Abstract
As the most diverse vertebrate group and a major component of a growing global aquaculture industry, teleosts continue to attract significant scientific attention. The growth in global aquaculture, driven by declines in wild stocks, has provided additional empirical demand, and thus opportunities, to explore teleost diversity. Among key developments is the recent growth in microbiome exploration, facilitated by advances in high-throughput sequencing technologies. Here, we consider studies on teleost gut microbiomes in the context of sustainable aquaculture, which we have discussed in four themes: diet, immunity, artificial selection and closed-loop systems. We demonstrate the influence aquaculture has had on gut microbiome research, while also providing a road map for the main deterministic forces that influence the gut microbiome, with topical applications to aquaculture. Functional significance is considered within an aquaculture context with reference to impacts on nutrition and immunity. Finally, we identify key knowledge gaps, both methodological and conceptual, and propose promising applications of gut microbiome manipulation to aquaculture, and future priorities in microbiome research. These include insect-based feeds, vaccination, mechanism of pro- and prebiotics, artificial selection on the hologenome, in-water bacteriophages in recirculating aquaculture systems (RAS), physiochemical properties of water and dysbiosis as a biomarker.
Keywords: fish, teleost, gut, microbiome, aquaculture, review
1. Introduction
Since its conception in the 1980s describing soil ecology [1], the term microbiome has evolved into an intensely studied area of research. In recent decades, this area has begun expanding from an anthropocentric and medically dominated field, into a taxonomically broad field, examining research questions in non-model species, from trees [2] to frogs [3], and increasingly, fish. The diversification in microbiome studies has been driven by increased access to next generation sequencing (NGS), a tool that is not reliant upon culture-based techniques, which often require previous knowledge of target microbes.
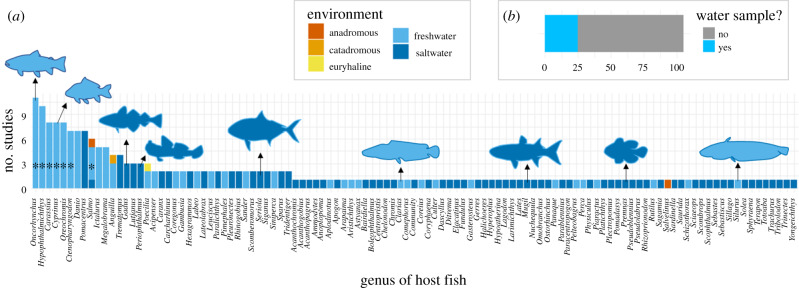
Currently, gut bacterial communities have been assessed in over 145 species of teleosts from 111 genera, representing a diverse range of physiology and ecology (figure 1a), often with similarities in bacterial phyla composition between fish species, dominated by Bacteroidetes and Firmicutes [5,6]. Non-model taxa from an array of aquatic ecosystems have had their gut microbiomes sequenced using NGS, with studies extending beyond species identification, into hypothesis testing which was once only feasible in model systems. Examples of studies on non-model teleost gut microbiomes range from those demonstrating rapid gut microbiome restructuring after feeding in clownfish (Premnas biaculeatus) [7] to the effect of differing environmental conditions, such as dissolved oxygen content, on the gut microbial diversity of blind cave fish (Astyanax mexicanus) [8]. Interest in the gut microbiome of fish has accelerated for many reasons, as not only do teleosts represent the most diverse vertebrate group [9], they are also of significant economic importance, including in aquaculture [10]. Aquaculture now provides over 45% of fish-based food products globally [11], and influence of the aquaculture industry on teleost gut microbiome research is demonstrated by the research questions tackled, with a clear bias towards salmonids (genera: Oncorhynchus and Salmo), carp (genera: Hypophthalmichthys, Carassius, Cyprinus and Ctenopharyngodon) and tilapia (genus: Oreochromis) (figure 2).
Figure 1.
(a) Number of studies on the gut microbiome using NGS broken down by the genus of fish that the study was conducted on, as well as the environment those fish same from. Asterisk represents salmonid, carp and talapia. (b) The number of studies that assessed the water microbial communities. Gut microbiome studies were compiled using Web of Science [4] and only include studies that implemented NGS. It is acknowledged that total microbiome research extends further than this. Further information on search terms and filtering can be found in the electronic supplementary material. (Online version in colour.)
Figure 2.
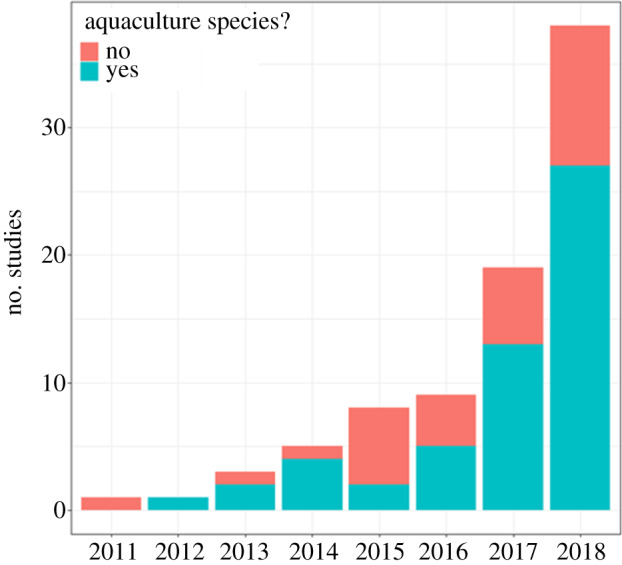
Growth in the studies using NGS on fish gut microbiomes, including food aquaculture species (aquaculture status taken from FishBase [12]). Further information on search terms and filtering can be found in the electronic supplementary material. (Online version in colour.)
Rapid growth of the aquaculture industry has led to mounting pressure to make it more sustainable [13], and here we discuss four key components relevant to its sustainability in the context of the teleost gut microbiome: diet, immunity, artificial selection and closed-loop systems. We highlight some key deterministic factors important to aquaculture, although as shown in figure 3, there are numerous interacting ecological processes. More in-depth reviews focusing on these specific interactions are available, for example, interactions between the gut microbiome and the immune system [14], energy homeostasis [15] and physiology [16]. Understanding and manipulating microbial–host–environmental interactions (figure 3a) and associated functional capacity in these areas could contribute substantially towards achieving a more sustainable aquaculture industry. We identify potential for future research, both methodological and conceptual. Other microbiomes are known to impact host function, in particular, the skin microbiome and its relationship to immunity [17], however, due to their differing ecology [18] and aquaculture applications [19], the gut microbiome will remain our focus here.
Figure 3.
(a) Schematic view of the deterministic processes that influence gut microbial communities in fish. Community assemblage of bacteria in the gut starts with inputs from the environment (green), such as the bacteria within the water column, or in solid particulates of biofilm, sediment and feed. Once ingested, these bacteria are influenced by interacting deterministic processes (brown) such as the host's abiotic gut environment, interaction with the hosts' physiology through the gut lining and its secretions, as well as interactions between other microbiomes. The outcome (red) is final community assembly, which can be characterized using an array of cutting-edge molecular techniques (purple). A subset of the boarder interactions is provided, with focus on (b) microbe–environment–host interactions, (c) host gut physiology and (d) behaviour. (Online version in colour.)
2. Diet
The gut microbiome has long been linked with diet, yielding insights into the commensal relationship between certain microbes and host. It has been shown that the teleost gut microbiome produces a range of enzymes (carbohydrases, cellulases, phosphatases, esterases, lipases and proteases) which contribute to digestion [10,20]. More intimate relationships also exist, for example, anaerobic bacteria in the teleost gut have a role in supplying the host with volatile fatty acids [21], an end product of anaerobic fermentation that provides energy for intestinal epithelial cells [22]. Gut microbes also synthesize vitamins and amino acids in the gut of aquatic vertebrates [23,24]. For example, the amount of vitamin B12 positively correlated with the abundance of anaerobic bacteria belonging to the genera Bacteroides and Clostridium, in Nile tilapia (Oreochromis niloticus) [25]. Here, we discuss this host–microbe relationship in the context of contemporary aquaculture, with a focus on two timely issues: fishmeal and starvation.
(a). Fishmeal
Fishmeal is an efficient energy source containing high-quality protein, as well as highly digestible essential amino and fatty acids [26], which is included in feed for a range of teleost species. Fish used in fishmeal production is, however, predominantly sourced from capture fisheries, putting pressure on already overfished stocks [13]. Despite a global decrease in fishmeal production, from an average of 6.0 million tonnes between 2001 and 2005 to 4.9 million tonnes between 2006 and 2010 [27], and growth in plant-based substitutes (e.g. wheat gluten, soya bean protein and pea protein), some aquaculture species still require a proportion of fish-sourced amino acids and proteins [28].
As dietary changes can alter the fish gut microbiome [29], there has been a considerable rise in the number of studies investigating the influence of alternative plant-protein sources on host–microbe interactions. Plant-protein sources have been shown to disturb the gut microbiota of some fish, with the production of antinutritional factors (factors that reduce the availability of nutrients) and antigens, impeding host resilience to stress [30], metabolism [31] and immune functioning [32]. Fish fed plant-protein-based diets can exhibit alterations in their intestinal morphology including disruption to the lamina propria and mucosal folds [33], which may modify attachment sites for commensal bacteria [34], and can therefore impact microbial composition [32,35].
Insect meal is increasingly used in aquafeed as a protein source with a high nutritional value [36], and several studies have demonstrated its potential use in manipulating the gut microbiome in fish [37,38]. As insects are chitin rich, these diets have been associated with prebiotic effects, through increased representation of beneficial commensal bacteria such as Pseudomonas sp. and Lactobacillus sp., which in turn improves performance and health in some fish [37]. Despite this, however, the beneficial effects of chitin are species specific, with Atlantic cod (Gadus morhua) and several cyprinid species demonstrating increased growth rates on diets with varying levels of chitin, whereas tilapia hybrids (O. niloticus × O. aureus) and rainbow trout (Oncorhynchus mykiss) both display decreased growth rates [39]. Chitin can therefore not be described as a probiotic for all species. The influence of insect meal on microbial-mediated functions also remains underexplored, with little known about the extent to which species-specific responses to a chitin-rich diet are microbially mediated [40], offering scope for future research.
(b). Starvation
Starvation is common in the production of valuable species such as salmon [41], sea bream [42], halibut [43] and cod [44], prior to handling, transportation and harvest, but is also used as a method to improve fillet quality. However, starvation is likely to have a substantial impact on host–microbe interactions (figure 3b). Gut microbial communities of the Asian seabass (Lates calcarifer), for example, shifted markedly in response to an 8-day starvation period, causing enrichment of the phylum Bacteroidetes, but a reduction of Betaproteobacteria, resulting in transcriptional changes in both host and microbial genes [45]. Perturbation to the gut microbiome could lead to the opening of niches for other commensal or even pathogenic bacteria [46], especially if this is combined with the compromised immune system of a stressed host [47] (figure 3d). Even if all fish are terminated shortly after starvation, gut microbial community changes before termination could cause long-term impacts to the microbial composition of water and biofilters in closed recirculating aquaculture systems (RAS). RAS systems will be discussed in greater detail later in this review.
3. Immunity
Gut microbial communities have strong links to immunity [48], which is pertinent in fish as they are in constant contact with water, a source of pathogenic and opportunistic commensal microbes [49]. In addition to this, fish cultured intensively are often stocked at high densities, allowing for easier transmission of microbes. Therefore, a microbially diverse gut microbiome in aquaculture is important to prevent unfavourable microbial colonization [50], and although the mechanisms are not fully understood, some key processes have been identified. For example, Bacillus and Lactobacillus, two common probiotic genera of bacteria used in aquaculture, are able to stimulate expression of inflammatory cytokines in the fish gut [51], increase the number of mucus layer producing goblet cells [52] and increase phagocytic activity [53]. Furthermore, comparison in gene expression between gnotobiotic zebrafish (Danio rerio) and conventionally reared zebrafish has shown bacteria induced expression of myeloperoxidase, an enzyme that allows neutrophil granulocytes to carry out antimicrobial activity [54]. Colonizing microbes can also modulate host gene expression to create favourable gut environments, thereby constraining invasion by pathogens [23], while also promoting expression of proinflammatory and antiviral mediators genes, leading to higher viral resistance [55]. Reducing viral and bacterial pathogens, such as Vibrio sp. and Aeromonas sp., is important for fish health in aquaculture, and will be discussed further in the context of closed-loop systems later in the review.
The interaction between the gut microbiome and the immune system is bilateral, for example, secretory immunoglobulins in fish recognize and coat intestinal bacteria to prevent them from invading the gut epithelium [56]. Similarly, in wild three-spined stickleback (Gasterosteus aculeatus), a causal chain (diet → immunity → microbiome) was discovered, demonstrating the impact of diet on fish immunity and thus the microbial composition of the gut [57]. Understanding microbial–host–environmental interactions like this are crucial for aquaculture, where, as previously discussed, diet is often manipulated.
(a). Antibiotics
As most antibiotics used in aquaculture display broad-spectrum activity, they can affect both pathogens and non-target commensal microbes [58]. Oxytetracycline is one of the most widely used veterinary antibiotics, with 1500 metric tonnes applied between 2000 and 2008 to salmon aquaculture in Chile [59]. However, oxytetracycline was seen to reduce gut microbial diversity in Atlantic salmon (Salmo salar), while enriching possible opportunistic pathogens belonging to the genus Aeromonas, and leading to a high prevalence of multiple tetracycline resistance-encoding bacterial genes [60]. Long-term exposure to oxytetracycline has also been reported to negatively affect growth, immunity and nutrient digestion/metabolism in Nile tilapia (O. niloticus) through antibiotic-induced disruption to the microbiota [61], causing considerable changes in the representation of Bacteroidetes and Firmicutes.
Vaccination has become a widespread prophylactic measure applied in aquaculture to improve immune functioning and disease resilience in farmed fish [62]. One study attempted to identify potential alterations in the microbiota structure and localized immune responses caused by a novel recombinant vaccine against Aeromonas hydrophila in grass carp (Ctenopharyngodon idella) [63]. Results from their study suggest that oral vaccines can target Aeromonas sp. through activation of innate and adaptive immune defences within the intestine without causing large disturbances in non-target microbiota populations. Given the importance of the immune response in regulating the gut microbiome [64], only a small number of studies have investigated the influence of vaccines on the resident microbiota composition and function in fish, providing grounds for future study.
(b). Pro- and prebiotic supplementation
In view of the challenges associated with antibiotics, studies have examined the impact of alternative, prophylactic measures such as pro- and prebiotics (figure 4a). As literature on the types of pro- and prebiotics used in aquaculture have been reviewed elsewhere [65,66], as well as their effectiveness [67,68], we focus here on the ability of these compounds to induce changes in host physiology and function through shifts in the gut microbiome. As has already been discussed, Bacillus sp. and Lactobacillus sp. have a beneficial effect on immunity and are suggested to provide an alternative approach to controlling disease in aquaculture. Targeted microbiota manipulation using these same bacteria have also been reported to exert beneficial effects on fish growth through (i) alterations in gut morphology [69], leading to improved digestion and metabolism [70] and (ii) microbial-mediated regulation of the genetic components involved in growth and appetite control [71,72]. Recently, the establishment of Lactobacillus probiotic bacteria within the gut microbiota was also associated with improved learning/memory capacity and changes in shoaling of zebrafish [73,74], indicating a potential gut–brain interaction pathway similar to what is described in higher vertebrates [75].
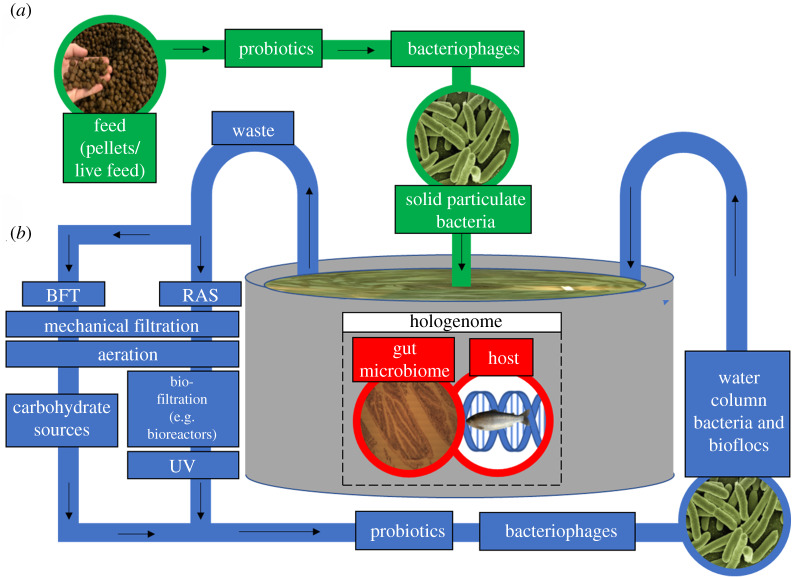
Figure 4.
Schematic diagram of (a) feed inputs (green), (b) water processing (both RAS and BFT) (blue) and the (c) species being cultivated, along with its gut microbiome (red). (Online version in colour.)
Research into the modulation of gut microbial communities using prebiotic compounds has expanded also. Certain dietary components have been reported to induce changes in gut morphology within the fish host, including vacuolation of enterocytes [76] and enhancing mucosal barrier integrity [77]. Improved mucosal protection and disease resilience are thought to be driven by microbes and associated microbial metabolites. Several prebiotics have been reported to manipulate the resident microbiota community of a host in favour of Firmicutes and short-chain fatty acid producing communities [78]. Mechanistic pathways remain elusive, however, with additional research required.
4. Artificial selection
Within aquaculture, selection has been applied routinely to increase production by enhancing desirable traits such as growth and disease resilience [79,80]. Recent evidence suggests, however, that host genetics plays a fundamental role in determining the gut microbiota in fish [81]. The ‘hologenome’ concept proposes that the host organism, along with their commensal microbial community, form one unit of selection [82]. Host physiology, for example, is determined in part by the host's genome and has the ability to shift gut microbiome composition, as demonstrated in zebrafish, whereby host neural activity and subsequent gut motility is able to destabilize microbial communities [46] (figure 3c). Although not described in teleosts, the reverse has also been seen, whereby microbial communities are able to regulate the host's gut through: (i) serotonin signalling [83,84], (ii) macrophages and enteric neurons interactions [85], (iii) metabolism of bile salts [86] and possibly, (iv) metabolism of short-chain fatty acids such as butyrate [87]. The host–microbe relationship means that traits selected during breeding programmes may be traits from the hologenome. Pyrosequencing studies have also shown significant changes in the microbial community composition of genetically improved fish compared with domesticated individuals [88,89]. Artificial selection has also been demonstrated on single species of bacteria, with Aeromonas veronii selected to exhibit greater colonization success in gnotobiotic zebrafish [90]. Environmental filtering of the reservoir of bacteria surrounding the fish generates the potential for improving colonization success of commensal bacteria. Currently, bacterial communities selected by breeding programmes could be neutral, sympathetic or antagonistic to the goals of artificial selection, and understanding this relationship will be vital in manipulating the hologenome.
5. Closed aquaculture systems
Many environmental problems plague current aquaculture practices. In addition to those already discussed, there are also issues with parasite transmission to wild fish [91], interactions between wild and escaped farmed fish [92], and release of faeces and excess feed into the environment [93]. One way to better control these problems is to remove aquaculture from ecosystems and bring it into a land-based setting [94].
(a). Manipulating environmental microbiota
RAS and biofloc technology (BFT) are forms of aquaculture which use microbial communities to minimize excess nutrients and pathogens in rearing water (figure 4). In these systems, microbial reconditioning of the rearing water is vital as fish are stocked at high densities, resulting in elevated levels of organic material, which can promote microbial growth [95]. Selection of competitive, slow-growing K-strategist bacteria shifts the community from autotrophy to heterotrophy activity. Such shifts allow for a microbial community which maintains both water quality, through nutrient recycling, and inhibits the growth of fast-growing, opportunistic r-strategists, which include many bacterial pathogens such as Aeromonas sp. [96,97]. RAS and BFT could therefore be combined with vaccination against bacterial pathogens such as Aeromonas sp., as previously discussed, to reduce infections. The selection of K-strategist microbial communities differ between RAS and BFT. In RAS; K-selection is achieved by passing rearing water through heterotrophic biofilters [98], whereas in BFT, a high carbon to nitrogen ratio within rearing water is conditioned by the addition of carbohydrate sources, favouring heterotrophic K-strategist bacteria [99]. High-carbon conditions in BFT systems also promote nitrogen uptake into microbial biomass, which forms protein-rich bacterial ‘flocs’ that supplement feed [100].
Manipulation of microbes associated with live feed cultures is critical to the production of fish larvae as live feeds often contain opportunistic pathogens (figure 4a), resulting in stochastic mortality [64]. While traditional approaches involve non-selective, temporary methods (i.e. physical/chemical disinfection [101]), more recent efforts have shifted towards targeted manipulation through probiotics, for example, the successful use of Phenylobacterium sp., Gluconobacter sp. and Paracoccus denitrificans in rotifer (Brachionus plicatilis) production [102]. Lytic bacteriophages have also proven somewhat successful in reducing the prevalence of opportunistic pathogens, such as Vibrio sp. [103–105]. Live feed also appears to play a critical role in the delivery and establishment of colonizing gut microbiota in fish larvae upon first feeding [106]. Supplementation of live feed cultures with beneficial microbes, such as the previously mentioned Lactobacillus spp. and Pediococcus sp., has become common practice in hatcheries, with beneficial effects on growth, mucosal immunity and stress tolerance of larvae [17,107,108]. Bacteriophages and probiotics have also been applied directly to tank water (figure 4b); probiotics such as Bacillus spp. preventing fish mortality from Vibrio spp. infections [109] and Flavobacterium columnare-infecting phages have been shown to persist in RAS for up to 21 days [110]. Far less is known about the application of probiotics directly to tank water when compared with feed application [111]; however, and the use of bacteriophages is still in its infancy, providing potential for future research.
(b). Controlling environmental variables
Changes in abiotic conditions in the water column propagate into the gut, as seen with dissolved oxygen concentration [8]. Such parameters are hard to control within the natural environment, but closed-loop systems provide consistent abiotic conditions, and allow for other variables, such as hologenome (figure 4c), to be manipulated with greater ease. The effect of many important physiochemical water properties (e.g. nitrate, ammonia and phosphate) on the teleost gut microbiome has not been studied, however, let alone how these properties interact [112]. Salinity is another important physiochemical property for the gut microbiome in many aquaculture species. When Atlantic salmon transition from freshwater to saltwater, individuals can experience a 100-fold increase in gut bacteria, combined with a shift in dominant microbial taxa [113]. Increasing salinity in RAS systems can, however, negatively impact nitrate removal in bioreactors [114], highlighting the importance of understanding interacting physiochemical properties.
(c). Dysbiosis as a stress biomarker
The use of closed-loop systems is a progression to a more intensive method of aquaculture, mirroring the progression seen in animal agriculture, and a crucial element to sustainable intensification is welfare. It is possible to measure fish welfare through physiological and behavioural indicators, with a current focus on identifying stress. The microbiome has been identified as another potential biomarker [64] due to its interaction with the host immune system, and its responsive nature to stressors [115,116]. Therefore, identifying imbalances in the gut microbiome, or dysbiosis, could be a useful predictor of stress-related syndromes, which could ultimately lead to mortality. Using non-invasive faecal samples could complement other non-invasive stress biomarkers, such as water cortisol [117], allowing for the optimization of husbandry, alerting operators to chemical (e.g. poor water quality, diet composition imbalance, accumulation of wastes), biological (e.g. overcrowding, social dominance, pathogens), physical (e.g. temperature, light, sounds, dissolved gases) or procedural (e.g. handling, transportation, grading, disease treatment) stressors [118]. More research is needed, however, in assessing the reliability and accuracy of faecal microbiome sampling in identifying stress.
6. Conclusion and future applications
The teleost gut microbiome has a clear role in the future of aquaculture, and although research has come a long way in recent decades, there are still many areas of gut microbiome research that require further development. As highlighted in figure 1b, there are still key elements lacking from many studies, particularly those assessing metacommunity composition, with the lack of water samples being particularly glaring. The ability to sample the environmental metacommunity with ease is one of the strengths of using a teleost model. Another methodological problem that will hinder comparability, reproducibility and metanalysis of fish gut microbiome datasets is the varying degree of sequencing platforms and markers (figure 5). A solution to this problem would be to focus on one marker, and one sequencing platform, with many metabarcoding microbiome studies adopting the V3 and V4 regions, sequenced on Illumina platforms. It is noted, however, that different markers and sequencing platforms work better in some systems with no simple fit-all approach. Therefore, tools that incorporate differences in taxonomic identification that arise through using different methodological approaches will be vital in comparing datasets.
Figure 5.
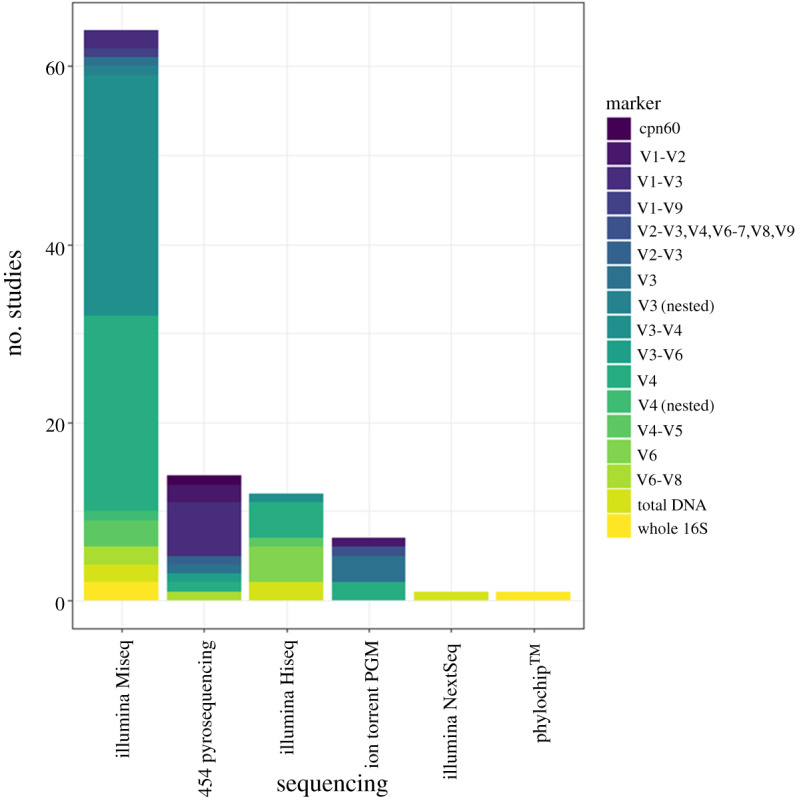
Methodological approaches used in high-throughput sequencing of fish gut microbiomes, broken down by the type of sequencing platform and genetic marker. Marker types are predominantly variable regions (V) within the 16S ribosomal RNA gene. Further information on search terms and filtering can be found in the electronic supplementary material. (Online version in colour.)
Current findings, as summarized here, show that the teleost gut microbiome plays an important role in aquaculture, however, the literature is dominated with studies performed on mammals, leading to limited data on functional capacity of fish gut microbiomes [64]. Furthermore, a knowledge gap exists between ascertaining the composition of the microbiome and understanding its function, partly due to the complexity and variability in the ecology of teleost gastrointestinal tracts [119] and unknown bacterial taxa. More specifically, however, it has been caused by the lack of synthesis between multiple cutting-edge molecular techniques. Progression in teleost gut microbiome research will depend on combining function (RNA sequencing), composition (metabarcoding and metagenomics) and spatial distribution (fluorescence in situ hybridization). Understanding host genetic diversity (population genomics) and expression (RNA sequencing) of that diversity, all while incorporating environmental variation, will also be vital.
Finally, there are many areas in which synergies between gut microbiomes and aquaculture can be made. These have been highlighted through the review, but, in summary, include a better understanding of the gut microbiome with respect to insect-based feeds, vaccination, mechanism of pro- and prebiotics, artificial selection on the hologenome, in-water bacteriophages in RAS/BFT, physiochemical properties of water and dysbiosis as a biomarker.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to Professor Gary Carvalho and Dr Martin Llewellyn for providing feedback on the review. This work was supported by the Natural Environment Research Council.
Data accessibility
All data collected in the systematic review can be found in the electronic supplementary material.
Authors' contributions
W.B.P. organized the creation of the review and facilitated communications between authors. W.B.P., E.L., R.K., C.J.P. and C.B. contributed to the concept and writing of the review.
Competing interests
No competing interests.
Funding
This study was funded by Natural Environment Research Council.
References
- 1.Whipp J, Lewis K, Cooke R. 1987. Fungi in biological control systems. Manchester, UK: Manchester University Press. [Google Scholar]
- 2.Denman S, et al. 2018. Microbiome and infectivity studies reveal complex polyspecies tree disease in Acute Oak Decline. ISME J. 12, 386–399. ( 10.1038/ismej.2017.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohl KD, Cary TL, Karasov WH, Dearingy MD. 2015. Larval exposure to polychlorinated biphenyl 126 (pcb-126) causes persistent alteration of the amphibian gut microbiota. Env. Toxicol. Chem. 34, 1113–1118. ( 10.1002/etc.2905) [DOI] [PubMed] [Google Scholar]
- 4.Reuters T. 2012. Web of Science Service for UK Education. See https://wok.mimas.ac.uk.
- 5.Sullam KE, Essinger SD, Lozupone CA, O'connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. 2012. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol. Ecol. 21, 3363–3378. ( 10.1111/j.1365-294X.2012.05552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Givens C, Ransom B, Bano N, Hollibaugh J. 2015. Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar. Ecol. Prog. Ser. 518, 209–223. ( 10.3354/meps11034) [DOI] [Google Scholar]
- 7.Parris DJ, Morgan MM, Stewart FJ. 2019. Feeding rapidly alters microbiome composition and gene transcription in the clownfish gut. Appl. Environ. Microbiol. 85, e02479-18. ( 10.1128/AEM.02479-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ornelas-García P, Pajares S, Sosa-Jiménez VM, Rétaux S, Miranda-Gamboa RA. 2018. Microbiome differences between river-dwelling and cave-adapted populations of the fish Astyanax mexicanus (De Filippi, 1853). PeerJ 2018, e5906 ( 10.7717/peerj.5906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravi V, Venkatesh B. 2008. Rapidly evolving fish genomes and teleost diversity. Curr. Opin. Genet. Dev. 18, 544–550. ( 10.1016/J.GDE.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Ren Y, Peng C, Hao Y, Xiong F, Wang G, Li W, Zou H, Angert ER. 2015. Metatranscriptomic discovery of plant biomass-degrading capacity from grass carp intestinal microbiomes. FEMS Microbiol. Ecol. 91, fiv107 ( 10.1093/femsec/fiv107) [DOI] [PubMed] [Google Scholar]
- 11.Longo SB, Clark B, York R, Jorgenson AK. 2019. Aquaculture and the displacement of fisheries captures. Conserv. Biol. 33, cobi.13295 ( 10.1111/cobi.13295) [DOI] [PubMed] [Google Scholar]
- 12.Froese R, Pauly D (eds). 2000. FishBase 2000: concepts, design and data sources. Laguna, Philippines: ICLARM. [Google Scholar]
- 13.Naylor RL, et al. 2000. Effect of aquaculture on world fish supplies. Nature 405, 1017–1024. ( 10.1038/35016500) [DOI] [PubMed] [Google Scholar]
- 14.Kelly C, Salinas I. 2017. Under pressure: interactions between commensal microbiota and the teleost immune system. Front. Immunol. 8, 559 ( 10.3389/fimmu.2017.00559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butt RL, Volkoff H. 2019. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. 10, 9 ( 10.3389/fendo.2019.00009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, Arockiaraj J. In press. Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. raq.12416 ( 10.1111/raq.12416) [DOI] [Google Scholar]
- 17.Azimirad M, Meshkini S, Ahmadifard N, Hoseinifar SH. 2016. The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immunol. 54, 516–522. ( 10.1016/j.fsi.2016.05.001) [DOI] [PubMed] [Google Scholar]
- 18.Sylvain FÉ, Cheaib B, Llewellyn M, Gabriel Correia T, Barros Fagundes D, Luis Val A, Derome N. 2016. PH drop impacts differentially skin and gut microbiota of the Amazonian fish tambaqui (Colossoma macropomum). Sci. Rep. 6, 32032 ( 10.1038/srep32032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llewellyn MS, et al. 2017. Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci. Rep. 7, 43465 ( 10.1038/srep43465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray AK, Ghosh K, Ringø E. 2012. Enzyme-producing bacteria isolated from fish gut: a review. Aquac. Nutr. 18, 465–492. ( 10.1111/j.1365-2095.2012.00943.x) [DOI] [Google Scholar]
- 21.Ramirez RF, Dixon BA. 2003. Enzyme production by obligate intestinal anaerobic bacteria isolated from oscars (Astronotus ocellatus), angelfish (Pterophyllum scalare) and southern flounder (Paralichthys lethostigma). Aquaculture 227, 417–426. ( 10.1016/S0044-8486(03)00520-9) [DOI] [Google Scholar]
- 22.Clements KD. 1997. Fermentation and gastrointestinal microorganisms in fishes. In Gastrointestinal microbiology (eds Mackie RI, White BA), pp. 156–198. Boston, MA: Springer. [Google Scholar]
- 23.Balcázar JL, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL. 2006. The role of probiotics in aquaculture. Vet. Microbiol. 113, 173–186. ( 10.1016/j.vetmic.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 24.Nayak SK. 2010. Role of gastrointestinal microbiota in fish. Aquac. Res. 41, 1553–1573. ( 10.1111/j.1365-2109.2010.02546.x) [DOI] [Google Scholar]
- 25.Sugita H, Miyajima C, Deguchi Y. 1990. The vitamin B12-producing ability of intestinal bacteria isolated from tilapia and channel catfish. Nippon SUISAN GAKKAISHI 56, 701 ( 10.2331/suisan.56.701) [DOI] [Google Scholar]
- 26.Cho JH, Kim IH. 2011. Fish meal: nutritive value. J. Anim. Physiol. Anim. Nutr. 95, 685–692. ( 10.1111/j.1439-0396.2010.01109.x) [DOI] [PubMed] [Google Scholar]
- 27.Shepherd CJ, Jackson AJ. 2013. Global fishmeal and fish-oil supply: inputs, outputs and markets. J. Fish Biol. 83, 1046–1066. ( 10.1111/jfb.12224) [DOI] [PubMed] [Google Scholar]
- 28.Pratoomyot J, Bendiksen EÅ, Bell JG, Tocher DR. 2010. Effects of increasing replacement of dietary fishmeal with plant protein sources on growth performance and body lipid composition of Atlantic salmon (Salmo salar L.). Aquaculture 305, 124–132. ( 10.1016/j.aquaculture.2010.04.019) [DOI] [Google Scholar]
- 29.Ingerslev HC, Strube ML, von Jørgensen LG, Dalsgaard I, Boye M, Madsen L. 2014. Diet type dictates the gut microbiota and the immune response against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 40, 624–633. ( 10.1016/j.fsi.2014.08.021) [DOI] [PubMed] [Google Scholar]
- 30.Batista S, Ozório ROA, Kollias S, Dhanasiri AK, Lokesh J, Kiron V, Valente LMP, Fernandes JMO. 2016. Changes in intestinal microbiota, immune- and stress-related transcript levels in Senegalese sole (Solea senegalensis) fed plant ingredient diets intercropped with probiotics or immunostimulants. Aquaculture 458, 149–157. ( 10.1016/j.aquaculture.2016.03.002) [DOI] [Google Scholar]
- 31.Gatesoupe FJ, Fauconneau B, Deborde C, Madji Hounoum B, Jacob D, Moing A, Corraze G, Médale F. 2018. Intestinal microbiota in rainbow trout, Oncorhynchus mykiss, fed diets with different levels of fish-based and plant ingredients: a correlative approach with some plasma metabolites. Aquac. Nutr. 24, 1563–1576. ( 10.1111/anu.12793) [DOI] [Google Scholar]
- 32.Miao S, Zhao C, Zhu J, Hu J, Dong X, Sun L. 2018. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 8, 1–10. ( 10.1038/s41598-017-18430-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Tao Q, Wang Z, Mai K, Xu W, Zhang Y, Ai Q. 2017. Effects of fish meal replacement by soybean meal with supplementation of functional compound additives on intestinal morphology and microbiome of Japanese seabass (Lateolabrax japonicus). Aquac. Res. 48, 2186–2197. ( 10.1111/are.13055) [DOI] [Google Scholar]
- 34.Ringø E, Gatesoupe F-J. 1998. Lactic acid bacteria in fish: a review. Aquaculture 160, 177–203. ( 10.1016/S0044-8486(97)00299-8) [DOI] [Google Scholar]
- 35.Desai AR, Links MG, Collins SA, Mansfield GS, Drew MD, Van Kessel AG, Hill JE. 2012. Effects of plant-based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 350–353, 134–142. ( 10.1016/j.aquaculture.2012.04.005) [DOI] [Google Scholar]
- 36.Magalhães R, Sánchez-López A, Leal RS, Martínez-Llorens S, Oliva-Teles A, Peres H. 2017. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 476, 79–85. ( 10.1016/j.aquaculture.2017.04.021) [DOI] [Google Scholar]
- 37.Bruni L, Pastorelli R, Viti C, Gasco L, Parisi G. 2018. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 487, 56–63. ( 10.1016/j.aquaculture.2018.01.006) [DOI] [Google Scholar]
- 38.Huyben D, Vidaković A, Werner Hallgren S, Langeland M. 2019. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 500, 485–491. ( 10.1016/j.aquaculture.2018.10.034) [DOI] [Google Scholar]
- 39.Ringø E, Zhou Z, Olsen RE, Song SK. 2012. Use of chitin and krill in aquaculture: the effect on gut microbiota and the immune system: a review. Aquac. Nutr. 18, 117–131. ( 10.1111/j.1365-2095.2011.00919.x) [DOI] [Google Scholar]
- 40.Fines BC, Holt GJ. 2010. Chitinase and apparent digestibility of chitin in the digestive tract of juvenile cobia, Rachycentron canadum. Aquaculture 303, 34–39. ( 10.1016/j.aquaculture.2010.03.010) [DOI] [Google Scholar]
- 41.Waagbø R, Jørgensen SM, Timmerhaus G, Breck O, Olsvik PA. 2017. Short-term starvation at low temperature prior to harvest does not impact the health and acute stress response of adult Atlantic salmon. PeerJ 5, e3273 ( 10.7717/peerj.3273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginés R, Palicio M, Zamorano MJ, Argüello A, López JL, Afonso JM. 2003. Starvation before slaughtering as a tool to keep freshness attributes in gilthead sea bream (Sparus aurata). Aquac. Int. 10, 379–389. ( 10.1023/A:1023365025292) [DOI] [Google Scholar]
- 43.Foss A, Imsland AK, Vikingstad E, Stefansson SO, Norberg B, Pedersen S, Sandvik T, Roth B. 2009. Compensatory growth in Atlantic halibut: effect of starvation and subsequent feeding on growth, maturation, feed utilization and flesh quality. Aquaculture 290, 304–310. ( 10.1016/j.aquaculture.2009.02.021) [DOI] [Google Scholar]
- 44.Bjørnevik M, Hansen H, Roth B, Foss A, Vikingstad E, Solberg C, Imsland AK. 2017. Effects of starvation, subsequent feeding and photoperiod on flesh quality in farmed cod (Gadus morhua). Aquac. Nutr. 23, 285–292. ( 10.1111/anu.12391) [DOI] [Google Scholar]
- 45.Xia J, et al. 2014. The intestinal microbiome of fish under starvation. BMC Genomics 15, 266 ( 10.1186/1471-2164-15-266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiles TJ, et al. 2016. Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol. 14, e1002517 ( 10.1371/journal.pbio.1002517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellison AR, Uren Webster TM, Rey O, Garcia de Leaniz C, Consuegra S, Orozco-terWengel P, Cable J.. 2018. Transcriptomic response to parasite infection in Nile tilapia (Oreochromis niloticus) depends on rearing density. BMC Genomics 19, 723 ( 10.1186/s12864-018-5098-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raulo A, et al. 2018. Social behaviour and gut microbiota in red-bellied lemurs (Eulemur rubriventer): in search of the role of immunity in the evolution of sociality. J. Anim. Ecol. 87, 388–399. ( 10.1111/1365-2656.12781) [DOI] [PubMed] [Google Scholar]
- 49.Ellis AE. 2001. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 25, 827–839. ( 10.1016/S0145-305X(01)00038-6) [DOI] [PubMed] [Google Scholar]
- 50.Balcázar JL, Decamp O, Vendrell D, De Blas I, Ruiz-Zarzuela I.. 2006. Health and nutritional properties of probiotics in fish and shellfish. Microb. Ecol. Health Dis. 18, 65–70. ( 10.1080/08910600600799497) [DOI] [Google Scholar]
- 51.He S, Ran C, Qin C, Li S, Zhang H, De Vos WM, Ringø E, Zhou Z.. 2017. Anti-infective effect of adhesive probiotic lactobacillus in fish is correlated with their spatial distribution in the intestinal tissue. Sci. Rep. 7, article 13195 ( 10.1038/s41598-017-13466-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popovic NT, et al. 2017. The effects of diet supplemented with Lactobacillus rhamnosus on tissue parameters of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 48, 2388–2401. ( 10.1111/are.13074) [DOI] [Google Scholar]
- 53.Chen SW, Liu CH, Hu SY. 2019. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 84, 59 ( 10.1016/j.fsi.2018.10.059) [DOI] [PubMed] [Google Scholar]
- 54.Rawls JF, Samuel BS, Gordon JI. 2004. From the cover: gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl Acad. Sci. USA 101, 4596–4601. ( 10.1073/pnas.0400706101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galindo-Villegas J, Garcia-Moreno D, de Oliveira S, Meseguer J, Mulero V.. 2012. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc. Natl. Acad. Sci. USA 109, E2605–E2614. ( 10.1073/pnas.1209920109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, Lapatra SE, Bartholomew J, Sunyer JO. 2010. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 11, 827–835. ( 10.1038/ni.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friberg IM, Taylor JD, Jackson JA. 2019. Diet in the driving seat: natural diet-immunity-microbiome interactions in wild fish. Front. Immunol. 10, 243 ( 10.3389/fimmu.2019.00243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ubeda C, Pamer EG. 2012. Antibiotics, microbiota, and immune defense. Trends Immunol. 33, 459–466. ( 10.1016/J.IT.2012.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buschmann AH, Tomova A, López A, Maldonado MA, Henríquez LA, Ivanova L, Moy F, Godfrey HP, Cabello FC. 2012. Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS ONE 7, e42724 ( 10.1371/journal.pone.0042724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarrete P, Mardones P, Opazo R, Espejo R, Romero J. 2008. Oxytetracycline treatment reduces bacterial diversity of intestinal microbiota of Atlantic salmon. J. Aquat. Anim. Health 20, 177–183. ( 10.1577/H07-043.1) [DOI] [PubMed] [Google Scholar]
- 61.Limbu SM, Zhou L, Sun S-X, Zhang M-L, Du Z-Y.. 2018. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 115, 205–219. ( 10.1016/J.ENVINT.2018.03.034) [DOI] [PubMed] [Google Scholar]
- 62.Sudheesh PS, Cain KD. 2017. Prospects and challenges of developing and commercializing immersion vaccines for aquaculture. Int. Biol. Rev. 1, 1–20. ( 10.18103/ibr.v1i1.1313) [DOI] [Google Scholar]
- 63.Liu L, Gong Y-X, Zhu B, Liu G-L, Wang G-X, Ling F. 2015. Effect of a new recombinant Aeromonas hydrophila vaccine on the grass carp intestinal microbiota and correlations with immunological responses. Fish Shellfish Immunol. 45, 175–183. ( 10.1016/J.FSI.2015.03.043) [DOI] [PubMed] [Google Scholar]
- 64.Llewellyn MS, Boutin S, Hoseinifar SH, Derome N. 2014. Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 5, 207 ( 10.3389/fmicb.2014.00207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hai NV. 2015. The use of probiotics in aquaculture. J. Appl. Microbiol. 119, 917–935. ( 10.1111/jam.12886) [DOI] [PubMed] [Google Scholar]
- 66.Dawood MAO, Koshio S. 2016. Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454, 243–251. ( 10.1016/J.AQUACULTURE.2015.12.033) [DOI] [Google Scholar]
- 67.Zorriehzahra MJ, Delshad ST, Adel M, Tiwari R, Karthik K, Dhama K, Lazado CC. 2016. Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet. Q. 36, 228–241. ( 10.1080/01652176.2016.1172132) [DOI] [PubMed] [Google Scholar]
- 68.Hoseinifar SH, Sun YZ, Wang A, Zhou Z. 2018. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 9, 2429 ( 10.3389/fmicb.2018.02429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MAO, Eltholth M. 2018. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 24, 1613–1622. ( 10.1111/anu.12797) [DOI] [Google Scholar]
- 70.Falcinelli S, et al. 2015. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 5, 8–10. ( 10.1038/srep09336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falcinelli S, Rodiles A, Unniappan S, Picchietti S, Gioacchini G, Merrifield DL, Carnevali O. 2016. Probiotic treatment reduces appetite and glucose level in the zebrafish model. Sci. Rep. 6, 1–13. ( 10.1038/srep18061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gioacchini G, Ciani E, Pessina A, Cecchini C, Silvi S, Rodiles A, Merrifield DL, Olivotto I, Carnevali O. 2018. Effects of Lactogen 13, a new probiotic preparation, on gut microbiota and endocrine signals controlling growth and appetite of Oreochromis niloticus juveniles. Microb. Ecol. 76, 1063–1074. ( 10.1007/s00248-018-1194-0) [DOI] [PubMed] [Google Scholar]
- 73.Borrelli L, et al. 2016. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci. Rep. 6, 1–9. ( 10.1038/srep30046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zang L, et al. 2019. Dietary Lactobacillus plantarum ST-III alleviates the toxic effects of triclosan on zebrafish (Danio rerio) via gut microbiota modulation. Fish Shellfish Immunol. 84, 1157–1169. ( 10.1016/j.fsi.2018.11.007) [DOI] [PubMed] [Google Scholar]
- 75.Mayer EA, Tillisch K, Gupta A, Mayer EA, Tillisch K, Gupta A. 2015. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938. ( 10.1172/JCI76304.Several) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cerezuela R, Fumanal M, Tapia-Paniagua ST, Meseguer J, Moriñigo M, Esteban M. 2013. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 34, 1063–1070. ( 10.1016/j.fsi.2013.01.015) [DOI] [PubMed] [Google Scholar]
- 77.Yang P, et al. 2018. Dietary stachyose altered the intestinal microbiota profile and improved the intestinal mucosal barrier function of juvenile turbot, Scophthalmus maximus L. Aquaculture 486, 98–106. ( 10.1016/j.aquaculture.2017.12.014) [DOI] [Google Scholar]
- 78.Piazzon MC, et al. 2017. Under control: how a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 5, 164 ( 10.1186/s40168-017-0390-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yáñez JM, Newman S, Houston RD. 2015. Genomics in aquaculture to better understand species biology and accelerate genetic progress. Front. Genet. 6, 128 ( 10.3389/978-2-88919-957-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zenger KR, Khatkar MS, Jones DB, Khalilisamani N, Jerry DR, Raadsma HW. 2019. Genomic selection in aquaculture: application, limitations and opportunities with special reference to marine shrimp and pearl oysters. Front. Genet. 10, 693 ( 10.3389/fgene.2018.00693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, et al. 2018. Genetic effects on the gut microbiota assemblages of hybrid fish from parents with different feeding habits. Front. Microbiol. 9, 2972 ( 10.3389/fmicb.2018.02972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zilber-Rosenberg I, Rosenberg E. 2008. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol. Rev. 32, 723–735. ( 10.1111/j.1574-6976.2008.00123.x) [DOI] [PubMed] [Google Scholar]
- 83.Yano JM, et al. 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. ( 10.1016/j.cell.2015.02.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Vadder F, Grasset E, Holm LM, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F.. 2018. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl Acad. Sci. USA 115, 6458–6463. ( 10.1073/pnas.1720017115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muller PA, et al. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313. ( 10.1016/j.cell.2014.04.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, Ahmed T, Gordon JI. 2015. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell 163, 95–107. ( 10.1016/j.cell.2015.08.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raja S, Batra V, Srinivasan S. 2018. The influence of microbiota on gastrointestinal motility. In Mechanisms underlying host–microbiome interactions in pathophysiology of human diseases (eds Sun J, Dudeja PK), pp. 113–127. Berlin, Germany: Springer US. [Google Scholar]
- 88.Kokou F, Sasson G, Nitzan T, Doron-Faigenboim A, Harpaz S, Cnaani A, Mizrahi I. 2018. Host genetic selection for cold tolerance shapes microbiome composition and modulates its response to temperature. Elife 7, 1–21. ( 10.7554/elife.36398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown RM, Wiens GD, Salinas I. 2019. Analysis of the gut and gill microbiome of resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 86, 497–506. ( 10.1016/j.fsi.2018.11.079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson CD, Klein HS, Murphy KD, Parthasarathy R, Guillemin K, Bohannan BJM. 2018. Experimental bacterial adaptation to the zebrafish gut reveals a primary role for immigration. PLoS Biol. 16, e2006893 ( 10.1371/journal.pbio.2006893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krkošek M, Lewis MA, Volpe JP. 2005. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proc. R. Soc. B 272, 689–696. ( 10.1098/rspb.2004.3027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glover KA, et al. 2017. Half a century of genetic interaction between farmed and wild Atlantic salmon: status of knowledge and unanswered questions. Fish Fish. 18, 890–927. ( 10.1111/faf.12214) [DOI] [Google Scholar]
- 93.Primavera JH. 2006. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 49, 531–545. ( 10.1016/j.ocecoaman.2006.06.018) [DOI] [Google Scholar]
- 94.Tal Y, Schreier HJ, Sowers KR, Stubblefield JD, Place AR, Zohar Y. 2009. Environmentally sustainable land-based marine aquaculture. Aquaculture 286, 28–35. ( 10.1016/j.aquaculture.2008.08.043) [DOI] [Google Scholar]
- 95.Aruety T, Brunner T, Ronen Z, Gross A, Sowers K, Zilberg D. 2016. Decreasing levels of the fish pathogen Streptococcus iniae following inoculation into the sludge digester of a zero-discharge recirculating aquaculture system (RAS). Aquaculture 450, 335–341. ( 10.1016/j.aquaculture.2015.08.002) [DOI] [Google Scholar]
- 96.Skjermo J, Salvesen I, Øie G, Olsen Y, Vadstein O. 1997. Microbially matured water: a technique for selection of a non-opportunistic bacterial flora in water that may improve performance of marine larvae. Aquac. Int. 5, 13–28. ( 10.1007/BF02764784) [DOI] [Google Scholar]
- 97.Ahmad HI, Verma AK, Babitha RAM, Rathore G, Saharan N, Gora AH. 2016. Growth, non-specific immunity and disease resistance of Labeo rohita against Aeromonas hydrophila in biofloc systems using different carbon sources. Aquaculture 457, 61–67. ( 10.1016/j.aquaculture.2016.02.011) [DOI] [Google Scholar]
- 98.Vadstein O, Attramadal KJK, Bakke I, Olsen Y. 2018. K-selection as microbial community management strategy: a method for improved viability of larvae in aquaculture. Front. Microbiol. 9, 1–17. ( 10.3389/fmicb.2018.02730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu H, Li H, Wei H, Zhu X, Han D, Jin J, Yang Y, Xie S. 2019. Biofloc formation improves water quality and fish yield in a freshwater pond aquaculture system. Aquaculture 506, 256–269. ( 10.1016/j.aquaculture.2019.03.031) [DOI] [Google Scholar]
- 100.Pérez-Fuentes JA, Hernández-Vergara MP, Pérez-Rostro CI, Fogel I. 2016. C: N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 452, 247–251. ( 10.1016/j.aquaculture.2015.11.010) [DOI] [Google Scholar]
- 101.Skjermo J, Vadstein O. 1999. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 177, 333–343. ( 10.1016/S0044-8486(99)00096-4) [DOI] [Google Scholar]
- 102.Qi Z, Dierckens K, Defoirdt T, Sorgeloos P, Boon N, Bao Z, Bossier P. 2009. Effects of feeding regime and probionts on the diverting microbial communities in rotifer Brachionus culture. Aquac. Int. 17, 303–315. ( 10.1007/s10499-008-9202-x) [DOI] [Google Scholar]
- 103.Karunasagar I, Shivu MM, Girisha SK, Krohne G, Karunasagar I. 2007. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 268, 288–292. ( 10.1016/j.aquaculture.2007.04.049) [DOI] [Google Scholar]
- 104.Higuera G, Bastías R, Tsertsvadze G, Romero J, Espejo RT. 2013. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 392–395, 128–133. ( 10.1016/j.aquaculture.2013.02.013) [DOI] [Google Scholar]
- 105.Kalatzis PG, Bastías R, Kokkari C, Katharios P. 2016. Isolation and characterization of two lytic bacteriophages, $φ$st2 and $φ$grn1; Phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 11, 1–18. ( 10.1371/journal.pone.0151101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reid HI, Treasurer JW, Adam B, Birkbeck TH. 2009. Analysis of bacterial populations in the gut of developing cod larvae and identification of Vibrio logei, Vibrio anguillarum and Vibrio splendidus as pathogens of cod larvae. Aquaculture 288, 36–43 ( 10.1016/j.aquaculture.2008.11.022) [DOI] [Google Scholar]
- 107.Carnevali O, et al. 2004. Administration of probiotic strain to improve sea bream wellness during development. Aquac. Int. 12, 377–386. ( 10.1023/B:AQUI.0000042141.85977.bb) [DOI] [Google Scholar]
- 108.Rollo A, Sulpizio R, Nardi M, Silvi S, Orpianesi C, Caggiano M, Cresci A, Carnevali O. 2006. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiol. Biochem. 32, 167–177. ( 10.1007/s10695-006-0009-2) [DOI] [Google Scholar]
- 109.Moriarty DJW. 1998. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164, 351–358. [Google Scholar]
- 110.Almeida GMF, Mäkelä K, Laanto E, Pulkkinen J, Vielma J, Sundberg L-R. 2019. The fate of bacteriophages in recirculating aquaculture systems (RAS)—towards developing phage therapy for RAS. Antibiotics 8, 192 ( 10.3390/antibiotics8040192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jahangiri L, Esteban MÁ. 2018. Administration of probiotics in the water in finfish aquaculture systems: a review. Fishes. 3, 33 ( 10.3390/fishes3030033) [DOI] [Google Scholar]
- 112.Ruiz P, Vidal JM, Sepúlveda D, Torres C, Villouta G, Carrasco C, Aguilera F, Ruiz-Tagle N, Urrutia H. 2019. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquac. 17, raq.12392 ( 10.1111/raq.12392) [DOI] [Google Scholar]
- 113.Rudi K, Angell IL, Pope PB, Vik JO, Sandve SR, Snipen L-G. 2018. Stable core gut microbiota across the freshwater-to-saltwater transition for farmed Atlantic salmon. Appl. Environ. Microbiol. 84, e01974–17 ( 10.1128/AEM.01974-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Ahnen M, Aalto SL, Suurnäkki S, Tiirola M, Pedersen PB.. 2019. Salinity affects nitrate removal and microbial composition of denitrifying woodchip bioreactors treating recirculating aquaculture system effluents. Aquaculture 504, 182–189. ( 10.1016/j.aquaculture.2019.01.068) [DOI] [Google Scholar]
- 115.Boutin S, Bernatchez L, Audet C, Derôme N. 2013. Network analysis highlights complex interactions between pathogen, host and commensal microbiota. PLoS ONE 8, e84772 ( 10.1371/journal.pone.0084772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Webster TMU, Rodriguez-Barreto D, Consuegra S, de Leaniz CG. 2019. Cortisol-induced signatures of stress in the fish microbiome. bioRxiv, 826503 ( 10.1101/826503) [DOI]
- 117.Fanouraki E, Papandroulakis N, Ellis T, Mylonas CC, Scott AP, Pavlidis M. 2008. Water cortisol is a reliable indicator of stress in European sea bass, Dicentrarchus la. Behaviour 145, 1267–1281. ( 10.1163/156853908785765818) [DOI] [Google Scholar]
- 118.Gabriel UU, Gabriel U, Akinrotimi A. 2011. Management of stress in fish for sustainable aquaculture development. Researcher 3, 28–38. [Google Scholar]
- 119.Egerton S, Culloty S, Whooley J, Stanton C, Ross RP. 2018. The gut microbiota of marine fish. Front. Microbiol. 9, 873 ( 10.3389/fmicb.2018.00873) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data collected in the systematic review can be found in the electronic supplementary material.