Abstract
Background
Insulin therapy is poorly accepted by patients with type 2 diabetes mellitus (T2DM). A needle-free insulin injector has been developed for patients who fear injections or are reluctant to initiate insulin therapy when it is clearly indicated. The objective of this trial was to evaluate the glucose-lowering effect, tolerability, patient satisfaction and compliance with insulin treatment via a needle-free insulin injector (NFII) compared with insulin treatment via a conventional insulin pen (CIP) in patients with T2DM.
Methods
A total of 427 patients with T2DM were enrolled in a prospective, multicenter, randomized, open-label study, and were randomly assigned 1:1 to receive 16 weeks’ treatment with basal insulin or premixed insulin administered either by a NFII or CIP.
Trial registration
ClinicalTrials.gov (NCT03243903).
Findings
In the 412 patients who completed the study, the adjusted mean reduction of HbA1c from baseline at week 16 in the NFII group was 0.55% (95% CI −0.71, −0.39), which was non-inferior and statistically superior to the HbA1c reduction in the CIP group (0.26%, 95% CI −0.42, −0.11). Patients in the NFII group showed significantly higher treatment satisfaction scores than those in the CIP group (mean scores, 8.17 ± 1.78 vs. 7.21 ± 2.22, respectively; p<0.0001). The occurrence of hypoglycemia was similar in the two groups, and the NFII group showed reduced incidences of skin scratches, indurations and lower VAS pain scores.
Interpretation
Insulin therapy through needle-free injector showed a non-inferior glycemic-lowering effect and a significantly enhanced level of patient satisfaction with insulin treatment compared with conventional insulin therapy through needle injections. In addition, the needle-free injector also had a better safety profile.
Funding
This study were funded by Beijing QS Medical Technology Co., Ltd, as well as The Major Chronic Non-communicable Disease Prevention and Control Research.
Keywords: Diabetes Mellitus, Efficacy, HbA1c, Insulin, Needle-free injector, Safety, Type 2
Research in context.
Evidence before this study
Insulin therapy is often poorly accepted by patients with type 2 diabetes when it is clearly indicated, and when insulin is administered, patients’ adherence to treatment is often poor, due to fear and anxiety about injection pain and other adverse effects. Needle-free insulin injection (NFII) was developed to promote insulin therapy acceptance and improve the effectiveness of insulin therapy through enhancing patient's adherence to insulin treatment. The NFII device has been shown to have an advantage in controlling post-prandial glucose levels due to its faster insulin absorption and more rapid effect than conventional insulin pen (CIP)-based injections.
Added value of this study
This 16-week prospective, multicenter, randomized, open-label, study was designed to evaluate the long-term efficacy and safety of insulin therapy administered via a needle-free insulin injector (NFII) as compared with a conventional insulin pen (CIP) in a relatively large number of patients (n=427) with poorly-controlled type 2 diabetes mellitus (some of whom were insulin-naïve while others had previously been treated with premixed or basal insulin for >3 months). The adjusted mean reduction of HbA1c from baseline in the NFII group at the end of the 16-week study was non-inferior and statistically superior to that in the CIP group. Although the occurrence of hypoglycemia was similar in the two groups, the NFII group also had reduced incidences of skin scratches, indurations and lower VAS pain scores than the CIP group.
Implications of all the available evidence
The NFII system has an advantage in controlling post-prandial glucose levels due to its faster insulin absorption and more rapid effect than CIP-based insulin injection. The NFII device increased patients’ satisfaction with treatment without increasing episodes of hypoglycemia, and it reduced the adverse effects associated with needle-based insulin injections. Consequently, this study has added evidence to support the view that the pharmacokinetic and pharmacodynamic benefits of the needle-free insulin injector can potentially improve glycemic control in type 2 diabetes patients treated with insulin, and it supports the application of this device across patients with type 2 diabetes who use premixed or basal insulin.
Alt-text: Unlabelled box
1. Introduction
China has the world's largest diabetes population, 109.6 million according to the International Diabetes Federation (IDF) diabetes atlas 2018, which imposes an intensive healthcare burden on society [1]. Diabetes is poorly controlled in China as less than half of patients diagnosed with the disease achieve the HbA1c target level of <7.0% (53 mmol/mol). Insulin is recommended in patients with suboptimal glycemic control taking oral antihyperglycemic drugs in both national and international diabetes management guidelines [2]. However, patients with type 2 diabetes are often reluctant to initiate insulin therapy and their adherence to insulin therapy is poor [3,4]. In China, the average HbA1c at the time of insulin initiation has been reported to be 9.6% in type 2 diabetes patients who had failed oral antihyperglycemics in a real-word setting [5], and the mean HbA1c in type 2 diabetes patients on insulin therapy was 8.5% [6]. Thus, methods to improve the acceptance of insulin therapy when it is necessary for managing hyperglycemia and enhance its effectiveness are imperative to improve glucose control in Chinese patients with diabetes.
One important way to promote insulin acceptance and improve the effectiveness of insulin therapy is to improve the method of insulin administration. Although insulin injection technology has improved in recent years, commonly used insulin injections through needles, especially inappropriate injections, can cause injection pain, psychological discomfort, subcutaneous lipohypertrophy, lipoatrophy, inflammation, skin reactions at the injection site, and needle breaks and retention in the skin, all of which may affect the acceptance of insulin by patients [7]. A large proportion of diabetes patients have ‘psychological insulin resistance’, which may be attributable to fear and anxiety related to injection pain and other adverse effects, leading to late initiation of insulin therapy when oral agents have failed to achieve glycemic control [8]. Needle-free injections, also known as jet injections, are based on the principle of pressure jets that push the liquid from micropores to form a very thin liquid column, which instantly penetrates through the skin to the subcutaneous tissue with a spray-like diffusion. As the injected depth with the needle-free injection is limited, nerve endings are stimulated only slightly, and the stinging feeling is not as obvious as with needle injections.
Needle-free injections of insulin have considerably enhanced insulin absorption and the consequent glucose-lowering effect, as well as achieving faster correction of hyperglycemia in short-term and small-scale trials in healthy volunteers and patients with diabetes [9,10]. However, whether these benefits can translate to better or equivalent glycemic control compared with needle-based insulin injections and improve patient satisfaction with treatment needs to be tested in larger and longer-term trials, since insulin treatment is a long-term therapy administered by patients themselves in ambulatory settings under the supervision of clinicians. In addition, the long-term safety of needle-free injections also needs to be evaluated. Consequently, the aim of this study was to evaluate the long-term efficacy, safety, and patient-reported outcomes (PROs) of insulin therapy delivered by a needle-free insulin injector compared with standard needle-based insulin delivery in a large sample of patients with type 2 diabetes mellitus (T2DM).
2. Methods
2.1. Study design and participants
The study was designed as a prospective, multicenter, randomized, open-label, parallel-group, non-inferiority trial, the protocol for which has been published previously [11]. Before the study was commenced, approval was received from the Ethics Committee of Peking University People's Hospital, and the study was conducted in accordance with the moral, ethical, and scientific principles of the declaration of Helsinki and the provisions of good clinical practice (GCP) in China. Written informed consent was obtained from all participants before any study-related procedures were implemented. The study was designed to test whether insulin administered by either a needle-free insulin injector (NFII) or a conventional insulin pen (CIP) for 16 weeks had similar efficacy and safety. The needle-free insulin injector used was the QS-M Needle-Free Injector (Beijing QS Medical Technology Co. Ltd) and the conventional insulin pens used included NovoPen® 5 (90 patients), HumaPen Ergo II® (17 patients), Gansulin Pen® (6 patients), Xiulin Pen® (19 patients), Dongbao Pen® (21 patients), Lantus SoloStar® (45 patients), and Unipen (8 patients).
Between August 2017 and July2018, patients with previously diagnosed T2DM were recruited at 10 research centers in China including Peking University People's Hospital, Beijing Hospital, General Hospital of Tianjin Medical University, Jilin University First Hospital, The First Affiliated Hospital of China Medical University, Tianjing Metabolic Diseases Hospital, Affiliated Hospital of Qingdao University, West China Hospital of Sichuan University, Zhongda Hospital of Southeast University, and Fuzhou General Hospital of Nanjing Command. Entry criteria included adults diagnosed with T2DM (according to the WHO 1999 criteria) who were aged 18–75 years, had a baseline HbA1c of 7.5–11.0% (58−97 mmol/mol), and a BMI ≤32 kg/m2 who had been receiving insulin (premixed insulin or basal insulin) for more than 3 months before enrollment without a recent (<1 month) dosage adjustment or taking oral antihyperglycemic agents for more than 3 months and were willing to initiate insulin therapy due to poor glycemic control. The major exclusion criteria included pregnant or lactating women; the presence of severe cardiovascular events; use of immunosuppressive agents; end-stage renal disease or a serum creatinine concentration >1.5 mg/dL (for male patients) or >1.4 mg/dL (for female patients); acute pancreatitis; acute liver disease with ALT or AST >3 times the upper limit of normal; a history of skin lesions; allergy to insulin; and anemia. Detailed inclusion and exclusion criteria are available in the appendix to this study and in the previously published protocol of the trial [11].
The trial was registered with ClinicalTrials.gov, identification number NCT03243903.
2.2. Randomization and masking
Treatment randomization to either the needle-free insulin injector (NFII) or a conventional insulin pen (CIP) was performed via an integrated web response system (IWRS). To avoid the influence of external factors such as the patients’ previous usage of insulin and the type of insulin, patients were stratified by whether or not they had previously used insulin and whether premixed or basal insulin was used. Patients were randomly assigned to the NFII and CIP groups in a 1:1 ratio at each center.
Since the injecting devices are quite distinct from each other, the study was an open-label one and neither the investigators nor the patients were blinded.
2.3. Interventions
Patients in both groups entered a 2-week screening period after providing their written informed consent. The serum HbA1c level was measured at each research center and also by a central laboratory at Peking University People's Hospital using an NGSP (National Glycohemoglobin Standardization Program) approved method. The patients’ demographic data, information on their medical and family history, physical examination findings, and quality-of-life data (obtained via the SF-36 questionnaire) were collected.
A run-in period from week 1 to the end of week 4 was implemented to allow treatment adjustment. Since regular insulin (RI) injected through a needle-free injector has shown a similar pharmacological profile to rapid-acting insulin [9,10], we reduced the insulin dosage when switching the insulin injecting device from a CIP to the NFII to avoid transient hyperinsulinemia, as this may lead to hypoglycemia. In patients previously using premixed insulin, those assigned to the NFII group had their insulin dosage reduced by 10%. In patients continuing on basal insulin, the insulin dosage was reduced by 20–25% in those with a fasting blood glucose (FBG) concentration of 7–10 mmol/L before treatment, and by 10–15% in those with a FBG concentration of 10–15 mmol/L, but the dosage was unadjusted in those with a FBG concentration >15 mmol/L. In patients who were newly started on insulin therapy, the insulin type and dosage was selected and adjusted by clinicians following the recommendations of the Chinese guideline for type 2 diabetes mellitus [2].
The insulin dosage in patients assigned to both the NFII and CIP groups was adjusted according to the blood glucose level as recommended in the CDS guideline [2]. Patients who continued insulin administration used the same type of insulin that they had previously received during the trial. The type of insulin and injection frequency was fixed during the trial.
Self-monitoring of blood glucose (SMBG) was performed on the first study day at baseline and thereafter at 2 days before each visit at week 1, 2, 4, 6, 8, 12, and 16. If the patient was randomly assigned to one treatment group on the screening day, the 7-point blood glucose level was considered as the baseline blood glucose concentration on the day of randomization.
After the 4-week run-in phase, the patients entered a 12-week treatment observation period. The insulin dosage was adjusted during the first 6 weeks of the observation period if there was a need to do so. It was assumed that patients would achieve steady glycemic control after the 4-week run-in phase and the 6-week insulin dosage optimization period. During week 11 to 16, if the SMBG concentration before breakfast or dinner was higher than 13.3 mmol/L, SMBG was performed again over the next 2 days.
Patients were advised to contact the researchers if their blood glucose concentration before breakfast or dinner was higher than 13.3 mmol/L for 3 consecutive days. The researcher then sought the possible reason for the uncontrolled glucose, e.g.: (1) whether or not the patient was complying with the recommended lifestyle recommendations; (2) whether the SMBG concentration was a fasting or post-prandial one; (3) whether the dosages of insulin and oral antihyperglycemic agents were appropriate; (4) whether there were any coexisting medical conditions that could influence blood glucose levels, e.g. infection; and (5) whether the patient was complying with the insulin administration instructions given by their clinicians. If these potential reasons for the lack of glucose control could explain the increased glucose concentration, the researcher then selected one or more of the following actions:
-
1.
An extra visit was arranged to reinforce lifestyle recommendations and another SMBG was requested.
-
2.
The insulin dosage was adjusted according to the research protocol.
-
3.
Coexisting conditions were treated.
-
4.
The need for treatment compliance by the patient was reinforced.
If the high SMBG (>13.3 mmol/L) was not caused by any of the above reasons or was not resolved when one or more of the appropriate actions were taken, the patient was advised to cease participating in the trial. At the end of the trial, quality-of-life information obtained via the SF-36 questionnaire, physical examination data, treatment satisfaction scores, and adverse event data were collected.
Assessment visits occurred at screening (week −2), baseline, and week 1, 2, 4, 6, 8, 12 and 16. Telephone follow-ups were also conducted at week 3, 5, 7, and 10. Information on clinical characteristics, 7-point SMBG concentrations (before and 2 h after breakfast, lunch, and dinner and at bedtime), and adverse events were collected at each of these visits (if applicable).
2.4. Endpoints
The primary endpoint was the change from baseline in the glycated hemoglobin (HbA1c) level after 16 weeks of treatment. Secondary endpoints included: (1) the profile of SMBG concentrations performed at baseline and thereafter at 2 days before each study visit (for each SMBG test, the 7-point blood glucose concentration was monitored before and at 2 hours after each meal and at bedtime); (2) the rate of achieving the HbA1c target (<7%) at the end of the trial; (3) the improvement from baseline in patients’ quality-of-life measured by the SF-36 questionnaire; (4) the change in the daily dosage of insulin administered (the insulin dose was recorded on the first day at baseline and then the day before each study visit); (5) patients’ compliance with insulin administration (assessed by checking patients’ records at every visit to determine missing injections); and (6) patients’ satisfaction with their treatment at the end of the trial (assessed on a scale of 0–10, with 10 as the most satisfactory score and 0 the least).
The safety analysis assessed vital signs and ECG changes, the frequency and severity of hypoglycemia, injection site reactions, changes in body weight, and other adverse events that occurred during the study.
2.5. Data analysis and statistics
PASS 13.0 was used to calculate sample size. A sample size of 167 evaluable patients per group was estimated to give 80% power for the upper confidence limit of the mean difference in the change of HbA1c levels between different insulin administrations not to exceed 0.4%, assuming that the standard change is 1.3% for a true difference of 0.0%. The sample size was adjusted to 210 subjects per group to allow for 20% dropouts.
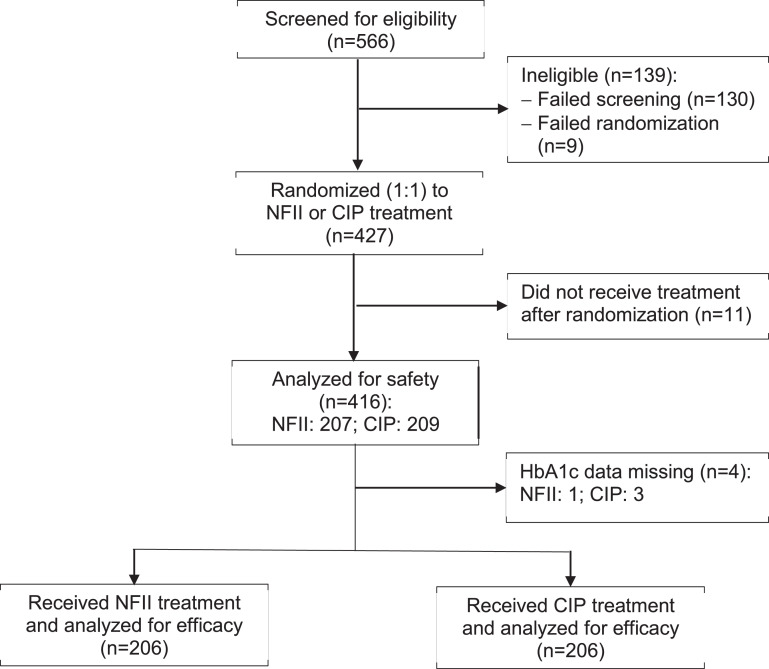
Mean and standard deviation (SD) data were reported for the primary and secondary endpoints, which had an approximately normal distribution. Primary efficacy and secondary endpoints were assessed in the intention-to-treat (ITT) study population, which was defined as all randomized participants who received at least one dose of study insulin, and had a baseline and at least one post-baseline assessment. Safety analyses included all randomized patients who were exposed to at least one dose of study insulin (Fig. 1).
Fig. 1.
Flow of patients in the trial. NFII = needle-free insulin injector; CIP = conventional insulin pen.
For the primary outcome, the differences between treatment groups were analyzed using an analysis of covariance (ANCOVA) model, after adjusting for HbA1c at baseline to assess the change of HbA1c from baseline. If non-inferiority was achieved, we further tested for the superiority of the NFII administration method. If the upper limit of the 95% confidence interval (CI) of the difference (NFII group − CIP group) of the least-square (LS) mean of the HbA1c decline was <0%, the conclusion that the NFII is superior to CIP could be drawn.
For secondary outcomes, continuous variables were evaluated by an ANCOVA model after adjusting the baseline value, and categorical variables were compared by a Chi-square test or Fisher's exact test, unless otherwise specified. All p-values reported were two-sided, and no formal adjustments for multiple comparisons were made. Significance was defined as p<0.05.
2.6. Role of funding source
This study and the article processing charges were funded by Beijing QS Medical Technology Co., Ltd. However, Beijing QS Medical Technology Co. was not involved in the study design, data collection, analysis, article writing, or the decision to submit for publication. The Major Chronic Non-communicable Disease Prevention and Control Research was the main funding source for Linong Ji and Leili Gao when they performed the trial.
3. Results
3.1. Study population
A total 566 patients were screened, 130 of whom failed screening, while 9 failed randomization, and 427 were randomized to either the NFII or CIP groups. Of the 412 patients who received insulin treatment and had HbA1c data available for analysis, 206 patients received insulin via the NFII and 206 received insulin via a CIP (Fig. 1). The majority of patients were treated with insulin before entering the trial, about half of them with premix insulin. Eleven patients (5.34%) in the NFII group and 10 in the CIP group (4.85%) were insulin-naïve before entering the trial. At baseline, the patients had a mean age around 58 years, the duration of diabetes was around 14 years, BMI was around 25 kg/m2, HbA1c around 8.2% (66.1 mmol/mol), and total insulin doses were around 30 units/day. There were no significant differences in baseline characteristics between the two groups (Table 1).
Table 1.
Baseline characteristics of the patients.
| Characteristic | NFII group (n=206) | CIP group (n=206) | p-Value |
|---|---|---|---|
| Age, years | 58.43 ± 10⋅05 | 57.41 ± 10.02 | 0.3022 |
| Male sex | 114 (55.34) | 107 (51.94) | 0⋅4892 |
| Body weight, kg | 70.30 ± 11.51 | 69.21 ± 12.09 | 0.2987 |
| BMI, kg/m2 | 25.38 ± 3⋅09 | 25.28 ± 2.84 | 0.7249 |
| SBP, mmHg | 131.08 ± 15.83 | 129.22 ± 15.53 | 0.8606 |
| Diabetes duration, years | 14.44 ± 7.52 | 13.79 ± 6.65 | 0.4299 |
| Insulin-naïve | 11 (5.34) | 10 (4.85) | 0.8228 |
| Initiated basal insulin | 5 | 8 | 0.5733 |
| Initiated premix insulin | 6 | 2 | 0.2834 |
| Using premix insulin during trial: | 118 (57.28) | 109 (52.91) | 0⋅3727 |
| Human insulin (30% or 50%) | 41 (34.75%) | 42 (38.53%) | 0.5830 |
| Insulin analogue (30%, 50% or 25%) | 77 (65.25%) | 67 (61.47%) | 0.5830 |
| Using basal insulin during trial: | 88 (42.72) | 97 (47.09) | 0.3727 |
| Glargine | 64 (72.73%) | 65 (67.01%) | 0.4265 |
| Determir | 15 (17.05%) | 21 (21.65%) | 0.4620 |
| NPH | 9 (10.23%) | 11 (11.34%) | 1.0000 |
| Daily insulin dosage, U/kg: | 0.44 ± 0.05 | 0.44 ± 0.06 | 0.8449 |
| Using premix | 0.54 ± 0.04 | 0.56 ± 0.03 | 0.5523 |
| Initiating premix | 0.55 ± 0.02 | 0.63 ± 0.18 | 0.8358 |
| Using basal | 0.31 ± 0.03 | 0.31 ± 0.05 | 0.8959 |
| Initiating basal | 0.24 ± 0.05 | 0.16 ± 0.00 | 0.4207 |
| HbA1c, % | 8.30 ± 1.01 | 8.19 ± 0.99 | 0.2565 |
| [mmol/mol] | [67.0 ± 11.0] | [66.0 ± 10.8] | |
| FBG, mmol/L | 8.76 ± 2.18 | 8.84 ± 2.35 | 0.7429 |
| BG after breakfast, mmol/L | 12.27 ± 3.20 | 12.19 ± 3.10 | 0.8042 |
| BG before lunch, mmol/L | 9.67 ± 2.88 | 9.90 ± 2.88 | 0.4159 |
| BG after lunch, mmol/L | 12.30 ± 3.08 | 12.35 ± 3.05 | 0.8719 |
| BG before dinner, mmol/L | 9.96 ± 2.78 | 10.⋅03 ± 2.85 | 0.8077 |
| BG after dinner, mmol/L | 11.91 ± 2.98 | 12.09 ± 3.16 | 0.5552 |
| G at bedtime, mmol/L | 10.69 ± 3.03 | 10.81 ± 3.11 | 0.6964 |
| Using metformin | 120 (57.97) | 97 (46.41) | 0.0183 |
| Using sulfonylureas | 33 (15.94) | 36 (17.22) | 0.7251 |
| Using α-glycosidase inhibitors | 100 (48.31) | 101 (48.33) | 0.9974 |
| Using DPP-4 inhibitors | 6 (2.90) | 8 (3.83) | 0.5993 |
| Using GLP-1RAs | 3 (1.45) | 0 (0.00) | 0.1223 |
| Using SGLT2Is | 1 (0.48) | 1 (0.48) | 1.000 |
| Diabetes retinopathy | 4 (1.94) | 1 (0.49) | 0.3719 |
| Diabetes kidney disease | 4 (1.94) | 3 (1.46) | 1.0000 |
| Diabetes neuropathy | 2 (0.97) | 3 (1.46) | 1.0000 |
| Hypertension | 74 (35.92) | 76 (36.89) | 0.8377 |
| Cerebrovascular disease | 3 (1.46) | 5 (2.43) | 0.7239 |
| Cardiovascular disease | 21 (10.19) | 22 (10.68) | 0.8720 |
| Dyslipidemia | 40 (19.42) | 35 (16.99) | 0.5232 |
Data are mean values ± SEM or n (%).
Abbreviations: BG, blood glucose concentration measured by patients themselves; BMI, body mass index; CIP, conventional insulin pen; DDP-4, dipeptidyl peptidase-4; FBG, fasting blood glucose concentration; GLP-1RAs, glucagon-like peptide-1 receptor agonists; NFII, needle-free insulin injector; NPH, neutral protamine Hagedorn; SBP, systolic blood pressure; SGLT2Is, sodium-glucose cotransporter-2 inhibitors.
3.2. Glycemic responses and insulin dosages
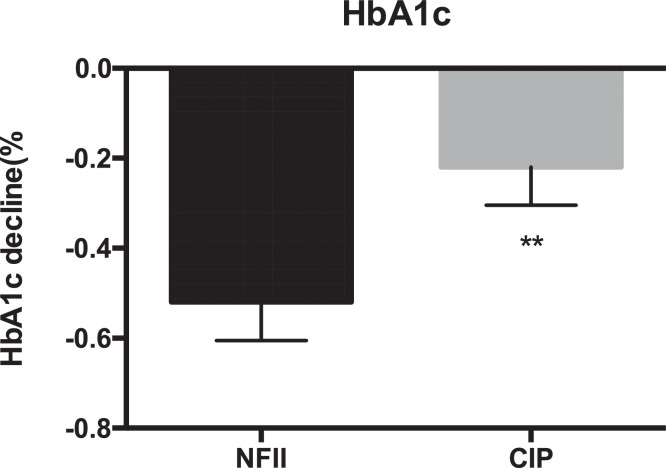
The adjusted mean reduction in HbA1c from baseline at week 16 in the NFII group was non-inferior to that in the CIP group. The least squares (LS) mean HbA1c reduction was −0.55% (95% CI −0.71, −0.39) in the NFII group and −0.26% (95% CI −0.42, −0.11) in the CIP group; p=0⋅0092; Fig. 2). The difference in the LS mean decline of the HbA1c level between the two groups was −0.29% (95% CI −0.50, −0.07) which reached statistical significance. However, there was no difference between the two groups in the percentages of patients achieving the HbA1c target of <7.0% (27.32% and 24.26%, respectively; p=0.486).
Fig. 2.
HbA1c decline from baseline (%) in the two groups after 16 weeks’ treatment.
NFII = needle-free insulin injector, CIP = conventional insulin pen. Data are presented as mean values ± SEM. **p<0.01 between the two groups.
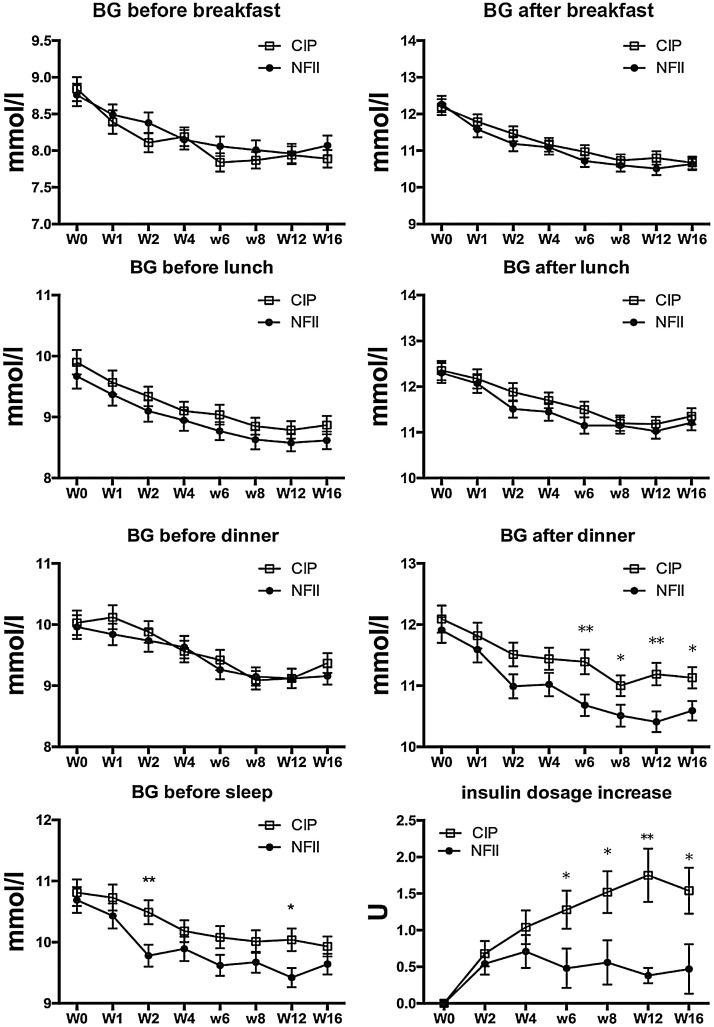
The self-measured blood glucose concentration declined in both groups (Fig. 3). A significantly greater decline from baseline in the blood glucose concentration after dinner was observed in the NFII group (LS mean, −1.59 mmol/L (95% CI −1.92,−1.26) in comparison with the CIP group (LS mean −1.00 mmol/L (95% CI −1.32, −0.68) [p=0.0077]. In the premix insulin users subgroup, the reduction of post-prandial glucose measured by SMBG after dinner was significantly larger in patients using NFII than in patients using CIP (Supplementary Table S1).
Fig. 3.
7-point SMBG values (mmol/L) and insulin dosages in the two groups from baseline to the end of trial. BG = blood glucose concentration; CIP = conventional insulin pen; NFII = needle-free insulin injector. Data are presented as mean values ± SEM.
In the NFII group, the mean daily dosage of insulin was 30.48 ± 14.30 U at baseline and 30.51 ± 14.59 U at the end of the trial (p=0.165). In the CIP group, the mean dosage increased from 29.76 ± 15.57 U at baseline to 31.23 ± 16.01 at the end of the trial (p<0.001). The daily insulin dosage increased less in patients treated via the NFII compared with those treated with a CIP from the 6th week of treatment (Fig. 3; lower right panel).
3.3. Treatment satisfaction and quality-of-life
The SF-36 questionnaire scores were increased from the baseline to week 16 and the magnitude of the increase was similar between the NFII and CIP groups (LS mean scores, 4.63 [95% CI 3.41, 5.85] vs. 4.78 [95% CI 3.59, 5.97], respectively; p=0.858). The increase in subsections of the SF-36 questionnaire scores was also similar between the two groups (data not shown).
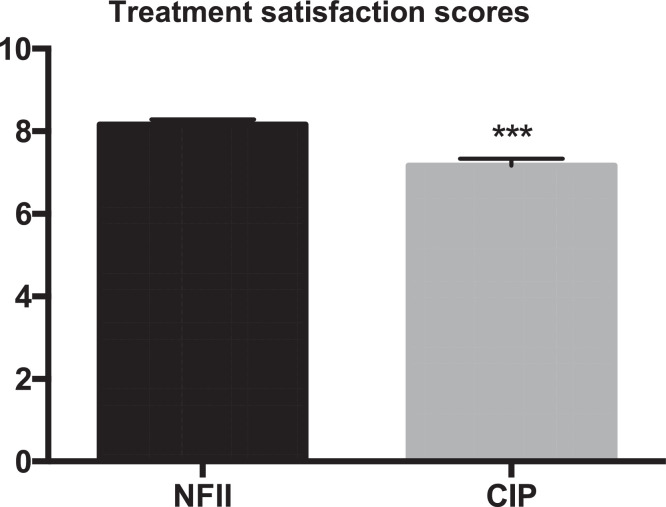
There was also no significant difference between the groups in compliance, because the rate of injections missed were similar (0.97% in the NFII group and 0% in the CIP group; p=1.000). However, patients in the NFII group had higher treatment satisfaction scores than those in the CIP group after 16 weeks of treatment (8.17 ± 1.78 vs. 7.21 ± 2.22; p<0.0001) (Fig. 4).
Fig. 4.
Treatment satisfaction scores in the two groups at the end of the trial. CIP = conventional insulin pen; NFII = needle-free insulin injector. Data are presented as mean values ± SEM. ***p<0.01 between the groups.
3.4. Adverse events
In the safety analysis, hypoglycemia occurred in 53 patients (25.6%) in the NFII group and 38 (18.18%) in the CIP group (p=0.0671). There was no significant difference in the rates of mild, moderate and severe hypoglycemia between the two groups.
Patients who received insulin by the NFII experienced less needle breaks (0 vs. 3 patients), significantly less injection site skin scratches (32 vs. 50 patients; p=0.0319) and subcutaneous induration (0 vs. 6 patients; p=0.015), and similar incidences of skin redness, subcutaneous hemorrhage, and ecchymosis compared with those in the CIP group. The visual analog scale (VAS) pain score was significantly less in the NFII group (1.11 ± 1.21 vs. 1.98 ± 1.99, respectively; p<0.001). Body weight increased by 0.54 ± 3.64 kg in the NFII group and 0.99 ± 3.55 kg in the CIP group after 16 weeks of treatment (p=0.2036).
The incidence of unexpected adverse events was similar between the groups (5.31% vs. 3.35%, respectively; p=0.3468). [Table 2]. No unexpected injection device-associated AEs were recorded, and all vital signs, including respiration rate, blood pressure, heart rate, body temperature, and ECG abnormities were equivalent in the two groups at all time points during the trial.
Table 2.
Summary of unexpected adverse events (AEs) in the two patient groups.
| Unexpected Adverse event | NFII group (n=207) | CIP group (n=209) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Episodes | Patients | % | Episodes | Patients | % | ||
| All adverse events | 14 | 11 | 5.31 | 9 | 7 | 3.35 | 0.3468 |
| Related to the device | 0 | 0 | 0.00 | 0 | 0 | 0.00 | 1.0000 |
| Unrelated to the device | 14 | 11 | 5.31 | 9 | 7 | 3.35 | 0.3468 |
| AEs leading to treat-ment withdrawal | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Severe adverse events (bone disorders) | 2 | 2 | 0.97 | 0 | 0 | 0.00 | 0.2470 |
| Unexpected adverse events: | |||||||
| All adverse events | 14 | 11 | 5.31 | 9 | 7 | 3.35 | 0.3468 |
| Bone disorders | 3 | 3 | 1.45 | 0 | 0 | 0.00 | 0.1223 |
| Upper respiratory tract infection | 3 | 3 | 1.45 | 3 | 3 | 1.44 | 1.0000 |
| Fever | 2 | 2 | 0.97 | 0 | 0 | 0.00 | 0.2470 |
| Hyperuricemia | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Abnormal liver function test | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Disc disorder | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Bacterial infection | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Diarrhea | 1 | 1 | 0.48 | 2 | 2 | 0.96 | 1.0000 |
| Angina | 1 | 1 | 0.48 | 0 | 0 | 0.00 | 0.4976 |
| Joint disorder | 0 | 0 | 0.00 | 1 | 1 | 0.48 | 1.0000 |
| Virus infection | 0 | 0 | 0.00 | 1 | 1 | 0.48 | 1.0000 |
| Toothache | 0 | 0 | 0.00 | 1 | 1 | 0.48 | 1.0000 |
| Spastic paralysis | 0 | 0 | 0.00 | 1 | 1 | 0.48 | 1.0000 |
4. Discussion
In this prospective, multicenter, randomized, open-label, parallel-group trial, we showed that 16-weeks of NFII-administered insulin therapy was non-inferior in controlling blood glucose to conventional needle-based insulin therapy in Chinese patients with type 2 diabetes. The NFII increased patients’ satisfaction with treatment without increasing episodes of hypoglycemia, and it reduced the adverse effects associated with needle-based insulin injection. To the best of our knowledge, our study is the first to evaluate the effectiveness and safety of the needle-free insulin injector using changes in HbA1c and hypoglycemia as the major endpoints in a randomized controlled clinical trial setting.
There was a non-inferior decline in HbA1c after 16 weeks of insulin treatment in the NFII group compared with the CIP group. In addition, we detected a statistically significantly greater reduction of HbA1c in the NFII group compared with the CIP group by following a pre-defined analysis plan. We also observed a greater reduction in the glucose level after dinner, as indicated by the SMBG glucose excursion profiles (Fig. 3), specifically in patients receiving premixed insulin (Supplementary Table S1). Moreover, the increase in the dose of insulin was less in the NFII group than in the CIP group (Fig. 3). These observations suggest the possibility of enhanced glycemic control with the needle-free injection. The QS-M NFII system has an advantage in controlling post-prandial glucose levels due to its faster insulin absorption and more rapid effect than CIP-based injection [12,13]. Rapid-acting insulin administered by a NFII has been reported to have a shorter time to achieve peak plasma insulin levels, and a reduced hyperglycemic burden during the first hour of treatment compared with conventional needle-based administration in both healthy volunteers and patients with type 2 diabetes [9,14]. Regular insulin (RI) administered by a NFII was found to exhibit a similar pharmacological profile to rapid-acting insulin administered by CIP [10]. Whether this benefit can be translated to better glycemic control in the long term is unclear. Nevertheless, our study has added evidence to support the view that these pharmacokinetic and pharmacodynamic benefits can potentially improve glycemic control in patients treated with insulin. The possibility of improving glycemic control by changing the pharmacodynamics of insulin administration is supported by a meta-analysis of large clinical trials and real-world data suggesting that the better glucose-lowering efficacy of rapid-acting insulin versus regular insulin is independent of diabetes type [15]. Although we demonstrated an improved post-dinner glucose level in our patients, there was no significant difference in the glucose level after breakfast or after lunch. To further elucidate the role of NFII administration on post-dinner glucose levels, a fixed insulin type should be used in the study.
This study also showed that NFII insulin treatment was as safe as CIP insulin treatment with similar episodes of hypoglycemia and other adverse effects. It also supported the safety of needle-free insulin therapy in the setting of use of mixed types of insulin. However, episodes of hypoglycemia increased in the NFII group, although this was without statistical significance. The increased episodes of hypoglycemia could be attributable to the increased pharmacokinetics/pharmacodynamics of insulin administered by jet injection.
Use of the NFII was associated with less injection-site associated pain and dermatological reactions, which possibly contributed to the improved patient satisfaction at the end of the trial. Initiating insulin therapy has been problematic in Chinese patients with type 2 diabetes, since the average HbA1c in patients initiating insulin therapy was found to be 9.6% (81.4 mmol/mol) [4]. Delayed insulin initiation could result in faster progression of diabetes complications. The ‘psychological resistance’ to insulin is attributable to the fear of pain, inconvenience of needles, and other psychological problems. In addition, 95% of Chinese patients are reluctant to change needles, which may contribute to a higher occurrence of injection site-related lesions such as lipohypertrophy [16,17]. The NFII can potentially help patients to accept insulin therapy more easily when insulin is needed for improving glycemic control.
A strength of our multicenter study is that it included a large number of patients who were either continuing insulin therapy or initiating insulin therapy, which supports the generalizability of our findings. It also enabled us to evaluate the efficacy and safety of the NFII device by comparing HbA1c changes and insulin dosage adjustments over a 16-week period in a well-powered study population. As the patients administered insulin and adjusted the dosage at home after appropriate training, no investigator-led optimization of insulin therapy took place, and the observed improvements in glucose outcomes can be solely attributed to use of the NFII device. The study's limitations include: (1) it was an open-label investigation and thus bias cannot be eliminated. Most of the intervention group started something new whereas the control group did not, which may affect treatment adherence and introduce intervention bias as the placebo effect of initiating a new therapy may contribute to measurable HbA1c reduction [18]; (2) the patients were not followed long enough to evaluate long-term changes in HbA1c and long-term complications; (3) as we were not able to include patients using a bolus-basal insulin regimen, the utility of this device in these patients is unknown. However, among patients taking oral antihyperglycemic drugs plus insulin, approximately 66% of Chinese patients use insulin in the form of premixed insulin and 17% use basal insulin [19]; (4) only a small proportion of patients (24−28%) achieved the HbA1c goal (<7.0%) suggesting ‘tardy dosage adjustment’ in the trial; (5) the safety of the NFII device was not evaluated in patients with robust insulin dosage; and (6) checking patient diaries for missing injections is not a precise measure of insulin treatment adherence. However, in this short trial, we checked the number of missing injections to estimate the adherence of patients using needle-free injectors in an easy and convenient manner.
In conclusion, the insulin therapy administered with the NFII demonstrated non-inferior efficacy in glycemic control compared with CIP-based insulin therapy in Chinese patients with type 2 diabetes. In addition it also exhibited an improved safety profile and increased patient satisfaction with treatment. This supports the application of this device across patients with type 2 diabetes who use premixed insulin or basal insulin.
Declaration of Competing Interest
Linong Ji receives consulting fees from Beijing QS Medical Technology Co. He also reports grants and personal fees from Novo Nordisk, Merck, Bristol-Myers Squibb, Novartis, Sanofi, MSD, AstraZeneca, and Roche, and personal fees from Eli Lilly, Takeda, Bayer, and Boehinger Ingelheim, outside the submitted work. Leili Gao reports grants from Beijing QS Medical Technology Co. Ltd, China, and grants from the Major Chronic Non-communicable Disease Prevention and Control Research during the conduct of the study. The other authors report grants from Beijing QS Medical Technology Co. Ltd during the conduct of the study.
Acknowledgments
Acknowledgments
Editorial assistance with the manuscript was provided by Content Ed Net Co., Shanghai, China. We thank all of the researchers and patients who participated in this study.
Funding
This study and the article processing charges were funded by Beijing QS Medical Technology Co., Ltd, China. The work was also supported by Grants 2016YFC1305600, 2016YFC1305603 from the Major Chronic Non-communicable Disease Prevention and Control Research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100368.
Appendix. Supplementary materials
References
- 1.IDF Diabetes Atlas. 2018. Available from:https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html(accessed 1 Jun, 2019).
- 2.Chinese Diabetes Society Chinese guideline for the prevention and treatment of type 2 diabetes mellitus (2017 edition) Chin J Diabetes Mell. 2018;10(1):4–67. [Google Scholar]
- 3.He X., Chen L., Wang K., Wu H., Wu J. Insulin adherence and persistence among Chinese patients with type 2 diabetes: a retrospective database analysis. Patient Prefer Adher. 2017;11:237–245. doi: 10.2147/PPA.S123389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji L., Feng B., Qing S.U. The safety and efficacy of initiating insulin therapy in Chinese patients with type 2 diabetes mellitus inadequately controlled with previous oral antidiabetic drugs. Chin J Diab. 2011;19(10):746–751. [Google Scholar]
- 5.Ji L., Zhang P., Zhu D. Observational Registry of Basal Insulin Treatment (ORBIT) in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic drugs: Real-life use of basal insulin in China. Diab Obes Metab. 2017;19(6):822–830. doi: 10.1111/dom.12886. [DOI] [PubMed] [Google Scholar]
- 6.Ji L., Su Q., Feng B. Glycemic control and self-monitoring of blood glucose in Chinese patients with type 2 diabetes on insulin: baseline results from the COMPASS study. Diab Res Clin Pract. 2016;12:82–87. doi: 10.1016/j.diabres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Frid A.H., Hirsch L.J., Menchior A.R., Morel D.R., Strauss K.W. Worldwide injection technique questionnaire study: injecting complications and the role of the professional. Mayo Clin Proc. 2016;91(9):1224–1230. doi: 10.1016/j.mayocp.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Nam S., Chesla C., Stotts N.A., Kroon L., Janson S.L. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diab Care. 2010;33(8):1747–1749. doi: 10.2337/dc10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engwerda E.E., Tack C.J., de Galan B.E. Needle-free jet injection of rapid-acting insulin improves early postprandial glucose control in patients with diabetes. Diab Care. 2013;36(11):3436–3441. doi: 10.2337/dc13-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engwerda E.E.C., Tack C.J., de Galan B.E. Pharmacokinetic and pharmacodynamic variability of insulin when administered by jet injection. J Diabetes Sci Technol. 2017;11(5):947–952. doi: 10.1177/1932296817699638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji L., Chen L., Wang Y., Ma Z., Ran X., Sun Z. Study protocol for a prospective, multicenter, randomized, open-label, parallel-group clinical trial comparing the efficacy and safety of a needle-free insulin injector and a conventional insulin pen in controlling blood glucose concentrations in Chinese patients with type 2 diabetes mellitus (the FREE Study) Adv Ther. 2019;36(6):1485–1496. doi: 10.1007/s12325-019-00951-4. [DOI] [PubMed] [Google Scholar]
- 12.Hu J., Shi H., Zhao C. Lispro administered by the QS-M Needle-Free Jet Injector generates an earlier insulin exposure. Expert Opin Drug Deliv. 2016;13(9):1203–1207. doi: 10.1080/17425247.2016.1198772. [DOI] [PubMed] [Google Scholar]
- 13.Cui X., You L., Zhu L. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism. 2018;78:95–105. doi: 10.1016/j.metabol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 14.de Wit H.M., Engwerda E.E., Tack C.J., de Galan B.E. Insulin administered by needle-free jet injection corrects marked hyperglycaemia faster in overweight or obese patients with diabetes. Diab Obes Metab. 2015;17(11):1093–1099. doi: 10.1111/dom.12550. [DOI] [PubMed] [Google Scholar]
- 15.Heller S., Bode B., Kozlovski P., Svendsen A.L. Meta-analysis of insulin aspart versus regular human insulin used in a basal-bolus regimen for the treatment of diabetes mellitus. J Diabetes. 2013;5(4):482–491. doi: 10.1111/1753-0407.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji L., Sun Z., Li Q. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diab Technol Ther. 2017;19(1):61–67. doi: 10.1089/dia.2016.0334. [DOI] [PubMed] [Google Scholar]
- 17.Baruah M.P., Kalra S., Bose S., Deka J. An audit of insulin usage and insulin injection practices in a large indian cohort. Indian J Endocrinol Metab. 2017;21(3):443–452. doi: 10.4103/ijem.IJEM_548_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wit H.M., Te Groen M., Rovers M.M., Tack C.J. The placebo response of injectable GLP-1 receptor agonists vs. oral DPP-4 inhibitors and SGLT-2 inhibitors: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(1):301–314. doi: 10.1111/bcp.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji L.N., Lu J.M., Guo X.H. Glycemic control among patients in China with type 2 diabetes mellitus receiving oral drugs or injectables. BMC Public Health. 2013;13:602. doi: 10.1186/1471-2458-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.