Abstract
Research on mechanisms underlying the phenomenon of developmental programming of health and disease has focused primarily on processes that are specific to cell types, organs and phenotypes of interest. However, the observation that exposure to suboptimal or adverse developmental conditions concomitantly influences a broad range of phenotypes suggests that these exposures may additionally exert effects through cellular mechanisms that are common, or shared, across these different cell and tissue types. It is in this context that we focus on cellular bioenergetics and propose that mitochondria, bioenergetic and signalling organelles, may represent a key cellular target underlying developmental programming. In this review, we discuss empirical findings in animals and humans that suggest that key structural and functional features of mitochondrial biology exhibit developmental plasticity, and are influenced by the same physiological pathways that are implicated in susceptibility for complex, common age-related disorders, and that these targets of mitochondrial developmental programming exhibit long-term temporal stability. We conclude by articulating current knowledge gaps and propose future research directions to bridge these gaps.
Keywords: mitochondria, developmental programming, bioenergetics, fetal programming, maternal–fetal–placental biology
1. Introduction
This review advances a novel hypothesis and conceptual framework regarding developmental programming of mitochondrial biology. Essentially, this hypothesis proposes that mitochondrial biology may represent a key common cellular pathway in the intergenerational transmission of the effects of maternal conditions, states and exposures prior to conception and/or during early and sensitive periods of embryonic/fetal development on structural and functional properties of cells, tissues and organ systems, with important implications for offspring health and disease risk. Our conceptual model, depicted in electronic supplementary material, figure S1, proposes that (i) intrauterine life represents a particularly sensitive time period when the effects of maternal conditions, states and exposures around conception and across pregnancy may be transmitted to the developing embryo/fetus; (ii) transmission occurs primarily via the effects of various maternal states and conditions on stress-related maternal–placental–fetal (MPF) oxidative, immune/inflammatory, endocrine and metabolic pathways that participate in the process of developmental programming of health and disease risk; (iii) the initial setting and function of the offspring mitochondrial biology system exhibits developmental plasticity and represents a key cellular target of such programming; (iv) this initial setting of offspring mitochondrial biology has important implications for health, aging and susceptibility for common age-related disorders; and (v) in addition to the prenatal period, exposures during the maternal pre-conception and during grandmaternal gestation (for female fetuses) periods may influence the germ pool of inherited mitochondria in oocytes and primordial germ cells (PGCs).
We begin this paper with a brief overview of the concept of developmental programming. We then summarize key features of mitochondrial biology, including an overview of mitochondrial structure and function, and the role of mitochondrial biology in health and disease. Next, we present the rationale for the proposition that mitochondrial biology may represent an important candidate pathway underlying the process of developmental programming. Specifically, we discuss and review empirical findings in animals and humans that suggest key structural and functional features of mitochondrial biology exhibit developmental plasticity and are impacted by the same physiological pathways that are implicated in the process of developmental programming, that these targets of mitochondrial programming during development exhibit long-term temporal stability, and that these features of mitochondrial biology likely influence health and susceptibility for age-related disorders. Finally, we conclude by articulating current knowledge gaps and future research directions.
2. The concept of developmental programming
A considerable and converging body of epidemiological, clinical and experimental evidence now suggests that the developing embryo/fetus responds to environmental conditions during sensitive periods of cellular proliferation, differentiation and maturation to shape various structural and functional characteristics of cells, tissues and organ systems [1,2]. This process, commonly referred to as developmental programming, is adaptive from an evolutionary perspective, but at the individual level may confer a trade-off favouring short-term survival/reproductive fitness at the long-term cost of disease susceptibility, particularly for complex, common disorders [3].
The health outcomes commonly implicated in developmental programming comprise a wide range of physical and mental disorders, including cardiovascular and metabolic disease, allergies and asthma, osteoporosis, neurodevelopmental disorders and psychopathology (reviewed in [1–3]). Research on mechanisms underlying developmental programming has focused primarily on processes that are specific to cell types, organs and phenotypes of interest, such as pancreatic β-cell mass and diabetes risk [4] or hippocampal methylation patterns and anxiety risk [5]. However, the observation that exposure to adverse intrauterine conditions such as excess stress or under- or over-nutrition concomitantly influences a broad range of phenotypes raises the possibility that these exposures may additionally (not instead) exert effects through cellular mechanisms that are shared across different cell and tissue types. It is in this context that we propose the mitochondrial biology system may represent a crucial cellular mechanism underlying the process of developmental programming.
3. The mitochondrial biology system
Mitochondria, maternally inherited bacterial endosymbionts, are cellular organelles central to the process of transforming energy into a form that can perform biological work [6]. Through the oxidation of food with oxygen from air, mitochondria generate cellular energy into the universal form used by cells and tissues––primarily adenosine triphosphate (ATP)––via the flow of protons in oxidative phosphorylation (OXPHOS). In OXPHOS, electrons derived from carbohydrates and fats are transferred through the tricarboxylic acid (TCA) cycle and β-oxidation pathways and feed into the electron transport chain (ETC). Energy is released as the electrons pass through a series of complexes in the ETC to the final recipient, oxygen, and this energy is harnessed in the form of a proton gradient, which generates an electrochemical trans-membrane potential (ΔΨ) across the inner mitochondrial membrane. The generation of this charge is coupled with the production of ATP, and the degree of coupling of these two sets of chemical reactions reflects the efficiency of mitochondrial function.
Mitochondria also serve as a signalling system [7] and regulate cell fate, differentiation and apoptosis––importantly through the control of Ca2+, cellular redox, and reactive oxygen species (ROS) production [8]. This signalling role may be particularly relevant in the context of development as recent findings suggest that mitochondria control the canonical developmental signalling pathways Notch, NF-κB and Wnt, which regulate the balance of core cellular processes that form tissues and organs at the correct time and place in embryonic/fetal life [8]. Additionally, mitochondria serve as a site of essential metabolism, including amino acid, steroid, cholesterol and phospholipid biosynthesis [9,10]. The approximately 102–103 mitochondria per cell are not static [11], but dynamically interact with one another and form comprehensive branched networks that work together to rapidly adapt to the cell's changing bioenergetic and biosynthetic needs through fission and fusion dynamics, mitochondrial biogenesis, mitophagy and cross-talk with nuclear DNA (nDNA) [12].
A unique and important feature of the mitochondrion is the possession of its own genome, known as mtDNA, which encodes 22 tRNAs, 2 rRNAs, 13 key protein subunits of the ETC and a novel class of endogenous peptides (with the remaining ETC subunits and mitochondrial proteins encoded by nDNA) [13,14]. Mitochondrial integrity is achieved and maintained by tight coordination between the nDNA and mtDNA expression (known as mito-nuclear cross-talk) [15]. This cross-talk is critical, as even subtle mismatches can result in an inefficient or backlogged ETC, causing increased electron leakage and ROS, decreased ATP production and blockage of the TCA cycle, thereby impairing the production of important biological intermediates and cellular biosynthesis [16]. The mitochondrial genome contains no histones, and despite some controversy, is not believed to be epigenetically regulated [17]. However, nDNA epigenetic modifications affect mitochondrial function, and, conversely, mitochondrial function and activity is a significant driver of nDNA gene expression via epigenetic modifications [18]. Over the course of evolution, it appears that the majority of mitochondrial genes have relocated to the nucleus. However, the continued persistence of mtDNA serves a functional significance to allow for very rapid and local energetic adaptations within the different regions of a cell (as opposed to the slower and more uniform energetic adaptation mediated via a singular nDNA molecule) [19]. Consequently, mitochondria are believed to represent the central environmental sensor in mediating the link between environmental perturbations and genomic responses [19], and mitochondrial production of ROS, metabolic intermediates or energetic compounds, such as α-ketoglutarate, ATP or acetyl-CoA, drives short- and long-term change in mtDNA and nDNA transcriptional activity through both signal transduction and epigenetic regulatory processes [7,20]. ROS production and the redox state of the cell are directly tied to epigenetic processes, particularly during development [21].
Given its critical importance, it has been argued that mitochondrial function determines the limits of an organism's adaptive capacity, and that deficits in mitochondrial content and bioenergetic function decrease biological adaptability and resilience to respond to a diverse range of physiological and psychological challenges [22]. Accordingly, the process of cellular aging is characterized, in part, by decreased mitochondrial bioenergetics, increased mitochondrial ROS production and mtDNA damage [23]. Mitochondrial dysfunction has been identified as a key component across a wide range of common age-related disorders such as diabetes, cancer, cardiovascular disease and several neurodegenerative conditions including Alzheimer's and Parkinson's disease [24]. Moreover, a causal role for the integrity of mitochondrial function in aging and disease susceptibility is supported in humans by prospective studies of oxidative damage, mtDNA mutations/deletions and mtDNA copy number that predict the onset of cardiovascular disease, diabetes and cancer [25–27], and in animals particularly by recent findings using the mtDNA mutator mouse model [28].
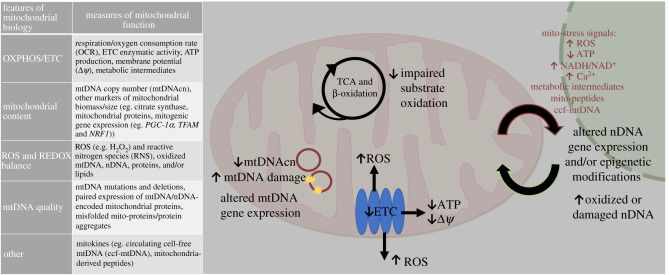
Lastly, it is important to note that because mitochondrial biology is a dynamic process, there is no single measure to quantify its structural and functional integrity [29]. Concurrent consideration of multiple measures is warranted, particularly those that use dynamic versus static measures, such as substrate oxidation or ETC enzymatic activities, and those that take into account the heterogeneity of mitochondria subpopulations across and within cells (see reviews [30–33]). Figure 1 depicts and describes commonly used measures of mitochondrial function and the concordant features of mitochondrial biology that they represent.
Figure 1.
Adverse/suboptimal maternal or grandmaternal gestational exposures can alter the essential aspects of offspring mitochondrial function in the following ways: (i) impaired oxidative phosphorylation; (ii) decreased mitochondrial content; (iii) increased ROS and impaired REDOX balance; and (iv) impaired mtDNA quality. There are multiple measures representing specific aspects of mitochondrial function, as depicted in this figure. Collectively, mitochondria can send out stress-related signals, such as increased ROS or Ca2+, decreased ATP, altered mitochondrial metabolic intermediates (e.g. NAD+, acetyl-CoA) and mito-peptides, that can alter nDNA gene expression or modify the epigenome via chromatin (methylation or acetylation of the DNA and/or histones), which can further alter mitochondrial function and bioenergetic capacity. In particular, mitochondrially generated ROS can have a major impact on this process. Nuclear and mitochondrial function are linked, such that one system regulates the other, and vice versa. The collective effect of these processes enables and regulates the flow of matter/energy in metabolic networks, and these regulate cellular information through signalling and transcriptional regulatory networks, which regulate the flow of energy. (Online version in colour.)
4. Developmental plasticity in the mitochondrial biology system
Mitochondrial structure and function is highly sensitive and responsive to environment conditions. Indeed, a wide range of socio-demographic, biophysical, clinical, behavioural, psychological and biological states––particularly those related to stress and stress-related conditions and processes––have been associated with variation in mitochondrial content, bioenergetics capacity and mtDNA quality (for reviews, see [22,34,35]). Of particular interest are the effects on mitochondrial biology of stress-related metabolic, oxidative, immune/inflammation and endocrine processes that [22,36], on one hand, are known to mediate the downstream effects on health and disease risk of a wide range of states and conditions, and on the other hand, are also known to impact the emergence of programmed phenotypic specificity (stress-metabolic MPF signals) [37]. Our model proposes that the mitochondrial biology system exhibits developmental plasticity. Based on empirical findings in animals and humans, we suggest that (i) the initial setting of the early-life mitochondrion is impacted by the same stress-related biological processes that mediate the effects of a range of suboptimal conditions on mitochondrial biology during adult life, and (ii) this initial setting exhibits temporal stability, thereby modulating subsequent susceptibility to cellular aging and health/disease risk over the life span.
(a). Gestational conditions induce variability in offspring mitochondrial structure and function
(i). Animal studies
Findings from in vivo and in vitro animal models converge to suggest that starting as early as during the first stages of life mitochondria in the fertilized oocyte are susceptible to damage from suboptimal gestational conditions (environmental stressors), that these acquired mitochondrial deficits persist into embryonic/fetal life [38–41] and beyond (i.e. they exhibit temporal stability), and that they are causally associated with the likelihood of developing disorders in later life, including obesity and metabolic disease [40–64]. Our review of the literature identified 23 published animal studies that represent the impact of various in vivo gestational exposures on offspring mitochondrial biology (electronic supplementary material, table S1) [41–64]. Common models of gestational exposures include maternal obesity and oxidative stress, and a smaller number relate to dietary quantity/quality and pollutant exposure. Changes to mitochondrial function have been demonstrated in the earliest stages of offspring development, from the fertilized oocyte to fetal tissue. Furthermore, these changes have been demonstrated in a variety of offspring tissues (pancreas, liver, adipose, brain, muscle, heart, placental and ovarian tissue) and are present in fetal tissue and through adulthood.
Across the various gestational exposures and tissues that were examined, changes in mitochondrial biology consistently demonstrated a decreased capacity to meet cellular energetic demands through either decreased mitochondrial content (e.g. mtDNA copy number (mtDNAcn)) or OXPHOS capacity and/or efficiency (e.g. ETC complex activity, substrate oxidation), and additionally, increased oxidative damage via increased mitochondrial ROS production, and/or imbalanced redox state. We note that some of the measures of mitochondrial content/function that were assessed appeared to exhibit improvement in response to adverse gestational exposures, which we suggest may reflect secondary compensation. For example, in the fetal tissue of non-human primates exposed to maternal obesity, mitochondrial content (i.e. mtDNAcn) and OXPHOS capacity and efficiency were reduced, and individual enzymes in the ETC demonstrated significantly higher activity, suggesting a compensatory increase in OXPHOS activity related to the lower mitochondrial content and function [50]. This compensation may, however, not be sufficient or entirely benign. For example, if damaged or inefficient mitochondrial systems increase OXPHOS activity, this may result in further ROS production [65]. Although the generation of a certain amount of ROS is a normal part of the ETC (ROS are even considered to be signalling molecules) [66], impaired mitochondrial function can produce elevations of ROS levels and result in excess generation of superoxide at the expense of ATP production [67], which, in turn, can damage mtDNA, nDNA and other cellular components [36,68] (e.g. two studies found an increase in mtDNA deletions in fetal and newborn tissue [42,59]). As seen in electronic supplementary material, table S1, all studies that assessed oxidative damage showed an increase in mitochondrially derived ROS, oxidized particles and/or the oxidized redox state of the cell [42,43,45,46,49,50,56–58,62], with one exception [64]. The measurement of ROS/oxidative stress is of particular interest; however, many commonly used methods for the direct measurement of ROS are not specific and can produce artefacts (e.g. dichlorodihydrofluorescein diacetate (DCFH-DA) in the above-reviewed animal studies, and MitoSox at greater than 2 µM in the humans studies reviewed below) [69]. Despite limitations in methods of direct ROS measurement, the majority of the reviewed studies also quantified end products related to ROS damage, and those were consistently elevated, supporting the presence and importance of gestationally induced elevations in ROS.
(ii). Human studies
We identified 31 published human studies to date on the association of various gestational exposures with offspring mitochondrial biology (electronic supplementary material, table S2) [70–100]. Common models of human gestational exposures include environmental pollutants and maternal obesity, and a smaller number relate to growth restriction and psychosocial stress exposure. The majority of studies assessed mitochondrial function in cord blood leucocytes or placental tissue at birth, and used mtDNAcn or mitochondrial-encoded gene expression as the primary mitochondrial biology outcome. A more recent and growing body of work focuses on methylation patterns of nDNA-encoded mitochondrial genes, particularly in association with gestational air pollution.
Similar to the animal findings, our review suggests that a broad range of suboptimal maternal conditions during pregnancy and resultant gestational exposures lead to parallel changes in mitochondrial biology, specifically a decreased capacity to meet the energetic demands of the offspring cell through either decreased mitochondrial content (e.g. mtDNAcn) or decreased OXPHOS capacity and/or efficiency, and additionally, increased oxidative damage. In humans, the earliest evidence for these changes to mitochondrial biology comes from in vitro work. Fertilized oocytes (through the blastocytes stage) from obese women exhibit decreased mitochondrial metabolism and impaired substrate oxidation [86]. The majority of in vivo human studies have used placental mtDNAcn (a marker of mitochondrial content) as the primary outcome measure, and they report an association between a range of gestational exposures and variation (primarily a decrease) in placental mtDNAcn in offspring of women exposed to pollutants [74,79,80,93], cigarette smoke [71], intrauterine growth restriction [85,87], pre-eclampsia [94], obesity [78,88] and psychosocial stress. [72,73] There are limitations in the interpretation of altered mtDNAcn [101]; however, one study of maternal obesity exposure included multiple measures of placental mitochondrial function and reported that in conjunction with decreased mtDNAcn, there were matched decreases in other measures of mitochondrial content (citrate synthase) and OXPHOS/ETC function and capacity (ATP levels, complex I–V protein levels and direct respiratory maximum, basal capacity) [88].
The next set of human studies examined mitochondrial outcomes at the time of delivery in cells and tissues from the umbilical cord or cord blood. These studies also report changes (primarily a decrease) in mtDNAcn. Specifically, infants of mothers exposed to higher levels of pollution during pregnancy, lead exposure and placental insufficiency exhibited alterations in cord blood mtDNAcn [73,89,91,92,95]. With respect to mitochondrial outcomes over and beyond mtDNAcn, five studies examined newborn umbilical cord mesenchymal stem cells (uMSCs) and/or endothelial cells collected soon after birth [70,75,81,99,100]. Specifically, gestational exposure to maternal obesity was associated with mitochondrial inefficiency and increased oxidative stress in skin epithelial cells [70] and down-regulation of several genes related to mitochondrial function and lipid metabolism in umbilical vein epithelial cells [75]. Newborns of mothers with gestational diabetes exhibited reduced expression of mitochondrial-encoded ETC genes (complexes I and V) and nuclear-encoded mitochondrial regulatory genes (TFAM, PGC-1α), decreases in all OXPHOS respiratory measures (basal respiration, maximum respiration, ATP synthesis, spare respiratory capacity, with a 30% reduction in basal oxygen consumption) and elevated ROS production in uMSCs [81]. Furthermore, uMSCs of newborns exposed to maternal obesity exhibited lower rates of complete fatty acid oxidation (FAO) that corresponded with hypermethylation and lower gene expression on a related FAO gene (CPTA1) and ETC complex II (SDHC) [99]. These multi-potent cells––uMSCs––eventually differentiate into a broad range of tissues, including muscle, bone and adipose cells, thereby increasing the generalizability of these findings. One study applied this principle and differentiated newborn uMSCs exposed to maternal obesity into cells of relevant tissue, adipocytes and myocytes, and demonstrated impaired FAO in uMSC myocytes and altered mitochondrial gene expression in uMSC adipocytes, which indicate higher ETC activity (complexes I–V), but lower mitochondrial biogenesis, mitophagy and elevated fission/fusion ratio [100]. Importantly, these findings parallel changes seen in these tissues (adipose/muscle) with age or disease and demonstrate primary alterations in mitochondrial function in otherwise healthy newborns that precede and likely increase subsequent susceptibility for common complex disorders.
Lastly, a large, unbiased meta-analysis of the effects of prenatal pollution exposure on the cord blood epigenome in a population of 1503 infants found genome-wide significant DNA methylation differences in nuclear genes related to mitochondrial function. [77] Moreover, one association appeared to persist (cg08973675 (SLC25A28)) in a subsequent look-up population of 4- and 8-year-old children. Two recent studies demonstrated 34 significant differentially methylated nDNA regions in relation to cord blood mtDNAcn [90] and an association between mtDNA methylation load and newly discovered mitochondrial peptides [97]. Although no human study appears to have prospectively followed mitochondrial function in individuals from birth to adulthood, mtDNA density is lower at birth in infants exposed to metabolic stress [78,88], positively associates with cord blood insulin [90,96], and is prospectively associated with insulin resistance [102], diabetes onset [26] and cardiovascular disease [25] in adulthood.
(b). Evidence for temporal stability of gestational condition-related variability in offspring mitochondrial biology
An important criterion in supporting the veracity of the developmental programming of mitochondrial biology hypothesis is the persistence (or temporal stability) of gestational exposure-related effects in mitochondrial biology that are evident during the embryonic, fetal, newborn or early infancy periods of life. The multiple studies that demonstrate that mitochondrial changes induced by gestational exposure persist into adulthood [40,42,44–49,51–53,56,62], or across generations [41,62,103,104], are informative in this regard. Trans-generational transmission of mitochondrial dysfunction consequent to adverse F0 gestational exposure has been demonstrated in the somatic as well as germline tissue across three generations of mice (F1–F3) [62]. It is important to note that this third generation transmission (F3) provides strong evidence of developmental programming, because none of the cells of the F3 generation could have been directly exposed to the gestational insult (F1 embryo and F2 PGCs can be directly exposed to any F0 gestational exposure). InFewer the above-referenced studies, mitochondrial function is commonly assessed in adult animals, with a gap between exposure and assessment of function; however, a small but important set of animal studies have performed serial assessment of mitochondrial function from embryonic/fetal to adult life [44,45,56], and support the persistence (stability) of features of the initial setting of mitochondria function.
It is important to note that the process of developmental programming primarily produces a shift in future disease susceptibility, as opposed to overt disease. This concept of susceptibility rather than determinism is consistent with the fundamentally dynamic nature of mitochondrial biology. Such dynamics could amplify mitochondrial dysfunction in a susceptible system (as discussed above), particularly with further adverse exposures across the lifetime (e.g. metabolic stress), or these dynamic systems could potentially serve to correct and/or diminish dysfunction over time. This emphasizes the need for serial measurement of mitochondrial function across multiple tissues, and for the careful consideration of gestationally induced changes in the context of further post-natal adverse and/or protective exposures.
(c). Germline tissue mitochondrial biology and developmental programming: special consideration
Mitochondria are maternally inherited and physically passed from the mother's oocyte to the fertilized zygote and developing embryo. At the point of fertilization, human oocytes have approximately 106 mitochondria, which is substantially more than the range seen in somatic cells (103–104) [105]. This large population of mitochondria is necessary since mitochondrial biogenesis is suppressed through the hatched blastocyte stage, and the pool of oocyte mitochondria is divided among the early embryonic cells [105,106]. Furthermore, PGCs of females are formed during this early stage of embryonic development, and as a result, there are only approximately 10 mitochondria per PGC. Fewer than 0.1% of maternal mitochondria are passed on to any given gamete in a female fetus, thus forming a unique inheritance bottleneck [105]. This process is important to consider in developmental programming research, as the entire future embryonic/fetal mitochondrial population and its function are determined by the inheritance of a relatively small number of mitochondria in the womb of the maternal grandmother.
Several studies have shown that various environmental stressors can produce deleterious effects on germ cell mitochondrial number and function. Specifically in mice [41,43,58,59,104,107–109] and humans [86], variations in maternal diet and metabolic health during the pre-conceptional and early gestational windows can affect mitochondrial function, with decreased oocyte quality and fertility across at least two subsequent generations. These findings suggest mitochondrial function in germ cells is sensitive and responsive to the gestational environment, and that these effects may persist in somatic tissues and alter long-term health and disease risk across multiple generations. Therefore, in addition to considering the intergenerational (mother to child) effects of gestational exposures (described in §§4.1 and 4.2), research on developmental programming of mitochondrial biology should consider the grandmaternal and pre-conceptional periods as well. Furthermore, mitochondria are heterogeneous within cells, and there is evidence in PGC/oocyte development and early embryogenesis that these subpopulations asymmetrically segregate during cellular division and across cellular subtypes [33]. A more rigorous evaluation of these subpopulations could provide critical information on the molecular mechanisms of developmental programming of mitochondrial biology [33].
(d). Magnitude and consequences of effects of gestational exposures on mitochondrial biology
We posit that the magnitude and/or consequences of effects of exposures during development (e.g. the gestational period) on mitochondrial biology are more pronounced than of those occurring later in life for the following overlapping reasons: (i) Since the long-term consequence on health and disease risk is a cumulative function over time, it stands to reason that any alterations (relative deficits) in cellular biology that occur earlier in the life span likely have greater consequences than those (of the same magnitude) that occur later in life [110]. Adverse gestational exposure could lead to a self-reinforcing cycle of progressive dysfunction in mitochondrial function, starting with disrupted ETC function, limited ATP supply and increased ROS, leading to mtDNA damage, which then causes further ROS leak and mitochondrial dysfunction [56]. (ii) It also is likely that the magnitude of effects of equivalent levels of potentially detrimental exposures that occur during development is greater than that of those that occur after the developmental period, because the systems under consideration are more vulnerable during development, when they are relatively immature and are undergoing rapid change [111]. For example, in a pre-clinical model of gestational hypoxia, the resultant disruption of mitochondrial energy flow and ROS production during a critical window of development lead to permanent organ or tissue structural changes, specifically in the pancreatic islet cells, and adult development of diabetes [56].
5. Mitochondrial biology mechanisms underlying developmental programming
It appears that mitochondria serve a dual role as sensors as well as transducers in the process of transmission of the effects of environmental states and exposures. Mitochondria represent a key site of sensing multiple stress-related biological signals (e.g. glucose/lipids, ROS, inflammation, glucocorticoids) and also transducing the effects of these signals through short- and long-term changes to mitochondrial structure, function and mtDNA integrity, and in interaction with nDNA signalling. We postulate that the mechanisms underlying programming of the mitochondrial biology system and its subsequent long-term effects may be mediated, in part, by early alterations in PGC and oocyte biology (as previously reviewed in §4c), by direct changes to mtDNA integrity, by the the production of stable epigenetic alterations in embryonic and fetal nDNA, and/or secondary to the programming on the telomere biology system.
(a). mtDNA quality
mtDNA is more susceptible than nDNA to damage and demonstrates a higher intrinsic mutation rate [112]. The accumulation of mtDNA defects impairs mitochondrial bioenergetics, and such changes are generally stable and amplified over time [113]. While the tissue burden of mtDNA deletion/mutation is relatively low, even modest levels of heteroplasmy can have broad and pervasive effects on mitochondrial function, and the quality of mtDNA may be a marker for an organism's fitness, resilience and adaptability [23]. Thus, the developmental programming of mtDNA quality (deletion and mutation) provides a plausible mechanism by which phenotypes of mitochondrial dysfunction in embryonic/fetal life may persist over time and confer long-term effects on health and disease risk. Two empirical studies in animals provide evidence that adverse gestational exposure can induce variation in mtDNA quality, with implications for organismal fitness. Specifically, gestational exposure to smoking and maternal obesity -induced mtDNA deletions in fetal tissue [59] with persistent effects of gestational exposure present in adult tissue [42].
(b). Mitochondria and the epigenome
Generally, one of the key mechanisms/pathways that confer temporal stability (i.e. long-term consequences) of the effects of developmental programming is mediated via the induction of a suite of stable epigenetic alterations to the nuclear genome [1]. Although mtDNA epigenetic modification is controversial [17], approximately 97% of genes that regulate mitochondrial biology are located in the nucleus. Thus, the programming of epigenetic characteristics of nuclear-encoded mitochondrial genes provides one likely underlying mechanism by which phenotypes of mitochondrial dysfunction in embryonic/fetal life may persist over time and confer long-term effects on health and disease risk. For example, methylation differences have been observed in mitochondrial fatty acid oxidation genes in offspring exposed to gestational obesity [99], and genome-wide methylation studies of human placentae exposed to maternal smoking have reported that the top canonical pathways show differential methylation in genes underlying OXPHOS and mitochondrial function [114].
Mitochondria engage in close cross-talk with the nucleus, and it is increasingly evident that mitochondria exert a substantial influence on nuclear gene expression and epigenetic regulatory processes [7,18,21]. While it is widely acknowledged that suboptimal gestational exposures can programme the fetal epigenome, the underlying pathways/mechanisms remain largely unclear [1]. Mitochondria serve as the heart of cellular bioenergetics, and the cell and its plastic nuclear epigenome have evolved tight molecular sensitivity to mitochondria's biochemical signals [34]. Among these, ROS and antioxidant balance are believed to represent key signals initiating and perpetuating epigenetic control of gene expression during development [21]. We suggest it is plausible that mitochondria could serve as one of the key sensors and transducers of fetal epigenome programming.
(c). Mitochondrial–telomere axis
Based on study findings that have established extensive regulation and counter-regulation between key aspects of mitochondrial and telomere biology, a model integrating these cellular systems has been formulated and has been referred to as the mitochondrial–telomere signalling axis [115]. Work in animal models has uncovered shared pathways that regulate the mitochondrial–telomere signalling axis, particularly through the activity of the reverse transcriptase enzyme telomerase, and expression of p53 [115]. The connection between these two systems is further supported by human studies showing a tight correlation between mtDNAcn and telomere length [116,117]. We have previously proposed that the initial setting of the telomere biology system exhibits developmental plasticity and likely exerts a long-term influence on cellular processes underlying health, aging, disease susceptibility and longevity [118]. It is plausible that the effects of developmental programming of the telomere biology system could, in part, serve as another underlying mechanism for the development of the initial setting and stability of mitochondrial function.
6. Conclusion and future research directions
Mitochondria ‘sense' information about the cellular energetic environment and signal the nuclear genome through metabolic intermediates and ROS to drive acute and chronic changes in gene expression that, in turn, regulate fundamental cellular activities such as cell division, growth, size and cell death [7]. Based on the considerations that the initial settings of the mitochondrial biology system exhibit developmental plasticity in response to pre-conceptional and gestational conditions, demonstrate subsequent long-term temporal stability and implicate the same mitochondrial phenotypes that are known to underlie health, aging and disease susceptibility, we submit that mitochondria biology represents a promising candidate cellular mechanism underlying the process of developmental programming of health and disease risk. The consequences of alterations in mitochondrial phenotypes in response to developmental conditions may include transient changes that limit cellular energy flow during critical periods of growth and differentiation to produce structural changes in the architecture of tissues and organ systems, or stable changes that impact cell function (particularly energy flow and ROS production), or both.
Important knowledge gaps remain, including: the magnitude and duration of the long-term effects of developmental conditions on the initial settings of mitochondrial function; the clinical significance of these observed effects on subsequent health and disease susceptibility-related outcomes over the life span; the molecular mechanism(s) underlying long-term effects; the elucidation of informative biomarkers of mitochondrial function across tissues; and the influence of sex differences on mitochondrial function and its consequences. These questions and issues provide a framework for future research. Prospective, longitudinal studies are warranted in humans that serially track the effects of early-life conditions on mitochondrial biology across different cell and tissues types and across sexes, from prenatal life and birth through infancy and childhood until adult life. To address the issue of confounding that is inherent in observational studies, such studies could be complemented by ones that use quasi-experimental family-based studies [119] or Mendelian randomization. Furthermore, studies using newborn cells (e.g. uMSCs) offer great promise in the context of developmental programming, as ex vivo experimental challenge paradigms using ecologically relevant stimuli will be particularly informative in interrogating aspects of mitochondrial function. The selection of individuals from whom such cells are obtained (e.g. gestational diabetes mellitus newborns) may provide further insights regarding the direction and magnitude of the effects of specific gestational exposures.
In conclusion, the process of developmental programming of mitochondrial biology may represent an important avenue by which population health disparities are propagated to influence the health and well-being of individuals and their offspring across the life span and over generations. A better understanding of these processes is important for the formulation and testing of interventions for primary and secondary prevention of disorders that confer the major societal burden of disease.
Supplementary Material
Supplementary Material
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the National Institute on Aging (grant no. AG050455), National Institute of Mental Health (grant no. MH105538) and National Institute of Child Health and Human Development (grant nos HD060628 and HD097302).
References
- 1.Wadhwa PD, Buss C, Entringer S, Swanson J. 2009. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 27, 358–368. ( 10.1055/s-0029-1237424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD. 2011. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog. Biophys. Mol. Biol. 106, 272–280. ( 10.1016/j.pbiomolbio.2010.12.008) [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Buklijas T. 2010. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 1, 6–18. ( 10.1017/S2040174409990171) [DOI] [PubMed] [Google Scholar]
- 4.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. 2001. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int. J. Exp. Diabetes Res. 2, 139–143. ( 10.1155/EDR.2001.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suderman M, Mcgowan PO, Sasaki A, Huang TCT, Hallett MT, Meaney MJ, Turecki G, Szyf M. 2012. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl Acad. Sci. USA 109, 17 266–17 272. ( 10.1073/pnas.1121260109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane N. 2017. Serial endosymbiosis or singular event at the origin of eukaryotes? J. Theor. Biol. 434, 58–67. ( 10.1016/j.jtbi.2017.04.031) [DOI] [PubMed] [Google Scholar]
- 7.Chandel NS. 2015. Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206. ( 10.1016/j.cmet.2015.05.013) [DOI] [PubMed] [Google Scholar]
- 8.Kasahara A, Scorrano L. 2014. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 24, 761–770. ( 10.1016/j.tcb.2014.08.005) [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos V, Miller WL. 2012. Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 26, 771–790. ( 10.1016/j.beem.2012.05.002) [DOI] [PubMed] [Google Scholar]
- 10.Cheng Z, Ristow M. 2013. Mitochondria and metabolic homeostasis. Antioxid. Redox Signal. 19, 240–242. ( 10.1089/ars.2013.5255) [DOI] [PubMed] [Google Scholar]
- 11.Boldogh IR, Pon LA. 2007. Mitochondria on the move. Trends Cell Biol. 17, 502–510. ( 10.1016/j.tcb.2007.07.008) [DOI] [PubMed] [Google Scholar]
- 12.Chan DC. 2012. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287. ( 10.1146/annurev-genet-110410-132529) [DOI] [PubMed] [Google Scholar]
- 13.Anderson S, et al. 1981. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465. ( 10.1038/290457a0) [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Kim KH, Cohen P. 2016. MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic. Biol. Med. 100, 182–187. ( 10.1016/j.freeradbiomed.2016.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff JN, Ladoukakis ED, EnrãQuez J, Dowling DK. 2014. Mitonuclear interactions: evolutionary consequences over multiple biological scales. Phil. Trans. R Soc. Lond. B 369, 20130443 ( 10.1098/rstb.2013.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane N. 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays 33, 860–869. ( 10.1002/bies.201100051) [DOI] [PubMed] [Google Scholar]
- 17.Mechta M, Ingerslev LR, Fabre O, Picard M, Barrès R.. 2017. Evidence suggesting absence of mitochondrial DNA methylation. Front. Genet. 8, 166 ( 10.3389/fgene.2017.00166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minocherhomji S, Tollefsbol TO, Singh KK. 2012. Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics 7, 326–334. ( 10.4161/epi.19547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DC. 2016. Genetics: mitochondrial DNA in evolution and disease. Nature 535, 498–500. ( 10.1038/nature18902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard M, et al. 2014. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl Acad. Sci. USA 111, E4033–E4042. ( 10.1073/pnas.1414028111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchler MJ, Domann FE. 2007. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 43, 1023–1036. ( 10.1016/j.freeradbiomed.2007.06.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picard M, Juster RP, McEwen BS. 2014. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310. ( 10.1038/nrendo.2014.22) [DOI] [PubMed] [Google Scholar]
- 23.Wallace DC. 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407. ( 10.1146/annurev.genet.39.110304.095751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas RH. 2019. Mitochondrial dysfunction in aging and diseases of aging. Biology (Basel) 8, 48 ( 10.3390/biology8020048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashar FN, et al. 2017. Association of mitochondrial DNA copy number with cardiovascular disease. JAMA Cardiol. 2, 1247–1255. ( 10.1001/jamacardio.2017.3683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, Koh C-S. 1998. Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pract. 42, 161–167. ( 10.1016/S0168-8227(98)00110-7) [DOI] [PubMed] [Google Scholar]
- 27.Lan Q, Lim U, Liu C-S, Weinstein SJ, Chanock S, Bonner MR, Virtamo J, Albanes D, Rothman N. 2008. A prospective study of mitochondrial DNA copy number and risk of non-Hodgkin lymphoma. Blood 112, 4247–4249. ( 10.1182/blood-2008-05-157974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trifunovic A, et al. 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. ( 10.1038/nature02517) [DOI] [PubMed] [Google Scholar]
- 29.Kurz FT, Kembro JM, Flesia AG, Armoundas AA, Cortassa S, Aon MA, Lloyd D. 2017. Network dynamics: quantitative analysis of complex behavior in metabolism, organelles, and cells, from experiments to models and back. Wiley Interdiscip. Rev. Syst. Biol. Med. 9, e1352 ( 10.1002/wsbm.1352) [DOI] [PubMed] [Google Scholar]
- 30.Brand MD, Nicholls DG. 2011. Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312. ( 10.1042/BJ20110162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanza IR, Nair KS. 2010. Mitochondrial metabolic function assessed in vivo and in vitro. Curr. Opin. Clin. Nutr. Metab. Care 13, 511–517. ( 10.1097/MCO.0b013e32833cc93d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen RC, Boyle KE. 2019. Microplate assays for spectrophotometric measurement of mitochondrial enzyme activity. Methods Mol. Biol. 1978, 355–368. ( 10.1007/978-1-4939-9236-2_22) [DOI] [PubMed] [Google Scholar]
- 33.Woods DC, Khrapko K, Tilly JL. 2018. Influence of maternal aging on mitochondrial heterogeneity, inheritance, and function in oocytes and preimplantation embryos. Genes (Basel) 9, 265 ( 10.3390/genes9050265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard M, Turnbull DM. 2013. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–678. ( 10.2337/db12-1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picard M, McEwen BS. 2018. Psychological stress and mitochondria: a systematic review. Psychosom. Med. 80, 141–153. ( 10.1097/PSY.0000000000000545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill BG, Benavides GA, Lancaster JR, Ballinger S, Dell'Italia L, Zhang J, Darley-Usmar VM. 2012. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 393, 1485–1512. ( 10.1515/hsz-2012-0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Entringer S, Buss C, Wadhwa PD. 2015. Prenatal stress, development, health and disease risk: a psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology 62, 366–375. ( 10.1016/j.psyneuen.2015.08.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thouas GA, Trounson AO, Jones GM. 2006. Developmental effects of sublethal mitochondrial injury in mouse oocytes. Biol. Reprod. 74, 969–977. ( 10.1095/biolreprod.105.048611) [DOI] [PubMed] [Google Scholar]
- 39.McConnell JM, Petrie L. 2004. Mitochondrial DNA turnover occurs during preimplantation development and can be modulated by environmental factors. Reprod. Biomed. Online 9, 418–424. ( 10.1016/S1472-6483(10)61277-1) [DOI] [PubMed] [Google Scholar]
- 40.Zander-Fox DL, Fullston T, McPherson NO, Sandeman L, Kang WX, Good SB, Spillane M, Lane M. 2015. Reduction of mitochondrial function by FCCP during mouse cleavage stage embryo culture reduces birth weight and impairs the metabolic health of offspring. Biol. Reprod. 92, 124 ( 10.1095/biolreprod.114.123489) [DOI] [PubMed] [Google Scholar]
- 41.Andreas E, Reid M, Zhang W, Moley KH. et al. 2019. The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol. Hum. Reprod. 25, 717–728. ( 10.1093/molehr/gaz049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, Ballinger SW. 2013. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE−/− mice. PLoS ONE 8, e66835 ( 10.1371/journal.pone.0066835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aiken CE, Tarry Adkins JL, Penfold NC, Dearden L, Ozanne SE. et al. 2016. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 30, 1548–1556. ( 10.1096/fj.15-280800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruin JE, Petre MA, Raha S, Morrison KM, Gerstein HC, Holloway AC. 2008. Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS ONE 3, e3371 ( 10.1371/journal.pone.0003371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterside IE, Selak MA, Simmons RA. 2003. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am. J. Physiol. Endocrinol. Metab. 285, E1258–E1266. ( 10.1152/ajpendo.00437.2002) [DOI] [PubMed] [Google Scholar]
- 46.Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden A, Ozanne SE. 2014. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R26–R34. ( 10.1152/ajpregu.00049.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Velasco PC, et al. 2017. Maternal intake of trans-unsaturated or interesterified fatty acids during pregnancy and lactation modifies mitochondrial bioenergetics in the liver of adult offspring in mice. Br. J. Nutr. 118, 41–52. ( 10.1017/S0007114517001817) [DOI] [PubMed] [Google Scholar]
- 48.Shelley P, Martin-Gronert MS, Rowlerson A, Poston L, Heales SJR, Hargreaves IP, Mcconnell JM, Ozanne SE, Fernandez-Twinn DS. 2009. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R675–R681. ( 10.1152/ajpregu.00146.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, Hargreaves IP, Neergheen V, Aiken CE, Ozanne SE. 2016. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis. Model Mech. 9, 1221–1229. ( 10.1242/dmm.026591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCurdy CE, et al. 2016. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 1, e86612 ( 10.1172/jci.insight.86612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borengasser SJ, Faske J, Kang P, Blackburn ML, Badger TM, Shankar K. 2014. In utero exposure to prepregnancy maternal obesity and postweaning high-fat diet impair regulators of mitochondrial dynamics in rat placenta and offspring. Physiol. Genomics 46, 841–850. ( 10.1152/physiolgenomics.00059.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mdaki KS, Larsen TD, Wachal AL, Schimelpfenig MD, Weaver LJ, Dooyema SDR, Louwagie EJ, Baack ML. 2016. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 310, H681–H692. ( 10.1152/ajpheart.00795.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgueno AL, Cabrerizo R, Gonzales Mansilla N, Sookoian S, Pirola CJ. 2013. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J. Nutr. Biochem. 24, 6–13. ( 10.1016/j.jnutbio.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 54.Mayeur S, et al. 2013. Maternal calorie restriction modulates placental mitochondrial biogenesis and bioenergetic efficiency: putative involvement in fetoplacental growth defects in rats. Am. J. Physiol. Endocrinol. Metab. 304, E14–E22. ( 10.1152/ajpendo.00332.2012) [DOI] [PubMed] [Google Scholar]
- 55.Jones AK, Brown LD, Rozance PJ, Serkova NJ, Hay WW Jr, Friedman JE, Wesolowski SR. 2019. Differential effects of intrauterine growth restriction and a hypersinsulinemic-isoglycemic clamp on metabolic pathways and insulin action in the fetal liver. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R427–R440. ( 10.1152/ajpregu.00359.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons RA, Suponitsky-Kroyter I, Selak MA. 2005. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to β-cell failure. J. Biol. Chem. 280, 28 785–28 791. ( 10.1074/jbc.M505695200) [DOI] [PubMed] [Google Scholar]
- 57.Ferreira DJS, Da Silva Pedroza A, Braz GRF, Da Silva-Filho RC, Lima TA, Fernandes MP, Doi SQ, Lagranha CJ. 2016. Mitochondrial bioenergetics and oxidative status disruption in brainstem of weaned rats: immediate response to maternal protein restriction. Brain Res. 1642, 553–561. ( 10.1016/j.brainres.2016.04.049) [DOI] [PubMed] [Google Scholar]
- 58.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, Mcconnell J. 2010. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 5, e10074 ( 10.1371/journal.pone.0010074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu LL, et al. 2015. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 142, 681–691. ( 10.1242/dev.114850) [DOI] [PubMed] [Google Scholar]
- 60.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen J-R, Borengasser SJ, Ronis MJJ, Badger TM. 2011. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 152, 4158–4170. ( 10.1210/en.2010-1078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McPherson NO, Bell VG, Zander-Fox DL, Fullston T, Wu LL, Robker RL, Lane M. 2015. When two obese parents are worse than one! Impacts on embryo and fetal development. Am. J. Physiol. Endocrinol. Metab. 309, E568–E581. ( 10.1152/ajpendo.00230.2015) [DOI] [PubMed] [Google Scholar]
- 62.Saben JL, et al. 2016. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 16, 1–8. ( 10.1016/j.celrep.2016.05.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJJ, Badger TM, Shankar K. 2011. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS ONE 6, e24068 ( 10.1371/journal.pone.0024068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMurray F, MacFarlane M, Kim K, Patten DA, Wei-LaPierre L, Fullerton MD, Harper ME. 2019. Maternal diet-induced obesity alters muscle mitochondrial function in offspring without changing insulin sensitivity. FASEB J. 33, 13 515–13 526. ( 10.1096/fj.201901150r) [DOI] [PubMed] [Google Scholar]
- 65.Korshunov SS, Skulachev VP, Starkov AA. 1997. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18. ( 10.1016/S0014-5793(97)01159-9) [DOI] [PubMed] [Google Scholar]
- 66.D'Autreaux B, Toledano MB. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824. ( 10.1038/nrm2256) [DOI] [PubMed] [Google Scholar]
- 67.Pitkanen S, Robinson BH. 1996. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest. 98, 345–351. ( 10.1172/JCI118798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. 2009. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 37, 2539–2548. ( 10.1093/nar/gkp100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dikalov SI, Harrison DG. 2014. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox. Signal. 20, 372–382. ( 10.1089/ars.2012.4886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham M, Collins CA, Flewelling S, Camazine M, Cahill A, Cade WT, Duncan JG. 2018. Mitochondrial inefficiency in infants born to overweight African-American mothers. Int. J. Obes. 42, 1306–1316. ( 10.1038/s41366-018-0051-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouhours-Nouet N, et al. 2005. Maternal smoking is associated with mitochondrial DNA depletion and respiratory chain complex III deficiency in placenta. Am. J. Physiol. Endocrinol. Metab. 288, E171–E177. ( 10.1152/ajpendo.00260.2003) [DOI] [PubMed] [Google Scholar]
- 72.Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, Wright RO, Baccarelli A, Wright RJ. 2017. Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. Am. J. Epidemiol. 186, 1227–1236. ( 10.1093/aje/kwx183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunst KJ, et al. 2018. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ. Int. 112, 49–58. ( 10.1016/j.envint.2017.12.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clemente DB, et al. 2016. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ. Health Perspect. 124, 659–665. ( 10.1289/ehp.1408981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costa SM, Isganaitis E, Matthews TJ, Hughes K, Daher G, Dreyfuss JM, Da Silva GAP, Patti M-E. 2016. Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int. J. Obes. 40, 1627–1634. ( 10.1038/ijo.2016.142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grevendonk L, Janssen BG, Vanpoucke C, Lefebvre W, Hoxha M, Bollati V, Nawrot TS. 2016. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ. Health 15, 10 ( 10.1186/s12940-016-0095-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruzieva O, et al. 2017. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ. Health Perspect. 125, 104–110. ( 10.1289/EHP36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hastie R, Lappas M. 2014. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 35, 673–683. ( 10.1016/j.placenta.2014.06.368) [DOI] [PubMed] [Google Scholar]
- 79.Janssen BG, Byun H-M, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. 2015. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: an ENVIRONAGE birth cohort study. Epigenetics 10, 536–544. ( 10.1080/15592294.2015.1048412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janssen BG, et al. 2012. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ. Health Perspect. 120, 1346–1352. ( 10.1289/ehp.1104458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, et al. 2015. Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev. 24, 575–586. ( 10.1089/scd.2014.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lambertini L, Chen J, Nomura Y. 2015. Mitochondrial gene expression profiles are associated with maternal psychosocial stress in pregnancy and infant temperament. PLoS ONE 10, e0138929 ( 10.1371/journal.pone.0138929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lassance L, Haghiac M, Leahy P, Minium J, Zhou J, Reider M, Catalano PM, Hauguel-de Mouzon S. 2015. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am. J. Obstet. Gynecol. 212, 647-e1–647-e111. ( 10.1016/j.ajog.2015.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lassance L, Haghiac M, Minium J, Catalano P, Hauguel-De Mouzon S. 2015. Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J. Clin. Endocrinol. Metab. 100, E11–E18. ( 10.1210/jc.2014-2792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, Cetin I. 2008. Higher mitochondrial DNA content in human IUGR placenta. Placenta 29, 1029–1033. ( 10.1016/j.placenta.2008.09.012) [DOI] [PubMed] [Google Scholar]
- 86.Leary C, Leese HJ, Sturmey RG. 2015. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 30, 122–132. ( 10.1093/humrep/deu276) [DOI] [PubMed] [Google Scholar]
- 87.Mando C, et al. 2014. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am. J. Physiol. Endocrinol. Metab. 306, E404–E413. ( 10.1152/ajpendo.00426.2013) [DOI] [PubMed] [Google Scholar]
- 88.Mele J, Muralimanoharan S, Maloyan A, Myatt L. 2014. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 307, E419–E425. ( 10.1152/ajpendo.00025.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Novielli C, Mandò C, Tabano S, Anelli GM, Fontana L, Antonazzo P, Miozzo M, Cetin I. 2017. Mitochondrial DNA content and methylation in fetal cord blood of pregnancies with placental insufficiency. Placenta 55, 63–70. ( 10.1016/j.placenta.2017.05.008) [DOI] [PubMed] [Google Scholar]
- 90.Reimann B, et al. 2019. The cord blood insulin and mitochondrial DNA content related methylome. Front. Genet. 10, 325 ( 10.3389/fgene.2019.00325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosa MJ, et al. 2017. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ. Int. 98, 198–203. ( 10.1016/j.envint.2016.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanchez-Guerra M, et al. 2019. Altered cord blood mitochondrial DNA content and pregnancy lead exposure in the PROGRESS cohort. Environ. Int. 125, 437–444. ( 10.1016/j.envint.2019.01.077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vriens A, et al. 2017. Neonatal exposure to environmental pollutants and placental mitochondrial DNA content: a multi-pollutant approach. Environ. Int. 106, 60–68. ( 10.1016/j.envint.2017.05.022) [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Walsh SW. 1998. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 19, 581–586. ( 10.1016/S0143-4004(98)90018-2) [DOI] [PubMed] [Google Scholar]
- 95.Xu Y, et al. 2019. Associations of blood mercury and fatty acid concentrations with blood mitochondrial DNA copy number in the Seychelles child development nutrition study. Environ. Int. 124, 278–283. ( 10.1016/j.envint.2019.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vriens A, et al. 2018. Cord blood leptin and insulin levels in association with mitochondrial DNA content. J. Transl. Med. 16, 224 ( 10.1186/s12967-018-1599-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Breton CV, et al. 2019. Effects of air pollution on mitochondrial function, mitochondrial DNA methylation, and mitochondrial peptide expression. Mitochondrion 46, 22–29. ( 10.1016/j.mito.2019.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clemente DBP, et al. 2017. Prenatal ambient air pollution exposure, infant growth and placental mitochondrial DNA content in the INMA birth cohort. Environ. Res. 157, 96–102. ( 10.1016/j.envres.2017.05.018) [DOI] [PubMed] [Google Scholar]
- 99.Boyle KE, et al. 2017. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol. Metab. 6, 1503–1516. ( 10.1016/j.molmet.2017.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baker PR, Patinkin ZW, Shapiro ALB, De La Houssaye BA, Janssen RC, Vanderlinden LA, Dabelea D, Friedman JE. 2017. Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Scient. Rep. 7, 18095 ( 10.1038/s41598-017-17588-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, Burelle Y, Epel ES. 2018. A mitochondrial health index sensitive to mood and caregiving stress. Biol. Psychiatry 84, 9–17. ( 10.1016/j.biopsych.2018.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song J, Oh JY, Sung Y-A, Pak YK, Park KS, Lee HK. 2001. Peripheral blood mitochondrial DNA content is related to insulin sensitivity in offspring of type 2 diabetic patients. Diabetes Care 24, 865–869. ( 10.2337/diacare.24.5.865) [DOI] [PubMed] [Google Scholar]
- 103.Hanafi MY, Abdelkhalek TM, Saad MI, Saleh MM, Haiba MM, Kamel MA. 2016. Diabetes-induced perturbations are subject to intergenerational transmission through maternal line. J. Physiol. Biochem. 72, 315–326. ( 10.1007/s13105-016-0483-7) [DOI] [PubMed] [Google Scholar]
- 104.Aiken CE, Tarry-Adkins JL, Ozanne SE. 2015. Transgenerational developmental programming of ovarian reserve. Scient. Rep. 5, 16175 ( 10.1038/srep16175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shoubridge EA, Wai T. 2007. Mitochondrial DNA and the mammalian oocyte. Curr. Top. Dev. Biol. 77, 87–111. ( 10.1016/S0070-2153(06)77004-1) [DOI] [PubMed] [Google Scholar]
- 106.St John J. 2014. The control of mtDNA replication during differentiation and development. Biochim. Biophys. Acta 1840, 1345–1354. ( 10.1016/j.bbagen.2013.10.036) [DOI] [PubMed] [Google Scholar]
- 107.Wang Q, Ratchford AM, Chi MM-Y, Schoeller E, Frolova A, Schedl T, Moley KH. 2009. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 23, 1603–1612. ( 10.1210/me.2009-0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Q, Moley KH. 2010. Maternal diabetes and oocyte quality. Mitochondrion 10, 403–410. ( 10.1016/j.mito.2010.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reynolds KA, Boudoures AL, Chi MM-Y, Wang Q, Moley KH. 2015. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod. Fertil. Dev. 27, 716–724. ( 10.1071/RD14251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Godfrey KM, Gluckman PD, Hanson MA. 2010. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol. Metab. 21, 199–205. ( 10.1016/j.tem.2009.12.008) [DOI] [PubMed] [Google Scholar]
- 111.Gluckman PD, Hanson MA. 2007. Developmental plasticity and human disease: research directions. J. Internal. Med. 261, 461–471. ( 10.1111/j.1365-2796.2007.01802.x) [DOI] [PubMed] [Google Scholar]
- 112.Yakes FM, Van Houten B. 1997. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl Acad. Sci. USA 94, 514–519. ( 10.1073/pnas.94.2.514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Picard M, Vincent AE, Turnbull DM. 2016. Expanding our understanding of mtDNA deletions. Cell Metab. 24, 3–4. ( 10.1016/j.cmet.2016.06.024) [DOI] [PubMed] [Google Scholar]
- 114.Suter M, Ma J, Harris AS, Patterson L, Brown KA, Shope C, Showalter L, Abramovici A, Aagaard-Tillery KM. 2011. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 6, 1284–1294. ( 10.4161/epi.6.11.17819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sahin E, Depinho RA. 2010. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528. ( 10.1038/nature08982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pieters N, Janssen BG, Valeri L, Cox B, Cuypers A, Dewitte H, Plusquin M, Smeets K, Nawrot TS. 2015. Molecular responses in the telomere-mitochondrial axis of ageing in the elderly: a candidate gene approach. Mech. Ageing Dev. 145, 51–57. ( 10.1016/j.mad.2015.02.003) [DOI] [PubMed] [Google Scholar]
- 117.Tyrka AR, Carpenter LL, Kao H-T, Porton B, Philip NS, Ridout SJ, Ridout KK, Price LH. 2015. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp. Gerontol. 66, 17–20. ( 10.1016/j.exger.2015.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Entringer S, De Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: an update. Phil. Trans. R Soc. B 373, 20170151 ( 10.1098/rstb.2017.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D'Onofrio BM, Class Q, Lahey B, Larsson H. 2014. Testing the developmental origins of health and disease hypothesis for psychopathology using family-based quasi-experimental designs. Child Dev. Perspect. 8, 151–157. ( 10.1111/cdep.12078) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.