3) sex-specific growth trajectories with size and tempo parameters for WFL.
The following sections outline the adjusted results of the growth trajectory analyses for the three anthropometric outcomes: weight (kg), length (cm) and WFL (g/cm).
Weight trajectories: size, tempo and velocity
After including all covariates in the model, GA was the only characteristic associated with any weight growth parameters. In the pooled sample, GA was significantly associated with the weight tempo parameter (−2.01, 95% CI –2.98 to –1.70), indicating a leftward shift of about 2 days for each additional week in GA. This indicates earlier timing of weight gain in infants who were born with higher GA (table 2). There was no substantive difference in this association in the sex-stratified analyses.
Table 2.
Sociodemographic predictors and association with weight SuperImposition by Translation and Rotation growth parameter* †, stratified by sex of child in the Santiago Longitudinal Study, 1991–1996
| Males | Females | Total | ||||||||||||||||
| Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | |||||||||||||
| Characteristic | Size | Tempo | Velocity | Size | Tempo | Velocity | Size | Tempo | Velocity | Size | Tempo | Velocity | Size | Tempo | Velocity | Size | Tempo | Velocity |
| Gest age | 0.59 (−0.12 to 1.31) | −2.28 (−3.15 to −1.41) | −0.81 (−2.28 to 0.66) | NA | −1.96 (−3.15 to −1.40) | NA | 0.76 (−0.02 to 1.54) | −2.38 (−3.32 to −1.45) | −2.14 (−3.87 to −0.42) | 0.45 (−0.32 to 9.88) | −2.23 (−3.35 to −1.47) | −1.58 (−3.85 to 0.08) | 0.64 (0.10 to 1.18) | −2.35 (−2.98 to −1.71) | −1.53 (−2.70 to −0.37) | 0.53 (−0.05 to 1.15) | −2.01 (−2.98 to −1.70) | −1.06 (−2.67 to 0.01) |
| Maternal age | 0.11 (−0.00 to 0.23) | −0.06 (−0.20 to 0.09) | −0.07 (−0.31 to 0.17) | 0.07 (−0.11 to 0.21) | −0.06 (−0.21 to 0.26) | −0.06 (−0.33 to 0.82) | 0.21 (0.07 to 0.34) | −0.02 (−0.18 to 0.15) | −0.41 (−0.71 to −0.12) | 0.19 (−6.13 to 0.22) | 0.01 (−2.29 to 0.13) | −0.36 (−0.67 to −0.04) | 0.16 (0.07 to 0.25) | −0.03 (−0.14 to 0.07) | −0.20 (−0.39 to −0.00) | 0.15 (−0.78 to 0.22) | −0.01 (−0.16 to 0.83) | −0.18 (−0.56 to 0.22) |
| Maternal education | −0.03 (−0.32 to 0.26) | 0.14 (−0.21 to 0.49) | −0.04 (−0.62 to 0.55) | NA | NA | NA | −0.01 (−0.31 to 0.29) | 0.06 (−0.30 to 0.43) | −0.03 (−0.69 to 0.64) | 0.00 (−Inf to −0.41) | 0.12 (−0.95 to 0.52) | NA | −0.03 (−0.24 to 0.19) | 0.10 (−0.15 to 0.36) | −0.04 (−0.50 to 0.41) | 0.00 (−10.67 to 0.04) | 0.04 (−1.59 to 1.58) | −0.05 (−0.75 to 4.42) |
| Graffar Index§ | −0.12 (−0.23 to −0.01) | −0.13 (−0.27 to 0.01) | −0.15 (−0.39 to 0.08) | −0.08 (−0.22 to 0.07) | −0.13 (−0.28 to 0.03) | −0.13 (−0.41 to 0.28) | −0.07 (−0.19 to 0.06) | 0.12 (−0.03 to 0.28) | 0.28 (0.00 to 0.57) | −0.03 (−5.15 to 0.23) | 0.13 (−0.24 to 0.29) | 0.23 (−0.16 to 0.52) | −0.09 (−0.18 to −0.00) | −0.01 (−0.11 to 0.09) | 0.06 (−0.12 to 0.25) | −0.06 (−0.83 to 0.04) | −0.00 (−0.06 to 3.49) | 0.02 (−1.66 to 0.32) |
*Size units are percentage change in log(weight) from average, tempo units are time (days), velocity units in per cent change from average.
†Bold values indicate significance with Bonferroni correction at alpha level of 0.05.
‡Adjusted linear regression models only include non-zero coefficients from lasso regression models that include all covariates in full model. NA indicates the variable is not included in the adjusted analysis.
§Higher Graffar Index values indicate lower socioeconomic status.
Length trajectories: tempo and velocity
When evaluating the relationship between deviations from the average length growth characteristics and sociodemographic predictors, we found associations for SEP and GA. In the pooled group, the coefficient of association between the Graffar Index and the velocity parameter (−0.22, 95% CI –0.31 to –0.13; table 3) indicated that for each unit increase in the Graffar Index, lower values indicating higher SEP, there was a −0.22% decline from the average length velocity. Conversely, this association reflects a positive relationship between the length velocity parameter and SEP. This coefficient was not substantively different in the sex-stratified analyses, all of which indicated faster linear (length) growth with higher SEP. In contrast to the sex-stratified analyses, all covariates remained in the pooled adjusted model with less than 5% change from the unadjusted SEP coefficient (−0.23, 95% CI –0.31 to –0.15). Similar to SEP, GA was also positively associated with the length velocity parameter, demonstrating a 0.61% (95% CI 0.06% to 1.15%) increase from the average length velocity in the pooled sample for every unit increase in GA (weeks). GA was inversely associated with the length tempo parameter in the pooled sample (−2.94, 95% CI –3.51 to –2.41), indicating a leftward shift of about 3 days of the trajectory on the time scale, and a faster start to length growth, for each 1 week increase in GA (table 3).
Table 3.
Sociodemographic predictors and association with length SuperImposition by Translation and Rotation growth parameters* †, stratified by sex of child in the Santiago Longitudinal Study, 1991–1996
| Males | Females | Both | ||||||||||
| Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | |||||||
| Characteristic | Tempo | Velocity | Tempo | Velocity | Tempo | Velocity | Tempo | Velocity | Tempo | Velocity | Tempo | Velocity |
| Gest age | −3.33
(−4.09 to −2.56) |
0.99
(0.29 to 1.68) |
−3.05
(−4.10 to −2.55) |
NA | −2.57
(−3.36 to −1.79) |
0.25 (−0.52 to 1.02) |
−2.53
(−3.33 to −1.77) |
NA | −2.97
(−3.52 to −2.42) |
0.64
(0.12 to 1.15) |
−2.94
(−3.51 to −2.41) |
0.61
(0.06 to 1.15) |
| Maternal age | −0.04 (−0.18 to 0.09) |
0.09 (−0.03 to 0.20) |
−0.01 (−0.10 to 1.64) |
NA | −0.17
(−0.30 to −0.03) |
0.01 (−0.13 to 0.14) |
−0.15 (−0.29 to 0.01) |
NA | −0.10 (−0.19 to −0.00) |
0.05 (−0.04 to 0.14) |
−0.07 (−0.17 to 0.06) |
0.02 (−0.35 to 0.10) |
| Maternal education | 0.06 (−0.26 to 0.38) |
0.12 (−0.16 to 0.40) |
NA | NA | −0.18 (−0.49 to 0.13) |
0.28 (−0.01 to 0.58) |
−0.14 (−0.45 to 0.52) |
0.16 (−0.35 to 0.52) |
−0.05 (−0.28 to 0.17) |
0.20 (−0.00 to 0.40) |
−0.06 (−0.27 to 0.73) |
0.13 (−0.21 to 0.34) |
| Graffar Index§ | 0.06 (−0.06 to 0.19) |
−0.26
(−0.37 to −0.15) |
0.05 (−0.25 to 0.36) |
−0.21
(−0.37 to −0.14) |
0.16
(0.03 to 0.29) |
−0.19
(−0.32 to −0.07) |
0.13 (−0.03 to 0.26) |
−0.17
(−0.31 to −0.05) |
0.11
(0.02 to 0.20) |
−0.23
(−0.31 to −0.15) |
0.09 (−0.02 to 0.18) |
−0.22
(−0.31 to −0.13) |
*Size units are percentage change in log(length) from average, tempo units are time (days), velocity units in per cent change from average.
†Bold values indicate significance with Bonferroni correction at alpha level of 0.05.
‡Adjusted linear regression models only include non-zero coefficients from lasso regression models that include all covariates in full model. NA indicates the variable is not included in the adjusted analysis.
§Higher Graffar Index values indicate lower socioeconomic status.
WFL trajectories: size and tempo
Evaluations of shifts in WFL size and tempo from the average indicated associations with SEP and GA. Increases in the Graffar Index, equivalent to lower SEP, were associated with a positive shift in the WFL tempo parameter for females (0.25, 95% CI 0.05 to 0.42). This estimate approximates a rightward shift in time (days) relative to the average growth curve indicating later growth timing with lower SEP.
Similar to weight and length trajectory analyses, an increase in GA was inversely associated with a decline in tempo from the average in the pooled sample (−1.99, 95% CI –2.83 to –1.49) (table 4) indicating about a 2-day shift to the left on the time scale from the average growth curve for every 1 week increase in GA. Similar values were found in the sex-stratified analyses, all indicating earlier timing of WFL growth with higher GA.
Table 4.
Sociodemographic predictors and association with weight-for-length (WFL) SuperImposition by Translation and Rotation growth parameters* † ‡ stratified by sex of child in the Santiago Longitudinal Study, 1991–1996
| Males | Females | Both | ||||||||||
| Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | |||||||
| Characteristic | Size | Tempo | Size | Tempo | Size | Tempo | Size | Tempo | Size | Tempo | Size | Tempo |
| Gest age | 0.09 (−0.55 to 0.73) | −2.03 (−2.91 to −1.15) | NA | −1.58 (−2.90 to −1.11) | 0.05 (−0.58 to 0.69) | −2.34 (−3.35 to −1.32) | NA | −2.32 (−3.35 to −1.33) | 0.07 (−0.38 to 0.52) | −2.17 (−2.84 to −1.51) | NA | −1.99 (−2.83 to −1.49) |
| Maternal age | 0.07 (−0.03 to 0.18) | −0.09 (−0.23 to 0.06) | 0.04 (−0.23 to 0.16) | −0.08 (−0.24 to 0.17) | 0.02 (−0.09 to 0.13) | −0.18 (−0.36 to −0.00) | NA | −0.13 (−0.36 to 0.14) | 0.05 (−0.03 to 0.12) | −0.13 (−0.24 to −0.02) | 0.03 (−0.16 to 0.12) | −0.11 (−0.22 to 0.03) |
| Maternal education | −0.09 (−0.35 to 0.16) | 0.08 (−0.27 to 0.44) | NA | NA | −0.10 (−0.35 to 0.14) | 0.00 (−0.40 to 0.40) | NA | 0.07 (−2.11 to 0.42) | −0.10 (−0.28 to 0.08) | 0.04 (−0.22 to 0.31) | NA | NA |
| Graffar Index§ | −0.08 (−0.18 to 0.02) | −0.07 (−0.21 to 0.07) | −0.05 (−0.17 to 0.15) | −0.08 (−0.24 to 0.17) | 0.08 (−0.02 to 0.19) | 0.26 (0.10 to 0.43) | 0.04 (−0.21 to 0.18) | 0.25 (0.05 to 0.42) | −0.01 (−0.08 to 0.07) | 0.08 (−0.03 to 0.19) | NA | 0.06 (−0.14 to 0.17) |
*Size units are percentage change in log(WFL) from average, tempo units are time (days) and velocity units in per cent change from average.
†Bold values indicate significance with Bonferroni correction at alpha level of 0.05.
‡Adjusted linear regression models only include non-zero coefficients from lasso regression models that include all covariates in full model. NA indicates the variable is not included in the adjusted analysis.
§Higher Graffar Index values indicate lower socioeconomic status.
The post hoc analysis examining the association between odds of exclusive or any breast feeding at 5 months and the continuous SEP measure (the Graffar Index) did not find a substantive or significant association (data not shown).
Discussion
In this research, we found that lower SEP, measured by the Graffar Index, was inversely associated with length growth characteristics—but not weight—in the first 5 months. Lower SEP was associated with later timing of WFL growth as reflected by the positive association between the Graffar Index and the WFL tempo parameter. These higher tempo values translate to a rightward shift in growth relative to the average growth curve as well as a later age at peak velocity.33 This delay in growth can be considered an unfavourable outcome associated with lower SEP.
Maternal age was not associated with any of the three adjusted growth parameters for length, weight or WFL. GA was inversely associated with the tempo growth parameters for length, weight and WFL indicating that higher GA is associated with earlier timing of these three measures. GA is also positively associated with length velocity in the pooled sample indicating faster length change with increasing GA.
Of three previous studies investigating associations between sociodemographic predictors and infant growth before 6 months, two studies found a significant and fully adjusted positive association between length (linear) growth and maternal education,8 10 used as a proxy for SEP. Only one study found an inverse association with length growth,12 which was close to null on adjustment. Many studies including age ranges exceeding 6 months of age up to 5 years of age demonstrated a positive association between maternal education and length/height growth.7 8 10 The majority of these studies support the conclusion that lower SEP is associated with slower length (linear) growth in infancy and early childhood.
Several prior studies representing high-income European countries have noted that their findings of no evidence of a relationship7 12 between SEP and length (linear) growth prior to 6 months may not generalise to low-income to middle-income countries. Deviations from the Western diet and lifestyle were one of the reasons given for this limitation. Chile, the country from which our data were collected, offers an interesting context in this respect. The recruitment period for this study, 1991–1996, occurred as Chile was transitioning from a low-income to an upper middle-income country. In 1990, 40% of the Chilean population was below the poverty line34; by 2012, WHO classified Chile as an upper middle-income country.35 There were nutrition and epidemiological transitions36 37 beginning in the 1970s and continuing during the 1990s when study infants were enrolled. Specifically, consumption of high-calorie food, accompanied by a sedentary lifestyle, resulted in rising obesity prevalence across all socioeconomic levels. In the context of an emerging western diet and lifestyle, we found that lower SEP was associated with poorer length (linear) growth in early infancy. Of course, contemporary generations in Chile experience lower SEP in a new context of overnutrition and higher levels of sedentary behaviour. Thus, current studies in Chile may find distinct relationships between SEP and early growth when compared with generations born 20 years ago.
Plausible biological mechanisms, linked to modifiable factors, have been proposed for the observed association between lower SEP and length growth in the first 5 postnatal months. Breast feeding and maternal smoking are two commonly proposed mechanisms, although evidence is limited. In our sample, breast feeding was close to universal38 39 and not associated with infant weight change in the first year. We did not evaluate maternal smoking in this study given the large proportion of missing information. However, prior studies did not find that either prenatal or postnatal maternal smoking substantially altered the association between SEP and growth.11 12 16
Maternal age was the only sociodemographic predictor positively associated with the unadjusted SITAR size growth parameter for weight. This was similarly reported in another cohort from the same geographic area of Santiago, Chile, the Growth and Obesity Cohort Study,13 which started a decade later and studied ages between birth and 2 years. Our findings add to this work. Through our intense focus on the first 5 postnatal months, our results demonstrate that the association between SEP and weight growth appears earlier in the postnatal period than previously documented.
Other potential mechanisms relating to SEP could include gestational weight gain and maternal nutrient status. Size at birth, considered a proxy for these two factors and represented in these analyses by the size SITAR parameter, was not associated with any of the sociodemographic measures. Further research will be useful in clarifying the biological mechanisms behind the association between SEP and early infant growth.
Strengths of this study include the combination of an analytical approach to growth that better captures the non-linear characteristic of growth in the first 5 months of life with a detailed measure of SEP appropriate to the context of a lower income setting. Another strength is the monthly anthropometric measures collected in the first 5 postnatal months. We also note several limitations. The sample size (n=1412) is smaller than other studies with sample sizes in the thousands or tens of thousands.5 13 14 Our study, therefore, may not have been powered to detect some effects reported in larger studies. Another limitation is that the Graffar Index, developed to assess differences in low-income to middle-income populations, limits the generalisability of our findings to higher income groups.
This investigation examined various growth characteristics from birth to 5 months and their association with sociodemographic factors in a Chilean infancy cohort. We found associations between lower SEP and slower length (linear) growth, which are similar in direction to previous findings for maternal education that span periods of time greater than the first 6 months and up to 5 years of age.7 8 10 12 The association between maternal age and weight size, in our study, was similar to findings in other studies of growth between birth and 2 years of age.13 In sum, our results extend findings from previous research by showing that sociodemographic factors affect infant growth even in the first 5 months of growth and in relatively homogenous low-income to middle-income populations.
Supplementary Material
Acknowledgments
The authors thank the Santiago Longitudinal Study participants.
Footnotes
Contributors: AVH designed the study, conducted the data analysis and wrote the first draft of the paper. KEN and SG supervised and contributed to the study design, interpretation of results and draft revisions. EB helped acquire the data. All authors contributed to revisions of the draft for intellectual content and approved the final version of the manuscript.
Funding: AVH was supported by an American Heart Association Mid-Atlantic Affiliate predoctoral fellowship (award number 16PRE29200008). KEN was supported by AHA grant 15GRNT25880008. The data for this work were supported by grants from the National Institutes of Health R01-HL-088530 and R01-HD-033487
Disclaimer: AHA had no role in the study design, data collection, and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The Santiago Longitudinal Study had been approved by Institutional Review Boards from (1) University of Michigan Medical Center, Ann Arbor, (2) Institute of Nutrition and Food Technology, Chile and (3) University of California, San Diego. The Office of Human Research Ethics at the University of North Carolina, Chapel Hill exempted this current research using existing anonymous data from review under the 45 CFR 46.101(b) regulatory category.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1. Gillman MW. The first months of life: a critical period for development of obesity. Am J Clin Nutr 2008;87:1587–9. 10.1093/ajcn/87.6.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leunissen RWJ, Kerkhof GF, Stijnen T, et al. . Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009;301:2234. 10.1001/jama.2009.761 [DOI] [PubMed] [Google Scholar]
- 3. Touwslager RNH, Houben AJHM, Tan FES, et al. . Growth and endothelial function in the first 2 years of life. J Pediatr 2015;166:666–71. 10.1016/j.jpeds.2014.11.059 [DOI] [PubMed] [Google Scholar]
- 4. Plagemann A, Harder T, Schellong K, et al. . Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract Res Clin Endocrinol Metab 2012;26:641–53. 10.1016/j.beem.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 5. McCrory C, O'Leary N, Fraga S, et al. . Socioeconomic differences in children's growth trajectories from infancy to early adulthood: evidence from four European countries. J Epidemiol Community Health 2017;71:981–9. 10.1136/jech-2016-208556 [DOI] [PubMed] [Google Scholar]
- 6. Murasko JE. Associations between household income, height and BMI in contemporary US children: infancy through early childhood. Ann Hum Biol 2014;41:488–96. 10.3109/03014460.2014.885081 [DOI] [PubMed] [Google Scholar]
- 7. Howe LD, Tilling K, Galobardes B, et al. . Socioeconomic differences in childhood growth trajectories: at what age do height inequalities emerge? J Epidemiol Community Health 2012;66:143–8. 10.1136/jech.2010.113068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel R, Tilling K, Lawlor DA, et al. . Socioeconomic differences in childhood length/height trajectories in a middle-income country: a cohort study. BMC Public Health 2014;14:932. 10.1186/1471-2458-14-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Queiroz VAO, Assis AMO, Pinheiro SMC, et al. . Predictors of linear growth in the first year of life of a prospective cohort of full term children with normal birth weight. J Pediatr 2012;88:79–86. 10.2223/JPED.2143 [DOI] [PubMed] [Google Scholar]
- 10. Matijasevich A, Howe LD, Tilling K, et al. . Maternal education inequalities in height growth rates in early childhood: 2004 Pelotas birth cohort study. Paediatr Perinat Epidemiol 2012;26:236–49. 10.1111/j.1365-3016.2011.01251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herngreen WP, van Buuren S, van Wieringen JC, van BS, van WJ, et al. . Growth in length and weight from birth to 2 years of a representative sample of Netherlands children (born in 1988-89) related to socioeconomic status and other background characteristics. Ann Hum Biol 1994;21:449–63. 10.1080/03014469400003472 [DOI] [PubMed] [Google Scholar]
- 12. Silva LM, van Rossem L, Jansen PW, et al. . Children of low socioeconomic status show accelerated linear growth in early childhood; results from the generation R study. PLoS One 2012;7:e37356. 10.1371/journal.pone.0037356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pizzi C, Cole TJ, Richiardi L, et al. . Prenatal influences on size, velocity and tempo of infant growth: findings from three contemporary cohorts. PLoS One 2014;9:e90291. 10.1371/journal.pone.0090291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hui LL, Leung GM, Cowling BJ, et al. . Determinants of infant growth: Evidence from Hong Kong's "Children of 1997" birth cohort. Ann Epidemiol 2010;20:827–35. 10.1016/j.annepidem.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 15. Fuemmeler BF, Wang L, Iversen ES, et al. . Association between prepregnancy body mass index and gestational weight gain with size, tempo, and velocity of infant growth: analysis of the newborn epigenetic study cohort. Child Obes 2016;12:210–8. 10.1089/chi.2015.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wijlaars LPMM, Johnson L, van Jaarsveld CHM, van JCHM, et al. . Socioeconomic status and weight gain in early infancy. Int J Obes 2011;35:963–70. 10.1038/ijo.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lejarraga H. Growth in infancy and childhood : Human growth and development. Elsevier, 2012: 23–56. [Google Scholar]
- 18. Lozoff B, De Andraca I, Castillo M, et al. . Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54. [PubMed] [Google Scholar]
- 19. Lozoff B, Kaciroti N, Walter T. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr 2006;84:1412–21. 10.1093/ajcn/84.6.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graffar M. Une methode de classification sociales d’echantillons de population. Courrier 1956;6:445–59. [Google Scholar]
- 21. Alvarez ML, Muzzo S, Ivanović D. [Scale for measurement of socioeconomic level, in the health area]. Rev Med Chil 1985;113:243–9. [PubMed] [Google Scholar]
- 22. Cole TJ, Donaldson MDC, Ben-Shlomo Y. SITAR--a useful instrument for growth curve analysis. Int J Epidemiol 2010;39:1558–66. 10.1093/ije/dyq115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beath KJ. Infant growth modelling using a shape invariant model with random effects. Stat Med 2007;26:2547–64. 10.1002/sim.2718 [DOI] [PubMed] [Google Scholar]
- 24. Pinheiro J, Bates D, R Core Team . nlme: linear and nonlinear mixed effects models, 2017. [Google Scholar]
- 25. Tibshirani R. Regression shrinkage and selection via the LASSO: a retrospective. J R Stat Soc Series B Stat Methodol 2011;73:273–82. 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- 26. Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am J Epidemiol 2008;167:523–9. 10.1093/aje/kwm355 [DOI] [PubMed] [Google Scholar]
- 27. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer, 2015. [Google Scholar]
- 28. Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. 2 edn New York: Springer, 2017. [Google Scholar]
- 29. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22. 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tibshirani R, Tibshirani R, Taylor J, et al. . SelectiveInference: Tools for post-selection inference [R package version 1.2.4], 2017. [Google Scholar]
- 31. Cole TJ, Altman DG. Statistics notes: percentage differences, symmetry, and natural logarithms. BMJ 2017;358:j3683. 10.1136/bmj.j3683 [DOI] [PubMed] [Google Scholar]
- 32. Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med 2000;19:3109–25. [DOI] [PubMed] [Google Scholar]
- 33. Cole TJ, Kuh D, Johnson W, et al. . Using Super-Imposition by translation and rotation (SITAR) to relate pubertal growth to bone health in later life: the medical Research Council (MRC) national survey of health and development. Int J Epidemiol 2016;45:dyw134–134. 10.1093/ije/dyw134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiménez J, Romero MI. Reducing infant mortality in Chile: success in two phases. Health Aff 2007;26:458–65. 10.1377/hlthaff.26.2.458 [DOI] [PubMed] [Google Scholar]
- 35. Gitlin LN, Fuentes P. The Republic of Chile: an upper middle-income country at the crossroads of economic development and aging. Gerontologist 2012;52:297–305. 10.1093/geront/gns054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Albala C, Vio F, Kain J, et al. . Nutrition transition in Latin America: the case of Chile. Nutr Rev 2001;59:170–6. 10.1111/j.1753-4887.2001.tb07008.x [DOI] [PubMed] [Google Scholar]
- 37. Albala C, Vio F, Kain J, et al. . Nutrition transition in Chile: determinants and consequences. Public Health Nutr 2002;5:123–8. 10.1079/PHN2001283 [DOI] [PubMed] [Google Scholar]
- 38. Kang Sim DE, Cappiello M, Castillo M, et al. . Postnatal growth patterns in a Chilean cohort: the role of Ses and family environment. Int J Pediatr 2012;2012:1–8. 10.1155/2012/354060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khuc K, Blanco E, Burrows R, et al. . Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr 2012;2012:1–6. 10.1155/2012/478610 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033695supp001.pdf (214.3KB, pdf)