Abstract
Background
Maternal and neonatal thyroid function is critical for growth and neurodevelopment. Exposure to individual per- and polyfluoroalkyl substances (PFAS) can alter circulating thyroid hormone levels, but few studies have investigated effects of combined exposure to multiple PFAS.
Objectives
Estimate associations of exposure to multiple PFAS during early pregnancy with maternal and neonatal thyroid function.
Methods
The study population consisted of 726 mothers and 465 neonates from Project Viva, a Boston, Massachusetts area longitudinal pre-birth cohort. We measured six PFAS [perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoate (PFNA), perfluorohexane sulfonate (PFHxS), 2-(N-ethyl-perfluorooctane sulfonamido)acetate (EtFOSAA), and 2-(N-methyl-perfluorooctane sulfonamido)acetate (MeFOSAA)] and thyroxine (T4), Free T4 Index (FT4I), and thyroid stimulating hormone (TSH) in maternal plasma samples collected during early pregnancy, and neonatal T4 in post-partum heel sticks. We estimated individual and joint effects of PFAS exposure with thyroid hormone levels using weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR), and evaluated potential non-linearity and interactions among PFAS using BKMR.
Results
Higher concentrations of the PFAS mixture were associated with significantly lower maternal FT4I, with MeFOSAA, EtFOSAA, PFOA, and PFHxS contributing most to the overall mixture effect in BKMR and WQS regression. In infants, higher concentrations of the PFAS mixture were associated with lower T4 levels, primarily in males, with PFHxS and MeFOSAA contributing most in WQS, and PFHxS contributing most in BKMR. The PFAS mixture was not associated with maternal T4 or TSH levels. However, in maternal BKMR analyses, ln-PFOS was positively associated with T4 levels (Δ25th to 75th percentile:0.21 μg/dL; 95% credible interval: −0.03, 0.47) and ln-PFHxS was associated with a non-linear effect on TSH levels.
Conclusions
These findings support the hypothesis that there may be combined effects of prenatal exposure to multiple PFAS on maternal and neonatal thyroid function, but the direction and magnitude of these effects may vary across individual PFAS.
Keywords: PFAS, thyroid, pregnancy, endocrine disrupting chemicals, chemical mixtures
1. INTRODUCTION
Proper maternal and neonatal thyroid function is critical for growth and neurodevelopment. During early pregnancy, the fetus is completely dependent on maternal thyroid hormones until fetal thyroid hormone production increases around 18 to 20 weeks gestation (Fisher 1997). After birth, normal thyroid function is necessary to regulate continued growth and neurodevelopment during childhood, as well as metabolism and other major bodily systems throughout adulthood (Miller et al. 2009). Alterations in maternal thyroid function during pregnancy are associated with adverse fetal outcomes including preterm delivery, insufficient fetal growth, and neurodevelopmental deficits (de Escobar et al. 2004). Additionally, neonatal thyroid dysfunction has been associated with impaired cognition and neurodevelopment (Lyall et al. 2016; Rose et al. 2006; Simic et al. 2009a).
Recent epidemiological studies suggest that prenatal exposure to per- and polyfluoroalkyl substances (PFAS) may alter maternal and neonatal thyroid function (Ballesteros et al. 2016). PFAS are a group of fluorinated synthetic compounds commonly used in consumer and industrial products such as stain-resistant and non-stick coatings, firefighting foams, food packaging, upholstery, and carpeting (Lindstrom et al. 2011). Because of the strength of the carbon-fluorine bond, PFAS are environmentally persistent, and many also have relatively long elimination half-lives in humans (Olsen et al. 2007). Exposure to PFAS contaminated drinking water is an emerging issue of concern, particularly in areas near industrial contamination or use of aqueous film forming foam (Barton et al. 2019; Daly et al. 2018; Hu et al. 2019; Sunderland et al. 2019). In addition, substantial exposure of the general population to PFAS also occurs through diet and the indoor environment (Gebbink et al. 2015; Hu et al. 2016; Makey et al. 2017; Sunderland et al. 2019). Widespread exposure to PFAS has resulted in ubiquitous detection of PFAS in human serum in the U.S. general population (Calafat et al. 2007).
Toxicology studies have demonstrated that PFAS can disrupt thyroid function in animals, often leading to a hypothyroid-like effect, with reduced levels of circulating total thyroxine (T4) or free T4, with or without an increase in thyroid stimulating hormone (TSH) (Boas et al. 2012; Zoeller 2010). Previous epidemiologic studies assessing associations between PFAS exposure and thyroid hormone levels in pregnant women and neonates have been inconsistent, but have generally found associations between higher PFAS serum or plasma concentrations and lower free or total T4 levels and/or increased TSH levels (Ballesteros et al. 2016). In our previous work in the Project Viva cohort located in the Boston, Massachusetts area, we reported that prenatal plasma concentrations of some PFAS were inversely associated with maternal Free T4 Index (FT4I) levels and neonatal T4 levels, when assessing PFAS individually (Preston et al. 2018). However, the majority of previous epidemiologic studies, including our own, have assessed each PFAS-thyroid hormone association individually, without evaluating possible combined effects of exposure to multiple PFAS.
There is growing recognition of the need to investigate health effects of exposure to chemical mixtures, understanding that individuals are exposed to many different environmental chemicals (Braun et al. 2016; CDC 2017; Rosofsky et al. 2017). To address this need, multiple novel statistical methods have been developed to assess associations between mixtures of correlated exposures and health outcomes (Taylor et al. 2016), including weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR).
The goal of this study was to assess individual and combined effects of exposure to multiple PFAS with maternal and neonatal thyroid hormones in a prospective cohort of pregnant women from the Boston, MA area, using both WQS regression and BKMR, and to compare the results across these novel statistical methods.
2. MATERIALS & METHODS
2.1. Study participants
The study population is described in detail in Preston et al. (2018). Briefly, the study population consisted of a subset of pregnant women and their neonates enrolled in the Project Viva prospective pre-birth cohort study between 1999 and 2002. Project Viva enrolled women at their first prenatal visit (median 9.6 weeks gestation), collecting plasma samples and detailed participant information. Of the 2,128 singleton births in Project Viva, 768 had both plasma PFAS and maternal thyroid hormone measurements, and 505 had both plasma PFAS and neonatal T4 data. We excluded women from our analysis if they were using thyroid-altering medication and/or had a prior or current diagnosis of thyroid disease at enrollment (maternal, n=36; neonatal, n=25) or had missing covariate information (maternal, n=6; neonatal, n=15). The final study population consisted of 726 pregnant women and 465 neonates.
The Centers for Disease Control and Prevention (CDC) laboratory’s involvement did not constitute engagement in human subjects research. The Institutional Review Boards of all other participating institutions approved all study protocols and all participating women provided written informed consent.
2.2. PFAS quantification
PFAS quantification is described in detail in Preston et al. 2018. Briefly, maternal plasma samples, collected at a median 9.6 weeks gestation, were analyzed at the Division of Laboratory Sciences at the CDC (Atlanta, GA) for concentrations of eight PFAS [perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoate (PFNA), perfluorohexane sulfonate (PFHxS), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA; also known as Et-PFOSA-AcOH), 2-(N-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA; also known as Me-PFOSA-AcOH), perfluorodecanoate (PFDA; also known as PFDeA), perfluorooctane sulfonamide (FOSA)] (Kato et al. 2011). PFAS were detected in 99–100% of plasma samples with the following limits of detection (LOD): PFOS, 0.2 ng/mL; all other PFAS, 0.1 ng/mL. PFDA and FOSA were detected in <50% of samples and were not included in further analyses. Concentrations of all other PFAS below the LOD were replaced with the value of the LOD/√2 for analysis
2.3. Thyroid hormone measures
As described in Preston et al. (2018), we quantified levels of TSH, total thyroxine (T4), and triiodothyronine (T3) resin uptake (T3U) in the same maternal blood samples used for PFAS quantification, using the Bayer Advia Centaur assay (Centaur; Bayer Diagnostics, Tarrytown, NY) at the Boston University School of Medicine. We calculated free T4 index (FT4I) using total T4 and T3U levels, which is a standard clinical estimate of circulating free T4 levels, accounting for potential changes in thyroid binding protein levels such as increased levels of thyroid binding globulin (TBG) during pregnancy and is therefore less prone to bias than traditional immunoassay methods (Lee et al. 2009). TSH levels below the method LOD (0.01 mIU/L; n=7) were replaced with values of 0.01/√2 for this analysis and an additional 8 women with missing TSH data from TSH models only.
As previously described (Oken et al. 2009; Preston et al. 2018), Project Viva obtained neonatal T4 data from post-partum heel sticks as part of the New England Newborn Screening Program (NENSP).
2.4. Statistical Analysis
We calculated summary statistics and distributions of PFAS concentrations and thyroid hormone levels in mothers and neonates and used Spearman rank correlation coefficients to calculate the degree of correlation among PFAS. Building upon our previous research estimating individual PFAS-hormone associations using multivariable linear regression models in (Preston et al. 2018), we used WQS regression and BKMR (described below) to examine individual (BKMR) and joint (WQS and BKMR) associations of exposure to all six PFAS with maternal and neonatal thyroid hormones. All models were adjusted for potential confounding covariates. Maternal models included: maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, and fish intake; neonatal models included: maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, child sex, age at heel stick, gestational age at birth, and cesarean delivery. Descriptions of variable selection and inclusion are described in detail in Preston et al. (2018). We modeled thyroid hormone levels as continuous outcomes and ln-transformed FT4I and TSH levels in all models.
2.4.1. WQS regression
Using WQS regression, we estimated joint associations of exposure to all six PFAS with each thyroid hormone. A detailed description of WQS regression can be found in Carrico et al. (2015). Briefly, WQS regression simultaneously estimates empirical weights for each PFAS concentration based on their association with the outcome using a bootstrap step in the training subset of the data. The weighted mean of the bootstrap weights is then applied to plasma concentration quantiles and summed to create an index of the combined exposure, the weighted quantile sum. The exposure index is then used to assess the association between the WQS index and the outcome of interest using the hold-out validation subset of the data with the following model:
| [Equation 1] |
Where β0 is the intercept, z’ is a vector of covariates, φ is a vector of corresponding regression coefficients, and g represents the link function, the identity function in our models, linking the predictor variables to the continuous outcome mean, μ. represents the weighted index of all six (c) PFAS for each individual, where wi is the weight for each exposure and PFASqi, is the variable for the ith PFAS scored into quantiles. Weights for all PFAS are constrained from 0 ≤ wi ≤ 1, and must sum to 1 . β1 is the regression coefficient for the weighted index and can be interpreted as the combined effect of exposure to PFAS mixtures on the outcome, while the individual exposure weights (wi) can be interpreted as the relative contribution of that PFAS to the association with the outcome. We used a threshold of ≥ (100%/n) for weights, where n represents the number of exposure variables, as a guide to determine which PFAS were the greatest contributors to the overall mixture effect, as well as qualitatively discussing their contributions to the overall mixture effect in relation to each other. WQS regression assumes linear exposure-outcome associations across quantiles of each exposure and is a first-order approximation of non-additivity. Additionally, WQS regression focuses inference in a single direction at a time with constrained optimization of the beta parameter. Effects can be estimated for both directions individually by conducting separate analyses constraining the analyses in positive and negative directions.
We created WQS indexes based on concentrations of all six PFAS categorized into deciles. We split the data into training (40%) and validation (60%) datasets, estimating empirical weights in the training dataset, averaging weights for each PFAS over 500 bootstrapped samples, and testing the association between the resulting WQS index and our outcome in the validation dataset. We scaled WQS β1 estimates by the interquartile range (IQR) of the WQS index for greater ease of interpretation. Based on our previous work (Preston et al. 2018), we hypothesized that PFAS would be negatively associated with maternal T4, FT4I, and neonatal T4 levels, and positively associated with maternal TSH levels. However, we created WQS indexes in both positive and negative directions for all thyroid hormone outcomes in order to account for potential disparate directions of effect across PFAS.
In our previous work modeling PFAS individually, we found differential effects on neonatal T4 based on infant sex (Preston et al. 2018). Therefore, we assessed heterogeneity in effects by child sex by estimating sex-specific weights within the full neonatal dataset, rather than stratifying our sample to optimize the sample size when splitting the dataset for testing and validation. We did this by creating female and male exposure variables (e.g. “PFOA_male” and “PFOA_female” variables), which were used to simultaneously estimate weights for each PFAS variable (Brunst et al. 2017). The results included twelve sex-specific PFAS weights, six male and six female, all constrained from 0 ≤ wi ≤ 1, and to sum to 1 , and an overall beta estimate for the combined effect of exposure to the PFAS mixture in all neonates based on the weighted exposures.
2.4.2. BKMR analyses
BKMR is a statistical method that can be used to flexibly model the individual and joint effects of exposure to mixtures of chemicals by using a kernel function (Bobb et al. 2018; Bobb et al. 2015). Unlike WQS, BKMR allows the user to visualize individual exposure-response functions, while accounting for the other exposures and allowing potential non-linear relationships and/or differential directions of effect among exposures. This allows the user to identify independent effects of individual PFAS in addition to the overall combined mixture effect. In addition, BKMR allows the user to assess potential interactions among exposures.
We created separate BKMR models for all maternal thyroid hormone outcomes, and sex-stratified models for neonatal T4, based on the model below:
| [Equation 2] |
Where Yi is the continuous thyroid hormone outcome, h() is the exposure-response function, which can incorporate both non-linear relationships and interactions among exposures, zi is a vector of covariates. PFAS were ln-transformed and scaled for BKMR analyses. BKMR can also be used as a variable selection tool, identifying exposure variables that are important to the overall effect of the exposure mixture by estimating posterior inclusion probabilities (PIPs) for each exposure variable. Similar to the interpretation of the WQS weights, PIPs can be used to identify the relative importance of individual exposure variables to the overall mixture effect. However, unlike WQS weights, PIPs are not constrained to sum to 1.
Results from these models were then be used to 1) provide a visual representation of individual PFAS exposure-response functions, h(), and their uncertainty, while holding all other PFAS at a given value (e.g. median); 2) calculate specific point estimates for the difference in outcome levels for a change in individual PFAS concentration between the 25th and 75th percentile, with a corresponding 95% credible interval; 3) Estimate the overall joint effect of exposure to the chemical mixture by providing an estimate of the difference in outcome levels holding concentrations of all six PFAS at various percentiles, compared to holding all PFAS at their median concentrations. Interactions between PFAS can also be assessed by estimating the change in outcome level associated with a change in individual PFAS concentrations, at varying levels (e.g. 25th, 50th, 75th percentile) of one or more additional PFAS. Further details on post-estimation visualizations and statistics from BKMR analyses are discussed in more detail in Bobb et al. 2018.
We used SAS (version 9.4; SAS Institute, Inc) to calculate summary statistics and used R (version 3.5.2; R Development Core Team) for all other analyses including WQS (gWQS package) and BKMR (bkmr package).
3. RESULTS
3.1. Participant characteristics
Study population characteristics are described in detail in Preston et al. (2018). Briefly, maternal participants were primarily white (77%), nonsmoking (70%), college graduates (74%), with a mean age of 32.5 years. Neonates were evenly split between males (51%) and females (49%) and had a mean gestational age of 39.5 weeks. Neonatal heel sticks were performed at a mean of 2.0 days post-partum.
3.2. PFAS concentrations and thyroid hormone levels
Table 1 summarizes the distributions of prenatal plasma PFAS concentrations, as well as maternal and neonatal thyroid hormone levels. Prenatal plasma PFAS concentrations were comparable in maternal and neonatal subsets (data not shown).
Table 1.
Prenatal plasma PFAS (ng/mL) and maternal (n=726) and neonatal (n=465) thyroid hormone distributions
| Analyte | Detection frequencya | Min | Percentile |
Max | ||
|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||
| Prenatal plasma PFAS (ng/mL) | ||||||
| PFOS | 100 | 2.8 | 17.6 | 23.9 | 32.6 | 115.0 |
| PFOA | 100 | 0.3 | 3.9 | 5.6 | 7.7 | 36.7 |
| PFHxS | 98.5 | <LOD | 1.6 | 2.4 | 3.7 | 43.2 |
| PFNA | 98.6 | <LOD | 0.5 | 0.6 | 0.8 | 6.0 |
| EtFOSAA | 99.6 | <LOD | 0.7 | 1.1 | 1.7 | 33.6 |
| MeFOSAA | 100 | 0.1 | 1.2 | 1.8 | 2.9 | 29.7 |
| Thyroid hormones | ||||||
| Maternal (n=726) | ||||||
| Total T4 (μg/dL) | 3.9 | 8.7 | 9.9 | 11.2 | 24.4 | |
| Free T4 Index | 1.3 | 1.9 | 2.1 | 2.3 | 6.0 | |
| TSH (mIU/mL)b | <LOD | 0.7 | 1.2 | 1.9 | 19.3 | |
| Neonatal (n=465) | ||||||
| Total T4 (μg/dL)c | 3.73 | 14.8 | 17.3 | 20.2 | 35.7 | |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamide) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate
Limits of detection (LOD) were 0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS
n=718, 8 participants with missing TSH values
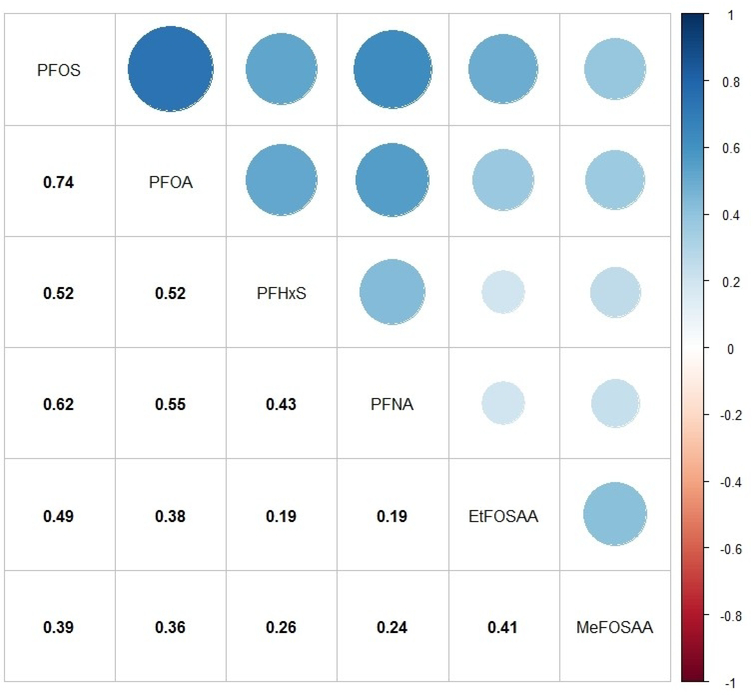
Figure 1 illustrates the Spearman rank correlation coefficients among prenatal plasma PFAS concentrations. PFAS were moderately to highly correlated with each other (rs range: 0.19–0.74; p<0.0001), with PFOS and PFOA having the highest correlation (rs = 0.74, p<0.0001).
Figure 1.
Spearman rank correlation matrix for maternal PFAS concentrations (ng/mL) measured in early pregnancy plasma samples (n=726). All p-values <0.0001. Abbreviations: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamide) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate.
3.3. WQS regression
3.3.1. Maternal thyroid hormones
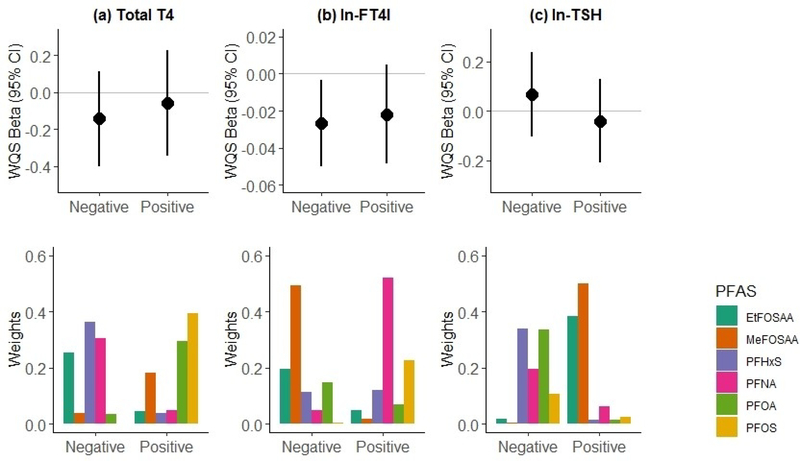
Figure 2 summarizes the results of the WQS regression analysis of covariate-adjusted associations between maternal plasma PFAS concentrations and thyroid hormone levels. The results include the beta estimate and corresponding 95% CI and p-value for an IQR increase in WQS index (WQSpfas) and the corresponding weighted mean of the estimated weights across the 500 bootstrap samples for each individual PFAS. An IQR increased in the WQSPFAS index was not associated with maternal T4 levels (Fig 2a) or TSH levels (Fig 2c), in either the positive or negative direction, indicating that exposure to the PFAS mixture was not associated with maternal T4 or TSH levels. We observed a significant inverse association between an IQR increase in the WQSPFAS index and lower maternal ln-FT4I (WQSPFAS β= −0.03; 95% CI: −0.05, −0.003) when constraining the analysis in the negative direction (Fig 2b). Based on the weighted mean empirical weights for each PFAS, MeFOSAA (49%), EtFOSAA (20%), and to a lesser extent PFOA (15%) and PFHxS (11%), contributed to the overall association, while PFOS (0%) and PFNA (4%) did not appear to contribute.
Figure 2.
Associations of combined PFAS exposure with maternal thyroid hormone levels based on weighted quantile sum (WQS) regression analysis (n=726), adjusting for maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, and fish intake. We calculated two separate WQS indices for associations with each hormone, one in the positive direction of effect and one in the negative direction of effect. Results of each analysis include the WQS beta and 95% confidence interval for the effect of combined exposure to the PFAS mixture on (a) total T4 (μg/dL), (b) ln-FT4I, and (c) ln-TSH levels (mIU/mL), with corresponding weights for the contributions of individual PFAS to the overall effect.
3.3.2. Neonatal T4
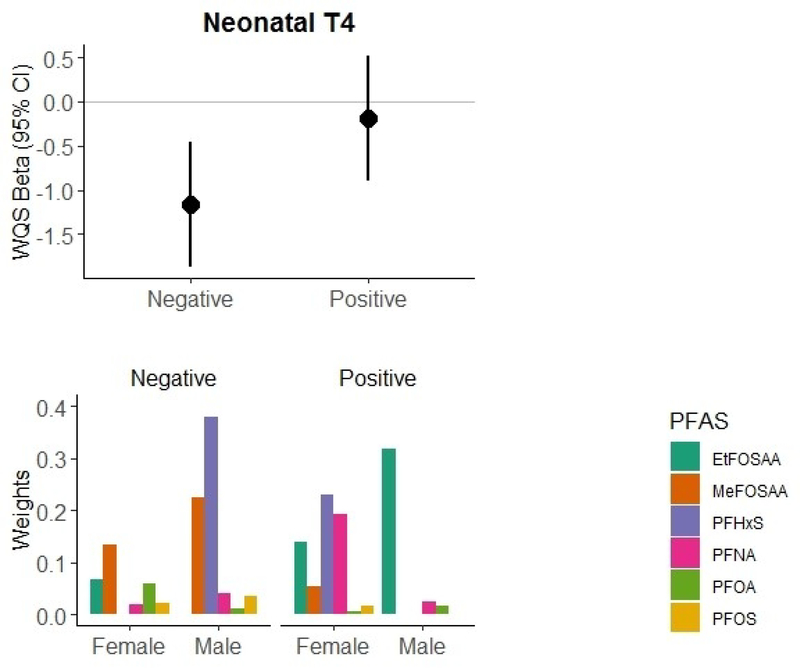
Figure 3 summarizes the results of the WQS regression analysis of the covariate-adjusted association between prenatal plasma PFAS concentrations and neonatal T4 levels, estimating sex-specific weights for each PFAS. An IQR increase in the WQSPFAS index was significantly inversely associated with lower neonatal T4 levels (WQSPFAS β= −1.17 μg/dL; 95% CI: −1.88, −0.45). Male infants received the majority of the weights (69%) associated with the decreased T4 levels, with PFHxS (38%) and MeFOSAA (22%) receiving the highest weights. However, MeFOSAA in female infants also received a contributing weight (13%).
Figure 3.
Associations of combined PFAS exposure with neonatal T4 levels based on weighted quantile sum (WQS) regression analysis (n=465), adjusting for maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, age at heel stick, gestational age at birth, and cesarean delivery. We calculated two separate WQS indices for associations with each hormone, one in the positive direction of effect and one in the negative direction of effect. Results of each analysis include the WQS beta and 95% confidence interval for the effect of combined exposure to the PFAS mixture on T4 levels (μg/dL), with corresponding sex-specific weights for the contributions of individual PFAS to the overall effect.
Results from the WQS regression analysis of infants without sex-specific weights were similar. An IQR increase in the WQSPFAS index was associated with a −0.80 μg/dL (95% CI: −1.53, −0.06) decrease in neonatal T4 levels, with MeFOSAA (46%), PFOS (15%), PFHxS (14%), and PFOA (14%) receiving the highest weights (Table S1).
3.4. BKMR Analyses
3.4.1. Maternal thyroid hormones
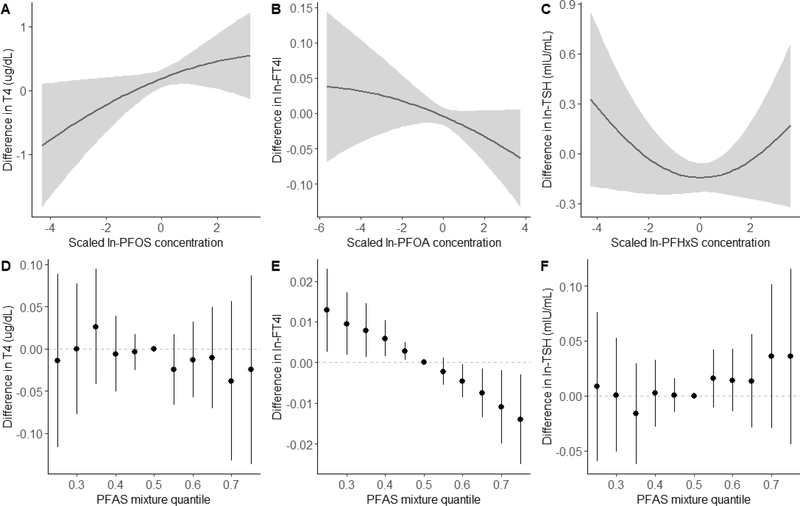
Figure 4 shows the results of covariate-adjusted BKMR analyses for maternal thyroid hormones. Figures 4A–C show the univariate exposure-response relationship and 95% credible intervals for select PFAS and maternal thyroid hormones based on the estimated kernel function, controlling for all other PFAS by holding them at their median concentrations. Other PFAS not included in the figure were not individually associated with thyroid hormone levels (Table S2). Figures 4D–F represent the overall effects of the PFAS mixture, showing the estimated differences in thyroid hormone levels and 95% credible intervals when all PFAS concentrations are held at a certain percentile compared to when all PFAS are held at their median concentrations. We did not observe an overall effect of exposure to the PFAS mixture on maternal total T4 levels (Fig 4D) or ln-TSH levels (Fig 4F). However, BKMR did identify an individual U-shaped association between PFHxS concentrations and ln-TSH levels (Fig 4C), as well as an individual positive association between PFOS concentrations and total T4 levels (Fig 4A), while holding all other PFAS at their median concentrations. A change in PFOS concentrations from the 25th to 75th percentile was associated with a 0.21 μg/dL increase in total T4 levels (95% credible interval: −0.03, 0.47).
Figure 4.
Combined effects of the PFAS mixture on maternal thyroid hormone levels (n=726) estimated by Bayesian Kernel Machine Regression (BKMR), adjusting for maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, and fish intake. Univariate exposure-response function and 95% confidence bands for (A) ln-PFOS and total T4 (μg/dL), (B) ln-PFOA and ln-FT4I, and (C) ln-PFHxS and ln-TSH levels (mIU/mL) holding all other PFAS at the median. Overall effect of the PFAS mixture on (D) total T4, (E) ln-FT4I, and (F) ln-TSH levels. These plots show the estimated difference in hormone levels and 95% credible intervals when all PFAS concentrations are held at a certain percentile compared to when PFAS concentrations are held at their medians, representing the exposure-response relationship of the PFAS mixture with hormone levels.
We observed a significant inverse association between exposure to the entire PFAS mixture and ln-FT4I (Fig 4E). Holding all PFAS at the 75th percentile compared to the 50th percentile was associated with a −0.01 decrease in ln-FT4I (95% credible interval: −0.02, −0.003). Based on the estimated PIPs, BKMR identified PFOA (0.07), PFHxS (0.05), EtFOSAA (0.03), and MeFOSAA (0.02) as being important contributors to the overall association. However, only PFOA was independently associated with ln-FT4I (Fig 4B); a change in PFOA concentration from the 25th to 75th percentile was associated with a −0.02 decrease in ln-FT4I (95% credible interval: −0.04, 0.01). We saw no evidence of interactions among PFAS in any of our maternal BKMR analyses (data not shown).
3.4.2. Neonatal T4
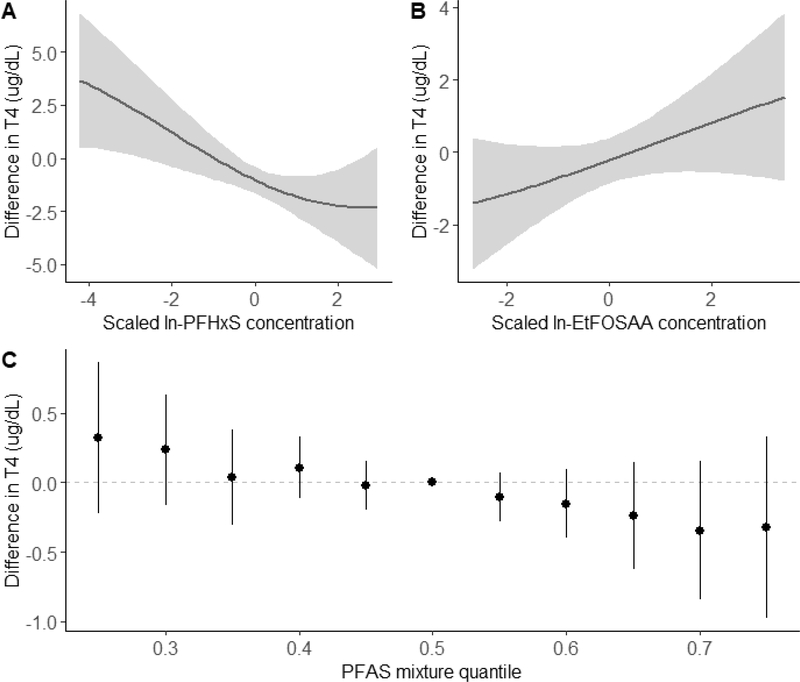
Figure 5 shows the results of BKMR our sex-stratified analyses for total T4 in male infants. We observed a non-significant inverse association between exposure to the overall PFAS mixture and T4 levels in male infants (Fig 5C); associations were null in female infants (Table S3Based on the PIPs, PFHxS (0.97) was the primary contributor to the overall association, while the other PFAS did not seem to contribute to the association (PIPs: EtFOSAA=0.30, PFOS=0.25, PFOA=0.22, PFNA=0.20, MeFOSAA=0.20). When evaluating individual associations, we found a significant inverse association between PFHxS concentrations and T4levels (Fig 5A). A change in ln-PFHxS concentrations from the 25th to 75th percentile was associated with a −0.89 μg/dL decrease in T4 levels (95% credible interval: −1.64, −0.15), holding all other PFAS at their medians. Conversely, BKMR also identified a suggestive positive linear association between EtFOSAA concentrations and T4 levels in male infants (Fig 5B), with an estimated 0.68 μg/dL increase in T4 levels (95% credible interval: −0.15, 1.51) associated with a change in EtFOSAA concentrations from the 25th to 75th percentile, while holding all other PFAS at their medians. We saw no evidence of interactions among PFAS in our neonatal analysis (data not shown).
Figure 5.
Combined effects of the PFAS mixture on total T4 levels (μg/dL) in male infants (n=236) estimated by Bayesian Kernel Machine Regression (BKMR), adjusting for maternal age, race/ethnicity, smoking habits, parity, gestational week at blood draw, age at heel stick, gestational age at birth, and cesarean delivery). Univariate exposure-response functions and 95% confidence bands for (A) ln-PFHxS and (B) ln-EtFOSAA holding all other PFASs at their medians. (C) Overall effect of the PFAS mixture on T4 levels in male infants. This plot shows the estimated difference in total T4 levels and 95% credible intervals when all PFAS concentrations are held at a certain percentile compared to when all PFAS concentrations are held at their medians, representing the exposure-response relationship of the PFAS mixture with T4 levels.
Results from BKMR in all infants were similar in direction of effect to those in male infants. We observed a non-significant trend of higher levels of the overall PFAS mixture associated with lower neonatal T4 levels (Figure S1) and a suggestive individual association between a change in ln-PFHxS concentrations from the 25th to 75th percentile and lower neonatal T4 levels (−0.37 μg/dL; 95% CI: −0.88, 0.13) (Table S3).
4. DISCUSSION
The aims of this study were to assess the individual and combined effects of prenatal exposure to multiple PFAS on maternal and neonatal thyroid hormone levels, using two different statistical approaches. In this relatively large cohort of pregnant women and their neonates, the PFAS mixture was inversely associated with maternal FT4I and neonatal T4 levels in male infants, using both BKMR and WQS regression. However, the PFAS mixture was not associated with maternal T4 or TSH levels in either WQS regression or BKMR analyses. Additionally, our BKMR results identified multiple individual PFAS-hormone associations, while accounting for exposures to the other PFAS. In moms, higher PFOS concentrations was associated with higher total T4 levels, while higher PFOA concentrations were associated with lower FT4I, and PFHxS concentrations were non-linearly associated with TSH levels. In infants, higher PFHxS concentrations were associated with lower T4 levels and higher EtFOSAA concentrations were suggestively associated with higher T4 levels in male, but not female infants.
Overall, results from BKMR and WQS regression analyses were relatively consistent. Table 2 summarizes the results of the maternal FT4I and male neonatal T4 analyses from BKMR, WQS, and our previous linear regression analysis (Preston et al. 2018). Both WQS and BKMR identified associations of the PFAS mixture with decreased maternal FT4I and neonatal T4, primarily in male infants. Neither method identified combined effects of the PFAS mixture with maternal total T4 or TSH levels or neonatal T4 in females. When comparing which individual PFAS were identified as important contributors to these combined effects, BKMR and WQS regression identified PFOA, PFHxS, EtFOSAA, and MeFOSAA as contributing to the negative joint association of PFAS exposure with maternal FT4I. Conversely, WQS identified both PFHxS and MEFOSAA exposure in males as contributing to the negative joint association of PFAS exposure with neonatal T4 levels, while BKMR only identified PFHxS as an important contributor to the suggestive negative association with T4 levels. However, in our WQS regression analysis we were able to create sex-specific weights while still utilizing the entire neonatal dataset, while for BKMR analyses we analyzed male and female infants separately, which could have led to slightly different results. Additionally, the directionality assumption of WQS may have been violated in our neonatal analysis, as BKMR identified disparate directions of effect for PFHxS and EtFOSAA on T4 levels in male infants, which likely contributed to the slightly different results from WQS and BKMR analyses. While the PFAS mixture was associated with neonatal T4 levels and maternal FT4I, it was not associated with maternal total T4 levels. The differences between neonatal and maternal hormone analyses could be due to increased variability in maternal T4 levels during pregnancy, which could have limited our ability to detect subtle associations.
Table 2.
Summary of results from statistical methods estimating individual and joint associations of PFAS concentrations with maternal FT4I and male neonatal T4 levels
| Analysis | PFAS Mixture | PFOS | PFOA | PFHxS | PFNA | EtFOSAA | MeFOSAA |
|---|---|---|---|---|---|---|---|
| Maternal FT4I | |||||||
| Linear regressiona | NA | ↓ | ↓ | ↓ | |||
| BKMR | ↓ | ↓ | x | x | x | ||
| WQS | ↓ | x | x* | x | x | ||
| Neonatal T4 – males | |||||||
| Linear regressiona | NA | ↓ | ↓ | ↓ | |||
| BKMR | ↓ | ↓ | ↑ | ||||
| WQS | ↓ | x | x | ||||
Note: Arrows represent individual associations with the outcome; “x” represents PFAS identified as contributing to overall mixture effect with high PIPs (BKMR) or high weights (WQS)
Results from Preston et al. 2018 – modeling single PFAS-hormone associations using individual covariate-adjusted linear regression models, where PFAS concentrations were modeled as quartiles.
WQS weight < 100%/n
In our previous analysis modeling individual PFAS-hormone associations in the Project Viva cohort, we observed individual inverse associations between PFOA, PFHxS, and MeFOSAA with maternal FT4I (Preston et al. 2018), which were all also identified here in both WQS and BKMR as contributing to the joint effect of PFAS exposure on maternal FT4I levels (Table 2). However, only PFOA was individually associated with FT4I in BKMR analyses. The consistency across traditional regression and novel mixtures methods indicates that there was not significant confounding among PFAS of the association with individual PFAS and maternal FT4I. In our previous neonatal analyses, we observed individual inverse associations between PFOS, PFOA, and PFHxS with T4 levels in male infants (Preston et al. 2018). However, in our WQS regression and BKMR analyses, PFOS and PFOA did not contribute to the overall joint associations with neonatal T4 levels, and were also not individually associated with neonatal T4 in BKMR analyses. MeFOSAA was not associated with neonatal T4 levels in our previous analyses, but was an important contributor to the overall mixture effect in both WQS and BKMR analyses. Differences in findings across the analyses may be due to the relatively high correlations among these exposures, and/or confounding among PFAS in our previous single- PFAS analyses.
While we did not see consistent associations between exposures to multiple PFAS and T4 or TSH in our analyses, reductions in maternal FT4I (free T4) alone have been consistently associated with impaired fetal growth and neurodevelopment (de Escobar et al. 2004; Henrichs et al. 2013; Morreale de Escobar 2001). Effects of low neonatal T4 on subsequent growth and development are less well characterized, but altered neonatal thyroid function has been associated with reductions in IQ, attention, and neurocognitive tests (American Academy of Pediatrics et al. 2006; Lyall et al. 2017; Simic et al. 2009b).
While there is growing evidence from both the epidemiology and toxicology literature that exposure to PFAS alters thyroid function, the mechanism(s) of effect and potential differences across PFAS remain unclear. Proposed mechanisms include reduced responsiveness to the hypothalamic-pituitary-thyroid axis, increased hepatic clearance of T4, increased conversion of T4 to T3 by type 1 deiodinase, and competitive binding to thyroid hormone binding proteins (Long et al. 2013; Weiss et al. 2009; Yu et al. 2009).
Toxicology studies investigating effects of PFAS exposure on thyroid function have primarily investigated effects of PFOS or PFOA, but less is known about the toxicological effects of other PFAS and even less is known about exposure to PFAS mixtures. Studies including a range of PFAS have demonstrated varying levels of effect based on PFAS structure (Long et al. 2013; Ren et al. 2016; Ren et al. 2015; Weiss et al. 2009). For example, PFAS binding affinity to the thyroid hormone transport protein, transthyretin, varies based on PFAS alkyl chain length and functional group (Ren et al. 2016; Weiss et al. 2009). Similarly, Ren et al. (2015) demonstrated that there is a structure-dependent binding relationship between PFAS and the human thyroid receptor (TR), where longer-chain PFAS with acid end groups had the highest binding affinity to TR. Long et al. (2013) showed differences in effects on the thyroid hormone system using the T-screen assay, across seven PFAS. While the specific differences in action across PFAS described above may not directly explain why we observed that only certain PFAS were responsible for the joint effects on maternal FT4I and neonatal T4 levels, they demonstrate the potential for different toxicities and mechanisms of action across different PFAS. There is a critical lack of data on the joint effects of PFAS on thyroid function. However, limited evidence from studies investigating other endpoints (e.g. cytotoxicity) have reported both additive and more complex interaction effects between exposure to multiple PFAS (Ding et al. 2013; Hoover et al. 2019).
Results from previous studies of associations between PFAS exposure and maternal and neonatal thyroid hormone levels have been inconsistent, likely due to the extreme heterogeneity in study design, population demographics, PFAS and thyroid hormone analysis techniques, statistical analysis, and PFAS concentration distributions (Ballesteros et al. 2016). The most consistent findings have been a positive association between PFAS and TSH in both maternal and neonatal analyses, with some studies also reporting an inverse association between PFAS and maternal and/or neonatal free or total T4 levels (Ballesteros et al. 2016; Berg et al. 2015; Berg et al. 2016; Wang et al. 2014; Wang et al. 2013). The inverse association between PFAS and maternal FT4I was also reported (fT4) in single-PFAS models in a Taiwanese cohort (Wang et al. 2014) and in TPOAb positive women in Canadian (Webster et al. 2014) and Japanese (Itoh et al. 2019) cohorts. Inverse associations between PFAS concentrations and neonatal T4 levels have also been reported in single-PFAS models in previous studies (Kim et al. 2011; Wang et al. 2014). Previous studies have also reported sex-specific effects of PFAS exposure on thyroid hormones. However, the mechanism behind these effects remains unclear. Male infants may be more susceptible to alterations in thyroid function due to their generally lower levels of T4 compared to female infants (Chan et al. 2011; Herbstman et al. 2008; Kuppens et al. 2011; Preston et al. 2018). While in the opposite direction of our findings, a recent Chinese cohort reported positive associations between multiple PFAS and free T4 levels among male, but not female infants (Aimuzi et al. 2019). Conversely, Wen et al. (2013) observed a similar sex-specific association as seen in our study, reporting higher serum PFHxS concentrations associated with lower free T4 levels in male, but not female adult participants in NHANES.
Few previous studies have examined the combined effects of exposure to multiple PFAS, and those that have done so have primarily used traditional regression models containing multiple PFAS in a single model (Chan et al. 2011; Shah-Kulkarni et al. 2016). Berg et al. (2016) used principle components analysis and hierarchical clustering methods to assess associations between multiple pollutants, including several PFAS, and maternal thyroid hormone levels, due to high correlations among the chemicals. However, these methods group exposures based on correlations with each other rather than with the outcome. Thus, individual exposures within a component or correlated group may vary in respect to their association with the outcome, without a clear method of disentangling these relationships within the components. A recent Chinese study (Aimuzi et al. 2019) used sparse partial least squares (SPLS) regression, a novel method proposed to identify the dominant exposures related to the outcome of interest in a group of highly correlated exposures (Lenters et al. 2015), to assess associations of prenatal PFAS concentrations with neonatal thyroid hormone levels. However, the majority of these methods do not estimate the overall joint effects of exposure to multiple PFAS. While toxicological data on the joint effects of PFAS is extremely limited, additive and even non-additive interactions between PFAS have been reported (Ding et al. 2013; Hoover et al. 2019). Because we know that individuals are ubiquitously exposed to multiple PFAS, understanding the joint effects of these exposures on thyroid function and other endpoints is critically important and should be considered in future studies.
The major strength of the present study was the use of BKMR and WQS regression, which allowed us to examine both the joint and individual effects of exposure to multiple PFAS on maternal and neonatal thyroid hormone levels, accounting for moderately to highly correlated PFAS concentrations. Additionally, the use of BKMR allowed us to evaluate potential non-linear exposure-response functions and interactions among PFAS. Other strengths of the present study include the relatively large sample size, which allowed us to assess these complex relationships while controlling for multiple potential confounding factors and stratifying by neonatal sex, the prospective nature of our neonatal analysis, and the collection of maternal blood samples during early pregnancy. Unlike many previous studies, we collected maternal blood samples during the first trimester of pregnancy, a period when offspring are particularly susceptible to the potential adverse neurodevelopmental effects of alterations in maternal thyroid hormone levels (de Escobar et al. 2004; Henrichs et al. 2013). Physiological changes such as plasma volume expansion and increased glomerular filtration rate, which can affect both plasma PFAS concentrations and thyroid hormone levels, are less likely to be significant confounding factors during early pregnancy compared to later pregnancy. As previously reported in the Project Viva cohort, controlling for these factors using plasma albumin and estimated GFR levels, in addition to gestational week at blood draw, did not affect the observed associations and were therefore not included in the present analyses (Preston et al. 2018).
There are several potential limitations to the present study. WQS regression assumes a linear relationship between concentration quantiles and the outcome of interest. We were able to assess the linearity of these relationships in our BKMR analysis. While most exposure-response associations appeared to be relatively linear, we observed a non-linear association between PFHxS concentrations and maternal TSH levels, indicating that WQS may not have been an appropriate method to assess the joint effects of PFAS on this hormone. WQS requires the exposures to all act in the same direction of effect on the outcome for a given analysis. However, users can perform separate analyses in both directions to assess disparate directions of effect across exposures, as done in the present study. The direction of associations did vary for some PFAS in our analyses. For example, in our BKMR analysis, we observed a significant inverse association between PFHxS and T4 levels, but a suggestive positive association between EtFOSAA and T4 levels in male neonates. WQS does not directly account for differences in concentration ranges across exposures as exposure concentrations are categorized into quantiles. In addition, WQS assumes that there are no greater than additive interactions among exposure variables, but we were able to validate this assumption in our BKMR analysis.
There are several additional limitations of the present study. The Project Viva study population is of higher socioeconomic status and less racially diverse than the general population, potentially limiting the generalizability of our study results. However, concentrations of PFAS in Project Viva were comparable to those in NHANES during the corresponding sampling time period (Sagiv et al. 2015). In both maternal and neonatal analyses, we did not have a direct measure of iodine status, an important potential confounder, but we did have information on dietary intake of iodine rich foods. Our maternal analysis was cross-sectional, limiting our ability to determine temporality of exposures in relation to the outcomes, but is potentially offset by the relatively long half-lives of many PFAS in plasma, measured in years. In neonatal analyses, we only had data on T4 levels and were not able to explore associations with other neonatal thyroid hormone levels. While we were able to assess potential confounding by numerous demographic and physiological factors as well as PFASevaluated, we cannot rule out potential residual confounding from uncontrolled factors such as demographic factors or other exposures to additional thyroid disrupting chemicals. We also cannot rule out potential interactions between PFAS and other unmeasured chemical exposures.
5. CONCLUSION
We examined the effect of exposure to multiple PFAS on maternal and neonatal thyroid function using two novel statistical methods, WQS regression and BKMR. We found that exposure to the PFAS mixture was associated with lower maternal FT4I and lower neonatal T4, particularly in male infants, with PFOA, PFHxS, EtFOSAA, and MeFOSAA primarily contributing to the joint effect on maternal FT4I, and PFHxS and MeFOSAA primarily contributing to the joint effect on T4 levels in male infants. To our knowledge, this is one of the first epidemiologic studies to use WQS regression and/or BKMR to assess the joint and individual effects of exposure to multiple PFAS on maternal and neonatal thyroid hormone levels. Further studies are needed to confirm these results in other populations and to better understand the toxicological mechanism(s) behind the observed differential effects of individual PFAS.
Supplementary Material
HIGHLIGHTS.
We examined associations of PFAS mixtures with maternal and neonatal thyroid function
Results from WQS regression and BKMR analyses were relatively comparable
The PFAS mixture was inversely associated with maternal FT4I and neonatal T4
PFOA, PFHxS, EtFOSAA, and MeFOSAA primarily contributed to the joint effect on FT4I
PFHxS and MeFOSAA primarily contributed to the joint effect on neonatal T4 levels
ACKNOWLEDGEMENTS
The authors thank the Project Viva participants and staff, as well as Kayoto Kato, Ayesha Patel, Tao Jie, and the late Xiaoyun Ye (Centers for Disease Control and Prevention, CDC) for PFAS measurements, the late Lewis E. Braverman for assistance in measurement and interpretation of thyroid hormone levels, and Anne M. Comeau of the New England Newborn Screening Program for assistance in obtaining newborn T4 results.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Funding: This work was supported by the National Institutes of Health (R01ES021447, T32ES014562, T32ES007069, K23ES024803, R01ES030101, R01HD034568, R00ES022986, R01ES027880, and UH3OD023286).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aimuzi R; Luo K; Chen Q; Wang H; Feng L; Ouyang F; Zhang J Perfluoroalkyl and polyfluoroalkyl substances and fetal thyroid hormone levels in umbilical cord blood among newborns by prelabor caesarean delivery. Environment international 2019;130:104929. doi: 10.1016/j.envint.2019.104929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics.; Rose SR; Section on E; Committee on Genetics ATA; Brown RS; Public Health Committee LWPES; Foley T; Kaplowitz PB; Kaye CI; Sundararajan S; Varma SK Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006;117:2290–2303. doi: 10.1542/peds.2006-0915 [DOI] [PubMed] [Google Scholar]
- Ballesteros V; Costa O; Iniguez C; Fletcher T; Ballester F; Lopez-Espinosa MJ Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environment international 2016; doi: 10.1016/j.envint.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Barton KE; Starling AP; Higgins CP; McDonough CA; Calafat AM; Adgate JL Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. International journal of hygiene and environmental health 2019; doi: 10.1016/j.ijheh.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V; Nøst TH; Hansen S; Elverland A; Veyhe AS; Jorde R; Odland JØ; Sandanger TM Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environment international 2015;77:63–69. doi: 10.1016/j.envint.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Berg V; Nost TH; Pettersen RD; Hansen S; Veyhe AS; Jorde R; Odland JO; Sandanger TM Persistent Organic Pollutants and the Association with Maternal and Infant Thyroid Homeostasis: A Multipollutant Assessment. Environmental health perspectives 2016; doi: 10.1289/EHP152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M; Feldt-Rasmussen U; Main KM Thyroid effects of endocrine disrupting chemicals. Molecular and Cellular Endocrinology 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Bobb JF; Claus Henn B; Valeri L; Coull BA Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environmental health : a global access science source 2018;17:67. doi: 10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF; Valeri L; Claus Henn B; Christiani DC; Wright RO; Mazumdar M; Godleski JJ; Coull BA Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics (Oxford, England) 2015;16:493–508. doi: 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM; Gennings C; Hauser R; Webster TF What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environmental health perspectives 2016;124:A6–9. doi: 10.1289/ehp.1510569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ; Sanchez Guerra M; Gennings C; Hacker M; Jara C; Bosquet Enlow M; Wright RO; Baccarelli A; Wright RJ Maternal Lifetime Stress and Prenatal Psychological Functioning and Decreased Placental Mitochondrial DNA Copy Number in the PRISM Study. American journal of epidemiology 2017;186:1227–1236. doi: 10.1093/aje/kwx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM; Kuklenyik Z; Reidy JA; Caudill SP; Tully JS; Needham LL Serum concentrations of 11 polyfluoroalkyl compounds in the US population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental science & technology 2007;41:2237–2242. doi: 10.1021/es062686m [DOI] [PubMed] [Google Scholar]
- Carrico C; Gennings C; Wheeler DC; Factor-Litvak P Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 2015;20:100–120. doi: 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, C.f.D.C.a.P. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2017, Volume One 2017 [Google Scholar]
- Chan E; Burstyn I; Cherry N; Bamforth F; Martin JW Perfluorinated acids and hypothyroxinemia in pregnant women. Environmental research 2011;111:559–564. doi: 10.1016/j.envres.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Daly ER; Chan BP; Talbot EA; Nassif J; Bean C; Cavallo SJ; Metcalf E; Simone K; Woolf AD Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. International journal of hygiene and environmental health 2018;221:569–577. doi: 10.1016/j.ijheh.2018.02.007 [DOI] [PubMed] [Google Scholar]
- de Escobar GM; Obregon MJ; del Rey FE Maternal thyroid hormones early in pregnancy and fetal brain development. Best practice & research Clinical endocrinology & metabolism 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Ding G; Zhang J; Chen Y; Wang L; Wang M; Xiong D; Sun Y Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch Environ Contam Toxicol 2013; 64(4):668–675. doi: 10.1007/s00244-012-9864-2 [DOI] [PubMed] [Google Scholar]
- Fisher DA Fetal thyroid function: Diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol 1997;40:16–31. doi:Doi 10.1097/00003081-199703000-00005 [DOI] [PubMed] [Google Scholar]
- Gebbink WA; Glynn A; Darnerud PO; Berger U Perfluoroalkyl acids and their precursors in Swedish food: The relative importance of direct and indirect dietary exposure. Environ Pollut 2015;198:108–115. doi: 10.1016/j.envpol.2014.12.022 [DOI] [PubMed] [Google Scholar]
- Henrichs J; Ghassabian A; Peeters RP; Tiemeier H Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–162. doi: 10.1111/cen.12227 [DOI] [PubMed] [Google Scholar]
- Herbstman JB; Sjodin A; Apelberg BJ; Witter FR; Halden RU; Patterson DG; Panny SR; Needham LL; Goldman LR Birth delivery mode modifies the association between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect 2008; 116(10):1376–1382. doi: 10.1289/ehp.11379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover G; Kar S; Guffey S; Leszynski J; Sepulveda MS In Vitro and silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 2019; 233:25–33. doi: 10.1016/j.chemosphere.2019.05.065 [DOI] [PubMed] [Google Scholar]
- Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental science & technology letters 2016;3:344–350. doi: 10.1021/acs.estlett.6b00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC; Tokranov AK; Liddie J; Zhang X; Grandjean P; Hart JE; Laden F; Sun Q; Yeung LWY; Sunderland EM Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environmental health perspectives 2019;127:67006. doi: 10.1289/ehp4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S; Araki A; Miyashita C; Yamazaki K; Goudarzi H; Minatoya M; Ait Bamai Y; Kobayashi S; Okada E; Kashino I; Yuasa M; Baba T; Kishi R Association between perfluoroalkyl substance exposure and thyroid hormone/thyroid antibody levels in maternal and cord blood: The Hokkaido Study. Environment International. 2019; 133(PtA):105139. doi: 10.1016/j.envint.2019.105139 [DOI] [PubMed] [Google Scholar]
- Kato K; Basden BJ; Needham LL; Calafat AM Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. Journal of chromatography A 2011;1218:2133–2137. doi: 10.1016/j.chroma.2010.10.051 [DOI] [PubMed] [Google Scholar]
- Kim S; Choi K; Ji K; Seo J; Kho Y; Park J; Kim S; Park S; Hwang I; Jeon J; Yang H; Giesy JP Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environmental Science and Technology 2011;45:7465–7472. doi: 10.1021/es202408a [DOI] [PubMed] [Google Scholar]
- Kuppens SM; Kooistra L; Wijnen HA; Vader HL; Hasaart TH Oei, S.G.; Vulsma, T.; Pop, V.J. Neonatal thyroid screening results are related to gestational maternal thyroid function. Clin Endocrinol (Oxf) 2011; 75(3):382–387. doi: 10.1111/j.1365-2265.2011.04083.x [DOI] [PubMed] [Google Scholar]
- Lee RH; Spencer CA; Mestman JH; Miller EA; Petrovic I; Braverman LE; Goodwin TM Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 2009;200:260 e261–266. doi: 10.1016/j.ajog.2008.10.042 [DOI] [PubMed] [Google Scholar]
- Lenters V; Portengen L; Smit LA; Jonsson BA; Giwercman A; Rylander L; Lindh CH; Spano M; Pedersen HS; Ludwicki JK; Chumak L; Piersma AH; Toft G; Bonde JP; Heederik D; Vermeulen R Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: a multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occupational and environmental medicine 2015;72:385–393. doi: 10.1136/oemed-2014-102264 [DOI] [PubMed] [Google Scholar]
- Lindstrom AB; Strynar MJ; Libelo EL Polyfluorinated Compounds: Past, Present, and Future. Environmental science & technology 2011;45:7954–7961. doi: 10.1021/es2011622 [DOI] [PubMed] [Google Scholar]
- Long M; Ghisari M; Bonefeld-Jorgensen EC Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environmental science and pollution research international 2013;20:8045–8056. doi: 10.1007/s11356-013-1628-7 [DOI] [PubMed] [Google Scholar]
- Lyall K; Anderson M; Kharrazi M; Windham GC Neonatal thyroid hormone levels in association with autism spectrum disorder. Autism Research 2016; doi: 10.1002/aur.1708 [DOI] [PubMed] [Google Scholar]
- Lyall K; Anderson M; Kharrazi M; Windham GC Neonatal thyroid hormone levels in association with autism spectrum disorder. Autism Res 2017;10:585–592. doi: 10.1002/aur.1708 [DOI] [PubMed] [Google Scholar]
- Makey CM; Webster TF; Martin JW; Shoeib M; Harner T; Dix-Cooper L; Webster GM Airborne Precursors Predict Maternal Serum Perfluoroalkyl Acid Concentrations. Environ Sci Technol 2017;51:7667–7675. doi: 10.1021/acs.est.7b00615 [DOI] [PubMed] [Google Scholar]
- Miller MD; Crofton KM; Rice DC; Zoeller RT Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environmental health perspectives 2009;117:1033–1041. doi: 10.1289/ehp.0800247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab 2001;14 Suppl 6:1453–1462. [PubMed] [Google Scholar]
- Oken E; Braverman LE; Platek D; Mitchell ML; Lee SL; Pearce EN Neonatal thyroxine, maternal thyroid function, and child cognition. The Journal of clinical endocrinology and metabolism 2009;94:497–503. doi: 10.1210/jc.2008-0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW; Burris JM; Ehresman DJ; Froehlich JW; Seacat AM; Butenhoff JL; Zobel LR Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives 2007;115:1298–1305. doi: 10.1289/ehp.10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV; Webster TF; Oken E; Claus Henn B; McClean MD; Rifas-Shiman SL; Pearce EN; Braverman LE; Calafat AM; Ye X; Sagiv SK Maternal Plasma per- and Polyfluoroalkyl Substance Concentrations in Early Pregnancy and Maternal and Neonatal Thyroid Function in a Prospective Birth Cohort: Project Viva (USA). Environmental health perspectives 2018;126:027013. doi: 10.1289/EHP2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XM; Qin WP; Cao LY; Zhang J; Yang Y; Wan B; Guo LH Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 2016;366–367:32–42. doi: 10.1016/j.tox.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Ren XM; Zhang YF; Guo LH; Qin ZF; Lv QY; Zhang LY Structure-activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Archives of toxicology 2015;89:233–242. doi: 10.1007/s00204-014-1258-y [DOI] [PubMed] [Google Scholar]
- Rose SR; Brown RS; Foley T; Kaplowitz PB; Kaye CI; Sundararajan S; Varma SK Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006;117:2290–2303. doi: 10.1542/peds.2006-0915 [DOI] [PubMed] [Google Scholar]
- Rosofsky A; Janulewicz P; Thayer KA; McClean M; Wise LA; Calafat AM; Mikkelsen EM; Taylor KW; Hatch EE Exposure to multiple chemicals in a cohort of reproductive-aged Danish women. Environmental research 2017;154:73–85. doi: 10.1016/j.envres.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK; Rifas-Shiman SL; Webster TF; Mora AM; Harris MH; Calafat AM; Ye X; Gillman MW; Oken E Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environmental science & technology 2015;49:11849–11858. doi: 10.1021/acs.est.5b02489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Kulkarni S; Kim B-M; Hong Y-C; Kim HS; Kwon EJ; Park H; Kim YJ; Ha E-H Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environment international 2016;94:−. doi:http://dx.doi. 10.1016/j.envint.2016.06.024 [DOI] [PubMed] [Google Scholar]
- Simic N; Asztalos EV; Rovet J Impact of Neonatal Thyroid Hormone Insufficiency and Medical Morbidity on Infant Neurodevelopment and Attention Following Preterm Birth. Thyroid : official journal of the American Thyroid Association 2009a;19:395–401. doi: 10.1089/thy.2008.0282 [DOI] [PubMed] [Google Scholar]
- Simic N; Asztalos EV; Rovet J Impact of neonatal thyroid hormone insufficiency and medical morbidity on infant neurodevelopment and attention following preterm birth. Thyroid : official journal of the American Thyroid Association 2009b;19:395–401. doi: 10.1089/thy.2008.0282 [DOI] [PubMed] [Google Scholar]
- Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of exposure science & environmental epidemiology 2019;29:131–147. doi: 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KW; Joubert BR; Braun JM; Dilworth C; Gennings C; Hauser R; Heindel JJ; Rider CV; Webster TF; Carlin DJ Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environmental health perspectives 2016;124:A227–a229. doi: 10.1289/ehp547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y; Rogan WJ; Chen PC; Lien GW; Chen HY; Tseng YC; Longnecker MP; Wang SL Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environmental health perspectives 2014;122:529–534. doi: 10.1289/ehp.1306925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y; Starling AP; Haug LS; Eggesbo M; Becher G; Thomsen C; Travlos G; King D; Hoppin J.a.; Rogan WJ; Longnecker MP Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environmental health : a global access science source 2013;12:76. doi: 10.1186/1476-069X-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM; Venners SA; Mattman A; Martin JW Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environmental research 2014;133:338–347. doi: 10.1016/j.envres.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Weiss JM; Andersson PL; Lamoree MH; Leonards PE; van Leeuwen SP; Hamers T Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicological sciences : an official journal of the Society of Toxicology 2009;109:206–216. doi: 10.1093/toxsci/kfp055 [DOI] [PubMed] [Google Scholar]
- Wen LL; Lin LY; Su TC; Chen PC; Lin CY Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007–2010. The Journal of clinical endocrinology and metabolism 2013;98:E1456–1464. doi: 10.1210/jc.2013-1282 [DOI] [PubMed] [Google Scholar]
- Yu WG; Liu W; Jin YH Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ Toxicol Chem 2009;28:990–996. doi: 10.1897/08-345.1 [DOI] [PubMed] [Google Scholar]
- Zoeller TR Environmental chemicals targeting thyroid. Hormones 2010;9:28–40. doi: 10.14310/horm.2002.1250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.